Abstract
Noonan syndrome (NS) is a mostly dominantly inherited disorder affecting 1:1000 to 1:2500 live births. The phenotype varies in severity and can involve multiple organ systems over a patient’s lifetime. Diagnosis is based on a combination of features, including typical facial features, short stature, skeletal abnormalities, presence of cardiac defects, mild developmental delay, cryptorchidism, lymphatic dysplasia and a family history of NS. The phenotype varies from oligosymptomatic adults without significant medical issues to severely affected neonates with life-threatening heart disease. Early, accurate diagnosis is important for individualised management and to optimise developmental and long-term outcomes, but mildly affected patients often go undiagnosed for both healthcare provider (HCP)-related and patient-related reasons. Lack of awareness of NS among HCPs means that some do not recognise the condition, particularly in mildly affected patients and families. Some families do not want to receive a diagnosis that medicalises a condition that may account for family traits (eg, distinctive facial features and short stature), particularly when a child’s physical and cognitive development may be satisfactory. As for any condition with lifelong effects on multiple organ systems, a multidisciplinary approach provides the best care. It is proposed that increasing awareness of NS among non-specialist HCPs and other professionals could help direct a parent/carer to seek specialist advice and increase the number of NS diagnoses, with the potential to optimise lifelong patient outcomes. Non-specialists do not need to become experts in either diagnosis or treatment; however, early recognition of NS and referral to an appropriate specialist is important.
Keywords: endocrinology, genetics, syndrome
What is already known on this topic?
Noonan syndrome is a relatively common genetic disease; early diagnosis and referral may improve patient outcomes.
The disparate signs and symptoms of Noonan syndrome can make diagnosis difficult, as patients may present to a variety of healthcare professionals.
What this study adds?
Increased awareness among non-specialist and non-healthcare professionals may improve diagnosis, leading to improved patient outcomes.
Provides a European perspective of the disease and its management.
Introduction
Noonan syndrome (NS) is a fairly common, mostly autosomal-dominant inherited disorder with a phenotype that varies in severity and can involve multiple organ systems over the patient’s lifetime.1 2 It has an estimated incidence of 1 in 1000–2500 live births.1 While many individuals have a de novo pathogenic variant, an affected parent (more commonly a female parent) is recognised in 20%–40% of patients. Although formal epidemiological studies are lacking, prevalence is assumed to be similar across all ethnicities.
Signs and symptoms
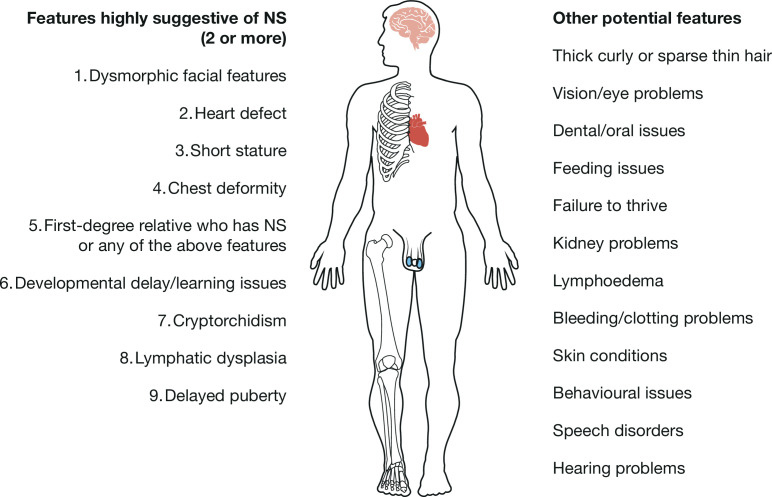
The syndrome is characterised by a number of features, as shown in figure 1.1–6 The emergence of these characteristics at different ages is considered in more detail later in this article.
Figure 1.
