Abstract
Background
The introduction of highly effective direct-acting antiviral therapy has changed the hepatitis C virus (HCV) treatment paradigm. However, a recent update on HCV epidemiology in incarcerated settings is necessary to accurately determine the extent of the problem, provide information to policymakers and public healthcare, and meet the World Health Organization's goals by 2030. This systematic review and meta-analysis were performed to determine the prevalence of HCV Ab and RNA in incarcerated settings.
Methods
For this systematic review and meta-analysis, we searched PubMed, Embase, Scopus, and Web of Science for papers published between January 2013 and August 2021. We included studies with information on the prevalence of HCV Ab or RNA in incarcerated settings. A random-effects meta-analysis was done to calculate the pooled prevalence and meta-regression to explore heterogeneity.
Results
Ninety-two unique sources reporting data for 36 countries were included. The estimated prevalence of HCV Ab ranged from 0.3% to 74.4%. HCV RNA prevalence (available in 46 sources) ranged from 0% to 56.3%. Genotypes (available in 19 sources) 1(a) and 3 were most frequently reported in incarcerated settings. HCV/HIV coinfection (available in 36 sources) was highest in Italy, Estonia, Pakistan, and Spain. Statistical analysis revealed that almost all observed heterogeneity reflects real differences in prevalence between studies, considering I2 was very high in the meta-analysis.
Conclusions
HCV in incarcerated settings is still a significant problem with a higher prevalence than in the general population. It is of utmost importance to start screening for HCV (Ab and RNA) in incarcerated settings to give clear, reliable and recent figures to plan further treatment. This is all in the context of meeting the 2030 WHO targets which are only less than a decade away.
Trial registration
PROSPERO: CRD42020162616
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-022-14623-6.
Keywords: Hepatitis c, Incarcerated setting, Prevalence, Global health, Meta-analysis
Background
Chronic hepatitis C virus (HCV) infection remains a global health problem prompting the World Health Organization (WHO) to define elimination goals by 2030 (by reducing new infections by 90% and mortality by 65%) [1]. One of the key risk groups for HCV infection is people with a history of (injecting) drug use, with the majority having an opioid use disorder [2, 3]. These individuals often have a lower social health status (e.g., low education level, unstable housing) compared to the general population and are often marginalized [4]. Subsequently, they are difficult to reach and have reduced access to healthcare services [5]. Since drug use is illegal in most countries, these individuals are more likely to end up in incarcerated settings. Prior studies indicate that individuals who are incarcerated are more likely to engage in HCV-related risk behavior such as unsterile tattooing, high-risk sexual behavior, and sharing paraphernalia [2, 6]. Worldwide, this has led to an increased prevalence of HCV in individuals who are incarcerated compared to the general population. The prevalence of HCV antibodies (Ab) in closed settings was estimated in 2013 by Larney et al. to be 30% in Western Europe and varied worldwide with prevalences up to 35% in Australia and 38% in Central Asia [6, 7]. In 2016, Dolan et al. showed a decrease in the estimated pooled HCV Ab prevalence in Western European incarcerated settings (15.5%) [6]. In contrast, HCV Ab is present in 2–3% of the global population [8]. Given that at any moment, an estimated 1.6 million people are incarcerated in Europe and even 10.7 million worldwide, individuals who are incarcerated are a key group to combat for HCV elimination [9].
The high occurrence of HCV infection in individuals who are incarcerated and the substantial risks associated with untreated HCV infection underline the need for HCV screening and access to treatment in individuals who are incarcerated [10]. The WHO, therefore, recommends that all individuals who are incarcerated should be tested for HCV at least once during their stay [11]. However, adherence to WHO guidelines for HCV screening in incarcerated settings is currently inadequate. In Europe, only ten (34.4%) of 29 surveyed countries reported HCV screening programs for individuals who are incarcerated in 2010 [10, 12]. Since then, only scarce data on HCV prevalence in individuals who are incarcerated have been published in Europe or other regions [13]. Also, chronic HCV infection leads to cirrhosis and potentially hepatocellular carcinoma if untreated and is one of the leading causes of liver transplantations in high-income countries [14]. However, neither Larney et al. nor Dolan et al. reported data on HCV RNA or HCV genotype. Nevertheless, they suggested that HCV screening and subsequent antiviral treatment in this group of patients is necessary and will reduce the global HCV transmission rate [15, 16].
To determine the extent of the problem and provide guidance to policymakers and public healthcare, a better understanding of the epidemiology of HCV infection in individuals who are incarcerated is necessary. A valuable meta-analysis on HCV prevalence in closed settings worldwide was published in 2013. This review showed high heterogeneity among prevalence estimates from the different data sources [7]. To reach the WHO goals by 2030, we need to systematically map this global health problem in all risk groups, including individuals who are incarcerated.
Elimination of HCV (reducing new infections by 90% and 80% of eligible patients being treated), even in incarcerated settings, is possible as the Trap HepC study from Iceland demonstrates. They offered screening and (simultaneous) treatment for HCV to all individuals who were incarcerated. If released during treatment, the individual who was incarcerated was followed at one of the TraP HepC treatment sites to facilitate the elimination of HCV in formerly incarcerated persons in the community [17].
Therefore, we performed an update and a new systematic review and meta-analysis on HCV in incarcerated settings worldwide. This review will report not only data on HCV Ab but, for the first time, also on HCV RNA and genotype in incarcerated settings. HCV Ab prevalence will shed light on the overall problem and the need for testing and monitoring. HCV RNA prevalence indicates the necessity of antiviral treatment. Genotyping is essential for countries that do not have access to pangenotypic antivirals. Since HIV is a risk factor for HCV, we also aim to assess the extent to which HCV/HIV coinfection is present in incarcerated settings worldwide.
Materials and methods
Search strategy
This systematic review and meta-analysis is reported in line with the PRISMA checklist [see Additional file A1] [18] and registered at PROSPERO (CRD42020162616). To assess HCV prevalence in individuals who are incarcerated worldwide, a structured approach was followed. For this purpose, PubMed, Embase, Scopus, and Web of Science databases were used.
The following terms and keywords alone and/or in appropriate combinations were included in the search: “hepatitis C,” “HCV,” “antibodies,” “RNA,” “jail,” “prevalence,” and “prison.” The search was limited to full articles or abstracts in the English language and published between January 2013 and August 2021 [see Additional file A2]. This date limit was set as in 2013, Larney et al. published an essential meta-analysis on HCV prevalence in closed settings worldwide [7].
All search results were systematically screened by the first author and last author and documented using EndNote X8. The snowball method was used to enrich the results, meaning that reference lists of included articles were screened for relevant articles.
Data extraction
A quality assessment of the found sources was carried out. To grade and select studies for inclusion, available methodological information was used, and the principles defined in Nelson et al. were used to grade the data [19]. Ungraded studies (i.e., unknown methodology) were excluded [see Additional file A3].
We selected HCV prevalence studies in a incarcerated setting (prison, jail, or pre-trial detention center). Studies using self-reported HCV status, saliva, dried blood spot sampling or RNA testing only were also included though they were graded lower [see Additional file A3]. Participants in the included studies were defined as individuals who are incarcerated at a criminal justice facility with any sentence duration and aged 18 years or older. We explicitly excluded studies with a study population consisting of individuals who are incarcerated not by criminal justice (war prisoners, concentration camps, persons in police custody, and persons in migrant centers), individuals with a history of incarceration or ex-convicts, and individuals who are incarcerated aged 17 years or younger. Publication types such as guidelines, perspectives, correspondence, systematic reviews or meta-analyses were excluded as well.
We extracted the following data from the articles included in this study: study sample, enrolment dates, country, number of subjects studied, number of persons positive for HCV Ab and/or HCV RNA, HCV genotypes, and HIV/HCV coinfection. If the article reported risk factors significantly associated with HCV prevalence, these were also extracted and are shown in [see Additional file A5].
Statistical analyses
All statistical analyses were performed in R version 3.6.1. [20]. In this meta-analysis, studies in individuals who are incarcerated selected for the systematic review were included if they estimated HCV prevalence. If the exact number of cases was unknown, this was computed from prevalence and sample size and rounded upward. Sensitivity analysis was performed by rounding these numbers downward. Classic random-effects meta-analysis (i.e., inverse variance method) with double arcsine transformed proportions was used. Since the analysis results using double arcsine transformed proportions may be seriously misleading when the individual sample sizes vary (in this study ranging from 133 to 101,727), logit transformed proportions were used as a sensitivity analysis. A generalized linear mixed model (GLMM; random-intercept logistic regression model, with logit transformed proportions) was also used as recommended by Schwarzer et al. [21].
Heterogeneity was investigated using the I2 measure, which ranges from 0 to 100% and reflects the proportion of the observed variance that reflects true differences in prevalence estimates not attributable to chance [22]. To investigate possible sources of between-study heterogeneity, subgroup analyses based on region (Table 1), publication year, study size, type of study, single vs. multi-site were performed, and to whether people who inject drugs (PWID) or HIV-coinfected population were included. Next, a meta-regression analysis was performed. First, univariate models were used to examine which study characteristics could explain some of the between-study variability. Study characteristics included were region, publication year, sample size, study type, single- or multi-centric, the proportion of HIV coinfected individuals, the proportion of PWID, and the proportion of males. All characteristics with a p-value < 0.200 were then included in a multivariable meta-regression model, and backward selection was applied, removing all non-significant covariates in a stepwise manner.
Table 1.
