Abstract
In the murine model of Lyme disease, C3H/He mice exhibit severe arthritis while C57BL/6N mice exhibit mild lesions when infected with Borrelia burgdorferi. Joint tissues from these two strains of mice harbor similar concentrations of B. burgdorferi, suggesting that the difference in disease severity reflects differences in the magnitude of the inflammatory response to B. burgdorferi lipoproteins. Stimulation of bone marrow macrophages from C3H/HeN mice with the B. burgdorferi lipoprotein OspA resulted in higher-level production of the inflammatory mediators tumor necrosis factor alpha, nitric oxide, and interleukin-6 (IL-6) than that of macrophages from C57BL/6N mice. In contrast, macrophages from C57BL/6N mice consistently produced larger amounts of the anti-inflammatory cytokine IL-10 than did C3H/HeN macrophages. Addition of recombinant IL-10 suppressed the production of inflammatory mediators by macrophages from both strains. IL-10 was found to modulate B. burgdorferi-induced inflammation in vivo, since C57BL/6J mice deficient in IL-10 (IL-10−/−) developed more severe arthritis than wild-type C57BL/6J mice. The increase in arthritis severity was associated with a 10-fold decrease in the number of B. burgdorferi organisms present in ankle tissues from IL-10−/− mice. These findings suggest that in C57BL/6 mice, IL-10-dependent regulation of arthritis severity occurs at the expense of effective control of bacterial numbers.
Lyme disease is a multisystem disorder caused by infection with Borrelia burgdorferi (11, 28, 49). The spirochetes are transmitted to a mammalian host via the bite of an infected Ixodes tick and subsequently disseminate from the site of inoculation. In the absence of antibiotic therapy, B. burgdorferi can infect multiple tissues, but it appears to have some predilection for joints, skin, and heart tissue (48, 49, 62). Lyme arthritis is distinct from other types of arthritis in that a bacterial presence appears to be necessary to elicit lesions and in that arthritis is not dependent on T or B lymphocytes (6, 9, 48, 57, 58, 62, 74). This subacute arthritis appears to be caused by the host inflammatory response to invasion of joints by the spirochete and is characterized by edema, synovial thickening, tendonitis, and a leukocytic infiltration consisting mainly of neutrophils and mononuclear cells (4).
B. burgdorferi produces a number of outer membrane lipoproteins which possess potent inflammatory potential and are believed to be responsible for the inherent inflammatory properties attributed to this spirochete (15, 40, 56, 58). These lipoproteins are capable of activating a wide variety of cell types, including macrophages (38, 40, 55, 56, 63), neutrophils (45), and endothelial cells (13, 18, 59, 70), which results in the production of a broad spectrum of pro- and anti-inflammatory mediators that have been linked to inflammatory disease. The receptor responsible for mediating lipoprotein signaling is distinct from that for lipopolysaccharide (LPS)-mediated signaling (40, 50); however, the stimulatory properties of these lipoproteins are potentiated by CD14 (23, 60, 71), the coreceptor for LPS (64, 72), suggesting that these lipoproteins might be directed to receptors on responsive cell types. These and other findings suggest that host responses to these lipoproteins are directly responsible for creating distinctive inflammatory lesions of Lyme disease (25, 51).
The lesions exhibited by B. burgdorferi-infected mice are similar to those seen in human disease, making the mouse an excellent model system for studying Lyme disease (4). Infection of different inbred mouse strains with B. burgdorferi can result in different but distinct disease outcomes, similar to the variability seen in the human population (5, 37, 49, 74). Infection with a range of inoculum doses of B. burgdorferi elicits severe arthritis in C3H/HeN mice but mild to moderate arthritis in C57BL/6N mice at all spirochete concentrations tested (37). When B. burgdorferi levels in ankles are quantified by PCR, the two mouse strains possess similar numbers of spirochetes, regardless of the concentration of the infectious dose and the disease severity displayed. One interpretation of these findings is that C57BL/6N mice are better able to regulate inflammation in response to B. burgdorferi lipoproteins than C3H/HeN mice, resulting in a less intense inflammatory response and decreased arthritis severity. In the present study, we tested this hypothesis by stimulating macrophages from C3H/HeN and C57BL/6N mice with a prototypic B. burgdorferi lipoprotein, OspA, and comparing the levels and scopes of inflammatory mediators produced. Differences in the balance of pro- and anti-inflammatory mediators produced by macrophage cultures from the two mouse strains were observed. The role of one cytokine, interleukin-10 (IL-10), that showed regulatory effects in vitro was assessed by infecting IL-10-deficient (IL-10−/−) mice with B. burgdorferi. The effect of this gene on disease severity is reported.
MATERIALS AND METHODS
Mice.
Female C3H/HeNCr and C57BL/6NCr mice were obtained from the National Cancer Institute, and female C57BL/6J and C57BL/6-IL-10tm/Cgn were obtained from The Jackson Laboratory (Bar Harbor, Maine). The C57BL/6-IL-10tm/Cgn mice (IL-10−/−) contain an IL-10 gene that has been inactivated by targeted mutation (34) and backcrossed into C57BL/6J mice. Mice were housed in the Animal Resource Center at the University of Utah Medical Center according to National Institutes of Health guidelines for the care and use of laboratory animals.
Bacteria and lipoproteins.
The N40 isolate of B. burgdorferi, provided at passage 3 from the mouse by Steve Barthold (University of California at Davis), was used in all infection studies (5). Passage 4 cultures were stored at −70°C. These frozen stocks were subsequently grown in BSK-H medium containing 6% rabbit serum (Sigma Chemical, St. Louis, Mo.) for 3 to 5 days at 32°C prior to injection. Lipidated recombinant OspA (rOspA) from the B31 strain of B. burgdorferi was a gift from Robert Huebner (Connaught Laboratories, Swiftwater, Pa.). rOspA contained less than 0.3 endotoxin units/500 ng of protein as determined by the Limulus amoebocyte lysate assay (Associates of Cape Cod, Woods Hole, Mass.) and has been shown to possess stimulatory properties similar to those of native OspA purified from B. burgdorferi (67).
Reagents.
Paired monoclonal antibodies and recombinant standards for murine tumor necrosis factor alpha (TNF-α), IL-6, and IL-10 were purchased from Pharmingen (San Diego, Calif.). Polyclonal goat anti-mouse immunoglobulin (Ig) and horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG1, IgG2a, IgG2b, and IgG3 were purchased from Zymed (San Francisco, Calif.). Purified murine IgG, IgM, and polyclonal HRP-conjugated antibodies specific for murine IgG and IgM were purchased from Sigma. Avidin-HRP was purchased from Vector Laboratories (Burlingame, Calif.). LPS from Salmonella typhi 14901 was obtained from Sigma.
