Abstract
The development of combined vaccines constitutes one of the priorities in modern vaccine research. One of the most successful combined vaccines in use is the diphtheria-pertussis-tetanus vaccine. However, concerns about the safety of the pertussis arm have led to decreased acceptance of the vaccine but also to the development of new, safer, and effective acellular vaccines against pertussis. Unfortunately, the production cost of these new vaccines is significantly higher than that of previous vaccines. Here, we explore the potential of live recombinant Mycobacterium bovis BCG producing the hybrid protein S1-TTC, which contains the S1 subunit of pertussis toxin fused to fragment C of tetanus toxin, as an alternative to the acellular vaccines. S1-TTC was produced in two different expression systems. In the first system its production was under the control of the 85A antigen promoter and signal peptide, and in the second system it was under the control of the hsp60 promoter. Although expression of the hybrid antigen was obtained in both cases, only the second expression system yielded a recombinant BCG strain able to induce both a specific humoral immune response and a specific cellular immune response. The antibodies generated were directed against the TTC part and neutralized toxin activity in an in vivo challenge model, whereas interleukin-2 production was specific for both parts of the molecule. Since protection against tetanus is antibody mediated and protection against pertussis may be cell mediated, this constitutes a first promising step towards the development of a cost-effective, protective, and safe combined vaccine against pertussis, tetanus, and tuberculosis.
Diphtheria, pertussis, and tetanus (DPT) vaccines have contributed tremendously to the decrease of childhood mortality over the past decades (31). However, despite this success, these three diseases remain among the most significant infectious childhood diseases, especially in the developing world. In addition, the safety of DPT vaccines has been questioned in several countries in recent years, especially with regard to the pertussis arm, a whole-cell vaccine component (11). As a consequence, these vaccines have suffered a decrease in acceptance, resulting in the reappearance of pertussis epidemics.
A significant improvement has recently been accomplished through the development of new, acellular pertussis vaccines. In large phase III trials, these vaccines have been shown to be highly efficacious and of negligible reactogenicity (1a, 14, 15, 37). While the acellular vaccines tested varied substantially in antigen composition, all contained at least pertussis toxin (PTX), considered to be the main protective antigen against pertussis. Although PTX-neutralizing antibodies are able to protect against Bordetella pertussis infection (35), several observations suggest that cellular immunity may play an important role in protection against pertussis.
The role of cellular immunity against pertussis has been demonstrated in mouse models (28), and spleen cells isolated from convalescent mice produce high levels of gamma interferon (IFN-γ) and interleukin-2 (IL-2), indicative of a Th1-type response. In addition, CD4+ clones of infected human subjects also secrete mainly IFN-γ and IL-2 but little IL-4 (30). Considering that natural infection usually induces a longer-lasting protection against subsequent infection by B. pertussis than does vaccination (5, 19, 25), it is possible that a Th1-type cellular immune response rather than a Th2-type antibody response mediates long-lasting protection.
Mycobacterium bovis Bacillus Calmette-Guérin (BCG) is known to induce essentially a Th1-type response, and infection of live recombinant BCG producing foreign antigens has been shown to elicit cell-mediated immunity directed toward the heterologous antigens (2, 39). In addition to inducing a cellular immune response, recombinant BCG can also induce significant levels of antibodies against foreign antigens (23). Furthermore, BCG has been widely used as a vaccine against tuberculosis, is generally considered safe, can be administered at or any time after birth, and is substantially less expensive than most other vaccine formulations (8).
With the long-term goal to develop a combined pertussis-tetanus-tuberculosis vaccine, we therefore wished to evaluate the immune responses elicited by a recombinant BCG strain that produces a hybrid protein composed of the protective part of PTX fused to the protective part of tetanus toxin (TTX). This protein, named S1-TTC, contains at its N-terminal moiety the S1 subunit of PTX and at its C-terminal moiety fragment C of TTX (TTC). We have previously shown that it can be produced at high levels in Escherichia coli and that, in its purified form, it retains TTC-specific receptor-binding activity to the ganglioside GT1b and antigenicity to neutralizing anti-S1 antibodies which specifically recognize conformational epitopes (9). In this study, we demonstrate that S1-TTC can be produced in BCG and that the recombinant BCG is able to induce a specific T-cell response as well as an antibody response against S1-TTC which is able to neutralize TTX activity.
MATERIALS AND METHODS
Animals.
Eight-week-old BALB/c (H-2d) female mice (Iffa Credo, l’Arbresle, France) were used for immunization with the recombinant BCG. The mice were immunized intraperitoneally (i.p.) or intravenously (i.v.) with 5 × 106 nontransformed BCG or BCG cells producing S1-TTC.
Bacterial strains and growth conditions.
All cloning steps were performed in E. coli XL1-Blue (Stratagene, La Jolla, Calif.). S1-TTC production was carried out in M. bovis BCG (vaccine strain 1173P2) and in Mycobacterium smegmatis mc2 155 (38). Mycobacterial transformation and culture were done as described by Kremer et al. (21).
