Abstract
Background
The primary aim of our study was to investigate the association between intubation timing and hospital mortality in critically ill patients with coronavirus disease 2019 (COVID-19)-associated respiratory failure. We also analysed both the impact of such timing throughout the first four pandemic waves and the influence of prior noninvasive respiratory support on outcomes.
Methods
This is a secondary analysis of a multicentre, observational and prospective cohort study that included all consecutive patients undergoing invasive mechanical ventilation due to COVID-19 from across 58 Spanish intensive care units (ICUs) participating in the CIBERESUCICOVID project. The study period was between 29 February 2020 and 31 August 2021. Early intubation was defined as that occurring within the first 24 h of ICU admission. Propensity score matching was used to achieve a balance across baseline variables between the early intubation cohort and those patients who were intubated after the first 24 h of ICU admission. Differences in outcomes between early and delayed intubation were also assessed. We performed sensitivity analyses to consider a different time-point (48 h from ICU admission) for early and delayed intubation.
Results
Of the 2725 patients who received invasive mechanical ventilation, a total of 614 matched patients were included in the analysis (307 for each group). In the unmatched population, there were no differences in mortality between the early and delayed groups. After propensity score matching, patients with delayed intubation presented higher hospital mortality (27.3% versus 37.1%; p=0.01), ICU mortality (25.7% versus 36.1%; p=0.007) and 90-day mortality (30.9% versus 40.2%; p=0.02) compared with the early intubation group. Very similar findings were observed when we used a 48-h time-point for early or delayed intubation. The use of early intubation decreased after the first wave of the pandemic (72%, 49%, 46% and 45% in the first, second, third and fourth waves, respectively; first versus second, third and fourth waves p<0.001). In both the main and sensitivity analyses, hospital mortality was lower in patients receiving high-flow nasal cannula (HFNC) (n=294) who were intubated earlier. The subgroup of patients undergoing noninvasive ventilation (n=214) before intubation showed higher mortality when delayed intubation was set as that occurring after 48 h from ICU admission, but not when after 24 h.
Conclusions
In patients with COVID-19 requiring invasive mechanical ventilation, delayed intubation was associated with a higher risk of hospital mortality. The use of early intubation significantly decreased throughout the course of the pandemic. Benefits of such an approach occurred more notably in patients who had received HFNC.
Short abstract
In patients who require intubation and invasive mechanical ventilation due to COVID-19-associated acute respiratory failure, delays in implementation may increase the risk of mortality https://bit.ly/3fZLCIP
Introduction
Debate has arisen relating to determining when to start invasive mechanical ventilation in critically ill patients with coronavirus disease 2019 (COVID-19) presenting with respiratory failure [1–3]. Clinicians who advocate treating such patients with noninvasive respiratory support (i.e. high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) and continuous positive airway pressure (CPAP)) argue that this strategy may avoid intubation and minimise the likelihood of well-known complications associated with invasive mechanical ventilation [2, 3]. On the other hand, increasing time on noninvasive support with spontaneous ventilation has been shown to possibly put patients at risk of self-inflicted lung injury and increase their chances of death if invasive mechanical ventilation is finally needed [4, 5]. In randomised clinical trials, NIV and CPAP have been shown to reduce intubation requirements in patients with COVID-19-associated acute respiratory failure [6, 7]. However, patients in whom such interventions failed presented a longer time elapsing since the start of noninvasive respiratory support to intubation [6]. It remains unknown whether this delay in invasive mechanical ventilation start worsens clinical outcomes. While there have been investigations exploring intubation timing and clinical outcomes, results have been conflicting. Some studies in which intubation was delayed have reported an association with worse outcomes [5, 8], whereas others have found no association [9].
The main objective of this study was to compare the risk of hospital mortality between patients intubated within the first 24 h of intensive care unit (ICU) admission (early intubation) and those intubated after that time frame (delayed intubation). As a secondary objective, we analysed changes in these practices and their impact throughout the initial four pandemic waves in Spain. Furthermore, we investigated whether the prior use of NIV or HFNC had any influence on mortality between the early and delayed intubation groups. Finally, we performed a sensitivity analysis considering a different time-point (intubation within or after 48 h from ICU admission) for early and delayed intubation.
Materials and methods
Study design
This is a secondary analysis of a multicentre, observational and prospective cohort study that included all consecutive patients undergoing invasive mechanical ventilation due to COVID-19 from across 58 Spanish ICUs participating in the CIBERESUCICOVID project (ClinicalTrials.gov: NCT04457505) (details of participating centres are provided in supplementary table S1). The study period was between 29 February 2020 and 31 August 2021. Two cohorts were established: early intubation and delayed intubation. The former was defined as those patients receiving the procedure within the first 24 h of ICU admission, while the latter was defined as those receiving the procedure after the first day of ICU admission. Clinical outcomes were compared between the early and delayed intubation groups. Moreover, we analysed the influence of the Spanish pandemic waves on the proportion of patients intubated within or after 24 h of ICU admission, determining whether these different periods had any impact on outcomes. We also performed a subgroup analysis based on the noninvasive respiratory support used prior to intubation and performed sensitivity analyses considering a 48-h time-point (intubation within or after 48 h from ICU admission) to explore other understandings of early or delayed intubation. Supplementary table S2 lists the periods that comprised each wave.
The study was approved by the Hospital Clínic de Barcelona's Internal Review Board (Comité Ètic d'Investigació Clínica; registry number HCB/2020/0370) and informed consent was obtained from either patients or their relatives. De-identified data were collected and stored in a Research Electronic Data Capture (REDCap) database. Trained local researchers incorporated data from patients’ medical records into a separate database. Prior to statistical analyses, three independent and experienced data collectors trained in critical care (A. Motos and two other collaborators) reviewed the data; in cases of query, site investigators were contacted. Missing analyses were performed, and site investigators were approached to obtain as much reliable and complete data as possible.