Cell culture.
Murine macrophages were obtained from femur bone marrow as previously described (38). Briefly, bone marrow cells were cultured in RPMI medium supplemented with L929-conditioned medium for 7 days at 37°C. Macrophages were recovered with ice-cold phosphate-buffered saline and replated in 12-well culture dishes at a density of 5 × 105 per well in serum-free medium containing 1% Nutridoma (Boehringer Mannheim, Indianapolis, Ind.). After an overnight incubation at 37°C, nonadherent cells were removed and various agonists were added. Addition of polymyxin B effectively blocked activation of cells by LPS but had no effect on cultures stimulated with OspA and so was included in all OspA samples. Supernatants were harvested from these cultures at various times and frozen at −20°C until assayed.
Cytokine and nitric oxide assays.
All cytokines were assayed from frozen macrophage supernatants except TNF-α, which was assayed immediately upon collection. Supernatants were assayed for TNF-α, IL-6, and IL-10 content by sandwich enzyme-linked immunosorbent assay (ELISA) using paired monoclonal antibodies. Cytokine levels were determined by using a biotinylated detection antibody and avidin-HRP. Values were obtained by comparison to data for appropriate recombinant standards.
Nitrite levels in macrophage supernatants were assayed as previously described (17). Briefly, 100 μl of Griess reagent [equal volumes of 1% sulfanilamide in 30% acetic acid and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in 60% acetic acid) was added to 50 μl of thawed supernatant. Samples were read with an ELISA reader at 570 nm, and values were determined by using sodium nitrite as a standard.
Infection of mice.
B. burgdorferi numbers were determined after 3 to 5 days in culture by darkfield microscopy using a Petroff-Hauser chamber. Spirochetes were diluted in sterile medium such that there were 2 × 103 B. burgdorferi cells per 20-μl dose. Mice 6 to 7 weeks of age were infected by intradermal injection into shaven backs; this protocol closely mimics tick infection and has been shown to consistently elicit infection (3, 52). Infection was confirmed by ankle swelling, culturing of viable spirochetes from ear tissue, production of B. burgdorferi-specific Ig, and the presence of B. burgdorferi DNA in tissues as determined by PCR. Mock-infected animals received 20 μl of sterile BSK-H medium by intradermal injection and tested negative for B. burgdorferi by the above criteria.
Measurement of ankle joints.
Ankle joint measurements were performed as described previously (73) and provided a method of following the course of infection. Mice were anesthetized with methoxyflurane before measurement of the rear ankle joints by the use of a metric caliper. Weekly measurements were performed in a blinded fashion on the thickest portion (anterior-posterior) of the ankle, with the joint extended. Since normal joints from different strains of mice can differ in size, findings are reported as the increase in swelling relative to mock-infected animals for each mouse strain.
Lesion assessment of ankle joints.
Histologic analyses were performed on the rear ankle joint that exhibited the greatest amount of swelling when mice were sacrificed at 2 or 4 weeks postinfection. Joints were fixed in 10% neutral-buffered formalin, decalcified, embedded in paraffin, and sectioned at 5 μm thickness, and sections were stained with hematoxylin and eosin. Sections were viewed in a blinded fashion and assessed for various lesions of disease, including edema, neutrophil- and/or mononuclear-cell-infiltration, tendon sheath thickening, and reactive and reparative responses. Each lesion was issued a score ranging from 0 to 5, with 5 representing the most severe lesion and 0 indicating normal tissue. Scores for individual lesions were incorporated into the overall lesion scores reported for this study.
DNA preparation.
The contralateral rear ankle joint, the entire heart, and ear tissues were harvested from experimental animals sacrificed at 4 weeks postinfection, and DNA was prepared as previously described (37). Briefly, individual tissue specimens were incubated in 0.1% collagenase at 37°C overnight before addition of an equal volume of 0.2-mg/ml proteinase K (Sigma). After an overnight incubation at 55°C, DNA was recovered by phenol-chloroform extraction and ethanol precipitation. Following digestion with DNase-free RNase (Sigma) at 1 mg/ml, samples were again extracted and DNA was recovered by precipitation. This precipitate was resuspended in 1.5 ml of water, and the DNA content was determined by measuring the absorbance at 260 nm.
Quantification of B. burgdorferi by continuous monitoring of PCR.
PCR analyses were performed in a fluorescence temperature cycler (LightCycler LC24; Idaho Technology, Idaho Falls, Idaho) as previously described (44). Briefly, amplification was performed on 200 ng of sample DNA in a 10-μl final volume containing 50 mM Tris (pH 8.3), 3 mM MgCl2, 4.5 μg of bovine serum albumin, 200 μM deoxynucleoside triphosphates, a 1:30,000 dilution of SYBR Green I (Molecular Probes, Eugene, Oreg.), 5 μM each primer, 0.5 U of Taq polymerase (GIBCO BRL, Gaithersburg, Md.), and 110 ng of TaqStart antibody (ClonTech, Palo Alto, Calif.). Amplification was performed at 40 cycles, with each cycle consisting of heating at 20°C/s to 95°C with a 1-s hold, cooling at 20°C/s to 60°C with a 1-s hold, and heating at 1°C/s to 84°C. This technique continuously monitors the cycle-by-cycle accumulation of fluorescently labeled product. The cycle at which the product is first detected is used as an indicator of the relative starting copy numbers present in the sample. Copy numbers for mouse nidogen gene and B. burgdorferi recA were calculated by using the LightCycler software, and recA values were corrected by normalization based on the nidogen gene copy number. The oligonucleotide primers used to detect mouse nidogen were nido.F (5′-CCA GCC ACA GAA TAC CAT CC-3′) and nido.R (5′-GGA CAT ACT CTG CTG CCA TC-3′). The oligonucleotide primers used to detect B. burgdorferi recA were nTM17.F (5′-GTG GAT CTA TTG TAT TAG ATG AGG CTC TCG-3′) and nTM17.R (5′-GCC AAA GTT CTG CAA CAT TAA CAC CTA AAG-3′).
Immunoglobulin quantification.