Plasmids and DNA manipulation.
pUC18 and pUC19 were purchased from New England Biolabs (Beverly, Mass.). pUC4K (Pharmacia LKB, Uppsala, Sweden) was used to isolate the kanamycin resistance (Kanr) gene. pEC001 and pRR3ΔKan were described by Kremer et al. (21), pS1-tC encoding the S1-TTC hybrid protein was described by Boucher et al. (9), and pUC::hsp60 was described by Kremer et al. (22). Restriction enzymes, T4 DNA polymerase, Klenow fragment, and other DNA modifying enzymes were purchased from Boehringer Mannheim Corp. (Mannheim, Germany). All DNA manipulations were performed by standard protocols as described by Sambrook et al. (34).
Purification of S1-TTC.
The procedure used for the purification of S1-TTC was described by Boucher et al. (9). Briefly, two liters of culture containing E. coli JM109(pS1-tC) were grown to an optical density at 600 nm of 0.4. Expression of the S1-TTC-encoding gene was then induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. After an additional incubation at 37°C for 5 h, the cells were harvested by centrifugation, washed, and resuspended in 40 ml of lysis buffer (25 mM Tris-HCl [pH 7.5], 25 mM NaCl, 1 mM EDTA, 0.5 mM phenylmethylsulfonylfluoride). The cells were then disrupted by sonication, and the lysate was clarified by centrifugation. The supernatant was applied onto a DEAE-cellulose column (Whatman DE-52; 2.5 by 30 cm), and after washing, the bound material was eluted with lysis buffer containing 50 mM NaCl. The fractions containing S1-TTC were pooled, adjusted to 1 M NaCl, and loaded onto a Phenyl-Sepharose (Pharmacia) column. After being washed, the protein was eluted with 10 mM Tris-HCl (pH 9.5), 10% glycerol, 0.5 mM phenylmethylsulfonyl fluoride. The fractions containing S1-TTC were pooled, immediately adjusted to pH 7.5 by the addition of 1 M Tris-HCl (pH 7.0), dialyzed overnight against several changes of phosphate-buffered saline (PBS; K2HPO4 [pH 7.5], 0.15 M NaCl), concentrated with a Millipore CX-10 concentrator to 1 mg/ml, and then stored at −80°C until further use.
Construction of expression vectors.
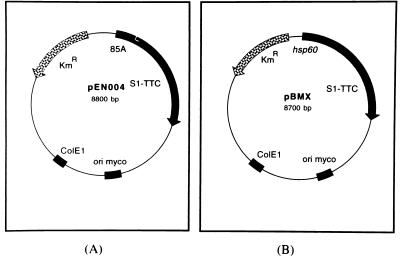
pEN004 was constructed as follows. The promoter and signal peptide coding sequence of the antigen 85A gene were isolated from pEC001 by digesting the plasmid with HindIII, blunt ending it with Klenow fragment, and then redigesting it with BamHI. This fragment was ligated into SmaI/BamHI-digested pS1-tC, generating pEC007. The 1.3-kb HindIII fragment corresponding to the Kanr gene was isolated from pUC4K and ligated into pEC007 previously digested with HindIII and blunt ended with Klenow fragment. This yielded pEC012, which was further digested with PvuII, and the resulting 4.2-kb fragment was ligated into the blunt-ended ScaI site of pRR3ΔKan, generating the final shuttle vector, pEN004 (Fig. 1A).
FIG. 1.
Schematic representation of pEN004 and pBMX. The following relevant features of pEN004 (A) and pBMX (B) are shown. The mycobacterial and E. coli origins of replication are indicated by the black boxes labelled ori myco and ColE1, respectively. The Kanr gene is indicated by the stippled arrow labelled KmR. The black boxes labelled hsp60 and 85A denote the hsp60 promoter and the antigen 85A promoter and signal peptide coding sequence, respectively. The grey boxes correspond to the S1-TTC-coding sequence.
pBMX was constructed as follows. First, the 1.3-kb PstI fragment corresponding to the Kanr gene was isolated from pUC4K, blunt ended with T4 DNA polymerase, and cloned into pUC::hsp60, previously digested with BamHI and blunt ended with Klenow fragment. The resulting plasmid, named pUChspK, was then digested with NcoI and XbaI and blunt ended with Klenow fragment. The 1.7-kb fragment containing the Kanr gene and the hsp60 promoter was cloned into the unique SmaI site of pS1-tC, giving rise to pBM02, which was in turn digested with PvuII. The resulting 3.4-kb fragment was finally inserted into the ScaI site of pRR3ΔKan, yielding the shuttle vector pBMX (Fig. 1B).
Immunoblotting of recombinant proteins.
