Abstract
Objective
To assess the comparative effectiveness of exercise, antidepressants and their combination for alleviating depressive symptoms in adults with non-severe depression.
Design
Systematic review and network meta-analysis.
Data sources
Embase, MEDLINE, PsycINFO, Cochrane Library, Web of Science, Scopus and SportDiscus.
Eligibility criteria
Randomised controlled trials (1990–present) that examined the effectiveness of an exercise, antidepressant or combination intervention against either treatment alone or a control/placebo condition in adults with non-severe depression.
Study selection and analysis
Risk of bias, indirectness and the overall confidence in the network were assessed by two independent investigators. A frequentist network meta-analysis was performed to examine postintervention differences in depressive symptom severity between groups. Intervention drop-out was assessed as a measure of treatment acceptability.
Results
Twenty-one randomised controlled trials (n=2551) with 25 comparisons were included in the network. There were no differences in treatment effectiveness among the three main interventions (exercise vs antidepressants: standardised mean differences, SMD, −0.12; 95% CI −0.33 to 0.10, combination versus exercise: SMD, 0.00; 95% CI −0.33 to 0.33, combination vs antidepressants: SMD, −0.12; 95% CI −0.40 to 0.16), although all treatments were more beneficial than controls. Exercise interventions had higher drop-out rates than antidepressant interventions (risk ratio 1.31; 95% CI 1.09 to 1.57). Heterogeneity in the network was moderate (τ2=0.03; I2=46%).
Conclusions
The results suggest no difference between exercise and pharmacological interventions in reducing depressive symptoms in adults with non-severe depression. These findings support the adoption of exercise as an alternative or adjuvant treatment for non-severe depression in adults.
Systematic review registration
PROSPERO CRD4202122656.
Keywords: Sports medicine
WHAT ARE THE FINDINGS?
Exercise alleviates symptoms of depression to a similar extent as antidepressant treatments alone or in combination with exercise.
The drop-out rates of exercise studies was higher than that of antidepressant studies.
HOW MIGHT IT IMPACT ON CLINICAL PRACTICE IN THE FUTURE?
These results suggest that exercise may be used as an alternative treatment approach for the management of non-severe depression in adults.
This study adds to the body of evidence for the benefits of exercise in managing depression and will inform future mental health treatment guidelines regarding the protective role of exercise for non-severe depression.
Introduction
Depression is a leading cause of disability worldwide and is estimated to affect over 320 million people.1 The burden of depression negatively affects role functioning and quality of life,2 and is estimated to cost over US$920 billion due to lost productivity alone.3 Depression has a lifetime prevalence of up to 19% and is highly associated with the onset of other somatic and psychiatric disorders.4 5
Currently, second-generation antidepressant medications are one of the first-line treatments for depression.6 However, the evidence on their effectiveness remains controversial because the immediate and short-term benefits may be small and the long-term balance between benefit and harm is poorly understood.7 Individual-level meta-analyses have found a direct relationship between the magnitude of depressive symptoms and the effectiveness of antidepressants.8–10 Thus, the benefits of antidepressants in non-severe depression have been argued to be minimal.8 11 This is concerning considering that most patients with depression report symptoms below the threshold for severe depression.12 13 In addition, high costs, fear of addiction and possible adverse effects limit the applicability of antidepressants in some real-life settings.14 Reluctance to use antidepressants may also be found in patients with non-severe symptoms due to low perceived need or effectiveness of the medication and/or social stigmatisation.15 Although there is evidence that antidepressants have some beneficial effects on milder forms of depression compared with placebo,16 concerns over the risk-to-benefit ratio and the availability of alternative treatments raise questions about the appropriateness of pharmacological treatments for non-severe depression.
Recently, lifestyle interventional strategies incorporating diet, sleep and physical activity have been recognised as protective treatments for depression.17 18 Specifically, the use of exercise as an alternative treatment for non-severe depression is endorsed by several treatment guidelines (EPA in Europe, CANMAT in Canada, NICE in the UK and RANZCP in Australia).19–22 In contrast, the clinical practice guidelines provided by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) support exercise therapy only when antidepressants or psychotherapy treatments are ineffective or unacceptable.6 Moreover, the DSM-5 guidelines state that there is a lack of evidence to recommend exercise as an official treatment. This contradicts the report by the European Psychiatric Association that states that there is sufficient data supporting exercise for the management of mild-to-moderate depression.19 The contrasting statements conveyed by international treatment guidelines preclude drawing definitive conclusions regarding the role of exercise as a treatment for non-severe depression.