Results of a systematic review on hepatitis C virus prevalence in incarcerated settings
| Author | Publication year | Sample size | Enrolment dates | Prevalence HCV (%) | ||
|---|---|---|---|---|---|---|
| Ab | RNA | |||||
| Eastern Europe | ||||||
| Azerbaijan | Azbel L et al. [23] | 2015 | 510 | 2014 | 38.2 | - |
| Bosnia and Herzegovina | Hodzic H et al. [24] | 2017 | 200 | 2013 | 13.0 | - |
| Estonia | Kivimets K et al. [25] | 2018 | 1,845 | 2014–2015 | - | 56.3 |
| Georgia | Bergen-Cico D et al. [26] | 2017 | 500 | 2016 | 60.0 | - |
| Harris AM et al. [27] | 2019 | 13,500 | 2013–2015 | 38.0 | 28.4 | |
| Hungary | Vanya M et al. [28] | 2017 | 200 | 2014 | 1.0 | - |
| Macedonia | Jovanovska T et al. [29] | 2014 | 200 | - | 20.0 | - |
| Turkey | Keten D et al. [30] | 2016 | 266 | 2014–2015 | 17.7 | 8.6 |
| Kose S et al. [31] | 2019 | 360 | - | 0.5 | - | |
| Ukraine | Azbel L et al. [32] | 2013 | 402 | 2011 | 60.2 | - |
| Western Europe | ||||||
| Austria | Silbernagl M et al. [33] | 2018 | 133 | - | 74.4 | 45.0 |
| Belgium | Busschots D et al. [34] | 2021 | 886 | 2019–2020 | 5.0 | 2.1 |
| Denmark | Soholm J et al. [35] | 2019 | 801 | 2016–2017 | 7.4 | 4.2 |
| France | Semaille C et al. [36] | 2013 | 2,154 | 2010 | 4.8 | 2.5 |
| Roux P et al. [37] | 2014 | 5,957 | 2004–2010 | 5.2 | - | |
| Jacomet C et al. [38] | 2016 | 342 | 2012–2013 | 4.7 | 1.5 | |
| Izquierdo L et al. [39] | 2019 | 1,093 | 2017 | 2.9 | 1.1 | |
| Ireland | Crowley D et al. [40] | 2019 | 422 | 2017 | 22.8 | 5.5 |
| Italy | Brandolini M et al. [41] | 2013 | 695 | 2006–2008 | 22.4 | 19.4 |
| Foschi A et al. [42] | 2016 | 3,400 | - | 10.0 | 6.0 | |
| Masarone M et al. [43] | 2020 | 458 | 2018–2019 | 12.7 | 10.0 | |
| Fiore V et al. [44] | 2021 | 2,376 | 2019 | 10.4 | 4.3 | |
| Spain | Marco, A et al. [45] | 2014 | 2,377 | 1992–2011 | - | 4.9 |
| Cuadrado A et al. [46] | 2018 | 847 | 2016–2017 | 13.0 | 10.2 | |
| Jiménez Galan G et al. [47] | 2019 | 1,200 | 2015 | 12.4 | - | |
| Cabezas J et al. [48] | 2021 | 548 | 2019–2021 | 8.0 | 2.9 | |
| United Kingdom | Taylor A et al. [49] | 2013 | 4,904 | 2010–2011 | 19.0 | - |
| Aisyah D et al. [50] | 2018 | 511 | 2011–2013 | 4.0 | 3.1 | |
| Morey S et al. [51] | 2019 | 1,495 | 2016–2017 | 6.4 | 3.1 | |
| Perrett S et al. [52] | 2019 | 256 | 2016 | 3.1 | - | |
| Bhandari R et al. [53] | 2020 | 8,538 | 2016–2020 | 7.2 | 4.4 | |
| Mohamed Z et al. [54] | 2020 | 2,442 | 2017–2019 | 3.7 | 2.6 | |
| Perrett S et al. [55] | 2020 | 6,949 | 2015–2017 | 11.0 | - | |
| Gahrton C et al. [56] | 2019 | 471 | 2017 | 17.0 | 11.5 | |
| Switzerland | Chacowry P et al. [57] | 2018 | 273 | 2011–2013 | 6.2 | - |
| Northern America | ||||||
| Canada | Courtemanche Y et al. [58] | 2018 | 1,315 | 2014–2015 | 12.9 | - |
| United States | Alvarez KJ et al. [59] | 2013 | 2,788 | 2009–2013 | - | 10.1 |
| Cocoros N et al. [60] | 2014 | 596 | 2009–2011 | 20.5 | - | |
| Wenger PJ et al. [61] | 2014 | 304 | 2012–2013 | 16.4 | - | |
| Kuncio D et al. [62] | 2015 | 51,562 | 2011–2012 | 3.0 | - | |
| Beckwith C et al. [63] | 2016 | 249 | 2012–2014 | 9.2 | 6.0 | |
| Mahowald M et al. [64] | 2016 | 101,727 | 2004–2012 | 18.7 | 5.2 | |
| Schoenbachler B et al. [65] | 2016 | 893 | 2012–2014 | 13.2 | 7.4 | |
| Stockman LJ et al. [66] | 2016 | 1,239 | 2014–2015 | 12.5 | 8.9 | |
| Akiyama M et al. [67] | 2017 | 10,856 | 2013–2014 | 20.6 | - | |
| de la Flor C et al. [68] | 2017 | 3042 | 2015–2016 | 16.4 | - | |
| Hochstatter K et al. [69] | 2017 | 22,918 | 2015 | - | 13.6 | |
| Assoumou SA et al. [70] | 2019 | 24,567 | 2012–2016 | 20.0 | 7.0 | |
| Abe C et al. [71] | 2020 | 4,089 | 2017 | 17.3 | 10.1 | |
| Chan J et al. [72] | 2020 | 40,219 | 2014–2017 | - | 11.6 | |
| Qureshi N et al. [73] | 2021 | 80,681 | 2000–2019 | 34.6 | - | |
| Latin America | ||||||
| Argentina | Adaszko D et al. [74] | 2018 | 2,181 | 2015–2017 | 3.3 | - |
| Mendizabal M et al. [75] | 2020 | 1,141 | 2018–2020 | - | 1.1 | |
| Brazil | Barros LA et al. [76] | 2013 | 148 | 2007–2008 | 6.1 | 3.4 |
| Falquetto T et al. [77] | 2013 | 730 | 2010 | 1.0 | 0.8 | |
| El Maerrawi I et al. [78] | 2015 | 680 | 2007 | 5.3 | - | |
| Puga M et al. [79] | 2017 | 3,368 | 2013–2014 | 2.4 | 1.5 | |
| Felisberto M et al. [80] | 2019 | 147 | 2015 | 5.4 | - | |
| Machado F et al. [81] | 2019 | 349 | 2016–2017 | - | 8.3 | |
| Do Nascimento CT et al. [82] | 2020 | 37,497 | 2017–2018 | - | 0.2 | |
| Mexico | Bautista-Arredondo S et al. [83] | 2015 | 17,296 | 2010 | 3.2 | - |
| Belaunzaran-Zamudio P et al. [84] | 2017 | 3,192 | 2011–2012 | 4.8 | - | |
| Silverman-Retana O et al. [85] | 2017 | 391 | 2010–2011 | 3.3 | - | |
| Central Asia | ||||||
| Kyrgyzstan | Azbel L et al. [86] | 2016 | 368 | 2014 | 42.4 | - |
| East Asia | ||||||
| Taiwan | Yang TH et al. [87] | 2020 | 824 | 2019 | 33.5 | 23.2 |
| South Asia | ||||||
| India | Ramamoorthy M et al. [88] | 2016 | 1,381 | 2015 | 1.2 | - |
| Tyagi SK et al. [89] | 2018 | 1,611 | 2016 | 10.4 | - | |
| Indonesia | Prasetyo AA et al. [90] | 2013 | 375 | 2009 | 34.1 | |
| Pakistan | Pervaiz A et al. [91] | 2015 | 5,894 | 2007–2009 | - | 14.6 |
| Wali A et al. [92] | 2019 | 346 | 2017 | 10.4 | - | |
| Sri Lanka | Niriella MA et al. [93] | 2015 | 393 | - | 6.9 | 0.5 |
| West Asia | ||||||
| Iran | Salem F et al. [94] | 2013 | 3,000 | 2008–2009 | 0.7 | - |
| Ziaee M et al. [95] | 2014 | 881 | 2009–2010 | - | 7.7 | |
| Khajedaluee M et al. [96] | 2016 | 1,114 | 2008 | 24.5 | 19.1 | |
| Moradi G et al. [97] | 2018 | 6,200 | 2015 | 9.5 | ||
| Ghafari S et al. [98] | 2019 | 300 | 2016 | 8.0 | - | |
| Khademi N et al. [99] | 2019 | 1,034 | - | 22.2 | - | |
| Moradi G et al. [100] | 2019 | 6,481 | 2016 | 8.2 | - | |
| Sharafi H et al. [101] | 2019 | 1,788 | 2017–2018 | 5.9 | - | |
| Hariri S et al. [102] | 2020 | 3,485 | 2017–2018 | 5.2 | 3.4 | |
| Hariri S et al. [103] | 2020 | 1,892 | 2018 | 6.9 | 4.8 | |
| Australasia | ||||||
| Australia | Reekie JM et al. [104] | 2014 | 1,393 | 2004–2007-2010 | 29.8 | - |
| Cunningham E et al. [105] | 2017 | 320 | 2005–2014 | 29.1 | - | |
| Snow K et al. [106] | 2017 | 1,315 | 2008–2010 | 33.8 | - | |
| Hajarizadeh B et al. [107] | 2021 | 3?691 | 2018 | - | 19.0 | |
| Sullivan RP et al. [108] | 2021 | 12,153 | 2003–2017 | 4.7 | - | |
| West Africa | ||||||
| Senegal | Jaquet A et al. [109] | 2016 | 333 | 2014 | 0.6 | - |
| Togo | Jaquet A et al. [109] | 2016 | 347 | 2013 | 0.3 | - |
| Nigeria | Okafor IM et al. [110] | 2020 | 142 | 2018 | 29.6 | - |
| Sub Saharan Africa | ||||||
| Ethiopia | Wakjira K et al. [111] | 2017 | 156 | 2016 | 2.6 | - |
| Kassa Y et al. [112] | 2021 | 339 | 2020 | 1.2 | - | |
| Middle East and North Africa | ||||||
| Egypt | Mohamed HI et al. [113] | 2013 | 500 | - | 15.8 | 12.2 |
Prevalence HCV RNA is the prevalence relative to the total sample size
Abbreviations: HCV hepatitis c virus, Ab antibody
Results
The data search resulted in 306 potential data sources found on Embase, 333 on PubMed, 421 on Scopus, and 409 on Web of Science. The abstracts of these articles were systematically screened. Whereas 1,340 articles were excluded, 129 were thoroughly reviewed. While reviewing these articles, the snowball method was applied and another four articles and two abstracts were included. Twenty-nine articles were identified as reviews or meta-analyses, and 14 articles were duplicates and therefore excluded. This resulted in 90 eligible articles and two abstracts, reporting data for 36 different countries (Fig. 1) [see Additional file A4].