Ten milliliters of each mycobacterial culture was harvested at mid-log phase by centrifugation. The cells were then resuspended in 1.5 ml of PBS and disrupted for 10 min with a Branson Sonifier 450 at half-maximal constant output. The resulting M. bovis BCG or M. smegmatis crude extracts were then subjected to polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and with a 12% polyacrylamide gel as described by Laemmli (24). After electrophoresis, the proteins were transferred onto a Hybond-C Extra membrane (Amersham France). The membrane was then saturated with 5% dry milk in PBS containing 0.1% Tween 20 (PBS-T). The antibodies used for the detection of recombinant proteins were a monoclonal antibody (1B7) directed against the S1 subunit of PTX (36) and rabbit polyclonal antibodies directed against tetanus toxoid (9), both kindly provided by H. Sato and Y. Sato (National Institute of Health, Tokyo, Japan). The immunoblots were developed with goat anti-mouse alkaline phosphatase-conjugated antibodies or goat anti-rabbit alkaline phosphatase-conjugated antibodies, purchased from Promega (Madison, Wis.).
Enzyme-linked immunosorbent assay (ELISA).
The wells of polystyrene microdilution plates (catalog no. 4-39454; Nunc, Roskilde, Denmark) were coated overnight with 100 μl of the ganglioside GT1b at a concentration of 1 μg/ml in PBS. The plates were washed three times with PBS, and 50 μl of purified S1-TTC was then added at a concentration of 2 μg/ml in PBS in each well. After incubation for 2 h at room temperature, the plates were washed three times with PBS and the sera were then added in PBS-T containing 0.5% bovine serum albumin (PBS-T-BSA) in twofold serial dilutions. The plates were incubated for 2 h and washed with PBS-T-BSA, and horseradish peroxidase-conjugated anti-immunoglobulin G1 (IgG1) (1/10,000), anti-IgG2a (1/10,000), anti-IgG2b (1/12,000), or anti-IgG3 (1/10,000) was added. Alternatively, biotinylated anti-mouse IgG (catalog no. RPN.1001; Amersham) was added at a 1/500 dilution, incubated for 1 h in PBS-T, washed three times with PBS-T, and then incubated with the streptavidin-biotinylated horseradish peroxidase complex (catalog no. RPN.1051; Amersham) at a dilution of 1/1,000 for 30 min in PBS-T. The plates were finally washed three times with PBS-T and then developed with 0.4 mg of O-phenylenediamine dihydrochloride (catalog no. P-4664; Sigma)/ml and 1 μl of hydrogen peroxide/ml in 0.1 M citrate buffer (pH 4.5) at 37°C in the dark. The plates were read with a Nunc NJ 2000 immunoreader at 490 nm.
TTX neutralization.
To test for in vivo TTX-neutralizing activity of the anti-S1-TTC antisera, we used the standard techniques of the European Pharmacopoeia (1974) at the level L+/1,000, as described previously (40). The results are expressed in international units per milliliter.
Spleen cell cytokine production.
Two months after i.p. immunization of mice with recombinant BCG producing S1-TTC, the mice were sacrificed by cervical dislocation and the spleens were removed aseptically. Spleen cells were isolated by using a loosely fitting Dounce homogenizer, washed, adjusted to a concentration of 4 × 106 per ml, and grown in flat-bottomed microwell plates (Nunc) in RPMI 1640 medium supplemented with HEPES, glutamine, 5 × 10−5 M 2-mercaptoethanol, antibiotics, and 10% heat-inactivated fetal calf serum. The purified S1-TTC or PTX, purchased from List Biologicals (Campbell, Calif.), was added to the cultures at 10 μg/ml and 1 μg/ml, respectively. The cells were incubated at 37°C in a humidified incubator at 5% CO2. After 24 h the supernatants were harvested for the determination of IL-2 production as described previously (17). Each experiment was repeated at least three times, and the results of one representative experiment are shown.
RESULTS
Production of S1-TTC under the control of the antigen 85A promoter and signal peptide.
We have previously shown that the antigen 85A promoter and signal peptide can be used for heterologous expression and protein secretion in mycobacteria (21). In a first attempt, therefore, we used these expression and secretion signals for the production of S1-TTC in BCG by constructing pEN004, as described in Materials and Methods. In addition to the S1-TTC expression cassette, this vector contains a mycobacterial origin of replication and a Kanr gene (Fig. 1A).
