Abstract
Background
The use of robotics in medicine may enable increased technical accuracy, reduced procedural time and radiation exposure, and remote completion of procedures. We have previously described the first-in-human, robotic-assisted cerebral aneurysm treatment using the CorPath GRX Robotic System. In this report we discuss our early experiences and outcomes using this robotic device for endovascular treatment of intracranial aneurysms using stent-assisted coil embolization and flow diversion.
Methods
The patient and disease characteristics, procedural details, and follow-up imaging and clinical outcomes of consecutive patients undergoing robotically-assisted intracranial aneurysm embolization between November 2019 and February 2020 are presented.
Results
Six patients underwent robotically-assisted embolization of intracranial aneurysms. Four of the patients were treated with a neck-bridging stent (with or without coiling) and two patients were treated with a flow-diverting stent. Two patients were treated in the subacute period of subarachnoid hemorrhage and four patients were treated electively. All of the procedures could be completed robotically and there was no need for unplanned manual intervention. The technical success rate of the procedures was 100%. There was no morbidity or mortality associated with the procedures. One year follow-up imaging showed that four aneurysms were completely obliterated (Raymond-Roy Occlusion Classification (RROC) class I) and the remaining two were occluded with a residual neck (RROC class II).
Conclusions
The Corpath GRX Robotic System demonstrated a precise control over the microcatheter, wire and stent during aneurysm treatment. Robotic neuro-procedures seem to be safe and effective and demonstrate stable occlusion results in the midterm follow-up.
Keywords: aneurysm, brain, coil, stent, technology
Introduction
Despite the 21st century drive to develop ‘intelligent’ robots, the use of robots in medicine today is primarily assistive to human control. Robotic-assisted surgery may enable increased technical accuracy, reduced procedural time and radiation exposure, and remote completion of procedures.1 The first robotic-assisted medical procedure was in 1983 when the ‘Arthrobot’ device was used to position a patient’s limbs during orthopedic surgery. Shortly thereafter, in 1985, the industrial robotic arm PUMA 560 was used to guide a needle under CT guidance into the brain.2 Since then, robotic technology has been used for procedural assistance in an increasing number of neurosurgical and interventional specialties.3–6 The second-generation CorPath platform (Corindus, A Siemens Healthineers Company, Waltham, MA) is currently the only commercially available robotic device for endovascular surgery.7 The CorPath platform has been in use since 2012 for percutaneous coronary intervention and was also approved in the USA for peripheral vascular intervention.8 The system has subsequently undergone a number of engineering and software modifications to facilitate the use of neurovascular microcatheters and longer working lengths necessary for intracranial access and neurointervention.9
We have previously described the first case of in-human, robotic-assisted neuroendovascular intervention.10 In this report we discuss our early experience, clinical outcomes, and imaging follow-up using the CorPath GRX Robotic System for endovascular treatment of intracranial aneurysms using coil embolization, stents and flow diverters.
Methods
This was a retrospective single-institution series. The study was approved by the institutional medical ethics board (REB study ID: 20–5121). A series of adult patients with a cerebral aneurysm were included and treated with robotic-assisted neurointervention at our institution between November 2019 and February 2020. We considered patients with large neck aneurysms eligible for stent and/or stent-assisted coiling that could benefit from the robotic precision for the stent placement step of the procedure. Small aneurysms (<5 mm) and those requiring treatment in less than 48 hours were excluded (to allow for special access approval). The CorPath GRX Robotic System is currently cleared for percutaneous coronary and peripheral vascular interventions in the USA, European Union (EU) and other select countries, and for neuroendovascular intervention in the EU, Australia and New Zealand. As the robotic system is not currently approved for use in Canada, the cases presented here were performed with approval for off-label use under a ‘special access’ from Health Canada. Informed consent was obtained before the procedure for all patients.