Features of Noonan syndrome (NS).3 4 6
Genetic causes
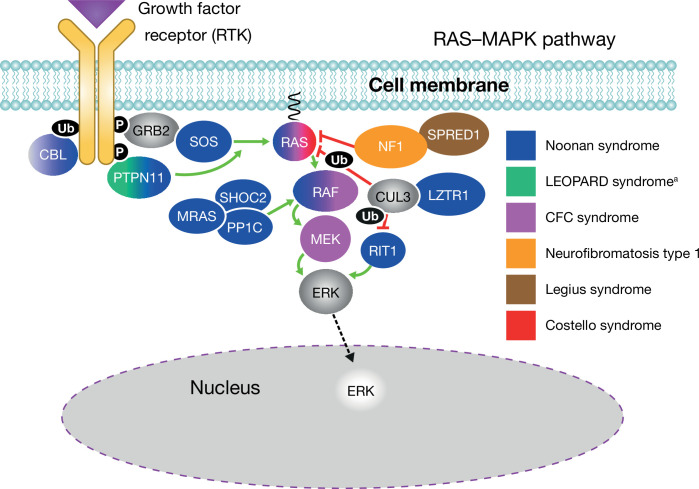
NS is caused primarily by gain-of-function (activating) mutations in genes encoding components or regulators of the RAS/mitogen-activated protein kinase (MAPK) signal transduction pathway, which is essential for cell cycle differentiation, growth and senescence (figure 2).1 2 4 As such, NS is one of a family of phenotypically overlapping genetic disorders caused by dysregulation of the RAS/MAPK pathway—the ‘RASopathies’—that also includes cardiofaciocutaneous syndrome, Costello syndrome, neurofibromatosis type 1, Legius syndrome, NS with multiple lentigines, Noonan-like syndrome with loose anagen hair, and capillary malformation/arteriovenous malformation syndrome.6 7 The family of RASopathies constitutes one of the largest groups of multiple congenital anomaly diseases.
Figure 2.
The RAS–MAPK signalling pathway showing mutations that may lead to diseases such as Noonan syndrome (reproduced with permission from Zenker, 2020). Some of the pathway components exist in multiple isoforms (eg, RAS: KRAS, HRAS, NRAS, RRAS; RAF: RAF1, BRAF; SOS: SOS1, SOS2, etc). aAlso known as Noonan syndrome with multiple lentigines. CFC, cardiofaciocutaneous; MAPK, mitogen-activated protein kinase; P, proline; RTK, receptor tyrosine kinase enzyme; Ub, ubiquitin.
Genes implicated in NS include PTPN11 (in about half of patients with NS), SOS1, RAF1, RIT1, KRAS, NRAS, BRAF, LZTR1, SOS2 6 8 9 and others. Robust epidemiological data and mutation detection rates are lacking; however, it is likely that approximately 80% of patients with a clinical diagnosis of NS have a mutation in one of these genes.9 The causative mutations remain unidentified in 10%‒20% of patients, and de novo mutations account for a majority of NS cases.7 Genetic heterogeneity partly explains the observed phenotypical variability,1 2 7 10 and a number of clinically relevant genotype–phenotype correlations have been identified. For example, PTPN11 and SOS1 mutations are more often associated with pulmonary valve stenosis, while RAF1 and RIT1 mutations are particularly associated with hypertrophic cardiomyopathy,11 12 and specific PTPN11 mutations confer increased risk of juvenile myelomonocytic leukaemia.13 Genetic testing, therefore, can help with risk assessment and patient management.14 Genetic testing is recommended at the time of clinical diagnosis or suspicion. The patient can be referred to a clinical geneticist or genetic testing can be performed or may be ordered after appropriate counselling of the patient/family by the clinician in charge of the patient. Given the genetic heterogeneity of NS, multigene panel sequencing of known RASopathy genes should be preferred before serial single gene testing. More comprehensive genomic testing, including exome sequencing or genome sequencing, may be considered if multigene panel testing fails to confirm the diagnosis in an individual with features of NS.15
Why is early, accurate diagnosis of NS important?
The NS phenotype varies from oligosymptomatic adults without significant medical issues to severely affected neonates with life-threatening heart disease or lymphatic issues. While patients with NS with severe manifestations tend to be diagnosed early, more mildly affected patients may remain undiagnosed.1 4 Early, accurate diagnosis of NS is important because it has an impact on individual management and prognosis.4 7 16 More specifically, earlier diagnosis would aid clinical management, and optimise developmental and long-term outcomes.16–18 Psychological disorders and symptoms are common, including hyperactivity/impulsivity and anxiety/depressive symptoms that can add to variable cognitive deficits, and social and behavioural problems.18 19 Hearing/speech and vision/ocular problems may also contribute to learning issues.2 17 The clinical implications of the neuropsychological and behavioural aspects of NS mean that most children with NS will need support at some stage of development (eg, physiotherapy, speech therapy, occupational therapy),20 but the overall prognosis is good and many patients live independently as adults.2 3 16 It is assumed that the broad availability of genetic testing for NS (in some countries) has led to increasing and earlier diagnosis of NS, but no systematic studies have so far addressed this point.