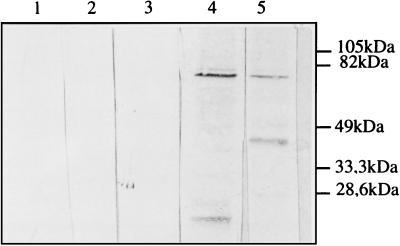
M. smegmatis and BCG were transformed with pEN004, and the recombinant mycobacteria were selected on solid Middlebrook medium containing 25 μg of kanamycin/ml. Kanamycin-resistant colonies were tested for their plasmid content by the electroduction method described by Baulard et al. (6). Recombinant mycobacteria containing the expected plasmid were then grown in liquid medium containing 25 μg of kanamycin/ml for approximately 5 days in the case of M. smegmatis and for 2 weeks in the case of BCG. Mycobacterial cell extracts were then prepared and analyzed by immunoblotting with anti-S1 monoclonal antibody 1B7 and an anti-TTC polyclonal antibody. As shown in Fig. 2, only the recombinant BCG containing pEN004 produced an immunoreactive band, corresponding to an approximately 75-kDa protein. The size of the protein was as expected, and it was recognized by both anti-S1 and anti-TTC antibodies, indicating that it contained both antigenic determinants. In addition to the 75-kDa protein, a smaller, approximately 37-kDa protein was also detected by the anti-TTC antibodies. This protein was not recognized by 1B7, suggesting that it is a proteolytic breakdown product containing essentially the C-terminal portion of the hybrid protein. Similar results were obtained with M. smegmatis (not shown).
FIG. 2.
Immunoblot analysis of recombinant BCG(pEN004). Recombinant BCG(pEN004) (lanes 3 to 5) was analyzed with anti-S1 monoclonal antibody 1B7 (lanes 1 to 4) and anti-TTC polyclonal antibodies (lane 5). Both whole-cell extracts (lanes 4 and 5) and culture supernatant (lane 3) were analyzed. Lane 1 contains a whole-cell extract, and lane 2 contains culture supernatant of nonrecombinant BCG as a control. The sizes of the molecular mass markers are given in the right margin.
Production of S1-TTC under the control of the hsp60 promoter.
A second expression system used in this study was driven by the M. bovis BCG hsp60 promoter to produce intracellular S1-TTC via the construction of pBMX. This plasmid had characteristics similar to those of pEN004, except that the antigen 85A promoter and signal-peptide coding sequence were replaced by the BCG hsp60 promoter (Fig. 1B).
After transformation and selection of recombinant M. smegmatis and BCG, cell extracts were prepared and analyzed by immunoblotting with the monoclonal antibody 1B7 and the polyclonal anti-TTC antibodies. With either reagent, an approximately 75-kDa protein was again readily detectable in extracts of BCG containing pBMX (Fig. 3). Similar results were obtained for M. smegmatis (not shown). This protein was not present in the BCG control extracts, indicating that the pBMX expression system also yielded an immunoreactive hybrid protein containing both the PTX and TTX antigenic determinants. Expression levels appeared to be significantly higher than in BCG(pEN004) extracts, and several smaller peptides were detected with anti-TTC antibodies in BCG(pBMX) extracts, whereas a single major breakdown product was found in BCG(pEN004) lysates. When compared on a quantitative basis by densitometric scanning of the immunoblots, BCG(pBMX) was found to produce approximately 10-fold more S1-TTC than BCG(pEN004) (approximately 5 ng of S1-TTC/μg of total protein versus approximately 0.4 ng of S1-TTC/μg of total protein). As expected, no extracellular S1-TTC was detected in culture supernatants of BCG(pBMX).
FIG. 3.
Immunoblot analysis of recombinant BCG(pBMX). Recombinant BCG(pBMX) (lanes 2 and 3) was analyzed with anti-S1 monoclonal antibody 1B7 (lane 3) and anti-TTC polyclonal antibodies (lane 2). Lane 1 contains a whole-cell extract of nonrecombinant BCG as a control. The sizes of the molecular mass markers are given in the right margin.
Antibody responses after immunization with BCG(pEN004) or BCG(pBMX).
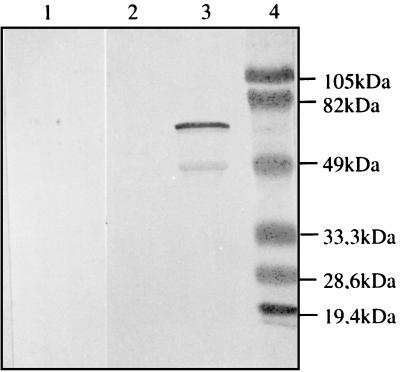
Groups of five BALB/c mice were immunized with 5 × 106 BCG(pEN004), BCG(pBMX), or nonrecombinant control BCG cells either i.v. or i.p. Two months later, the mice were boosted with 5 × 106 cells of the same BCG strains. At different time intervals after the boost, sera were collected, pooled per group of mice, and then tested for the presence of anti-BCG and anti-S1-TTC antibodies by immunoblotting and ELISA. All groups of mice produced anti-BCG antibodies at similar levels (data not shown). However, only those mice that had been immunized i.p. with BCG(pBMX) produced antibodies directed against S1-TTC (Fig. 4). These antibodies did not react with purified S1, suggesting that they were directed against the TTC part of the protein. No such antibodies were found in the sera of mice that were immunized i.p. with BCG(pEN004) or with the control BCG or those that were immunized i.v. with any strain.