Serum obtained by retro-orbital bleeding of experimental animals was assayed by ELISA to determine the total Ig as well as the B. burgdorferi-specific Ig content. Microtiter plates were coated with either sonicated B. burgdorferi or goat antibodies to mouse IgG, IgM, and IgA. Serum dilutions were added to plates for 90 min at 37°C, and bound murine Ig was detected by addition of HRP-conjugated antibodies to murine IgG or IgM. Ig content was determined by comparison to standard curves constructed by using purified IgG or IgM. B. burgdorferi-specific Ig subclasses were assessed by determining the titers of serum samples on plates coated with sonicated B. burgdorferi, using isotype-specific HRP-conjugated secondary antibodies.
Statistical analysis.
The degrees of statistical significance of the quantitative differences between sample groups were determined by application of Student’s t test.
RESULTS
Differential production of inflammatory mediators by macrophages from C3H/HeN and C57BL/6N mice.
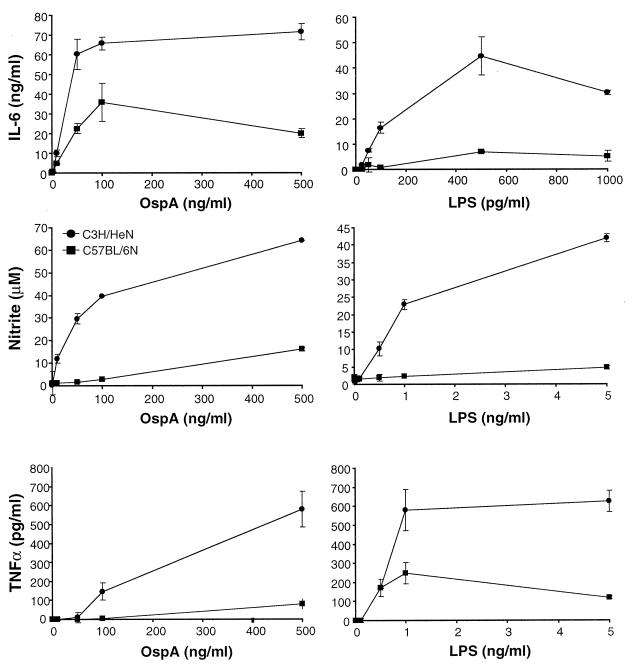
Previous studies have shown that C3H (both C3H/HeN and C3H/HeJ) and C57BL/6N mice exhibit different degrees of arthritis severity when infected with equivalent doses of B. burgdorferi and yet their affected tissues contain similar numbers of spirochetes (37). This finding suggests that the differences in arthritis severity between the two mouse strains are related to differences in the intensity of the inflammatory response to B. burgdorferi and its lipoproteins. To address this issue, bone marrow macrophages from C3H/HeN and C57BL/6N mice were cultured in the presence of the B. burgdorferi lipoprotein OspA and supernatants were assayed for the presence of representative inflammatory mediators. Macrophages from C3H/HeN mice were found to produce substantial amounts of IL-6 in response to OspA, with maximum production elicited in response to 50 to 500 ng of lipoprotein/ml (Fig. 1). Macrophages from C57BL/6N mice secreted significantly less IL-6 in response to OspA than macrophages from C3H/HeN mice. Similar differences in IL-6 production were seen with macrophages cultured in the presence of LPS, indicating that this distinction is not lipoprotein specific.
FIG. 1.
Production of inflammatory mediators by macrophages in response to OspA. Bone marrow-derived macrophages from C3H/HeN (circles) and C57BL/6N (squares) mice were stimulated with the indicated doses of OspA or LPS. Supernatants were assayed by ELISA (TNF-α and IL-6) or Griess assay (nitric oxide) at 6 h (TNF-α) or 24 h (nitric oxide and IL-6) after stimulation. Results for nitric oxide reflect supernatants in which agonists were added in the presence of 2 U of gamma interferon/ml. Data points represent duplicate samples, and results are representative of six experiments.
Other inflammatory mediators were also produced at higher levels by macrophages from C3H/HeN mice than by macrophages from C57BL/6N mice. C3H/HeN macrophages produced 2- to 10-fold-higher levels of nitric oxide and TNF-α than C57BL/6N mice (Fig. 1). This bias was not specific for lipoproteins, since both nitric oxide and TNF-α levels elicited in response to LPS were also higher in macrophage cultures from C3H/HeN mice than in those from C57BL/6N mice. This disparity was not related to differences in sensitivity to the agonist concentration, since the two macrophage populations responded to similar concentrations of OspA and LPS. Additionally, these differences were not due to production of higher baseline levels of inflammatory mediators by C3H/HeN macrophages, since the two cell types secrete similar quantities of mediators in the absence of an agonist.
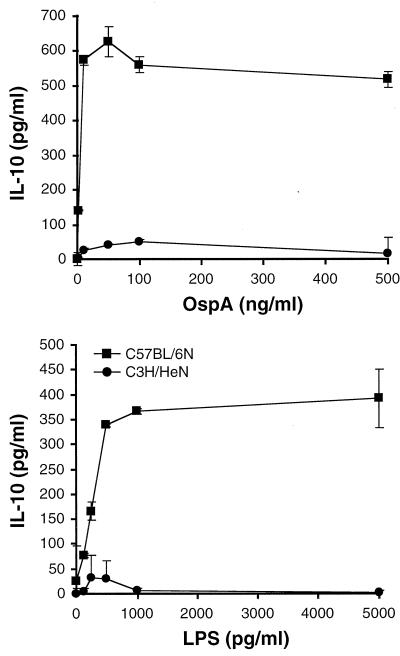
Diminished levels or the absence of anti-inflammatory cytokines such as IL-10 has been linked to increased severity of inflammatory disease (32, 36). Analysis of culture supernatants indicated that macrophages from C57BL/6N mice produced up to 10-fold-higher amounts of IL-10 than did macrophages from C3H/HeN mice (Fig. 2); this finding is the opposite of the trend seen with secretion of proinflammatory mediators. These differences were also not lipoprotein specific, since IL-10 levels produced in response to LPS were much higher in macrophage culture supernatants from C57BL/6N mice than in those from C3H/HeN mice. Collectively, the C57BL/6N macrophage cultures that produced small quantities of inflammatory mediators did produce high levels of IL-10 (Fig. 1 and 2 and data not shown), demonstrating that the diminished production of inflammatory mediators was not due to decreased fitness of the C57BL/6N cultures. These data indicate that in response to the B. burgdorferi lipoprotein OspA, macrophages from C3H/HeN mice produce substantially higher levels of inflammatory mediators and smaller amounts of an anti-inflammatory cytokine than macrophages from C57BL/6N mice.
FIG. 2.
