Abstract
During early neurodevelopment of infant, myelination plays an essential role in brain connectivity and emergence of behavioral and cognitive function. Early life nutrition is an important factor to shape myelination and consequently cognitive appearance. To analyze the effects of additive nutrients, including 2'-fucosyllactose (2'-FL), osteopontin (OPN), docosahexaenoic acid (DHA), on neurocognitive function and brain structure, the current study evaluated the effects of different composition of breast milk nutrients on oligodendrocyte progenitor cells (OPCs) myelination with a neural primary cell model in vitro. The study showed that the three nutrients promoted the proliferation, maturation and differentiation of OPCs into mature oligodendrocytes (OLs) in each phage of the cell growth, and the effect of the nutrients blend is obviously stronger than that of the nutrient treatment alone, showing a synergistic effect in promotion of OPCs. The results of this experiment clarified the effects of 2′-FL OPN and DHA to promote myelination development of neural cells, and laid an experimental basis for further optimization of infant formula.
Keywords: myelination, 2'-fucosyllactose, osteopontin, docosahexaenoic acid, neural primary cell
Introduction
Early life nutrition plays a critical role in neurodevelopmental processes such as neuronal maturation, synaptogenesis, and myelination (1). Myelination is the process of specialized glial cells oligodendrocytes (OLs) in the central nervous system (CNS), to form myelin sheaths around axons, which is essential for normal brain cells connectivity (2, 3). Myelin sheath, composed of several condensed lipid bilayer membranes (4), increases axonal conduction velocity and the maturation of cognitive function by reducing the capacitance of the axonal membrane and allows jumping currents (5–7). Oligodendrocyte progenitor cells (OPCs) are the main glial cell population in the central nervous system, accounting for 5–8% of the total cell population (8). Post-mitotic OPCs differentiate into myelinated OLs, and these OLs expand a number of processes, establishing contacts with axons of different neurons and initiating myelination (9, 10). During their maturation, OLs produce different components of myelin as lipids (cholesterol, galactolipids, and phospholipids) and myelin-specific proteins. The types of myelin proteins expressed by OLs, such as myelin-associated glycoprotein (MAG) and myelin basic protein (MBP), closely correlate with its maturity (11, 12). Myelinated OLs express MAG, and the expression of MAG gradually increases during the maturation of OLs. MAG is a sialic acid-binding immunoglobulin-like lectin, and although it constitutes only a small fraction of the total protein content of myelin, it is predominantly expressed in the peri-axonal region of myelin (13). It appears to play an important role in oligodendrocyte-axon interactions and mediate bidirectional signaling between axons and OLs to support myelination (14). MBP is expressed in mature myelinated OLs and is one of the main components of myelin. MBP appears to play an active role in myelination and compaction. In fact, MBP aggregates and forms a cohesive reticulin network, which is essential for hopping currents (15, 16).
In the CNS, every step of myelination, including the proliferation of OPCs, the differentiation and maturation of OPCs into myelinating OLs, and myelination, is highly regulated by both external and internal factors. In particular, different nutrients have different effects on myelination, suggesting that early life nutrition may have important implications for the regulation of myelination. Therefore, identifying early-life nutritional factors that support myelination is critical for optimal brain and cognitive development.
Osteopontin (OPN), 2′-fucosyllactose (2′-FL), and docosahexaenoic acid (DHA) are essential nutrients in breast milk and infant formula. Many studies have proved that OPN plays an important role in organism, especially in the process of immune activation, bone damage repair, vascular regeneration and bone remodeling (17). 2′-FL, a breast milk oligosaccharide, possesses physiological functionalities of prebiotics effect, antiadhesive antimicrobials, immunomodulation and promotion of brain development (18). DHA is a key nutritional n-3 PUFA and was found to have a strong influence on brain health (19). Though having promotion on neurocognitive function and brain structure, the underlying mechanism of the three essential nutrients on development of neural cells remains unknown by now. In the current study, an in vitro model of primary cell cultures containing neurons and OLs was used to evaluate the effects of a composition of breast milk nutrients on myelination. We hoped to add nutrients to mixed cell cultures to promote the proliferation, maturation and differentiation of OPCs into mature OLs and/or the myelinating properties of OLs. The density of OPCs was firstly evaluated after 12 days of in vitro culture. Then, OPCs differentiation into OLs and OLs maturation and myelination were assessed by quantifying MAG-positive cells and MBP-positive cells at 18 and 30 days, respectively.
Methods, materials, and instruments
Materials and reagents
2′-FL, OPN and DHA were purchased from Beijing Jinkangpu Food Science & Technology Co., Ltd (Beijing, China). Neural cell culture medium was obtained from GibcoTM (Life Technologies Inc., Grand Island, NY, USA). A2B5 antibody (Lot. MAB312RX) and MAG antibody (Lot. MAB1567) were got from Merck Co., Inc., (NJ, USA). MBP antibody (Lot. NBP1-05204) was obtained from Novus Biologicals.