FIG. 4.
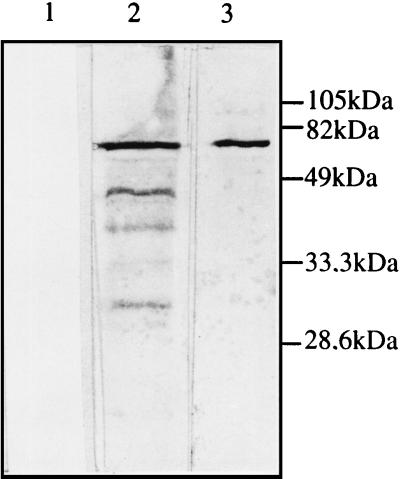
Immunoblot analysis of sera of mice immunized with BCG(pBMX). Ten weeks after the first immunization, the sera of mice immunized with BCG(pBMX) (lanes 2 and 3) or with nonrecombinant BCG (lane 1) was analyzed by immunoblotting with purified S1-TTC (lanes 1 and 3) or recombinant S1d (lane 2) as an antigen. Lane 4 contains the molecular mass markers, the sizes of which are given in the right margin.
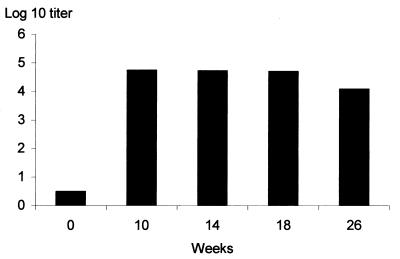
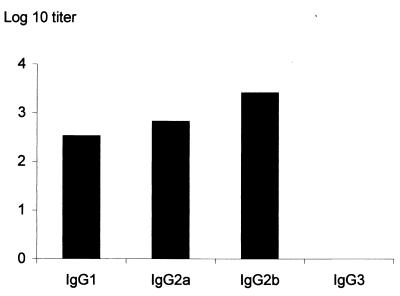
As shown in Fig. 5, the anti-S1-TTC antibody titers of the mice that had received BCG(pBMX) stayed high for at least 26 weeks after the boost. The analysis of the isotype profile indicated that specific anti-S1-TTC IgG1, IgG2a, and IgG2b were produced (Fig. 6), suggestive of a mixed antibody response. The antibodies were exclusively directed against the TTC part of the hybrid protein, since no anti-PTX antibodies were detected by ELISA (not shown).
FIG. 5.
Kinetics of anti-S1-TTC antibody titers of mice immunized with BCG(pBMX). The total anti-S1-TTC IgG titers of mice immunized with BCG(pBMX) were determined at 0, 10, 14, 18, and 26 weeks after the first immunization, as indicated. The titers correspond to the reciprocals of the highest serum dilutions giving optical density values that are at least threefold over background levels.
FIG. 6.
Isotype profile of anti-S1-TTC antibodies of mice immunized with BCG(pBMX). Pooled sera of mice immunized with BCG(pBMX) were analyzed for their anti-S1-TTC IgG1, IgG2a, IgG2b, and IgG3 titers. The titers were estimated 18 weeks after the first immunization and correspond to the reciprocals of the highest serum dilutions giving optical density values that are at least threefold over background levels.
To test for the functional activities of these anti-TTX antibodies, the in vivo toxin-neutralizing activities of the sera were measured by using the reference method of the European Pharmacopoeia. At 2 weeks after the first immunization with BCG(pBMX) the sera contained 0.01 to 0.02 IU/ml. This level of neutralizing antibodies rose to 0.06 to 0.08 IU/ml after the boost, whereas the neutralizing activities of sera of mice immunized with nonrecombinant BCG remained below detectable levels. Since levels above 0.01 IU/ml are considered protective in humans, a single immunization with the recombinant strain thus induced protective levels of anti-TTX antibodies.
IL-2 production after immunization with BCG(pBMX).
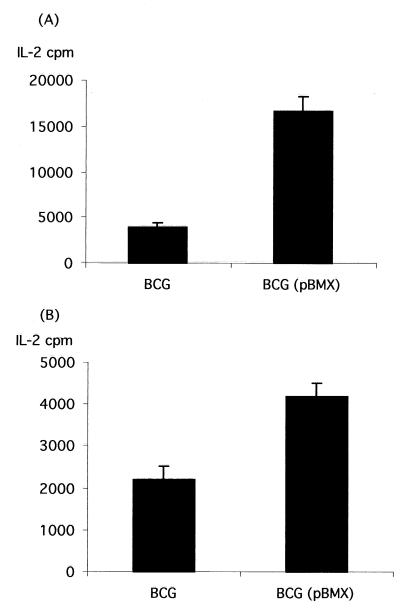
The spleens of BALB/c mice immunized and boosted with either BCG(pEN004), BCG(pBMX), or nonrecombinant BCG were tested for the secretion of IL-2 by using purified S1-TTC for stimulation of the spleen cells. As shown in Fig. 7A, high levels of S1-TTC-specific IL-2 secretion were detected only for spleen cells isolated from mice that were immunized with BCG(pBMX), whereas BCG-specific IL-2 secretion was also detected for cells isolated from nonrecombinant BCG-infected mice.