Procedural details
All cases were reviewed by a neurovascular team consisting of medical professionals trained in endovascular, microvascular and neuroimaging techniques. The general set-up of the robotic system has been described previously.8 Before using the robot in clinical practice, the treating physician and team spent more than 100 hours familiarizing themselves with the system using simulation. When preparing for cases it was often found useful to rehearse the procedure with a patient-specific three-dimensionally printed model (EVIAS, Biomodex, Paris, France) based on the patient’s CT or three-dimensional rotational angiography data.11
During the procedure an assisting neurointerventionalist and technologist were beside at the operation table and the primary operator was at the mobile, radiation-shielded control cockpit. In all of the procedures in our series, the workstation was situated within the angiography procedure room, but it may also be operated from the control room. The primary neurointerventionalist (VMP) operated the robot using the control console which uses both joysticks and touchscreen controls. In our setup it was not necessary for the robotic operator to wear lead protective clothing. The assisting neurointerventionalist and technologist wore lead but were able to distance themselves from the patient bedside and stand behind further lead shielding, if they so pleased, during the intracranial robotic portion of the procedure. Procedures were performed under general anesthesia with systemic intravenous heparinization. Common femoral or radial artery access was obtained and guide catheters were placed into the parent artery, with or without an intermediate catheter, by the assisting neurointerventionalist. When in position, the articulating robotic arm was moved to bring the drive system and a single-use cassette into position to attach to the microcatheter and microwire to assist the intracranial portion of the procedure. The cassette is the mechanical transmission module that translates the real-time commands issued from the remote physician unit’s joysticks to manipulate the device. This can enable the operator to advance, retract, rotate, and deploy microcatheters, wires, stents, and coils. Automated functions, such as ‘Active Device Fixation’, ‘Rotate on Retract’ and ‘Limited Speed’ were used for precise control of the microwire and microcatheter. For example, ‘Active Device Fixation’ is equivalent to the manual maneuver of pinning the microwire and advancing the microcatheter. It works by retracting the wire an equal travel distance to the forward distance the microcatheter is advanced. At the operating table, a technologist with specialized robotic training and a neurointerventionalist managed the loading and exchange of devices within the robotic system while maintaining communication with the main operator. Devices such as microcatheters, 0.014 inch guidewires, stent systems, and coiling systems were loaded into the appropriate tracks of the cassette, which served as the sterile interface between the robotic system and the patient. With the current generation platform, it is only possible to operate a single wire device (such as microwire or coil) and a single catheter simultaneously. Post-procedure, patients were maintained normotensive and monitored for at least 24 hours after embolization for neurological changes.
Outcomes
The primary outcomes were angiographic aneurysm occlusion as categorised by the Raymond-Roy Occlusion Classification (RROC) and adverse events (class I: complete obliteration; class II: residual neck; class IIIa: contrast opacification within the coil interstices of a residual aneurysm; class IIIb: contrast opacification outside the coil interstices, along the residual aneurysm wall).12 Other data collected included patient demographics, disease characteristics, technical details of treatment, further treatments performed, imaging follow-up and clinical follow-up. Follow-up MR angiography (MRA) examinations were performed using a 1.5 Tesla MR scanner (GE Medical Systems, Milwaukee, WI) with a standard eight channel head coil. The MRA examinations all included a contrast-enhanced MRA using Gadovist (Bayer, Germany) injected at a rate of 1.5 mL/s to a total of 15 mL followed immediately by a flush of 30 mL of saline.13
Results
Patient demographics and procedural details
Patient demographics, clinical presentation, and details of treatment are listed in table 1. Six patients underwent robotic-assisted aneurysm treatment between November 2019 and February 2020. The median age was 64 years (range 63–84 years). The ratio of men to women was 1:5. Two patients (33%) were treated in the subacute period of subarachnoid hemorrhage (during the same admission but not as the first endovascular treatment). The remaining patients had unruptured aneurysms that were incidentally discovered. The mean pre-treatment modified Rankin Scale (mRS) was 1.3. The most common site was the basilar artery (three patients, 50%) followed by the posterior communicating artery (two patients, 33%), and paraophthalmic internal carotid artery segment (one patient, 16.5%). All of the aneurysms were of saccular morphology. The mean size of the treated aneurysms was 8.8 mm (range 6.2–11 mm). Transfemoral access was employed in five cases and transradial in one case. All patients were loaded pre-procedurally with dual antiplatelet therapy. This constituted aspirin (81 mg daily per day) in addition to one of either: ticagrelor (90 mg twice daily), clopidogrel (75 mg daily), or prasugrel (10 mg daily). As a guide catheter, a 6 French (6 F) Neuronmax (Penumbra) was used in four cases, and a 6 F Shuttle (Cook) and a 5 F Sofia (Microvention) were used in one case each. An intermediate catheter was used in five cases: a 5 F Sofia in three cases and 5 F Catalyst in two cases. A Headway-17 (Microvention) and a XT27 (Stryker) microcatheter were used once each. In all other cases, SL-10 (Stryker) microcatheters were used. A 300 cm 0.014 inch Synchro (Stryker) microwire was used in all cases.