Production of IL-10 by macrophages in response to OspA. Bone marrow-derived macrophages from C3H/HeN and C57BL/6N mice were stimulated with the indicated doses of OspA or LPS. Supernatants were assayed by ELISA at 24 h after stimulation. Data points represent duplicate samples, and results are representative of six experiments.
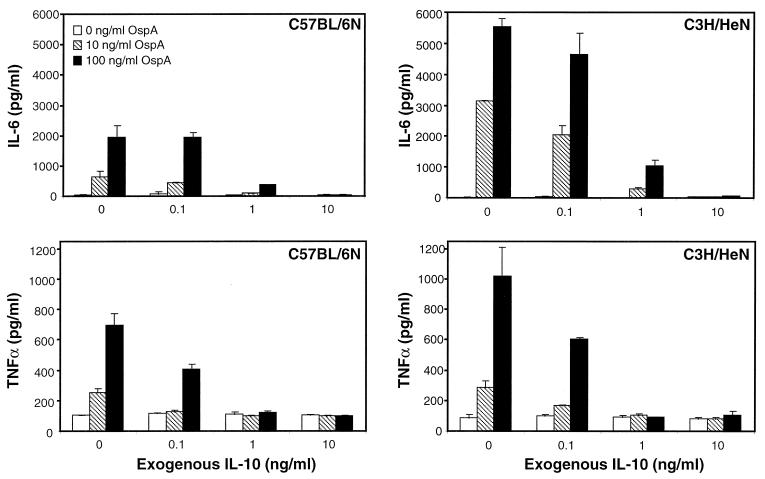
Exogenous IL-10 reduces the levels of inflammatory mediators produced in response to OspA.
Previous studies have shown that IL-10 can downregulate a wide range of inflammatory mediators produced by macrophages in response to LPS (43). To determine if IL-10 can also suppress the levels of inflammatory mediators produced in response to B. burgdorferi lipoproteins, macrophages from both C3H/HeN and C57BL/6N mice were cultured in the presence of OspA and increasing amounts of rIL-10. In the absence of exogenous IL-10, macrophages from C3H/HeN mice produced two- to threefold more IL-6 than did macrophages from C57BL/6N mice (Fig. 1 and 3). The addition of rIL-10 resulted in a >90% decrease in IL-6 production by macrophages from either mouse strain, such that little IL-6 was detectable in the presence of 1 to 5 ng of rIL-10/ml. The high concentrations of TNF-α produced by C3H/HeN macrophages in response to OspA were also reduced to levels similar to those produced by C57BL/6N macrophages in the presence of exogenous IL-10 (Fig. 3). More than 90% of cells from all cultures excluded trypan blue, and mRNA could be recovered (data not shown), suggesting that these decreases were not due to killing of macrophages by the added exogenous IL-10. It was interesting that the addition of low levels of rIL-10 (≤1 ng/ml) caused the production of IL-6 and TNF-α by macrophages from C3H/HeN mice to drop to levels similar to those of C57BL/6N macrophages (Fig. 3).
FIG. 3.
Effects of exogenous IL-10 on production of inflammatory mediators in response to OspA. Bone marrow-derived macrophages from C3H/HeN and C57BL/6N mice were stimulated with the indicated doses of OspA in the presence of different concentrations of exogenous IL-10. Supernatants were assayed by ELISA at 6 h (TNF-α) or 24 h (IL-6) after stimulation. Data points represent duplicate samples, and results are representative of four experiments.
IL-10-deficient C57BL/6J mice have more B. burgdorferi-induced lesions than wild-type C57BL/6J mice.
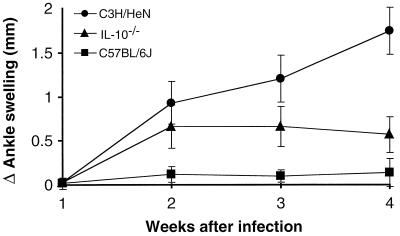
The previous findings suggested that IL-10 production by C57BL/6N mice might play a role in controlling B. burgdorferi-induced inflammation in vivo. To test this hypothesis, IL-10−/− mice were infected with B. burgdorferi and evaluated for disease severity. The IL-10−/− disruption is in a C57BL/6J substrain background, as opposed to the C57BL/6N mice that were used in the previous in vitro studies (Fig. 1 to 3). When these in vitro experiments were repeated with macrophages from both C57BL/6 substrains, similar results were generated (data not shown). We have observed a higher degree of variability in arthritis severity in the C57BL/6J substrain mice, ranging from mild or moderate to severe in a small percentage of animals. The greater severity occurs more frequently in male mice than in females (unpublished observation); therefore, female mice were used in this experiment. Bone marrow-derived macrophages from IL-10−/− mice produced concentrations of pro- and anti-inflammatory mediators similar to those of wild-type C57BL/6J macrophages in response to OspA or LPS in vitro (data not shown). Infected wild-type C57BL/6J displayed mostly mild to moderate arthritis, with one animal displaying severe arthritis (see Fig. 5). Infection was confirmed by culturing the tissue from an ear from each animal at the time of sacrifice; all ear cultures of infected animals produced viable B. burgdorferi after 2 weeks in culture (data not shown). Disease progression was monitored by weekly measurement of rear ankle joints. C3H/HeN mice showed greatly increased ankle swelling within 2 weeks and maximum swelling at 3 to 4 weeks postinfection (Fig. 4). C57BL/6J mice displayed little ankle swelling after infection with B. burgdorferi, consistent with previous reports (37). IL-10−/− C57BL/6J mice showed greater ankle swelling as early as 2 weeks postinfection and maximum swelling at 3 to 4 weeks. Ankle swelling for the infected IL-10−/− mice was not as pronounced as that in C3H/HeN mice but was significantly greater than that of the wild-type C57BL/6J mice (P < 0.01). These findings indicate that in the absence of IL-10, B. burgdorferi-infected C57BL/6J mice display increased ankle swelling.
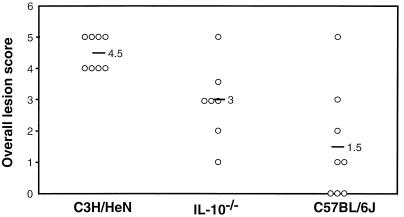
FIG. 5.
Lesion scores of joints from mice infected with B. burgdorferi. The indicated mouse strains were infected by intradermal injection of 2,000 B. burgdorferi organisms, and rear ankle joints were assessed for lesions. Each open circle represents the overall lesion score for an individual animal, and each black bar indicates the average score for the eight mice in a group. Uninfected controls exhibited normal histology (data not shown). These results are representative of two separate experiments.
