Abstract
Thermosensitive liposomes (TSL) have been used for localized temperature-responsive release of chemotherapeutics into solid cancers, with a minimum of one invention currently in clinical trials (phase III). In this study, TSL was designed using a lipid blend comprising 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG-2000) (molar ratio of 88:9:2.8:0.2). Either nedaplatin (ND) or p-sulfonatocalix[4]arene-nedaplatin was encapsulated in the aqueous inner layer of TSL to form (ND-TSL) or p-SC4-ND-TSL, respectively. The hydrophobic platinum-based drug picoplatin (P) was loaded into the external lipid bilayer of the TSL to develop P-TSL. The three nanosystems were studied in terms of size, PDI, surface charge, and on-shelf stability. Moreover, the entrapment efficiency (EE%) and release % at 37 and 40 °C were evaluated. In a 30 min in vitro release study, the maximum release of ND, p-SC4-ND, and picoplatin at 40 °C reached 74, 79, and 75%, respectively, compared to approximately 10% at 37 °C. This demonstrated temperature-triggered drug release from the TSL in all three developed systems. The designed TSL exhibited significant in vitro anticancer activity at 40 °C when tested on human mammary gland/breast adenocarcinoma cells (MDA-MB-231). The cytotoxicity of ND-TSL, p-SC4-ND-TSL, and P-TSL at 40 °C was approximately twice those observed at 37 °C. This study suggests that TSL is a promising nanoplatform for the temperature-triggered release of platinum-based drugs into cancer cells.
Introduction
More than 50% of cancer patients treated with chemotherapeutics receive platinum-based drugs (PBDs).1 The antitumor activity of cisplatin was first discovered unintentionally by Barnett Rosenberg in 1965, and was approved by the U.S. Food and Drug Administration in 1978.2 Since its approval, several other PBDs have been developed, such as oxaliplatin,3 carboplatin,4 and satraplatin,5 aiming to improve anticancer activity and reduce the systemic adverse effects of cisplatin. These include ND and P, with the former being a water-soluble second-generation platinum-based drug, with remarkable antineoplastic effects as compared to cisplatin in numerous clinical trials conducted on lung, neck, head, cervical, and testicular cancer6 and has been approved for use in Japan since 1995. Picoplatin is designed to overcome intrinsic resistance caused by high levels of thiols in cancer cells and demonstrated anticancer activity when tested against colorectal, refractory prostate, ovarian, lung, and small and nonsmall cell lung cancers. Picoplatin is hydrophobic and hence shows significant bioavailability upon oral administration7 and is currently in phase III clinical trials.3 Systemic adverse effects, such as the potential risk of nephrotoxicity, present significant hurdles to the global acceptance of ND and picoplatin.
Through reformulation, considerable efforts have been made to improve chemotherapeutic drugs, including PBDs. These efforts aim to provide delivery systems that increase drug targeting and accumulation in cancer cells while reducing side effects. Such drug delivery systems may revive interest in chemotherapeutic drugs with limited acceptance such as ND and picoplatin.8 Several nanoplatforms have been reported to accommodate chemotherapeutic agents such as polymeric nanocapsules, dendritic nanosystems, macromolecules, and nanovesicles (niosomes and liposomes).8,9 Recently, supramolecular host molecules, including p-sulfonatocalix[4]arine (p-SC4), have exhibited a significant influence on the targeted delivery of chemotherapeutics.10P-SC4 is biodegradable, hydrophilic, and at doses up to 105 μg/kg is compatible with human cells .10−12 Considerable efforts have also been devoted to developing intelligent liposomal nanosystems that selectively release their cargo into cancer cells upon exposure to a particular stimulus, such as hyperthermia, ultrasound, or pH change.13−15 Thermosensitive liposomes (TSL) have been used for the localized temperature-responsive release of chemotherapeutics into solid cancers. ThermoDox is a TSL loaded with doxorubicin (DOX) and comprised of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphatidylcholine (MSPC), and N-(carbonylmethoxypolyethylene glycol2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (mPEG2000-DSPE) at a molar ratio of 86:10:4. ThermoDox has reached phase III clinical trials against hepatocellular carcinoma (HCC); however, it was declared unsuccessful in reaching its principal end point in terms of progression-free survival. The failure of this formulation might be attributed to the choice of chemotherapeutic drug, doxorubicin (DOX), which is known to have modest anticancer activity against HCC, as well as the scarcity of previous preclinical data on DOX against HCC.16 We believe the formulations suggested here might have more successful due to the fact that ND and picoplatin are both typically more effective against breast cancer cells (MDA-MB-231). In addition, our liposomal formulations have shown better encapsulation capacity than ThermoDoX, which allows for more drug payload. Finally, our use of supramolecular complexing agents such as p-SC4 has shown a significant increase in overall anticancer activity, most likely due to the increased solubility of the complexes formed. Previous studies reported augmentation of the anticancer effects of chemotherapeutics when combined with local hyperthermia (39–41 °C).17 This is achieved by increasing the accumulation of TSL loaded with drugs onto cancer cells by improving the permeability of the loaded drugs through loose cancer blood vessels.18,19 Also, when TSL is exposed to slight hyperthermia (39–42 °C), a phase transition of the liposomal lipid bilayer from a solid gel organized phase (Lβ) to a fluid scrambled stage (Lα) takes place. This increases the permeability of the liposomal membranes, enabling selectively triggered diffusion of the loaded drug.19−21 Several studies have reported the temperature-responsive liposomal delivery of chemotherapeutic agents, including paclitaxel, DOX, cisplatin, and methotrexate.22−26 Thermosensitive polymers, such as chitosan, gelatin, poly(N-isopropylacrylamide), and poly(N-vinylalkylamides), are also employed to create TSL. Thermosensitive polymers are water-soluble at body temperature, and upon application of the triggering temperature, they are converted into a hydrophobic state. Consequently, disruption of the liposomal membranes occurs, leading to cargo release.27−30 Radiolabeled liposomes also show considerable potential for cancer therapy and diagnosis. The incorporation of α- or β-emitting radionuclides into liposomal lipid bilayers or their entrapment in liposomal inner aqueous cavities has been previously reported for their potential use in cancer therapy.31 β-Emitters have been reported to allow much higher penetration into cancer tissues than α-emitters.32 Despite this fact, α-emitters have been proven effective in inducing DNS double-strand breaks in different cancer cells, where significant cytotoxic activity against leukemia cells resistant to anticancer drugs and β-emitters has been reported.33−35 The use of radionuclides is promising in cancer therapy and diagnosis; however, their in vivo behavior has not been thoroughly investigated.36
In this study, we reformulated some members of the newer generation PBDs, being ND and P (nedaplatin and picoplatin) using thermosensitive liposomes to revitalize interest in these promising chemotherapeutics by improving their anticancer activities while minimizing the off-target toxic effects. In this context, the first TSL formulations loaded with either ND, p-sulfonatocalix[4]arine-nedaplatin (p-SC4-ND) complex, or picoplatin were prepared. The physicochemical properties and entrapment efficiencies of these systems are presented. Controlled release of the drugs and improved cytotoxic activities in human mammary gland/breast adenocarcinoma cells (MDA-MB-231) were observed at physiological body temperature and at 40 °C.