Table 1.
Patient demographics, clinical presentation, and details of treatment
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
| Presentation | Symptomatic—vertigo | Incidental | SAH | SAH | Incidental | Incidental |
| Acute versus elective | Elective | Elective | Subacute | Subacute | Elective | Elective |
| Previous treatment? | No | No | Yes | Yes | No | No |
| Aneurysm parent artery | Basilar | Basilar | Pcom | Pcom | Basilar | ICA |
| Aneurysm artery segment | Body (side wall) | Tip | Communicating | Communicating | Tip | Paraophthalmic |
| Morphology | Saccular | Saccular | Saccular | Saccular | Saccular | Saccular |
| Max aneurysm size (mm) | 11.0 | 6.9 | 14.4 | 6.2 | 6.7 | 7.9 |
| Medication regimen | Aspirin/ticagrelor | Aspirin/clopidogrel | Aspirin/ticagrelor | Prasugrel/aspirin | Aspirin/ticagrelor | Aspirin/prasugrel |
| Medication post-procedure for maintenance | Aspirin/ticagrelor | Aspirin/clopidogrel | Aspirin/ticagrelor | Aspirin/prasugrel | Aspirin/ticagrelor | Aspirin/prasugrel |
| Access site | RCFA | RCFA | RRA | RCFA | RCFA | RCFA |
| Guide catheter | Neuronmax | 6 F Shuttle | 5 F Sofia | Neuronmax | Neuronmax | Neuronmax |
| Intermediate catheter | 6 F Sofia | 6 F Sofia | n/a | CAT5 | 6 F Sofia | CAT5 |
| Microcatheter | SL-10 | SL-10 | Headway-17 | SL-10 | SL-10 | XT-27 |
| Microwire | Synchro-14 | Synchro-14 | Synchro-14 | Synchro-14 | Synchro-14 | Synchro-14 |
| Angioplasty required? | No | No | No | No | No | No |
| Stent or flow diverter | 4.5×21 mm Neuroform Atlas | 3×24 mm Neuroform Atlas | 2.75×10 mm Silk Vista Baby | 4.5×21 mm Neuroform Atlas | 4×21 mm Neuroform Atlas | 4.5×20 mm Surpass Evolve |
| Coils deployed? | Yes | Yes | No | No | Yes | No |
| Procedure time | 2 hours 23 min | 2 hours 01 min | 2 hours 46 min | 1 hour 23 min | 2 hours 57 min | 1 hours 58 min |
F, French; ICA, internal carotid artery; Pcom, posterior communicating artery; RCFA, right common femoral artery; RRA, right radial artery; SAH, subarachnoid hemorrhage.