FIG. 4.
Ankle swelling in different mouse strains infected with B. burgdorferi. The indicated mouse strains were infected by intradermal injection of 2,000 B. burgdorferi organisms, and ankles were measured weekly as described in Materials and Methods. Data points represent the averages and standard deviations of values for eight infected animals, and results are representative of two separate experiments. Mock-infected animals showed no increase in ankle swelling at any time point (data not shown).
To further assess the effect of IL-10 on arthritis development, one ankle joint from each animal was sectioned and evaluated histopathologically. When the overall lesion scores of the different mouse strains were compared, infected C3H/HeN mice were found to have significantly more lesions than infected C57BL/6J mice (Fig. 5), similar to previous findings with the C57BL/6N substrain (37). Again, the arthritis phenotype in the C57BL/6J substrain was more variable than previously identified for the C57BL/6N substrain (37). Lesion scores in infected IL-10−/− mice were also significantly lower than those of C3H/HeN ankles (P < 0.01) but were substantially higher than those of wild-type C57BL/6J mice (P = 0.08). When the individual traits that make up the overall lesion scores were compared, no single lesion trait appeared to be linked to the absence of IL-10, but all were somewhat increased. These findings indicate that genetic ablation of IL-10 results in more overall inflammatory lesions; however, the absence of IL-10 alone is not enough to produce arthritis of severity equivalent to that exhibited by infected C3H/HeN mice.
IL-10 deficiency results in decreased numbers of B. burgdorferi in tissues.
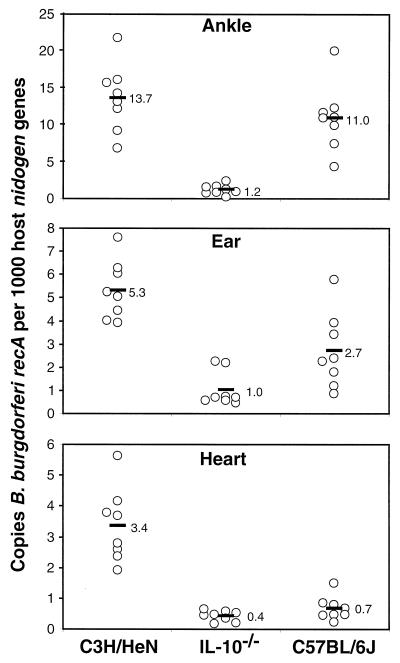
It has been previously shown that C3H/HeN and C57BL/6N mice exhibit very different levels of arthritis severity when infected with B. burgdorferi yet have quite similar numbers of bacteria in their joint tissues (37). To determine if IL-10 deficiency has any effect on bacterial persistence, DNA from various infected mouse tissues was purified and the numbers of B. burgdorferi organisms present were determined by continuous monitoring of quantitative PCR (46). This technique has been previously shown to allow comparison of various tissues and mouse strains and is based on normalization to the single-copy-number mouse nidogen gene. Ankle tissues from C3H/HeN mice taken 4 weeks postinfection were found to contain an average of 14 B. burgdorferi recA molecules per 1,000 mouse nidogen gene copies (Fig. 6). These numbers were similar to those found in ankles of infected C57BL/6J mice and confirm the trend observed in previous studies using C57BL/6N mice (37). Interestingly, when ankle tissues from infected IL-10−/− mice were examined, every animal contained small numbers of B. burgdorferi organisms, and the average spirochete numbers were 10-fold lower than that of the wild-type C57BL/6J mice. Also, there was much less variance in B. burgdorferi numbers among infected IL-10−/− mice, with the highest values still being lower than the lowest values of the other mouse strains. When ear tissues were assessed for spirochete numbers, infected C3H ears contained twofold more B. burgdorferi organisms than did ears from C57BL/6J mice (P ≤ 0.05) (Fig. 6). Infected IL-10−/− mouse ear tissues showed a further threefold decrease in B. burgdorferi numbers from that of their wild-type partners (P ≤ 0.05), although the ranges were overlapping. Hearts from infected C3H/HeN mice contained fivefold more B. burgdorferi than did hearts from C57BL/6J (Fig. 6), also consistent with results of previous studies using C57BL/6N mice (37). There was little further decrease in spirochete numbers in IL-10−/− mouse hearts from the already-low levels in wild-type C57BL/6J mice. All tissues isolated from uninfected controls of all three mouse strains contained no detectable B. burgdorferi DNA (data not shown). These findings document that in the absence of IL-10, infected animals are better able to control spirochete numbers in ankle and ear tissues.
FIG. 6.
Detection of B. burgdorferi DNA in tissues from infected mice. Mice were infected by intradermal injection of 2,000 B. burgdorferi organisms, and the indicated tissues were harvested 4 weeks postinfection. Tissues were assessed for B. burgdorferi-specific DNA by continuously monitored PCR of the B. burgdorferi recA gene, and results were normalized to those of the host nidogen gene. Each data point represents the B. burgdorferi-specific DNA content of an individual infected tissue relative to nidogen, and each black bar indicates the average score for the eight mice in a group. Uninfected controls contained no B. burgdorferi-specific DNA (data not shown). These results are representative of two separate experiments.
Absence of IL-10 results in increased antibody production.
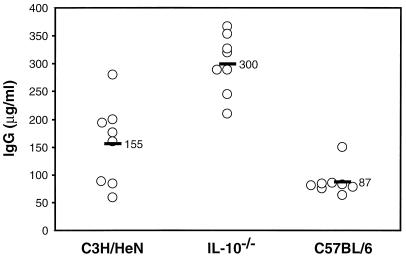
One mechanism by which IL-10−/− animals might better control bacterial infection would be through increased production of B. burgdorferi-specific antibodies. When B. burgdorferi-specific antibodies from the serum of infected mice were assessed, C3H/HeN mice were found to possess twofold more antibodies than C57BL/6J mice (Fig. 7). Serum from infected IL-10−/− mice contained twofold more specific antibody than C3H/HeN mice and fourfold more than the wild-type mice. Comparison of total IgG levels of the three mouse strains revealed that IL-10−/− mice produced larger amounts of antibody than C3H/HeN or C57BL/6J mice (data not shown). Therefore, the increased antibody levels seen in the sera of IL-10−/− mice appear to be a general trend that is consistent with known effects of IL-10 (34). B. burgdorferi-specific IgG1 levels were substantially higher in serum from infected C3H/HeN mice than in that from C57BL/6J mice (Table 1). Serum from infected IL-10−/− mice contained larger amounts of B. burgdorferi-specific IgG1 than did that from wild-type C57BL/6J, with titers approaching those present in C3H/HeN serum. B. burgdorferi-specific IgG2b and IgG3 levels in serum from infected C3H/HeN mice were also higher than or similar to those in C57BL/6J serum. Serum from infected IL-10−/− mice contained larger amounts of B. burgdorferi-specific IgG3 and, especially, IgG2b than serum from the other mouse strains. (We were unable to assess IgG2a levels in IL-10−/− or wild-type C57BL/6 mice because they lack the gene for IgG2a and instead express the novel IgG2c isotype [30, 41, 42]. Commercially available anti-IgG2a sera do not consistently cross-react with IgG2c, and reagents specific for IgG2c are unavailable at this time.) These results indicate that infected animals produce larger quantities of B. burgdorferi-specific Ig in the absence of IL-10 and that this increase is not isotype specific.
