Abstract
This study describes the construction and analysis of three in vivo-inducible promoter expression plasmids, containing pnirB, ppagC, and pkatG, for the delivery of foreign antigens in the ΔaroAD mutant of Salmonella enterica var. Typhimurium (hereafter referred to as S. typhimurium). The reporter genes encoding β-galactosidase and firefly luciferase were used to assess the comparative levels of promoter activity in S. typhimurium in vitro in response to different induction stimuli and in vivo in immunized mice. It was determined that the ppagC construct directed the expression of more β-galactosidase and luciferase in S. typhimurium than the pnirB and pkatG constructs, both in vitro and in vivo. The gene encoding the C fragment of tetanus toxin was expressed in the aroAD mutant of S. typhimurium (BRD509) under the control of the three promoters. Mice orally immunized with attenuated S. typhimurium expressing C fragment under control of the pagC promoter [BRD509(pKK/ppagC/C frag)] mounted the highest tetanus toxoid-specific serum antibody response. Levels of luciferase expression in vivo and C-fragment expression in vitro from the pagC promoter appeared to be equivalent to if not lower than the levels of expression detected with the constitutive trc promoter. However, mice immunized with BRD509(pKK/ppagC/C frag) induced significantly higher levels of tetanus toxoid-specific antibody than BRD509(pKK/C frag)-immunized mice, suggesting that the specific location of foreign antigen expression may be important for immunogenicity. Mutagenesis of the ribosome binding sites (RBS) in the three promoter/C fragment expression plasmids was also performed. Despite optimization of the RBS in the three different promoter elements, the expression levels in vivo and overall immunogenicity of C fragment when delivered to mice by attenuated S. typhimurium were not affected. These studies suggest that in vivo-inducible promoters may give rise to enhanced immunogenicity and increase the efficacy of S. typhimurium as a vaccine vector.
The relative success of various attenuated Salmonella enterica var. Typhimurium (hereafter referred to as S. typhimurium) and Salmonella enterica var. Typhi strains to act safely and efficaciously as anti-salmonella vaccines has encouraged the development of these strains as vaccine vectors. At present, Salmonella vaccine vectors are experimental and the vast majority of vector studies have been conducted in the murine typhoid (i.e., S. typhimurium) model. To date, numerous different genes from bacteria, viruses, parasites, and other organisms of eucaryotic origin have been expressed and delivered in attenuated Salmonella to various levels of success (for reviews, see references 17 and 30).
A number of different strategies have been developed to achieve stable foreign antigen expression in S. typhimurium vaccine vectors. One strategy, the asd+ vector/Δasd host lethal system developed by Nakayama et al. (24), relies on stabilizing the expression plasmid, whereas another approach avoids the use of plasmids by integrating the foreign gene onto the Salmonella chromosome (16, 34). One of the most effective strategies to overcome the problem of plasmid instability is the use of in vivo-inducible promoters (6, 13, 21, 31). The rationale behind the use of these promoters is that the level of foreign antigen expression will be low until the vector bacterium receives an environmental stimulus in vivo, which then results in enhanced foreign antigen expression. The regulation of foreign antigen expression should also minimize loss of the expression plasmid during vaccine production. Chatfield et al. (6) constructed a novel in vivo-regulated expression system based on the nirB promoter from Escherichia coli. The nirB promoter from E. coli represents one of the best characterized in vivo-regulated promoters in attenuated S. typhimurium. A single oral dose of an S. typhimurium ΔaroAD mutant expressing C fragment from the in vivo-inducible nirB promoter protected mice against lethal tetanus toxin challenge (6).
In this study, three promoters from S. typhimurium and E. coli were evaluated with respect to the ability to stabilize foreign gene expression and potentially enhance the immunogenicity and efficacy of an S. typhimurium vaccine. The three environmentally regulated promoters, pnirB, ppagC, and pkatG, were selected on the basis of their potential ability to be induced in environments such as those encountered by an attenuated S. typhimurium inside the vaccinated host. The in vivo-inducible promoter expression system based on the pagC promoter was shown to provide stable, high-level expression of a heterologous antigen which, when delivered by attenuated S. typhimurium, was highly immunogenic.
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. typhimurium BRD509 is an aroA aroD mutant of SL1344 and was a gift from G. Dougan, Imperial College, London, United Kingdom. All DNA manipulations were carried out with the E. coli laboratory strain JM101 (38). The expression plasmids were transferred into the r− m+ strain S. typhimurium LB5010 (4) by electroporation and were then transduced into BRD509 by using bacteriophage P22 (Int−) (32). Bacterial strains were routinely cultured in Luria-Bertani (LB) broth or LB agar overnight at 37°C without antibiotic or with 75 μg of ampicillin per ml.
DNA manipulations.
Restriction endonuclease digestions, ligations, alkaline phosphatase treatment, agarose gel electrophoresis, and transformations (32) were performed, and miniprep plasmid DNA was prepared (14), by using standard techniques. All enzymes were used as specified by the manufacturer (Promega, Madison, Wis.). DNA fragments to be ligated were purified by Geneclean (Bio 101 Inc., Vista, Calif.) or phenol-chloroform extraction and ethanol precipitation (32).
Construction of reporter genes and promoter expression plasmids.
