Abstract
COVID-19 remains a major public health concern, with large resurgences even where there has been widespread uptake of vaccines. Waning immunity and the emergence of new variants will shape the long-term burden and dynamics of COVID-19. We explore the transition to the endemic state, and the endemic incidence in British Columbia (BC), Canada and South Africa (SA), to compare low and high vaccination coverage settings with differing public health policies, using a combination of modelling approaches. We compare reopening (relaxation of public health measures) gradually and rapidly as well as at different vaccination levels. We examine how the eventual endemic state depends on the duration of immunity, the rate of importations, the efficacy of vaccines and the transmissibility. These depend on the evolution of the virus, which continues to undergo selection. Slower reopening leads to a lower peak level of incidence and fewer overall infections in the wave following reopening: as much as a 60% lower peak and a 10% lower total in some illustrative simulations; under realistic parameters, reopening when 70% of the population is vaccinated leads to a large resurgence in cases. The long-term endemic behaviour may stabilize as late as January 2023, with further waves of high incidence occurring depending on the transmissibility of the prevalent variant, duration of immunity, and antigenic drift. We find that long term endemic levels are not necessarily lower than current pandemic levels: in a population of 100,000 with representative parameter settings (Reproduction number 5, 1-year duration of immunity, vaccine efficacy at 80% and importations at 3 cases per 100K per day) there are over 100 daily incident cases in the model. Predicted prevalence at endemicity has increased more than twofold after the emergence and spread of Omicron. The consequent burden on health care systems depends on the severity of infection in immunized or previously infected individuals.
Keywords: COVID-19, Endemic mode, Reopening, Evolution, Vaccination
1. Introduction
SARS-CoV-2, the pathogen that causes COVID-19, is still spreading rapidly in many countries across the globe. There are indications that the disease will eventually become endemic rather than be eliminated. Natural questions to ask are: how will factors such as vaccination coverage, vaccine efficacy, duration of immunity and disease importation interplay to determine how and when endemic mode will be reached, and how can the transition happen without major resurgence of cases?
Despite the widespread use of vaccines that are highly efficacious at preventing severe disease and hospitalization, vaccines alone have failed to control transmission in many countries. Therefore physical distancing and other non-pharmaceutical interventions (NPIs) are still widely used to control the spread of COVID-19. These restrictions often come at a cost to the economy (Atalan, 2020), and individuals’ physical and mental well-being (Rossi et al., 2020, Greyling et al., 2020). Previous studies that have investigated the impact, on COVID-19 cases, of public health measure relaxation, all agree that some level of restrictions will still be required to keep cases under control (Moore et al., 2021b, Mulberry et al., 2021, Childs et al., 2021). Since then many jurisdictions have lifted NPIs and later re-introduced them when cases surged. But at some point in the near future, it is likely they will wish to implement some level of further reopening once again. Jurisdictions will need to determine the correct level and appropriate speed of reopening to sufficiently prevent negative outcomes such as cases, hospitalizations, or deaths, in light of their vaccine uptake.
The emergence of new variants of SARS-CoV-2 virus, often called variants of concern (VOCs), is another immediate challenge for COVID-19 pandemic response. Currently identified VOCs are more transmissible than the wild-type SARS-CoV-2, and have ability to evade host immunity to COVID-19 acquired from vaccines or infection (Snell et al., 2021, Moore et al., 2021a, Davies et al., 2021, Far, 2021, Naveca et al., 2021, Abdool Karim and de Oliveira, 2021, Bernal et al., 2021). SARS-CoV-2 is expected to undergo continuous adaptation, and new variants may continue to emerge as long as transmission remains high. Jurisdictions, therefore, need to factor in phenotypic changes in SARS-CoV-2 to their COVID-19 response plan, because there are serious implications for the short-term healthcare burden and longer-term health of the population.
It is important to study, and put in the context of real populations, how various factors such as choices related to reopening (relaxation of public health restrictions), vaccination coverage, viral evolution, waning immunity, and vaccine efficacy will shape short term case trajectories, and also determine the path from COVID-19 pandemic to endemic mode. We use two models, that we validate with a fit to data, to address several relevant issues such as SARS-CoV-2 evolution, how fast the current restrictions can be lifted without causing resurgence of cases, and the impact of high vaccination coverage on SARS-CoV-2 resurgence.
Mathematical modelling continues to play an important role in informing the COVID-19 response plan in many jurisdictions. The SIR-type model and its variants, which have a long history spanning over 200 years (Dietz and Heesterbeek, 2000, Kermack and McKendrick, 1927), are commonly used to understand COVID-19 dynamics. A number of modelling studies have analysed possible long term dynamics of COVID-19. Earlier on in the pandemic Kissler et al. (2020) used data from other coronaviruses to inform a model for SARS-CoV-2 in the USA, and predicted that social distancing measures may continue to be required intermittently up to as late as 2022. While emphasizing the role of acquired immunity, cross-immunity between coronaviruses and therapeutic interventions, they predict that resurgence may continue to occur up until 2025. Other studies on the long term dynamics of COVID-19 include (Saad-Roy et al., 2020), and more recently (Lavine et al., 2021a, Li et al., 2021, Lavine et al., 2021b). The studies suggest that duration of acquired immunity will modulate the transition from pandemic to endemic mode. In this work, we consider the impact of various reopening scenarios, and explicitly model the impact of new variants in reducing vaccine efficacy over time and the consequences for COVID-19 endemicity. Some studies that have investigated the impact of emergence and spread of immune evading variants and other variants of concern in the long term, as well as the impact of social networks and reopening policies, on the disease dynamics, include (Leng et al., 2020, Dyson et al., 2021, Thompson et al., 2021, Ryckman et al., 2021)
Since those studies were published, SARS-CoV-2 variants have continued to emerge: the most recent being Omicron and its sub-lineages, with indications of both increased transmissibility and immune escape (Karim and Karim, 2021). It is therefore important to continue to update knowledge of COVID-19 transition to endemic mode as the pandemic unfolds.
In this study, we first use an age and contact structured model to assess near-term dynamics of COVID-19 under several reopening and vaccination coverage scenarios. We calculate the herd immunity threshold from an age and contact structured model, as compared to equivalent calculations in a more simple SIR model, and find good agreement. These matching estimates, together with the fact that we must now accommodate immune escape (vaccine breakthrough infections and reinfections of those who have recovered), motivate us to develop a simple SVEIRS model to investigate how factors including vaccination efficacy against infection, infection importation rate, waning rate of acquired immunity, and the emergence of high transmission variants will impact the endemic state of COVID-19. The simpler model allows us to obtain a closed-form solution for the endemic steady state, and predict as well as analyse case incidence at endemicity. We also explore how antigenic “drift” and “shift” compare in this model, in terms of reduction in vaccine efficacy and the resulting impact on COVID-19 case numbers. We compare pre- and post-Omicron wave predictions of infections at endemic mode in British Columbia (BC), Canada and South Africa (SA).