Study population and data collection
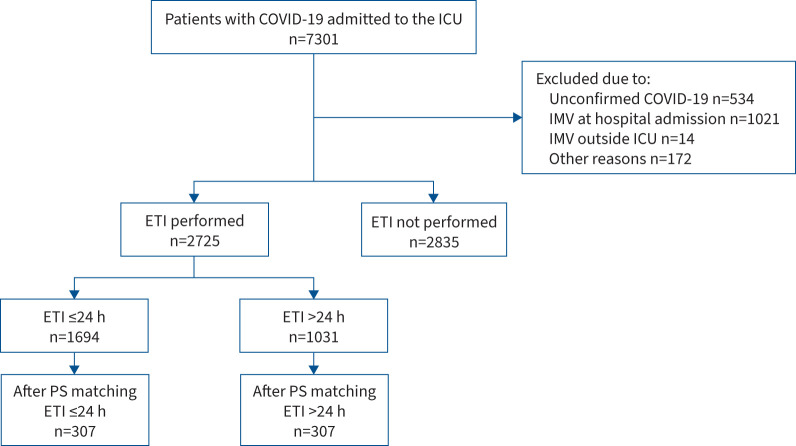
Inclusion criteria comprised the following characteristics: age ≥18 years, ICU admission and a confirmed diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by real-time reverse transcriptase quantitative PCR testing on nasopharyngeal swabs or lower respiratory tract aspirates. Exclusion criteria included: no requirement for orotracheal intubation, requirement for emergent intubation at hospital admission or outside of the ICU, no available data at either baseline or hospital discharge and ICU admission due to other reasons (figure 1).
FIGURE 1.
Study flowchart. Inclusion period was from 29 February 2020 to 31 August 2021. Propensity score (PS) matching was performed with the following variables: sex, age, respiratory rate at hospital admission, arterial oxygen tension/inspiratory oxygen fraction at hospital admission (categorised as >300, 200– ≤300, 100– ≤200 or ≤100 mmHg), time from hospital admission to intensive care unit (ICU) admission (categorised as ≤2 or >2 days), immunodepression, corticosteroid treatment and COVID-19 wave. ETI: endotracheal intubation; IMV: invasive mechanical ventilation.
After enrolment, prior epidemiological data regarding demographics, comorbidities, clinical symptoms and disease chronology were recorded. Site researchers subsequently collected data acquired at hospital and ICU admission. Follow-up was extended to death or hospital discharge. Data registered included vital signs, noninvasive respiratory support devices (i.e. conventional oxygen, HFNC and NIV), use of adjunctive therapies (i.e. prone position), laboratory findings and arterial blood gases. Furthermore, we collected data on pharmacological treatments administered at and during hospital or ICU admission until either ICU or hospital discharge, or death. Worst event values were preferentially recorded.
Objectives and outcomes
The primary aim of this study was to evaluate hospital mortality in relation to intubation timing implemented (i.e. early or delayed intubation). For secondary objectives, we analysed differences in ICU and 90-day mortality, duration of both ICU admission and invasive mechanical ventilation, and the need for rescue therapies. We also evaluated changes in intubation timing and their respective effects throughout the pandemic. Finally, we assessed both primary and secondary outcomes considering the type of noninvasive respiratory support used before intubation.
Statistical analysis
Number (percentage) of patients was reported for categorical variables, while median (interquartile range) was reported for continuous variables. Percentages were calculated excluding missing data. Categorical variables were compared using either the Chi-squared test or Fisher's exact test, whereas continuous variables were compared using the nonparametric Mann–Whitney U-test or parametric t-test.
Propensity score matching was used to achieve a balance between the early and delayed intubation groups [10, 11]. To match the two cohorts in both the 24- and 48-h time-point analyses, we used a 1:1 nearest-neighbour matching, without replacement and within a caliper width of 0.005 for the general population and 0.05 for subgroup analyses. The propensity score was determined (irrespective of outcome) using a multivariable logistic regression to predict the influence of several predetermined variables on early/delayed intubation. Variables were chosen for inclusion in propensity score calculations according to methods set forth by Brookhart et al. [12]. Criteria to include variables in this model were based on those that could affect the likelihood of outcome occurrence and study treatments to be received. When determining independent variables to predict the likelihood of intubation within the first 24 h of ICU admission or afterwards, we selected age, sex, chronic immunosuppression, respiratory rate at hospital admission, arterial oxygen tension (PaO2)/inspiratory oxygen fraction (FIO2) at hospital admission (categorised as >300, 200– ≤300, 100– ≤200 or ≤100 mmHg), time from hospital admission to ICU admission (≤2 or >2 days), treatment with corticosteroids for COVID-19 and COVID-19 wave. An adequate model fit with calibration of the propensity score was demonstrated by logistic modelling that included covariates (24-h time-point: goodness-of-fit, p=0.28 for general population, p=0.16 for HFNC and p=0.13 for NIV; 48-h time-point: goodness-of-fit, p=0.07 for general population, p=0.53 for HFNC and p=0.08 for NIV). Proper adjustment was assessed with standardised mean differences (SMDs) in the matched population, while covariate imbalance was defined with an SMD threshold >0.2 [13].
First, to evaluate the effect of timing of intubation on in-hospital mortality, a logistic regression model was used in the matched population; odds ratios and 95% confidence intervals were calculated. Then, to describe in-hospital mortality, we also employed a competing risks model [14], considering hospital discharge as a competing risk for mortality. Survival curves for patients with early and delayed intubation were obtained and compared using the cumulative incidence function and Gray's test, respectively [15]. Patients who were transferred to another hospital were censored in the survival analyses.
Finally, we performed exploratory subgroup analyses for the type of noninvasive respiratory support used before intubation and each pandemic wave in Spain.
The level of significance was set at 0.05 (two-tailed). All statistical analyses were performed with Python version 3.7 (www.python.org) and R version 4.0.3 (www.r-project.org).
Results
During the study period, 7301 patients required admission to participating ICUs. Of these, 2835 were not intubated; 1741 were excluded for other reasons (figure 1). The study, therefore, included a total of 2725 subjects, of whom 1694 received early intubation and 1031 received delayed intubation.
Characteristics of the population
Median (IQR) age was 64 (56–71) years and most patients were male (71.2%). More than half of the cohort (57.9%) was recruited during the first wave of the pandemic. The most frequent comorbidity was hypertension (52.6%). Chronic immunosuppression was present in 523 (19.1%) patients and most patients (86.5%) received corticosteroids at ICU admission for COVID-19. Despite this, patients exhibited a high inflammatory response and lymphopenia. At hospital admission, median (IQR) PaO2/FIO2 and respiratory rate were 219 (128–281) mmHg and 24 (20–30) breaths·min−1, respectively. At this time-point, 576 (21.6%) and 346 (12.8%) patients received support with HFNC and NIV, respectively. Table 1 describes the characteristics of the cohort according to intubation timing. In summary, those receiving early intubation were older and less chronically immunocompromised. They also presented a slightly lower body mass index. At hospital admission, PaO2/FIO2 of patients intubated early was slightly higher and respiratory rate mildly lower. Time since symptom onset to intubation was shorter in the early intubation group.