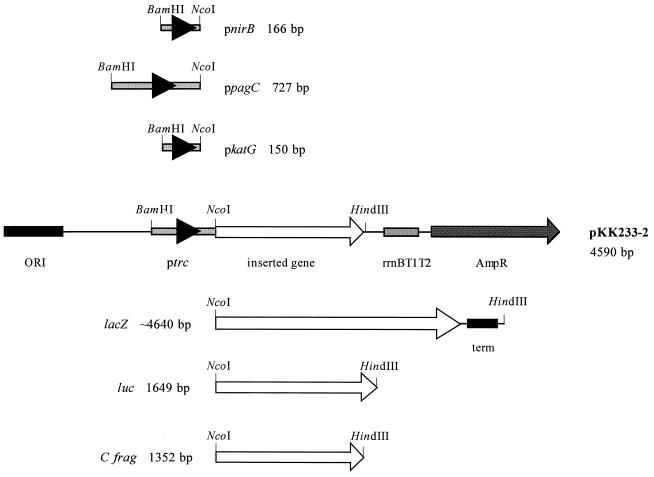
Oligonucleotide primers were used to PCR amplify the region upstream of the nirB gene from E. coli and the region upstream of the pagC and katG genes from S. typhimurium (Table 1). The genes encoding β-galactosidase, firefly luciferase, and C fragment were PCR amplified from the E. coli chromosome, pGL2 (Promega), and pTETtac4 (8), respectively. The oligonucleotide primers used to PCR amplify lacZ do not amplify the complete lacZ gene. The complete lacZ gene was generated by cloning the truncated lacZ, which was PCR amplified from the E. coli chromosome to the remaining portion of the lacZ gene derived from plasmid pMU2386 (gift from J. Pittard, The University of Melbourne, Parkville, Victoria, Australia). All oligonucleotides contained the recognition sequences of specific restriction enzymes to facilitate cloning of the PCR products. The PCR was performed with a GeneAmp PCR system 9600 as specified by the manufacturer (Perkin-Elmer Corp., Norwalk, Conn.). The PCR products were initially ligated to pBluescript DNA, and the nirB, pagC, and katG promoter regions were sequenced by using a PRISM dye terminator cycle sequencing ready reaction kit and an Applied Biosystems 373A DNA sequencer (Applied Biosystems, Scoresby, Victoria, Australia). The DNA containing the promoter regions and the reporter genes was excised from the pBluescript clones and ligated to the expression vector pKK233-2 (Fig. 1). This strategy enabled the construction of 12 expression plasmids (based on plasmid pKK233-2), with the reporter genes encoding β-galactosidase, firefly luciferase, and C fragment cloned downstream of the four promoter regions, pnirB, ppagC, pkatG, and ptrc (Fig. 1).
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotidea | Nucleotide sequenceb | Description |
|---|---|---|
| 450 | 5′-CGTTGGATCCAGCTGTCCGCAGGCG-3′ | nirB upstream region, 5′ PCR primer |
| 451 | 5′-CTGACTGCAGCCATGGTTGCCTCGATTTC-3′ | nirB upstream region, 3′ PCR primer |
| 663 | 5′-CTCTGGATCCTAATGGGTTTTATAGCG-3′ | pagC upstream region, 5′ PCR primer |
| 664 | 5′-AAAGAATTCTTTCCATGGCAACTCCTTAAT-3′ | pagC upstream region, 3′ PCR primer |
| MU1 | 5′-CCGGGATCCAGCTGTTACAACTCATATTG-3′ | katG upstream region, 5′ PCR primer |
| MU2 | 5′-ATCGTCTGCAGTGCCCATGGCTCAGCTCCCTTTTA-3′ | katG upstream region, 3′ PCR primer |
| MU5 | 5′-ACGGATCCTACCATGGAAGACGCCAAAAAC-3′ | Firefly luciferase, 5′ PCR primer |
| MU6 | 5′-CGTCATCGCTGGATCCAGTAAGCTTTTACAATTTGGACTTTCC-3′ | Firefly luciferase, 3′ PCR primer |
| MU7 | 5′-CGGGATCCCATGGAAAATCTGGATTGTTGGG-3′ | C fragment, 5′ PCR primer |
| 2947 | 5′-AAACTGCAGTTAGTCGTTGGTCCAACCTTC-3′ | C fragment, 3′ PCR primer |
| 3009 | 5′-CACGCTCATCGATAATTTCACC-3′ | Truncated β-galactosidase, 3′ PCR primer |
| 3013 | 5′-CCGCCGCTCGAGGCCCATGGCCATGATTACGGATTCACTG-3′ | Truncated β-galactosidase, 5′ PCR primer |
MU series oligonucleotides were synthesized in the Department of Microbiology and Immunology, The University of Melbourne, Parkville, Victoria Australia; all other oligonucleotides were synthesized and purchased from the Microbial Biotechnology and Diagnostic Unit, Monash University, Clayton, Victoria Australia.
Restriction enzyme sites are underlined.
FIG. 1.
Expression plasmids constructed. The promoter regions of nirB, pagC, and katG and the genes encoding β-galactosidase, firefly luciferase, and C fragment were obtained by PCR and ligated to pKK233-2. The four different promoter cassettes combined with the three genes generated 12 different expression plasmids.
Detection of protein expression by using reporter genes.
β-Galactosidase assays (22) were performed on noninduced and induced cultures. To induce the nirB promoter, log-phase bacteria were incubated without aeration at 37°C for 4 h (some cultures were flushed with nitrogen and sealed prior to static incubation). To induce the pagC promoter, log-phase bacteria were washed once in N medium (10), then resuspended in N medium plus supplements (0.1% Casamino Acids, 38 mM glycerol, aromatic supplements [15], and 0.001 to 100 mM MgCl2), and grown at 37°C for 2.5 h. To induce the katG promoter, log-phase bacteria were resuspended in LB broth containing H2O2 (1 to 100 mM) and grown at 37°C for 2.5 h. Luciferase assays were performed by using the Promega luciferase assay system according to the manufacturer’s specifications and were quantified with a Lumat LB9501 luminometer (Berthold Australia, Bundoora, Victoria, Australia). To prepare homogenized organ samples, the spleen, Peyer’s patches (PPs), and mesenteric lymph nodes (MLNs) were removed from S. typhimurium-immunized mice. Single-cell suspensions were prepared by passing organs through small sieves into 1 ml of phosphate-buffered saline (PBS); cells were collected by centrifugation (265 × g for 10 min) and resuspended in PBS (2 ml). Two hundred microliters of organ homogenate was used to assess the number of viable bacteria, and the remaining 1.8 ml was centrifuged (265 × g for 10 min), resuspended in 500 μl of lysis buffer (Promega) containing 1 mg of bovine serum albumin per ml, and sonicated (two bursts of 30 s each). Cell debris was pelleted (15,000 × g for 5 min), and the luciferase assay was performed on 100-μl aliquots of the supernatant. The relative light units per organ and viable count of bacteria per organ were calculated and expressed as relative light units per 105 bacteria.