2. Methods
In this study we use two Susceptible–Exposed–Infectious–Recovered models to answer important public health questions about the impact on the path to COVID-19 endemicity of vaccination coverage, public health measure relaxation plans, viral evolution, and immunity waning rates. The first model (Model 1), which we describe here (in the main text) with detailed results in the supplement, is an age and contact structured deterministic model. This allows us to explore how near-term dynamics are impacted by reopening and vaccination, as these factors will be significantly impacted by local population heterogeneity. We use the second, simpler, model (Model 2) for projections over the longer term, where a homogeneous model is more reasonable but we critically must incorporate waning immunity and viral evolution. These are not included in the age-structured model. Both models are set up to reflect the pandemic trajectory in BC, with a population of just over 5 million people. Moreover, Model 2 is set up for SA, providing a basis for comparison of the path to endemicity in two populations with different levels of exposure and vaccination coverage. This gives insight into how the infections at endemic mode may differ in different settings. We present results as a rate per 100k population for infections and hospitalizations. Model code and data for both models are openly available in: https://github.com/Yexuan-Song/End-Game.git
2.1. Model 1: Age and contact structured model for reopening scenarios and short term projections
We adapted an earlier published deterministic Susceptible–Exposed–Infectious–Recovered model (Mulberry et al., 2021) by parameterizing it to explore the impact, on infections and hospitalizations, of speed of relaxation of lockdown restrictions and vaccination coverage levels at the time of relaxation. Furthermore, we calibrated the model using COVID-19 data in BC. In the model the population is stratified into 15 sub-populations: by age and work status . Groups with superscript denote an “essential worker” group. The model has a contact matrix, that we took from Mulberry et al. (2021) which was based on an initially published data by Prem et al. (2017) before the COVID-19 pandemic. The contact matrix represents the contact probability between each age and “essential worker” status group. We further modified the contact matrix to match model fit to data on specific dates. The reproduction number reflects the effective reproductive number in the absence of vaccination but in the presence of NPIs (social distancing, quarantine, school opening etc.). The model tracks vaccination status, but only whether an individual takes a vaccine or not, neglecting the details of number of doses and time until the vaccine is effective. There is no waning of acquired immunity in the model, and we assume that vaccines can still prevent hospitalization and death even when they fail to prevent infection. We therefore use this model to explore short-to-medium term dynamics of COVID-19, primarily the impact of relaxation of NPIs and of vaccine coverage. We model gradual reopening (relaxation of NPIs) by increasing in a linear fashion from over a 300 day window, while rapid reopening is modelled by an all-at-one increase of . This age and contact structured model was presented in Mulberry et al. (2021) and is based on an earlier model of Bubar et al. (2021). Further details on the methodology used for Model 1 as well as the related results are presented in the supplement. Although most of the analyses and results from Model 1 are obtained a few months ago before the emergence and spread of Omicron, the key findings are still relevant as jurisdictions, with varying degrees of vaccination coverage level, will have to relax public health measures sooner or later. Therefore, these results can help inform best reopening strategies in such jurisdictions during COVID-19 and/or future pandemics.
2.1.1. Model 1 calibration
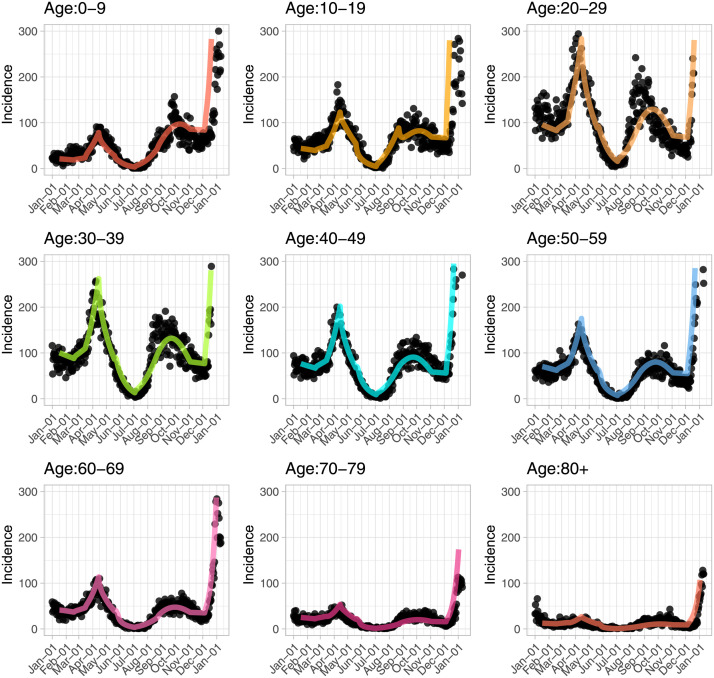
We validate the age and contact structured model by matching the model predicted case counts (including vaccine uptake and rollout as detailed above) to reported cases by age in BC from January 2021 to January 2022 (Fig. 1). On ‘important dates’, the model contact matrix and reproduction number are modified until a good fit to the reported case counts by age is obtained. This matching was done visually. A list of the identified ‘important dates’ is included in Supplementary Table S1.
Fig. 1.
Model calibration with age distribution of cases. Black dots represent actual reported cases by age from January 2021 to January 2022 in BC; coloured lines are the model predicted case counts by age. There is a sharp drop in testing in those younger than 70 years old starting from end of December, while testing is relatively consistent in those who are older than 70 years.
2.2. Model 2: Exploring endemic state with a simple SVEIRS model
Key determinants of COVID-19’s endemic state are: viral evolution — which will determine the overall transmissibility of infection and the antigenic drift and/or shift of the virus over time, infection importation rates, vaccine uptake and vaccine efficacy at preventing infection, as well as the duration of acquired immunity. To investigate how these factors interplay in determining the path to COVID-19 endemicity, we develop a simple Susceptible–Vaccinated–Exposed–Infectious–Recovered–Susceptible compartmental model to analyse and predict the endemic state for the COVID-19 pandemic in BC and SA. The model is described with a system of first order ordinary differential equations.
| (1) |
where .
In the model, susceptible individuals are vaccinated at a rate per day, and we assume vaccine efficacy against infection. Both vaccine induced and infection induced immunity wane at a rate , where is the duration of acquired immunity.
A quantity models the impact of control interventions at time on transmissibility by reducing or increasing the transmission rate to reflect changes in disease transmission when measures are implemented or relaxed in response to either resurgence of cases or to ease the negative impact of lockdown restrictions when cases are deemed to be under control. Exposed individuals become infectious after an average days, and they eventually recover, or are removed, after an average days. To model importations, the model allows susceptibles per unit time to be replaced by individuals who have already been infected but are not yet symptomatic or infectious, modelling the impact of travel-associated introductions without net changes in the population size. We use the following baseline parameter values: per day, , and (Davies et al., 2020). Note that is the per day rate for renewing vaccination (through boosters, for example) after individuals’ immunity has waned. We explore several scenarios that could determine the path of COVID-19 from pandemic to endemic in the two jurisdictions.
The prevalence () at endemic steady state is obtained analytically and analysed as a function of various parameters representing the aforementioned factors that will determine the number of active cases when the disease becomes endemic. For simplicity, we assume that at endemic state (no physical distancing measures, but we explore a range of transmission parameters) and that the population is constant over time. The full analytic solution is provided in the Supporting Information. To model the Omicron wave, with increased capacity to evade immunity, we modified model 2 by introducing parameters and which account for breakthrough infections and extra protection for those newly recovered against breakthrough infections, respectively. This modifies a subset of the model equations such that:
We use modified model 2 to assess the impact of evolution of SARS-CoV-2 on disease dynamics. We analyse mutations in the context of reduction of vaccine efficacy against the virus over time. We considered antigenic “drift” (gradual mutation) and “shift” (abrupt mutation) by allowing vaccine efficacy to vary with time non-linearly following a sigmoid curve. The speed of decline in vaccine efficacy is captured by the slope of the curve — slow decline for antigenic “drift” and faster decline for antigenic “shift”.