FIG. 7.
Detection of B. burgdorferi-specific Ig from infected mice. Serum was collected from the indicated mouse strains at 4 weeks after infection with 2,000 B. burgdorferi spirochetes. B. burgdorferi-specific Ig levels were determined by ELISA. Each data point represents the serum Ig content of an individual animal, and the black bars indicate the average scores. Uninfected controls contained no detectable B. burgdorferi-specific Ig (data not shown). These results are representative of two separate experiments.
TABLE 1.
Titers of different B. burgdorferi-specific IgG subclasses in sera from infected micea
| Mouse strain | IgG subclass titer
|
||
|---|---|---|---|
| IgG1 | IgG2b | IgG3 | |
| C3H/HeN | 3,200 | 6,400 | 3,200 |
| IL-10−/− | 3,200 | 25,600 | 6,400 |
| C57BL/6J | 800 | 3,200 | 3,200 |
The indicated mouse strains were infected as described in Materials and Methods. Serum was collected 4 weeks postinfection, and B. burgdorferi-specific IgG subclasses were assessed by ELISA. Numbers represent the inverse values for the lowest serum dilutions that produced an optical density of ≥0.1 and reflect the average values for eight infected animals. Values for serum from mock-infected animals at a 1:100 dilution were ≤0.1 (data not shown). These results are representative of two separate experiments.
DISCUSSION
The murine model of Lyme disease has proven beneficial in the elucidation of host factors responsible for the development of Lyme arthritis (58). Such studies have indicated that susceptibility to severe subacute arthritis is not linked to genes involved in mediating the acquired immune responses but is more likely due to differences in the inflammatory responses of these strains (1, 6, 31, 68). B. burgdorferi membrane lipoproteins directly activate a number of inflammatory cell types, and differential induction of inflammatory and anti-inflammatory molecules could modulate inflammatory arthritis. In this study, we found that the anti-inflammatory cytokine IL-10 has a major influence on the degree of inflammation and severity of arthritis in B. burgdorferi-infected mice. This finding was documented both by an increase in ankle swelling following infection with B. burgdorferi in mice deficient in IL-10 and histopathological scoring of lesions for ankle joints taken from mice 4 weeks postinfection. Both measures of injury strongly indicated that genetic ablation of the IL-10 gene in an arthritis-resistant mouse strain resulted in an increase in the severity of arthritis. This observation suggests that IL-10-dependent regulation of the inflammatory response to B. burgdorferi defines a major pathway controlling arthritis severity in C57BL/6 mice. The fact that arthritis was not as severe in the IL-10−/− C57BL/6 mouse as in the C3H/HeN mouse indicates that additional pathways may also be functioning to modify inflammation in C57BL/6 mice.
While inflammation is an essential component of host defenses, an excessive inflammatory response can lead to detrimental outcomes such as arthritis and septic shock. Macrophage-derived proinflammatory cytokines have been detected in joints of patients with rheumatoid arthritis (10, 20), and the presence of such mediators has been shown to directly influence much of the tissue damage which occurs in arthritis (26, 33, 54, 69). IL-10, which is secreted by macrophages and other immune cells, serves as an important negative regulator of inflammatory responses (43). IL-10 has been shown to downregulate many inflammatory mediators produced by macrophages both in vitro and in vivo (7, 8, 16, 19), apparently by downregulating NF-κB activation (35). Subsequently, it has been shown that IL-10 concentrations in the ankle joints can alter the course of arthritic disease. Addition of exogenous IL-10 to synovial fluid mononuclear cells from patients with rheumatoid arthritis was shown to inhibit production of inflammatory cytokines in vitro, while addition of IL-10-neutralizing antibody caused a significant increase in inflammatory cytokine levels (27). Intraperitoneal injection of rIL-10 (29, 66) and adenovirus-mediated transfer of an Epstein-Barr virus-encoded IL-10 gene (2, 39) were shown to reduce disease severity in a murine model of collagen-induced arthritis. Taken together, these data indicate that increased IL-10 levels in tissues affected by different types of arthritis could suppress inflammatory responses and subsequently decrease disease severity.
Outer membrane lipoproteins produced by B. burgdorferi have been shown to potently activate macrophages (38, 40, 55, 56, 63), endothelial cells (13, 18, 59, 61, 70), neutrophils (45), and B cells (40, 63) to produce many of the same proinflammatory mediators that have been implicated in different models of arthritis. Although the expression of different lipoproteins by B. burgdorferi is regulated by the environment inhabited by this organism (15), OspA has served as the prototypic lipoprotein for most in vitro studies. The B. burgdorferi genome encodes over 100 proteins containing the signal peptidase II consensus sequence for Pam3Cys modification that is responsible for the stimulatory properties of lipoproteins (22). Therefore, it is possible that an excessive inflammatory response to these outer membrane lipoproteins is related to more-severe arthritis. We found that a higher ratio of inflammatory to anti-inflammatory products was induced by OspA in macrophages from C3H/HeN mice and that the opposite was true for macrophages from C57BL/6 mice. Recently, Giambartolomei et al. have shown that heat-killed B. burgdorferi and OspA can stimulate IL-10 production in peripheral blood mononuclear cells from humans and rhesus monkeys in vitro (24). The main source of IL-10 in B. burgdorferi-stimulated human peripheral blood mononuclear cells was subsequently identified as the monocyte (23). Furthermore, we have shown that addition of physiologic amounts of IL-10 to macrophage cultures suppresses the production of OspA-induced TNF, nitric oxide, and IL-6 (Fig. 3). These results suggest the following mechanism for the regulatory influence of IL-10 on arthritis development in B. burgdorferi-infected mice: IL-10 serves to depress the intensity of the inflammatory response to B. burgdorferi lipoproteins that are expressed in vivo. This process results in less-intense inflammatory responses in C57BL/6 mice, since their cells appear to innately produce more IL-10 than those of C3H/HeN mice. The removal of IL-10 from C57BL/6J mice by genetic ablation interrupts the regulation of inflammation and results in an increase in arthritis severity.