Neural primary cell acquisition
The animal experiment was approved by the Laboratory Animal Center of Peking University Health Science Center (Beijing, China). To get primary mixed cultures of neurons and OLs (20), forebrains of neonatal rat were taken out on ice and trypsinized for 20 min at 37°C (Trypsin EDTA 1X, PAN BIOTECH). The reaction was stopped by the addition of Dulbecco's modified Eagle's medium (DMEM, PAN BIOTECH) containing DNAase II (0.1 mg/ml, PAN BIOTECH) and 10% fetal bovine serum (FCS, GIBCO). Cells were mechanically dissociated three times by 10 ml pipette and centrifuged at 515 g for 10 min at 4°C, and then were seeded in plates (2 × 104 cells/well) pre-coated with poly-L-lysine (BD Falcon) and laminin (Sigma) in a humidified incubator. The medium consisted of Neurobasal (GIBCO) supplemented with 2% B27 (GIBCO), 2 mM L-glutamine (L-Glu, PAN BIOTECH), 2% P/S solution (PAN BIOTECH), 1% FCS and 10 ng/ml of platelet-derived growth factor (PDGF-AA, PAN BIOTECH).
Neural cell culture
Cells were seeded in 48-well-plates at a density of 2 × 104 cells/well and added mix or individual nutrients in fresh medium 6 h later. The incubations were replaced with half of the medium containing the same mix or individual nutrients every other day, and stop at 12, 18, or 30 days for immunohistochemistry analysis.
Immunohistochemistry assay
Immunocytochemistry was carried out as previous report (20) with minor modification. After incubation with the nutrients for 12, 18 and 30 days, the cells were fixed with a cold mixture of 95% ethanol and acetic acid (5%) for 5 min. Non-specific sites were then blocked with 0.1% saponin (Sigma) and 1% FCS (GIBCO) in PBS for 15 min at room temperature.
At 12 days, the cells were incubated with mouse monoclonal anti-A2B5 conjugated Alexa fluor 488 (1/200, MAB312RX) in PBS containing 1% FCS and 0.1% saponin for 2 h at room temperature. After washing with PBS for 3 times, the cells were incubated with a rabbit anti-neurofilament antibody (1/500, N4142) PBS containing 1% FCS and 0.1% saponin for 2 h at room temperature. Neurofilament (NF) was stained with a secondary goat anti-rabbit CF568 antibody (1/400, SAB4600084, SIGMA) containing 1% FCS and 0.1% saponin in PBS for 1 h at room temperature.
At 18 days, the cells were incubated with a mouse monoclonal Anti-MAG (1/400, MAB1567, Millipore) and rabbit anti-NF antibody (1/500, N4142, SIGMA) in PBS containing 1% FCS and 0.1% saponin for 2 h. After washing with PBS for 3 times, the cells were incubated with a secondary goat anti-mouse CF488A antibody (1/400, SAB4600042, SIGMA) and goat anti-rabbit CF 568 antibody (dilution: 1/400, SIGMA, SAB4600084) in PBS containing 1% FCS and 0.1% saponin at room temperature for 1 h. For all conditions, the cell nuclei were stained using a Hoechst solution (SIGMA, B1155).
At 30 days, the cells were incubated with a mouse monoclonal anti-MBP (1/1000, NBP1-05204, NOVUS) and a rabbit anti-Neurofilament antibody (1/500, N4142, SIGMA) in PBS containing 1% FCS and 0.1% saponin for 2 h. After washing with PBS for 3 times, the cells were incubated with goat anti-mouse CF488A antibody (1/800, SAB4600042, SIGMA) and goat anti-rabbit CF568 antibody (1/400, SAB4600084, SIGMA) in PBS containing 1% FCS and 0.1% saponin at room temperature for 1 h.
Microscopic analysis
Digital images were collected at 20x magnification using ImageXpress equipped with LED lights (excitation 360/480/565 and emission 460/535/620). All images were acquired with the same settings. The number of OPCs was calculated by quantifying the number of A2B5-expressing cells at 12 days, and the results were expressed as the average number of A2B5-expressing cells per well. Differentiation of OPCs to OLs was assessed by counting the number of MAG-positive cells at 18 days. Results are expressed as the average number of cells per well. At 30 days, the maturity of OLs was estimated by counting the number of MBP-positive cells.
Cell experiment grouping and dosages
To evaluate the function of nutrients in promoting the proliferation, maturation and differentiation of neuronal OPCs into mature OLs and/or myelination of OLs, the effects of different doses of nutrients mixture were studied using the isolated primary neuronal cells (Table 1). The density of OPCs, OPCs differentiation into OLs and OLs maturation, and level of myelination were assessed after 12, 18, and 30 days of in vitro culture, respectively.
Table 1.