Materials and Methods
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-hosphocholine (DSPC) (Lipoid GmbH, Germany), 1,2-distearoyl-sn-glycero-3-phosphoe-thanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG-2000), and cholesterol were obtained from Avanti Polar Lipids, Inc., USA. Polycarbonate membranes were obtained from Nuclepore (Whatman, United Kingdom). ND and picoplatin were purchased from BIOZOL Diagnostica Vertrieb GmbH, Germany, and Abcam, Germany, respectively. p-SC4 was purchased from Sigma-Aldrich (Germany). All other analytical-grade chemicals were obtained from AppliChem GmbH (Darmstadt, Germany) and Sigma-Aldrich (Germany).
Preparation of Stealth Thermosensitive Liposomes (TSL)
Drug Solutions
A 4.3 × 10–3 M solution of p-SC4-ND was prepared in a molar ratio of 1:1 in 20 × 10–3 M HEPES buffer (pH 7.4), according to our previously reported method.37
Lipid Composition
The lipid composition is a critical factor in designing optimal thermosensitive liposomes. Temperature-responsive release of the loaded drugs improves their efficiency while minimizing their potential adverse effects on healthy cells.22 The liposomal lipid bilayer undergoes structural deformations at temperatures equal to or lower than the melting phase transition temperature (Tm) of lipids and hence controls the release of loaded drugs outside the TSL membranes at the target organ.22 DPPC has a Tm of approximately 41 °C that is marginally higher than average body temperature; hence, it is utilized in designing TSL systems. However, DPPC was reported to obstruct drug release at the site of action and may lead to instability of liposomal membranes.38 Thus, in this study, DSPC, cholesterol, and DSPE-PEG-2000 were also used with DPPC at predetermined molar ratios to overcome the shortcomings of DPPC and improve the physicochemical properties of TSL. In this regard, DSPC with a Tm = 55 °C was used to prevent premature drug leakage at physiological body temperature.22 Cholesterol is involved in improving liposomal membrane stability and reducing drug diffusion across the lipid bilayer.39 In addition, DSPE-PEG-2000 was included to extend the residence time of the liposomes in systemic circulation by decreasing liposomal uptake by the reticuloendothelial system by generating steric hindrance around the liposomal membranes. DSPE-PEG-2000 was also reported to improve drug diffusion at the tumor site when exposed to local hyperthermia.40,41
In this study, TSL formulations were loaded with either ND, p-SC4-ND-TSL, or picoplatin to study the effect of this liposomal system on improving the cytotoxic activity of different PBDs. Either ND or p-SC4-ND-TSL was encapsulated in the aqueous inner layer of TSL to form ND-TSL or p-SC4-ND-TSL, respectively. The hydrophobic PBD picoplatin was loaded into the external lipid bilayer of the TSL to develop P-TSL. The physicochemical properties, entrapment efficiency, in vitro release, and cytotoxicity of the three systems were studied.
Preparation of ND-TSL and p-SC4-ND-TSL
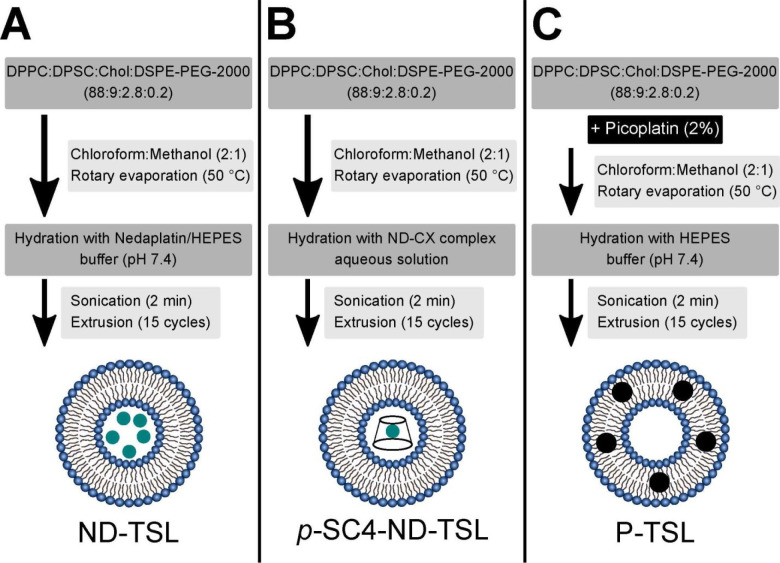
ND-TSL and p-SC4-ND-TSL were produced using the thin-film hydration technique followed by extrusion as described elsewhere42−45 with some modifications (Figure 1, panels A and B). Briefly, a lipid mixture comprising DPPC, DSPC, cholesterol, and DSPE-PEG-2000 was utilized in a molar ratio of 88:9:2.8:0.2, which is considered optimal for fabricating efficient TSL, and dissolved in a methanol/chloroform mixture (1:2, v/v). The obtained solutions were evaporated under reduced pressure for 1 h at 50 °C, which is close to the lipid transition temperature, Tm, employing a Laborota 4000 rotary evaporator (Heidolph Instruments, Schwabach, Germany) equipped with a vacuum pump (KNF Neuberger GmbH, Freiburg, Germany), to form a thin lipid film. The produced thin film was hydrated using a 20 × 10–3 M HEPES buffer (pH 7.4) containing either 2 mg/mL ND or p-SC4-ND solution in a rotary evaporator under normal pressure for 1 h at 50 °C to reach a final TSL concentration of 10 mg/mL. The obtained suspensions (multilamellar liposomes) were sonicated for 2 min using a bath sonicator (Elmasonic P30 H, Elma Hans Schmidbauer, Singen, Germany) and then were extruded through a 100 nm pore size polycarbonate membrane for 15 cycles at 50 °C (AvantiMini-Extruder (Avanti Polar Lipids, Inc., USA) to generate monodispersed liposomes. Unentrapped ND and p-SC4-ND were removed via size-exclusion chromatography as described below. The same procedure was employed to prepare void liposomes, where 20 mM HEPES buffer (pH 7.4) were used instead of ND and p-SC4-ND solutions. The prepared thermosensitive liposomes were stored at 4 °C until further studies.
Figure 1.
Schematic diagram summarizing the steps employed in preparing (A) ND-TSL, (B) p-SC4-ND-TSL, and (C) P-TSL.