Expression of C fragment by S. typhimurium.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of whole-cell proteins was performed with 12.5% acrylamide gels (19). Western blot analyses were performed (36) with rabbit tetatus toxoid antiserum (Calbiochem, San Diego, Calif.) and a sheep anti-rabbit–horseradish peroxidase conjugate (Silenus Laboratories, Hawthorn, Victoria, Australia). C-fragment-specific antibody was detected with 4-chloro-1-naphthol (Bio-Rad, Hercules, Calif.) and H2O2. Cultures were induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (ptrc) or 1 mM H2O2 (pkatG), by growth in LB broth containing MgCl2 (ppagC), or by growth in a nonaerated LB broth (pnirB).
Immunization of BALB/c mice.
Female 6- to 8-week-old BALB/c mice (The University of Melbourne Department of Microbiology and Immunology animal facility) were immunized orally via a 4-cm gastric lavage needle with approximately 1010 bacteria (200 μl/mouse) previously grown at 37°C for 24 h, without shaking. Thirty minutes prior to oral inoculation, mice were administered 100 μl of 10% sodium bicarbonate to neutralize stomach acidity.
S. typhimurium colonization of mice and plasmid stability in vivo.
Following removal, spleens, PPs, and MLNs were homogenized separately in sterile plastic bags (Starstedt, Ingle Farm, South Australia, Australia) containing 5 ml of PBS, using a Stomacher 80 (Seward Medical, London, United Kingdom) homogenizer. The total number of salmonellae and the number of plasmid-bearing S. typhimurium present in each organ was determined by viable count on LB agar plates and on LB agar plates containing an antibiotic for selection, respectively.
Measurement of serum antibody responses by ELISA.
The titers of antibody present in mouse sera were determined by a standard endpoint enzyme-linked immunosorbent assay (ELISA). Immunoplates (Nunc A/S, Kamstrup, Denmark) were coated with either 10 μg of S. typhimurium lipopolysaccharide (LPS; Sigma, St. Louis, Mo.) per ml in PBS or 2 Lf of tetanus toxoid (CSL Ltd., Parkville, Victoria, Australia) per ml in carbonate coating buffer (pH 9.6). Bound antibody was detected by adding sheep anti-mouse immunoglobulin–horseradish peroxidase conjugate (Silenus Laboratories). ELISAs were developed by using Immunopure o-phenylenediamine (Pierce, Rockford, Ill.) with H2O2, and absorbance was read at 492 nm (Titertek Multiskan MCC; Titertek Multiskan, Helsinki, Finland). The titer was designated as the reciprocal of the dilution of specific antibody that generated an optical density at 492 five times the value obtained for preimmune sera.
Statistical analysis.
Unrelated groups of data were compared by the unpaired Student t test. A P value of less than 0.05 indicated that the groups were significantly different.
RESULTS
PCR amplification of the promoters and reporter genes and construction of the promoter expression plasmids.
Twelve expression plasmids that contained the genes encoding β-galactosidase, firefly luciferase, and C fragment under pnirB, ppagC, pkatG, or ptrc control were constructed (Fig. 1).
Promoter-driven expression of the lacZ gene is affected by environmental stimuli.
The lacZ reporter gene was used to confirm that each promoter construct contained the sequence necessary to drive gene expression and to assess the promoters’ responsiveness to environmental signals likely to influence their activity. The nirB promoter was induced in vitro by reducing the amount of oxygen available to the cells. The aerated culture exhibited a low level of β-galactosidase activity which increased approximately eightfold when the culture was incubated statically (Fig. 2A). The addition of 2.5 mM sodium nitrite and 20 mM sodium nitrate to the culture medium further increased β-galactosidase activity of BRD509(pKK/pnirB/lacZ) (Fig. 2A). BRD509(pKK/ppagC/lacZ) cultures were supplemented with MgCl2 to mediate repression of the pagC promoter. MgCl2 concentration affected expression of the lacZ gene by the pagC promoter, with the maximum level of β-galactosidase being detected in the BRD509(pKK/ppagC/lacZ) cultures supplemented with <1 mM MgCl2 (Fig. 2B). As the concentration of MgCl2 increased, the level of β-galactosidase activity decreased, suggesting that high concentrations of MgCl2 were inhibitory for β-galactosidase expression. BRD509(pKK/pkatG/lacZ) cultures were induced in vitro by the addition of H2O2, with the optimal concentration of H2O2 for pkatG induction determined as 1 mM (Fig. 2C). β-Galactosidase activity was markedly less at concentrations as high as 10 to 100 mM, perhaps due to toxic effects of the H2O2 on the S. typhimurium cells.
FIG. 2.
Detection of β-galactosidase expression in vitro. (A)
β-Galactosidase activity of BRD509(pKK/pnirB/lacZ) was
determined after incubation of cultures at 37°C either shaken,
static, or static flushed with nitrogen and sealed. Nitrate (20 mM)
( ),
nitrite (2.5 mM) (░⃞), or no supplement (■) was added to the growth
media. (B) BRD509(pKK/ppagC/lacZ) cultures were induced for
2.5 h by the addition of 0.001 to 100 mM MgCl2. (C)
BRD509(pKK/pkatG/lacZ) cultures were induced for 2.5 h
by the addition of 0.001 to 100 mM H2O2.