Comparing the effects of exercise and antidepressants is essential to elucidate whether exercise is a suitable non-pharmacological treatment approach to manage non-severe depression, and to inform current international treatment guidelines about the protective role of exercise in depression. We therefore conducted a systematic review and network meta-analysis to determine the comparative effectiveness of exercise and antidepressants on depressive symptoms in adults with non-severe depression. In addition, we examined the effect of combination treatment versus either treatment alone to explore the potential synergistic action of exercise and antidepressants. We also set to compare the drop-out rates of participants among interventions as a measure of treatment acceptance.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension guidelines for network meta-analyses23 and was registered in PROSPERO (identifier CRD42021226561).
Search strategy and selection criteria
We searched seven electronic databases (Embase, MEDLINE (PubMed), PsycINFO, Cochrane Library, Web of Science, Scopus and SportDiscus) from January 1990 to January 2022 for relevant articles published in any language. A detailed description of the search strategy is provided in online supplemental eappendix 1. We handsearched references of previous meta-analyses and articles of interest to identify further eligible studies. Two independent researchers performed the search using pre-established criteria. In case of disagreement, a third author was consulted, and the disagreement was resolved by consensus.
bjsports-2022-105964supp001.pdf (1.3MB, pdf)
We included randomised controlled trials investigating the effect of (1) exercise versus antidepressants, (2) either exercise or antidepressants versus a control condition and (3) exercise combined with antidepressants versus either treatment alone, during an initial treatment attempt in adults with non-severe depression. Non-severe depression was defined as a diagnosis of major depressive disorder with mild-to-moderate symptoms. We included studies that recruited participants with a clinical diagnosis of depression assessed using standard diagnostic criteria and determined to have mild-to-moderate symptoms using a psychiatric interview or the cut-off score of a validated rating scale. If studies did not specify the severity of depression for recruitment, they were included if (1) mild symptoms were reported as minimum inclusion criteria and (2) mean baseline depression scores were of moderate severity or lower.
We defined exercise according to the American College of Sports Medicine guideline as ‘planned, structured and repetitive bodily movement aimed to improve and/or maintain one or more components of physical fitness’.24 Studies were excluded if they involved mind-body practices such as yoga or Tai Chi, as these comprise a number of behavioural techniques that might confound the effect of physical exercise. Studies on antidepressants were included if they assessed the effectiveness of a second-generation antidepressant that was approved by the Food and Drug Administration (FDA) and that was administered in doses within the standard therapeutic range.25 To ensure homogeneity among participant and trial characteristics, studies where all participants had treatment-resistant depression or a primary comorbidity, as well as studies where exercise or antidepressants were added to another treatment (eg, psychotherapy), were excluded. Studies with the intervention lasting less than 4 weeks were also excluded.
Outcomes
The primary outcome was depressive symptoms severity, defined as the score on a depression scale at the primary endpoint. When multiple scales were used, we applied a hierarchical protocol based on the most frequently employed scale. The secondary outcome was treatment acceptability, defined as the number of participants who withdrew from the study before the end of the intervention. We used overall drop-out as a measure of acceptability, as this encompasses all possible reasons for discontinuation, including tolerance and satisfaction to the intervention.
Data extraction
Two authors independently extracted sample sizes, postintervention mean scores of depressive symptoms, SD and number of drop-outs in each study. Disagreements were resolved by consensus. When SD were missing, we calculated them using previously validated methods.26 27 If SD could not be computed from the available data and the authors were unreachable, these were imputed. Sensitivity analyses were performed for studies where SD were imputed. If dichotomous data for the number of drop-outs was not clearly reported, we computed the drop-outs based on the difference between participants randomised at baseline and those who completed the intervention. If data were missing, the authors were directly contacted to request additional information. If the authors were unresponsive or unreachable the study was excluded.
We extracted information on participants (ie, mean age, sex and baseline symptoms severity) and trial characteristics (ie, first author, publication date, type of intervention, type of control, outcome assessment and intervention duration) using a data extraction form embedded in an Excel spreadsheet.
We assessed risk of bias using the Cochrane risk of bias assessment tool (RoB-2).28 We used the Confidence in Network Meta-Analysis (CINeMA) model to assess the confidence of the entire network.29 Additional information regarding RoB-2 and CINeMA can be found in online supplemental eappendix 2.