Preparation of P-TSL
The design of the P-TSL followed the same protocol as described above (see Figure 1, panel C). However, 0.2 mg/mL picoplatin was dissolved in methanol (2% of the total lipid concentration) before its addition to the lipid mixture,46 and the hydration step was performed using 20 × 10–3 M HEPES buffer (pH 7.4), to obtain a final concentration of TSL of 10 mg/mL. The hydrophobic drug picoplatin has been reported to be incorporated within liposomal bilayers by binding to their hydrophobic tails.47
Determination of Average Particle Size, Polydispersity Index (PDI), and Zeta-Potential
The different average particle size of the liposomal systems, PDIs, and zeta potentials were studied using dynamic scattering employing Zetasizer Nano ZS (Malvern Instruments Herrenberg, Germany) at 25 °C.48
Entrapment Efficiency
The Entrapment Efficiency of ND-TSL and p-SC4-ND TSL
Size-exclusion chromatography was used to separate free ND and p-SC4-ND from previously reported liposomal formulations, with slight modifications.49 A Sephadex filled chromatographic column C10/20 G-25 Superfine (Amersham Biosciences, Sweden) was used in this study. Each liposomal formulation (500 μL) was separated from the unloaded drug by using isotonic HEPES (pH 7.4) as the eluent. The ND and p-SC4-ND concentrations were spectrophotometrically quantified at 283 nm using a UV/vis spectrophotometer-1240 mini, SHIMADZU after hydrolyzing the liposomes with Triton X-100 (10% w/v).
Entrapment Efficiency of P-TSL
To determine the entrapment efficiency, EE, of P-TSL, 500 μL of P-TSL formulation was centrifuged for 2 h at 14,000 rpm using an Eppendorf Centrifuge 5418 (Eppendorf GmbH, Wesseling-Berzdorf, Germany). The obtained residue was diluted in 300 μL of methanol and 300 μL of HEPES buffer (pH 7.4), and 300 μL of the supernatant was diluted with 300 μL of methanol.50 The concentration of picoplatin in both samples was determined spectrophotometrically at 291 nm.
Calculating the Entrapment Efficiency of TSL Systems
The entrapment efficiencies were computed using eq 1, as follows:
| 1 |
Morphology
Surface analysis of the prepared TSL formulations, diluted 1:10 with deionized water, was carried out using atomic force microscopy (AFM) utilizing a Nanowizard 3 Nanoscience AFM (JPK Instruments, Berlin, Germany) in intermittent contact mode with an aluminum-coated silicon nitride probe (HQ: NSC14/Al BS, μmasch, Tallinn, Estonia) at scan rates between 0.5 and 1 Hz.51 The JPKSPM data processing software (JPK Instruments) was used to process the generated raw images.
In Vitro Temperature-Triggered Drug Release from TSL
The % drug released was evaluated at 37 and 40 °C, respectively. Briefly, 500 μL of each formulation was inserted into seperate 2 mL Eppendorf tubes and heated in a water bath at 37 and 40 °C, respectively, for predetermined time intervals, ranging from 0 to 30 min. At each time interval, the tubes were removed and immediately centrifuged using an Eppendorf centrifuge 5418 (Eppendorf GmbH, Wesseling-Berzdorf, Germany).45 The drug concentration for each liposomal formulation was determined spectrophotometrically as described previously.
The % release of ND, p-SC4-ND, and picoplatin was determined using eq 2.
| 2 |
Effect of Storage on the TSL Systems
The effect of storage on the TSL systems was evaluated where their average particle sizes and PDIs were measured after 1, 7, 14, 21, and 28 days of storage at 4 °C.52
Cell Culture
Human mammary gland/breast adenocarcinoma cells (MDA-MB-231) derived from metastatic sites were obtained from the American Type Culture Collection (ATCC, Manassas, VA). MDA-MB-231 cells were cultivated at 37 °C and 7% CO2 under humid conditions in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, exposed to γ irradiation (Capricorn Scientific, Ebsdorfergrund, Germany). Cells were grown as monolayers and passaged upon reaching 80–90% confluence.
Cytotoxicity Assay of ND-TSL, p-SC4-ND-TSL, and P-TSL Systems
To investigate the in vitro antineoplastic activity of void TSL, p-SC4, ND-TSL, p-SC4-ND-TSL, P-TSL, and free ND, MDA-MB-231 cells were exposed to varying concentrations of loaded liposomal systems, free drug solutions, void liposomes, and p-SC4 solutions in triplicate. The viability of MDA-MB-231 cells was evaluated via MTT assay at 37 and 40 °C using our previously reported method.30
Statistical Analysis
All experiments were performed in triplicate, and the results are expressed as mean ± standard deviation. A two-tailed t-test was performed, and statistical significance was set at p < 0.05.
Results and Discussion
Particle Size, Polydispersity Index (PDI), ζ-Potential, And Entrapment Efficiency
The average particle size and PDI of the TSL systems were determined by dynamic light scattering. Table 1 shows that the average particle size of ND-TSL was close to that of the void liposomes at 112.84 ± 2.14 nm (PDI = 0.155 ± 0.039). The average sizes of p-SC4-ND-TSL and P-TSL were 122.93 ± 1.96 nm (PDI = 0.16 ± 0.01) and 120.73 nm ± 1.33 nm (PDI = 0.18 ± 0.02), respectively. The incorporation of either the p-SC4-ND complex inside the aqueous inner layer of liposomes or the of picoplatin in the outer lipid bilayer was accompanied by an increase in the average particle size. These results are in line with those of previously reported studies.40,46 The presented data also suggested the formation of monodispersed unilamellar liposomes with sizes of 112–123 nm for all formulations produced here. As illustrated in Figure 2 all liposomal formulations showed spherical shapes and homogeneous size distribution. The size of the TSL, together with its lipid composition, play an essential role in controlling drug release. TSL formulations with average sizes ranging from 50 to 200 nm were previously reported to be ideal for temperature-triggered cancer therapy and the bioavailability of encapsulated drugs.22,41 Liposomal membrane permeability is increased by decreasing the membrane size owing to the high membrane curvatures, which typically leads to more structural defects.41
Table 1. Hydrodynamic Diameter, PDI, ζ-Potential, and Entrapment Efficiency of Void TSL, ND-TSL, p-SC4-ND-TSL, and P-TSL.
| formula | hydrodynamic diameter (nm) | PDI | ζ-potential (mV) ± SD | entrapment efficiency (%) ± SD |
|---|---|---|---|---|
| void TSL | 114.39 ± 0.94 | 0.09 ± 0.03 | –3.86 ± 0.70 | |
| ND-TSL | 112.84 ± 2.14 | 0.15 ± 0.04 | –3.68 ± 0.59 | 74.19 ± 0.49 |
| p-SC4-ND TSL | 122.93 ± 1.96 | 0.16 ± 0.01 | –15.46 ± 0.37 | 91.32 ± 0.63 |
| P-TSL | 120.73 ± 1.33 | 0.18 ± 0.02 | –5.11 ± 0.15 | 95.75 ± 0.49 |
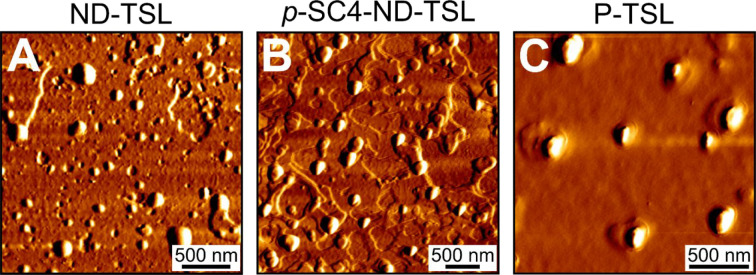
Figure 2.