2.2.1. Model 2 calibration
Model calibration involves matching to model output, COVID-19 reported cases in BC, from February to November 23, 2021 (Delta wave) and from late November 2021 to late March 2022 (Omicron wave) in BC and SA. The model matching was done by varying the reproduction number over a range of values and visually matching model output to reported cases until a reasonable fit is achieved. The reproduction number was calculated using the next-generation matrix method (Diekmann et al., 2010). The vaccination rate is set such that the vaccination coverage in the model largely resembles vaccination uptake in the two jurisdictions during that period. We convert model predicted incident cases to reported cases by assuming a constant ascertainment probability of 24% in BC and 5% in SA (Gu et al., 2021). In practice, though, the ascertainment fraction likely varies over time. Ordinarily, ascertainment probability does not have any direct impact on the number of infections, but in our model, because of the fit to data, the ascertainment probability that we assume has some consequences for transmission in the model. For instance, it will require higher transmission rate to fit the data when the ascertainment probability is low, and vice versa. Consequently, lower ascertainment rates will mean an earlier and higher peak due to increased transmission. The reverse effect occurs under a higher ascertainment probability. Therefore, when we assume a much higher ascertainment probability, the infections settle to endemic levels more quickly than for lower ascertainment. And these effects are only substantial when the ascertainment probability changes significantly. In BC, during the Omicron wave, there was a drastic reduction in testing in those younger than 70, whereas testing was more consistent for those who are 70 years and older. We model an 80% reduction in ascertainment to account for this by using testing rates in the older cohort to correct for the testing deficit in the younger age group. The model fit to data is shown in Fig. 3 and in Figure S11 of the supplement. The model is also fitted to reported case data from SA for the Omicron wave, where to our knowledge, testing was not hugely changed during the Omicron wave (Fig. 4). We used openly available data published by the Data Science for Social Impact research group, at the University of Pretoria, SA (Marivate et al., 2020).
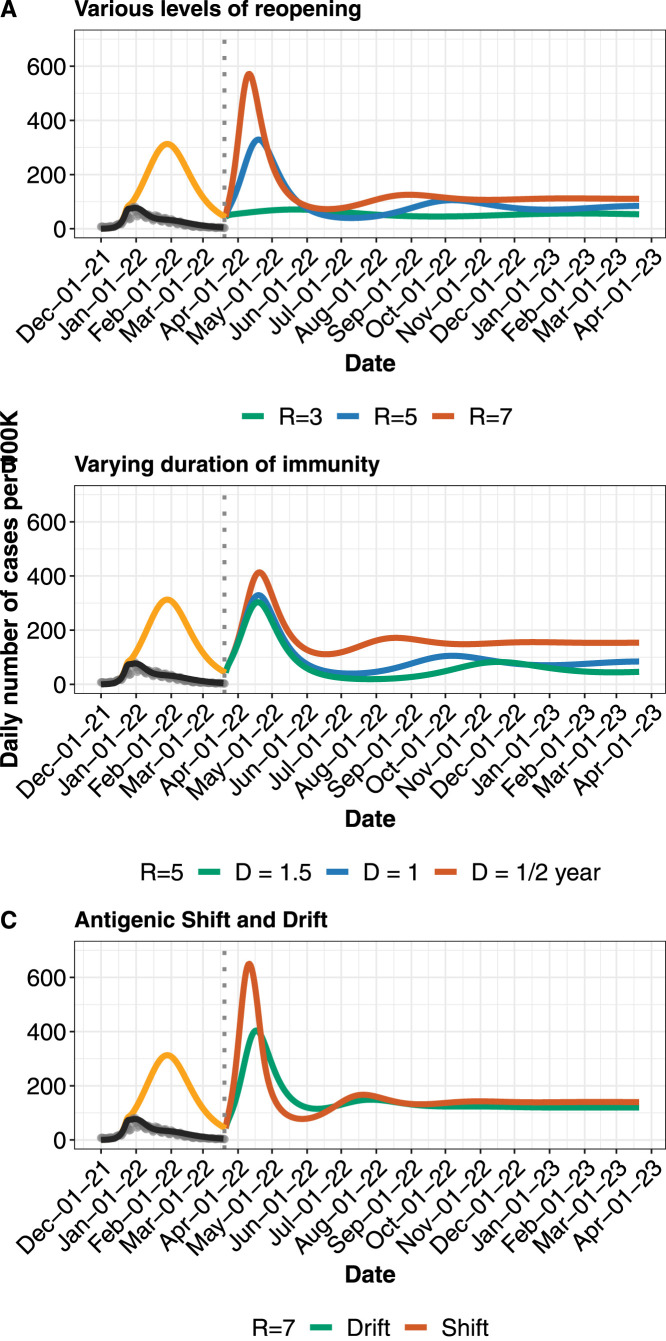
Fig. 3.
Near-future model 2 projections and various possible paths to COVID-19 endemicity in BC. A. Projected daily cases for different levels of reopening (shown by changing R), assuming reopening occurs in ending of March 2022. B. Projected daily cases for different lengths of duration of immunity (0.5–1.5 years), at . C. Comparing antigenic drift and shift. Gradual decrease in vaccine efficacy (“drift”) over a 1 year period versus a sudden decrease (“shift”) by 50% over a few days, at . Model output, corrected for testing constraints (black line), is matched to reported cases (grey dots) in BC from December 2021 to March 2022. Orange line indicates reportable cases without further testing constraint. Dotted vertical line indicates further reopening. Where not varied, importation rate is fixed at 2 cases per 100k per day, and duration of immunity at 1 year, additional protection for newly-recovered at 90%, and immune-escape capacity at 75%. Table 1 contains other parameter values and their sources.
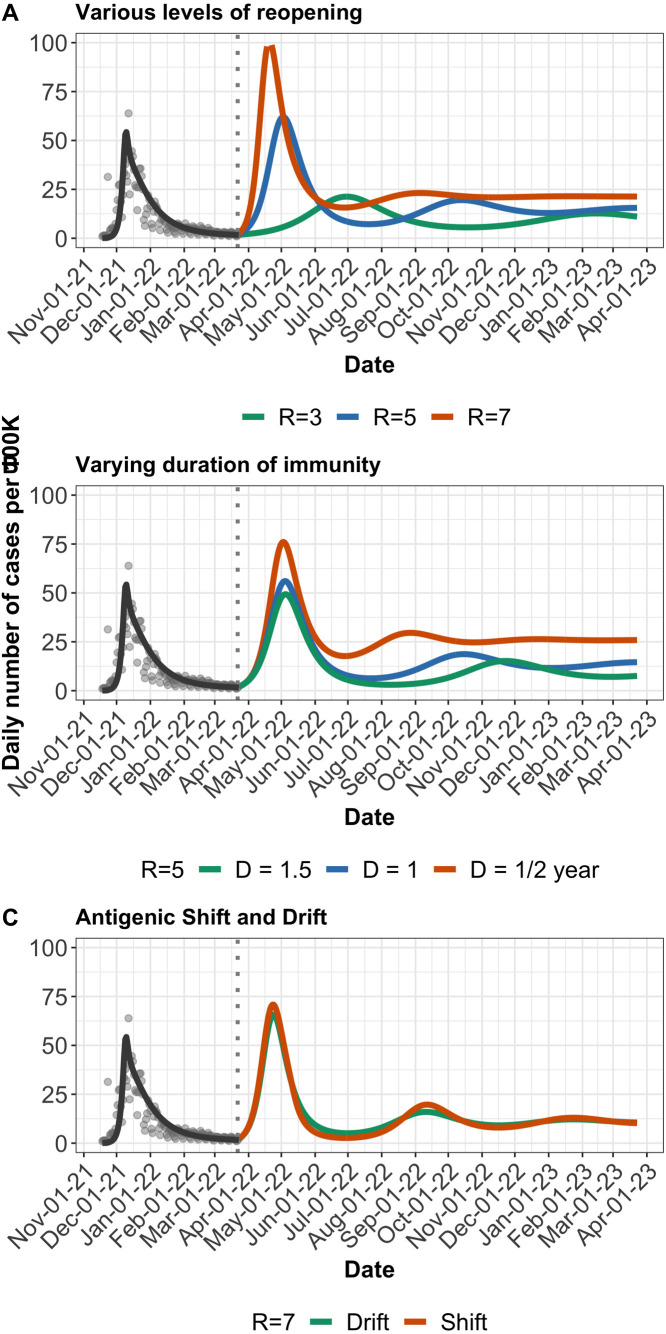
Fig. 4.