The average procedure time from groin puncture to closure was 134±14 min (range 83–177 min), which included diagnostic angiographic imaging and procedure planning. The robotic intracranial intervention was on average 85±15 min from when the microcatheter was first connected to when it was removed from the system. Four patients were treated with a neck-bridging Neuroform Atlas stent (Stryker). Two patients were treated with flow-diverting stents (one Surpass Evolve and one Silk Vista Baby). Three patients (50%) underwent additional coiling after stent deployment. Table 2 lists outcomes of treatment.
Table 2.
Treatment characteristics and outcomes
| Pt | 30-day complications | Long-term complications | Pre-Tx RROC class | Post-Tx RROC class | mRS pre-Tx | mRS post-Tx | Mortality | Time to imaging f/u (months) | F/u imaging modality | F/u RROC class | Time to clinical f/u (months) | Clinical f/u mRS |
| 1 | Nil | Nil* | n/a | II | 1 | 1 | No | 13 | MRI | I | 15 | 1 |
| 2 | Nil | Nil | n/a | I | 1 | 1 | No | 14 | MRI | I | 8 | 0 |
| 3 | Nil | Nil | III | II | 3 | 3 | No | 12 | MRI | II | 11 | 1 |
| 4 | Nil | Nil | II | II | 1 | 1 | No | 12 | MRI | II | 4 | 1 |
| 5 | Nil | Nil | n/a | I | 1 | 1 | No | 12 | MRI | I | 9 | 1 |
| 6 | Nil | Nil | n/a | III | 1 | 1 | No | 14 | MRI | I | 13 | 0 |
*This patient had a brainstem infarct 2 months after the intervention (related to the aneurysm occlusion but unrelated to the robotic intervention) of which they fully recovered.
f/u, follow up; mRS, modified Rankin score; Pt, patient; RROC, Raymond Roy occlusion classification; Tx, treatment.
Treatment efficacy and adverse events
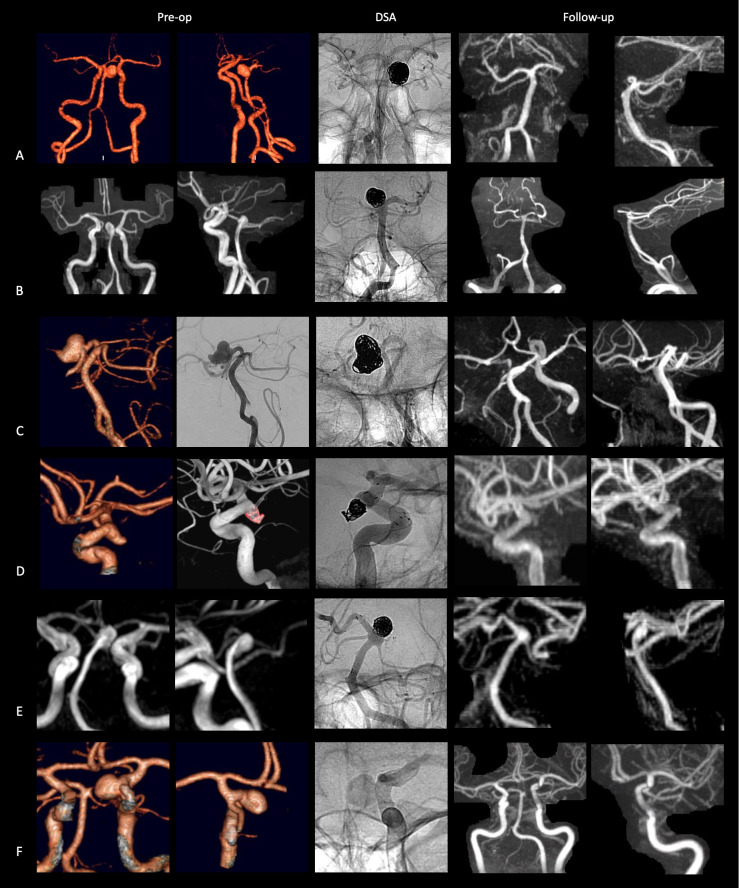
All procedures could be completed robotically without unplanned manual intervention. The mean RROC at the end of the procedure was 1.8 and the mean mRS at the end was unchanged at 1. All patients underwent MRI follow-up with a mean most recent follow-up time of 13±1 months (range 12–14 months). The mean RROC at follow-up was 1.3. All patients achieved an RROC class of I or II. Figure 1 demonstrates the CT/MRI work-up images, final angiogram and follow-up MRI for all patients. All six patients had achieved at least one clinical follow-up at the time of manuscript processing (average most recent follow-up: 10.0±1.6 months) and all reported being in good health without symptoms or procedure-related morbidity at latest follow-up. Three patients improved from baseline mRS at follow-up and the remaining patients were unchanged; however, one patient had a brainstem infarct 2 months after the intervention from which they fully recovered. The cause of the brainstem infarct was from a perforator that emerged from the transition between the neck and the sac that was patent after the procedure but probably occluded with the aneurysm sac in a delayed fashion. There were no cases of intra- or post-procedural aneurysm hemorrhage and no need for subsequent endovascular or surgical treatment. There were no deaths, permanent neurological deficits or other robotic-related complications.
Figure 1.
Patient imaging. Preoperative CT or MRI (left two columns), perioperative digital subtraction angiography (middle column), and follow-up contrast-enhanced MRI (right two columns) for all six patients (rows A–F) treated using the robotic system.
Discussion
