Abstract
In recent years, there has been increasing interest in research showing positive results in antimicrobial photodynamic therapy (aPDT) and laser therapy (LT) in dentistry. The authors of this review tried to answer the question: “Is the effectiveness of lasers and aPDT in the elimination of intraoral halitosis possible?” For this purpose, the electronic database of PubMed and Cochrane Library were searched until September 2021 using a combination of different keywords: (bad breath OR fetor ex ore OR halitosis OR oral malodor) AND (laser OR PDT OR PACT OR photodynamic inactivation OR photodynamic therapy OR photodynamic antimicrobial chemotherapy). Initially, 83 studies were identified. A total of 9 articles were qualified after the application of the eligibility criteria. Eight works concerned aPDT treatment, and only one dedicated to the Er,Cr:YSGG laser. A significant reduction in halitosis occurred immediately after both LT and aPDT. The review found the confirmation of the effectiveness of laser therapy in reducing the number of volatile sulfur compounds (VSC) and the amount of anaerobic bacteria responsible for VSC formation. In most studies, a positive effect was observed for a 1-week follow-up. Laser therapy (aPDT, Er,Cr:YSGG) effectively eliminates microorganisms that produce volatile compounds and can effectively eliminate bad breath for the longer period of time than traditional methods of combatting this ailment.
Keywords: Bad breath, Fetor ex ore, Halitosis, Laser, Oral malodor
Introduction
Halitosis describes any unpleasant odor of exhaled air, regardless of its source. Other commonly used names are oral malodor, bad breath, and fetor ex ore. The incidence of this disease in the population amounts to 31.8%, with 85–90% of cases having its origins in the oral cavity [1, 2]. Halitosis affects the quality of social life, leading to embarrassment and psychological withdrawal. It is caused by the accumulation of decomposed food debris on the back of the tongue, in its pits and between the filamentous papillae. It is also affected by salivary proteins, exfoliating epithelium that is broken down by bacteria in the mouth. The classification of intraoral halitosis (IOH) consists of three basic groups: true, pseudohalitosis, and halitophobia [3]. True halitosis has been divided into the physiological one, in which there is no disease process, and the pathological one, which occurs in inflammation of the gums, periodontium, tonsils, xerostomia, and tooth decay. Pseudohalitosis is a condition in which patients experience an unpleasant odor, but no one else confirms it. Halitophobia is a consequence of the treatment of halitosis and is associated with the fear of recurrence of unpleasant symptoms [3].
The main components of bad breath are volatile sulfur compounds (VSC), i.e., hydrogen sulfide, methyl mercaptan, dimethyl sulfide, and other volatile organic compounds resulting from metabolic changes. VSC in the oral cavity are produced mainly by anaerobic bacteria, which are found, among others, on the dorsum of the uvula (51%), in the gingival pockets, in the interdental plate, and tonsils [4]. Bacteria, including Solobacterium morei, are responsible for biofilm formation on the tissues in the oral cavity and the breakdown of amino acids, mainly methionine and cysteine, from which VSC are secreted [4]. Other bacteria involved in halitosis include Treponema denticola, Porphyromonas gingivalis, Fusobacterium nucleatum, Capnocytophaga gingivalis, Prevotella intermedia, and Peptostreptococcus micros [4]. Measurements of VSC quantity in ppb (part per billion) are carried out with devices such as Halimeter, Breathtron, and oral OralChroma [5, 6]. Until the outbreak of the COVID-19 pandemic, the subjective organoleptic method introduced by Rosenberg and McCulloch [7], involving the patient blowing air from the mouth into a tube, and the air odor being judged by the use of the sense of smell of the evaluator (clinician) was the gold standard for assessing halitosis.