Groups and dosages of the nutrition.
| Groups | Nutrition composition | 2-FL(mg/ml) | OPN(mg/ml) | DHA(mg/ml) |
|---|---|---|---|---|
| Control | Vehicle | 0 | 0 | 0 |
| Positive | Olesoxime | 0 | 0 | 0 |
| 1 | 2-FL(Ha) | 10 | 0 | 0 |
| 2 | OPN(Ha) | 0 | 1 | 0 |
| 3 | DHA(Ha) | 0 | 0 | 5 |
| 4 | 2-FL(Lb)+OPN(Lb) | 0.1 | 0.01 | 0 |
| 5 | 2-FL(Lb)+OPN(Ha) | 0.1 | 1 | 0 |
| 6 | 2-FL(Mc)+OPN(Mc) | 1 | 0.1 | 0 |
| 7 | 2-FL(Ha)+ OPN(Lb) | 10 | 0.01 | 0 |
| 8 | 2-FL(Ha)+OPN(Ha) | 10 | 1 | 0 |
| 9 | 2-FL(LMd)+OPN(LMd) | 0.5 | 0.05 | 0 |
| 10 | 2-FL(MHe) +OPN(MHe) | 5 | 0.5 | 0 |
| 11 | 2-FL(Lb)+OPN(Lb)+DHA(Lb) | 0.1 | 0.01 | 0.05 |
| 12 | 2-FL(Lb)+OPN(Mc)+DHA(Mc) | 0.1 | 1 | 0.5 |
| 13 | 2-FL(Ha)+OPN(Ha)+DHA(Ha) | 10 | 1 | 5 |
| 14 | 2-FL(LMd)+OPN(LMd)+DHA(LMd) | 0.5 | 0.05 | 0.25 |
| 15 | 2-FL(MHe)+OPN(MHe)+DHA(MHe) | 5 | 0.5 | 2.5 |
a, high dose; b, low dose; c, medium dose; d, low to medium dose; e, medium to high dose.
Statistical analysis
The results were expressed as mean ± standard error (mean ± SEM), and SPSS software (version 26.0, IBM, Armonk, NY, USA) was used for T-test and one-way ANOVA test. A value of P < 0.05 was considered statistically significant, while it was judged to be extremely significant when p < 0.01.
Results and discussion
Pro-proliferative effect of the nutrients on OPCs
To measure the effect of mixed nutrition or nutrient treatment alone on OPCs, the number of A2B5-labeled positive cells were assessed after 12 days to estimate the number of OPCs.
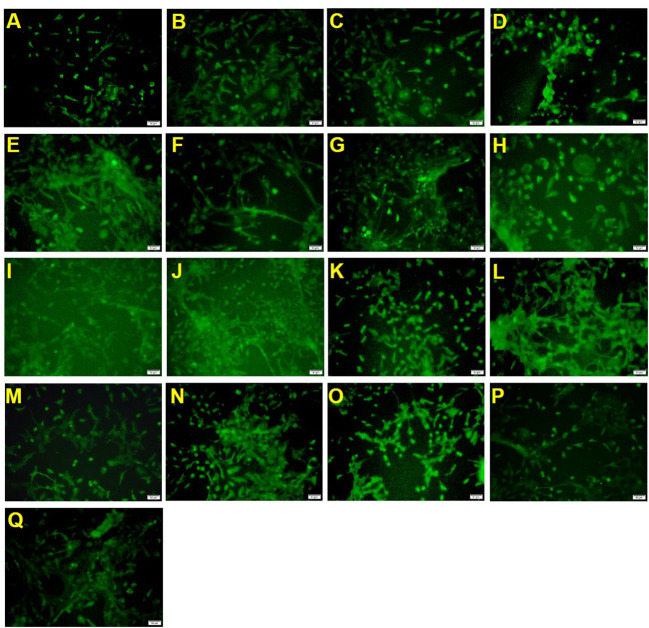
The sample processing results (Table 2 and Figure 1) showed that the positive drug olesoxime increased the number of A2B5 positive cells compared to the control group. 2′-FL, OPN and DHA high-dose groups (groups 1, 2 and 3) could also significantly increase the number of A2B5 positive cells, indicating that the three components contributed to the proliferation of oligodendrocyte precursor cells. However, the 2′-FL+ OPN and 2′-FL+ OPN + DHA low dose groups (groups 4 and 11) failed to increase the number of A2B5 positive cells, which suggested that the low-dose group set could not effectively induce the proliferation of oligodendrocyte precursor cells. The effects of 2′-FL + OPN middle dose group (group 6), 2′-FL + OPN high dose group (group 8), 2′-FL + OPN middle to high dose group (group 10), and 2′-FL + OPN + DHA high dose group (group 13) and 2′-FL + OPN + DHA medium to high dose group (group 15) were all significantly higher than those of the high dose groups of 2′-FL, OPN or DHA (groups 1, 2 and 3), indicating that these combinations have a synergistic effect on the growth of OPCs.
Table 2.
Effects of different nutritional compositions containing 2'-FL, OPN and/or DHA on the number of A2B5 positive cells.