A striking finding from this study was that infected IL-10−/− mice, which displayed larger ankle lesions, contained 10- and 3-fold-fewer spirochetes in ankle and ear tissues, respectively than infected C57BL/6J mice. This was surprising, since previous studies of arthritis-resistant BALB/c mice demonstrated that arthritis resistance could be overcome by a high inoculum dose and that this dose was associated with an increase in the number of spirochetes in ankle tissues (37). Similarly, Pennington et al. showed that a serotype of Borrelia turicatae that caused severe arthritis was present at sevenfold-higher levels in affected tissues than a serotype that caused less-severe disease (53). In this study, the increase in arthritis severity in IL-10−/− mice was correlated with decreased numbers of B. burgdorferi spirochetes in ankle tissues. These results indicate that IL-10-dependent regulation of the inflammatory response coordinately reduces the effectiveness of the host defense against B. burgdorferi. Whether the reduction in B. burgdorferi numbers is mediated at the level of inhibiting spirochete dissemination to affected tissues or increased clearance of the spirochetes after they reach these tissues has yet to be determined. Therefore, understanding the mechanism of action of IL-10 in this infectious process will provide insight into the normal host defense against B. burgdorferi.
There have been several reported cases in which IL-10 has been shown to reduce the antimicrobial activities of immune cells. Studies of infected IL-10−/− mice or mice in which IL-10 has been neutralized with antibodies have generally indicated that the elimination of pathogens is more effective when IL-10 is absent (14, 21, 47, 65, 75). In some cases, the effect of IL-10 has been at the level of CD4+-T-cell responses, with IL-10 modulating the type of help for antibody production. In this study, IL-10−/− mice produced higher levels of B. burgdorferi-specific IgG1, IgG2b, and IgG3 than wild-type mice at 4 weeks postinfection. However, increases in the levels of these Ig isotypes have not been directly linked to decreased bacterial numbers. Additionally, B. burgdorferi numbers were already reduced at 2 weeks postinfection, a time at which no obvious differences in B. burgdorferi-specific IgG or IgM levels were seen between mouse strains (data not shown). It has increasingly been recognized that a major target of IL-10 is the phagocytic cell and that suppression leads to an inhibition of the innate ability of this cell type to combat microbial infections (14, 21, 47, 65, 75). We hypothesize that the activity of neutrophils, monocytes, and/or other cell types involved in innate defenses may be suppressed by IL-10 in C57BL/6 mice and that in the absence of this cytokine there is more-efficient killing of the bacteria. Burns and Furie have shown that IL-10 treatment of endothelial monolayers causes a decrease in migration of monocytes in response to B. burgdorferi (12), suggesting that the anti-inflammatory effects of IL-10 may be mediated through the recruitment of inflammatory cells.
As previously mentioned, while the severity of arthritis in IL-10−/− mice was greater than that in wild-type C57BL/6J mice, it was not as great as that observed for the C3H/HeN mice. This finding suggests that genes within other regulatory pathways also contribute to arthritis severity. This hypothesis is consistent with published results from our mapping study, in which at least three chromosomal regions that regulate arthritis severity in C3H/HeN mice were identified (68). Since the IL-10 structural gene and its receptor map to regions distinct from those identified in our quantitative trait loci analysis, we expect that the arthritis-regulatory genes within these loci are involved in other pathways that regulate disease severity and that these other pathways are involved in the disease phenotype polymorphism seen in C3H/HeN and C57BL/6 mice.
In summary, increased production of IL-10 by C57BL/6 mice appears to be related to decreased arthritis severity. The anti-inflammatory effect of IL-10 appears to allow C57BL/6 mice to minimize inflammation produced in response to B. burgdorferi lipoproteins in infected joint tissues. However, this decreased inflammation does have a cost to the animal, since the numbers of persisting spirochetes in these tissues are large. This expense to the animal is documented by the fact that an IL-10-deficient mouse harbors significantly fewer spirochetes 4 weeks postinfection than does a wild-type C57BL/6 mouse. Therefore, it appears that C57BL/6 mice have established a balance in their innate responses to B. burgdorferi: the magnitude of inflammation in joints is regulated at the expense of persistence of large numbers of spirochetes in joint tissues.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI-32223 (J.J.W) and AR-43521 (J.J.W and C.T.), an Arthritis Foundation postdoctoral fellowship (R.M.W.), and grant 5P30-CA-42014 from the University of Utah.
We thank Robert Huebner for recombinant OspA and Kathy Seiler, Tom Morrison, and John Weis for guidance.