There are no standard methods of treating unpleasant odor from the mouth, as it can be a symptom associated with many general diseases (extraoral halitosis, EOH); however, approximately 10–15% can be related to a mouth disease only (IOH) [1]. Patients with halitosis use various odor-neutralizing agents. These are mouthwashes, chewing gums, lozenges, toothpaste containing mostly alcohol, chlorhexidine, zinc, and essential oils. The use of a tongue scraper is also recommended. However, these methods work efficiently for a short period of time only. Other safe methods of removing or decreasing the malodor are being sought. The high-power lasers, i.e., Er:YAG, Er,Cr:YSGG, and Nd:YAG, which are applicable for disinfection and cleansing tissues by evaporating the water they contain can be one of them. Also, antimicrobial photodynamic therapy (aPDT), which consists of the activation of the photosensitizer by an appropriate light wavelength, eliminates pathogens by using singlet oxygen or other reactive forms of oxygen [8–13]. Thanks to various laser systems, researchers and clinicians can reduce the number of microorganisms in the oral cavity [14].
This study aimed to provide a comprehensive literature review and evaluate the effectiveness of various laser wavelengths and aPDT in the treatment of halitosis in vivo in a human model.
Materials and methods
Focused question
The question posed by the authors of this review is as follows: “Is it possible to treat halitosis effectively with lasers and aPDT in healthy patients?”.
Protocol
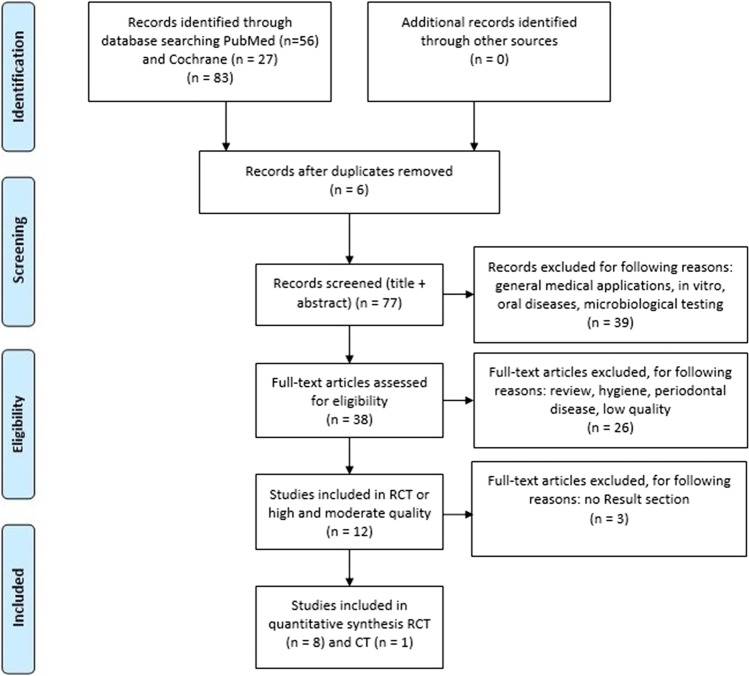
According to the PRISMA scheme, the details of the selection criteria are presented in Fig. 1
Fig. 1.
PRISMA flowchart presenting the criteria for the included studies
Eligibility criteria
The articles’ selection criteria for the review included studies of healthy subjects over 12 years of age participating in randomized clinical trials (RCT) or clinical trials (CT) with a minimum observation period of more than 7 days.
Non-English language articles, reviews, and opinions that did not consider laser treatment of bad breath were excluded. Articles related to the treatment of patients with systemic diseases accompanied by halitosis and to the cases of advanced periodontal disease were also excluded.
Inclusion criteria:
Use of a lasers or aPDT
Number of patients not less than 10 per group
Randomized clinical trial
Clinical trial
Laser treatment of halitosis in generally healthy subjects
The effect of the laser on the bacterial flora in the oral cavity
A minimum of one week of observation after laser/aPDT use
The use of various photosensitizers in aPDT
In vivo studies
Exclusion criteria:
Non-randomized studies
Examinations of patients after laser treatment with general diseases, except for MS (multiple sclerosis)
Number of patients less than 10 per group
Patients age less than 12 years
Laser treatment of advanced periodontal diseases and other oral diseases
Change of the bacterial flora in the oral cavity without the use of a laser
Duplicated articles
No use of a laser
In vitro studies
Search strategy
An electronic screening of PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) databases from 1994 to September 2021 was done. A following combination of keywords was used: (halitosis OR fetor ex ore OR bad breath OR oral malodor) AND (laser OR aPDT or PACT OR photodynamic inactivation OR photodynamic therapy OR photodynamic antimicrobial chemotherapy). The search strategy was limited to studies that met the eligibility criteria. Articles with fully available texts were taken into account.