While there appear to be no formal studies of the potential benefits of an early diagnosis in NS, it is possible that this would be beneficial in a number of areas. In cardiology, for example, a diagnosis of NS (with its increased risk of hypertrophic cardiomyopathy, arrhythmia and sudden cardiac death) would imply lifelong cardiological follow-up even in those patients in whom congenital structural heart defects had not been ascertained. With regard to malignancies, there are a few specific mutations that are considered high-risk variants for malignancies (eg, PTPN11 p.Thr73Ile for juvenile myelomonocytic leukaemia13), and cancer surveillance is recommended for individuals with these mutations, including those with NS. Some children with NS may have growth hormone (GH) deficiency, and in some countries, treatment with recombinant human GH is licensed for use in NS per se. While there is ample evidence that GH treatment leads to accelerated growth in NS, irrespective of the presence of GH deficiency, the long-term benefit on adult height is still unclear.21 More generally, early diagnosis of NS should lead to evaluation of the coagulation system to avoid bleeding complications during surgery, and an early genetic diagnosis would help distinguish NS from other RASopathy conditions with a separate risk profile, and avoid unnecessary examinations (eg, imaging, metabolic assessments). It can be speculated that, in future, a prospective observational study of the natural history of the syndrome in patients diagnosed young would help to clarify the natural history of facets of the syndrome, such as heart disease, and the severity of the bleeding disorders. Future studies could also explore the effect on outcomes of earlier recognition of cardiac and bleeding conditions, as well as malignant disease, and evaluation of patient and parent views on the benefits and disadvantages of early diagnosis could inform future clinical practice.
When should NS be suspected?
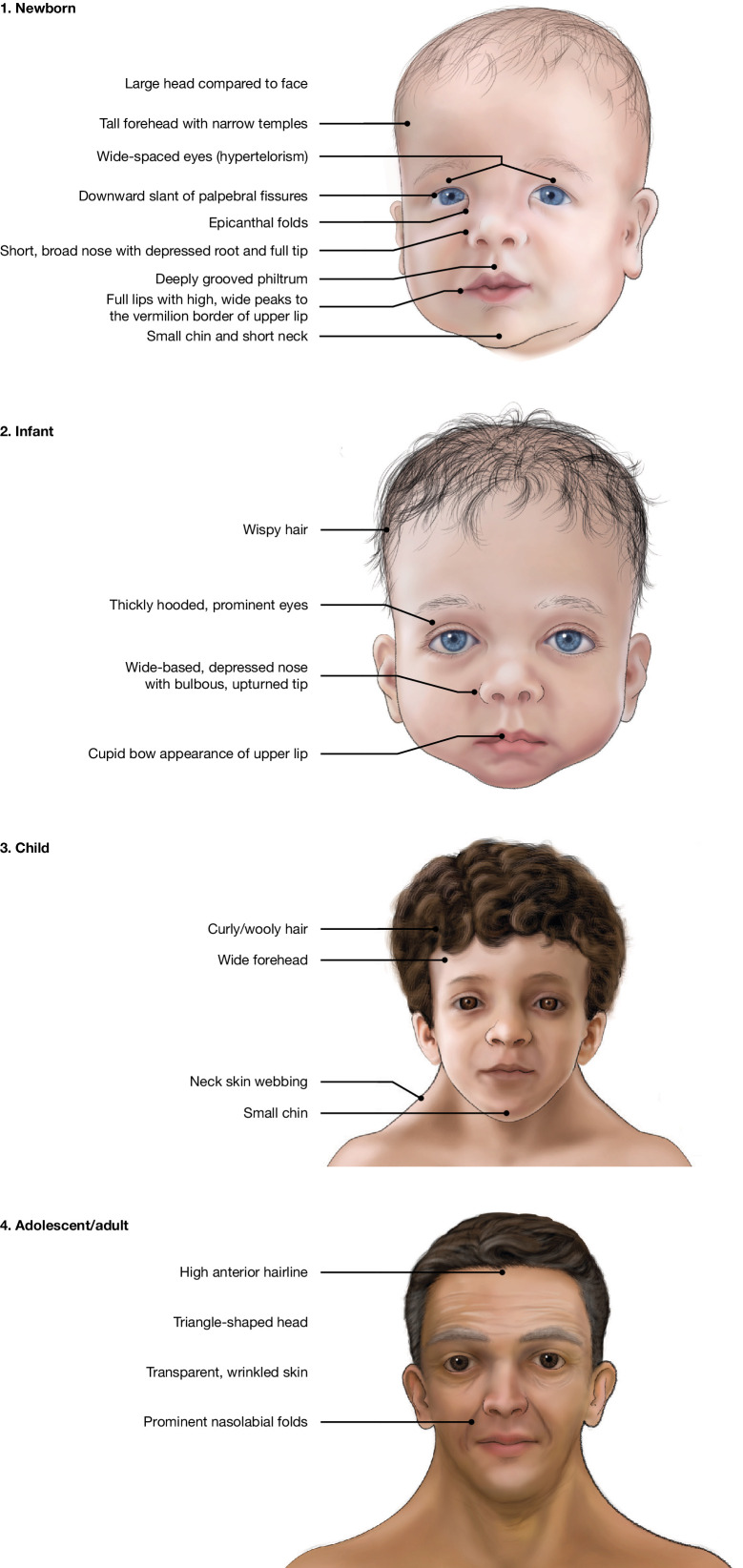
There are a number of major, easily identifiable clinical signs at each age that raise suspicion of NS. Of these signs, distinctive facial features are often the biggest clue to the presence of NS, including palpebral ptosis, widely spaced, down-slanting eyes, low-set posteriorly rotated ears and a short neck with low posterior hairline. The facial features of NS become less noticeable with age.4 7 The typical facial features of NS at different ages are shown in figure 3.