AFM images of (A) ND-TSL, (B) p-SC4-ND-TSL, and (C) P-TSL show near spherical shapes for all liposomal systems.
The zeta potential of the liposomal system was measured by laser Doppler velocimetry. As presented in Table 1, the ζ-potential values for void liposomes, ND-TSL, and P-TSL bear a lower negative charge at −13.86 ± 0.70, −10.31 ± 4.01, and −5.11 ± 0.15 mV, respectively relative to −16.03 ± 1.15 mV forp-SC4-ND-TSL. This was attributed to the presence of p-SC4 in the complex, which has a highly negative charge.40 The negative charge on all systems imparts high stability. The lipid composition also contributes to liposomal stability, thus cholesterol has been used to improve TSL stability and reduce drug permeability across the lipid bilayer.39
As shown in the entrapment studies summarized in Table 1, the entrapment efficiency of p-SC4-ND-TSL was higher than that of ND-TSL. This is partially because p-SC4 has a high hydrophobic interior cavity and significantly higher hydrophilicity and stability upon forming inclusion complexes with ND. These factors partially favor the entrapment of p-SC4 into the aqueous core of the TSL, hence the higher EE% which include encapsulated and surface-immobilized drugs. The formation of the inclusion complexes (between p-SC4 and ND) may also significantly reduce the leakage of ND outside the liposomal membranes, which results in the observed higher EE%.37,40,53 The interactions between ND, ND-TSL, or p-SC4-ND and lipid membranes are not well investigated and may include electrostatic attraction, hydrogen bonding, charge-assisted hydrogen bonding, and electron-donor–acceptor interactions. The high EE% of ND in both ND-TSL and p-SC4-ND TSL is possibly partially attributed to the electrostatic interactions between the cationic aquated ND species, formed by the aquation of ND in solution, and the anionic liposomal system. Previous studies reported the critical role of such electrostatic interactions in binding the cationic drugs with negatively charged liposomal surface.54 Moreover, the presence of DSPC,Tm = 55 °C, and cholesterol in the TSL most likely hindered the premature drug leakage across the lipid bilayer and enhanced the liposomal membrane stability under physiological temperature.39 The entrapment efficiency of P-TSL (>95%) was higher than that of ND-TSL and p-SC4-ND-TSL, which is consistent with previous studies.47 This is because of the hydrophobic nature of picoplatin, which stimulates its interaction with the phospholipid bilayer via hydrophobic interactions.55
In Vitro Temperature-Triggered Drug Release from TSL
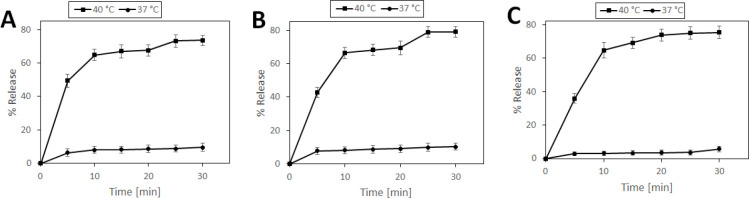
The release patterns of ND from ND-TSL and p-SC4-ND-TSL as well as of picoplatin from P-TSL, all at 37 and 40 °C are shown in Figure 3 (panels A, B, and C, respectively). At 37 °C, being below the lipid Tm, only 10% of ND, p-SC4-ND, and picoplatin were released within 30 min from the TSL systems. On the other hand, at 40 °C, the maximum release of ND, p-SC4-ND, and picoplatin at 30 min were 74, 79, and 75%, respectively. Thus, the release of the drugs was accelerated significantly when the temperature was increased to 40 °C, being above the lipid Tm. These values align with our previous study that reported the ability of TSL to release more than 40% of its content in 30 min at 40 °C.56 At this temperature, the lipid bilayer is transformed from the solid gel assembled phase to a liquid disassembled phase, increasing the liposomal lipid bilayer permeability and fluidity. This leads to the temperature-triggered release of the loaded hydrophilic and hydrophobic drugs.57 Additionally, DSPE-PEG-2000 enhanced drug diffusion upon exposure to local hyperthermia.40,41
Figure 3.
In vitro temperature-triggered drug release at 37 and 40 °C for (A) ND-TSL, (B) p-SC4-ND-TSL, and (C) P-TSL.
Storage Study
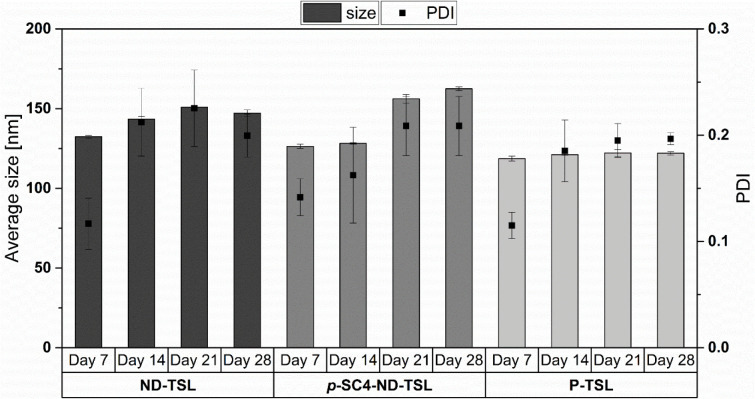
Figure 4 shows that all three systems did not significantly increase in size or PDI within the test period. The sizes of ND-TSL, p-SC4-ND-TSL, and P-TSL were observed to increase, compared to their initial sizes, by about 15, 35, and 4 nm, respectively. These size increases are modest for ND-TSL and p-SC4-ND-TSL and nearly negligible for the P-TSL formulation. This may be attributed to a greater amount of surface-immobilized ND in the first two formulations relative to the corresponding surface-immobilized picoplatin in the P-TSL formulation. Surface-immobilized drugs may, in turn, interact with the water molecules in the solvent, thus increasing the effective hydrodynamic sizes as measured by dynamic light scattering. Picoplatin, on the other hand, is well settled inside the lipid bilayer due to its hydrophobic nature and its formulations has shown insignificant size changes. These findings show acceptable stability of the prepared TSL upon storage for up to 28 days.
Figure 4.
Average size and PDI of ND-TSL, p-SC4-ND-TSL, and P-TSL after 7, 14, 21, and 28 days at 4 °C.