TABLE 1.
Characteristics and outcomes of critically ill patients with COVID-19 according to intubation timing from intensive care unit (ICU) admission: early (≤24 h) and delayed (>24 h)
| Before propensity score matching (n=2725) | After propensity score matching (n=614) | |||||
| Early intubation (n=1694) | Delayed intubation (n=1031) | p-value | Early intubation (n=307) | Delayed intubation (n=307) | p-value | |
| Age, years | 65 (57–72) | 63 (55–71) | <0.001 | 64 (56–71) | 64 (57–71) | 0.83 |
| Female | 499 (29.4) | 285 (27.6) | 0.31 | 100 (32.5) | 87 (28.3) | 0.29 |
| BMI, kg·m−2 | 28.4 (25.7–31.8) | 29.1 (26.2–32.3) | 0.01 | 29.4 (26.4–33.5) | 29.3 (26.1–32.3) | 0.19 |
| Hypertension | 885 (52.2) | 549 (53.2) | 0.63 | 165 (53.7) | 169 (54.2) | 0.80 |
| Diabetes mellitus | 416 (24.5) | 265 (25.7) | 0.52 | 78 (25.4) | 84 (27.3) | 0.64 |
| Chronic cardiac failure | 209 (12.3) | 133 (12.9) | 0.67 | 37 (12) | 45 (14.6) | 0.40 |
| COPD | 164 (9.4) | 98 (9.5) | 0.89 | 38 (12.3) | 35 (11.4) | 0.80 |
| Immunodepression | 286 (16.8) | 237 (22.9) | <0.001 | 53 (17.2) | 45 (14.6) | 0.44 |
| Received corticosteroids | 1425 (84.9) | 910 (89.2) | 0.002 | 281 (91.5) | 274 (89.2) | 0.41 |
| Clinical characteristics at hospital admission | ||||||
| PaO2/FIO2, mmHg | 230.1 (156.7–290.4) | 190.7 (98.6–267.7) | <0.001 | 214.2 (142.8–267.2) | 216.6 (114–275.7) | 0.97 |
| Respiratory rate, breaths·min−1 | 24 (20–29) | 25 (20–31) | <0.001 | 25 (20–31.5) | 25 (20–30) | 0.43 |
| PaCO2, mmHg | 33.2 (30–38) | 34 (30.3–38) | 0.18 | 34 (30–38) | 33.6 (29.9–37.7) | 0.46 |
| pH | 7.45 (7.41–7.47) | 7.45 (7.41–7.47) | 0.37 | 7.45 (7.42–7.48) | 7.45 (7.42–7.48) | 0.63 |
| HFNC#,+ | 836 (61.7) | 876 (91.1) | <0.001 | 178 (71.7) | 263 (91.9) | <0.001 |
| NIV¶,+ | 588 (35.8) | 375 (36.6) | 0.53 | 90 (29.7) | 127 (41.6) | 0.002 |
| CRP, mg·dL−1 | 12.5 (6.9–20.3) | 12.6 (6.9–21.4) | 0.31 | 13.5 (8–20.7) | 13.7 (7.9–20.8) | 0.66 |
| Lymphocytes, ×109 cells·L−1 | 0.8 (0.58–1.1) | 0.8 (0.58–1.09) | 0.73 | 0.8 (0.6–1.1) | 0.8 (0.6–1.06) | 0.49 |
| Platelets, ×109 cells·L−1 | 179 (143–231) | 186 (142–239) | 0.18 | 189 (154–233) | 178 (145–233) | 0.12 |
| D-dimer, mg·L−1 | 0.67 (0.4–1.11) | 0.64 (0.37–1.17) | 0.29 | 0.63 (0.38–1.1) | 0.63 (0.37–1.16) | 0.83 |
| Creatinine, mg·dL−1 | 0.98 (0.8–1.2) | 0.95 (0.7–1.2) | 0.61 | 0.96 (0.8–1.21) | 0.93 (0.7–1.2) | 0.62 |
| Lactate, mg·dL−1 | 12.6 (9–17.1) | 13.4 (9.9–17.1) | 0.31 | 12.61 (9–16.2) | 11.71 (9–16) | 0.88 |
| Disease chronology | ||||||
| Time since symptom onset to ICU admission, days | 10 (7–13) | 8 (6–11) | <0.001 | 9 (7–11) | 8 (7–11) | 0.38 |
| Patients spending >2 days in hospital before ICU admission | 1002 (59.18) | 359 (34.82) | <0.001 | 108 (35.18) | 100 (32.57) | 0.55 |
| Time since symptom onset to IMV, days | 10 (7–13) | 11 (8–14) | <0.001 | 9 (7–11) | 11 (8–14) | <0.001 |
| Time since hospital admission to IMV, days | 3 (2–5) | 4 (2–6) | <0.001 | 2 (1–3) | 4 (2–6) | <0.001 |
| Time since ICU admission to IMV, days | 0 (0–0) | 2 (1–3) | <0.001 | 0 (0–0) | 2 (1–3) | <0.001 |
| COVID-19 wave | ||||||
| First wave | 1148 (67.7) | 430 (41.7) | <0.001 | 150 (48.8) | 145 (47.2) | 0.74 |
| Second wave | 369 (21.7) | 383 (37.1) | <0.001 | 102 (33.2) | 122 (39.7) | 0.11 |
| Third wave | 139 (8.2) | 157 (15.2) | <0.001 | 50 (16.2) | 38 (12.3) | 0.20 |
| Fourth wave | 20 (1.18) | 24 (2.3) | 0.02 | 5 (1.6) | 2 (0.6) | 0.45 |
| Outcomes | ||||||
| IMV duration, days§ | 13 (8–25) | 14 (8–29) | 0.22 | 13 (8–24) | 18 (9–31.5) | 0.01 |
| ICU duration, days§ | 17.5 (11–33) | 23 (14–40) | <0.001 | 17 (11–32) | 27 (16–44) | <0.001 |
| Prone position | 1292 (76.6) | 797 (77.6) | 0.57 | 220 (71.6) | 220 (78.4) | 0.06 |
| Neuromuscular blockade | 1442 (85.2) | 860 (83.6) | 0.27 | 247 (80.7) | 262 (85.3) | 0.13 |
| ECMO | 27 (1.5) | 31 (3) | 0.01 | 6 (1.9) | 6 (1.9) | 1 |
| ICU mortality | 598 (35.3) | 360 (35) | 0.86 | 79 (25.7) | 111 (36.1) | 0.007 |
| Hospital mortality | 641 (37.8) | 384 (37.2) | 0.77 | 84 (27.3) | 114 (37.1) | 0.01 |
| 90-day mortality | 636 (41) | 381 (40.5) | 0.83 | 85 (30.9) | 113 (40.2) | 0.02 |
Data are presented as median (interquartile range) or n (%), unless otherwise stated; percentages calculated with nonmissing data only. BMI: body mass index; PaO2: arterial oxygen tension; FIO2: inspiratory oxygen fraction; PaCO2: arterial carbon dioxide tension; HFNC: high-flow nasal cannula; NIV: noninvasive ventilation; CRP: C-reactive protein; IMV: invasive mechanical ventilation; ECMO: extracorporeal membrane oxygenation. Variables used to perform propensity score matching included age, sex, respiratory rate at hospital admission, PaO2/FIO2 at hospital admission (categorised as >300, 200– ≤300, 100– ≤200 or ≤100 mmHg), time from hospital admission to ICU admission (categorised as ≤2 or >2 days), chronic immunosuppression, corticosteroid treatment and COVID-19 wave. After excluding patients with missing values, we had a population of n=1117. Supplementary table S4 shows standardised mean differences in baseline covariates from the matched population. #: HFNC with or without NIV; ¶: NIV with or without HFNC; +: received at least one session of NIV and/or HFNC since hospital admission before intubation; §: analysed only in survivors. Bold indicates statistical significance.