Assistive robotic technologies have the potential to expand the current limitations of neurointervention. In this pilot study of six patients, we demonstrated the feasibility of this technology for embolization of intracranial aneurysms. The high degree of accuracy and control over the microcatheters, wires and stents was noted to be exceptional, and allowed for precise millimetric movements that would be difficult to achieve manually. In particular, stent and flow diverter deployment could be performed with a very high degree of accuracy.
This is the largest series of robotically-assisted intracranial aneurysm repair procedures to date. In addition to the feasibility, we demonstrated the long-term imaging results and occlusion rates of aneurysms treated with robotic assistance. We were able to control multiple devices, stents and coils and achieve an angiographic result that was stable in the long term. The supposition of some, that a robot could underperform or compromise the completeness of the procedure, is not confirmed by our study.14 On the contrary, complex wide neck aneurysms usually associated with high recanalization rates were successfully treated with long-term (1 year) stable occlusion results. This is a first-generation technology that will certainly evolve further and expand the scope of procedures. We appreciated the precision of stent placement, including both a laser cut stent with unsheathing technique and a flow diverter with a more sophisticated deployment. Sometimes, to reach complete occlusion or a desired coil packing, we had to use rather complex maneuvers such as changing the microcatheter position inside the sac or re-entering the aneurysm after being pushed out. For the flow diverter procedures, we were able to re-enter the stent and perform massaging maneuvers to improve its apposition to the vessel wall. We were able to execute the procedure with the robot similarly to how we would manually. We think it is important to mention that controlling the slack and tension on the catheter system was often better performed with the robotic system compared with a manual procedure. There was a selection bias with the cases included in our study. We included aneurysms larger than 5 mm that could wait at least 48 hours for the treatment to obtain special access approval, which therefore excluded most of the ruptured cases. We recommend that the learning curve and first cases be performed on larger aneurysms that will carry a lower procedural risk compared to aneurysms with a smaller sac, particularly with regard to aneurysm catheterization and coiling.
The use of this new technology brings potential benefits not only to the patient, but also to the operator. Numbers of certain neurointerventional procedures, such as thrombectomy, have expanded greatly in recent years. In addition, certain procedures, such as those utilizing transradial access, require closer positioning of the interventionalist to the radiation source. It has been previously demonstrated that interventionalists have a threefold risk of cataract-type opacities compared with age-matched controls.15 In a study of robotic coronary intervention, radiation exposure for the primary operator was reduced by 95% compared with the usual position next to the operating table.8 Robotic procedures could also eliminate the necessity of wearing lead protection and the consequent morbidity associated with increased physical load on the spine and weight-bearing joints.16 Lastly, it is postulated that errors during protracted procedures might be reduced due to diminished fatigue induced by performing repetitive physically-taxing actions.