Near-future model 2 projections and various possible paths to COVID-19 endemicity in SA. A. Projected daily cases for different levels of reopening (shown by changing R), assuming reopening occurs in March 2022. B. Projected daily cases for different lengths of duration of immunity (0.5–1.5 years), at . C. Comparing antigenic drift and shift. Gradual decrease in vaccine efficacy (“drift”) over a 1 year period versus a sudden decrease (“shift”) from 80% to 40% over a few days, at . Model output (black line) is matched to reported cases (grey dots) in SA from November 2021 to March 2022. Dotted vertical line indicates further reopening. Where not varied, importation rate is fixed at 3 cases per 100k per day, all other parameters assume same values used for BC in Fig. 3.
3. Results
Model 1 calibration shows in Fig. 1 that the model output matches reasonably well the reported case numbers.
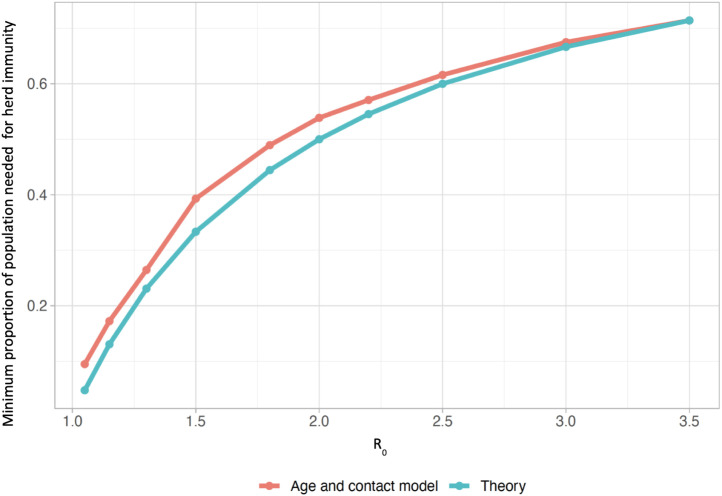
We compare the age and contact structured model’s results to those obtained from a theoretical (SIR) model of “herd immunity”, which we use here in the classic infectious disease modelling sense, namely the fraction of the population that must be in the “recovered” class in order for the number of infections to begin to decrease.
Fig. 2 illustrates Model 1’s prediction for the fraction of the population protected at the herd immunity threshold is similar to the theoretical prediction from the simple model. We can therefore estimate whether a given level of immunity obtained through vaccination is sufficient to stop the spread of SARS-CoV-2 using the classic relationship in SIR models between minimum herd immunity fraction and reproductive number : . Consider this simple theoretical example: in a jurisdiction where 20% of the population declines the vaccine, 10% are not eligible and we have a vaccine that is 80% effective against infection, the fraction of the population that is immune from vaccination alone is . The to which that fraction confers herd immunity, , is then . Accounting for approximately 5% of the population having had COVID-19 but some overlap between past infection and vaccination, , with corresponding of 2.5. Higher values lead to rises in cases. This theoretical framework thus provides a crude estimate of how resilient a population will be to resurgence of cases for a given value. It is noteworthy that there could be differing outcomes for achieving herd-immunity either through vaccination or through unabated transmission of infection. The latter may lead to significant further infections after falls below 1, due to overshoot caused by high levels of residual infection (Handel et al., 2020), whereas largely vaccine-induced herd immunity may lead to fewer infections in the population without as much burden on public health facilities.
Fig. 2.
Comparison between herd immunity estimates from age and contact structured model (Model 1) and a simple SIR model. The age and contact structured model is very similar to a simple SIR model in terms of the fraction of the population that must either be infected naturally (in the , or classes) or vaccinated successfully in order for the number of infections to begin to decline. This is the so-called “herd immunity” fraction. The theoretical result (blue) is simply . The model result is obtained by running a simulation at the given , as always defined in the absence of vaccination, detecting when infections begin to decline, and obtaining the portion of the population either infected or successfully vaccinated at that time. (Recall that Model 1 does not account for immune evasion).
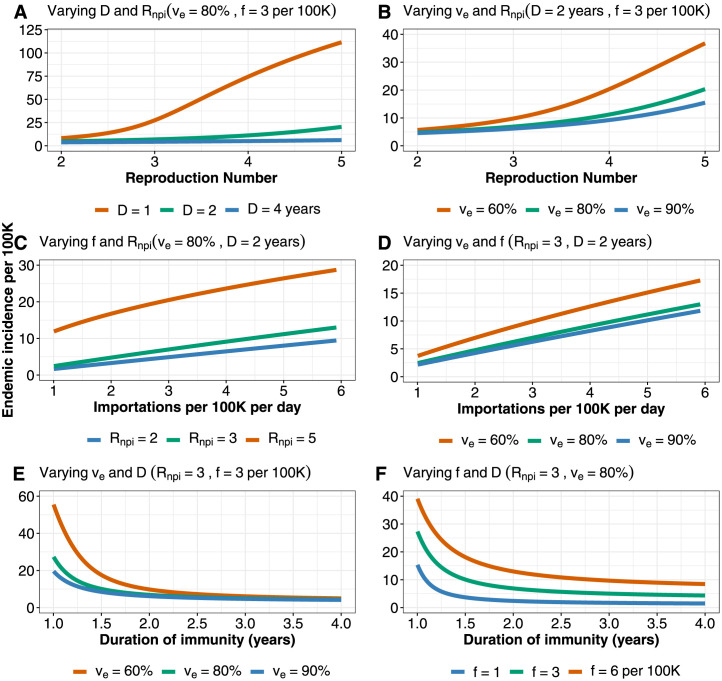
Given the motivation of similar behaviour in the age and contact structured model (Model 1) and the SIR model above, we drop the age and contact structure as they add considerable complexity. We explore several long-term scenarios using the SVEIRS model (Model 2). While this model does not include age and contact structure, it does include waning immunity, breakthrough infections and reinfections. We first consider reopening to various values, whilst also allowing for importation of infected cases. Model 2 yields a reasonable fit to reported case data (Fig. 3, Fig. 4). We find that in this model, there may be multiple waves of COVID-19 cases before it eventually becomes endemic. The frequency and peaks of the waves will depend on the duration of immunity and whether or not the vaccination campaign will continue to be supplemented with booster doses. When or greater, cases rebound to cause another wave of infection. In contrast, if is below 4, reopening will not lead to a major wave before becoming endemic. This is under the assumption that booster doses will be used to maintain relatively high population immunity (Fig. 3A). Furthermore, we study several immunity waning regimes (Fig. 3B). The endemic state is sensitive to the duration of acquired immunity, even under continual boosting after immunity wanes. Reopening to where immunity lasts for 1 year will lead to resurgence of cases, with endemic incidence below 100 reported infections per 100k per day. The picture becomes more optimistic as immunity lasts longer (Fig. 3B), with endemic incidence less than 50 reported cases per 100k per day under years immunity. However, if booster doses are suspended and immunity wanes, the projections become pessimistic (See Figure S11 in the Supporting Information). This will be compounded if high transmission and immune escape variants continue to emerge. In BC, infections at endemic state has increased compared to the pre-Omicron projections which predicted 40 cases per 100k per day for and 1 year immunity waning period (Fig. 5).
