Abstract
Objective
The aim of this study was to evaluate the change in fasting blood sugar (FBS) over time and its determinants in diabetic patients.
Methods
A longitudinal data analysis retrospective‐based study was considered with a sample of 312 patients, and the linear mixed effect model was applied.
Results
Based on the linear mixed model, the 3‐month change in time decreases the average FBS level by 0.0111. An increase of one unit of body mass index (BMI) increases the FBS level by 0.0434. Similarly, an increase in blood pressure (DBP) per unit increased the average log FBS level by 0.0005. Secondary and higher education levels lower log FBS levels by 99.41% and 99.45%, respectively, compared with noneducated individuals.
Conclusion
The study showed that hypertension history, type of diet, age, status of education, type of drug, body mass index, diastolic blood pressure, and time were statistically significant factors.
Implications
According to the study, eating a healthy diet, maintaining a healthy body weight, and a low blood sugar level are essential to controlling blood sugar and preventing long‐term complications. The government should build an educational institution proportional to the population and open programs to increase awareness about the prevention mechanism of diabetes in communities.
Keywords: diabetes mellitus, fasting blood sugar, linear mixed model, longitudinal data analysis
1. INTRODUCTION
Diabetes mellitus (DM) is a lifelong disease that affects human health whether the pancreas does not produce enough insulin (the hormone that controls blood sugar), or the body cannot use insulin effectively. 1 DM is a chronic and progressive disease with an increase in blood sugar levels. 2 Diabetes can affect many parts of the body, including blood vessels, eyes, the heart, kidneys, and nerves. This also increases the overall risk of dying. 1 , 2 , 3 , 4 The typical signs of diabetes disease include inflated urine, water thirst, persistent hunger, loss of weight, changes in vision, and fatigue. 5
Diabetic diseases are divided into three main categories according to their origin and cause: gestational diabetes, Type 2 diabetes, and Type 1 diabetes. 6 When the body does not produce the necessary insulin, Type 1 diabetes develops, on the other hand, Type 1 diabetes develops when the body produces an elevated level of blood sugar or sugar due to errors in insulin synthesis or inadequate insulin. 6 Unlike the above two, gestational diabetes is a disease of diabetes that occurs when the mother becomes pregnant 7 , 8 This study focused on the first two types of diabetes, specifically Type 1 and Type 2 diabetes. The number of people with diabetes has continuously increased in recent decades due to population growth, an aging population, and an increase in the prevalence of diabetes at all ages. 1
Currently, 463 million people between the ages of 20 and 79 in the world have diabetes. 9 In 2019, out of the 463 million people with diabetes (Type 2 diabetes), 50% or 231.9 million, were unaware that they had the disease. Around 79.4% of diabetic patients worldwide reside in low‐ and middle‐income countries, and the majority are between the ages of 40 and 59.
The South East Asia and Western Pacific regions have the largest numbers of diabetes patients, and represent half of the diabetes cases in the world. 10 , 11 According to estimates, 4.2 million people aged 20 to 79 died from diabetes in 2019. This indicates that a person with diabetes dies every 8 s. Among all death causes in the world, diabetes scored 11.3%. Almost half (46.2%) of the deaths associated with diabetes are in people under 60 years of age in the working age group. 12
According to a recent report, 19.4 million adult Africans between the ages of 20 and 79 had diabetes. 12 Similarly, in this region, 45.9% of people with diabetes live in low‐income countries, and 54.1% of diabetics live in middle‐income countries. 12 In Africa, adults aged 65 to 69 account for 8.8% of the prevalence of diabetes, and 59.7% of the diseased individuals are ignorant of it. 12
In Ethiopia, the number of people with diabetes consistently increases. For example, according to the Global Estimated Prevalence of Diabetes, the prevalence of diabetes among adult Ethiopians increased and was 4.4% in 2013. 1 According to the report of the International Diabetes Federation (IDF) report, there were 2,652,129 cases of diabetes in 2017 in Ethiopia. This placed Ethiopia among the top five countries in Africa. 5 Likewise, the International Diabetes Federation Atlas (IDFA) in 2019 reported that there were 1.9 million diabetes cases whose age rolled from 20 to 79 years, and 1.2 million of them were diabetic patients from rural areas. In the same way, 34,262 deaths related to diabetes occurred. 12 Additionally, the frequency of diabetes among the towns of Ethiopia is increasing dangerously. 13
The development of diabetes‐related diagnostic and treatment tools has led to a reduction in diabetes deaths and related complications. Managing blood sugar includes managing heart disease, stroke, retinal diseases, loss of vision, chronic kidney diseases and high blood pressure. However, it is impossible to prevent all complications completely. 14
According to Belete et al. 15 the metabolic syndrome affects more than a quarter of people with Type 1 diabetes. Furthermore, another study investigating the effects of diabetes on bone health showed that long‐term exposure to diabetes affected bone metabolism, and it changes the microarchitecture of bone through various molecular and structural mechanisms. 16 Moreover, a study also indicated that hospital mortality is more severe due to diabetes. 17 In Greece, a study compared six meals a day with three meals a day. The authors of this study conclude that six versus six meals a day can increase glycemic control in obese patients with early stage. Type 2 diabetes improves and stabilizes postpregnancy sugar regulation in prediabetic subjects. 18 Patient education is the most important factor associated with diabetes. Education is used as the exchange of tools and information practices that meet the needs of the patient, and it is the most crucial factor associated with diabetics that could be achieved through education. 19