REFERENCES
- 1.Anguita J, Samanta S, Barthold S W, Fikrig E. Ablation of interleukin-12 exacerbates Lyme arthritis in SCID mice. Infect Immun. 1997;65:4334–4336. doi: 10.1128/iai.65.10.4334-4336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160:5213–5220. [PubMed] [Google Scholar]
- 3.Barthold S W. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991;163:419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- 4.Barthold S W. Lyme borreliosis in the laboratory mouse. J Spirochetal Tick-Borne Dis. 1996;3:22–44. [Google Scholar]
- 5.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 7.Bessis N, Chiocchia G, Kollias G, Minty A, Fournier C, Fradelizi D, Boissier M C. Modulation of proinflammatory cytokine production in tumour necrosis factor-alpha (TNF-α)-transgenic mice by treatment with cells engineered to secrete IL-4, IL-10 or IL-13. Clin Exp Immunol. 1998;111:391–396. doi: 10.1046/j.1365-2249.1998.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown C R, Reiner S L. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect Immun. 1999;67:1967–1973. doi: 10.1128/iai.67.4.1967-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchan G, Barrett K, Turner M, Chantry D, Maini R N, Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988;73:449–455. [PMC free article] [PubMed] [Google Scholar]
- 11.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease: a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 12.Burns M J, Furie M B. Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms. Infect Immun. 1998;66:4875–4883. doi: 10.1128/iai.66.10.4875-4883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns M J, Sellati T J, Teng E I, Furie M B. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secretion IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect Immun. 1997;65:1217–1222. doi: 10.1128/iai.65.4.1217-1222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai W J, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 15.de Silva A M, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Investig. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 18.Ebnet K, Brown K D, Siebenlist U K, Simon M M, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–3292. [PubMed] [Google Scholar]
- 19.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 20.Firestein G S, Alvaro-Gracia J M, Maki R, Alvaro-Garcia J M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- 21.Flesch I E, Hess J H, Oswald I P, Kaufmann S H. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 22.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 23.Giambartolomei G H, Dennis V A, Lasater B L, Philipp M T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giambartolomei G H, Dennis V A, Philipp M T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect Immun. 1998;66:2691–2697. doi: 10.1128/iai.66.6.2691-2697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondolf K B, Mihatsch M, Curschellas E, Dunn J J, Batsford S R. Induction of experimental allergic arthritis with outer surface proteins of Borrelia burgdorferi. Arthritis Rheum. 1994;37:1070–1077. doi: 10.1002/art.1780370713. [DOI] [PubMed] [Google Scholar]
- 26.Hom J T, Bendele A M, Carlson D G. In vivo administration with IL-1 accelerates the development of collagen-induced arthritis in mice. J Immunol. 1988;141:834–841. [PubMed] [Google Scholar]
- 27.Isomaki P, Luukkainen R, Saario R, Toivanen P, Punnonen J. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum. 1996;39:386–395. doi: 10.1002/art.1780390306. [DOI] [PubMed] [Google Scholar]
- 28.Johnson R C, Schmid G P, Hyde F W, Steigwalt A G, Brenner D J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme Disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 29.Joosten L A, Lubberts E, Durez P, Helsen M M, Jacobs M J, Goldman M, van den Berg W B. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 30.Jouvin-Marche E, Morgado M G, Leguern C, Voegtle D, Bonhomme F, Cazenave P A. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29:92–97. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 31.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasama T, Strieter R M, Lukacs N W, Lincoln P M, Burdick M D, Kunkel S L. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Investig. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 35.Lentsch A B, Shanley T P, Sarma V, Ward P A. In vivo suppression of NF-κB and preservation of IκBα by interleukin-10 and interleukin-13. J Clin Investig. 1997;100:2443–2448. doi: 10.1172/JCI119786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubberts E, Joosten L A, Helsen M M, van den Berg W B. Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine. 1998;10:361–369. doi: 10.1006/cyto.1997.0298. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y, Seiler K P, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y, Seiler K P, Tai K-F, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Thornton S, Duwel L E, Boivin G P, Giannini E H, Leiden J M, Bluestone J A, Hirsch R. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J Immunol. 1998;161:1516–1524. [PubMed] [Google Scholar]
- 40.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin R M, Lew A M. Is IgG2a a good Th1 marker in mice? Immunol Today. 1998;19:49. doi: 10.1016/s0167-5699(97)87499-3. [DOI] [PubMed] [Google Scholar]
- 42.Martin R M, Silva A, Lew A M. The Igh-1 sequence of the non-obese diabetic (NOD) mouse assigns it to the IgG2c isotype. Immunogenetics. 1997;46:167–168. doi: 10.1007/s002510050258. [DOI] [PubMed] [Google Scholar]
- 43.Moore K W, O’Garra A, de Waal Malefyt R, Viera P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 44.Morrison T B, Ma Y, Weis J H, Weis J J. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–992. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison T B, Weis J H, Weis J J. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J Immunol. 1997;158:4838–4845. [PubMed] [Google Scholar]
- 46.Morrison T B, Weis J J, Wittwer C T. Quantification of low copy transcripts by continuous SYBR Green I monitoring during amplification. BioTechniques. 1998;24:954–962. [PubMed] [Google Scholar]
- 47.Murray P J, Wang L, Onufryk C, Tepper R I, Young R A. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 48.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 49.Nocton J J, Steere A C. Lyme disease. Adv Intern Med. 1995;40:69–117. [PubMed] [Google Scholar]
- 50.Norgard M V, Arndt L L, Akins D R, Curetty L L, Harrich D A, Radolf J D. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves transcriptional activator NF-κB. Infect Immun. 1996;64:3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norgard M V, Riley B S, Richardson J A, Radolf J D. Dermal inflammation elicited by synthetic analogs of Treponema pallidum and Borrelia burgdorferi lipoproteins. Infect Immun. 1995;63:1507–1515. doi: 10.1128/iai.63.4.1507-1515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pachner A R, Delaney E, Ricalton N S. Murine Lyme borreliosis: route of inoculation determines immune response and infectivity. Reg Immunol. 1992;4:345–351. [PubMed] [Google Scholar]
- 53.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettipher E R, Higgs G A, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci USA. 1986;83:8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 56.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis: analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 57.Schaible U E, Gay S, Museteanu C, Kramer M D, Zimmer G, Eichmann K, Museteanu U, Simon M M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 58.Seiler K P, Weis J J. Immunity to Lyme disease: protection, pathology and persistence. Curr Opin Immunol. 1996;8:503–509. doi: 10.1016/s0952-7915(96)80038-0. [DOI] [PubMed] [Google Scholar]
- 59.Sellati T J, Abrescia L D, Radolf J D, Furie M B. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–3187. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sellati T J, Bouis D A, Kitchens R L, Darveau R P, Pugin J, Ulevitch R J, Gangloff S C, Goyert S M, Norgard M V, Radolf J D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 61.Sellati T J, Burns M J, Ficazzola M A, Furie M B. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sigal L H. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 63.Tai K-F, Ma Y, Weis J J. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 65.Wagner R D, Maroushek N M, Brown J F, Czuprynski C J. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walmsley M, Katsikis P D, Abney E, Parry S, Williams R O, Maini R N, Feldmann M. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 67.Weis J J, Ma Y, Erdile L F. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect Immun. 1994;62:4632–4636. doi: 10.1128/iai.62.10.4632-4636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weis J J, McCracken B A, Ma Y, Fairbairn D, Roper R J, Morrison T B, Weis J H, Zachary J F, Doerge R W, Teuscher C. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–956. [PubMed] [Google Scholar]
- 69.Williams R O, Feldmann M, Maini R N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wooten R M, Modur V R, McIntyre T M, Weis J J. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 71.Wooten R M, Morrison T B, Weis J H, Wright S D, Thieringer R, Weis J J. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- 72.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 73.Yang L, Ma Y, Schoenfeld R, Griffiths M, Eichwald E, Araneo B, Weis J J. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect Immun. 1992;60:3033–3041. doi: 10.1128/iai.60.8.3033-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X, Hay-Glass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]