| Groups | Nutrition composition | Density of A2B5 positive cells (Mean ±SEM) |
|---|---|---|
| Control | Vehicle | 77.333 ± 5.181 |
| Positive | Olesoxime | 113.833 ± 4.708*** |
| 1 | 2-FL(Ha) | 102.167 ± 6.036** |
| 2 | OPN(Ha) | 95.50 ± 4.595* |
| 3 | DHA(Ha) | 100.333 ± 5.327* |
| 4 | 2-FL(Lb)+OPN(Lb) | 85.833 ± 5.009 |
| 5 | 2-FL(Lb)+OPN(Ha) | 101.00 ± 7.832** |
| 6 | 2-FL(Mc)+OPN(Mc) | 114.50 ± 8.563***∧▽ |
| 7 | 2-FL(Ha)+ OPN(Lb) | 110.333 ± 4.256*** |
| 8 | 2-FL(Ha)+OPN(Ha) | 122.667 ± 5.823***∧∧▽▽▽ |
| 9 | 2-FL(LMd)+OPN(LMd) | 108.167 ± 5.023*** |
| 10 | 2-FL(MHe)+OPN(MHe) | 116.00 ± 4.531***∧▽▽ |
| 11 | 2-FL(Lb)+OPN(Lb)+DHA(Lb) | 92.667 ± 5.155* |
| 12 | 2-FL(Lb)+OPN(Mc)+DHA(Mc) | 114.50 ± 6.474***▽ |
| 13 | 2-FL(Ha)+OPN(Ha)+DHA(Ha) | 130.167 ± 8.867***∧∧▽▽▽## |
| 14 | 2-FL(LMd)+OPN(LMd)+DHA(LMd) | 107.667 ± 7.315** |
| 15 | 2-FL(MHe)+OPN(MHe)+DHA(MHe) | 128.50 ± 8.086***∧∧▽▽▽## |
*p < 0.05 compared with blank control group, **p < 0.01 compared with blank control group, ***p < 0.001 compared with blank control group.
∧Compared with 2′-FL high-dose group p < 0.05, ∧∧compared with 2′-FL high-dose group p < 0.01.
▽Compared with OPN high-dose group p < 0.05, ▽▽ compared with OPN high-dose group p < 0.01, ▽▽▽ compared with OPN high-dose group p < 0.001.
## Compared with DHA high-dose group p < 0.01.
a, high dose; b, low dose; c, medium dose; d, low to medium dose; e, medium to high dose.
Figure 1.
A2B5 immunostaining of neural primary cells after treatment with 2′-FL and OPN and DHA for 12 days. (A), control group; (B), positive drug; (C), 2′-FL(H); (D), OPN(H); (E), DHA(H); (F), 2′-FL(L)+OPN(L); (G), 2′-FL(L)+OPN(H); (H), 2′-FL(M)+OPN(M); (I), 2′-FL(H)+OPN(L); (J), 2′-FL(H)+OPN(H); (K), 2′-FL(LM)+OPN(LM); (L), 2′-FL(MH) +OPN(MH); (M), 2′-FL(L)+OPN(L)+DHA(L); (N), 2′-FL(L)+OPN(M)+DHA(M); (O), 2′-FL(H)+OPN(H)+DHA(H); (P), 2′-FL(LM)+OPN(LM)+DHA(LM); (Q), 2′-FL(MH)+OPN(MH)+DHA(MH). Scale bar: 50 μm.
Nutrition promote differentiation of OPCs into mature OLs
To measure the effect of mixed nutrition or nutrition treatment alone on the myelination of OPCs, we assessed the number of MAG-labeled positive cells after treatment for 18 days.
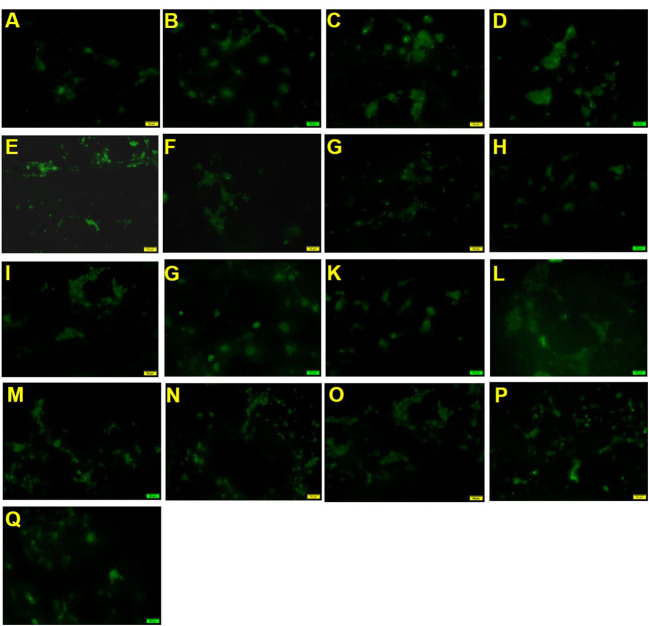
As shown in Figure 2 and Table 3, the positive drug olesoxime showed an increase in the number of MAG-positive cells compared to the control group. The 2′-FL OPN and DHA groups (groups 1, 2 and 3) could significantly increase the number of MAG-positive cells, indicating that both components contributed to the differentiation of OPCs into mature OLs. In addition, MAG-positive cells in 2′-FL + OPN middle dose group (group 6), 2′-FL + OPN high dose group (group 8), 2′-FL + OPN middle to high dose group (group 10), and 2′-FL + OPN + DHA high dose (group 13) and 2′-FL + OPN + DHA medium to high dose group (group 15) group were all significantly higher than those of the high dose groups of 2′-FL, OPN and DHA (groups 1, 2 and 3), which suggesting that the combinations of nutrition have synergistic effect to promote differentiation of OPCs into mature OLs.
Figure 2.