Fig. 1.
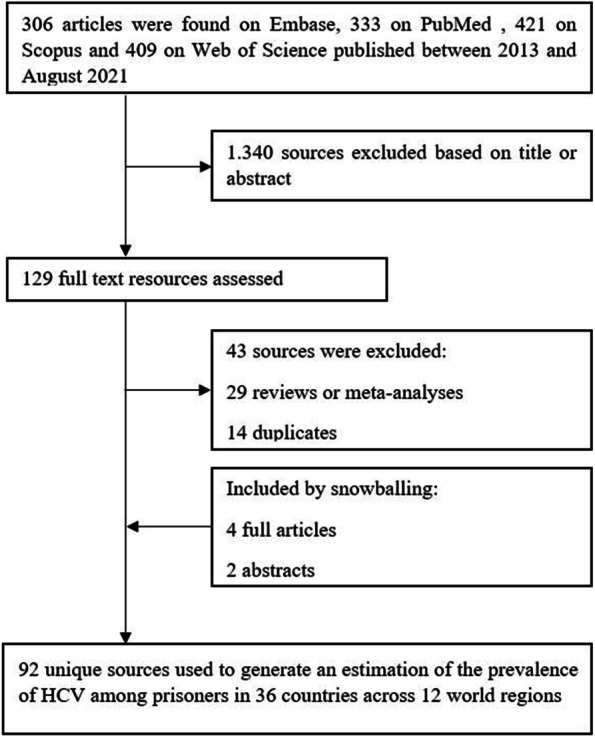
Search process and flowchart
Results of the quality assessment showed that half of the articles were rated A, B1, or B2, respectively 4 (4.3%), 38 (41.3%) and 2 (2.2%). The other half was rated C, D or E, respectively 35 (38.0%), 7 (7.6%) and 6 (6.5%).
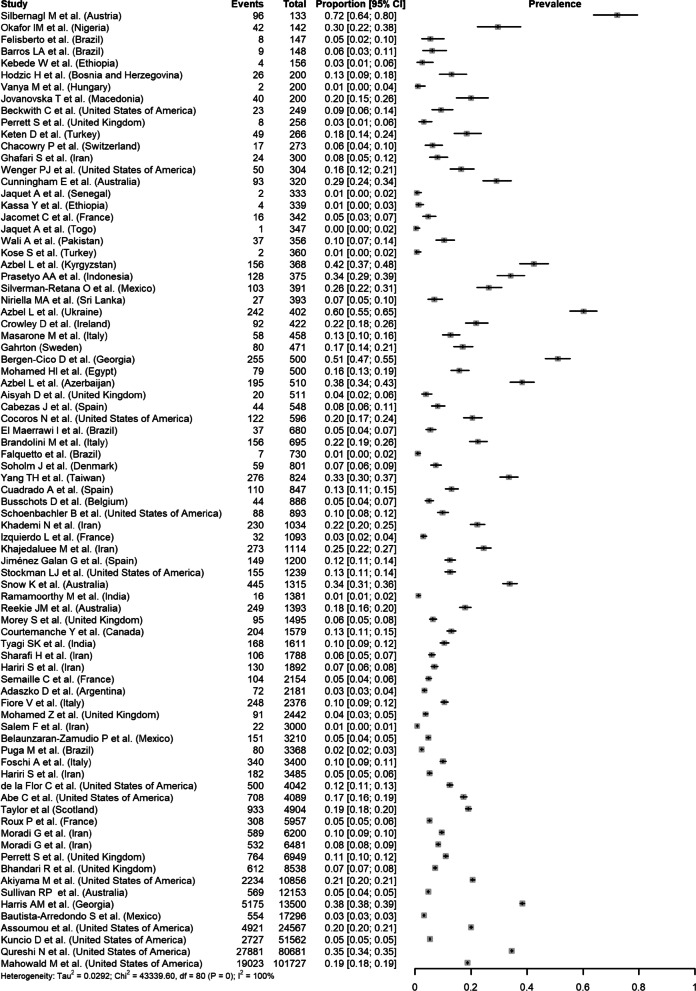
The estimated prevalence of HCV Ab ranged from 0.3% to 74.4%. Jaquet et al. (Fig. 2) reported the lowest prevalence in 347 individuals who are incarcerated held in the state prison of Lome, Togo (Table 1) [109]. None of the HCV Ab positives individuals in Lome reported a history of intravenous drug use (IVDU) and information on other transmission routes was unavailable. The same authors found similar results for a prison in Dakar, Senegal (0.6%).
Fig. 2.
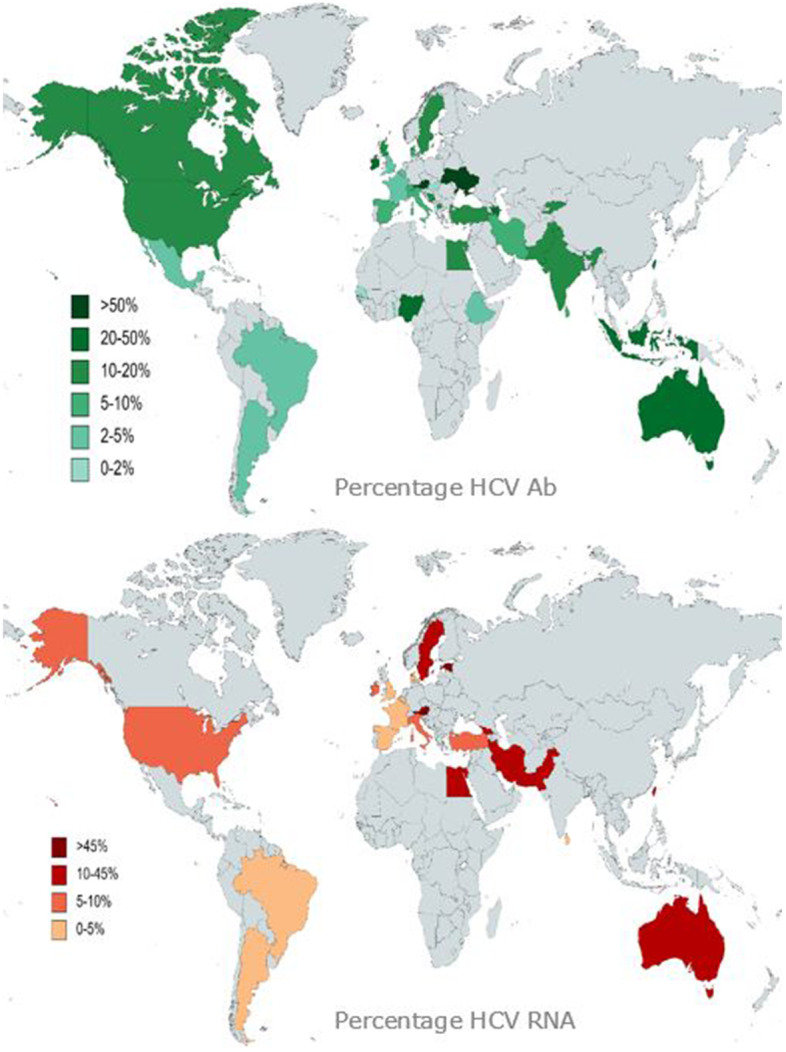
The global prevalence of hepatitis C in incarcerated settings
Generated with data from the studies that were best graded as in [see Additional file A3]
In contrast, the highest prevalence of HCV Ab was reported in Silbernagl et al. in the smallest study, with 133 individuals who are incarcerated receiving opioid agonist therapy (OAT) in four different prisons in Austria [33]. In this study, HCV Ab positivity was related to lifetime IVDU and younger age at first IVDU. The second highest prevalence (60.2%) was found in Ukraine in soon-to-released individuals who are incarcerated [32].