Information sources, search strategy, and study selection
Two reviewers (A. W., J. M.) independently extracted data from articles that met the inclusion criteria, and the third one (K. G. L.) checked the accuracy of the selection and resolved the disputed decisions. The following data was used: first author, year of publication, title, study design, laser type, laser parameters, photosensitizer type and concentration, incubation time, study groups, study results, and changes in the amount of VSC in exhaled air before and after treatment. The extracted data was saved in a standard Excel sheet.
Quality assessment
Two blinded reviewers filtered the studies individually and independently to assess the quality of each included study. The study analysis and implementation were based on the following criteria: description of laser parameters and laser type, the use of laser power meter to standardize lasers parameters, detailed treatment protocol, randomization, blinding, and control group, at least 1-month follow-up. The scoring range was from 0 to 9 points. The higher the result, the higher the quality of the test. Any disagreements were resolved through discussion until reaching consensus.
Risk of bias
The scores of each study were calculated, and overall the estimate risk of bias (low, moderate, high) was made for each included study, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions [15]. The risk of error based on sums of answers: yes—1 or no—0 was determined.
The total number of 1-yes answers shows us the degree of systematic error, assessed with scoring limits: 0–3 high risk, 4–6 moderate risk, and 7–9 low risk. The higher the result, the higher the quality of the test.
Results
Study selection
The main aim of this review was to evaluate the effectiveness of various laser wavelengths and aPDT in the treatment of halitosis in vivo in a human model.
Fifty-six articles had been found in the PubMed search engine and 27 in the Cochrane Library one. Six repeating research papers from both search engines were excluded. After using filters for randomized trials (RCT) and clinical trials (CT), the number of articles were reduced to 9. The review of articles included 8 randomized clinical trials [16–23] and 1 clinical trial [24]. In the treatment of halitosis, 8 studies concerned the use of diode lasers with a photosensitizer [16–21, 23, 24], while 1 of them involved the Er,Cr:YSGG laser [22].
The best results in reducing VSCs were shown in papers evaluating tongue scraper and aPDT [16, 19, 20, 23]. Most studies used methylene blue as a photosensitizer [16–21, 24], activated with a 660 nm wavelength. One article described the use of bixa orellana (other names urucum, arnota proper) as a yellow photosensitizer activated with a laser with a wavelength of 395–480 nm [23].
The influence of Er,Cr:YSGG on the level of VSC in patients without periodontal disease was described in one study [22] (Table 1).
Table 1.
Characteristics of lasers used for treatments
| No | Laser type | Wavelength (nm) | Power (W) | Laser therapy | Reference number |
|---|---|---|---|---|---|
| 1 | Diode laser | 660 | 0.1 | aPDT | Costa da Mota et al. [16] |
| 2 | Diode laser | 395–480 | 0.48 | aPDT | Goncalves et al. [23] |
| 3 | Diode laser | 660 | 0.1 | aPDT | Llanos do Vale et al. [17] |
| 4 | Diode laser | 660 | 0.1 | aPDT | Romero et al. [18] |
| 5 | Diode laser | 660 | 0.4 | aPDT | Ciarcia et al. [19] |
| 6 | Diode laser | 660 | 0.1 | aPDT | Goncalves et al. [24] |
| 7 | Diode laser | 660 | 0.1 | aPDT | Lopes et al. [20] |
| 8 | Diode laser | 660 | 0.1 | aPDT | Labban et al. [21] |
| 9 | Er,Cr:YSGG laser | 2780 | 4 | Vaporization/debridement | Krespi et al. [22] |
Results of individual studies
The study by Romero et al. [18] with the longest follow-up showed a reduction in halitosis after applying aPDT and a tongue scraper, and the effect was maintained throughout the 3-month observation period. Most studies reported an effect of reducing malodor immediately after treatment, and it lasted for a week [16–18, 20, 21, 23, 24]. Labban et al. [21] described the reduction in Porphyromonas gingivalis and VSC over 1 month of follow-up.