FIG. 7.
Antigen-specific IL-2 production in mice immunized with BCG(pBMX). The IL-2 contents in culture supernatants of splenocytes isolated from mice immunized with nonrecombinant BCG (left) or BCG(pBMX) (right) were estimated as described in Materials and Methods either after stimulation with purified S1-TTC (A) or with PTX (B). The error bars indicate the standard deviations.
When PTX was used to stimulate the spleen cells, IL-2 secretion was also detected only when the spleen cells were isolated from mice immunized with BCG(pBMX) (Fig. 7B).
DISCUSSION
BCG is one of the most widely used vaccines available today and is the only live bacterial vaccine recommended for use in children by the World Health Organization. Although its efficacy against tuberculosis still remains a matter of considerable debate (12), BCG has a remarkably low incidence of severe side effects. This makes it one of the most promising bacterial live vectors for the production of heterologous antigens to develop cost-effective vaccines able to protect against several diseases simultaneously. Consequently, several mycobacterial expression systems have been developed over the last few years (2, 23, 29, 39). In some instances protection against challenge has been achieved against the heterologous pathogen when recombinant BCG was given as a vaccine (13, 16, 26, 27). Different laboratories have used different expression systems based on several promoters. The most widely used promoter is that of the hsp60 gene. However, other promoters have also been used, such as the pAN promoter from Mycobacterium paratuberculosis (29) or the promoters of the genes encoding the antigen 85 complex (20, 21). The antigen 85 complex contains several major mycobacterial secreted proteins, named 85A, 85B, and 85C. The use of the promoter of the 85B gene controlling the production of the hybrid protein antigen 85B fused to the V3 epitope of the human immunodeficiency virus led to a recombinant BCG strain that was able to induce protective immune responses in small animals (16). In a comparative study with the same reporter gene, we have recently shown that the 85A promoter is stronger than the 85B promoter (1). The 85A promoter has been used successfully for the expression of several heterologous antigens (7, 21).
In this study, we compared the immunogenicity of recombinant BCG strains producing a PTX-TTX hybrid protein (S1-TTC) under the control of either the hsp60 promoter or the 85A promoter and signal peptide. The recombinant protein was readily detected in both expression systems. When the chimeric gene was expressed under the control of the hsp60 promoter, the expression level was approximately 10-fold higher than when the 85A promoter and signal peptide were used. The comparison of the anti-S1-TTC immune responses indicated that only the BCG strain producing the antigen under the control of the hsp60 promoter induced anti-S1-TTC antibodies. No immune response was detected with the 85A expression system, even after three immunizations. This finding was somewhat surprising, since, although the hsp60 promoter induced stronger expression of the foreign gene, the difference with the 85A expression system was only about 10-fold in vitro. However, it may be conceivable that the expression levels are much more divergent in vivo. In Salmonella the hsp gene is induced in vivo (10). When chromosome-borne, the BCG hsp60 promoter is also induced by stress; however, this regulation is apparently lost when the hsp60 promoter is plasmid-borne (39). Although there is no evidence yet that the antigen 85 complex genes are regulated in vivo, mice or humans infected by M. tuberculosis show a strong immune response against the antigen 85 complex (18), indicating that these proteins are produced in vivo.
Another difference between the two expression systems described here is the presence of the signal peptide in the 85A system. Although this system has been shown to allow for secretion of recombinant proteins (7, 21), no S1-TTC was found in the culture supernatant of the recombinant BCG strains. It is most likely that this is due to the relatively large size (75 kDa) of the antigen compared to antigens successfully secreted in the previous studies. Indeed, progressive shortening of the S1-TTC hybrid protein by the 3′ truncation of its gene gradually increased its secretion efficiency (7a). Nevertheless, it can be assumed that in the 85A expression system S1-TTC is located at the external side of the membrane, since the estimated size is consistent with that of the mature protein after removal of the signal peptide. Because of its relatively large size, the antigen is probably trapped in the cell wall. Since it has been shown by others that secretion of the foreign antigen by recombinant BCG may increase its immune response, we are currently constructing new vectors combining the hsp60 promoter with the 85A signal peptide coding region in an effort to distinguish between the role of secretion or hsp60-dependent expression in the induction of antibody responses against S1-TTC.