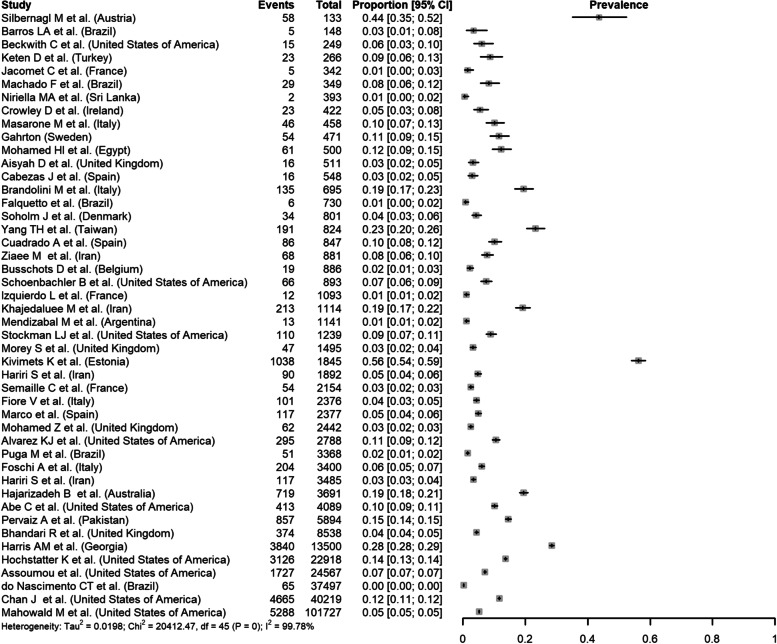
HCV RNA was available in 46/92 (50.0%) sources and ranged from 0% to 56.3% (Fig. 2). The lowest prevalence (0.2%) was reported by do Nascimento et al. in 37,497 individuals who are incarcerated in Brazil. Kivimets et al. reported the highest prevalence among 1,845 newly incarcerated individuals who are incarcerated in Estonia [25, 82]. Only 19 (41.3%) of these articles mentioned the HCV genotypes. The genotypes most frequently reported in incarcerated settings were 1(a) and 3 (Table 2).
Table 2.
Distribution of genotypes in incarcerated settings (%)
| COUNTRY | 1 | 1A | 1B | 1C | 2 | 2A/C | 3 | 4 | 6 |
|---|---|---|---|---|---|---|---|---|---|
| Estonia | 17.0 | 35.2 | 2.6 | 44.4 | 0.8 | ||||
| Georgia | 22.7 | 28.8 | 47.7 | ||||||
| Turkey | 2.1 | 68.0 | |||||||
| Austria | 52.8 | 3.8 | 1.6 | 35.8 | 7.5 | ||||
| France | 50.0 | 41.7 | 8.3 | ||||||
| Ireland | 58.7 | 41.3 | |||||||
| Italy | 48.0 | 4.0 | 26.0 | 10.0 | |||||
| 45.6 | 8.7 | 6.5 | 32.6 | 6.5 | |||||
| 35.6 | 6.9 | 1.0 | 44.6 | 11.9 | |||||
| Spain | 29.0 | 13.0 | 40.6 | 17.4 | |||||
| 18.8 | 12.5 | 6.2 | 6.2 | 6.2 | |||||
| United Kingdom | 38.0 | 57.0 | |||||||
| 46.4 | 5.3 | 3.6 | 41.1 | 3.6 | |||||
| United States | 76.6 | 9.3 | 11.7 | 1.4 | |||||
| 65.4 | 8.0 | 4.3 | 9.7 | ||||||
| Argentina | 50.0 | 25.0 | 8.3 | 16.7 | |||||
| Brazil | 87.5 | 12.5 | |||||||
| Taiwan | 22.0 | 14.1 | 10.5 | 10.5 | 39.3 | ||||
| Indonesia | 46.7 | 3.3 | 16.7 | 26.7 | 6.7 |
The proportion of PWID was studied and reported in 49 articles and ranged from 0.6% to 100% [see Additional file A5]. Thirty-five out of 92 studies reported analyses on risk factors associated with HCV seroprevalence. In 24 of these studies, IVDU was present as a risk factor significantly associated with HCV seroprevalence. HCV Ab prevalence in anincarcerated setting appears to be increased when IDU prevalence is higher within that incarcerated setting [see Additional file A6]. HIV coinfection was reported in 36 sources and varied between 0% in Australia, Belgium, Egypt, Ethiopia, Switzerland, and one study in Missouri, USA, to 42.7% in a Spanish prison [see Additional file A5].
In the meta-analysis, regardless of which transformation was used, I2 was high, for example 99.8% (95%CI 99.8–99.8%) when using the double arcsine transformation. In the GLMM, I2 was still high (99.9%). Analyses excluding the smallest study (Silbernagl et al., 2018), which had a very high prevalence estimate (72%; 95%CI 64–80%), did not affect the results. This indicates that almost all observed heterogeneity reflects true differences in estimates between studies, and a pooled prevalence estimate cannot be obtained (Fig. 3). Subgroup analyses revealed a significant difference regarding the study region (p = 0.004). Based on results from univariate analyses, study region (p = 0.022), proportion of PWID (p < 0.001), and proportion of males (p = 0.119), as well as their two-way interactions, were included in the multivariable meta-regression model. The final meta-regression model included significant interactions between region and proportion of PWID, as well as between proportion of PWID and proportion of males, and explained 76.0% of the between-study heterogeneity among the 42 studies reporting PWID that were included in this model.
Fig. 3.
Results of the meta-analyses for HCV Ab prevalence in incarcerated settings
Regarding HCV RNA prevalence in inmates, 46 studies were included in a meta-analysis. I2 was 99.8% (95%CI 99.8–99.8%), indicating that almost all observed heterogeneity reflects true differences in prevalence between studies (Fig. 4). Subgroup analyses revealed a significant difference between regions (p < 0.001). Based on results from univariate analyses, study region (p = 0.002), single- vs multi-site study (p = 0.064), proportion HIV (p = 0.001), and proportion of PWID (p < 0.001) would be included in the multivariable meta-regression model. However, due to the limited number of studies reporting on HIV (n = 24) and PWID (n = 22) these were not included in the multivariable model. The final meta-regression model included only region (p = 0.002) and explained 67.1% of the between-study heterogeneity among studies reporting HCV RNA prevalence.
Fig. 4.
Results of the meta-analyses for HCV RNA prevalence in incarcerated settings
Discussion
Gathering data on HCV prevalence in different settings is key to tracking the path to micro-elimination by 2030. Micro-elimination is a practical approach involving population segmentation to tailor concentrated elimination efforts to specific subgroups of the population. In addition, mapping the prevalence of HCV RNA is crucial to assess the need for treatment within incarcerated settings. This is the first meta-analysis to determine the HCV RNA prevalence in incarcerated settings worldwide. Despite the efforts, the data were too heterogeneous to establish a reliable pooled prevalence.
As expected, HCV Ab prevalence was higher than in the general population, identifying the population in an incarcerated setting as a risk group to target for micro-elimination. This elevated HCV prevalence could impose a financial burden on the health care system. It is striking that the prevalence of HCV Ab varies widely, even within a region of high-income countries such as Western Europe. The high numbers from Italy, Ireland, Scotland (the United Kingdom), and Sweden stand out. As the studies from Italy and Ireland are monocentric studies, they are not nationally representative. This is confirmed by the fact that the other Italian studies reported a substantially lower prevalence (10.0%-12.7% compared to 22.4%) [41, 42]. In this study, we also attempted to collect data on HCV RNA prevalence in incarcerated settings. However, only half of the studies reported these data, which is important for treatment and ultimately eliminating the virus.
It is crucial to obtain qualitatively strong and thus multicenter data on HCV prevalence that are representative of a country. Just under half of the studies were multicenter, and the quality assessment showed that half of all articles were rated A, B1, or B2.
Reporting HCV genotype is also essential as there are still countries where pangenotypic treatment is not yet available [114]. From the data collected on HCV genotypes, it appears that genotypes 1 and 3 are the most prevalent in an incarcerated setting. This finding supports the argument that HCV in individuals who are incarcerated is acquired primarily by IVDU since genotypes 1a and 3 are more prevalent in the PWID population worldwide than in the general population [115]. HCV/HIV coinfection varied between 0% and 42.7% and was highest in Italy, Estonia, Pakistan, and Spain. Dolan et al. associated higher HIV prevalence within an incarcerated setting with higher prevalence of PWID, but we lack sufficient data to support this argument.
Moreover, treatment for chronic HCV infection in closed settings can be delivered with sustained virologic response rates similar to those in community settings [116]. Treatment in closed settings would benefit individuals who are incarcerated and provide significant public health benefits, including reduced transmission and lowering the disease burden associated with chronic HCV infection. However, the high cost of treatment often remains a burden to implement it widely. After all, it places a heavy financial burden on the healthcare budgets of closed institutions [7].
This meta-analysis has several limitations and demonstrates the pitfalls of this kind of study. First, we only searched for articles published from 2013 onwards. Larney et al. concluded that many countries lacked epidemiological data on the extent of HCV infection in detained populations and that more efforts were needed. The available data were too heterogeneous and would have no added value in the current meta-analysis [7]. In addition, we did not include grey literature or unpublished results. Second, selection bias was a potential problem in some studies due to high non-response rates or flaws in the study design. Further, variables that could explain between-study heterogeneity, such as duration of incarceration, sexual behavior, and the number of previous incarcerations, were not analyzed because consistently reported data were mainly lacking. Thereby, even in the articles mentioning high-risk behavior in incarcerated settings such as IVDU, not everything may have been reported. Given that admitting particular risk behavior may have social implications for the individual who is incarcerated, self-reported data might not be reliable, leading to underreporting of high-risk behavior [115, 117]. As in the study of Larney et al., we could not include data from countries with a large population of incarcerated people, such as China or Russia. This study also did not include information on the incarcerated setting and incarceration policies in a country. These items could explain the wide variation in prevalence between different countries within a given region.
Conclusions
To conclude, HCV in incarcerated settings is still a significant problem with a higher prevalence than in the general population. In addition, data on HCV RNA are scarce, while these data are critical for HCV micro-elimination within the an incarcerated setting. Therefore, more studies need to be conducted to estimate the actual global burden of HCV in incarcerated settings. Future studies should consistently report more information on study design, type and size of the incarcerated setting, specific high-risk population included (e.g., PWID and people living with HIV), and use a better study method (e.g., multicenter and/or multiple sample types) to ensure data quality. Finally, it is of utmost importance to start screening for HCV (Ab and RNA) in incarcerated settings to give clear, reliable and recent figures to plan further treatment. This is all in the context of meeting the 2030 WHO targets which are only less than a decade away.