Figure 3.
The face of Noonan syndrome over time. Public domain images from the National Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland, USA.
In the fetus
Prenatal features are frequent in fetuses with NS, including increased nuchal translucency, persistent nuchal fold, distended jugular lymphatic sacs, cystic hygroma, hydrops fetalis, pleural or pericardial effusion, polyhydramnios, cardiac and renal anomalies, and specific dysmorphic facial features.22–25 In prospective genetic studies, mutations of genes implicated in NS have been reported in up to 16% of fetuses with an increased nuchal translucency and a normal karyotype.23 Prenatal genetic testing is offered in some countries. However, antenatal diagnosis is not systematically proposed even when ultrasound features are found, considering the impossibility of predicting phenotypical expression precisely and the fact that most individuals with NS have a quite favourable prognosis. Ultrasonographers and obstetricians are well placed to recognise these characteristics, but it is currently unknown what proportion of fetuses with NS actually show prenatal anomalies.
In the neonatal period
The perinatal period is a particularly important time for NS diagnosis, but the condition is not always easy to recognise. Neonatologists should keep in mind the suggestive prenatal history (see above) and the distinctive facial features of NS (figure 3).2 Other features that may be present at that age include heart defects (especially pulmonary valve stenosis and hypertrophic cardiomyopathy), cryptorchidism in boys1 4 and feeding difficulties/failure to thrive.26 Lymphatic dysplasia may manifest with congenital chylothorax or hydrops.
In childhood
Monitoring of growth and developmental milestones from birth to mid-childhood (7 years) provides repeated opportunities for the identification of NS. The distinctive facial features of NS remain, and facial shape may become more triangular with age as the face lengthens (figure 3). In addition, children with NS may present with skeletal abnormalities (pectus carinatum/pectus excavatum and scoliosis),17 heart diseases (eg, pulmonary valve stenosis and hypertrophic cardiomyopathy),1–6 abnormal bruising or bleeding,17 27 deafness, varying degrees of developmental delay/learning issues (eg, delayed speech or motor milestones)19 20 28 and short stature,29 whereas feeding difficulties/failure to thrive often resolve after the first years of life. In the case of short stature, children with NS often have normal birth measurements but usually show a decline in their growth curve during the first years of life, so the typical growth curve of infants and children with NS may provide a clue to the diagnosis.2 29 With regard to hypertrophic cardiomyopathy in childhood, adolescence or adulthood, the finding of certain additional cardiac lesions (eg, mitral valve dysplasia, pulmonary stenosis) or particular cardiac phenotypes (eg, biventricular hypertrophy) should also raise suspicion of NS.
In adolescence
In addition to the characteristic skeletal, cardiac, bruising/bleeding, neurological and developmental features of NS, the distinctive facial features of NS are often florid in adolescents (figure 3).2 In addition, puberty may be delayed, particularly in boys, and the pubertal growth spurt may be attenuated or absent. As mentioned above, the finding in adolescents of certain additional cardiac lesions or particular cardiac phenotypes should also raise suspicion of NS.
In adulthood
While the characteristic skeletal, cardiac, bruising/bleeding, and neurological features may be present in adults, the facial features of NS are often difficult to recognise (figure 3).2 As growth during childhood is often parallel but below the third centile, individuals with NS often have short stature, with a mean final adult height of 63–66 inches (160–168 cm) in men and 59–61 inches (150–155 cm) in women.4 Fertility problems may occur in men, while fertility tends to be normal in women. Again, the finding in adults of certain additional cardiac lesions or particular cardiac phenotypes should also raise suspicion of NS.
Why is it difficult for patients with NS to get a diagnosis?
Despite the existence of diagnostic guidelines/criteria,2 3 16 diagnosis is difficult in less severely affected individuals for a number of reasons, including both healthcare provider (HCP)-related and patient-related factors. As recognition of the typical facial gestalt is a key point in proposed diagnostic criteria, making the diagnosis remains difficult for someone who has little experience with the disease.