β-Galactosidase activity is expressed in Miller units. The results
presented are representative of a number of separate experiments.
),
nitrite (2.5 mM) (░⃞), or no supplement (■) was added to the growth
media. (B) BRD509(pKK/ppagC/lacZ) cultures were induced for
2.5 h by the addition of 0.001 to 100 mM MgCl2. (C)
BRD509(pKK/pkatG/lacZ) cultures were induced for 2.5 h
by the addition of 0.001 to 100 mM H2O2.
β-Galactosidase activity is expressed in Miller units. The results
presented are representative of a number of separate experiments.
Induction of the promoter constructs in BRD509 was also investigated in activated mouse peritoneal macrophages. S. typhimurium strains harboring the expression plasmids were capable of expressing detectable amounts of β-galactosidase inside peritoneal macrophages. The pagC promoter was found to direct the highest level of β-galactosidase expression, approximately 10-fold more than the katG promoter and approximately 100-fold more than the nirB promoter (data not shown).
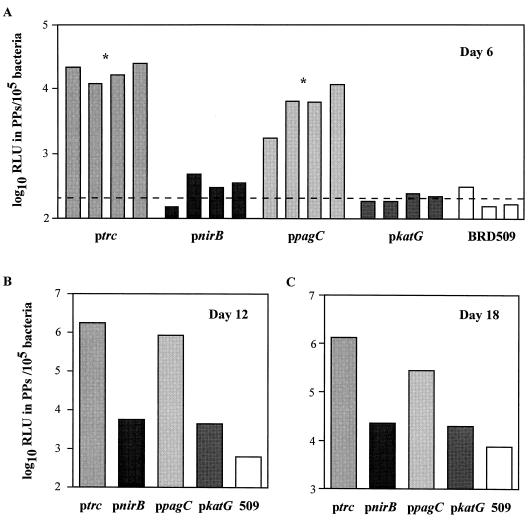
Detection of firefly luciferase in the PPs of immunized mice.
To quantitate reporter gene expression in vivo, mice were orally immunized with BRD509(pKK/pnirB/luc), BRD509(pKK/ppagC/luc), BRD509(pKK/pkatG/luc), and BRD509(pKK/luc). Six days after immunization, luciferase activity was detected in the PPs of mice immunized with BRD509(pKK/ppagC/luc) and BRD509(pKK/luc) (Fig. 3A). The level of luciferase detected was significantly greater than the background level of luciferase detected in the BRD509-immunized control mice (P < 0.05). The level of luciferase in the PPs of mice immunized with BRD509(pKK/pnirB/luc) and BRD509(pKK/pkatG/luc) was equivalent to the background level of luciferase detected in BRD509-immunized mice (P > 0.05) (Fig. 3A). On days 12 and 18 postimmunization, the highest amount of luciferase was detected in the PPs of BRD509(pKK/luc)- and BRD509(pKK/ppagC/luc)-immunized mice, whereas 10- to 100-fold less luciferase activity was detected in PPs of BRD509(pKK/pnirB/luc)- and BRD509(pKK/pkatG/luc)-immunized mice (Fig. 3B and C). Luciferase activity could not be detected in the organs of naive mice. Attempts to detect in vivo expression of luciferase in the MLNs and spleens of mice immunized with luciferase-expressing bacteria were unsuccessful. Despite the differences observed in luciferase expression in the PPs of mice, all constructs were capable of expressing luciferase in bacterial culture (data not shown).
FIG. 3.
Detection of luciferase in the PPs of mice. Groups of mice were immunized orally with BRD509(pKK/pnirB/luc) (pnirB), BRD509(pKK/ppagC/luc) (ppagC), BRD509(pKK/pkatG/luc) (pkatG), BRD509(pKK/luc) (ptrc), and BRD509 (509). On days 6, 12, and 18 postimmunization, mice were killed and their PPs were removed. The number of bacteria (calculated by viable count) and the amount of luciferase (by luciferase assay) were determined in each PP sample. The luciferase activity was represented as the number of relative light units (RLU) in PPs per 105 bacteria. Each bar represents the luciferase activity detected in the PPs of one mouse (A) or that detected in a pool of PPs from five mice (B and C). The dashed line on the day 6 graph denotes the mean of the luciferase activities detected in PPs of the control group, mice immunized with BRD509 alone. ∗, the level of luciferase activity is significantly different from that detected in the PPs of mice immunized with BRD509 alone (P < 0.05).
Expression of C fragment in S. typhimurium.
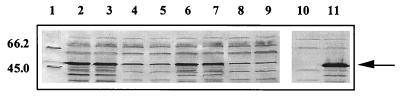
C-fragment expression was determined in S. typhimurium strains containing plasmids pKK/pnirB/C frag, pKK/ppagC/C frag, pKK/pkatG/C frag, and pKK/C frag by Western blotting. A protein of approximately 50 kDa corresponding to C fragment was detected in all S. typhimurium strains containing the promoter expression plasmids and was absent from the S. typhimurium strain alone (Fig. 4).
FIG. 4.
Expression of C fragment in S. typhimurium. Western blot analysis showing the expression of C fragment by the four promoter cassettes in S. typhimurium BRD509. C-fragment expression was detected by the use of polyclonal anti-tetanus toxoid antiserum and is indicated by the arrows. Lane 1, low-molecular-weight markers; lanes 2 and 3, BRD509(pKK/C frag); lanes 4 and 5, BRD509(pKK/pnirB/C frag); lane 6 and 7, BRD509(pKK/ppagC/C frag); lane 8 and 9, BRD509(pKK/pkatG/C frag); lane 10, BRD509 alone; lane 11, BRD509(pTETtac4) (positive control) (8). Sizes are indicated in kilodaltons.