Fig. 5.
Endemic incidence (Model 2) as a function of: A. The reproduction number () and duration of immunity (). B. and vaccine efficacy (). C. Importation rate () and . D. and . E. and . F. and . We set baseline parameter values such that vaccine induced immunity and immunity due to infection last for 2 years, and boosters are given 4 months after immunity wanes. Parameter values are: cases per 100k per day, vaccination rate per day, years, 80%, and . In each subplot three parameters are varied at the same time: the two parameters declared in the subplot title and the parameter presented in the horizontal axis of the subplot, while the remaining two parameters are fixed with their values declared in the subplot title. Numbers are not adjusted for ascertainment. These results are from pre-Omicron model predictions.
We compare the impact on COVID-19 dynamics of gradual changes (or small mutations) in the virus that make vaccines less effective against them over time, compared to more abrupt mutation(s) that reduce vaccine efficacy more rapidly. Borrowing terminology from influenza viruses, we term these “drift” and “shift”, though the biological mechanisms will differ. One rationale for considering lower efficacy is the continued emergence and spread of VOCs that may undermine vaccination as a COVID-19 control strategy. At the current time, evidence suggests that antibody neutralization is not as effective for VOC B.1.351 (Wu et al., 2021) and P.1 (Dejnirattisai et al., 2021) as it is for the SARS-CoV-2 variants we have seen to date (including B.1.1.7) — although vaccines’ population-level effectiveness against VOCs are still relatively high (Nasreen et al., 2021). At the time of writing there is clear evidence that Omicron and its sub-lineages shows a marked decrease in antibody neutralization (Wilhelm et al., 2021, Cele et al., 2021). We model “drift” and “shift” by reducing vaccine efficacy 1- gradually decrease by 50% over a 500-day period, and a sudden (all-at-once) change, respectively. We find that a sudden shift leads to a worse outcome in the model, with a steep rise and fall before the system settles to endemic equilibrium (Fig. 3C).
Table 1.
Parameter description, values and sources.
| Parameter | Description | Value and source | |
|---|---|---|---|
| (BC) | Mortality rate | 1/(82 years) | Canada life expectancy (Mazzuco and Campostrini, 2022) |
| (SA) | Mortality rate | 1/(64 years) | SA life expectancy (Malakar and Lagdhyan) |
| Importations per 100k per day | 2 | Arino et al. (2020) | |
| Recovery rate | 1/6 | Lima (2020) | |
| Rate of progression from to | 1/3 | Tan et al. (2020) | |
We present similar analyses for SA (Fig. 3A–C). Despite differing vaccination coverage levels, both jurisdictions are still susceptible to rebound in infections either due to complete relaxation of public health measures or emergence of a high-transmission immune-escape variant. The rebound in cases is more marked in BC but the number of cases at endemic mode is fairly comparable in both jurisdictions (Fig. 4A–B). The difference in “Shift” and “Drift” is not as pronounced in SA (Fig. 4C) and this is largely due to relatively low vaccine uptake, as of March ending 2022.
In the pre-Omicron analysis we explore the impact of four endemicity-determining factors on the endemic incidence: reproduction number, immunity duration, vaccine efficacy and importation rate (Fig. 5A to F).
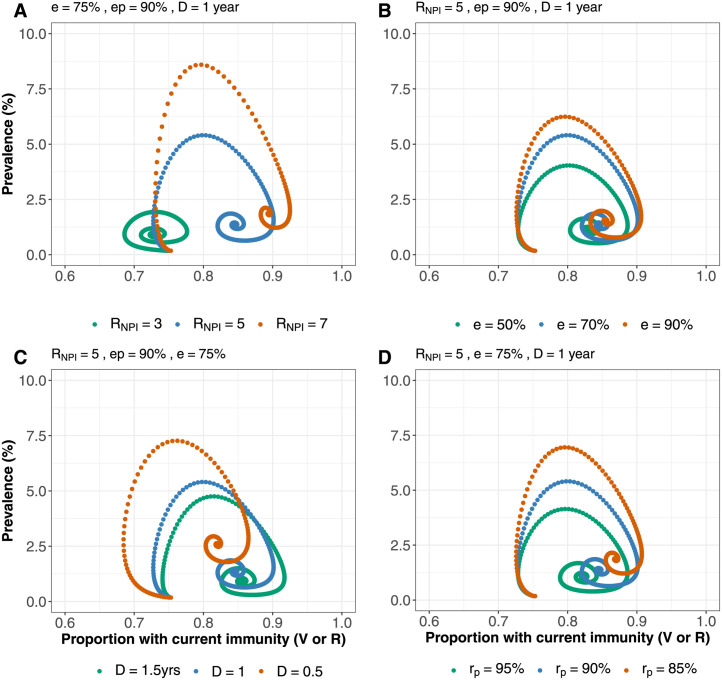
Using modified model 2 we present phase plane analysis for COVID-19 dynamics in BC and SA (Fig. 6, Fig. 7) – showing the prevalence and the proportion vaccinated or recovered. We varied various parameters that will determine the prevalence at endemicity, which are transmissibility , immune-escape capacity , duration of immunity and protection for newly recovered . Although vaccination coverage is higher in BC, at endemic mode, population exposure to infection pre-Omicron wave is higher in SA (Madhi et al., 2022) compared to BC (Watts et al., 2022) post-Omicron wave. Therefore BC is less resilient to resurgence compared to SA because of its higher proportion of recovered individuals (in ). However, overall BC has higher level of population immunity (in ) than SA, making SA more sensitive to changes in the varied parameters, as it will take longer to reach steady state equilibrium.
Fig. 6.
Phase portrait between disease prevalence and proportion vaccinated or previously exposed (Those currently in V or R) in BC . Prevalence and proportion immune at endemic mode for: A. various levels of . B. different values of immune-escape capacity . C. varying duration of immunity D. different levels of protection for newly-recovered .
Fig. 7.
Phase portrait between disease prevalence and proportion vaccinated or previously exposed (Those currently in V or R) in SA. Prevalence and proportion immune at endemic mode for: A. various levels of . B. different values of immune-escape capacity . C. varying duration of immunity . D. different levels of protection for newly-recovered .
4. Discussion
We used two modelling frameworks to study the impact of vaccination coverage and vaccine efficacy on resurgence of COVID-19 cases after public health measures are relaxed. The first model (Model 1), which has age and contact structure, provided insights on case distribution among different age groups under optimistic scenarios for duration of immunity, when public health measures are lifted either gradually or abruptly, at different vaccination coverage levels. On the other hand, the simpler model (Model 2), which incorporates reinfection and waning immunity, demonstrated how simpler models can provide similar insights to more complex ones, and also shows how various factors will interplay to shape incidence of COVID-19 infections at endemic mode. Model 1 is set up for only BC, while Model 2 is set up for both BC and SA.