A much vaunted potential benefit of robotic neurointervention is the possibility of remote intra-arterial thrombectomy in the future. Remote intervention has been performed successfully for percutaneous coronary intervention17 and coronary stenting,18 but still requires significant development for neurointerventional procedures. Deploying robot technology in smaller, more remote hospitals might allow faster brain reperfusion and solve the problem of training multiple specialists each potentially treating a low volume of cases. Earlier and faster access to thrombectomy has the potential to increase access to care, improve functional outcomes, and have a positive socioeconomic impact.
There remain limitations to the current technology of robotic-assisted intervention. Current generation robots lack tactile feedback. We did not find this to be a major challenge as we were able to detect obstacles and friction visually by watching for subtle changes in the shape and motion of devices. This visual feedback was sufficient to compensate for the altered sensory profile; however, this could be more challenging for less experienced operators who rely more on tactile feedback as opposed to visual feedback. Nevertheless, the addition of force-sensing and feedback technology could be a useful addition. Additionally, the robotic system can only control one microcatheter and one microwire or device at one time, which means the guide or intermediate catheter cannot be controlled during the procedure and a bedside practitioner must first obtain vascular access and place the guide catheter into the carotid or vertebral arteries before hooking up to the robot to perform the intracranial portion of the procedure. These challenges are currently being tackled in new experimental robots. In the beginning, procedures may be prolonged due to extra time required for adapting the set-up to the robot. For example, it took us extra time to set-up the patient’s arm for our first radial access case. Recently, however, these set-ups have become easier and faster as we gained more experience. Extra time may also be incurred due to time taken to communicate between the robotic operator and remote bedside team and for loading and exchanging devices within the robotic cassette. Also, the use of robots will require additional training for existing practitioners and new trainees. When robotic intervention becomes widespread it may be that current conventional manual skills become less widespread. Finally, robotic systems will add to the procedure cost. Materials and equipment may introduce additional costs as the technology becomes more sophisticated.
Conclusion
In this series, we demonstrated the feasibility and 1 year stable results of robotic-assisted embolization of intracranial aneurysms using the CorPath GRX system. The preciseness of control possible over the microcatheter, wire, and stent deployment, in particular, was noted to be exceptional. All intracranial steps of the procedure were completed robotically and successful results were achieved as confirmed with available follow-up. Radiation exposure to the primary operator was eliminated with the reduction in the morbidity associated with wearing heavy lead gowns.
neurintsurg-2021-017865supp001.pdf (837.4KB, pdf)
Acknowledgments
We would like to thank the Michael's family for their generous donation to the RADIS lab that helped support this work.
Footnotes
Contributors: All authors substantially contributed to the manuscript. VMP and NMC designed the work and performed all procedures; JL, NMC and VMP drafted the original manuscript; and all authors were involved in interpretation of data for the work, critical revision for important intellectual content, final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: VMP is an unpaid member of the neuro-advisory board of Corindus. KD, JMS and RT work at Corindus (robotic company).
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by University Health Network Research Ethics Board (REB) study ID: 20-5121. Participants gave informed consent to participate in the study before taking part.