Anti-S1-TTC antibody titers remained high for at least 6 months after immunization with the recombinant BCG producing the antigen under the control of the hsp60 promoter. Isotyping indicated that the mice produced IgG1, IgG2a, and IgG2b antibodies, suggestive of a mixed response. These antibodies, however, were mostly directed against the TTC part of the molecule, since no antibodies reactive against PTX were detected in the mouse sera. Instead, the mice immunized with this recombinant BCG strain mounted a weak but significant cellular immune response against PTX, as evidenced by the secretion of antigen-specific IL-2. This finding is particularly interesting with respect to the development of a combined vaccine against tetanus and pertussis, since recent evidence strongly suggests that the mechanism of protective immunity against pertussis in mice involves IL-2-secreting T cells (28). In addition, Th1 CD4+ cells probably also play a major role in protective immunity in humans, since convalescent patients with whooping cough produce B. pertussis-specific T cells that secrete high levels of IFN-γ and IL-2 but very little IL-5 or IL-4 (32, 33), and it is well known that infection with B. pertussis provides better and longer-lasting protection against whooping cough than does vaccination. Since immunization with recombinant BCG producing S1-TTC induces anti-TTX antibodies and anti-PTX-specific T cells of the Th1 subpopulation, this may constitute a promising approach for the development of a novel, effective, and safe combined vaccine against tetanus and whooping cough, affordable even for the developing world.
However, several points remain to be addressed. Disseminated BCG infections have been reported in patients with AIDS, and the use of recombinant BCG may be particularly counterindicated in areas where the human immunodeficiency virus prevalence is high. Recent efforts to genetically engineer BCG strains so that they can be used in immunocompromised hosts have provided some encouraging initial results (3), and it will be interesting to evaluate the S1-TTC construct for its immunogenicity in some of those strains. In addition, the immune response to the PTX part of the molecule induced after administration of the recombinant BCG strain is rather weak, and future constructs may help to increase its immunogenicity, either by the coexpression of cytokines or by simply redesigning the hybrid protein. Using similar S1-TTC constructs expressed in Salmonella typhi vaccine strains, Barry et al. (4) have shown that the S1 subunit fused to the N terminus of TTC was less effective than S1 fused to the C terminus in generating anti-PTX antibodies. Finally, antipertussis and antitetanus vaccines are usually administered together with antidiphtheria vaccines. We are therefore constructing recombinant BCG strains coexpressing protective diphtheria toxin domains together with the S1-TTC hybrid protein.
ACKNOWLEDGMENTS
We thank H. and Y. Sato for antibodies, A. Baulard for helpful discussion in the initial stages of the project, and E. Fort for photography.
The work was supported by INSERM, Institut Pasteur de Lille, Région Nord-Pas de Calais et Ministère de l’Enseignement Supérieur et de la Recherche. B.A. holds a fellowship from CNOUS.
REFERENCES
- 1.Abomoelak, B., et al. Unpublished data.
- 1a.Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet. 1988;i:955–960. . (Erratum, Lancet i:1238). [PubMed] [Google Scholar]
- 2.Aldovini A, Young R A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991;351:479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- 3.Bange F-C, Brown A M, Jacobs W R., Jr Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect Immun. 1996;64:1794–1799. doi: 10.1128/iai.64.5.1794-1799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry E M, Gomez-Duarte O, Chatfield S, Rappuoli R, Pizza M, Losonsky G, Galen J, Levine M M. Expression and immunogenicity of pertussis toxin S1 subunit-tetanus toxin fragment C fusions in Salmonella typhi vaccine strain CVD908. Infect Immun. 1998;64:4172–4181. doi: 10.1128/iai.64.10.4172-4181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass J W, Stephenson S R. The return of pertussis. Pediatr Infect Dis J. 1987;6:141–144. doi: 10.1097/00006454-198702000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Baulard A, Jourdan C, Mercenier A, Locht C. Rapid mycobacterial plasmid analysis by electroduction between Mycobacterium spp. and Escherichia coli. Nucleic Acids Res. 1992;20:4105. doi: 10.1093/nar/20.15.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baulard A, Kremer L, Supply P, Vidaud D, Bidart J-M, Bellet D, Locht C. A new series of mycobacterial expression vectors for the development of live recombinant vaccines. Gene. 1996;176:149–154. doi: 10.1016/0378-1119(96)00239-9. [DOI] [PubMed] [Google Scholar]
- 7a.Baulard, A., L. Kremer, and C. Locht. Unpublished data.