Our results show that contrary to the expectation that wide spread infection due to the Omicron wave coupled with high vaccine uptake might have provided sufficient immunity for the pandemic to become endemic with low prevalence, some of our model estimates of endemic incidence are similar to the peak incidence observed in BC and SA during the pandemic so far as of ending of March 2022. On the path between the current state of the pandemic and the eventual endemic state, the speed and peak of case resurgence will be modulated by how fast we reopen, vaccination coverage and vaccine efficacy, as well as the transmissibility, the level of protection against infection among those newly recovered, and the immune escape capacity of the dominant variant at the time of reopening.
In the pre-Omicron phase, endemic incidence is sensitive to all of these unknown factors, but is most sensitive to the combination of the underlying transmission , vaccine efficacy and the duration of immunity. The model’s endemic incidence is not always markedly lower than peak incidence levels in the pandemic to date (approximately 20 per 100k per day). High endemic levels occur if immunity wanes rapidly (in under 1.5 years), if for the combination of virus and long-term measures is above 3, if there are over 6 imported infections per 100k per day, if efficacy is low and for various combinations. We model the true incidence; reported incidence would be lower, and would depend on the surveillance system that is in place and on the extent to which infection caused symptoms and severe disease.
We note that when is relatively low and vaccine coverage is substantially high, our model predicts no incident cases without importations, and in this sense it is an optimistic baseline from which to explore. In practice, heterogeneity in the population, continued viral evolution, introductions from animal reservoirs and other factors not included would likely mean that instead there would be some very low level of endemic incidence at our baseline parameters. Moreover, we considered infection blocking vaccine efficacy in this work, whereas since the emergence and spread of Omicron with increased capacity to evade immunity and cause breakthrough infection, vaccine efficacy at preventing infection has reduced (Suah et al., 2022). However, vaccines are still relatively effective at preventing severe disease (Andrews et al., 2022). The implication of this for our model prediction is that our model projections can potentially underestimate actual infections.
Pre- and post-Omicron model projections for BC and SA suggest that SARS-CoV-2 cases will rebound after restrictions are lifted completely. This occurs even under optimistic assumptions that immunity is continuously boosted. Also, the emergence and spread of Omicron and its sub-lineages, with increased capacity to escape acquired immunity, has increased model predicted infections at endemic state from around 0.5% prevalence (pre-Omicron) to 1.25% (post-Omicron) for , and 1 year duration of immunity.
At the time of writing, many EU countries are experiencing surges in SARS-CoV-2 cases, after many of the countries reopened at 70% vaccine coverage. Large resurgences are to be expected under those circumstances as they reopen further, because vaccine effectiveness is not 100% and the transmission rate of the Omicron variant is very high. An earlier study conducted in a low prevalence setting concluded similarly that SARS-CoV-2 infections will rebound if NPIs, including travel restrictions, are fully relaxed (Sachak-Patwa et al., 2021). In Austria, for instance, where only 65% (Our world in data, 2021) of the population are fully vaccinated in December, daily cases are at all time high, as of mid March 2022, with more than 53k reported cases per day.
Prior to the emergence of Omicron, vaccine effectiveness (while not 100%) was high against infection (85% (95% CI 80–90) for mRNA-vaccines by Pfizer-BioNTech and Moderna) and disease (97% (95% CI 96.7–97.2)) (Braeye et al., 2021). However, the SARS-CoV-2 virus is increasingly facing large vaccinated populations, and has primarily experienced selection in favour of enhanced transmission to prior to Omicron (Otto et al., 2021). Indeed, this selection played a role in the rapid emergence of several pre-Omicron VOC including both Alpha and Gamma (Otto et al., 2021). Furthermore, as populations across the world become vaccinated or have prior infection, SARS-CoV-2 will face increased selection in favour of immune escape. SARS-CoV-2 remains a relatively new virus, and we should anticipate that it will evolve further. Accordingly, Omicron and other currently-known VOC will not likely remain the key threats to vaccination’s effectiveness in ending the pandemic. We found that sudden shifts in efficacy are more dangerous than slower drift, in causing significant setbacks in the COVID-19 response. The sudden emergence of the Omicron variant (B.1.1.529) which has 32 mutations in the spike protein along with mutations in other regions of the genome (see preliminary data Rao and Singh, 2021) may indicate that SARS-Cov-2 is capable of sudden changes in immune evasion. Similar patterns have been observed for type A influenza viruses, which experience both antigenic shift and drift and are more likely to cause major outbreaks than type B viruses that experience only antigenic drift (Centers for Disease Control and Prevention, 2021).
The large peaks predicted by the simpler model may not be observed, because it is unlikely that cases will be allowed to grow excessively before some public health measures are re-introduced. Nonetheless, our results have policy-relevance through highlighting the high potential impact of NPI’s such as travel restrictions or physical distancing measures that could be re-implemented. Booster doses could further reduce population susceptibility (Government of British Columbia, 2021). On the other hand, there may be limited motivation to curb transmission if SARS-CoV-2 ultimately presents as mild disease in most people, for example due to cross immunity and/or residual immunity from vaccination or previous exposure (Antia and Halloran, 2021). If measures are not implemented, the predicted high peaks could occur. We assumed constant ascertainment over time, but ascertainment probability can change rapidly, as occurred in many jurisdictions including BC in late December 2022. This will depend on the surveillance system that is put in place, on test-seeking behaviour, and on the likelihood of symptoms and severe disease given infection.
We did not explicitly model differences in transmission due to symptomatic and asymptomatic individuals. The different time scales in transmission in those individuals may have some consequences for disease dynamics, and not capturing these dynamics is one of the limitations of our modelling framework. Although we varied the importation rate in several experiments, assuming a constant rate of importation over time simplifies the model, but it is also one of the limitations of the model framework, because in practice, importation rate may change over time — depending on many factors such as border control policies and infection prevalence in other jurisdictions.
The endemic prevalence of infection will determine the endemic demand for hospital and acute care resources, though both ascertainment and the relationship between infections and hospitalizations may change, with its attendant consequences for policy direction. Eventually, many of the infections will occur in those who were either immunized or previously exposed, and with B-cell mediated immunological memory that is long-lasting, it is to be hoped that 100 per 100k incidence (Fig. 5A) will not present a burden to the health care system so strong as to require widespread NPI measures. However, throughout the pandemic in BC until at least July 2021, reported COVID-19 cases were hospitalized at a relatively constant rate around 9% (British Columbia Centre for Disease Control, 2021). Early observations suggest that disease-blocking immunity wanes more slowly than infection-blocking immunity (Antia and Halloran, 2021, Skowronski et al., 2021). If this is the case, we can expect the rate at which cases are hospitalized to decrease at endemic state. However, if the endemic incidence is high (over 30 incident infections per 100k per day), even a reduction in overall severity (such as an 80% reduction) would leave on average just under 30 daily hospitalizations. Current conditions suggest that this would place a burden on the health care system in BC, particularly if it were enhanced by seasonal variation, and if capacity were impacted by other seasonal infections such as influenza.