Primary care physicians may see patients with NS only rarely. Specialists treating individual manifestations of the syndrome in isolation may not be aware of their association with NS or may be unaware of the patient’s additional clinical features that would suggest this diagnosis. As the physician with an overview of all disciplines involved with a patient’s care, the general paediatrician is best placed to identify the pattern of features associated with NS and to refer on for a definitive diagnosis to be made. The challenge is to recall this relatively rare diagnosis, which may present with a diverse range of symptoms and signs that vary with severity and throughout life (NS has been referred to as the ‘changing phenotype’30).
In particular, the facial features of NS may be subtle, often florid during childhood and adolescence but difficult to recognise in adults (figure 3).2 5 10 Characteristics may also be attributed to family traits, as the condition is often dominantly inherited; short stature, for example, may be attributed to parental height in an undiagnosed parent, which is correct but misses the opportunity to make the diagnosis in both parent and child. In some cases, a parent with NS is only diagnosed after a more severely affected child is born.5 In other cases, the correct clinical diagnosis may be hampered or delayed by the presence of a more severe/complex phenotype (eg, the newborn with hydrops and cardiomyopathy, where a metabolic disorder is suspected).
A recent comprehensive analysis of biobank data suggested that a substantial fraction of individuals harbouring pathogenic variants in RASopathy genes remain undiagnosed, some with partial phenotypical matches to a RASopathy, and some not meeting diagnostic criteria.31 This means that there are many cases with minor, or in some cases, no significant medical problems. Diagnosis and optimal follow-up of such cases is unclear and there remains a need for wider prospective studies of the natural history of NS, and better prediction of rare complications in mild disease.
The importance of increased awareness of the subtleties of NS among specialists, such as endocrinopathies among endocrinologists, has been suggested.32 Our proposal, however, goes further and wider and suggests that efforts to increase awareness of NS are extended to include non-specialist HCPs/and other professionals who could help direct a parent/carer to seek specialist advice and a possible NS diagnosis.
Accompanying this proposal, however, is the caveat that diagnosis of NS can raise ethical questions. Although genotype–phenotype relationships are described, the severity of the clinical features of NS cannot be predicted from the patient’s genotype, and a child’s physical and cognitive development may be satisfactory.33 While children with NS often have learning issues, intellectual disability (defined as IQ <70) is only found in 6%–23% of patients,28 and they often outgrow this and have an IQ within the normal range as adults.34 Most individuals with NS finish their schooling and go on to employment.7 Some mildly affected patients/families may not be interested in receiving a diagnosis, considering this to be medicalisation of family traits, and aware of the potential stigma associated with a genetic diagnosis, unless there are significant benefits for the affected individuals. There may also be implications for obtaining medical and life insurance,35 varying with healthcare systems in different countries.
Who needs to know about NS?
A variety of HCPs and non-HCPs may encounter individuals with undiagnosed NS. Specialists such as paediatric endocrinologists, cardiologists and geneticists are generally familiar with NS, its diagnosis and management. However, other HCPs may only see NS very rarely, and may not recognise the features of NS. Monitoring of infant and child health in Europe potentially provides other opportunities for recognition of individuals with undiagnosed NS, although such programmes do vary by country. Short stature is one of the most consistent features of NS, and NS should be one of the diagnoses considered in the evaluation of any child concerned about their height and short or slowly growing children identified through growth surveillance programmes.
Other HCPs may encounter undiagnosed patients with NS with non-specific health issues, which, when accompanied by other signs (eg, facial characteristics), may help these specialists to consider a possible diagnosis of NS. Such HCPs might include:
Dermatologists for abnormal pigmentation, including multiple pigmented naevi, café au lait spots, lentigines, and keratosis pilaris of the upper arms and face.1
Orthopaedic specialists/surgeons for upper carinatum and lower excavatum (the classic sternal change in NS) and scoliosis.17
Haematologists for easy bruising or bleeding, monocytosis, and, in rare cases, juvenile myelomonocytic leukaemia.17 27
Ophthalmologists for strabismus, amblyopia, refractive errors and nystagmus.2 17 36
Otolaryngologists and speech therapists for feeding problems, articulation deficiencies, otitis media and hearing loss.37
Clinical/educational psychologists for neuropsychological impairments, language delays, ADHD (attention deficit hyperactivity disorder), behavioural problems, impaired memory and social skills, autism spectrum disorders and special educational needs.18–20 28 38–40
The characteristic features of NS may also be recognised by primary care or general practitioners, practice nurses and midwives/health visitors. Primary care professionals and general paediatricians should also be mindful of information that might come from educational professionals (eg, teachers, school nurses and doctors, nursery and kindergarten teachers, and educational psychologists) who are in a position to observe NS characteristics, enabling them to ‘join the dots’ relating to information from different stakeholders.
Non-specialists do not need to become experts in either diagnosis or treatment; they just need to have enough knowledge to raise suspicion of the possibility of NS. The diagnostic features of NS—and the relative importance of the different features—are shown in table 1.3
Table 1.