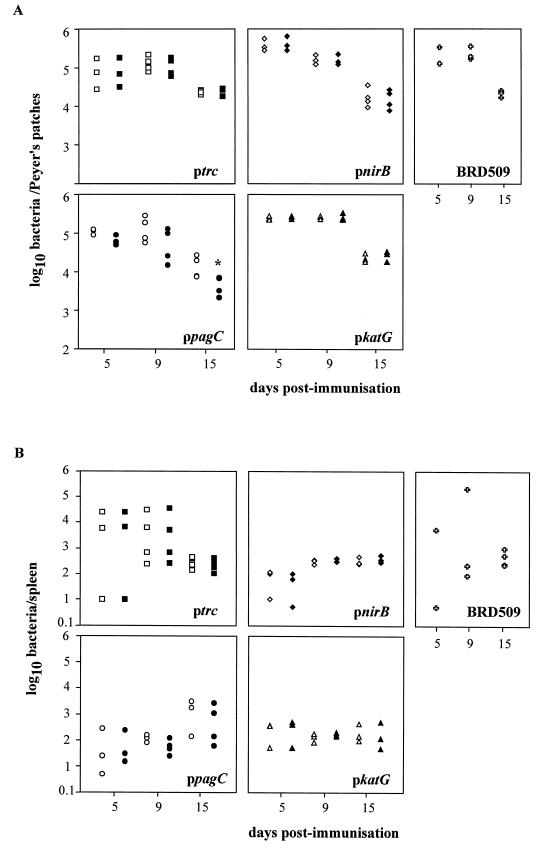
In vivo plasmid stability of promoter constructs expressing C fragment in immunized mice.
S. typhimurium strains harboring the four C-fragment expression constructs were capable of colonizing the spleen, PPs (Fig. 5), and MLNs (data not shown) of orally immunized mice and persisted until at least day 15. Over the first 15 days postinfection, the number of bacteria recovered from the PPs decreased, whereas the amount of bacteria detected in the spleen remained relatively constant, BRD509(pKK/C frag) being the exception. Throughout the 15-day time course experiment, all bacterial strains except BRD509(pKK/ppagC/C frag) could retain their plasmids in vivo at levels approaching 100%. Loss of the pKK/ppagC/C frag plasmid was observed in bacteria recovered from the PPs (Fig. 5A) and the MLNs (data not shown) but not the spleen on day 15.
FIG. 5.
In vivo plasmid stability and colonization of S. typhimurium. Groups of mice were orally immunized with BRD509(pKK/pnirB/C frag) (pnirB), BRD509(pKK/ppagC/C frag) (ppagC), BRD509(pKK/pkatG/C frag) (pkatG), BRD509(pKK/C frag) (ptrc), and BRD509. On days 5, 9, and 15 postimmunization, the numbers of bacteria present in the PPs (A) and spleen (B) were determined by isolating bacteria on LB agar (open shapes) or LB agar containing ampicillin (closed shapes). Each point represents the number of bacteria isolated from an individual mouse. ∗, the number of bacteria isolated on LB agar containing ampicillin was significantly lower than the number of bacteria isolated on the corresponding LB agar (P < 0.05).
Humoral immune response induced by immunization with S. typhimurium containing promoter constructs expressing C fragment.
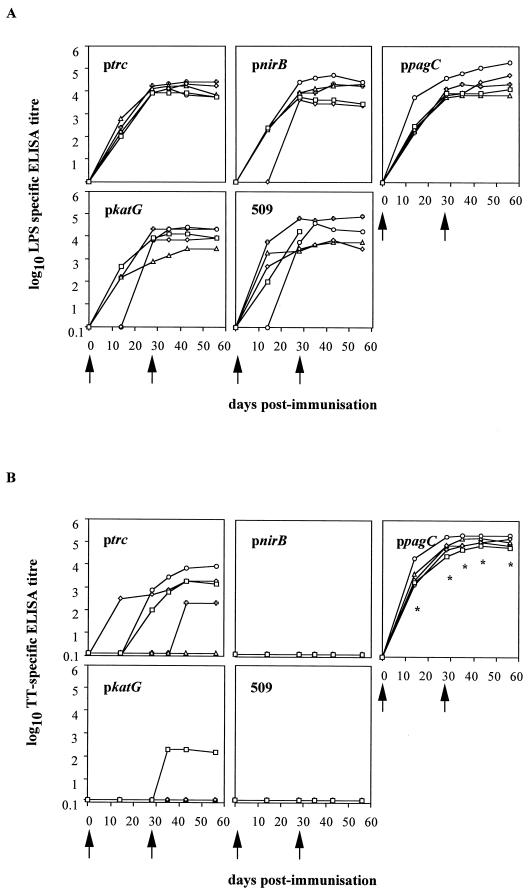
LPS-specific serum antibody was detected in all mice immunized with S. typhimurium BRD509 regardless of which expression plasmid they harbored (Fig. 6A). The LPS response peaked by day 28 and plateaued at that level until the last time point assayed, at day 56. From these results it appears that C-fragment expression in S. typhimurium BRD509 does not affect the strains’ ability to induce an antibody response to S. typhimurium LPS. S. typhimurium containing the pagC promoter expression construct was the only strain to induce a tetanus toxoid-specific antibody response in all five mice; four of five mice immunized with BRD509(pKK/C frag) had tetanus toxoid-specific antibody in their sera (Fig. 6B). The level of anti-tetanus toxoid antibody induced by BRD509(pKK/C frag) was significantly lower (P < 0.05) than that induced by BRD509(pKK/ppagC/C frag), with one mouse responding only after the day 28 boost and one mouse not responding at all. In the group of mice immunized with BRD509(pKK/pkatG/C frag), one animal of five produced a low level of antibody against tetanus toxoid after receiving the day 28 boost immunization. No mice in the groups immunized with either BRD509(pKK/pnirB/C frag) or BRD509 alone raised any anti-tetanus toxoid antibodies (Fig. 6B). When this experiment was repeated, the results reflected those seen in the first experiment.