Krespi et al. [22] in his research on the Er,Cr:YSGG laser described a reduction in VSC in exhaled air, the amount of anaerobic and aerobic bacteria in a microbiological study, and an improvement in the appearance of the tongue. The positive results were maintained until the end of the 1-month observation period (Table 2).
Table 2.
Characteristics and results of included studies
| Authors | Study type/number of patients/level of VSC | Groups | Laser (nm) + photosensitizer | Protocol aPDT | Study observation—time | Results |
|---|---|---|---|---|---|---|
| 1. Costa da Mota et al. [16] |
RCT 46 patients |
G1-aPDT (n = 15), G2- tongue scraper (n = 15) G3 (n = 16) |
660 nm + methylene blue MB (0.05 mg/ml) | P = 100 mW, E = 9 J, t = 90 s per point, density P = 3.5 W/cm2, density E = 320 J/cm2, 6 points, applicator 0.028 cm2, BM- incubation 5 min, irradiation distance: 1 cm from tongue | 1 session, 1 week, the treatment microbiological analysis before and immediately after |
G3—highest reduction in VSC, G2- lowest reduction in VSC After 1 week, there were no significant differences in quantity of VSC in particular groups. No significant differences in the microbiological analysis |
| 2. Goncalves et al. [23] |
RCT 44 patients |
G1 = 15, G2 = 14, G3 = 15 G1 = aPDT + BO (bixa orellana) G2 = tongue scraper G3 = aPDT + scraper |
395–480 nm BO (bixa orellana), 20% solution |
P = 480 mW, E = 9.6 J, t = 20 s/per point, density P = 153 mW/cm2, density E = 6.37 J/cm2, applicator 3.14 cm2, irradiation points = 6, contact method, incubation on the tongue for 2 min | 1 session, immediately after the treatment, 1 week | Quantity of VSC diminished in all groups. There were no significant differences in G1 and G3 groups. One week after the treatment, no significant differences were noticed with respect to the level of VSC |
| 3. Llanos do Vale et al. [17] |
RCT 45 patients |
G1 = 22 G2 = 23 G1- tongue scraper G2- aPDT |
660 nm, methylene blue—MB (0.005%) | P = 100 mW, E = 9 J, t = 90 s, density P = 35,368 mW/cm2, density E = 3183 J/cm2, 6 points irradiated with a contact method, applicator = 0.00287 cm2, MB—5 min on the tongue and rinsed with saline |
1 session 1 week (VSC level) |
aPDT significantly lowered levels of VSC |
| 4. Romero et al. [18] |
RCT 40 patients Single-blind trial |
G1 = 20 G2 = 20 G1- aPDT G2- tongue scraper |
660 nm methylene blue 0,005% |
P = 100 mW E = 9 J per point t = 1 min density P = 3537 mW/cm2 density E = 318 J/cm2 6 points |
1 session, results immediately after the treatment 1 week 90 days (VSC measurement) |
The VSC values were 2 (scraper) or 3 (aPDT) times lower than the baseline value after 1 week and after 3 months. The most significant improvement immediately after the treatment was observed |
| 5. Ciarcia et al. [19] |
RCT 39 patients |
G1 = 13 G2 = 13 G3 = 13 G1-aPDT G2- tongue scraper G3- aPDT i scraper |
660 nm methylene blue 0,005% |
P = 400 mW E = 36 J t = 90 s P = 1039.4 mW/cm2 density E = 93.5 J/cm2 4 irradiated points, distance: 2 cm, for 2 min |
1 session, immediately after the treatment 1 week 14 days 30 days (VSC level) |
Directly after the treatment the significant reduction in VSC was observed No data available after 7, 14, and 30 days |
| 6. Goncalves et al. [24] |
CT 60 patients |
G1 = 20 G2 = 20 G1-with MS G2—healthy |
660 nm methylene blue 0.005% |
P = 100 mW E = 9 J t = 90 s for a point density P = 3537 mW/cm2 density E = 320 J/cm2 applicator- 0.094 cm2 6 points irradiated with contact method MB on the tongue for 5 min |
1 session examination immediately after the treatment |
Level of VSC diminished in both groups |
| 7. Lopes et al. [20] |
RCT 45 patients |
G1 = 16 G2 = 15 G3 = 14 G1-aPDT G2- tongue scraper G3- aPDT + scraper |
660 nm methylene blue 0.005% |
P = 100 mW E = 9 J T = 90 s W/cm2 density E = 317.43 J/cm2 Applicator -0.02835 cm2 6 points separated by 1 cm were irradiated with the contact method, P = 3537 mW/cm2 Methylene blue 5 min. on the tongue |