Diagnostic features of Noonan syndrome (NS) and the relative importance of these features3
| Feature | A=major | B=minor | ||
| 1. Facial | Typical face* | Suggestive face | ||
| 2. Cardiac | Pulmonary valve stenosis and/or hypertrophic cardiomyopathy | Other cardiac defect | ||
| 3. Height | <3rd centile | <10th centile | ||
| 4. Chest wall | Pectus carinatum/excavatum | Broad thorax | ||
| 5. Family history | First-degree relative with definite NS | First-degree relative suggestive of NS | ||
| 6. Other | Mild developmental delay, cryptorchidism AND lymphatic dysplasia | Mild developmental delay, cryptorchidism OR lymphatic dysplasia | ||
| Definitive NS= | Criterion 1A (typical face) PLUS |
Criterion 1B (suggestive face) PLUS |
||
| 1×major criterion (2A‒6A) |
2×minor criteria (2B‒6B) |
2×major criteria (2A‒6A) |
3×minor criteria (2B‒6B) |
|
*Facial features of NS vary over time and may have only subtle differences.
Multidisciplinary team care for optimal NS management
As mentioned previously, there is currently no specific treatment for NS; each patient requires an individualised management approach and has a different prognosis depending on symptoms and severity.4 As for any condition that involves multiple organ systems affected over the patient’s lifetime, a multidisciplinary team approach provides the best care for patients with NS.7 41 The NS phenotype changes significantly over time, and the transition from adolescent to adult (and possibly to parent) represents a particularly vulnerable time for these individuals.42 43 While not as well studied in adolescence and adulthood as in children, NS-related clinical issues include cardiac issues (eg, hypertropic cardiomyopathy and arrhythmias/conduction defects), easy bruising, gastrointestinal issues, scoliosis, chronic joint pain, lymphoedema, anxiety and depression, Chiari malformation, and osteopenia/osteoporosis.43
Guidelines are available for NS management,1 7 16 although detailed discussion of these guidelines is outside the scope of this article. Optimised management, however, requires a diagnosis, and increased awareness of NS among non-specialist HCPs and other professionals as proposed here could help address this need (online supplemental information 1; Noonan syndrome infographic for distribution).
archdischild-2021-322858supp001.pdf (156.8KB, pdf)
Conclusions
It is proposed that increased awareness of NS among non-specialist HCPs and other professionals could help direct a parent/carer to seek specialist advice and possible NS diagnosis, increasing the potential for optimising lifelong patient outcomes. Guiding individuals with possible NS to a diagnosis must take ethical issues and patient wishes into consideration and be balanced against potential harm. Non-specialists do not need to become experts in either diagnosis or treatment; they just need to have enough knowledge to raise suspicion of the possibility of NS.
Acknowledgments
Under the direction of the authors, Sarah Smith, for Caudex, Oxford, UK, provided medical writing assistance in the form of development of the first draft and collation of author feedback, funded by Novo Nordisk Healthcare. Dr Maria Giulia Gagliardi (Bambino Gesù Children’s Hospital, Rome) took part in discussing the concept of this paper at the advisory board meeting.
Footnotes
Contributors: All authors contributed to the concept for this article during discussions at an advisory board meeting. All authors reviewed and revised interim drafts of the article and approved the final version.
Funding: This work was supported by Novo Nordisk Healthcare. Medical writing support for the development of this paper was provided by Sarah Smith, for Caudex, McCann Health Medical Communications, Oxford, funded by Novo Nordisk Healthcare.
Competing interests: The concept for this paper came about during discussions at an advisory board meeting organised and supported by Novo Nordisk Health Care, for which all attendees (JCB, MC, TE, MZ) received an honorarium. MZ has nothing further to disclose. TE passed his honorarium to the INSERM unit UMR 1043. He received research funding from Pfizer. JCB is a member of the Publication Steering Committee of a research study funded by Novo Nordisk Health Care and has received payment for this. She has received consultancy fees, honoraria and support to attend scientific meetings, also from Novo Nordisk Health Care. MC has had consultancy agreements with COR2ED, Merck Serono, Novo Nordisk Health Care, BlubirdBio and Ipsen. He has received honoraria for lectures from Novo Nordisk Health Care, Eli Lilly and Ipsen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study does not involve human participants.
References
- 1. Roberts AE, Allanson JE, Tartaglia M, et al. Noonan syndrome. Lancet 2013;381:333–42. 10.1016/S0140-6736(12)61023-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stevenson DA. Bmj best practice: Noonan syndrome: BMJ, 2018. Available: https://bestpractice.bmj.com/topics/en-us/1193 [Accessed 09 Dec 2019].