FIG. 6.
LPS- and tetanus toxoid-specific serum antibody responses. Groups of mice were immunized orally with BRD509(pKK/pnirB/C frag) (pnirB), BRD509(pKK/ppagC/C frag) (ppagC), BRD509(pKK/pkatG/C frag) (pkatG), BRD509(pKK/C frag) (ptrc), and BRD509 (509). The amounts of S. typhimurium LPS-specific (A) and tetanus toxoid-specific (B) total immunoglobulin present in serum were determined. Each point represents a serum titer from an individual mouse. The arrows indicate the times of oral immunization. ∗, the anti-tetanus toxoid antibody titer detected in the sera of BRD509(pKK/ppagC/C frag)-immunized mice was significantly different (P < 0.05) from the antibody titer detected in BRD509(pKK/C frag)-immunized mice.
Modification of promoter constructs to alter the RBS by PCR.
To attribute the differences in protein expression and more importantly the outcome of immunization to promoter activity and not translation, variants of pnirB, ppagC, and pkatG expression plasmids containing identical optimal ribosome binding sites (RBS) were constructed by using PCR. The optimal RBS that was incorporated into the original promoter constructs was the complement of the 16S rRNA sequence from E. coli (33). The spacer region between the start codon and RBS was the optimal size of 8 bp (29) and was identical in all promoter constructs. C-fragment expression was detected in S. typhimurium harboring these expression constructs, and these strains were capable of colonizing the organs of immunized mice (data not shown). The addition of the optimal RBS in the expression constructs had no effect on the ability of the strains to induce antibody to the heterologous antigen following oral immunization of mice (data not shown).
DISCUSSION
A number of quite different approaches to stabilize expression of heterologous antigens in bacterial vaccine vectors have been investigated. One of these approaches involves the use of in vivo-inducible promoters. Here we describe the construction of three in vivo-inducible promoter cassettes and their comparative ability to promote immunogenic levels of foreign antigen expression. The three in vivo-inducible promoters were able to direct expression of β-galactosidase in vitro, in response to induction stimuli, and direct expression of luciferase in vivo, in the PPs of immunized mice. The gene encoding the heterologous antigen C fragment was cloned downstream of the four promoter expression cassettes and delivered to mice in S. typhimurium ΔaroAD mutants. Two strains, BRD509(pKK/ppagC/C fragment) and BRD509(pKK/C fragment), induced anti-tetanus toxoid antibody in the sera of immunized mice.
Chatfield et al. (6) first reported the application of an in vivo-inducible promoter in attenuated S. typhimurium. A single-dose oral tetanus vaccine was developed based on the expression of C fragment by the nirB promoter in plasmid pTETnir15. A range of antigens, and antigens fused to C fragment, have been expressed from the nirB promoter, and some of them have induced protective immunity (1, 2, 5, 9, 12, 18, 20).
The inability of the nirB promoter expression cassette generated in this study to direct expression of immunogenic levels of C fragment is inconsistent with previously reported findings. The nirB promoter cassette constructed in this study is identical to the native promoter region upstream of the nirB gene on the E. coli chromosome. In contrast, the nirB promoter present in the previously characterized pTETnir15 (6) was generated by synthetic oligonucleotides (26). Four main sequence differences exist between the nirB promoter described here and the nirB promoter in pTETnir15: (i) the FNR binding site, (ii) the distance and sequence between the RBS and the transcriptional start point, (iii) the RBS and spacer region between it and the ATG, and (iv) the putative NarL binding site (26, 27, 37). All of these changes could possibly explain the differences between the two nirB expression constructs. The FNR binding site in pTETnir15 is derived from F2 DR25X (3) and is homologous to neither the native pnirB FNR binding site nor the consensus FNR binding site (26). However, this should not account for the observed differences in levels of C-fragment expression, as Bell et al. (3) demonstrated that the FNR binding site F2 DR25X was no more efficient at upregulating β-galactosidase expression from the nirB promoter than the native pnirB FNR binding site. Newton et al. (25) reported that the expression of a protein was influenced by the distance between the nirB promoter and the RBS, and the longer the distance the higher the level of expression. In pTETnir15 the distance between the nirB promoter and the RBS is longer than in the native sequence on the E. coli chromosome. This could account for differences in immunogenic levels of C-fragment expression from pTETnir15 and pKK/pnirB/C frag. The ability of the pnirB cassette, generated in this study, to be further upregulated in anaerobic conditions by the addition of nitrite provides evidence that a NarL homolog is present and functioning in S. typhimurium. Tyson et al. (37) reported that nirB promoter activity in E. coli is partially dependent on NarL, as removal of NarL, either by mutation or by the alteration of the NarL binding site, led to a decrease in anaerobic expression.
This study addressed whether poor translation was responsible for the lack of immune response in mice immunized with BRD509(pKK/pnirB/C frag). An optimal RBS sequence was incorporated into the nirB promoter construct. The improved RBS sequence was complementary to the 16S rRNA sequence from E. coli, and the spacer region between the RBS and ATG was increased to the optimal size of 8 bp (29, 33). The addition of the optimal RBS had no effect on the anti-tetanus toxoid antibody generated in mice immunized with BRD509(pKK/pnirBR/C frag), suggesting that the lack of immunization from the nirB promoter cassette is probably not due to poor translation initiation. The addition of the optimal RBS to each promoter cassette also standardized the sequences required for translation initiation in each construct and thus allowed for a direct comparison of the upstream regions containing the individual promoters. The addition of the optimal RBS had no affect on the abilities of any of the Salmonella strains containing the promoter cassettes to induce a C-fragment-specific response in immunized mice, suggesting that the level of translation from the original RBS was not limiting.