1 session 1 h and 24 h after treatment (VSC level) |
There was a significant reduction in VSC in all groups, the highest result of the reduction of VSC was shown in group G3 |
| 8. Labban et al. [21] |
RCT 40 patients |
G1 = 20 G2 = 20 G1- tongue scraper G2-scraper + aPDT |
Laser 660 nm, methylene blue 0.005% |
P = 100 mW, density P = 3527 mW/cm2, E = 9 J 6 points on the tongue and on the prosthesis, distance – 1 cm, MB – 5 min |
1 session, 5 days after, 15 days after, 30 days after treatment, the level of VSC was measured, microbiological test for the presence of Porphyromonas gingivalis was performed, OHIP-14 profile | Decrease in the level of H2S, which was sustained during the month of observation, decrease in the amount of P. gingivalis (highest after 5 days) |
| 9. Krespi et al. [22] |
RCT 60 patients |
G1 = 30 G2 = 30 G1- tongue scraper G2 laser |
2780 nm Er,Cr:YSGG |
P = 4 W E = 100 mJ f = 40 Hz t = 60 s Pulse width—60 µs Air -10% Water—5% Tip—MC12 distance from the tongue 3 mm Density E = 3 J/cm2 10 passes |
1 session/1 month The level of VSC was measured; a microbiological test has been performed, the appearance of the tongue was assessed |
Decrease in VSC and the number of anaerobic and aerobic bacteria, improvement in the appearance of the tongue, sustained throughout the observation period |
RCT, randomized clinical trial; aPDT, antimicrobial photodynamic therapy; P, power; E, energy; MS, multiple sclerosis
Quality assessment
Seven articles showed a low 7–9 bias [16, 18–23]. Two studies were in the moderate range of 4–6 of bias [17, 24]. None of the articles was classified as of a high risk of error (Table 3).
Table 3.
Quality assessment of the included studies
| Criteria | Authors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Costa da Mota [16] | Goncalves [23] | Llanos do Vale [17] | Romero [18] | Ciarcia [19] | Goncalves [24] | Lopes [22] | Labban [21] | Krespi [22] | |
| Randomization | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| Blinding | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Sample size calculation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Control group | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Laser parameters: (power, energy, density) applicator type | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Power meter use | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| Laser type (wavelength) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Detailed treatment protocol | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| At least 1-month follow-up | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| Total score | 8 | 7 | 6 | 8 | 7 | 5 | 7 | 8 | 8 |
| Risk of bias | low | low | medium | low | low | medium | low | low | low |
Discussion
All of the included studies reported the results of the reduction of bad breath coming from the analysis of volatile sulfur components detection devices [16–24]. The most commonly used method of VSC measurement was gas chromatography (Oral Chroma TM Abilit, Japan) [16–21, 23, 24] and halimetric analysis of hydrogen sulfide (Halimeter, Interscan Corporation, Chatsworth, USA) [22]. Additionally, Krespi et al. [22] determined breath quality by analyzing patient-perceived results using a visual analog scale (VAS) and the tongue’s appearance before and after treatment. The results of included studies were reported immediately after treatment [16–24], and the maximum follow-up was 3 months [18]. Most included studies showed a benefit of using aPDT in generally healthy patients with halitosis [16–21, 23, 24]. Only in one study by Krespi et al. [22] the application of high-level laser (Er,Cr:YSGG) reduced the amount of VSC, the number of anaerobic and aerobic bacteria, and improved the tongue’s appearance.