Mortality according to intubation timing
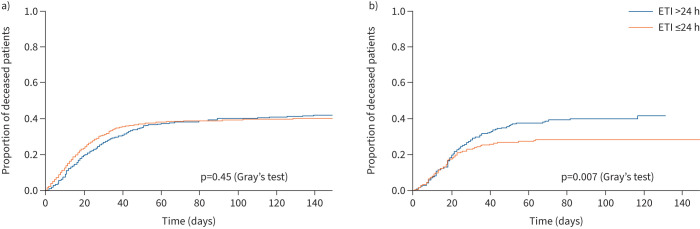
Overall hospital mortality was 37.6%. The unmatched analysis found no differences in hospital mortality: 37.8% for patients receiving early intubation and 37.2% for those receiving delayed intubation (p=0.77). Patients with a similar probability of belonging to the early or delayed intubation group were selected based on the variables chosen for propensity score matching. After excluding patients with missing values for the variables used for the propensity score, we identified a cohort of 307 cases and 307 controls. Time since both symptom onset and hospital admission to intubation was 2 days more in the delayed intubation group. In the matched cohort, hospital mortality in patients receiving delayed intubation was significantly higher (37.1% versus 27.3%; p=0.01). Logistic regression analyses revealed that, compared with delayed intubation, intubation within the first 24 h of ICU admission was associated with a reduction in hospital mortality risk (OR 0.63, 95% CI 0.45–0.89; p=0.01). Figure 2 shows survival curves obtained by the cumulative incidence function. Similarly, 90-day mortality was 30.9% and 40.2% in the early and delayed intubation groups, respectively (p=0.02). ICU mortality was also higher in patients intubated after 24 h of ICU admission (25.7% in the early intubation group versus 36.1% in the delayed intubation group; p=0.007). In the sensitivity analyses, we considered intubation within the first 48 h from ICU admission as early. We found that hospital mortality was higher in the delayed intubation group (43.27% versus 27.07%; p<0.001). Times since symptom onset and hospital admission to intubation were 3 days more in the delayed intubation group (supplementary table S3)
FIGURE 2.
Survival curves for the a) overall cohort and b) adjusted population, as obtained by propensity score matching. In total, 81 out of 614 (13.19%) patients were transferred to another hospital and censored from the survival analysis. ETI: endotracheal intubation.
Changes in intubation timing throughout the pandemic
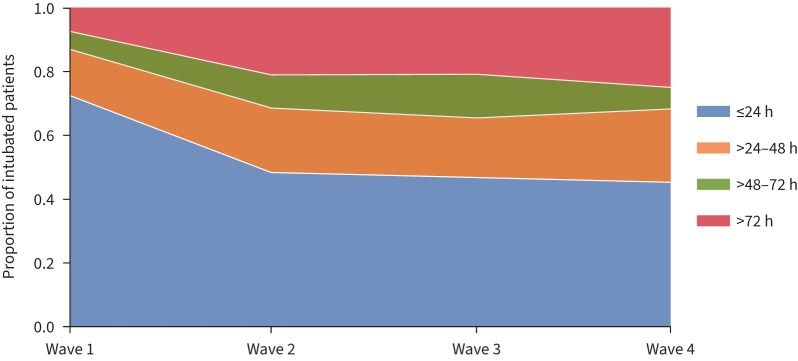
During the first wave of the pandemic, most patients receiving mechanical ventilation were intubated within the first 24 h of ICU admission (n=1148 (72%)). The mortality rate in this early intubation cohort was higher than in those receiving delayed intubation; however, these patients were older and the time since symptom onset to intubation was not shorter than that in patients with delayed intubation (supplementary table S6). In the subsequent waves, the number of patients receiving early intubation progressively decreased (49% in the second wave, 46% in the third wave and 45% in the fourth wave; p<0.001 versus the first wave) (figure 3). The mortality rate also changed: the second wave saw a significant decrease in death in patients who underwent early intubation. In the third and fourth waves, mortality was also lower in the early intubation cohort; however, this was not statistically significant. Findings about the impact of early intubation on mortality remained intact when we balanced cohorts and considered the wave during which patients received the intervention (table 1).
FIGURE 3.
Proportion of patients with COVID-19 intubated ≤24, >24–48, >48–72 or >72 h since intensive care unit (ICU) admission in the first, second, third and fourth waves in Spain. Proportion of patients intubated ≤24 h since ICU admission in the first, second, third and fourth waves: first versus second, third and fourth waves p<0.001.