Data analysis
We conducted a network meta-analysis with a frequentist framework using the netmeta package in the statistical software R (V.4.0.3). We designed a network including (1) exercise, (2) antidepressants and (3) exercise plus antidepressants as direct comparisons. If studies included control interventions, these were grouped together and added as a further comparison. We performed random effects pairwise meta-analyses with the Hartung-Knapp-Sidik-Jonkman method30 for direct comparisons to estimate standardised mean differences (SMD) from continuous outcomes and risk ratios (RR) from dichotomous outcomes. Indirect evidence was assessed using the whole network. The random effects netmeta model was used to control for the effect of multiarm trials.
Results from the primary outcome (depressive symptoms severity) were expressed as SMD, and results from the secondary outcome (acceptability) were expressed as RR. The 95% CI were provided whenever possible.
We used the Cochran’s Q statistic to determine the pairwise between-study heterogeneity. In addition, τ2 was calculated to determine the level of variance between studies. We used I2 to evaluate the percentage of variance caused by between-study heterogeneity. It was assumed that heterogeneity was common across the entire network.
We assessed the transitivity in the network by a visual inspection of study characteristics: mode participant age (≥60 or <60), mode proportion of women (≥50% or <50%), mean intervention duration and mode depression scale used.31 32 We performed meta-regression analyses within comparisons to assess the potential influence of the study characteristics on the effect sizes.31 We measured inconsistency in the network with local and global approaches using netsplit and decomp.design functions, respectively. The former was used to assess inconsistency between direct and indirect evidence within each comparison; the latter was used to assess inconsistency between comparisons. We presumed that every participant fitting our eligibility criteria could potentially be randomised to any of the treatments compared. We used the P-score proposed by Rücker and Schwarzer to rank the treatments within the network.33
Owing to an insufficient number of studies in each comparison group, subgroup and meta-regression analyses, as well as the assessment of publication bias, could not be performed to explore potential sources of heterogeneity across the network. Sensitivity analyses were performed by excluding studies with high indirectness and risk of bias, studies with participants older than 60, studies with interventions longer than 12 weeks and studies that used an attention/active or passive control comparison.
Results
The literature search identified 23 209 potential studies. After exclusion of studies by title and abstract, 329 full-text records were screened and 21 were included in the main analysis (online supplemental material efigure 1). All studies were written in English.
Study characteristics
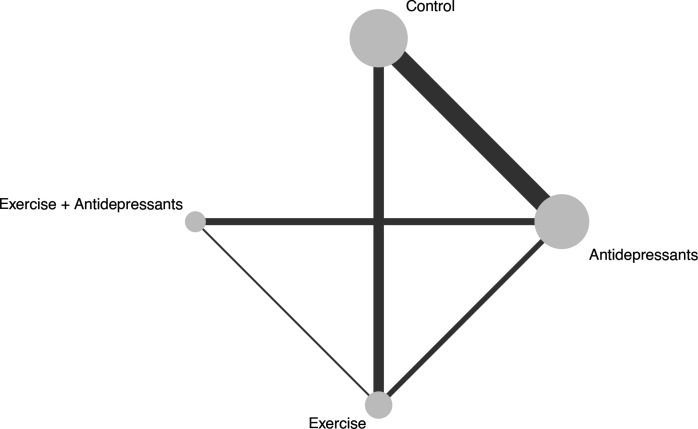
Overall, 2551 participants in 25 pairwise comparisons were assigned to one of the three treatments and control groups (figure 1). The comparison studies included: antidepressants versus controls (n=11), exercise versus controls (n=6), combined treatments versus antidepressants (n=4) and combined treatments versus exercise (n=1). Three studies provided direct evidence on the comparative effects of antidepressants and exercise. Control interventions included placebo (n=11), attention control (n=2), stretching (n=2), no intervention (n=1) and wait-list (n=1). We visually assessed the distribution of study characteristics across direct comparisons (table 1, online supplemental material etable 1). Participant age, the proportion of women and the outcome measure used were balanced across comparisons. The duration of the intervention differed among comparisons, with the exercise versus antidepressants comparison having the mean longest intervention (19 weeks), and the antidepressants versus control comparison having the shortest (9 weeks). Nonetheless, meta-regression analyses suggest that none of the study characteristics influenced the treatment effect (online supplemental material etable 2). Overall, we considered the assumption of transitivity to be valid.
Figure 1.
Geometry of the network. The size of the node represents the number of participants in each intervention. The thickness of the edges represents the number of studies in each treatment comparison.
Table 1.