Almost all of the included studies indicated aPDT as an efficient method in treating malodor [16–21, 23, 24]. Antibacterial photodynamic therapy is a process in which non-toxic photosensitizing substances and oxygen are combined with the appropriate wavelength of light [9, 11]. That phenomenon leads to the formation of reactive oxygen species that are deadly against bacteria, viruses, and fungi [11, 25]. PDT appears to represent an efficacious alternative modality for treating localized microbial infections through the in situ application of the photosensitizer followed by irradiation of the photosensitizer-loaded infected area [14]. In most studies on the effectiveness of aPDT, methylene blue (MB) and a laser with a wavelength of 660 nm were used [16–21, 23, 24]. One study focused on the use of bixa orellana with a laser wavelength of 395–480 nm [22]. All laser wavelengths combined with both photosensitizers (methylene blue and bixa orellana) used in included studies allowed for a significant reduction of the VCS amount.
Many studies confirmed that halitosis is caused by volatile sulfur components, which are the product of metabolic changes in anaerobic bacteria [4, 26–28]. A significant number of these bacteria are located on the back of the tongue. Mechanical cleaning of the tongue with a scraper reduces the amount of bacterial wastes, but does not significantly reduce the amount of microbes [29]. In their study, Mantilla et al. [29] did not observe a relationship between the appearance of the tongue and salivary bacterial load. The elimination of the number of bacteria and the change in their quality have had an impact on the reduction of unpleasant breath (malodor) [30]. In their study, Labban et al. [21] proved a significant decrease in the amount of Porphyromonas gingivalis (highest after five days). Moreover, Krespi et al. [22] observed a decrease in the total number of bacteria when using Er,Cr:YSGG laser. The laser treatment was significantly more effective immediately after the treatment than the tongue scarper at diminishing both anaerobic and aerobic cultures [22].
Last but not least, only one article assessed the influence of the Er,Cr:YSGG high-power laser on the level of VSC in the oral cavity. Krespi et al. [22] reported positive outcomes for all tested variables after laser treatment. The authors pointed out that the sustained reduction of VSC concentration due to laser treatment compared to that of the tongue scraping was significant. The results were maintained for one month of follow-up. The microbiological analysis of the tongue, its appearance, and the patient’s subjective feelings were assessed by using the HALT test. The decrease in total anaerobic bacteria from baseline to 1 month remained significantly higher for the laser treatment group than for the control group. The Er,Cr:YSGG laser effectively removed biofilm through light and water dual action [22]. This physical property damages water-rich cells, which is essential in eradicating pathogens [10]. Further studies should be conducted to examine whether laser-assisted halitosis treatment combined with various lasers (Er:YAG, Nd:YAG) improves the VCS and total microbial count.
Conclusions
Laser therapy (aPDT, Er,Cr:YSGG) effectively eliminates microorganisms that produce volatile compounds and it can effectively eliminate bad breath for the longer period of time than traditional methods of curing this ailment. Halitosis is an underestimated problem of the global population. It has a significant impact on quality of life and social withdrawal. There is a need to resolve this social problem, looking for minimally invasive treatments.
Declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Agnieszka Woźniak, Email: theagnieszkawozniak@gmail.com.
Jacek Matys, Email: jacek.matys@wp.pl.
Kinga Grzech-Leśniak, Email: kgl@periocare.pl.