- 3. van der Burgt I, Burgt vander. Noonan syndrome. Orphanet J Rare Dis 2007;2:4. 10.1186/1750-1172-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhambhani V, Muenke M. Noonan syndrome. Am Fam Physician 2014;89:37–43. [PMC free article] [PubMed] [Google Scholar]
- 5. Kruszka P, Porras AR, Addissie YA, et al. Noonan syndrome in diverse populations. Am J Med Genet A 2017;173:2323–34. 10.1002/ajmg.a.38362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tajan M, Paccoud R, Branka S, et al. The RASopathy family: consequences of germline activation of the Ras/MAPK pathway. Endocr Rev 2018;39:676–700. 10.1210/er.2017-00232 [DOI] [PubMed] [Google Scholar]
- 7. Romano AA, Allanson JE, Dahlgren J, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics 2010;126:746–59. 10.1542/peds.2009-3207 [DOI] [PubMed] [Google Scholar]
- 8. Grant AR, Cushman BJ, Cavé H, et al. Assessing the gene-disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Hum Mutat 2018;39:1485–93. 10.1002/humu.23624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capri Y, Flex E, Krumbach OHF, et al. Activating mutations of RRAS2 are a rare cause of Noonan syndrome. Am J Hum Genet 2019;104:1223–32. 10.1016/j.ajhg.2019.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gurovich Y, Hanani Y, Bar O, et al. Identifying facial phenotypes of genetic disorders using deep learning. Nat Med 2019;25:60–4. 10.1038/s41591-018-0279-0 [DOI] [PubMed] [Google Scholar]
- 11. Pandit B, Sarkozy A, Pennacchio LA, et al. Gain-Of-Function Raf1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet 2007;39:1007–12. 10.1038/ng2073 [DOI] [PubMed] [Google Scholar]
- 12. Kouz K, Lissewski C, Spranger S, et al. Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation. Genet Med 2016;18:1226–34. 10.1038/gim.2016.32 [DOI] [PubMed] [Google Scholar]
- 13. Strullu M, Caye A, Lachenaud J, et al. Juvenile myelomonocytic leukaemia and Noonan syndrome. J Med Genet 2014;51:689–97. 10.1136/jmedgenet-2014-102611 [DOI] [PubMed] [Google Scholar]
- 14. Prendiville TW, Gauvreau K, Tworog-Dube E, et al. Cardiovascular disease in Noonan syndrome. Arch Dis Child 2014;99:629–34. 10.1136/archdischild-2013-305047 [DOI] [PubMed] [Google Scholar]
- 15. Allanson JE, Roberts AE. Noonan Syndrome. In: Gene reviews, 2001. https://www.ncbi.nlm.nih.gov/books/NBK1124/ [Google Scholar]
- 16. Noonan Syndrome Guideline Development Group . Management of Noonan syndrome: a clinical guideline Manchester. UK: DYSCERNE, 2010. https://rasopathiesnet.org/wp-content/uploads/2014/01/265_Noonan_Guidelines.pdf [Google Scholar]
- 17. Sharland M, Burch M, McKenna WM, et al. A clinical study of Noonan syndrome. Arch Dis Child 1992;67:178–83. 10.1136/adc.67.2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pierpont EI, Tworog-Dube E, Roberts AE. Attention skills and executive functioning in children with Noonan syndrome and their unaffected siblings. Dev Med Child Neurol 2015;57:385–92. 10.1111/dmcn.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perrino F, Licchelli S, Serra G, et al. Psychopathological features in Noonan syndrome. Eur J Paediatr Neurol 2018;22:170–7. 10.1016/j.ejpn.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 20. Wingbermuehle E, Egger J, van der Burgt I, et al. Neuropsychological and behavioral aspects of Noonan syndrome. Horm Res 2009;72 Suppl 2:15–23. 10.1159/000243774 [DOI] [PubMed] [Google Scholar]
- 21. Rodríguez F, Gaete X, Cassorla F. Etiology and treatment of growth delay in Noonan syndrome. Front Endocrinol 2021;12:691240. 10.3389/fendo.2021.691240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myers A, Bernstein JA, Brennan M-L, et al. Perinatal features of the RASopathies: Noonan syndrome, cardiofaciocutaneous syndrome and Costello syndrome. Am J Med Genet A 2014;164A:2814–21. 10.1002/ajmg.a.36737 [DOI] [PubMed] [Google Scholar]
- 23. Croonen EA, Nillesen WM, Stuurman KE, et al. Prenatal diagnostic testing of the Noonan syndrome genes in fetuses with abnormal ultrasound findings. Eur J Hum Genet 2013;21:936–42. 10.1038/ejhg.2012.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baldassarre G, Mussa A, Dotta A, et al. Prenatal features of Noonan syndrome: prevalence and prognostic value. Prenat Diagn 2011;31:949–54. 10.1002/pd.2804 [DOI] [PubMed] [Google Scholar]