Characteristics of the included studies
| Study | Mean age (SD) | Gender (M/F) | Baseline score (SD) | Depression scale | Intervention | Duration (weeks) |
| Bjerkenstedt, 2005* | 50 (12) | 23M 86F | 24.5 (4) | HAM-D | Fluoxetine Placebo |
4 |
| Blumenthal et al. 1999† ‡ § | 57 (7) | 43M 113F | 17.9 (7.2) | HAM-D | Exercise Sertraline Aerobic exercise + sertraline |
16 |
| Blumenthal et al. 2007* ¶ † | 52 (8) | 35M 114F | 17 (4) | HAM-D | Exercise Sertraline Placebo |
16 |
| Danielsson, 2014‡ | 46 (14) | 10M 32F | 24 (4.6) | MADRS | Exercise + antidepressants Antidepressants + advice | 10 |
| Detke, 2002* | 41 (14) | 83M 184F | 20.4 (3.4) | HAM-D | Duloxetine Placebo |
9 |
| Detke, 2004* | 45 (12) | 47M 139F | 20.1 (3.5) | HAM-D | Duloxetine Placebo |
8 |
| Dunn, 2005¶ | 34 (7) | 9M 21F | 19.8 (2.1) | HAM-D | Exercise Stretching |
12 |
| Fava, 2005* | 37 (11) | 37M 53F | 19.7 (3.2) | HAM-D | Fluoxetine Placebo |
12 |
| Gastpar, 2006* | 49 (12) | 80M 177F | 21.9 (1.2) | HAM-D | Citalopram Placebo |
6 |
| Goldstein, 2004* | 41 (13) | 67M 113F | 17.6 (4.9) | HAM-D | Duloxetine Placebo |
8 |
| Hemat-Far, 2012¶ | 18–25 | 20F | 24.4 (5) | BDI | Exercise No-treatment |
8 |
| Hidalgo et al. 2021† | > 65 | 67M 246F | 15.5 (4.3) | MADRS | Exercise Antidepressants |
24 |
| Krogh, 2012¶ | 42 (11) | 38M 77F | 18.9 (4.4) | HAM-D | Exercise Stretching |
12 |
| Mao, 2015* | 44 (15) | 19M 18F | 15 (3) | HAM-D | Sertraline Placebo |
12 |
| Mather, 2002‡ | 65 (NA) | 27M 59F | 17.2 (6.5) | HAM-D | Exercise + antidepressants Antidepressants + attention control | 10 |
| McNeil, 1991¶ | NA | 20 M/F | 15.9 (2.8) | BDI | Exercise Wait-list |
6 |
| Moreno, 2006* | 41 (11) | 8M 38F | 16.1 (4.8) | HAM-D | Fluoxetine Placebo |
8 |
| Perahia, 2006* | 45 (11) | 60 142F | 21 (4.1) | HAM-D | Duloxetine Placebo |
8 |
| Philipp, 1999* | 46 (12) | 40M 117F | 22.5 (4.1) | HAM-D | Imipramine Placebo |
8 |
| Sadeghi, 2016¶ | 21 (1) | 24M 6F | 22.9 (4.2) | BDI-II | Exercise Attention control |
8 |
| Siqueira, 2016‡ | 39 (11) | 16M 41F | 19.8 (3.1) | HAM-D | Exercise + sertraline Sertraline |
4 |
*Included in the antidepressants versus control pairwise comparison.
†Included in the exercise versus antidepressants comparison.
‡Included in the exercise plus antidepressants versus antidepressants comparison.
§Included in the exercise plus antidepressants versus exercise comparison.
¶Included in the exercise versus control pairwise comparison.
BDI, Beck Depression Inventory; HAM-D, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; NA, not applicable.
Risk of bias was determined to be low in 5 studies, moderate in 15 studies and high in 1 study (online supplemental material etable 3). Most of the network evidence relied on moderate risk of bias and low-moderate indirectness (online supplemental material efigure 2, 3 and online supplemental material etable 4). The confidence in the network was moderate-to-high for all comparisons of interest (online supplemental material etable 5).
Depressive symptoms severity
At the end of the interventions, exercise (SMD, −0.45; 95% CI −0.67 to −0.23), antidepressants (SMD, −0.33; 95% CI −0.48 to −0.19) and combined treatments (SMD, −0.45; 95% CI −0.76 to −0.14) were superior in reducing depressive symptoms compared with controls (table 2). There were no differences among the main treatments. Exercise had a similar beneficial effect to that of antidepressants (SMD, −0.12; 95% CI −0.33 to 0.10), and the effect of combined treatments was similar to the effect of exercise (SMD, 0.00; 95% CI −0.33 to 0.33) and antidepressants (SMD, −0.12; 95% CI −0.40 to 0.16) alone. The ranking of treatments based on the P-score is reported in online supplemental material etable 6.