Supplementary Information
Additional file 1. A1. PRISMA 2020 Checklist.
Additional file 2. A2. Year of publication of included sources.
Additional file 3. A3. Classification system for assessment of study methodologies.
Additional file 4. A4. Country of origin of included sources.
Additional file 5. A5. Included prevalence sources with additional risk factors and the prevalence of HIV coinfection, people who inject drugs and male incarcerated individuals (n=92; 36 countries).
Additional file 6. A6. Dissemination of the prevalence hepatitis C antibodies and the prevalence of people who inject drugs (PWID) within the different studies.
Acknowledgements
The PhD authors of this review are part of the ‘Limburg Clinical Research Center (LCRC), supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital. D.B., C.K., N.H., F.N. and G.R. are part of the project G0B2317N funded by the Fund of Scientific Research—Flanders (FWO).
Abbreviations
- HCV
Hepatitis C virus
- WHO
World Health Organization
- Ab
Antibody
- GLMM
Generalized linear mixed model
- IVDU
Intravenous drug use
- OAT
Opioid agonist therapy
- PWID
People who inject drugs
Authors’ contributions
D.B. and G.R. designed the study. D.B. and G.R. collected the data. C.K. and N.H. conducted the statistical analyses. D.B. drafted the first version of the paper. All co-authors made substantial contributions to the interpretation of the data, critically revised the article and approved the final version, including the authorship list.
Funding
Not applicable
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declarations
Ethics approval and patient consent
Not applicable
Consent for publication
Not applicable
Competing interests
D.B. has received travel grants from AbbVie and Gilead Sciences; R.B. has received travel grants from AbbVie, Gilead Sciences and Merck Sharp & Dohme (MSD) and research grants from Gilead and MSD; O.K. has received a travel grant from Gilead Sciences and his institution received research grants from Gilead Sciences and CyTuVax BV; F.N. has received research grants, consultancy agreements and travel grants from UCB, Ipsen, Roche, Astellas, Ferring, Novartis, Janssen-Cilag, Abbvie, Gilead, CAF, Intercept, Gore, Bristol-Myers Squibb (BMS), MSD, Promethera Biosciences, Ono Pharma, Durect; N.H. reports grants from GlaxoSmithKline, grants from Johnson & Johnson pharmaceuticals and grants from Pfizer; G.R. has received research grants from AbbVie, Janssen Pharmaceuticals, MSD, and consultancy agreements for AbbVie, BMS, Gilead Sciences and MSD. All other co-authors report no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global Hepatitis Report, 2017. WHO: Geneva, April, 2017. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf.
- 2.Bielen R, Stumo SR, Halford R, Werling K, Reic T, Stover H, Robaeys G, Lazarus JV. Harm reduction and viral hepatitis C in European prisons: a cross-sectional survey of 25 countries. Harm Reduct J. 2018;15(1):25. doi: 10.1186/s12954-018-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stover H, Meroueh F, Marco A, Keppler K, Saiz de la Hoya P, Littlewood R, Wright N, Nava F, Alam F, Walcher S, et al. Offering HCV treatment to prisoners is an important opportunity: key principles based on policy and practice assessment in Europe. BMC Public Health. 2019;19(1):30. doi: 10.1186/s12889-018-6357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright N, Reimer J, Somaini L, Roncero C, Maremmani I, Simon N, Krajci P, Littlewood R, D'Agnone O, Alho H, et al. Are we ready to treat hepatitis C virus in individuals with opioid use disorder: assessment of readiness in European countries on the basis of an expert-generated model. Eur J Gastroenterol Hepatol. 2017;29(11):1206–1214. doi: 10.1097/MEG.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 5.Roose RJ, Cockerham-Colas L, Soloway I, Batchelder A, Litwin AH. Reducing barriers to hepatitis C treatment among drug users: an integrated hepatitis C peer education and support program. J Health Care Poor Underserved. 2014;25(2):652–662. doi: 10.1353/hpu.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolan K, Wirtz AL, Moazen B, Ndeffo-Mbah M, Galvani A, Kinner SA, Courtney R, McKee M, Amon JJ, Maher L, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet (London, England) 2016;388(10049):1089–1102. doi: 10.1016/S0140-6736(16)30466-4. [DOI] [PubMed] [Google Scholar]
- 7.Larney S, Kopinski H, Beckwith CG, Zaller ND, Jarlais DD, Hagan H, Rich JD, van den Bergh BJ, Degenhardt L. The incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology (Baltimore, MD) 2013;58(4):1215–1224. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology (Baltimore, MD) 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 9.World Prison Population List (12 th edition) [https://www.prisonstudies.org/sites/default/files/resources/downloads/wppl_12.pdf].
- 10.Arain A, Robaeys G, Stover H. Hepatitis C in European prisons: a call for an evidence-informed response. BMC Infect Dis. 2014;14(Suppl 6):S17. doi: 10.1186/1471-2334-14-S6-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galea G, Enggist S, Udesen C, Møller L. World Health Organization. 2014. Prisons and Health; pp. 165–170. [Google Scholar]
- 12.ECDC . Surveillance and prevention of hepatitis B and C in Europe. Stockholm: European Centre for Disease Prevention and Control; 2010. [Google Scholar]
- 13.Hagan LM, Schinazi RF. Best strategies for global HCV eradication. Liver Int. 2013;33(Suppl 1):68–79. doi: 10.1111/liv.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsoulfas G, Goulis I, Giakoustidis D, Akriviadis E, Agorastou P, Imvrios G, Papanikolaou V. Hepatitis C and liver transplantation. Hippokratia. 2009;13(4):211–215. [PMC free article] [PubMed] [Google Scholar]
- 15.Rich JD, Allen SA, Williams BA. Responding to hepatitis C through the criminal justice system. N Engl J Med. 2014;370(20):1871–1874. doi: 10.1056/NEJMp1311941. [DOI] [PubMed] [Google Scholar]
- 16.Larney S, C GB, N DZ, B TM, Rich J. "Seek, test, treat and retain" for hepatitis C in the United States criminal justice system. Int J Prison Health 2014;10(3):164-171. [DOI] [PMC free article] [PubMed]
- 17.Olafsson S, Fridriksdottir RH, Love TJ, Tyrfingsson T, Runarsdottir V, Hansdottir I, Bergmann OM, Björnsson ES, Johannsson B, Sigurdardottir B, et al. Cascade of care during the first 36 months of the treatment as prevention for hepatitis C (TraP HepC) programme in Iceland: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(8):628–637. doi: 10.1016/S2468-1253(21)00137-0. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) 2021;74(9):790–799. doi: 10.1016/j.rec.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet (London, England) 2011;378(9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R: A language and environment for statistical computing [https://www.R-project.org].
- 21.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rucker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476–483. doi: 10.1002/jrsm.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges L, Higgings J, Rothstein H: Introduction to Meta-analysis. In. Edited by Ltd JWS. UK; 2009.
- 23.Azbel L, Wickersham JA, Wegman MP, Polonsky M, Suleymanov M, Ismayilov R, Dvoryak S, Rotberga S, Altice FL. Burden of substance use disorders, mental illness, and correlates of infectious diseases among soon-to-be released prisoners in Azerbaijan. Drug Alcohol Depend. 2015;151:68–75. doi: 10.1016/j.drugalcdep.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodzic H, Bajramovic A, Obradovic Z, Mahmic-Kaknjo M. Intravenous drugs abuse as the main risk factor of increasing hepatitis C infection prevalence in prisoners in Zenica, Bosnia and Herzegovina. Med Glas (Zenica) 2017;14(1):73–78. doi: 10.17392/880-16. [DOI] [PubMed] [Google Scholar]
- 25.Kivimets K, Uusküla A, Lazarus JV, Ott K. Hepatitis C seropositivity among newly incarcerated prisoners in Estonia: data analysis of electronic health records from 2014 to 2015. BMC Infect Dis. 2018;18:339. doi: 10.1186/s12879-018-3242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergen-Cico D, Sikharulidze K, Ivanashvili N, Ivanishvili M, Keshelava T. Hepatitis C Risk and Protective Factors Associated With Drug Policies in the Republic of Georgia. World Med Health Policy. 2017;9(1):45–64. [Google Scholar]
- 27.Harris AM, Chokoshvili O, Biddle J, Turashvili K, Japaridze M, Burjanadze I, Tsertsvadze T, Sharvadze L, Karchava M, Talakvadze A, et al. An evaluation of the hepatitis C testing, care and treatment program in the country of Georgia's corrections system, December 2013 - April 2015. BMC Public Health. 2019;19(Suppl 3):466. doi: 10.1186/s12889-019-6783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanya M, Szili K, Magori K, Krisztina V. Skin diseases and sexually transmitted infection in a Hungarian prison. Med Microbiol. 2017;28:95–96. [Google Scholar]