An attenuated S. typhimurium vaccine vector would encounter a number of different environments once inside the immunized host. These environmental stimuli may either be inductive or repressive for environmentally regulated promoters such as pagC. Here, as well as elsewhere (10), it was demonstrated that the pagC promoter cassette directed protein expression in response to low Mg2+ concentrations in vitro. Garcia Vescovi et al. (10) identified Mg2+ as a physiological signal controlling the PhoP/PhoQ regulatory system. The induction of the pagC promoter may occur in phagosomal compartments of eucaryotic cells, as in vitro this environment has been shown to contain low concentrations of Mg2+ (11). Conversely, the pagC promoter may be repressed in extracellular fluid and the cytosol of cells, as the levels of Mg2+ in these environments have been shown to be high (10, 28).
In this study, the pagC promoter cassette was the most efficient at directing immunogenic levels of heterologous antigen expression in S. typhimurium BRD509. High-titer antibody responses to C fragment were detected in the sera of mice immunized with BRD509(pKK/ppagC/C frag). Previously, Hohmann et al. (13) had successfully used the pagC promoter in an S. typhimurium vaccine vector and demonstrated that protein expression directed from this promoter induced an antibody response against the heterologous antigen whereas expression from a constitutive promoter did not, even though the constitutive promoter produced a higher amount of heterologous antigen in vitro (13). Our data are consistent with the findings of Hohmann et al. (13). The most interesting observation described here is that a significantly higher titer of antibody against the heterologous antigen C fragment was detected in mice immunized with BRD509(pKK/ppagC/C frag) than in mice immunized with BRD509(pKK/C frag). In vitro however, the in vivo-inducible promoter construct appeared to express equivalent amounts of, if not less, C fragment than the constitutive promoter construct, as determined by Western blotting. This was also demonstrated quantitatively by the amount of luciferase detected in the PPs of mice, as the amount of luciferase produced in mice immunized with BRD509(pKK/ppagC/luc) was either equivalent to or lower than the amount produced in mice immunized with BRD509(pKK/luc). These data suggest that the total amount of heterologous antigen that a vaccine strain produces does not necessarily correlate with the strain’s ability to elicit an antibody response to that antigen. Future studies could address the hypothesis that the successful immunization of mice with BRD509(pKK/ppagC/C fragment) is due to a combination of the location and timing of C fragment expression in vivo.
The pagC promoter cassette generated in this study may be a useful tool in the further development of Salmonella vaccine vectors. The capacity of the pagC promoter to be induced in vivo may also overcome the need for large amounts of constitutively expressed antigen, potentially avoiding protein toxicity and expression construct instability. Repression of ppagC-driven heterologous protein expression by the addition of high concentrations of Mg2+ to the growth medium may also be beneficial to vaccine production in vitro.
The use of the katG promoter to direct expression of a heterologous antigen in S. typhimurium has not previously been reported. The rationale behind the choice of this promoter was that the katG promoter, which is positively activated by OxyR, is upregulated in the presence of H2O2 (7, 23, 35). Hydrogen peroxide is one of the oxidizing agents produced by the respiratory burst of a phagocyte in response to bacterial infection. Induction of heterologous antigen expression in this location in vivo may enhance the immune response elicited. In this study, no antigen-specific antibody was detected in sera when C fragment was expressed from the katG promoter cassette and delivered to the murine immune system in attenuated S. typhimurium. The location and timing of C-fragment expression by the katG promoter, or the poor strength of the promoter, may be responsible for the production of subimmunogenic levels of C fragment.
In summary, a number of variables, such as the location, timing, and level of antigen expression, as well as the inherent properties of the antigen to be expressed and the type of immune response required, are factors that when combined may affect the efficacy of a multivalent vaccine based on attenuated Salmonella.
ACKNOWLEDGMENTS
BRD509 was generously donated by G. Dougan (Imperial College, London, United Kingdom).
S.J.D. was a recipient of an Australian Postgraduate Award. This study was supported in part by the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Anderson R, Dougan G, Roberts M. Delivery of the Pertactin/P.69 polypeptide of Bordetella pertussis using an attenuated Salmonella typhimuriumvaccine strain: expression levels and immune response. Vaccine. 1996;14:1384–1390. doi: 10.1016/s0264-410x(96)00036-9. [DOI] [PubMed] [Google Scholar]
- 2.Barry E M, Gomez-Duarte O, Chatfield S, Rappuoli R, Pizza M, Losonsky G, Galen J, Levine M M. Expression and immunogenicity of pertussis toxin S1 subunit-tetanus toxin fragment C fusions in Salmonella typhivaccine strain CVD 908. Infect Immun. 1996;64:4172–4181. doi: 10.1128/iai.64.10.4172-4181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell A I, Cole J A, Busby S J. Molecular genetic analysis of an FNR-dependent anaerobically inducible Escherichia colipromoter. Mol Microbiol. 1990;4:1753–1763. doi: 10.1111/j.1365-2958.1990.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 4.Bullas L R, Ryu J I. Salmonella typhimurium LT2 strains which are r− m+for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabalgoity J A, Harrison J A, Esteves A, Demarco de Hormaeche R, Ehrlich R, Khan C M, Hormaeche C E. Expression and immunogenicity of an Echinococcus granulosus fatty acid-binding protein in live attenuated Salmonellavaccine strains. Infect Immun. 1997;65:2402–2412. doi: 10.1128/iai.65.6.2402-2412.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonellaoral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 7.