MAG immunostaining of neural primary cells after treatment with 2′-FL and OPN and DHA for 18 days. (A), control group; (B), positive drug; (C), 2′-FL(H); (D), OPN(H); (E), DHA(H); (F), 2′-FL(L)+OPN(L); (G), 2′-FL(L)+OPN(H); (H), 2'-FL(M)+OPN(M); (I), 2′-FL(H)+OPN(L); (J), 2′-FL(H)+OPN(H); (K), 2′-FL(LM)+OPN(LM); (L), 2′-FL(MH) +OPN(MH); (M), 2′-FL(L)+OPN(L)+DHA(L); (N), 2′-FL(L)+OPN(M)+DHA(M); (O), 2′-FL(H)+OPN(H)+DHA(H); (P), 2′-FL(LM)+OPN(LM)+DHA(LM); (Q), 2′-FL(MH)+OPN(MH)+DHA(MH). Scale bar: 50 μm.
Table 3.
Effects of different nutritional compositions containing 2'-FL, OPN and/or DHA on the number of MAG positive cells.
| Groups | Nutrition composition | Density of MAG positive cells (Mean ±SEM) |
|---|---|---|
| Control | Vehicle | 29.333 ± 3.612 |
| Positive | Olesoxime | 62.00 ± 5.520*** |
| 1 | 2-FL(Ha) | 51.167 ± 4.624* |
| 2 | OPN(Ha) | 52.333 ± 4.944* |
| 3 | DHA(Ha) | 47.50 ± 6.999* |
| 4 | 2-FL(Lb)+OPN(Lb) | 43.00 ± 5.323* |
| 5 | 2-FL(Lb)+OPN(Ha) | 55.167 ± 7.213** |
| 6 | 2-FL(Mc)+OPN(Mc) | 60.167 ± 7.441*** |
| 7 | 2-FL(Ha)+ OPN(Lb) | 56.00 ± 5.422** |
| 8 | 2-FL(Ha)+OPN(Ha) | 67.833 ± 7.305***∧▽# |
| 9 | 2-FL(LMd)+OPN(LMd) | 52.833 ± 4.045** |
| 10 | 2-FL(MHe) +OPN(MHe) | 69.00 ± 7.878***∧▽# |
| 11 | 2-FL(Lb)+OPN(Lb)+DHA(Lb) | 46.50 ± 6.402* |
| 12 | 2-FL(Lb)+OPN(Mc)+DHA(Mc) | 59.167 ± 7.596** |
| 13 | 2-FL(Ha)+OPN(Ha)+DHA(Ha) | 73.833 ± 9.250***∧▽## |
| 14 | 2-FL(LMd)+OPN(LMd)+DHA(LMd) | 57.167 ± 6.838** |
| 15 | 2-FL(MHe)+OPN(MHe)+DHA(MHe) | 72.00 ± 9.926***∧▽# |
*p < 0.05 compared with blank control group, **p < 0.01 compared with blank control group, ***p < 0.001 compared with blank control group.
∧ Compared with 2'-FL high-dose group p < 0.05.
▽Compared with OPN high-dose group p < 0.05.
# Compared with DHA high-dose group p < 0.05, ## Compared with DHA high-dose group p < 0.01.
a, high dose; b, low dose; c, medium dose; d, low to medium dose; e, medium to high dose.
Nutrition promotes maturation and myelination of OPCs
To measure the effect of mixed nutrient or nutrient treatment alone on the cell maturation and myelination of OPCs, we assessed the number of positive cells labeled MBP after treatment for 30 days.
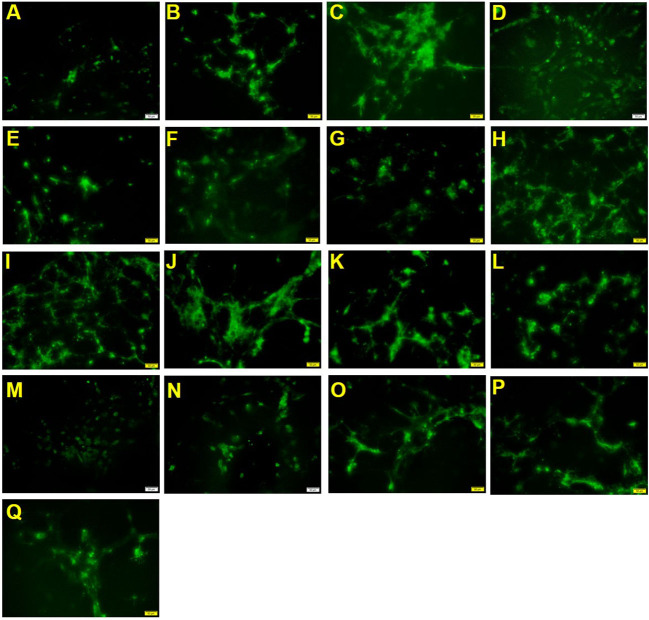
According to Figure 3 and Table 4, the positive drug olesoxime increased the number of MBP-positive cells compared with the blank control group. The 2′-FL, OPN and DHA groups (groups 1, 2 and 3) also could significantly increase the number of MBP-positive cells, which suggests that the components contributed to the maturation of OPCs. For mixed nutrition, 2′-FL + OPN high dose group (group 8), 2′-FL + OPN middle to high dose group (group 10), 2′-FL low dose + OPN medium dose + DHA medium dose (group 12), 2′-FL + OPN + DHA high dose (group 13), and 2′-FL + OPN + DHA medium to high dose (group 15) were significantly higher than 2′-FL, OPN or DHA single dose, indicating that these combinations have synergistic effect in maturation and myelination promotion of OPCs.