Table 2.
Results on the comparative effectiveness of the interventions from the network and pairwise meta-analyses
| Combination | 0.18 (−0.20 to 0.55) (N=1; I2=NA*) |
−0.13 (−0.63 to 0.36) (N=4, I2=41%) |
NA† |
| −0.00 (−0.33 to 0.33) (N=21; I2=46%) |
Exercise | −0.08 (−0.29 to 0.12) (N=3; I2=0%) |
−0.58 (−1.14 to −0.01) (N=6; I2=69%) |
| −0.12 (−0.40 to 0.16) (N=21; I2=46%) |
−0.12 (−0.33 to 0.10) (N=21; I2=46%) |
Antidepressants | −0.33 (−0.49 to −0.16) (N=11; I2=38%) |
| −0.45 (−0.76 to −0.14) (N=21; I2=46%) |
−0.45 (−0.67 to −0.23) (N=21; I2=46%) |
−0.33 (−0.48 to −0.19) (N=21; I2=46%) |
Control |
Results of the network meta-analyses are presented in grey and results of the pairwise meta-analyses are presented in white. Estimates are displayed as column versus row for the network meta-analyses and row versus column for the pairwise meta-analyses. Results are expressed as standardised mean differences (SMD). A negative SMD indicates a superiority of the first treatment over the comparison treatment.
*No evidence on I2 is available as there was only one study for that comparison.
†No studies compared combination treatment versus no treatment.
N, number of studies in the comparison; NA, not available.
The network meta-analysis showed that there was moderate heterogeneity (τ2=0.03; I2=46.2%), which was mostly caused by the comparison of exercise versus control (Q=15.80; df=4; p=0.003). All other comparisons showed no evidence of heterogeneity. Inconsistency was assessed both within and between comparisons. There was no evidence of inconsistency within any of the comparisons of interest (online supplemental material etable 7). Similarly, no inconsistency between comparisons was observed (Q=5.62, df=5, p=0.34).
The pairwise meta-analysis supported the findings of the network meta-analysis, with both exercise (SMD, −0.58; 95% CI −1.14 to −0.01) and antidepressants (SMD, −0.33; 95% CI −0.49 to −0.16) showing greater improvements over the controls. The comparison of exercise and antidepressants showed no evidence of the superiority of one treatment over the other (SMD, −0.08; 95% CI −0.29 to 0.12). Similarly, there was no evidence of the superiority of combined treatments over exercise (SMD, 0.18; 95% CI −0.20 to 0.55) and antidepressants (SMD, −0.13; 95% CI −0.63 to 0.36) alone (online supplemental eappendix 3).
Sensitivity analyses confirmed the robustness of the results (online supplemental material etable 8).
Acceptability
Four studies were excluded from the secondary outcome analyses as they failed to report the drop-out rate in the intervention groups and data could not be extracted from the text or there were no drop-outs in any of the interventions of interest. Drop-out rates in the exercise group were greater than in the antidepressant group (RR 1.31; 95% CI 1.09 to 1.57) and control group (RR 1.29; 95% CI 1.03 to 1.61) across all studies (table 3). No other differences were observed among the interventions.
Table 3.
Results on the comparative acceptability of the interventions from the network and pairwise meta-analyses
| Combination | 0.76 (0.38 to 1.52) (N=1; I2=NA*) |
1.03 (0.40 to 2.65) (N=3, I2=0%) |
NA† |
| 0.75 (0.47 to 1.21) (N=17; I2=0%) |
Exercise | 1.40 (1.13 to 1.72) (N=3; I2=0%) |
0.84 (0.27 to 2.58) (N=3; I2=14%) |
| 0.99 (0.62 to 1.57) (N=17; I2=0%) |
1.31 (1.09 to 1.57) (N=17; I2=0%) |
Antidepressants | 0.97 (0.84 to 1.13) (N=11; I2=0%) |
| 0.97 (0.60 to 1.58) (N=17; I2=0%) |
1.29 (1.03 to 1.61) (N=17; I2=0%) |
0.98 (0.84 to 1.15) (N=17; I2=0%) |
Control |
Results of the network meta-analyses are presented in grey and results of the pairwise meta-analyses are presented in white. Estimates are displayed as column versus row for the network meta-analyses and row versus column for the pairwise meta-analyses. Results are expressed as risk ratios (RRs). RRs that are smaller than one indicate a superiority of the first treatment over the comparison treatment.