Subgroup analysis of patients treated with NIV versus HFNC
Before intubation, whereas 963 (35.5%) underwent NIV with or without HFNC, 1712 (73.95%) patients received at least one session of HFNC with or without NIV and 1082 (39.5%) patients exclusively received HFNC (without NIV) (supplementary table S7). After propensity score matching, we obtained a population of 294 patients treated only with HFNC and 214 patients with NIV (table 2). We identified a higher mortality risk in patients with HFNC and delayed intubation (21.7% versus 34.6%; p=0.01). However, we did not observe such a difference in those patients receiving NIV before intubation (32.7% versus 39.2%; p=0.39). In the sensitivity analyses, we found higher mortality in patients receiving both HFNC and NIV and with delayed intubation. In patients treated with HFNC, hospital mortality was 37.3% in those with delayed intubation and 19.1% in those with early intubation (p=0.003). For patients with NIV, it was 46.6% and 30.5% in the delayed and early intubation groups, respectively (p=0.01) (supplementary tables S8 and S9).
TABLE 2.
Characteristics and outcomes of critically ill patients with COVID-19 receiving either early (≤24 h) or delayed (>24 h) intubation from intensive care unit (ICU) depending on prior use of high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) (matched population)
| HFNC (n=294) | NIV with or without HFNC (n=214) | |||||
| Early intubation (n=147) | Delayed intubation (n=147) | p-value | Early intubation (n=107) | Delayed intubation (n=107) | p-value | |
| Age, years | 63 (56–69) | 66 (57–71) | 0.23 | 64 (56–70) | 64 (58–71) | 0.73 |
| Female | 46 (31.2) | 44 (29.9) | 0.89 | 29 (27.1) | 23 (21.5) | 0.42 |
| BMI, kg·m−2 | 28.5 (26–31.6) | 28.3 (25.9–31.9) | 0.71 | 30.9 (27.5–34.1) | 29.8 (26.5–32) | 0.06 |
| Hypertension | 65 (44.2) | 77 (52.3) | 0.19 | 62 (57.9) | 57 (53.2) | 0.58 |
| Diabetes mellitus | 34 (23.1) | 34 (23.1) | 1 | 29 (27.1) | 29 (27.1) | 1 |
| Chronic cardiac failure | 15 (10.2) | 18 (12.2) | 0.71 | 13 (12.1) | 14 (13) | 1 |
| COPD | 16 (10.8) | 18 (12.2) | 0.85 | 13 (12.1) | 14 (13) | 1 |
| Immunodepression | 20 (13.6) | 19 (12.9) | 1 | 23 (21.5) | 20 (18.6) | 0.73 |
| Received corticosteroids | 133 (90.4) | 134 (91.1) | 1 | 98 (91.5) | 95 (88.7) | 0.64 |
| Clinical characteristics at hospital admission | ||||||
| PaO2/FIO2, mmHg | 230 (172–290.4) | 231.4 (146.5–307.3) | 0.80 | 194.2 (130.9–263.5) | 207.1 (114–271.1) | 0.81 |
| Respiratory rate, breaths·min−1 | 25 (20–30) | 25 (20–30) | 0.99 | 24 (20–32) | 25 (22–30) | 0.88 |
| PaCO2, mmHg | 34 (30–37) | 34 (30.1–38) | 0.41 | 33 (30–37) | 32 (29–37) | 0.46 |
| pH | 7.45 (7.42–7.48) | 7.45 (7.43–7.47) | 0.75 | 7.45 (7.41–7.48) | 7.46 (7.43–7.48) | 0.35 |
| CRP, mg·dL−1 | 13.9 (8.4–21.2) | 13 (7–18.5) | 0.11 | 14.1 (8.2–21) | 14.6 (10.3–24.8) | 0.11 |
| Lymphocytes, ×109 cells·L−1 | 0.8 (0.6–1) | 0.8 (0.6–1) | 0.85 | 0.8 (0.6–1.13) | 0.7 (0.5–1.01) | 0.13 |
| Platelets, ×109 cells·L−1 | 188 (145–238) | 176 (139–232) | 0.34 | 179 (147–220) | 193 (152–233) | 0.82 |
| D-dimer, mg·L−1 | 0.57 (0.38–0.9) | 0.54 (0.38–0.99) | 0.74 | 0.65 (0.42–1) | 0.71 (0.39–1.29) | 0.31 |
| Creatinine, mg·dL−1 | 0.94 (0.8–1.16) | 0.92 (0.79–1.17) | 0.56 | 0.99 (0.81–1.28) | 0.95 (0.79–1.3) | 0.75 |
| Lactate, mg·dL−1 | 12.7 (9–16.8) | 10.8 (8.9–16.2) | 0.31 | 12 (9.9–14.4) | 11.4 (9.2–15.5) | 0.79 |
| Disease chronology | ||||||
| Time since symptom onset to ICU admission, days | 9 (7–11) | 8 (7–11) | 0.057 | 9 (7–13) | 9 (8–12) | 0.92 |
| Patients spending >2 days in hospital before ICU admission | 52 (35.3) | 50 (34) | 0.90 | 54 (50.4) | 51 (47.6) | 0.78 |
| Time since symptom onset to IMV, days | 9 (7–12) | 11 (8–13) | 0.001 | 9 (7–13) | 12 (9–15) | <0.001 |
| Time since hospital admission to IMV, days | 2 (1–3) | 4 (2–6) | <0.001 | 3 (2–5) | 6 (3–9) | <0.001 |
| Time since ICU admission to IMV, days | 0 (0–0) | 1 (1–3) | <0.001 | 0 (0–0) | 2 (1–4) | <0.001 |
| COVID-19 wave | ||||||
| First wave | 70 (47.6) | 59 (40.1) | 0.68 | 57 (53.2) | 51 (47.6) | 0.49 |
| Second wave | 55 (37.4) | 67 (45.5) | 0.19 | 30 (28) | 41 (38.3) | 0.14 |
| Third wave | 18 (12.2) | 18 (12.2) | 1 | 19 (17.7) | 15 (14) | 0.57 |
| Fourth wave | 4 (2.7) | 3 (2) | 1 | 1 (0.9) | 0 (0) | 1 |
| Outcomes | ||||||
| IMV duration, days# | 13 (8–24) | 15 (9–31) | 0.24 | 16 (9–27) | 18 (9–31) | 0.42 |
| ICU duration, days# | 17 (11–34) | 24 (14–42) | 0.004 | 22 (12–31) | 24 (15–39) | 0.08 |
| Prone position | 97 (66.4) | 117 (79.5) | 0.01 | 92 (85.9) | 87 (81.3) | 0.46 |
| ECMO | 3 (2) | 2 (1.3) | 1 | 5 (4.6) | 3 (2.8) | 0.72 |
| Neuromuscular blockade | 113 (76.8) | 123 (83.6) | 0.18 | 95 (89.6) | 94 (87.8) | 0.82 |
| ICU mortality | 31 (21) | 48 (32.6) | 0.003 | 34 (31.7) | 41 (38.3) | 0.39 |
| Hospital mortality | 32 (21.7) | 51 (34.6) | 0.01 | 35 (32.7) | 42 (39.2) | 0.39 |
| 90-day mortality | 31 (23.8) | 51 (37.7) | 0.01 | 36 (34.2) | 41 (40.2) | 0.39 |
Data are presented as median (interquartile range) or n (%), unless otherwise stated; percentages calculated with nonmissing data only. BMI: body mass index; PaO2: arterial oxygen tension; FIO2: inspiratory oxygen fraction; PaCO2: arterial carbon dioxide tension; CRP: C-reactive protein; IMV: invasive mechanical ventilation; ECMO: extracorporeal membrane oxygenation. Patients included in the subgroup analysis received at least one session of NIV and/or HFNC before intubation. Variables used to perform propensity score matching included age, sex, respiratory rate at hospital admission, PaO2/FIO2 at hospital admission (categorised as >300, 200– ≤300, 100– ≤200 or ≤100 mmHg), time from hospital admission to ICU admission (categorised as ≤2 or >2 days), chronic immunosuppression, corticosteroid treatment and COVID-19 wave. After excluding patients with missing values, we had a population of 455 patients treated only with HFNC and 396 for patients with NIV with or without HFNC. Supplementary table S5 shows standardised mean differences in baseline covariates from the matched population. #: analysed only in survivors. Bold indicates statistical significance.