Figure 3.
MBP immunostaining of neural primary cells after treatment with 2′-FL and OPN and DHA for 30 days. (A), control group; (B), positive drug; (C), 2′-FL(H); (D), OPN(H); (E), DHA(H); (F), 2′-FL(L)+OPN(L); (G), 2′-FL(L)+OPN(H); (H), 2'-FL(M)+OPN(M); (I), 2′-FL(H)+OPN(L); (J), 2′-FL(H)+OPN(H); (K), 2′-FL(LM)+OPN(LM); (L), 2′-FL(MH) +OPN(MH); (M), 2′-FL(L)+OPN(L)+DHA(L); (N), 2′-FL(L)+OPN(M)+DHA(M); (O), 2′-FL(H)+OPN(H)+DHA(H); (P), 2′-FL(LM)+OPN(LM)+DHA(LM); (Q), 2′-FL(MH)+OPN(MH)+DHA(MH). Scale bar: 50 μm.
Table 4.
Effects of different nutritional compositions containing 2'-FL, OPN and/or DHA on the number of MBP positive cells.
| Groups | Nutrition composition | Density of MBP positive cells (Mean ±SEM) |
|---|---|---|
| Control | Vehicle | 44.333 ± 4.447 |
| Positive | Olesoxime | 82.167 ± 6.226*** |
| 1 | 2-FL(Ha) | 79.00 ± 6.846** |
| 2 | OPN(Ha) | 73.167 ± 6.215* |
| 3 | DHA(Ha) | 79.833 ± 7.507** |
| 4 | 2-FL(Lb)+OPN(Lb) | 65.833 ± 8.479* |
| 5 | 2-FL(Lb)+OPN(Ha) | 82.333 ± 7.961*** |
| 6 | 2-FL(Mc)+OPN(Mc) | 97.667 ± 7.365***▽ |
| 7 | 2-FL(Ha)+ OPN(Lb) | 89.50 ± 8.936*** |
| 8 | 2-FL(Ha)+OPN(Ha) | 128.50 ± 8.221***∧∧∧▽▽▽### |
| 9 | 2-FL(LMd)+OPN(LMd) | 84.667 ± 7.830*** |
| 10 | 2-FL(MHe) +OPN(MHe) | 122.833 ± 8.518***∧∧∧▽▽▽ |
| 11 | 2-FL(Lb)+OPN(Lb)+DHA(Lb) | 72.667 ± 8.135* |
| 12 | 2-FL(Lb)+OPN(Mc)+DHA(Mc) | 102.00 ± 9.331***∧▽# |
| 13 | 2-FL(Ha)+OPN(Ha)+DHA(Ha) | 132.833 ± 10.663***∧∧∧▽▽▽### |
| 14 | 2-FL(LMd)+OPN(LMd)+DHA(LMd) | 97.333 ± 5.818***▽ |
| 15 | 2-FL(MHe)+OPN(MHe)+DHA(MHe) | 135.00 ± 9.913***∧∧∧▽▽▽### |
*p < 0.05 compared with blank control group, **p < 0.01 compared with blank control group, ***p < 0.001 compared with blank control group.
∧Compared with 2′-FL high-dose group p < 0.05, ∧∧∧ compared with 2′-FL high-dose group p < 0.001.
▽Compared with OPN high-dose group p < 0.05, ▽▽▽Compared with OPN high-dose group p < 0.001.
# Compared with DHA high-dose group p < 0.05, ### Compared with DHA high-dose group p81 < 0.001.
a, high dose; b, low dose; c, medium dose; d, low to medium dose; e, medium to high dose.
Human milk oligosaccharide (HMO) is one of the main components of human milk carbohydrates, which is closely related to the health benefits and nutrition of breastfed infants (21). As the most abundant fucosylated HMO, 2'-FL possesses various beneficial health effects as suppressing pathogen infection, regulating intestinal flora, and boosting immunity, making it has remarkable value in nutrition and medicine (22–25). Additionally, it was verified that the intake of 2′-FL affects the cognitive domain and improves the learning and memory ability of rodents (26). Thus, 2′-FL has lots of beneficial health activities. Due to its various physiological and biological effects, 2′-FL has been assessed and authorized as a new food additive to many foods. OPN is an acidic and highly phosphorylated glycoprotein, and expressed in a variety of tissues including liver, skeletal muscle, brain, and mammary gland (27, 28). Previous studies revealed that OPN is significantly involved in immunity system development and regulation. Evidence indicated that OPN plays a key role in some autoimmune, cancer and cardiovascular disease (29–31). Recently, further researches revealed an important function of OPN involving in the regulation of myelination in central nervous system (32, 33). OPN is relatively resistant to digestion, and orally ingested OPN can be absorbed into the circulatory system (33), therefore, making it plays essential roles in the development in early life and an ideal milk powder additive. DHA is well-known for its effects in intellectual development in early life. Although it plays a key role in the growth and maturation of the infant's brain, DHA cannot be synthesized efficiently in the body (34). During rapid phases of brain growth, large amounts of DHA is needed. Thus, adding DHA to milk powder as an external source has become an inevitable choice when the supply of breast milk is insufficient.