*No evidence on I2 is available as there was only one study for that comparison.
†No studies compared combination treatment versus no treatment.
N, number of studies in the comparison; NA, not available.
The pairwise meta-analyses were in line with the results of the network analysis, as the drop-out rates were higher in the exercise group than in the antidepressant group (RR 1.40; 95% CI 1.13 to 1.72). There was no evidence of heterogeneity among pairwise comparisons (table 3, online supplemental eappendix 3).
Discussion
This is the first network meta-analysis to comparatively assess the effectiveness of exercise, antidepressants and combined treatments on depressive symptoms in adults with non-severe depression. Results showed that all treatments had similar beneficial effects on depressive symptoms when compared with the controls, but no treatment was superior to another. Assessment of acceptability showed that antidepressant treatments induced fewer intervention drop-outs than exercise.
Our findings align with the recommendations provided by European, Canadian, Australian and UK treatment guidelines supporting the use of exercise as an alternative treatment for non-severe depression.19–22 These guidelines recommend exercise programmes consisting of 30–60 min sessions at moderate intensity performed 2–3 times weekly for 9–12 weeks, and delivered in groups by a competent practitioner. This is in contrast with the DSM-5 guidelines, which only suggest exercise therapy to people who are unresponsive to antidepressant or psychotherapy treatment, but not as a first-line option.6 Importantly, they do not categorise depression by severity of symptoms, but rather offer treatment advice based on several reviews that met quality criteria. All the included reviews used in their assessment, except one, exclusively focused on psychotherapy or pharmacotherapy treatments. Gartlehner et al 34 compared pharmacological versus non-pharmacological treatments, but only direct evidence was analysed. This resulted in the inclusion of two exercise studies and with the conclusion that there was insufficient evidence to promote exercise as a depression treatment. In this study, we gathered direct and indirect evidence and found no differences in treatment effectiveness between exercise and antidepressants.
Combination treatment did not demonstrate greater beneficial effects on depressive symptoms compared with either treatment alone, probably due to the limited number of studies included in our analyses. The effects of the combination of exercise and pharmacotherapy on depression remains largely unclear. Although some studies have attempted to explain the possible synergism between the two interventions,35 evidence of their combined effectiveness against pharmacological treatment alone is still inconclusive.36 37 Our findings do not support the synergistic effects of exercise and antidepressants; however, considering the various health benefits of physical exercise, using exercise as an adjunctive treatment to pharmacotherapy may counterbalance the side effects that are often associated with antidepressant use and promote a faster recovery.
Exercise interventions induced greater drop-outs than antidepressant treatments, although only one study reported a substantial difference in drop-out rates between the two interventions. In this study, 58% and 40% of participants withdrew from the exercise and antidepressant groups, respectively.38 This is considerably higher than what reported in the two other trials directly comparing exercise and antidepressants, where drop-out rates ranged from 14% to 26%.39 40 Despite the greater drop-out rates in the exercise group, the proportion of participants with adverse events was greater in the antidepressant group, with 22% reporting adverse events compared with 9% in the exercise group. In our study, we used overall drop-out as a measure of treatment acceptability. It is possible that an analysis of drop-out due to adverse events alone would have led to different results. Clearly, both interventions have limitations in securing treatment adherence. Exercise is physically demanding and harder to implement in comparison to standard pharmacological treatments. On the other hand, antidepressant treatments are associated with greater adverse effects, higher costs and social stigma.14 Although both interventions can effectively alleviate depressive symptoms, different strategies must be adopted to enhance treatment adherence in depressed individuals. Further research needs to address this topic, possibly differentiating between treatment satisfaction, adverse events and overall study withdrawal.