Cytotoxicity Assay
ND-TSL and p-SC4-ND-TSL
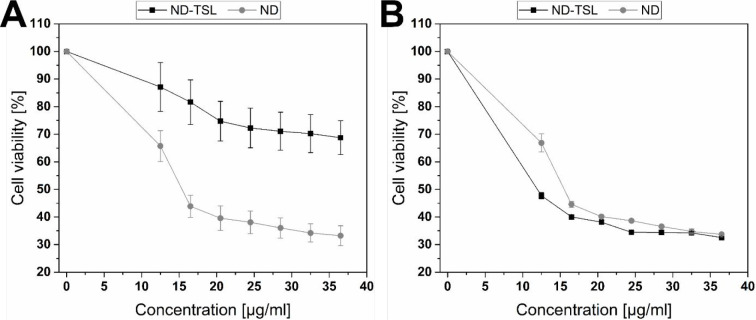
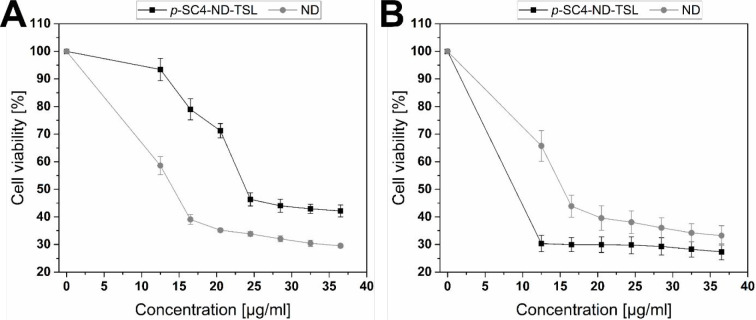
The cytotoxic effects of ND compared to void TSL, p-SC4, ND-TSL, and p-SC4-ND-TSL were examined individually against MDA-MB-231 cells using MTT assay at 37 and 40 °C. Void liposomes and p-SC4 were used as vehicle control and showed no significant decrease in cell viability (Figure S1). Figures 5 and 6 demonstrate the significance of triggering mild local hyperthermia in increasing the cytotoxic effects of both systems. Panels A in Figure 5 and Figure 6 show that, at 37 °C, the cytotoxicity of free ND is higher than ND-TSL and p-SC4-ND-TSL at all concentrations from 12.5–36.5 μg/mL. This strongly suggests that the TSL did not release its content at this physiological temperature. However, it was shown that the cytotoxicity of ND-TSL and p-SC4-ND-TSL at 40 °C were approximately twice that observed at 37 °C. This is likely due to the TSL releasing its content at that temperature, above the Tm of the lipids, as presented in panel B in Figures 5 and 6(58).
Figure 5.
Assessing cytotoxicity of ND-TSL and free ND at various concentrations (12.5–36.5 μg/mL) utilizing MTT assay and MDA-MB-231 cells at (A) 37 °C and (B) 40 °C for 24 h. An overall statistically significant decrease in cell viability was observed with ND-TSL compared to ND. Untreated cells were used as negative control and considered as 100%. All experiments were carried out in triplicate, and the mean values were calculated. Error bars represent ± standard deviation.
Figure 6.
Assessing cytotoxicity of p-SC4-ND-TSL and free ND at various concentrations (12.5–36.5 μg/mL) using MTT assay and MDA-MB-231 cells at (A) 37 and (B) 40 °C for 24 h. An overall statistically significant decrease in cell viability was observed with p-SC4-ND-TSL compared to ND. Untreated cells were used as the negative control and considered as 100%. All experiments were conducted in triplicate, and the mean values were calculated. Error bars represent ± standard deviation.
Figure 5 (panel B) shows the cytotoxicity of the thermosensitive liposomal formulation ND-TSL at 12.5 μg/mL, being the lowest concentration tested, at 40 °C was nearly twice that of an equivalent concentration of the free drug. Cell viability decreased as the concentrations of either the thermosensitive liposomal formulation or free drug increased, reaching 32.5 and 33.7%, respectively, at a maximum concentration of 36.5 μg/mL. Encapsulation of ND in TSL also permitted the anticancer effect of the drug to be exerted at a lower concentration than that of the free drug. These findings support previous studies showing the ability of liposomes to augment the cytotoxic activity of encapsulated anticancer drugs.59
The hybrid p-SC4-ND-TSL exhibited a notable cytotoxic activity compared to free ND and ND-TSL at 40 °C (Figure 6, panel B). The cytotoxicity of p-SC4-ND-TSL at 12.5 μg/mL, the lowest concentration tested, was nearly twice that of an equivalent free drug concentration and 1.5-fold higher than that of ND-TSL. The cell viability decreased as the concentrations of either the hybrid system or free drug increased, reaching 27.3 and 33.2%, respectively at the maximum concentration of 36.5 μg/mL. The increased anticancer activity of the hybrid p-SC4-ND system compared to that of free ND and ND-TSL may be partially attributed to the enhanced water solubility of ND upon complexation with p-SC4, thereby improving its bioavailability.37,60,61 Selective release of ND from p-SC4 in a controlled manner in a typical acidic extracellular fluid of cancer cells is consistent with previous reports.62 The observed cytotoxic activity of p-SC4-ND-TSL at a concentration of 12.5 μg/mL was nearly the same as that of the free drug at a concentration of 36.5 μg/mL, suggesting that the designed novel hybrid system can be used to reduce the therapeutic dose of ND and its systemic adverse effects. Notably, incorporating ND into the p-SC4 host molecule or thermosensitive liposomes loaded with these complexes remarkably enhanced their anticancer activity. However, loading the p-SC4-ND complexes inside liposomes led to an additional increase in the anticancer effect compared with free ND.
P-TSL
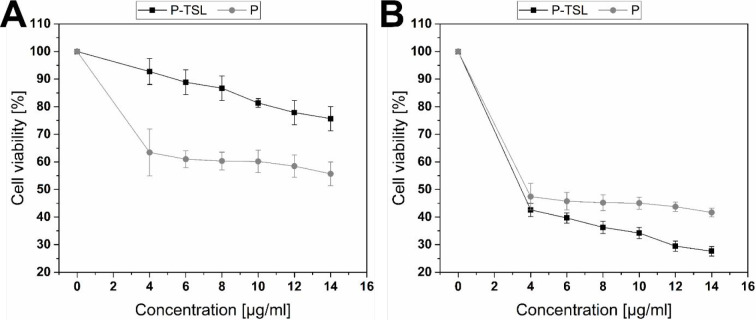
The antineoplastic activity of picoplatin compared to P-TSL was studied individually against MDA-MB-231 cells using MTT assay at 37 and 40 °C. Figure 7 shows the effect of the triggering temperature on enhancing the anticancer activity of the P-TSL formulation. Figure 7 (panel A) shows that at 37 °C the cytotoxicity of free picoplatin was higher than P-TSL at all tested concentrations (4–14 μg/mL). This is most likely because TSLs do not release their content at this temperature. However, it was shown that the cytotoxicity of P-TSL at 40 °C was approximately twice that at 37 °C. This is possibly because TSL releases its content at 40 °C, a temperature above the Tm of the lipids, as shown in Figure 7 (panel B). The cytotoxicity of the thermosensitive liposomal formulation at 4 μg/mL, which was the lowest concentration tested, was nearly twice that of the equivalent concentration of the free drug. The cell viabilities decreased as the concentrations of either the TSL formulation or free drug increased, reaching 27.6 and 41.7%, respectively, at a maximum concentration of 14 μg/mL.
Figure 7.
Assessing cytotoxicity of P-TSL and free P at various concentrations (4 to 14 μg/mL) utilizing MTT assay and MDA-MB-231 cells at (A) 37 °C and (B) 40 °C for 24 h. An overall statistically significant decrease in cell viability was observed with P-TSL compared to P. Untreated cells were used as the negative control and considered as 100%. All experiments were conducted in triplicate, and the mean values were calculated. Error bars represent ± standard deviation.