Although 2′-FL, OPN and DHA are important for cognitive development as the milk powder additives, the effect of the three components for neuronal maturation, synaptogenesis, and myelination still little is known. In this study, we evaluated the effect of the three nutrients on promoting the myelination, including the proliferation of OPCs, the differentiation and maturation of OPCs into myelinating OLs, of primary neuronal cells in vitro. The results showed that the three components contributed to the proliferation, differentiation, maturation and myelination of OPCs. They have similar effect with the positive nutrient olesoxime in different growth stages of OPCs. When the three components were used in pairs or in combination, these combinations showed a synergistic effect in promotion of OPCs, and the promotion effect was obviously stronger than that of the nutrition treatment alone or even the positive drug olesoxime.
In short, the current study described an in vitro model to test the effects of 2'-FL, OPN, DHA and a nutrient blend consisting of the three nutrients on increasing OPC maturation as well as OL myelination. This most promoting effect was driven by a combined net of nutrient blend, and individual nutrients did not exhibit the same positive effect on myelination, which showing the important of nutrients composition in infant formula. For excise mechanism, the results would need to be further verified with an in vivo experiment (35). Besides, studies of gene or protein expressions in OLs in response to the various individual nutrients as well as the nutrient blend are required to identify the signal pathways involved in the myelination related effects in the future (36).
Conclusions
As the most abundant constituents in human breast milk or infant formula, 2′-FL OPN and DHA have numerous beneficial health effects, especially during the intellectual development of infant. Our work elucidates a pro-myelinative effect of 2′-FL, OPN and DHA with neural primary cell in vitro. The potential mechanism of the nutrients included induction of oligodendrocyte precursor proliferation, differentiation, maturation and myelination via which this effect might be mediated. The study also showed that when the three nutrient components were used in in pair or in combination, their effect on promoting OPCs myelination was significantly better than that of the individual components. The results of this experiment clarified the mechanism of 2′-FL OPN and DHA to promote cognitive development, and provided a solid experimental basis for further optimization of infant formula.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Laboratory Animal Center of Peking University Health Science Center.
Author contributions
QX, YZ, MG, and QZ designed the study and manuscript writing. QX, JZ, and DC did the laboratory work in the expression and statistical analysis. JZ, DC, and QZ contributed to data analysis, interpretation, and the revision of articles critically for important intellectual content. All the authors read and approved the manuscript.
Funding
This research was funded by Technology Major Special Project of Heilongjiang Province: Dairy Products and Meat Processing (No. 2020ZX07B01), Beijing Municipal Excellent Talents Foundation (2018400685627G341), Fengtai Nova Program (KJXX201904), and Beijing Postdoctoral Research Foundation.
Conflict of interest
Authors QX and DC were employed by the company Heilongjiang Feihe Dairy Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. (2014) 72:267–84. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- 2.Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. (2014) 30:503–33. 10.1146/annurev-cellbio-100913-013101 [DOI] [PubMed] [Google Scholar]
- 3.Bercury KK, Macklin WB. Dynamics and mechanisms of CNS myelination. Dev Cell. (2015) 32:447–58. 10.1016/j.devcel.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanig J, Geeta N. Myelin: Structure, function, pathology, and targeted therapeutics. In: Handbook Dev Neurotoxicol (Chapter 4). Silver Spring, MD: Food and Drug Administration; (2018), p. 33–52. [Google Scholar]
- 5.Elizabeth E, Rogers MD, Susan R, Hintz MD. MS Epi Early neurodevelopmental outcomes of extremely preterm infants. Sem Perinatol. (2016) 40:497–509. 10.1053/j.semperi.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 6.Hughes AN, Appel B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat Commun. (2019) 10:4125. 10.1038/s41467-019-12059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson S, Pietsch M, Cordero-Grande L, Price AN, Hutter J, Xiao JX, et al. Development of human white matter pathways in utero over the second and third trimester. PNAS. (2021) 118:e2023598118. 10.1073/pnas.2023598118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. (2001) 24:39–47. 10.1016/S0166-2236(00)01691-X [DOI] [PubMed] [Google Scholar]
- 9.O'Meara RW, Ryan SD, Holly C, Kothary R. Derivation of enriched oligodendrocyte cultures and oligodendrocyte/neuron myelinating co-cultures from post-natal murine tissues. J Vis Exp. (2011) 54:3324. 10.3791/3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aranda-Anzaldo A. The post-mitotic state in neurons correlates with a stable nuclear higher-order structure. Commun Integr Biol. (2012) 5:134–9. 10.4161/cib.18761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prineas JW, Kwon EE, Sternberger NH, Lennon VA. The distribution of myelin-associated glycoprotein and myelin basic protein in actively demyelinating multiple sclerosis lesions. J Neuroimmunol. (1984) 6:251–64. 10.1016/0165-5728(84)90012-2 [DOI] [PubMed] [Google Scholar]