In the current review, evidence from studies that compared exercise and/or antidepressants to each other or to a control comparison was gathered. To our own surprise, only 6 and 11 studies comparing exercise and antidepressants to control were found, respectively. Several exercise studies were excluded because they did not include participants with a clinical diagnosis of depression, or because some but not all of participants randomised were taking antidepressants, thereby confounding the true effect of exercise. Most of the antidepressant studies were excluded because participants reported levels of depression that are indicative of moderate-to-severe depression. This was also reflected in a comprehensive network meta-analysis by Cipriani et al,41 who analysed over 500 antidepressant studies and found that 89% only recruited participants with moderate-to-severe symptoms (mean Hamilton Rating Scale score of 25.7). Compared with Cipriani et al, our study had tighter inclusion criteria to ensure that the transitivity assumption was not violated. This led to the exclusion of several antidepressant studies in mild-to-moderate depression. Overall, there is an imbalance between the proportion of studies assessing treatment effectiveness in mild-to-moderate depression and those focusing on severe depression. This imbalance has been suggested to be partly caused by the inclusion criteria used for FDA-funded trials, where higher cut-off scores are imposed at baseline to increase the sensitivity of the antidepressant versus placebo comparison.8 We hope that the current study will contribute to highlight the clinical importance of non-severe depression, and that more clinical trials on non-severe depression can be conducted in the future.
Limitations
There were some limitations in this study. First, the overall low number of studies available for each comparison precluded us to explore potential sources of heterogeneity and publication bias, and may have generated inaccurate estimates of between-study heterogeneity. This limited the interpretation of our findings, which need to be corroborated by further research. Second, the control groups in all comparisons were combined into a single node. While all antidepressant studies used a placebo comparison, exercise studies used various types of control. This might have contributed to the heterogeneity detected in the exercise-control comparison. Although our sensitivity analyses found no considerable changes in study effects after excluding studies with an active or passive control comparison, the effect of exercise might have been overestimated and needs to be validated by additional high-quality studies. Similarly, we combined exercise, antidepressant and combination treatments into their respective nodes without accounting for differences within interventions. This method was chosen because previous network meta-analyses showed little heterogeneity among antidepressant41 and exercise42 interventions. Yet, antidepressant studies can vary by type, dose and duration of antidepressant used, whereas exercise studies can vary by type, frequency, intensity and duration of the training sessions. These differences might have affected the overall heterogeneity of the network. Third, this study examined the comparative effectiveness of exercise and antidepressants in mild-to-moderate depression, but did not explore the potential benefits of exercise in individuals with more severe symptoms. This limited the interpretation of our findings, which cannot be extended to all depressed patients.
Conclusion
The meta-analytical evidence gathered through direct and indirect comparisons found no differences in treatment effectiveness between exercise, antidepressants and their combination. These findings support the use of exercise interventions as an alternative treatment option for non-severe depression. Results were corroborated through stringent sensitivity analyses that accounted for the quality of studies as well as types of participants and interventions. Further trials directly comparing the individual and synergistic action of exercise and antidepressants are warranted to corroborate the findings of this study.
Footnotes
Correction notice: This article has been corrected since it published Online First. The footnotes in tables 2 and 3 have been amended.
Contributors: All authors had full access to the data in the study and agreed to the decision to submit for publication. FR, CL and PS accessed and verified the data in this study. FR, CL and EC conducted the database search and data extraction. FR, DM and CPWC conducted the data analysis. All authors interpreted the data and wrote and edited the manuscript.
Funding: This work was supported by Health and Medical Research Fund (17182461) of Food and Health Bureau, Hong Kong SAR Government and the Seed Fund for Basic Research of The University of Hong Kong.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. World Health Organization . Depression and other common mental disorders: global health estimates. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Kessler RC. The costs of depression. Psychiatr Clin North Am 2012;35:1–14. 10.1016/j.psc.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chisholm D, Sweeny K, Sheehan P, et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry 2016;3:415–24. 10.1016/S2215-0366(16)30024-4 [DOI] [PubMed] [Google Scholar]
- 4. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 2018;75:336–46. 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996;276:293–9. 10.1001/jama.1996.03540040037030 [DOI] [PubMed] [Google Scholar]
- 6. American Psychological Association . Clinical practice guideline for the treatment of depression across three age cohorts. Guideline development panel for the treatment of depressive disorders, 2019. Available: https://www.apa.org/depression-guideline/guideline.pdf