References
- 1.Silva MF, Leite FRM, Ferreira LB, et al. Estimated prevalence of halitosis: a systematic review and meta-regression analysis. Clin Oral Investig. 2018;22(1):47–55. doi: 10.1007/s00784-017-2164-5. [DOI] [PubMed] [Google Scholar]
- 2.do Vale KL, Horliana ACRT, Romero SDS, et al. Evaluation of the treatment of halitosis with photodynamic therapy in older patients with complete denture: protocol for a randomized, controlled trial. Medicine (Baltimore) 2019;98(27):e16275. doi: 10.1097/MD.0000000000016275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc. 2000;66(5):e16275. doi: 10.12691/ijdsr-4-4-3. [DOI] [PubMed] [Google Scholar]
- 4.Hampelska K, Jaworska MM, Babalska ZŁ, Karpiński TM. The role of oral microbiota in intra-oral halitosis. J Clin Med. 2020;9(8):1–17. doi: 10.3390/jcm9082484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JG, Kim YJ, Yoo SH, et al. Halimeter ppb levels as the predictor of erosive gastroesophageal reflux disease. Gut Liver. 2010;4(3):320–325. doi: 10.5009/gnl.2010.4.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueno M, Shinada K, Yanagisawa T, et al. Clinical oral malodor measurement with a portable sulfide monitor. Oral Dis. 2008;14(3):264–269. doi: 10.1111/j.1601-0825.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg M, McCulloch CAG. Measurement of oral malodor: current methods and future prospects. J Periodontol. 1992;63(9):776–782. doi: 10.1902/jop.1992.63.9.776. [DOI] [PubMed] [Google Scholar]
- 8.Li YR, Trush M. Defining ROS in biology and medicine. React Oxyg Species. 2016;1(1):9. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grzech-Leśniak K, Nowicka J, Pajączkowska M, et al. Effects of Nd:YAG laser irradiation on the growth of Candida albicans and Streptococcus mutans: in vitro study. Lasers Med Sci. 2019;34(1):129–137. doi: 10.1007/s10103-018-2622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Świder K, Dominiak M, Grzech-Leśniak K, Matys J. Effect of different laser wavelengths on periodontopathogens in peri-implantitis: a review of in vivo studies. Microorganisms. 2019;7(7):189. doi: 10.3390/microorganisms7070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiench R, Skaba D, Matys J, Grzech-Leśniak K. Efficacy of toluidine blue—mediated antimicrobial photodynamic therapy on Candida spp.: a systematic review. Antibiotics. 2021;10(4):349. doi: 10.3390/antibiotics10040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matys J, Świder K, Flieger R. Laser instant implant impression method: a case presentation. Dent Med Probl. 2017;54(1):101. doi: 10.17219/dmp/66363. [DOI] [Google Scholar]
- 13.Dompe C, Moncrieff L, Matys J, et al. Photobiomodulation—underlying mechanism and clinical applications. J Clin Med. 2020;9(6):1724. doi: 10.3390/jcm9061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deeb JG, Smith J, Belvin BR, Grzech-Leśniak K, Lewis J. Er:YAG laser irradiation reduces microbial viability when used in combination with irrigation with sodium hypochlorite, chlorhexidine, and hydrogen peroxide. Microorganisms. 2019;7(12):612. doi: 10.3390/microorganisms7120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J, Thomas2 J (2021) Cochrane handbook for systematic reviews of interventions | Cochrane Training. https://training.cochrane.org/handbook/current. Accessed August 22, 2021
- 16.Costa da Mota AC, França CM, Prates R, et al. Effect of photodynamic therapy for the treatment of halitosis in adolescents – a controlled, microbiological, clinical trial. J Biophotonics. 2016;9(11-12):1337–1343. doi: 10.1002/jbio.201600067. [DOI] [PubMed] [Google Scholar]
- 17.Llanos do Vale K, Ratto Tempestini Horliana AC, Romero dos Santos S, et al. Treatment of halitosis with photodynamic therapy in older adults with complete dentures: a randomized, controlled, clinical trial. Photodiagnosis Photodyn Ther. 2021;33:102128. doi: 10.1016/j.pdpdt.2020.102128. [DOI] [PubMed] [Google Scholar]