Discussion
In this large cohort study focusing on the effects of intubation timing in patients with COVID-19, we identified a higher risk of hospital mortality in those individuals with delayed intubation (>24 h of ICU admission) compared with those intubated within 24 h of ICU admission. Likewise, we observed an increase in both ICU and 90-day mortality, ICU length of stay, and mechanical ventilation duration in those patients intubated after the first 24 h of ICU admission. Very similar findings were also confirmed when we considered early or delayed intubation with a different time-point (intubation within 48 h of ICU admission for early intubation).
The subgroup of patients treated with HFNC in whom intubation was delayed presented higher mortality irrespective of the time-point used for early or delayed intubation. However, the group of patients treated with NIV prior to intubation (delayed) presented with higher mortality when the time-point was considered as after 48 h of ICU admission. Finally, we also found that patients more frequently received intubation and invasive mechanical ventilation within 24 h of ICU admission in the first wave than in subsequent waves.
While the association between delayed intubation and mortality has been well documented in acute respiratory failure [5, 8, 16–18], it remains to be clarified in patients with COVID-19. In this population, the association between longer intubation timing and worse clinical outcomes has been controversial, given that several studies reported benefits from early intubation and others showed opposite results. A meta-analysis by Papoutsi et al. [9] that comprised 12 observational studies comparing early and delayed intubation (also defined as occurring within or after the first 24 h of ICU admission) did not report differences in mortality. However, most of the studies included had relevant limitations, such as retrospective nature [19–28] and lack of covariate adjustment [20–27], significant heterogeneity in study design and clinical characteristics [19–28], and analyses circumscribed to the first waves of each region [19–28]. On the other hand, similar to our results, one study reported higher chances of survival in patients in whom intubation and invasive mechanical ventilation were started within the first 48 h of noninvasive respiratory support [29]. Two important factors may explain the differences in outcomes noted in this study [29] and ours when compared with Papoutsi et al. [9]. First and foremost, to minimise confounding bias, we selected a population with a similar baseline risk of intubation and mortality using propensity score matching. We considered covariates that were known to play a significant role in both the intubation strategy and survival. Second, in propensity score matching, we accounted for time since hospitalisation to ICU admission. The resulting population, therefore, had a longer time (2 days) of spontaneous breathing from both symptom onset and hospital admission to intubation. Considering that patient self-inflicted lung injury is inevitably related to spontaneous breathing, this ensured that the delayed intubation group had longer exposure to such an event. This message has yet to be suggested in the literature and could prove useful in other types of acute respiratory failure.
Importantly, in patients intubated after 24 h of ICU admission, we found worse respiratory mechanics compared with those intubated earlier (higher driving and positive end-inspiratory pressures with lower tidal volumes and similar positive end-expiratory pressures). This finding is consistent with prior literature [30], and may suggest further lung damage as a result of longer exposure to uncontrolled and spontaneous ventilation. When we considered a 48-h time-point for early or delayed intubation, we also observed worse oxygenation during the first day of mechanical ventilation in patients belonging to the latter group. While this could partially explain differences observed in survival, the exact mechanisms that increase the risk of death due to a delayed start in invasive mechanical ventilation are not completely known.
We also examined the potential effect of the type of noninvasive respiratory support used before invasive mechanical ventilation. In the subgroup of patients treated exclusively with HFNC, we reported an increased risk of hospital mortality in those with delayed intubation, irrespective of the time-point used. However, we did not observe the same association in patients treated with NIV before intubation when we considered early intubation as that occurring within 24 h of ICU admission. The negative results in this subgroup should not be misinterpreted. The sensitivity analyses performed in the subgroup of patients with NIV, in whom we explored intubation ranges from a 48-h time-point perspective, showed higher mortality in those with delayed treatment. The lack of differences in mortality between the early and delayed intubation groups in the 24-h time-point analysis could be explained by the already high mortality of patients treated with NIV. This would suggest that NIV failure could increase the risk of mortality even if intubation is not delayed. The association of NIV failure and higher mortality has been widely reported in acute respiratory failure and has a strong physiological background [31–33]. The higher mortality found in patients treated with HFNC would rather suggest that spontaneous ventilation, even without the presence of positive inspiratory pressure, may also be deleterious.
In our study, we evaluated trends in intubation timing throughout the different waves included, finding a significant decrease in early intubation rates. The probable rationale for this finding is the increased confidence of clinicians in treating COVID-19-associated acute respiratory failure noninvasively. In fact, greater use of NIV and HFNC was reported in the later periods of the pandemic [34]. In light of our results and others [6, 7, 29], caution is warranted when assessing the potential benefits (avoidance of intubation) and risks (delayed intubation) of applying such noninvasive therapies in COVID-19.