- 12.Pronker MF, Lemstra S, Snijder J, Heck AJR, Thies-Weesie DME, Pasterkamp RJ, et al. Structural basis of myelin-associated glycoprotein adhesion and signaling. Nat Commun. (2016) 7:13584. 10.1038/ncomms13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng Y, Rademacher C. Nycholat CM, Futakawa S, Lemme K, Ernst B. High affinity sialoside ligands of myelin associated glycoprotein. Bioorg Med Chem Lett. (2011) 21:5045–9. 10.1016/j.bmcl.2011.04.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fruttiger M, Montag D, Schachner M, Martini R. Crucial role for the myelin-associated glycoprotein in the maintenance of axon-myelin integrity. Eur J Neurosci. (1995) 7:511–5. 10.1111/j.1460-9568.1995.tb00347.x [DOI] [PubMed] [Google Scholar]
- 15.Boggs JM. Myelin basic protein: a multifunctional protein. Cell Mol Life Sci CMLS. (2006) 63:1945–61. 10.1007/s00018-006-6094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahara J, Tan-Takeuchi K, Seiwa C, Yagi T, Aiso S, Kawamura K, et al. Myelin basic protein is necessary for the regulation of myelin-associated glycoprotein expression in mouse oligodendroglia. Neurosci Lett. (2001) 298:163–6. 10.1016/S0304-3940(00)01745-6 [DOI] [PubMed] [Google Scholar]
- 17.Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. (2018) 59:17–24. 10.1016/j.clinbiochem.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 18.Zhu YY, Wan L, Li W, Ni DW, Zhang WL, Yan X, et al. Recent advances on 2'-fucosyllactose: Physiological properties, applications, and production approaches. Crit Rev Food Sci Nutr. (2022) 62:2083–92. [DOI] [PubMed] [Google Scholar]
- 19.Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachère JC, Bégin ME, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostagl Leukot Ess Fatty Acids. (2006) 75:213–20. 10.1016/j.plefa.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 20.Hauser J, Sultan S, Rytz A, Steiner P, Schneider N. A blend containing docosahexaenoic acid, arachidonic acid, vitamin B12, vitamin B9, iron and sphingomyelin promotes myelination in an in vitro model. Nutr Neurosci. (2020) 23:931–45. 10.1080/1028415X.2019.1580918 [DOI] [PubMed] [Google Scholar]
- 21.Zhou WT, Jiang H, Wang LL, Liang XX, Mao XZ. Biotechnological production of 2'-fucosyllactose: a prevalent fucosylated human milk oligosaccharide. ACS Synth Biol. (2021) 10:447–58. 10.1021/acssynbio.0c00645 [DOI] [PubMed] [Google Scholar]
- 22.Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. (2015) 91:619–22. 10.1016/j.earlhumdev.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. (2005) 25:37–58. 10.1146/annurev.nutr.25.050304.092553 [DOI] [PubMed] [Google Scholar]
- 24.Autran CA, Schoterman MH, Jantscher-Krenn E, Kamerling JP, Bode L. Sialylated galacto-oligosaccharides and 2′-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br J Nutr. (2016) 116:294–9. 10.1017/S0007114516002038 [DOI] [PubMed] [Google Scholar]
- 25.Good M, Sodhi CP, Yamaguchi Y, Jia H, Lu P, Fulton WB, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr. (2016) 116:1175–87. 10.1017/S0007114516002944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vazquez E, Barranco A, Ramirez M, Gruart A, DelgadoGarcia JM, Martinez-Lara E, et al. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem. (2015) 26:455–65. 10.1016/j.jnutbio.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 27.Jiang R, Lönnerdal B. Biological roles of milk osteopontin. Curr Opin Clin Nutr Metab Care. (2016) 19:214–9. 10.1097/MCO.0000000000000275 [DOI] [PubMed] [Google Scholar]
- 28.Kahles F, Findeisen HM, Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. (2014) 3:384–93. 10.1016/j.molmet.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. (2008) 181:7480–8. 10.4049/jimmunol.181.11.7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rittling SR, Singh R. Osteopontin in immune-mediated diseases. J Dent Res. (2015) 94:1638–45. 10.1177/0022034515605270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waller AH, Sanchez-Ross M, Kaluski E, Klapholz M. Osteopontin in cardiovascular disease: a potential therapeutic target. Cardiol Rev. (2010) 18:125–31. 10.1097/CRD.0b013e3181cfb646 [DOI] [PubMed] [Google Scholar]
- 32.Selvaraju R, Bernasconi L, Losberger C, Graber P, Kadi L, Avellana-Adalid V, et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol Cell Neurosci. (2004) 25:707–21. 10.1016/j.mcn.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 33.Jiang RL, Lönnerdal B. Effects of milk osteopontin on intestine, neurodevelopment, and immunity. Nestle Nutr Inst Workshop Ser. (2020) 94:152–7. 10.1159/000505067 [DOI] [PubMed] [Google Scholar]
- 34.Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. (2016) 8:99. 10.3390/nu8020099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Zhao X, Li R, Yang HS. Integrated metabolomics and transcriptomics reveal the adaptive responses of Salmonella enterica serovar Typhimurium to thyme and cinnamon oils. Food Res Int. (2022) 157:111241. 10.1016/j.foodres.2022.111241 [DOI] [PubMed] [Google Scholar]
- 36.Li SB, Tian YF, Jiang P, Lin Y, Liu XL, Yang HS. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit Rev Food Sci Nutr. (2021) 61:1448–69. 10.1080/10408398.2020.1761287 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.