- 29.Jovanovska T, Kocic B, Stojcevska VP. Prevalence, attitudes and knowledge about HIV HBV and HCV infections among inmates in prisons Prilep and Bitola–a pilot study. Coll Antropol. 2014;38(2):417–422. [PubMed] [Google Scholar]
- 30.Keten D, Emin Ova M, Sirri Keten H, Keten A, Gulderen E, Tumer S, Caliskan A, Kulotu S. The Prevalence of Hepatitis B and C Among Prisoners in Kahramanmaras, Turkey. Jundishapur J Microbiol. 2016;9(2):e31598. doi: 10.5812/jjm.31598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kose S, Adar P, Gozaydin A, Kuzucu L, Akkoclu G. Hepatitis B and Hepatitis C in prisons: a prevalence study. Int J Prison Health. 2019;15(2):162–167. doi: 10.1108/IJPH-01-2018-0004. [DOI] [PubMed] [Google Scholar]
- 32.Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PLoS One. 2013;8(3):e59643. doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silbernagl M, Slamanig R, Fischer G, Brandt L. Hepatitis C infection and psychiatric burden in two imprisoned cohorts: Young offenders and opioid-maintained prisoners. Health Policy. 2018;122(12):1392–1402. doi: 10.1016/j.healthpol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Busschots D, Kremer C, Bielen R, Koc OM, Heyens L, Brixko C, Laukens P, Orlent H, Bilaey P, De Smet F, et al. A multicentre interventional study to assess blood-borne viral infections in Belgian prisons. BMC Infect Dis. 2021;21(1):708. doi: 10.1186/s12879-021-06405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soholm J, Holm DK, Mossner B, Madsen LW, Hansen JF, Weis N, Sauer AP, Awad T, Christensen PB. Incidence, prevalence and risk factors for hepatitis C in Danish prisons. PLoS One. 2019;14(7):e0220297. doi: 10.1371/journal.pone.0220297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semaille C, Le Strat Y, Chiron E, Chemlal K, Valantin MA, Serre P, Cate L, Barbier C, Jauffret-Roustide M. Prevalence of human immunodeficiency virus and hepatitis C virus among French prison inmates in 2010: a challenge for public health policy. Euro Surveill. 2013;18(28):20524. doi: 10.2807/1560-7917.es2013.18.28.20524. [DOI] [PubMed] [Google Scholar]
- 37.Roux P, Sagaon-Teyssier L, Lions C, Fugon L, Verger P, Carrieri MP. HCV seropositivity in inmates and in the general population: an averaging approach to establish priority prevention interventions. BMJ Open. 2014;4(10):e005694. doi: 10.1136/bmjopen-2014-005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacomet C, Guyot-Lénat A, Bonny C, Henquell C, Rude M, Dydymski S, Lesturgeon J-A, Lambert C, Pereira B, Schmidt J. Addressing the challenges of chronic viral infections and addiction in prisons: the PRODEPIST study. Euro J Public Health. 2016;26(1):122–8. doi: 10.1093/eurpub/ckv183. [DOI] [PubMed] [Google Scholar]
- 39.Izquierdo L, Mellon G, Buchaillet C, Fac C, Soutiere MP, Pallier C, Dulioust A, Roque-Afonso AM. Prevalence of hepatitis E virus and reassessment of HIV and other hepatitis virus seroprevalences among French prison inmates. PLoS One. 2019;14(6):e0218482. doi: 10.1371/journal.pone.0218482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley D, Lambert JS, Betts-Symonds G, Cullen W, Keevans M, Kelly E, Laird E, McHugh T, McKiernan S, Miggin SJ, et al. The seroprevalence of untreated chronic hepatitis C virus (HCV) infection and associated risk factors in male Irish prisoners: a cross-sectional study, 2017. Euro Surveill. 2019;24(14):1800369. doi: 10.2807/1560-7917.ES.2019.24.14.1800369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandolini M, Novati S, De Silvestri A, Tinelli C, Patruno SF, Ranieri R, Seminari E. Prevalence and epidemiological correlates and treatment outcome of HCV infection in an Italian prison setting. BMC Public Health. 2013;13:981. doi: 10.1186/1471-2458-13-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foschi A, Casana M, Radice A, Ranieri R, d'Arminio Monforte A. Hepatitis C management in prisons: an insight into daily clinical practice in three major Italian correctional houses. Hepatology (Baltimore, MD) 2016;64(5):1821–1822. doi: 10.1002/hep.28609. [DOI] [PubMed] [Google Scholar]
- 43.Masarone M, Caruso R, Aglitti A, Izzo C, De Matteis G, Attianese M, Pagano A, Persico M. Hepatitis C virus in jail: Difficult-to-reach, not to-treat. Results of a point-of-care screening and treatment program. Digest Liver Dis. 2020;52:541–546. doi: 10.1016/j.dld.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Fiore V, De Matteis G, Ranieri R, Saderi L, Pontali E, Muredda A, Ialungo AM, Caruso R, Madeddu G, Sotgiu G, et al. HCV testing and treatment initiation in an Italian prison setting: a step-by-step model to micro-eliminate hepatitis C. Int J Drug Policy. 2021;90:103055. doi: 10.1016/j.drugpo.2020.103055. [DOI] [PubMed] [Google Scholar]
- 45.Marco A, Gallego C, Caylà J. Incidence of Hepatitis C Infection among Prisoners by Routine Laboratory Values during a 20-Year Period. PloS One. 2014;9(2):e90560. doi: 10.1371/journal.pone.0090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuadrado A, Llerena S, Cobo C, Pallas JR, Mateo M, Cabezas J, Fortea JI, Alvarez S, Pellon R, Crespo J, et al. Microenvironment Eradication of Hepatitis C: A Novel Treatment Paradigm. Am J Gastroenterol. 2018;113(11):1639–1648. doi: 10.1038/s41395-018-0157-x. [DOI] [PubMed] [Google Scholar]
- 47.Jiménez Galán G, Alia Alia C, Vegue González M, García Berriguete RM, Fernández González F, Fernández Rodríguez CM, González Fernández M, Gutiérrez García ML, Losa JE, Velasco M, et al. The contribution of telemedicine to hepatitis C elimination in a correctional facility. Rev Esp Enferm Dig. 2019;111(7):550–555. doi: 10.17235/reed.2019.6152/2018. [DOI] [PubMed] [Google Scholar]
- 48.Cabezas J, Llerena S, Mateo M, Alvarez R, Cobo C, Gonzalez V, Martro E, Cuadrado A, Crespo J. Hepatitis C Micro-Elimination beyond Prison Walls: Navigator-Assisted Test-and-Treat Strategy for Subjects Serving Non-Custodial Sentences. Diagnostics (Basel) 2021;11(5):877. doi: 10.3390/diagnostics11050877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor A, Munro A, Allen E, Dunleavy K, Cameron S, Miller L, Hickman M. Low incidence of hepatitis C virus among prisoners in Scotland. Addiction (Abingdon, England) 2013;108(7):1296–1304. doi: 10.1111/add.12107. [DOI] [PubMed] [Google Scholar]
- 50.Aisyah DN, Shallcross L, Hayward A, Aldridge RW, Hemming S, Yates S, Ferenando G, Possas L, Garber E, Watson JM, et al. Hepatitis C among vulnerable populations: a seroprevalence study of homeless, people who inject drugs and prisoners in London. J Viral Hepat. 2018;25(11):1260–1269. doi: 10.1111/jvh.12936. [DOI] [PubMed] [Google Scholar]
- 51.Morey S, Hamoodi A, Jones D, Young T, Thompson C, Dhuny J, Buchanan E, Miller C, Hewett M, Valappil M, et al. Increased diagnosis and treatment of hepatitis C in prison by universal offer of testing and use of telemedicine. J Viral Hepat. 2019;26(1):101–108. doi: 10.1111/jvh.13017. [DOI] [PubMed] [Google Scholar]
- 52.Perrett SE, Waite TD. Exploring HIV infection in a UK vulnerable prisoner population in response to newly identified cases. Int J Prison Health. 2019;15(3):244–249. doi: 10.1108/IJPH-03-2018-0010. [DOI] [PubMed] [Google Scholar]
- 53.Bhandari R, Morey S, Hamoodi A, Thompson C, Jones D, Hewett M, Hunter E, Taha Y, McPherson S. High rate of hepatitis C reinfection following antiviral treatment in the North East England Prisons. J Viral Hepat. 2020;27(4):449–452. doi: 10.1111/jvh.13240. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed Z, Al-Kurdi D, Nelson M, Shimakawa Y, Selvapatt N, Lacey J, Thursz MR, Lemoine M, Brown AS. Time matters: Point of care screening and streamlined linkage to care dramatically improves hepatitis C treatment uptake in prisoners in England. Int J Drug Policy. 2020;75:102608. doi: 10.1016/j.drugpo.2019.102608. [DOI] [PubMed] [Google Scholar]
- 55.Perrett SE, Plimmer A, Shankar AG, Craine N. Prevalence of HCV in prisons in Wales, UK and the impact of moving to opt-out HCV testing. J Public Health (Oxf) 2020;42(2):423–428. doi: 10.1093/pubmed/fdaa022. [DOI] [PubMed] [Google Scholar]