The strengths of our study include the large population included, the assessment across different periods (four waves), the granularity of data with 58 ICUs included and the propensity score analyses performed. Our study also has limitations. First, it was not designed in the framework of a target-emulated trial. We excluded patients who were not intubated and a high proportion of individuals did not need this intervention. Therefore, as some patients may benefit from a wait-and-see approach, we cannot draw firm conclusions on the best strategy for intubation. Second, the arbitrary cut-off point of 24 h used to define early intubation may elicit critique. However, we defined this time-point in accordance with prior literature [9] and clinical prudence. Sensitivity analyses showed robustness of the findings. Third, as result of our design, immortal time bias may have occurred; patients intubated after 24 h of ICU admission had to survive to be included. Fourth, since treatment was not randomly allocated, both residual and unmeasured confounding are possible, even after careful covariate adjustment. Fifth, we did not have solid information about the clinical situation immediately before intubation, so we used data from hospital admission. Furthermore, we do not have the total time spent on neither noninvasive respiratory support nor in NIV and HFNC settings. Sixth, generalisation of the results may be hindered due to the prevalence of immunisation and changes made in clinical management after the patient recruitment period.
In conclusion, in patients with COVID-19 requiring invasive mechanical ventilation, delayed intubation was associated with a higher risk of hospital mortality when compared with earlier intubation. Patients undergoing HFNC before intubation presented an increased risk of mortality when intubation was delayed irrespective of the time-point used to consider early or delayed intubation. Patients with NIV presented a higher mortality risk when delayed intubation was that occurring after 48 h of ICU admission. Finally, more patients received intubation within 24 h of ICU admission in the first pandemic wave.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01426-2022.Supplement (430.1KB, pdf)
Shareable PDF
Footnotes
Individual members of the CIBERESUCICOVID group: Immaculada Salvador-Adell, Alexander Agrifoglio, María Aguilar Cabello, Luciano Aguilera, Victoria Alcaraz-Serrano, Cesar Aldecoa, Cynthia Alegre, Sergio Álvarez, Antonjo Álvarez Ruiz, Rut Andrea, José Ángel, Marta Arrieta, J. Ignacio Ayestarán, Joan Ramon Badia, Mariona Badía, Orville Báez Pravia, Ana Balan Mariño, Begoña Balsera, Laura Barbena, Tommaso Bardi, Patricia Barral Segade, Marta Barroso, José Ángel Berezo García, Judit Bigas, Rafael Blancas, María Luisa Blasco Cortés, María Boado, María Bodi Saera, Neus Bofill, María Teresa Bouza Vieiro, Leticia Bueno, Juan Bustamante-Munguira, Lucia Cachafeiro, David Campi Hermoso, Sandra Campos Fernández, Iosune Cano, Maria Luisa Cantón-Bulnes, Pablo Cardina Fernández, Laura Carrión García, Sula Carvalho, Núria Casacuberta-Barberà, Manuel Castellà, Andrea Castellví, Pedro Castro, Ramon Cicuendez Ávila, Catia Cillóniz, Luisa Clar, Cristina Climent, Jordi Codina, Pamela Conde, Sofía Contreras, María Cruz Martin, Raul de Pablo Sánchez, Diego De Mendoza, Cecilia del Busto Martínez, Yolanda Díaz, María Digna Rivas Vilas, Cristina Dólera Moreno, Irene Dot, Pedro Enríquez Giraudo, Inés Esmorís Arijón, Teresa Farre Monjo, Javier Fernández, Carlos Ferrando, Albert Figueras, Eva Forcadell-Ferreres, Lorena Forcelledo Espina, Nieves Franco, Àngels Furro, Felipe García, Beatriz García, Emilio García Prieto, Carlos García Redruello, Amaia García Sagastume, Maria Luisa Gascón Castillo, Gemma Gomà, Vanesa Gómez Casal, Silvia Gómez, Carmen Gómez Gonzalez, Jessica González, Federico Gordo, Maria Pilar Gracia, Alba Herraiz, Rubén Herrán-Monge, Mercedes Ibarz, Silvia Iglesias, Maria Teresa Janer, Gabriel Jiménez, Mar Juan Díaz, Karsa Kiarostami, Juan I. Lazo Álvarez, Miguel León, Alexandre López-Gavín, Ana López Lago, Desire Macias Guerrero, Nuria Mamolar Herrera, Rafael Mañez Mendiluce, Cecilia L. Mantellini, Gregorio Marco Naya, Pilar Marcos, Enrique Marmol Peis, Paula Martín Vicente, María Martínez, Carmen Eulalia Martínez Fernández, Maria Dolores Martínez Juan, Juan Fernando Masa Jimenez, Joan Ramon Masclans, Emilio Maseda, Eva María Menor Fernández, Mar Miralbés, Josman Monclou, Juan Carlos Montejo-González, Neus Montserrat, María Mora Aznar, Pedro Moral-Parras, Dulce Morales, Sara Guadalupe Moreno Cano, David Mosquera Rodríguez, Rosana Muñoz-Bermúdez, José María Nicolás, Ramon Nogue Bou, Rafaela Nogueras Salinas, Marta Ocón, Ana Ortega, Sergio Ossa, Pablo Pagliarani, Anna Parera Pous, Francisco Parrilla, Leire Pérez Bastida, Purificación Pérez, Gloria Pérez Planelles, Eva Pérez Rubio, David Pestaña Laguna, Esther Sauras-Colón, Javier Prados, Andrés Pujol, Núria Ramon Coll, Gloria Renedo Sanchez-Giron, Ferran Roche-Campo, Laura Rodriguez, Felipe Rodríguez de Castro, Silvia Rodríguez, Covadonga Rodríguez Ruiz, Jorge Rubio, Alberto Rubio López, Miriam Ruiz Miralles, Pablo Ryan Murúa, Eva Saborido Paz, Ana Salazar Degracia, Miguel Sanchez, Ana Sánchez, Bitor Santacoloma, Maria Teresa Sariñena, Marta Segura Pensado, Lidia Serra, Mireia Serra-Fortuny, Ainhoa Serrano Lázaro, Lluís Servià, Laura Soliva, Carla Speziale, Daniel Tognetti, Adrián Tormos, Mateu Torres, Sandra Trefler, Javier Trujillano, Alejandro Úbeda, Luis Urrelo-Cerrón, Estela Val, Luis Valdivia Ruiz, Montse Vallverdú, Maria Van der Hofstadt Martin-Montalvo, Sabela Vara Adrio, Nil Vázquez, Javier Vengoechea, Pablo Vidal Cortes, Clara Vilà-Vilardel, Judit Vilanova, Tatiana Villada Warrington, Hua Yang, Minlan Yang, Ana Zapatero and Jesús F. Bermejo-Martín.