References
- 1. Lechky O. Worlds first surgical robot in BC. The Medical Post 1985;21:92–3. [Google Scholar]
- 2. Kwoh YS, Hou J, Jonckheere EA, et al. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 1988;35:153–60. 10.1109/10.1354 [DOI] [PubMed] [Google Scholar]
- 3. De Benedictis A, Trezza A, Carai A, et al. Robot-assisted procedures in pediatric neurosurgery. Neurosurg Focus 2017;42:E7. 10.3171/2017.2.FOCUS16579 [DOI] [PubMed] [Google Scholar]
- 4. Gonen L, Chakravarthi SS, Monroy-Sosa A, et al. Initial experience with a robotically operated video optical telescopic-microscope in cranial neurosurgery: feasibility, safety, and clinical applications. Neurosurg Focus 2017;42:E9. 10.3171/2017.3.FOCUS1712 [DOI] [PubMed] [Google Scholar]
- 5. Ghasem A, Sharma A, Greif DN, et al. The arrival of robotics in spine surgery: a review of the literature. Spine 2018;43:1670–7. 10.1097/BRS.0000000000002695 [DOI] [PubMed] [Google Scholar]
- 6. Behnamfar O, Pourdjabbar A, Yalvac E, et al. First case of robotic percutaneous vascular intervention for below-the-knee peripheral arterial disease. J Invasive Cardiol 2016;28:E128–31. [PubMed] [Google Scholar]
- 7. Legeza P, Britz GW, Loh T, et al. Current utilization and future directions of robotic-assisted endovascular surgery. Expert Rev Med Devices 2020;17:919–27. 10.1080/17434440.2020.1814742 [DOI] [PubMed] [Google Scholar]
- 8. Weisz G, Metzger DC, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) study. J Am Coll Cardiol 2013;61:1596–600. 10.1016/j.jacc.2012.12.045 [DOI] [PubMed] [Google Scholar]
- 9. Britz GW, Panesar SS, Falb P, et al. Neuroendovascular-specific engineering modifications to the CorPath GRX robotic system. J Neurosurg 2019:1–7. 10.3171/2019.9.JNS192113 [DOI] [PubMed] [Google Scholar]
- 10. Mendes Pereira V, Cancelliere NM, Nicholson P, et al. First-in-human, robotic-assisted neuroendovascular intervention. J Neurointerv Surg 2020;12:338–40. 10.1136/neurintsurg-2019-015671.rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamaki VN, Cancelliere NM, Nicholson P, et al. Biomodex patient-specific brain aneurysm models: the value of simulation for first in-human experiences using new devices and robotics. J Neurointerv Surg 2021;13:272–7. 10.1136/neurintsurg-2020-015990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mascitelli JR, Moyle H, Oermann EK, et al. An update to the Raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015;7:496–502. 10.1136/neurintsurg-2014-011258 [DOI] [PubMed] [Google Scholar]
- 13. Agid R, Schaaf M, Farb R. CE-MRA for follow-up of aneurysms post stent-assisted coiling. Interv Neuroradiol 2012;18:275–83. 10.1177/159101991201800305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goyal M, Sutherland GR, Lama S, et al. Neurointerventional robotics: challenges and opportunities. Clin Neuroradiol 2020;30:203–8. 10.1007/s00062-020-00913-2 [DOI] [PubMed] [Google Scholar]
- 15. Jacob S, Boveda S, Bar O, et al. Interventional cardiologists and risk of radiation-induced cataract: results of a French multicenter observational study. Int J Cardiol 2013;167:1843–7. 10.1016/j.ijcard.2012.04.124 [DOI] [PubMed] [Google Scholar]
- 16. Goldstein JA, Balter S, Cowley M, et al. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv 2004;63:407–11. 10.1002/ccd.20201 [DOI] [PubMed] [Google Scholar]
- 17. Patel TM, Shah SC, Pancholy SB. Long distance tele-robotic-assisted percutaneous coronary intervention: a report of first-in-human experience. EClinicalMedicine 2019;14:53–8. 10.1016/j.eclinm.2019.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madder RD, VanOosterhout S, Parker J, et al. Robotic telestenting performance in transcontinental and regional pre-clinical models. Catheter Cardiovasc Interv 2021;97:E327–32. 10.1002/ccd.29115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2021-017865supp001.pdf (837.4KB, pdf)