- 25. Stuurman KE, Joosten M, van der Burgt I, et al. Prenatal ultrasound findings of rasopathies in a cohort of 424 fetuses: update on genetic testing in the NGS era. J Med Genet 2019;56:654–61. 10.1136/jmedgenet-2018-105746 [DOI] [PubMed] [Google Scholar]
- 26. Digilio MC, Lepri F, Baban A, et al. RASopathies: clinical diagnosis in the first year of life. Mol Syndromol 2011;1:282–9. 10.1159/000331266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niemeyer CM. Ras diseases in children. Haematologica 2014;99:1653–62. 10.3324/haematol.2014.114595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pierpont EI. Neuropsychological functioning in individuals with Noonan syndrome: a systematic literature review with educational and treatment recommendations. J Pediatr Neuropsychol 2016;2:14–33. 10.1007/s40817-015-0005-5 [DOI] [Google Scholar]
- 29. Cessans C, Ehlinger V, Arnaud C, et al. Growth patterns of patients with Noonan syndrome: correlation with age and genotype. Eur J Endocrinol 2016;174:641–50. 10.1530/EJE-15-0922 [DOI] [PubMed] [Google Scholar]
- 30. Allanson JE, Hall JG, Hughes HE, et al. Noonan syndrome: the changing phenotype. Am J Med Genet 1985;21:507–14. 10.1002/ajmg.1320210313 [DOI] [PubMed] [Google Scholar]
- 31. Wenger BM, Patel N, Lui M, et al. A genotype-first approach to exploring Mendelian cardiovascular traits with clear external manifestations. Genet Med 2021;23:94–102. 10.1038/s41436-020-00973-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Venugopal V, Romero CJ. Endocrine complications of Noonan syndrome beyond short stature. Pediatr Endocrinol Rev 2019;16:465–70. 10.17458/per.vol16.2019.vr.endocrinecomplicationsnoonan [DOI] [PubMed] [Google Scholar]
- 33. Gaudineau A, Doray B, Schaefer E, et al. Postnatal phenotype according to prenatal ultrasound features of Noonan syndrome: a retrospective study of 28 cases. Prenat Diagn 2013;33:238–41. 10.1002/pd.4051 [DOI] [PubMed] [Google Scholar]
- 34. Roelofs RL, Janssen N, Wingbermühle E, et al. Intellectual development in Noonan syndrome: a longitudinal study. Brain Behav 2016;6:e00479. 10.1002/brb3.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Genetic Alliance UK . Insurance and genetic conditions, 2016. Available: https://www.geneticalliance.org.uk/information/living-with-a-genetic-condition/insurance-and-genetic-conditions/ [Accessed 27 Jan 2020].
- 36. van Trier DC, van der Burgt I, Draaijer RW, et al. Ocular findings in Noonan syndrome: a retrospective cohort study of 105 patients. Eur J Pediatr 2018;177:1293–8. 10.1007/s00431-018-3183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geelan-Hansen K, Anne S. Otolaryngologic manifestations of Noonan syndrome. Ear Nose Throat J 2015;94:E4–6. [PubMed] [Google Scholar]
- 38. Garg S, Brooks A, Burns A, et al. Autism spectrum disorder and other neurobehavioural comorbidities in rare disorders of the Ras/MAPK pathway. Dev Med Child Neurol 2017;59:544–9. 10.1111/dmcn.13394 [DOI] [PubMed] [Google Scholar]
- 39. Aftab S, Dattani MT. Pathogenesis of growth failure in Rasopathies. Pediatr Endocrinol Rev 2019;16:447–58. 10.17458/per.vol16.2019.ad.pathogenesisrasopathies [DOI] [PubMed] [Google Scholar]
- 40. Selas M, Helland WA. Pragmatic language impairment in children with Noonan syndrome. Clin Linguist Phon 2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gravholt CH, Viuff MH, Brun S, et al. Turner syndrome: mechanisms and management. Nat Rev Endocrinol 2019;15:601–14. 10.1038/s41574-019-0224-4 [DOI] [PubMed] [Google Scholar]
- 42. Lin AE, Basson CT, Goldmuntz E, et al. Adults with genetic syndromes and cardiovascular abnormalities: clinical history and management. Genet Med 2008;10:469–94. 10.1097/GIM.0b013e3181772111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smpokou P, Tworog-Dube E, Kucherlapati RS, et al. Medical complications, clinical findings, and educational outcomes in adults with Noonan syndrome. Am J Med Genet A 2012;158A:3106–11. 10.1002/ajmg.a.35639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2021-322858supp001.pdf (156.8KB, pdf)