Jurisdictions differ in their approaches to COVID-19 management as well as in some of the main risk factors for severe COVID-19, in the overall severity, health care resources such as hospital bed capacity, as well as control strategies. For instance, SA has high HIV/AIDS and Tuberculosis burden (Karim et al., 2009, Adetokunboh and Are, 2020), which may indicate a relatively high sub-population of immunocompromised individuals with substantial predisposition to severe COVID-19. On the other hand, the SA population is young and may be more resilient to high disease severity. All these factors will interplay to determine whether the health care system will be overburdened, how much testing will be available and when public health measures will be implemented or suspended. Therefore, although model predicted prevalence is comparable for similar levels of transmission rates in BC and SA, the actual infections may differ.
Overall, the virus’ evolution and the nature of waning immunity will shape the relationships between infections and reported cases, and between infections and hospitalizations/health care burden. If immunity against infection wanes quickly while immunity against disease lasts longer, and testing criteria are largely symptom-based, then reported cases might be low even where there are ample infections, presenting the opportunity for immune-evading variants to emerge. Population-level screening and genomic surveillance will aid in the rapid detection of emerging types and the assessment of their phenotypes.
This study shows that the endemic mode can be reached without risking resurgence of cases, if restrictions are lifted slowly, and measures are taken to increase vaccine uptake, while closely monitoring disease importations and viral evolution to enable quick detection/identification of VOCs. However, without carefully planned and properly executed interventions, COVID-19 may continue to cause considerable public health disruption for several years to come.
CRediT authorship contribution statement
Elisha B. Are: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization, Investigation, Validation, Writing – review & editing. Yexuan Song: Data curation, Writing – original draft, Visualization, Investigation, Validation, Writing – review & editing. Jessica E. Stockdale: Conceptualization, Methodology, Writing – review & editing. Paul Tupper: Conceptualization, Methodology, Data curation, Writing – original draft, Supervision, Writing – review & editing. Caroline Colijn: Conceptualization, Methodology, Data curation, Writing – original draft, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding acknowledgement
EBA, YS, JES, and CC are funded by the Federal Government of Canada’s Canada 150 Research Chair program. PT and CC were supported by Natural Science and Engineering Research Council (Canada) Discovery Grants (RGPIN-2019-06911 and RGPIN-2019-06624).
Footnotes
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.jtbi.2022.111368.
Appendix A. Supplementary data
The following is the Supplementary material related to this article.
The supplementary information contains more details, rationales, and key results of Model 1, as well as supplementary results from model 2.
References
- Abdool Karim S.S., de Oliveira T. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N. Engl. J. Med. 2021;384(19):1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetokunboh O., Are E. Distribution and determinants of HIV prevalence in South Africa. Ann. Epidemiol. 2020;52:116. [Google Scholar]
- Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., Gallagher E., Thelwall S., Groves N., Dabrera G., et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N. Engl. J. Med. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antia R., Halloran M.E. Transition to endemicity: Understanding COVID-19. Immunity. 2021;54(10):2172–2176. doi: 10.1016/j.immuni.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino, J., Portet, S., Bajeux, N., Ciupeanu, A., 2020. Investigation of Global and Local COVID-19 Importation Risks. Report to the Public Health Risk Science Division of the Public Health Agency of Canada.
- Atalan A. Is the lockdown important to prevent the COVID-19 pandemic? Effects on psychology, environment and economy-perspective. Ann. Med. Surg. 2020;56:38–42. doi: 10.1016/j.amsu.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Tessier E., Groves N., Dabrera G., Myers R., et al. Effectiveness of COVID-19 vaccines against the b. 1.617. 2 variant. MedRxiv. 2021 doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeye T., Cornelissen L., Catteau L., Haarhuis F., Proesmans K., De Ridder K., Djiena A., Mahieu R., De Leeuw F., Dreuw A., et al. Vaccine effectiveness against infection and onwards transmission of COVID-19: Analysis of belgian contact tracing data, january-june 2021. Vaccine. 2021;39(39):5456–5460. doi: 10.1016/j.vaccine.2021.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Columbia Centre for Disease Control T. 2021. BCCDC COVID-19 Epidemiology App. https://bccdc.shinyapps.io/covid19_global_epi_app/, Accessed: 2021-11-12. [Google Scholar]
- Bubar K.M., Reinholt K., Kissler S.M., Lipsitch M., Cobey S., Grad Y.H., Larremore D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371(6532):916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cele S., Jackson L., Khan K., Khoury D.S., Moyo-Gwete T., Tegally H., Scheepers C., Amoako D., Karim F., Bernstein M., Lustig G., Archary D., Smith M., Ganga Y., Jule Z., Reedoy K., San J.E., Hwa S.-H., Giandhari J., Blackburn J., Gosnell B.I., Karim S.A., Hanekom W., NGS-SA, COMMIT-KZN Team, von Gottberg A., Bhiman J., Lessells R., Moosa M.-Y.S., Davenport M.P., de Oliveira T., Moore P.P., Sigal A. SARS-CoV-2 omicron has extensive but incomplete escape of pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. MedRxiv. 2021 Available from: https://www.medrxiv.org/content/early/2021/12/09/2021.12.08.21267417. [Google Scholar]
- Centers for Disease Control and Prevention S. 2021. How the flu virus can change:“Drift” and “Shift”. https://www.cdc.gov/flu/about/viruses/change.htm, Accessed: 2021-11-16. [Google Scholar]
- Childs L., Dick D.W., Feng Z., Heffernan J.M., Li J., Röst G. Modeling waning and boosting of COVID-19 in Canada with vaccination. MedRxiv. 2021 doi: 10.1016/j.epidem.2022.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A., Russell T.W., Tully D.C., Washburne A.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage b. 1.1. 7 in England. Science. 2021;372(6538) doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M., Tuekprakhon A., Nutalai R., et al. Antibody evasion by the p. 1 strain of SARS-CoV-2. Cell. 2021;184(11):2939–2954. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O., Heesterbeek J., Roberts M.G. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface. 2010;7(47):873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K., Heesterbeek J. Bernoulli was ahead of modern epidemiology. Nature. 2000;408(6812):513–514. doi: 10.1038/35046270. [DOI] [PubMed] [Google Scholar]
- Dyson L., Hill E.M., Moore S., Curran-Sebastian J., Tildesley M.J., Lythgoe K.A., House T., Pellis L., Keeling M.J. Possible future waves of SARS-CoV-2 infection generated by variants of concern with a range of characteristics. Nature Commun. 2021;12(1):1–13. doi: 10.1038/s41467-021-25915-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Far W.W.K.S. COVID-19 b. 1.1. 7 (501y. V1) variant of concern. Synthesis. 2021;2:17. [Google Scholar]
- Government of British Columbia W.W.K.S. 2021. Get your booster dose. https://www2.gov.bc.ca/gov/content/covid-19/vaccine/booster, Accessed: 2021-11-17. [Google Scholar]
- Greyling, T., Rossouw, S., Adhikari, T., 2020. A Tale of Three Countries: How did Covid-19 Lockdown Impact Happiness?. GLO Discussion Paper.