- 8.Bloom B R, Snapper S B, Kieser T, Jacobs W R., Jr Development of recombinant BCG vaccines. Semin Virol. 1990;1:21–30. [Google Scholar]
- 9.Boucher P, Sato H, Sato Y, Locht C. Neutralizing antibodies and immunoprotection against pertussis and tetanus obtained by use of a recombinant pertussis toxin-tetanus toxin fusion protein. Infect Immun. 1994;62:449–456. doi: 10.1128/iai.62.2.449-456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 11.Cody C L, Baraff L J, Cherry J D, Marcy S M, Manclark C R. Nature and rates of adverse reactions associated with DTP and DT immunization in infants and children. Pediatrics. 1981;68:650–660. [PubMed] [Google Scholar]
- 12.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Durdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 13.Connell N D, Medina-Acosta E, McMaster W R, Bloom B R, Russell D G. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guérin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi A E, Anemona A, Ciofi degli Atti M L, Giammanco A, Panei P, Blackwelder W C, Klein D L, Wassilak S G F the Progetto Pertosse Working Group. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med. 1996;334:341–348. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson L, Hallander H O, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 16.Honda M, Matsuo K, Nakasone T, Okamoto Y, Yoshizaki H, Kitamura K, Sugiura W, Watanabe K, Fukushima Y, Haga S, Katsura Y, Tasaka H, Komuro K, Yamada T, Asano T, Yamazaki A, Yamazaki S. Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guérin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proc Natl Acad Sci USA. 1995;92:10693–10697. doi: 10.1073/pnas.92.23.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, van Vooren J-P, De Leys R. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K, van Vooren J P, Turneer M, Bosmans R, Diercks P, De Bruyn J. Specific lymphoproliferation, gamma interferon production and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988;27:187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson D. Duration of effectiveness of pertussis vaccine: evidence from a 10 year community study. Br Med J. 1988;296:612–614. doi: 10.1136/bmj.296.6622.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazuhiro M, Yamaguchi R, Yamazaki A, Tasaka H, Terasaka K, Totsuka M, Kobayashi K, Yukitake H, Yamada T. Establishment of a foreign antigen secretion system in mycobacteria. Infect Immun. 1990;58:4049–4054. doi: 10.1128/iai.58.12.4049-4054.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kremer L, Baulard A, Estaquier J, Content J, Capron A, Locht C. Analysis of the Mycobacterium tuberculosis 85A antigen promoter region. J Bacteriol. 1995a;177:642–653. doi: 10.1128/jb.177.3.642-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremer L, Baulard A, Estaquier J, Poulain-Godefroy O, Locht C. Green fluorescent protein as a new expression marker in mycobacteria. Mol Microbiol. 1995b;17:913–922. doi: 10.1111/j.1365-2958.1995.mmi_17050913.x. [DOI] [PubMed] [Google Scholar]
- 23.Kremer L, Riveau G, Baulard A, Capron A, Locht C. Neutralizing antibody responses elicited in mice immunized with recombinant Bacillus Calmette-Guérin producing the Schistosoma mansoni glutathione S-transferase. J Immunol. 1996;156:4309–4317. [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lambert H J. Epidemiology of a small pertussis outbreak in Kent County, Michigan. Public Health Rep. 1965;80:365. [PMC free article] [PubMed] [Google Scholar]
- 26.Langermann S, Palaszynski S R, Burlein J E, Koenig S, Hanson M S, Briles D E, Stover C K. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guérin vaccines expressing pneumococcal surface protein A. J Exp Med. 1994;180:2277–2286. doi: 10.1084/jem.180.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langermann S, Palaszynski S R, Sadziene A, Stover C K, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing an outer-surface protein of Borrelia burgdorferi. Nature. 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 28.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muray A, Winter N, Lagranderie M, Hill D F, Rauzier J, Timm J, Leclerc C, Moriarty K M, Gheorghiu M, Gicquel B. Expression of Escherichia coli β-galactosidase in Mycobacterium bovis BCG using an expression system from Mycobacterium paratuberculosis which induced humoral and cellular immune responses. Mol Microbiol. 1992;6:3331–3342. doi: 10.1111/j.1365-2958.1992.tb02201.x. [DOI] [PubMed] [Google Scholar]
- 30.Peppoloni S, Nencioni L, Di Tommaso A, Tagliabue A, Parronchi P, Romagnani S, Rappuoli R, De Magistris T. Lymphokine secretion and cytotoxic activity of human CD4+ T-cell clones against Bordetella pertussis. Infect Immun. 1991;59:3768–3773. doi: 10.1128/iai.59.10.3768-3773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkin S A, Mortimer E A Jr, editors. Vaccines. Philadelphia, Pa: WB Saunders; 1994. [Google Scholar]
- 32.Ryan M, Gothefors L, Storsaeter J, Mills K H G. B. pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev Biol Stand. 1996;89:251–259. [PubMed] [Google Scholar]
- 33.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H G. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 Th cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory Manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sato H, Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990;58:3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato H, Ito A, Chiba J, Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984;46:422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato Y, Kimura M, Fukumi H. Development of a pertussis component in Japan. Lancet. 1984;i:122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- 38.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 39.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Jr, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 40.Turneer M, Marchal A. Pouvoirs immunogène et protecteur d’une anatoxine tétanique absorbée. Ann Méd Vét. 1983;127:635–642. [Google Scholar]