PBDs, including nedaplatin and picoplatin, exert their anticancer activity by first being bioactivated intracellularly through the aquation step forming highly activated cationic platinum species.63,64 These activated species exert their cytotoxic actions by binding the cancerous cells’ DNA forming thermodynamically stable platinum-DNA adducts that collapse the DNA structure via unwinding, bending, and destabilizing the DNA double helix. On the other hand, these cationic species are highly reactive toward healthy cells and blood components, leading to several undesired toxic side effects.63,64 Thus, in this work, we engineered the first reported triggerable thermosensitive liposomal nanoplatforms that can release PBDs at the target tumor cells in response to local mild hyperthermia.
As compared to individual chemotherapy, several studies reported synergistic anticancer effects when using local hyperthermia in combination with chemotherapeutics.65,66 The combination of local hyperthermia with TSL loaded with PBD is reported to enhance the anticancer activity via accumulation into the cancer cells through the enhanced permeability and retention effect. In addition, TSL increases the intravascular selective drug release at the tumor site upon exposure to a temperature above the Tm of the lipids.66 Furthermore, local hyperthermia has direct cytotoxic effects on tumor cells via stimulating apoptotic pathways and interfering with DNA repair mechanisms. Additionally, it was found that the cancer cells are more vulnerable to the cytotoxic effects of local hyperthermia than healthy cells because they are more stressed by the acidic environment, hypoxia, and lack of nutrients and hence will be more susceptible to the additional hyperthermia stress.66
Conclusions
In the present study, we engineered and characterized novel thermosensitive liposomes loaded with two members of the newer generation of PBDs . All systems produced here, showed good physicochemical properties and entrapment efficiency. The in vitro temperature-triggered drug release investigations of the prepared TSL have shown a significant increase in their release of their cargo upon reaching a temperature of 40 °C. Additionally, anticancer evaluation of TSL encapsulating ND, p-SC4-ND, or P demonstrated significantly higher cytotoxicity than the corresponding free drugs. This may reduce the off-target toxicity of platinum-based drugs under normal physiological conditions. Thus, we developed PBD delivery systems that selectively reach cancer cells, the fabricated thermosensitive liposomal nanoplatforms can protect the loaded PBDs from nonintended side reactions, which cause their deactivation while simultaneously increasing their targeting to the site of action. Thus, thermosensensitive liposomes loaded with PBDs and combined with local hyperthermia represent a promising approach for treating breast cancer.
Acknowledgments
The authors would like to thank the American University in Cairo and Philipps University in Marburg (Department of Pharmaceutics and Biopharmaceutics) for funding sponsorship and providing resources for the project.
Glossary
Abbreviations
- PBDs
platinum-based drugs
- ND
nedaplatin
- p-SC4
p-sulfonatocalix[4]arene
- TSL
thermosensitive liposomes
- p-SC4-ND
p-sulfonatocalix[4]arene-nedaplatin
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DSPC
1,2-distearoyl-sn-glycero-3-phosphocholine
- DSPE-PEG-2000
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (ammonium salt)
- p-SC4-ND-TSL
p-sulfonatocalix[4]arine-nedaplatin TSL
- Tm
melting phase transition temperature
- Chol
cholesterol
- ND-TSL
nedaplatin TSL
- p-SC4-ND-TSL
p-sulfonatocalix[4]arine-nedaplatin TSL
- P-TSL
picoplatin TSL
- PDI
polydispersity index
- SEC
size exclusion chromatography
- AFM
atomic force microscopy
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04525.
Average cell viability of MDA-MB-231 cells at 37 and 40 °C after treatment with void thermosensitive liposomes and p-SC4 for all runs for 24 h (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Galanski M.; Jakupec M. A.; Keppler B. K. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–94. 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]
- Rosenberg B.; VanCamp L.; Trosko J. E.; Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969, 222, 385–6. 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Fawzy I. M.; Sicilia E.; Bakowsky U.; Azzazy H.M.E.-S. Host-Guest Complexation of Oxaliplatin and Para-Sulfonatocalix[n]Arenes for Potential Use in Cancer Therapy. Molecules 2020, 25, 5926. 10.3390/molecules25245926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Sicilia E.; Azzazy H.M.E.-S. Experimental and Computational Investigations of Carboplatin Supramolecular Complexes. ACS Omega 2020, 5, 31456–31466. 10.1021/acsomega.0c05168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritacco I.; Al Assy M.; Abd El-Rahman M. K.; Fahmy S. A.; Russo N.; Shoeib T.; Sicilia E. Hydrolysis in Acidic Environment and Degradation of Satraplatin: A Joint Experimental and Theoretical Investigation. Inorg. Chem. 2017, 56, 6013–6026. 10.1021/acs.inorgchem.7b00945. [DOI] [PubMed] [Google Scholar]
- El-Shafie S.; Fahmy S. A.; Ziko L.; Elzahed N.; Shoeib T.; Kakarougkas A. Encapsulation of Nedaplatin in Novel PEGylated Liposomes Increases Its Cytotoxicity and Genotoxicity against A549 and U2OS Human Cancer Cells. Pharmaceutics 2020, 12, 863. 10.3390/pharmaceutics12090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberoi H. S.; Nukolova N. V.; Kabanov A. V.; Bronich T. K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv Rev. 2013, 65, 1667–85. 10.1016/j.addr.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.; Xiang J.; Wang G.; Xu H.; Piao Y.; Liu X.; Tang J.; Shen Y.; Zhou Z. Linear-Dendritic Polymer-Platinum Complexes Forming Well-Defined Nanocapsules for Acid-Responsive Drug Delivery. ACS Appl. Mater. Interfaces 2021, 13 (37), 44028–44040. 10.1021/acsami.1c12156. [DOI] [PubMed] [Google Scholar]
- Galanski Ma.; Keppler B. K.. Tumor-Targeting Strategies with Anticancer Platinum Complexes. In Drug Delivery in Oncology From Basic Research to Cancer Therapy; Kratz F., Senter P., Steinhagen H., Eds.; Wiley-VCH Verlag, 2012; pp 1605–1629. [Google Scholar]