- Gu X., Mukherjee B., Das S., Datta J. COVID-19 prediction IN SOUTH AFRICA: Estimating THE unascertained CASES-THE HIDDEN PART OF THE epidemiological iceberg. MedRxiv. 2021 [Google Scholar]
- Handel A., Miller J.C., Ge Y., Fung I.C.-H. If long-term suppression is not possible, how do we minimize mortality for COVID-19 and other emerging infectious disease outbreaks? MedRxiv. 2020 doi: 10.1017/dmp.2023.203. [DOI] [PubMed] [Google Scholar]
- Karim S.S.A., Churchyard G.J., Karim Q.A., Lawn S.D. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374(9693):921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack W.O., McKendrick A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. Ser. A. 1927;115(772):700–721. [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine J.S., Bjornstad O.N., Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371(6530):741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine J.S., Bjornstad O.N., Coombs D., Antia R. Severity of SARS-CoV-2 reinfections in second wave determines likelihood of mild endemicity. MedRxiv. 2021 [Google Scholar]
- Leng T., White C., Hilton J., Kucharski A., Pellis L., Stage H., Davies N.G., et al. The effectiveness of social bubbles as part of a Covid-19 lockdown exit strategy, a modelling study. Wellcome Open Res. 2020;5 doi: 10.12688/wellcomeopenres.16164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Metcalf C.J.E., Stenseth N.C., Bjørnstad O.N. A general model for the demographic signatures of the transition from pandemic emergence to endemicity. Sci. Adv. 2021;7(33):eabf9040. doi: 10.1126/sciadv.abf9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima C.M.A.d.O. Information about the new coronavirus disease (COVID-19) Radiol. Bras. 2020;53:V–VI. doi: 10.1590/0100-3984.2020.53.2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Kwatra G., Myers J.E., Jassat W., Dhar N., Mukendi C.K., Nana A.J., Blumberg L., Welch R., Ngorima-Mabhena N., et al. Population immunity and Covid-19 severity with omicron variant in south africa. N. Engl. J. Med. 2022;386(14):1314–1326. doi: 10.1056/NEJMoa2119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakar, S.N., Lagdhyan, K.-H.S., South Africa and BRICS. In: Locating BRICS in the Global Order. Routledge India, pp. 254–267.
- Marivate V., Arbi R., Combrink H., de Waal A., Dryza H., Egersdorfer D., Garnett S., Gordon B., Greyling L., Lebogo O., et al. 2020. Coronavirus disease (COVID-19) case data-South Africa. [Google Scholar]
- Mazzuco S., Campostrini S. Life expectancy drop in 2020. Estimates based on human mortality database. PLoS One. 2022;17(1) doi: 10.1371/journal.pone.0262846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Hill E.M., Dyson L., Tildesley M.J., Keeling M.J. Modelling optimal vaccination strategy for SARS-CoV-2 in the UK. PLoS Comput. Biol. 2021;17(5) doi: 10.1371/journal.pcbi.1008849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Hill E.M., Tildesley M.J., Dyson L., Keeling M.J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect. Dis. 2021;21(6):793–802. doi: 10.1016/S1473-3099(21)00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulberry N., Tupper P., Kirwin E., McCabe C., Colijn C. Vaccine rollout strategies: The case for vaccinating essential workers early. PLoS Glob. Public Health. 2021;1(10) doi: 10.1371/journal.pgph.0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen S., He S., Chung H., Brown K.A., Gubbay J.B., Buchan S.A., Wilson S.E., Sundaram M.E., Fell D.B., Chen B., et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. MedRxiv. 2021 [Google Scholar]
- Naveca F., Nascimento V., Souza V., Corado A., Nascimento F., Silva G., Costa Á., Duarte D., Pessoa K., Mejía M., et al. COVID-19 epidemic in the Brazilian state of amazonas was driven by long-term persistence of endemic SARS-CoV-2 lineages and the recent emergence of the new variant of concern p. 1. Res. Square. 2021 [Google Scholar]
- Otto S.P., Day T., Arino J., Colijn C., Dushoff J., Li M., Mechai S., Van Domselaar G., Wu J., Earn D.J., Ogden N.H. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021;31(14):R918–R929. doi: 10.1016/j.cub.2021.06.049. Available from: https://www.sciencedirect.com/science/article/pii/S0960982221008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our world in data S.P. 2021. Coronavirus (COVID-19) Vaccinations. https://ourworldindata.org/covid-vaccinations?country=AUT, Accessed: 2021-11-25. [Google Scholar]
- Prem K., Cook A.R., Jit M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017;13(9) doi: 10.1371/journal.pcbi.1005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Singh M. The newly detected b. 1.1. 529 (omicron) variant of SARS-CoV-2 with multiple mutations: Implications for transmission, diagnostics, therapeutics, and immune evasion. DHR Proc. 2021;1(S5):7–10. [Google Scholar]
- Rossi R., Socci V., Talevi D., Mensi S., Niolu C., Pacitti F., Di Marco A., Rossi A., Siracusano A., Di Lorenzo G. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front. Psychiatry. 2020;11:790. doi: 10.3389/fpsyt.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman T., Chin E.T., Prince L., Leidner D., Long E., Studdert D.M., Salomon J.A., Alarid-Escudero F., Andrews J.R., Goldhaber-Fiebert J.D. Outbreaks of COVID-19 variants in US prisons: a mathematical modelling analysis of vaccination and reopening policies. Lancet Public Health. 2021;6(10):e760–e770. doi: 10.1016/S2468-2667(21)00162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad-Roy C.M., Wagner C.E., Baker R.E., Morris S.E., Farrar J., Graham A.L., Levin S.A., Mina M.J., Metcalf C.J.E., Grenfell B.T. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020;370(6518):811–818. doi: 10.1126/science.abd7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachak-Patwa R., Byrne H., Dyson L., Thompson R. The risk of SARS-CoV-2 outbreaks in low prevalence settings following the removal of travel restrictions. Commun. Med. 2021;1(1):1–9. doi: 10.1038/s43856-021-00038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronski D.M., Setayeshgar S., Febriani Y., Ouakki M., Zou M., Talbot D., Prystajecky N., Tyson J.R., Gilca R., Brousseau N., et al. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: test-negative design studies from british columbia and quebec, Canada. MedRxiv. 2021 doi: 10.1093/cid/ciac290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell L.B., Wang W., Alcolea-Medina A., Charalampous T., Nebbia G., Batra R., de Jongh L., Higgins F., Wang Y., Edgeworth J.D., et al. First and second SARS-CoV-2 waves in inner London: A comparison of admission characteristics and the impact of the b. 1.1. 7 variant. MedRxiv. 2021 [Google Scholar]
- Suah J.L., Husin M., Tok P.S.K., Tng B.H., Thevananthan T., Low E.V., Appannan M.R., Zin F.M., Zin S.M., Yahaya H., et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int. J. Infect. Dis. 2022;119:69–76. doi: 10.1016/j.ijid.2022.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Wong L., Leo Y., Toh M. Does incubation period of COVID-19 vary with age? A study of epidemiologically linked cases in Singapore. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.N., Hill E.M., Gog J.R. SARS-CoV-2 incidence and vaccine escape. Lancet Infect. Dis. 2021;21(7):913–914. doi: 10.1016/S1473-3099(21)00202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A.W., Mâsse L.C., Goldfarb D.M., Irvine M.A., Hutchison S., Muttucomaroe L., Poon B., Barakauskas V., O’Reilly C., Bosman E.S., et al. SARS-CoV-2 seroprevalence among public school staff in metro vancouver after the first omicron wave in british columbia, Canada. MedRxiv. 2022 doi: 10.1136/bmjopen-2022-071228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., Metzler M., Kohmer N., Hoehl S., Helfritz F.A., Wolf T., Goetsch U., Ciesek S. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. MedRxiv. 2021 Available from: https://www.medrxiv.org/content/early/2021/12/08/2021.12.07.21267432. [Google Scholar]
- Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B., Colpitts T., Bennett H., Boyoglu-Barnum S., Shi W., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N. Engl. J. Med. 2021;384(15):1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary information contains more details, rationales, and key results of Model 1, as well as supplementary results from model 2.