- 18.Romero SS, do Vale KL, Remolina VG, et al. Oral hygiene associated with antimicrobial photodynamic therapy or lingual scraper in the reduction of halitosis after 90 days follow up: a randomized, controlled, single-blinded trial. Photodiagnosis Photodyn Ther. 2021;33:102057. doi: 10.1016/j.pdpdt.2020.102057. [DOI] [PubMed] [Google Scholar]
- 19.da CiarciaM ACC, Gonçalves MLL, Horliana ACRT, et al. Action of antimicrobial photodynamic therapy with red leds in microorganisms related to halitose: controlled and randomized clinical trial. Medicine (Baltimore) 2019;98(1):e13939. doi: 10.1097/MD.0000000000013939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes RG, da Mota ACC, Soares C, et al. Immediate results of photodynamic therapy for the treatment of halitosis in adolescents: a randomized, controlled, clinical trial. Lasers Med Sci. 2016;31(1):41–47. doi: 10.1007/s10103-015-1822-6. [DOI] [PubMed] [Google Scholar]
- 21.Labban N, Assery MK, Al-Kattan R, Al-Shibani N, Alfouzan AF, Al Taweel SM. Antimicrobial capacity of photodynamic therapy on oral health-related quality of life and halitosis among elderly patients wearing removal dentures. Photodiagnosis Photodyn Ther. 2020;32:102059. doi: 10.1016/j.pdpdt.2020.102059. [DOI] [PubMed] [Google Scholar]
- 22.Krespi YP, Kizhner V, Wilson KA, et al. Laser tongue debridement for oral malodor—a novel approach to halitosis. Am J Otolaryngol - Head Neck Med Surg. 2021;42(1):102458. doi: 10.1016/j.amjoto.2020.102458. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves MLL, da Mota ACC, Deana AM, et al. Antimicrobial photodynamic therapy with Bixa orellana extract and blue LED in the reduction of halitosis—a randomized, controlled clinical trial. Photodiagnosis Photodyn Ther. 2020;30:101751. doi: 10.1016/j.pdpdt.2020.101751. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves MLL, Kalil Bussadori S, Dadalti Fragoso Y, et al. Effect of photodynamic therapy in the reduction of halitosis in patients with multiple sclerosis: Clinical trial. J Breath Res. 2017;11(4):046004. doi: 10.1088/1752-7163/aa8209. [DOI] [PubMed] [Google Scholar]
- 25.Grzech-Leśniak K, Belvin BR, Lewis JP, Golob DJ. Treatment with Nd:YAG laser irradiation combined with sodium hypochlorite or hydrogen peroxide irrigation on periodontal pathogens: an in vitro study. Photobiomodulation, Photomedicine, Laser Surg. 2021;39(1):46–52. doi: 10.1089/photob.2019.4775. [DOI] [PubMed] [Google Scholar]
- 26.Ye W, Zhang Y, He M, Zhu C, Feng XP. Relationship of tongue coating microbiome on volatile sulfur compounds in healthy and halitosis adults. J Breath Res. 2020;14(1):016005. doi: 10.1088/1752-7163/ab47b4. [DOI] [PubMed] [Google Scholar]
- 27.Mogilnicka I, Bogucki P, Ufnal M. Microbiota and malodor—etiology and management. Int J Mol Sci. 2020;21(8):2886. doi: 10.3390/ijms21082886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nammour S, El Mobadder M, Maalouf E, et al. Clinical evaluation of diode (980 nm) laser-assisted nonsurgical periodontal pocket therapy: a randomized comparative clinical trial and bacteriological study. Photobiomodulation, Photomedicine, Laser Surg. 2021;39(1):10–22. doi: 10.1089/photob.2020.4818. [DOI] [PubMed] [Google Scholar]
- 29.Mantilla Gómez S, Danser MM, Sipos PM, et al. Tongue coating and salivary bacterial counts in healthy/gingivitis subjects and periodontitis patients. J Clin Periodontol. 2001;28(10):970–978. doi: 10.1034/j.1600-051x.2001.028010970.x. [DOI] [PubMed] [Google Scholar]
- 30.Salako NO, Philip L. Comparison of the use of the halimeter and the oral chroma™ in the assessment of the ability of common cultivable oral anaerobic bacteria to produce malodorous volatile sulfur compounds from cysteine and methionine. Med Princ Pract. 2010;20(1):75–79. doi: 10.1159/000319760. [DOI] [PubMed] [Google Scholar]