- Fahmy S. A.; Brüßler J.; Alawak M.; El-Sayed M. M. H.; Bakowsky U.; Shoeib T. Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy. Pharmaceutics. 2019, 11, 292. 10.3390/pharmaceutics11060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Fawzy I. M.; Sicilia E.; Bakowsky U.; Azzazy H.M.E.-S. Betaine host-guest complexation with a calixarene receptor: Enhanced in vitro anticancer effect. RSC Adv. 2021, 11, 24673–24680. 10.1039/D1RA04614D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Grande G.; Fawzy I. M.; Mandour A. A.; Sicilia E.; Azzazy H.M.E.-S. Synthesis, Characterization and Host-Guest Complexation of Asplatin: Improved In Vitro Cytotoxicity and Biocompatibility as Compared to Cisplatin. Pharmaceuticals 2022, 15, 259. 10.3390/ph15020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass C. R.; Walker T. L.; Burton M. A.; Decruz E. E. Enhanced anticancer therapy mediated by specialized liposomes. J. Pharm. Pharmacol. 2011, 49, 972–975. 10.1111/j.2042-7158.1997.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Schroeder A.; Honen R.; Turjeman K.; Gabizon A.; Kost J.; Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J. Controlled Release 2009, 137, 63–68. 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Karanth H.; Murthy R. S. pH-sensitive liposomes: principle and application in cancer therapy. J. Pharm. Pharmacol. 2010, 59, 469–483. 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- Dou Y.; Hynynen K.; Allen C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. Journal of controlled release. 2017, 249, 63–73. 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Wust P.; Hildebrandt B.; Sreenivasa G.; Rau B.; Gellermann J.; Riess H.; Felix R.; Schlag P. M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. 10.1016/S1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- Fahmy S. A.; Azzazy H.M.E.-S.; Schaefer J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13, 1345. 10.3390/pharmaceutics13091345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor A. A.; Lindner L. H.; Landon C. D.; Park J. Y.; Simnick A. J.; Dreher M. R.; Das S.; Hanna G.; Park W.; Chilkoti A.; Koning G. A.; ten Hagen T. L.; Needham D.; Dewhirst M. W. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T.; Porter T. M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Controlled Release 2013, 169, 112–125. 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokerse W. J. M.; Kneepkens E. C. M.; ten Hagen T. L. M.; Eggermont A. M. M.; Grüll H.; Koning G. A. In depth study on thermosensitive liposomes: optimizing systems for tumor specific therapy and in vitro to in vivo relations. Biomaterials 2016, 82, 138–150. 10.1016/j.biomaterials.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Grüll H.; Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Controlled Release 2012, 161, 317–327. 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Al-Ahmady Z.; Kostarelos K. Chemical components for the design of temperature responsive vesicles as cancer therapeutics. Chem. Rev. 2016, 116, 3883–3918. 10.1021/acs.chemrev.5b00578. [DOI] [PubMed] [Google Scholar]
- Li L.; ten Hagen T. L. M.; Schipper D.; Wijnberg T. M.; van Rhoon G. C.; Eggermont A. M. M.; Lindner L. H.; Koning G. A. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Controlled Release 2010, 143, 274–279. 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Li L.; ten Hagen T. L. M.; Bolkestein M.; Gasselhuber A.; Yatvin J.; Van Rhoon G. C.; Eggermont A. M. M.; Haemmerich D.; Koning G. A. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Controlled Release 2013, 167, 130–137. 10.1016/j.jconrel.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Dicheva B. M.; Ten Hagen T. L. M.; Schipper D.; Seynhaeve A. L. B.; Van Rhoon G. C.; Eggermont A. M. M.; Koning G. A. Targeted and heat-triggered doxorubicin delivery to tumors by dual targeted cationic thermosensitive liposomes. J. Controlled Release 2014, 195, 37–48. 10.1016/j.jconrel.2014.07.058. [DOI] [PubMed] [Google Scholar]
- Ward M. A.; Georgiou T. K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. 10.3390/polym3031215. [DOI] [Google Scholar]
- Kono K. Thermosensitive polymer-modified liposomes. Adv. Drug Delivery Rev. 2001, 53, 307–319. 10.1016/S0169-409X(01)00204-6. [DOI] [PubMed] [Google Scholar]
- Matanovic M. R.; Kristl J.; Grabnar P. A. Thermoresponsive polymers: insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275. 10.1016/j.ijpharm.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Abu Dayyih A.; Alawak M.; Ayoub A. M.; Amin M. U.; Abu Dayyih W.; Engelhardt K.; Duse L.; Preis E.; Brußler J.; Bakowsky U. Thermosensitive liposomes encapsulating hypericin: Characterization and photodynamic efficiency. Int. J. Pharm. 2021, 609, 121195. 10.1016/j.ijpharm.2021.121195. [DOI] [PubMed] [Google Scholar]
- van der Geest T.; Laverman P.; Metselaar J. M.; Storm G.; Boerman O. C. Radionuclide imaging of liposomal drug delivery. Expert Opinion on Drug Delivery 2016, 13 (9), 1231–1242. 10.1080/17425247.2016.1205584. [DOI] [PubMed] [Google Scholar]
- Phillips W. T.; Bao A.; Brenner A. J.; Goins B. A. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv. Drug Delivery Rev. 2014, 76, 39–59. 10.1016/j.addr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern A.; Apostolidis C.; Kratochwil C.; et al. An overview of targeted alpha therapy with 225Actinium and 213Bismuth. Curr. Radiopharm 2018, 11, 200–208. 10.2174/1874471011666180502104524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen C.; Glatting G.; Koop B.; et al. Breaking chemoresistance and radioresistance with [213 Bi]anti-CD45 antibodies in leukemia cells. Cancer Res. 2007, 67, 1950–1958. 10.1158/0008-5472.CAN-06-3569. [DOI] [PubMed] [Google Scholar]
- Seidl Ch. Targets for Therapy of Bladder Cancer. Seminars in Nuclear Medicine 2020, 50 (2), 162–170. 10.1053/j.semnuclmed.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Kgatle M. M.; Boshomane T. M. G.; Lawal I. O.; Mokoala K. M. G.; Mokgoro N. P.; Lourens N.; Kairemo K.; Zeevaart J. R.; Vorster M.; Sathekge M. M. Immune Checkpoints, Inhibitors and Radionuclides in Prostate Cancer: Promising Combinatorial Therapy Approach. Int. J. Mol. Sci. 2021, 22, 4109. 10.3390/ijms22084109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Abd El-Rahman M. K.; Russo N.; Sicilia E.; Shoeib T. Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug delivery. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 193, 528–536. 10.1016/j.saa.2017.12.070. [DOI] [PubMed] [Google Scholar]
- Yatvin M. B.; Weinstein J. N.; Dennis W. H.; Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978, 202, 1290–1293. 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- Sadeghi N.; Deckers R.; Ozbakir B.; Akthar S.; Kok R. J.; Lammers T.; Storm G. Influence of cholesterol inclusion on the doxorubicin release characteristics of lysolipid-based thermosensitive liposomes. Int. J. Pharm. 2018, 548, 778–782. 10.1016/j.ijpharm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Drakalska E.; Momekova D.; Manolova Y.; Budurova D.; Momekovd G.; Genova M.; Antonov L.; Lambov N.; Rangelov S. Hybrid liposomal PEGylated calix[4]arene systems as drug delivery platforms for curcumin. Int. J. Pharm. 2014, 472, 165–174. 10.1016/j.ijpharm.2014.06.034. [DOI] [PubMed] [Google Scholar]