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 8.Fairweather N F, Chatfield S N, Makoff A J, Strugnell R A, Bester J, Maskell D J, Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonellacarrier. Infect Immun. 1990;58:1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galen J E, Gomez-Duarte O G, Losonsky G A, Halpern J L, Lauderbaugh C S, Kaintuck S, Reymann M K, Levine M M. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhilive vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 10.Garcia Vescovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonellavirulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-del Portillo F, Foster J W, Maguire M E, Finlay B B. Characterization of the micro-environment of Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Duarte O G, Galen J, Chatfield S N, Rappuoli R, Eidels L, Levine M M. Expression of fragment C of tetanus toxin fused to a carboxyl-terminal fragment of diphtheria toxin in Salmonella typhiCVD 908 vaccine strain. Vaccine. 1995;13:1596–1602. doi: 10.1016/0264-410x(95)00094-h. [DOI] [PubMed] [Google Scholar]
- 13.Hohmann E L, Oletta C A, Loomis W P, Miller S I. Macrophage-inducible expression of a model antigen in Salmonella typhimuriumenhances immunogenicity. Proc Natl Acad Sci USA. 1995;92:2904–2908. doi: 10.1073/pnas.92.7.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 15.Homchampa P, Strugnell R A, Adler B. Molecular analysis of the aroA gene of Pasteurella multocida and vaccine potential of a constructed aroAmutant. Mol Microbiol. 1992;6:3585–3593. doi: 10.1111/j.1365-2958.1992.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 16.Hone D, Attridge S, van den Bosch L, Hackett J. A chromosomal integration system for stabilization of heterologous genes in Salmonellabased vaccine strains. Microb Pathog. 1988;5:407–418. doi: 10.1016/0882-4010(88)90002-2. [DOI] [PubMed] [Google Scholar]
- 17.Hormaeche C E, Khan C M A, Mastroeni P, Villarreal B, Dougan G, Roberts M, Chatfield S N. Salmonella vaccines: mechanisms of immunity and their use as carriers of recombinant antigens. In: Ala’Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. New York, N.Y: John Wiley & Sons Ltd.; 1995. pp. 119–153. [Google Scholar]
- 18.Khan C M, Villarreal R B, Pierce R J, Riveau G, Demarco de Hormaeche R, McNeill H, Ali T, Fairweather N, Chatfield S, Capron A, Dougan G, Hormaeche C E. Construction, expression, and immunogenicity of the Schistosoma mansoni P28 glutathione S-transferase as a genetic fusion to tetanus toxin fragment C in a live Aro attenuated vaccine strain of Salmonella. Proc Natl Acad Sci USA. 1994;91:11261–11265. doi: 10.1073/pnas.91.23.11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Londono L P, Chatfield S, Tindle R W, Herd K, Gao X M, Frazer I, Dougan G. Immunisation of mice using Salmonella typhimuriumexpressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine. 1996;14:545–552. doi: 10.1016/0264-410x(95)00216-n. [DOI] [PubMed] [Google Scholar]
- 21.McSorley S J, Xu D, Liew F Y. Vaccine efficacy of Salmonellastrains expressing glycoprotein 63 with different promoters. Infect Immun. 1997;65:171–178. doi: 10.1128/iai.65.1.171-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Morgan R W, Christman M F, Jacobson F S, Storz G, Ames B N. Hydrogen peroxide-inducible proteins in Salmonella typhimuriumoverlap with heat shock and other stress proteins. Proc Natl Acad Sci USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayama K, Kelly S M, Curtiss R I. Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonellavaccine strain. Bio/Technology. 1988;6:693–697. [Google Scholar]
- 25.Newton S M, Klebba P E, Hofnung M, Charbit A. Studies of the anaerobically induced promoter pnirBand the improved expression of bacterial antigens. Res Microbiol. 1995;146:193–202. doi: 10.1016/0923-2508(96)80275-0. [DOI] [PubMed] [Google Scholar]
- 26.Oxer M D, Bentley C M, Doyle J G, Peakman T C, Charles I G, Makoff A J. High level heterologous expression in E. coli using the anaerobically-activated nirBpromoter. Nucleic Acids Res. 1991;19:2889–2892. doi: 10.1093/nar/19.11.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peakman T, Crouzet J, Mayaux J F, Busby S, Mohan S, Harborne N, Wootton J, Nicolson R, Cole J. Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coliK-12 chromosome. Eur J Biochem. 1990;191:315–323. doi: 10.1111/j.1432-1033.1990.tb19125.x. [DOI] [PubMed] [Google Scholar]
- 28.Reinhart R A. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch Intern Med. 1988;148:2415–2420. doi: 10.1001/archinte.148.11.2415. [DOI] [PubMed] [Google Scholar]
- 29.Ringquist S, Shinedling S, Barrick D, Green L, Binkley J, Stormo G D, Gold L. Translation initiation in Escherichia coli: sequences within the ribosome-binding site. Mol Microbiol. 1992;6:1219–1229. doi: 10.1111/j.1365-2958.1992.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 30.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O’Hagan D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 27–58. [Google Scholar]
- 31.Roberts M, Li J, Bacon A, Chatfield S. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrApromoters. Infect Immun. 1998;66:3080–3087. doi: 10.1128/iai.66.7.3080-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Shine J, Dalgarno L. The 3′-terminal sequence of Escherichia coli16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strugnell R A, Maskell D, Fairweather N, Pickard D, Cockayne A, Penn C, Dougan G. Stable expression of foreign antigens from the chromosome of Salmonella typhimuriumvaccine strains. Gene. 1990;88:57–63. doi: 10.1016/0378-1119(90)90059-z. [DOI] [PubMed] [Google Scholar]
- 35.Tartaglia L A, Storz G, Ames B N. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J Mol Biol. 1989;210:709–719. doi: 10.1016/0022-2836(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 36.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyson K L, Bell A I, Cole J A, Busby S J. Definition of nitrite and nitrate response elements at the anaerobically inducible Escherichia colinirB promoter: interactions between FNR and NarL. Mol Microbiol. 1993;7:151–157. doi: 10.1111/j.1365-2958.1993.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 38.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]