- 56.Gahrton C, Westman G, Lindahl K, Öhrn F, Dalgard O, Lidman C, Nilsson LH, Said K, Duberg AS, Aleman S. Prevalence of Viremic hepatitis C, hepatitis B, and HIV infection, and vaccination status among prisoners in Stockholm County. BMC Infect Dis. 2019;19(1):955. doi: 10.1186/s12879-019-4581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chacowry Pala K, Baggio S, Tran NT, Girardin F, Wolff H, Getaz L. Blood-borne and sexually transmitted infections: a cross-sectional study in a Swiss prison. BMC Infect Dis. 2018;18(1):539. doi: 10.1186/s12879-018-3445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Courtemanche Y, Poulin C, Serhir B, Alary M. HIV and hepatitis C virus infections in Quebec's provincial detention centres: comparing prevalence and related risky behaviours between 2003 and 2014–2015. Can J Public Health. 2018;109(3):353–361. doi: 10.17269/s41997-018-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez KJ, Befus M, Herzig CT, Larson E. Prevalence and correlates of hepatitis C virus infection among inmates at two New York State correctional facilities. J Infect Public Health. 2014;7(6):517–521. doi: 10.1016/j.jiph.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cocoros N, Nettle E, Church D, Bourassa L, Sherwin V, Cranston K, Carr R, Fukuda HD, DeMaria A., Jr Screening for Hepatitis C as a Prevention Enhancement (SHAPE) for HIV: an integration pilot initiative in a Massachusetts County correctional facility. Public Health Rep. 2014;129(Suppl 1):5–11. doi: 10.1177/00333549141291S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenger PJ, Rottnek F, Parker T, Crippin JS. Assessment of hepatitis C risk factors and infection prevalence in a jail population. Am J Public Health. 2014;104(9):1722–1727. doi: 10.2105/AJPH.2014.301996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuncio DE, Newbern EC, Fernandez-Vina MH, Herdman B, Johnson CC, Viner KM. Comparison of risk-based hepatitis C screening and the true seroprevalence in an urban prison system. J Urban Health. 2015;92(2):379–386. doi: 10.1007/s11524-015-9945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beckwith CG, Kurth AE, Bazerman LB, Patry EJ, Cates A, Tran L, Noska A, Kuo I. A pilot study of rapid hepatitis C virus testing in the Rhode Island Department of Corrections. J Public Health (Oxf) 2016;38(1):130–137. doi: 10.1093/pubmed/fdv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahowald MK, Larney S, Zaller ND, Scharff N, Taylor LE, Beckwith CG, Noska A, Rich JD, Flanigan TP. Characterizing the Burden of Hepatitis C Infection Among Entrants to Pennsylvania State Prisons, 2004 to 2012. J Correct Health Care. 2016;22(1):41–45. doi: 10.1177/1078345815618384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schoenbachler BT, Smith BD, Sena AC, Hilton A, Bachman S, Lunda M, Spaulding AC. Hepatitis C Virus Testing and Linkage to Care in North Carolina and South Carolina Jails, 2012–2014. Public Health Rep. 2016;131(Suppl 2):98–104. doi: 10.1177/00333549161310S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stockman LJ, Greer J, Holzmacher R, Dittmann B, Hoftiezer SA, Alsum LE, Prieve A, Westergaard RP, Guilfoyle SM, Vergeront JM. Performance of Risk-Based and Birth-Cohort Strategies for Identifying Hepatitis C Virus Infection Among People Entering Prison, Wisconsin, 2014. Public Health Rep. 2016;131(4):544–551. doi: 10.1177/0033354916662212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akiyama MJ, Kaba F, Rosner Z, Alper H, Kopolow A, Litwin AH, Venters H, MacDonald R. Correlates of Hepatitis C Virus Infection in the Targeted Testing Program of the New York City Jail System. Public Health Rep. 2017;132(1):41–47. doi: 10.1177/0033354916679367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de la Flor C, Porsa E, Nijhawan AE. Opt-out HIV and Hepatitis C Testing at the Dallas County Jail: Uptake, Prevalence, and Demographic Characteristics of Testers. Public Health Rep. 2017;132(6):617–621. doi: 10.1177/0033354917732755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hochstatter KR, Stockman LJ, Holzmacher R, Greer J, Seal DW, Taylor QA, Gill EK, Westergaard RP. The continuum of hepatitis C care for criminal justice involved adults in the DAA era: a retrospective cohort study demonstrating limited treatment uptake and inconsistent linkage to community-based care. Health Justice. 2017;5(1):10. doi: 10.1186/s40352-017-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Assoumou SA, Wang J, Tasillo A, Eftekhari Yazdi G, Tsui JI, Strick L, Linas BP. Hepatitis C Testing and Patient Characteristics in Washington State's Prisons Between 2012 and 2016. Am J Prev Med. 2019;56(1):8–16. doi: 10.1016/j.amepre.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abe CM, Aguwa M, Zhao M, Sullivan J, Porsa E, Nijhawan AE. Hepatitis C Virus Infection in the Dallas County Jail: Implications for Screening, Prevention, and Linkage to Care. Public Health Rep. 2019;134(6):626–633. doi: 10.1177/0033354919874081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan J, Kaba F, Schwartz J, Bocour A, Akiyama MJ, Rosner Z, Winters A, Yang P, MacDonald R. The hepatitis C virus care cascade in the New York City jail system during the direct acting antiviral treatment era, 2014–2017. EClinicalMedicine. 2020;27:100567. doi: 10.1016/j.eclinm.2020.100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qureshi N, Tadesse M, Tran N, Henderson S: Establishing an Epidemiologic Profile of Hepatitis C Virus Infection at the Los Angeles County Jail. Public Health Rep 2021.10.1177/33354920988610. [DOI] [PMC free article] [PubMed]
- 74.Adaszko D, Sotelo J, Orlando M, Adaszko A, Angeleri P. HIV, hepatitis B en C, syphilis and Tuberculosis prevalence in people deprived of liberty for criminal reasons in Argentina. Final results of a national study. Int J Infect Dis. 2018;73:202. [Google Scholar]
- 75.Mendizabal M, Testa P, Rojas M, Colaci CS, Elías S, Nicolini P, Olguín S, Dunn C, Ronchi C, Barreiro M, et al. Pilot study using the ECHO model to enhance linkage to care for patients with hepatitis C in the custodial setting. J Viral Hepat. 2020;27(12):1430–1436. doi: 10.1111/jvh.13374. [DOI] [PubMed] [Google Scholar]
- 76.Barros LA, Pessoni GC, Teles SA, Souza SM, Matos MA, Martins RM, Del-Rios NH, Carneiro MA. Epidemiology of the viral hepatitis B and C in female prisoners of Metropolitan Regional Prison Complex in the State of Goias, Central Brazil. Rev Soc Bras Med Trop. 2013;46(1):24–29. doi: 10.1590/0037-868216972013. [DOI] [PubMed] [Google Scholar]
- 77.Falquetto TC, Endringer DC, de Andrade TU, Lenz D. Hepatitis C in prisoners and non-prisoners in Colatina, Espírito Santo. Brazil. Braz J Pharm Sci. 2013;49(4):737–744. [Google Scholar]
- 78.El Maerrawi I, Carvalho HB. Prevalence and risk factors associated with HIV infection, hepatitis and syphilis in a state prison of Sao Paulo. Int J STD AIDS. 2015;26(2):120–127. doi: 10.1177/0956462414531242. [DOI] [PubMed] [Google Scholar]
- 79.Puga MA, Bandeira LM, Pompilio MA, Croda J, Rezende GR, Dorisbor LF, Tanaka TS, Cesar GA, Teles SA, Simionatto S, et al. Prevalence and Incidence of HCV Infection among Prisoners in Central Brazil. PLoS One. 2017;12(1):e0169195. doi: 10.1371/journal.pone.0169195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felisberto M, Saretto AA, Wopereis S, Machado MJ, Spada C. Prevalence of HCV infection in a prison population of the greater Florianopolis area. Rev Soc Bras Med Trop. 2019;52:e20190143. doi: 10.1590/0037-8682-0143-2019. [DOI] [PubMed] [Google Scholar]
- 81.Machado F, Becker D, Fernando de Oliveira C, Possuelo L, Pollo Renner J. Seroprevalence of HIV, hepatitis B and C and syphilis infection in prisoners of the central region of Rio Grande do Sul. Brazil. Mundo da Saude. 2019;43(1):117–128. [Google Scholar]
- 82.do Nascimento CT, Pena DZ, Giuffrida R, Bandeira Monteiro FN, da Silva FA, Flores EF, Prestes-Carneiro LE. Prevalence and epidemiological characteristics of inmates diagnosed with infectious diseases living in a region with a high number of prisons in São Paulo state, Brazil. BMJ Open. 2020;10(9):e037045. doi: 10.1136/bmjopen-2020-037045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bautista-Arredondo S, Gonzalez A, Servan-Mori E, Beynon F, Juarez-Figueroa L, Conde-Glez CJ, Gras N, Sierra-Madero J, Lopez-Ridaura R, Volkow P, et al. A Cross-Sectional Study of Prisoners in Mexico City Comparing Prevalence of Transmissible Infections and Chronic Diseases with That in the General Population. PLoS One. 2015;10(7):e0131718. doi: 10.1371/journal.pone.0131718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belaunzaran-Zamudio PF, Mosqueda-Gomez JL, Macias-Hernandez A, Rodriguez-Ramirez S, Sierra-Madero J, Beyrer C. Burden of HIV, Syphilis, and Hepatitis B and C Among Inmates in a Prison State System in Mexico. AIDS Res Hum Retroviruses. 2017;33(6):524–533. doi: 10.1089/AID.2016.0271. [DOI] [PubMed] [Google Scholar]
- 85.Silverman-Retana O, Servan-Mori E, McCoy SI, Larney S, Bautista-Arredondo S. Hepatitis C antibody prevalence among Mexico City prisoners injecting legal and illegal substances. Drug Alcohol Depend. 2017;181:140–145. doi: 10.1016/j.drugalcdep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 86.Azbel L, Polonsky M, Wegman M, Shumskaya N, Kurmanalieva A, Asanov A, Wickersham JA, Dvoriak S, Altice FL. Intersecting epidemics of HIV, HCV, and syphilis among soon-to-be released prisoners in Kyrgyzstan: Implications for prevention and treatment. Int J Drug Policy. 2016;37:9–20. doi: 10.1016/j.drugpo.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang TH, Fang YJ, Hsu SJ, Lee JY, Chiu MC, Yu JJ, Kuo CC, Chen CH. Microelimination of Chronic Hepatitis C by Universal Screening Plus Direct-Acting Antivirals for Incarcerated Persons in Taiwan. Open Forum Infect Dis. 2020;7(8):ofaa301. doi: 10.1093/ofid/ofaa301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramamoorthy M, Venketeswaran A, Seenivasan P, Revathy M, Manimaran M, Chitra S, Malavizhi M, Frederick T. Risk factors and prevalence, hepatitis B virus and hepatitis C virus among prison inmates, Chennai, India, 2015. Int J Infect Dis. 2016;53:90. [Google Scholar]