- Hossann M.; Wang T.; Wiggenhorn M.; Schmidt R.; Zengerle A.; Winter G.; Eibl H.; Peller M.; Reiser M.; Issels R. D.; Lindner L. H. Size of thermosensitive liposomes influences content release. J. Controlled Release 2010, 147, 436–443. 10.1016/j.jconrel.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Levacheva I.; Samsonova O.; Tazina E.; Beck-Broichsitter M.; Levachev S.; Levachev B.; Baryshnikova M.; Oborotova N.; Oborotova A.; Bakowsky U. Optimized thermosensitive liposomes for selective doxorubicin delivery: Formulation development, quality analysis and bioactivity proof. Colloids and Surfaces B: Biointerfaces. 2014, 121, 248–256. 10.1016/j.colsurfb.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Nallamothu R.; Wood G. C.; Kiani M. F.; Moore B. M.; Horton F. P.; Thoma L. A Targeted Liposome Delivery system for Combretastatin A4: Formulation Optimization Through Drug Loading and In Vitro Release Studies. PDA J. Pharm. Sci. Technol. 2006, 60, 144–55. [PubMed] [Google Scholar]
- Zeng C.; Yu F.; Yang Y.; Chemg X.; Liu Y.; Zhang H.; Zhao S.; Yang Z.; Li M.; Li Z.; Mei X. Preparation and evaluation of oxaliplatin thermosensitive liposomes with rapid release and high stability. PLoS One 2016, 11 (7), e0158517 10.1371/journal.pone.0158517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Sabbagh C.; Tsapis N.; Novell A.; Calleja-Gonzalez P.; Escoffre J.; Bouakaz A.; Chacun H.; Denis S.; Vergnaud J.; Gueutin C.; Fattal E. Formulation and pharmacokinetics of thermosensitive stealth® liposomes encapsulating 5-fluorouracil. Pharm. Res. 2015, 32, 1585–1603. 10.1007/s11095-014-1559-0. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhang H.; Yang Y.; Xie X.; Yang Y.; Li Z.; Li Y.; Gong W.; Yu F.; Yang Z.; Li M.; Mei X. Preparation, characterization, and efficacy of thermosensitive liposomes containing paclitaxel. Drug Delivery. 2016, 23 (4), 1222–1231. 10.3109/10717544.2015.1122674. [DOI] [PubMed] [Google Scholar]
- Kunwar A.; Barik A.; Pandey R.; Priyadarsini K. I. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim. Biophys. Acta 2006, 1760, 1513–1520. 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Pinnapireddy S. R.; Duse L.; Strehlow B.; Schäfer J.; Bakowsky U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids and Surfaces B: Biointerfaces. 2017, 158, 93–101. 10.1016/j.colsurfb.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Fritze A.; Hens F.; Kimpfler A.; Schubert R.; Peschka-Suss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim. Biophys. Acta 2006, 1758, 1633–1640. 10.1016/j.bbamem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Duse L.; Pinnapireddy S. R.; Strehlow B.; Jedelská J.; Bakowsky U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. European Journal of Pharmaceutics and Biopharmaceutics. 2018, 126, 233–241. 10.1016/j.ejpb.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Sitterberg J.; Ozcetin A.; Ehrhardt C.; Bakowsky U. Utilising atomic force microscopy for the characterisation of nanoscale drug delivery systems. Eur. J. Pharm. Biopharm. 2010, 74, 2. 10.1016/j.ejpb.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Rahman S.; Cao S.; Steadman K.; Wei M.; Parekh H. S. Native and β-cyclodextrin-enclosed curcumin: entrapment within liposomes and their in vitro cytotoxicity in lung and colon cancer. Drug Delivery. 2012, 19 (7), 346–353. 10.3109/10717544.2012.721143. [DOI] [PubMed] [Google Scholar]
- Maestrelli F.; González-Rodríguez M. L.; Rabasco A. M.; Mura P. Preparation and characterisation of liposomes encapsulating ketoprofen-cyclodextrin complexes for transdermal drug delivery. International journal of pharmaceutics. 2005, 298 (1), 55–67. 10.1016/j.ijpharm.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Howell B. A.; Chauhan A. Interaction of cationic drugs with liposomes. Langmuir. 2009, 25 (20), 12056–65. 10.1021/la901644h. [DOI] [PubMed] [Google Scholar]
- Thanki K.; Gangwal R. P.; Sangamwar A. T.; Jain S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Controlled Release 2013, 170, 15–40. 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Alawak M.; Abu Dayyih A.; Mahmoud G.; Tariq I.; Duse L.; Goergen N.; Engelhardt K.; Reddy Pinnapireddy S.; Jedelska J.; Awak M.; Konig A. M.; Brußler J.; Bartsch J. W.; Bakowsky U. ADAM 8 as a novel target for doxorubicin delivery to TNBC cells using magnetic thermosensitive liposomes. European Journal of Pharmaceutics and Biopharmaceutics. 2021, 158, 390–400. 10.1016/j.ejpb.2020.12.012. [DOI] [PubMed] [Google Scholar]
- de Smet M.; Langereis S.; den Bosch S. v.; Grull H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. J. Controlled Release 2010, 143 (1), 120–127. 10.1016/j.jconrel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Alawak M.; Mahmoud G.; Dayyih A. A.; Duse L.; Pinnapireddy S. R.; Engelhardt K.; Awak I.; Wölk C.; König A. M.; Brüßler J.; Bakowsky U. Magnetic resonance activatable thermosensitive liposomes for controlled doxorubicin delivery. Materials Science and Engineering: C 2020, 115, 111116. 10.1016/j.msec.2020.111116. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv Rev. 2011, 63, 161–169. 10.1016/j.addr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Wang G.; Zhang H.; Ding F.; Liu Y. J. Preparation and characterization of inclusion complexes of topotecan with sulfonatocalixarene. Incl. Phenom. Macrocycl. Chem. 2011, 69, 85–89. 10.1007/s10847-010-9817-1. [DOI] [Google Scholar]
- Fahmy S. A.; Brüßler J.; Ponte F.; Abd El-Rahman M. K.; Russo N.; Sicilia E.; Bakowsky U.; Shoeib T. A study on the physicochemical properties and cytotoxic activity of p-sulfocalix[4]arene-nedaplatin complex. J. Phys.: Conf. Ser. 2019, 1310, 012011. 10.1088/1742-6596/1310/1/012011. [DOI] [Google Scholar]
- Abd Hamid S.; M Bunnori N.; Adekunle I. A.; Ali Y. Applications of calixarenes in cancer chemotherapy: Facts and perspectives. Drug Des. Devel. Ther. 2015, 9, 2831–2838. 10.2147/DDDT.S83213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zutphen S.; Reedijk J. Targeting platinum anti-tumour drugs: overview of strategies employed to reduce systemic toxicity. Coord. Chem. Rev. 2005, 249, 2845–2853. 10.1016/j.ccr.2005.03.005. [DOI] [Google Scholar]
- Oberoi H. S.; Nukolova N. V.; Kabanov A. V.; Bronich T. K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv Rev. 2013, 65, 1667–85. 10.1016/j.addr.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust P.; Hildebrandt B.; Sreenivasa G.; Rau B.; Gellermann J.; Riess H.; Felix R.; Schlag P. M. Hyperthermia in combined treatment of cancer. lancet oncology. 2002, 3 (8), 487–497. 10.1016/S1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- Ta T.; Porter T. M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. Journal of controlled release. 2013, 169 (1–2), 112–125. 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.