Due to their immunocompromised status, cancer patients have reduced immune responses against SARS-CoV-2 after dual-dose COVID-19 vaccination [1]. A third primary dose has been recommended for this population as various studies showed its ability to boost the humoural immune response against SARS-CoV-2 [2,3]. However, it is presumed that after this third primary vaccination dose, the vaccination-induced protection against SARS-CoV-2 will wane rapidly, as seen after dual-dose vaccination [2,4,5]. This in combination with the emergence of highly transmissible SARS-CoV-2 variants, such as omicron BA.1, suggested that a fourth vaccination doses against SARS-CoV-2 may be beneficial for cancer and other immunocompromised patients, aiming to improve the protection against COVID-19. It had been shown recently is observed that a fourth mRNA vaccination dose against SARS-CoV-2 is able to significantly boost antibody responses against SARS-CoV-2 in healthy individuals, elderly and patients with autoimmune rheumatic diseases [[6], [7], [8], [9]]. The aim of this current study (B-VOICE: EudraCT 2021-000300-38, and Tri-VOICE plus: EudraCT: 2021-003573-58) is to investigate the specific role of the fourth dose in cancer patients.
A group of 157 cancer patients (Supplementary Table 1) received a fourth dose of the BNT162b2 vaccine after dual-dose BNT162b2 or ChAdOx1 vaccination and a third primary dose BNT162b2. All subjects were assigned to a cohort based on the type of active treatment receiving at time of first dose administration. Here, we report data of five different cohorts: chemotherapy, immunotherapy, targeted/hormonal therapy, B-cell depleting therapy and other haematological cancer treatments. A fourth vaccination dose was administered within a time window of 7 months after third primary vaccination, in all cancer patients. The majority (93%) received a fourth vaccination dose within a time window of 4–6 months (122–183 ± 10 days) after administration of the third primary vaccination dose. Blood samples for analysis of the immune response were collected 28 days after third dose administration (D3_d28), on the day of and prior to the fourth vaccination (D4_d0), and 28 days afterwards (D4_d28). Anti-S1 IgG SARS-CoV-2 antibody titres were analysed using the Siemens Healthineers Atellica IM SARS-CoV-2 IgG (sCOVG) assay to assess humoural immunity. Patients with detectable antibodies were considered as seroconverters. Log-transformed antibody titres were compared using a random intercept linear mixed model including time, active treatment at time of sampling and the interaction between time and active treatment at time of sampling. A two-sided P value <0.05 after Bonferroni–Holm correction for multiple testing was considered statistically significant.
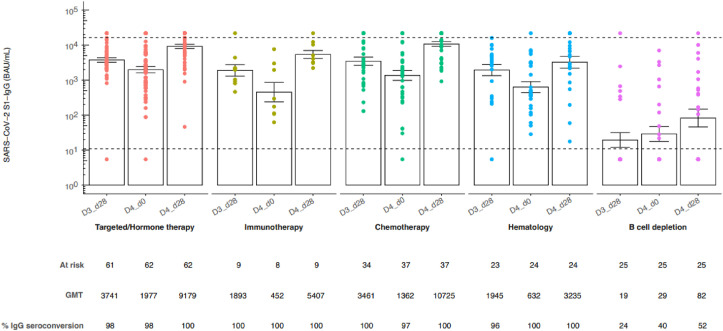
SARS-CoV-2 anti-S1 IgG antibody titres had significantly waned at D4_d0 compared to D3_d28 in patients receiving chemotherapy (GMT 1361.63 BAU/mL [95% CI 704.05–2633.36] versus GMT 3461.39 BAU/mL [95% CI 2012.74–5952.70, p adj. <0.05), immunotherapy (GMT 451.86 BAU/mL [95% CI 100.36–2034.36] versus GMT 1893.21 BAU/mL [95% CI 790.33–4535.14, p adj. <0.05), targeted/hormonal therapy (GMT 1977.19 BAU/mL [95% CI 1304.94–2995.76] versus GMT 3740.99 BAU/mL [95% CI 2776.15–5041.16, p adj. <0.05) and the haematology treatment cohort receiving other than B-cell depleting therapy (GMT 631.53 BAU/mL [95% CI 299.56–1331.40] versus GMT 1944.61 BAU/mL [95% CI 907.00–4169.23, p adj. <0.05) (Fig. 1 ). Waning humoural immunity after third vaccination dose was also observed in other studies, addressing both healthy individuals and immunocompromised patients, and was therefore expected [2,4,5]. Remarkably, waning of immunogenicity could not be observed in the group of haematological patients receiving B-cell depleting therapy since SARS CoV-2 anti-S1 IgG antibody titres did not change between D3_d28 and D4_d0. On the contrary, the percentage seroconverting patients in this cohort increases from 24% to 40% between D3_d28 and D4_d0.
Fig. 1.