- 7. Ioannidis JPA. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos Ethics Humanit Med 2008;3:14. 10.1186/1747-5341-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010;303:47–53. 10.1001/jama.2009.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med 2008;5:e45. 10.1371/journal.pmed.0050045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan A, Leventhal RM, Khan SR, et al. Severity of depression and response to antidepressants and placebo: an analysis of the food and drug administration database. J Clin Psychopharmacol 2002;22:40–5. 10.1097/00004714-200202000-00007 [DOI] [PubMed] [Google Scholar]
- 11. Jakobsen JC, Gluud C, Kirsch I. Should antidepressants be used for major depressive disorder? BMJ Evid Based Med 2020;25:130. 10.1136/bmjebm-2019-111238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shim RS, Baltrus P, Ye J, et al. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National health and nutrition examination survey (NHANES), 2005-2008. J Am Board Fam Med 2011;24:33–8. 10.3122/jabfm.2011.01.100121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zimmerman M, Posternak MA, Chelminski I. Symptom severity and exclusion from antidepressant efficacy trials. J Clin Psychopharmacol 2002;22:610–4. 10.1097/00004714-200212000-00011 [DOI] [PubMed] [Google Scholar]
- 14. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci 2012;9:41–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Andrade LH, Alonso J, Mneimneh Z, et al. Barriers to mental health treatment: results from the who world mental health surveys. Psychol Med 2014;44:1–15. 10.1017/S0033291713001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cipriani A, Barbui C, Butler R, et al. Depression in adults: drug and physical treatments. BMJ Clin Evid 2011;2011:1003. [PMC free article] [PubMed] [Google Scholar]
- 17. Opie RS, Jacka FN, Marx W, et al. Designing lifestyle interventions for common mental disorders: what can we learn from diabetes prevention programs? Nutrients 2021;13. doi: 10.3390/nu13113766. [Epub ahead of print: 25 Oct 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarris J, O'Neil A, Coulson CE, et al. Lifestyle medicine for depression. BMC Psychiatry 2014;14:107. 10.1186/1471-244X-14-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stubbs B, Vancampfort D, Hallgren M, et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and position statement from the European psychiatric association (EPA), supported by the International organization of physical therapists in mental health (IOPTMH). Eur Psychiatry 2018;54:124–44. 10.1016/j.eurpsy.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 20. Ravindran AV, Balneaves LG, Faulkner G, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. complementary and alternative medicine treatments. Can J Psychiatry 2016;61:576–87. 10.1177/0706743716660290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 2015;49:1087–206. 10.1177/0004867415617657 [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Clinical Excellence . Depression in adults: treatment and management NICE; 2022. [PubMed] [Google Scholar]
- 23. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 24. American College of Sports Medicine . ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Gartlehner GHR, Reichenpfader U, Kaminski A. Drug class review: second-generation antidepressants. update 5; 2011. [PubMed]
- 26. Higgins JPT TJ, Chandler J, Cumpston M, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020), 2020. [Google Scholar]
- 27. Furukawa TA, Barbui C, Cipriani A, et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7–10. 10.1016/j.jclinepi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 28. Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 29. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. Cinema: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17:e1003082. 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. IntHout J, Ioannidis JPA, Borm GF. The hartung-knapp-sidik-jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ho EK-Y, Chen L, Simic M, et al. Psychological interventions for chronic, non-specific low back pain: systematic review with network meta-analysis. BMJ 2022;376:e067718. 10.1136/bmj-2021-067718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta-analysis. BMJ 2021;372:n532. 10.1136/bmj.n532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gartlehner G, Gaynes BN, Amick HR, et al. Comparative benefits and harms of antidepressant, psychological, complementary, and exercise treatments for major depression: an evidence report for a clinical practice guideline from the American College of physicians. Ann Intern Med 2016;164:331–41. 10.7326/M15-1813 [DOI] [PubMed] [Google Scholar]
- 35. Guerrera CS, Furneri G, Grasso M, et al. Antidepressant drugs and physical activity: a possible synergism in the treatment of major depression? Front Psychol 2020;11:857. 10.3389/fpsyg.2020.00857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr 2014;19:496–508. 10.1017/S1092852913000953 [DOI] [PubMed] [Google Scholar]
- 37. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord 2016;202:67–86. 10.1016/j.jad.2016.03.063 [DOI] [PubMed] [Google Scholar]
- 38. Hidalgo JL-T, Sotos JR, DEP-EXERCISE Group . Effectiveness of physical exercise in older adults with mild to moderate depression. Ann Fam Med 2021;19:302–9. 10.1370/afm.2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007;69:587–96. 10.1097/PSY.0b013e318148c19a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med 1999;159:2349–56. 10.1001/archinte.159.19.2349 [DOI] [PubMed] [Google Scholar]
- 41. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet 2018;391:1357–66. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brupbacher G, Gerger H, Zander-Schellenberg T, et al. The effects of exercise on sleep in unipolar depression: a systematic review and network meta-analysis. Sleep Med Rev 2021;59:101452. 10.1016/j.smrv.2021.101452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2022-105964supp001.pdf (1.3MB, pdf)