- 89.Tyagi SK, Sovani V, Dias NP, Tyagi D, Saxena S. Prevalence and risk factors of HCV infection in a prison setting in Uttar Pradesh, India. Indian J Public Health Res Dev. 2018;9(9):346–352. [Google Scholar]
- 90.Prasetyo AA, Dirgahayu P, Sari Y, Hudiyono H, Kageyama S. Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J Infect Dev Ctries. 2013;7(6):453–467. doi: 10.3855/jidc.2965. [DOI] [PubMed] [Google Scholar]
- 91.Pervaiz A, Sipra FS, Rana TH, Qadeer I. PRE-DONATION SCREENING OF VOLUNTEER PRISONER BLOOD DONORS FOR HEPATITIS B AND C IN PRISONS OF PUNJAB, PAKISTAN. J Ayub Med Coll Abbottabad. 2015;27(4):794–797. [PubMed] [Google Scholar]
- 92.Wali A, Khan D, Safdar N, Shawani Z, Fatima R, Yaqoob A, Qadir A, Ahmed S, Rashid H, Ahmed B, et al. Prevalence of tuberculosis, HIV/AIDS, and hepatitis; in a prison of Balochistan: a cross-sectional survey. BMC Public Health. 2019;19(1):1631. doi: 10.1186/s12889-019-8011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niriella MA, Hapangama A, Luke HP, Pathmeswaran A, Kuruppuarachchi KA, de Silva HJ. Prevalence of hepatitis B and hepatitis C infections and their relationship to injectable drug use in a cohort of Sri Lankan prison inmates. Ceylon Med J. 2015;60(1):18–20. doi: 10.4038/cmj.v60i1.7288. [DOI] [PubMed] [Google Scholar]
- 94.Salem F, Hekmat S, Aghasadeghi M, Javadi F, Gholamz H, Mostafavi E. Prevalence and risk factors of hepatitis B virus genotype d amongst inmates in alborz province, Iran: a cross-sectional survey. Jundishapur J Microbiol 2013;6(6):e10221
- 95.Ziaee M, Sharifzadeh G, Namaee MH, Fereidouni M. Prevalence of HIV and Hepatitis B, C, D Infections and Their Associated Risk Factors among Prisoners in Southern Khorasan Province, Iran. Iran J Public Health. 2014;43(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- 96.Khajedaluee M, Babaei A, Vakili R, Valizade N, Homaei Shandiz F, Alavian SM, Seyed Nozadi M, Jazayeri SM, Hassannia T. Sero-Prevalence of Bloodborne Tumor Viruses (HCV, HBV, HTLV-I and KSHV Infections) and Related Risk Factors among Prisoners in Razavi Khorasan Province, Iran, in 2008. Hepat Mon. 2016;16(12):e31541. doi: 10.5812/hepatmon.31541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moradi G, Gouya MM, Azimizan Zavareh F, Mohamadi Bolbanabad A, Darvishi S, Aghasadeghi MR, Nabavi M, Alasvand R, Tashakorian M, Nouri B, et al. Prevalence and risk factors for HBV and HCV in prisoners in Iran: a national bio-behavioural surveillance survey in 2015. Trop Med Int Health. 2018;23(6):641–649. doi: 10.1111/tmi.13065. [DOI] [PubMed] [Google Scholar]
- 98.Ghafari S, Sharifzadeh G, Jamali S, Taji B, Javadmoosavi SY, Ziaee M. Prevalence of Hepatitis B and C among Drug-Abusing Male Prisoners in Birjand, South Khorasan, Iran. Arch Iran Med. 2019;22(9):501–504. [PubMed] [Google Scholar]
- 99.Khademi N, Skakiba E, Khodadoust M, Khoramdad M. Seroprevalence and Related Risk Behaviors of Hepatitis C, Hepatitis B and HIV Infections among Male Prisoners in Kermanshah, Iran. Arch Iran Med. 2019;22(10):588–591. [PubMed] [Google Scholar]
- 100.Moradi G, Jafari S, Zarei B, Mahboobi M, Azimian Zavareh F, Molaeipoor L, Mohamadi Bolbanabad A, Darvishi S, Aghasadeghi MR, Tashakorian M, et al. Prevalence and Risk Factors for Hepatitis B and Hepatitis C Exposure in Iranian Prisoners: A National Study in 2016. Hepat Mon. 2019;19(7):e91129. [Google Scholar]
- 101.Sharafi H, Poustchi H, Azimian F, Tamadoni B, Ramezani R, Gouya MM, Sheikh M, Hashemi F, Tashakorian M, Alasvand R, et al. Performance of a rapid diagnostic test for screening of hepatitis C in a real-life prison setting. J Clin Virol. 2019;113:20–23. doi: 10.1016/j.jcv.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Hariri S, Sharafi H, Sheikh M, Merat S, Hashemi F, Azimian F, Tamadoni B, Ramazani R, Gouya MM, Abbasi B, et al. Continuum of hepatitis C care cascade in prison and following release in the direct-acting antivirals era. Harm Reduct J. 2020;17(1):80. doi: 10.1186/s12954-020-00431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hariri S, Sharafkhah M, Alavi M, Roshandel G, Fazel A, Amiriani T, Motamed-Gorji N, Bazazan A, Merat S, Poustchi H, et al. A simple risk-based strategy for hepatitis C virus screening among incarcerated people in a low- to middle-income setting. Harm Reduct J. 2020;17(1):56. doi: 10.1186/s12954-020-00400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reekie JM, Levy MH, Richards AH, Wake CJ, Siddall DA, Beasley HM, Kumar S, Butler TG. Trends in HIV, hepatitis B and hepatitis C prevalence among Australian prisoners - 2004, 2007, 2010. Med J Aust. 2014;200(5):277–280. doi: 10.5694/mja13.11062. [DOI] [PubMed] [Google Scholar]
- 105.Cunningham EB, Hajarizadeh B, Bretana NA, Amin J, Betz-Stablein B, Dore GJ, Luciani F, Teutsch S, Dolan K, Lloyd AR, et al. Ongoing incident hepatitis C virus infection among people with a history of injecting drug use in an Australian prison setting, 2005–2014: The HITS-p study. J Viral Hepat. 2017;24(9):733–741. doi: 10.1111/jvh.12701. [DOI] [PubMed] [Google Scholar]
- 106.Snow KJ, Richards AH, Kinner SA. Use of multiple data sources to estimate hepatitis C seroprevalence among prisoners: a retrospective cohort study. PLoS One. 2017;12(7):e0180646. doi: 10.1371/journal.pone.0180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hajarizadeh B, Grebely J, Byrne M, Marks P, Amin J, McManus H, Butler T, Cunningham EB, Vickerman P, Martin NK, et al. Evaluation of hepatitis C treatment-as-prevention within Australian prisons (SToP-C): a prospective cohort study. Lancet Gastroenterol Hepatol. 2021;6(7):533–546. doi: 10.1016/S2468-1253(21)00077-7. [DOI] [PubMed] [Google Scholar]
- 108.Sullivan RP, Baird R, Freeman K, Heggie H, Davis JS, Marshall CS, Davies J. Viral hepatitis in correctional facilities in the Northern Territory of Australia 2003–2017. BMC Infect Dis. 2021;21(1):584. doi: 10.1186/s12879-021-06286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaquet A, Wandeler G, Tine J, Dagnra CA, Attia A, Patassi A, Ndiaye A, de Ledinghen V, Ekouevi DK, Seydi M, et al. HIV infection, viral hepatitis and liver fibrosis among prison inmates in West Africa. BMC Infect Dis. 2016;16:249. doi: 10.1186/s12879-016-1601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okafor IM, Ugwu SO, Okoroiwu HU. Hepatitis C virus infection and its associated factors among prisoners in a Nigerian prison. BMC Gastroenterol. 2020;20(1):360. doi: 10.1186/s12876-020-01504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wakjira K, Abdissa A, Seid Y, Mekonnen Z. Seroprevalence and risk factors of hepatitis B, hepatitis C and HIV infections among prisoners in Jimma Town, Southwest Ethiopia. Asia Pac J Trop Dis. 2017;7(5):270–275. [Google Scholar]
- 112.Kassa Y, Million Y, Biset S, Moges F. Hepatitis B and Hepatitis C Viral Infections and Associated Factors Among Prisoners in Northeast Ethiopia. J Blood Med. 2021;12:561–570. doi: 10.2147/JBM.S314556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohamed HI, Saad ZM, Abd-Elreheem EM, Abd-ElGhany WM, Mohamed MS, Abd Elnaeem EA, Seedhom AE. Hepatitis C, hepatitis B and HIV infection among Egyptian prisoners: seroprevalence, risk factors and related chronic liver diseases. J Infect Public Health. 2013;6(3):186–195. doi: 10.1016/j.jiph.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 114.WHO: Progress report on access to hepatitis C treatment: Focus on overcoming barriers in low- and middle-income countries. In. Edited by Organization WH. Geneva, Switzerland; 2018.
- 115.Robaeys G, Bielen R, Azar DG, Razavi H, Nevens F. Global genotype distribution of hepatitis C viral infection among people who inject drugs. J Hepatol. 2016;65(6):1094–1103. doi: 10.1016/j.jhep.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 116.Ocal S, Muir AJ. Addressing Hepatitis C in the American Incarcerated Population: Strategies for Nationwide Elimination. Curr HIV/AIDS Rep. 2020;17(1):18–25. doi: 10.1007/s11904-019-00476-z. [DOI] [PubMed] [Google Scholar]
- 117.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. A1. PRISMA 2020 Checklist.
Additional file 2. A2. Year of publication of included sources.
Additional file 3. A3. Classification system for assessment of study methodologies.
Additional file 4. A4. Country of origin of included sources.
Additional file 5. A5. Included prevalence sources with additional risk factors and the prevalence of HIV coinfection, people who inject drugs and male incarcerated individuals (n=92; 36 countries).
Additional file 6. A6. Dissemination of the prevalence hepatitis C antibodies and the prevalence of people who inject drugs (PWID) within the different studies.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.