Author contributions: A. Torres, J. Riera, E. Barbeta, A. Motos, L. Fernández-Barat and A. Ceccato participated in protocol development, study design, study management, statistical analysis and data interpretation. A. Tormos and D. García-Gasulla participated in statistical analysis and data interpretation. A. Palomeque, O. Peñuelas, J.Á. Lorente, C. Ferrando, R. Mellado-Artigas, F. Barbé and R. Ferrer participated in study design, study management and interpretation, and critical review of the first report draft. O. Roca participated in data interpretation and critical review of the manuscript. The CIBERESUCICOVID Consortium participated in data collection. All authors read and approved the final manuscript.
Conflicts of interest: O. Roca discloses a research grant from Hamilton Medical AG; speaker fees from Hamilton Medical AG, Ambu, Fisher & Paykel Ltd and Aerogen Ltd; and nonfinancial research support from Timpel and Masimo. R. Mellado-Artigas reports speaker fees from Medtronic and Fisher & Paykel, all outside the submitted work. The remaining authors declare no conflicts of interest.
Support statement: Financial support was provided by the Instituto de Salud Carlos III de Madrid (COV20/00110, ISCIII), Fondo Europeo de Desarrollo Regional (FEDER), “Una manera de hacer Europa” and the Centro de Investigación Biomedica En Red – Enfermedades Respiratorias (CIBERES). D. de Gonzalo-Calvo has received financial support from the Instituto de Salud Carlos III (Miguel Servet 2020: CP20/00041), co-funded by European Social Fund (ESF)/“Investing in Your Future”. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020; 323: 2329–2330. doi: 10.1001/jama.2020.6825 [DOI] [PubMed] [Google Scholar]
- 2.Tobin MJ, Laghi F, Jubran A. P-SILI is not justification for intubation of COVID-19 patients. Ann Intensive Care 2020; 10: 105. doi: 10.1186/s13613-020-00724-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care 2020; 10: 78. doi: 10.1186/s13613-020-00692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Uchiyama A, Matsuura N, et al. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit Care Med 2013; 41: 536–545. doi: 10.1097/CCM.0b013e3182711972 [DOI] [PubMed] [Google Scholar]
- 5.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med 2015; 41: 623–632. doi: 10.1007/s00134-015-3693-5 [DOI] [PubMed] [Google Scholar]
- 6.Perkins GD, Ji C, Connolly BA, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA 2022; 327: 546–558. doi: 10.1001/jama.2022.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grieco LD, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA 2021; 325: 1731–1743. doi: 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumas G, Lemiale V, Rathi N, et al. Survival in immunocompromised patients ultimately requiring invasive mechanical ventilation: a pooled individual patient data analysis. Am J Respir Crit Care Med 2021; 204: 187–196. doi: 10.1164/rccm.202009-3575OC [DOI] [PubMed] [Google Scholar]
- 9.Papoutsi E, Giannakoulis VG, Xourgia E, et al. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care 2021; 25: 121. doi: 10.1186/s13054-021-03540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011; 46: 399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 12.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006; 163: 1149–1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res 2011; 46: 119–151. doi: 10.1080/00273171.2011.540480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133: 601–609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- 16.Carrillo A, Gonzalez-Diaz G, Ferrer M. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med 2012; 38: 458–466. doi: 10.1007/s00134-012-2475-6 [DOI] [PubMed] [Google Scholar]
- 17.Esteban A, Frutos-Vivar F, Ferguson ND. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med 2004; 350: 2452–2460. doi: 10.1056/NEJMoa032736 [DOI] [PubMed] [Google Scholar]
- 18.Wood KA, Lewis L, Von Harz B, et al. The use of noninvasive positive pressure ventilation in the emergency department: results of a randomized clinical trial. Chest 1998; 113: 1339–1346. doi: 10.1378/chest.113.5.1339 [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med 2020; 48: E1045–E1053. doi: 10.1097/CCM.0000000000004600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020; 8: 853–862. doi: 10.1016/S2213-2600(20)30316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YH, Choi K-J, Choi SH, et al. Clinical significance of timing of intubation in critically ill patients with COVID-19: a multi-center retrospective study. J Clin Med 2020; 9: 2847. doi: 10.3390/jcm9092847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matta A, Chaudhary S, Bryan Lo K, et al. Timing of intubation and its implications on outcomes in critically Ill patients with coronavirus disease 2019 Infection. Crit Care Explor 2020; 2: e0262. doi: 10.1097/CCE.0000000000000262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roedl K, Jarczak D, Thasler L, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Aust Crit Care 2021; 34: 167–175. doi: 10.1016/j.aucc.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben SI, Ennouri E, Nachi R, et al. Very severe covid-19 in the critically ill in Tunisia. Pan Afr Med J 2020; 35: Suppl. 2, 136. doi: 10.11604/pamj.supp.2020.35.136.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siempos II, Xourgia E, Ntaidou TK, et al. Effect of early vs delayed or no intubation on clinical outcomes of patients with COVID-19: an observational study. Front Med 2020; 7: 614152. doi: 10.3389/fmed.2020.614152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, Hajage D, Demoule A, et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care 2021; 47: 60–73. doi: 10.1007/s00134-020-06294-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180: 1345–1355. doi: 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellado-Artigas R, Ferreyro BL, Angriman F, et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care 2021; 25: 58. doi: 10.1186/s13054-021-03469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González J, Benítez ID, de Gonzalo-Calvo D, et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: a prospective cohort study. Crit Care 2022; 26: 18. doi: 10.1186/s13054-021-03882-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ball L, Robba C, Herrmann J, et al. Early versus late intubation in COVID-19 patients failing helmet CPAP: a quantitative computed tomography study. Respir Physiol Neurobiol 2022; 301: 103889. doi: 10.1016/j.resp.2022.103889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 32.Bellani G, Laffey JG, Pham T, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med 2017; 195: 67–77. doi: 10.1164/rccm.201606-1306OC [DOI] [PubMed] [Google Scholar]
- 33.Coppola S, Chiumello D, Busana M, et al. Role of total lung stress on the progression of early COVID-19 pneumonia. Intensive Care Med 2021; 47: 1130–1139. doi: 10.1007/s00134-021-06519-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbonell R, Urgelés S, Rodríguez A, et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur 2021; 11: 100243. doi: 10.1016/j.lanepe.2021.100243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01426-2022.Supplement (430.1KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01426-2022.Shareable (947.6KB, pdf)