SARS-CoV-2 anti-S1 IgG antibody titres before and after a fourth dose BNT162b2 in cancer patients. SARS-CoV-2 anti-S1 IgG antibody titres 28 days and 6 months after third BNT162b2 dose and 28 days after fourth BNT162b2 COVID-19 vaccine in the different treatment cohorts. Subjects were assigned to treatment cohorts based on the therapy received at time of the third dose. The height of each bar represents the geometric mean titre (GMT) ± standard error of the mean. Anti-S1 IgG-class antibody titres were quantified using a SARS-CoV-2 Immunoassay, Siemens Healthineers Atellica IM SARS-CoV-2 IgG (sCOVG) assay for the detection of antibodies (BAU/mL). Measuring interval: 10.90–16350.00 BAU/mL. Values below the detection were imputed half of it (5.45 BAU/mL), values above the measuring interval were imputed 33% above the upper limit of detection (21,800 BAU/mL) with dotted line indicating LLQ and ULQ, respectively.
Administration of a fourth vaccination induced significantly higher SARS-CoV-2 anti-S1 IgG antibody titres at D4_d28 in comparison to D3_d28 in patients receiving chemotherapy (GMT 10725.43 BAU/mL [95% CI 7821.65–14707.22], versus GMT 3461.39 BAU/mL [95% CI 2012.74–5952.69], p adj. <0.05), targeted/hormonal therapy (GMT 9179.44 BAU/mL [95% CI 6970.13–12089.03], versus GMT 3740.99 BAU/mL [95% CI 2776.15–5041.16], p adj. <0.05) and B-cell depleting therapy (GMT 82.47 BAU/mL [95% CI 24.68–275.59], versus GMT 19.44 BAU/mL [95% CI 7.13–53.03], p adj. <0.05) (Fig. 1). This indicates that the immune system of cancer patients did not produce the highest possible level of antibodies after full primary vaccination (three vaccination doses) and that a fourth vaccination dose is able to further boost the production of SARS-CoV-2 anti-S1 IgG antibodies to higher levels.
This trend is also observed in hematological patients receiving B-cell depleting therapy, but SARS-CoV-2 anti-S1 IgG antibody titres after fourth vaccination dose are still significantly lower compared to all other treatment cohorts (Fig. 1). The findings about the negative impact of B-cell depleting therapy on the immune system after a fourth vaccination dose are similar to the observations of Aikawa et al. in patients with autoimmune rheumatic diseases [6]. We previously studied that a third primary vaccination dose barely induced a humuoral immune response in haematological patients receiving rituximab [2]. Therefore, the observation that in the group of patients receiving B-cell depleting therapy, the percentage of seroconverts increases after fourth vaccination dose is promising.
This study confirms that after expected waning, the humoural immune response against SARS-CoV-2 in cancer patients is boosted again after fourth vaccination dose administration. In conclusion, the waning immunity after third primary dose in combination with the increased risk for severe COVID-19 after SARS-CoV-2 infection [10] highlights the importance of a fourth vaccination dose in cancer patients.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors kindly thank the B-VOICE and Tri-VOICE plus patients for participation, the nursing staff members at the Day Care Unit of the Antwerp University Hospital, the staff members of the Biobank Antwerp and all recruiting physicians. The authors are grateful to Dr. Lise Verbruggen (Multidisciplinary Oncological Centre Antwerp (MOCA), Antwerp University Hospital) and Dr. Greetje Vanhoutte (Multidisciplinary Oncological Centre Antwerp (MOCA), Dr. Laure-Anne Teuwen, MD (Multidisciplinary Oncological Centre Antwerp (MOCA), Antwerp University Hospital) and Dr. Pieter Pannus (SD Infectious Diseases in Humans, Service Immune response, Sciensano) for logistic support and critical revision of the manuscript and The authors are thankful to the clinical biology study team for serological analysis. In addition, the authors thank the B-VOICE and TRI-VOICE plus study teams for patient inclusion and sample collection.
This work was supported by the Belgian Government through Sciensano [COVID-19_SC004, COVID-19_SC059, COVID-19_SC061, COVID-19_SC104, COVID-19_SC107, COVID-19_SC118] and Kom op tegen Kanker [KOTK_UZA/2020/12604/1].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2022.11.016.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Peeters M., Verbruggen L., Teuwen L., et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debie Y, Vandamme T, Goossens ME, van Dam PA, Peeters M. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in cancer patients. Eur J Cancer. 2022;163:177–179. doi: 10.1016/j.ejca.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrière J., Carles M., Audigier-Valette C., et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer. 2022;162:182–193. doi: 10.1016/j.ejca.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferdinands J.M., Rao S., Dixon B.E., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastawicki W., Juszczyk G., Gierczyński R., Zasada A.A. Comparison of anti-SARS-CoV-2 IgG and IgA antibody responses post complete vaccination, 7 months later and after 3rd dose of the BNT162b2 vaccine in healthy adults. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2022;152 doi: 10.1016/j.jcv.2022.105193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aikawa N.E., Kupa L.V.K., Silva C.A., et al. Strong response after 4th dose of mRNA COVID-19 vaccine in autoimmune rheumatic diseases patients with poor response to inactivated vaccine. Rheumatology (Oxford, England) 2022 doi: 10.1093/rheumatology/keac301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grewal R., Kitchen S.A., Nguyen L., et al. Effectiveness of a fourth dose of Covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ (Clinical Research ed) 2022;378 doi: 10.1136/bmj-2022-071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munro A.P.S., Feng S., Janani L., et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22(8):1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magen O., Waxman J.G., Makov-Assif M., et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garassino M.C., Vyas M., de Vries E.G.E., et al. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: vaccinate. Monitor. Educate. Ann Oncol. 2021;32(5):579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.