Low income, poor adherence, and poor glycemic control were found to be modifiable predictors of diabetes complications, while age and type of diabetes are determining factors that cannot be changed; they are determined as nonmodifiable determinants. 20 Diabetes is associated with high blood pressure, smoking, body fat, body mass index, and total cholesterol 21 , 22 As the prevalence of Type 2 diabetes increases compared to other diseases, it affects the shorter life expectancy of people. 23 Elderly people are more likely to develop diabetes related to obesity, high cholesterol, low lipoproteins, and high total cholesterol. 24 Another study showed that being a man, attaining education, monthly average income, and undergoing previous diabetes training are related to a high awareness of the diseases of the participants. 19 , 25
Similarly, a study conducted in Debre Berhan using a generalized linear mixed model reported that: age, sex, time, time to illiterates, time with primary, time with address, and time with age are statistically significant factors in the progression of fast blood sugar levels. 26 Similarly, a study using a linear mixed effects model conducted in a comprehensive specialized hospital of the University of Gondar showed that the household history of diabetes, food type, age, educational status, treatment, physical exercise, alcohol consumption, and body mass index are important factors in the random blood sugar level of diabetes over time 22 , 27 , 28 In the same way, a study of the incidence and risk factors of diabetes showed that smoking habits, high blood pressure, age, body mass index, waist circumference, and overall cholesterol are significant factors in diabetes mellitus. 29 A study conducted in Uganda showed that women between the ages of 61 and 65 become more diabetic. Variables such as diabetes history of the family, obesity, and overweight were identified as risk factors for type 2 diabetes. 30 Furthermore, a study conducted in Ethiopia investigated: behavioral factors (medication adherence and diabetic self‐care activity), sociodemographic factors (marital status, age, and residence), and clinical factors (such as diabetes mellitus complications and depression), that are caused by fasting blood sugar (FBS) are factors associated with diabetes diseases 31 , 32 The risk factors for developing diabetes were differ significantly among the population with BMI in Japanese populations. 33 Factors such as age, illiteracy, smoking, BMI ≥ 25, DM family history, hypertension history, and physical inactivity have been identified as risk factors for Type 2 DM. 34
Most of the earlier research, which were relied on cross‐sectional data, focused on the incidence and prevalence of diabetes. However, being unable to account for correlation between data leads to bias in inference and parameter estimation. Longitudinal studies are appropriate to model FBS variation and to identify associated risk factors over time taking into account the correlation of FBS levels within a patient. Although few longitudinal studies on fasting blood sugar change were conducted, for example, a study from Ghana 35 had conducted on the mixed effects model for the longitudinal study of Type 2 diabetes, but did not address sufficient baseline clinical/laboratory characteristics in the study that can significantly affect FBS change over time in diabetes patients. Therefore, the gap was addressed in this study with an appropriate variance covariance structure in the core of the linear mixed model. To our knowledge, no research has been conducted using longitudinal data analysis on fasting blood sugar change in diabetic patients, particularly within the Adama study area, Ethiopia. This is the research gaps that were filled in this study, with the research gap mentioned above. Therefore, the main objective of this study was to model the variation of fasting blood sugar over time among diabetic patients. The results of this study are providing information to public health professionals and personnel of the DM patient monitoring system. Furthermore, the study serves as the baseline for longitudinal FBS research for future research in the study area.
2. MATERIALS AND METHODS
2.1. Study area
The study area of this research was the diabetes clinic of Adama Hospital Medical College, which is located 99 km from southeast Addis Ababa, the capital city of Ethiopia. The target population of this study was all patients with newly diagnosed Type 1 and 2 DM who had been active in follow‐up treatment in a 3‐month interval from September 1, 2018, to August 30, 2019 at Adama Hospital. The medical records of the diabetic patients during the specified period were extracted from the patient's charts. All diabetic patients who were 18 years or older were coming to attend their treatment at Adama Hospital Medical College during the study period. However, only patients with at least two visits to the longitudinal response were eligible for this study.
2.2. Data
The samples were selected using a simple random sampling technique from a sampling frame of the identification number of DM patients. The number of measurements was not equal for all patients due to the time difference in the follow‐up. All patients who underwent measurements at baseline (time zero), 3, 6, 9, 12, 15, 18, 21, and 24 months, with a 3‐month gap between each measurement. Check considered the Diggle formula was used to determine the study sample size. 36
| (1) |
Where N is the total sample size, d is the effect size sample (0.8), m is the number of time points of repeated measurements, ρ is the correlation between repeated measurements, and σ2 is the variance of outcome variables all taken from the previous study. Assuming a significance level of 0.05, the power of the study was 0.8, m = 9, which is the number of time points for repeated measurements. ρ = 0.5 and effect size of 0.8, σ2 = 12.72 (random intercept model) 37 Using Zα/2 = 1.96, Zβ = 0.842 and inserting all quantities in the formula.
The outcome variable is the FBS level measured in milligrams per deciliter for each subject over a repeated time. Age, sex, marital status, residence, education level, exercise activity, frequency of meals, dietary type, history of hypertension, alcohol use, body mass index of patients, diastolic blood pressure, and comorbid condition were all predicted predictor variables. 38 , 39 , 40 , 41 , 42 , 43
2.3. Methods
For longitudinal data, there are two sources of variation: within‐subject variation, variation in measurements within each subject, which allows the study of changes over time and between‐subject variation, variation in data between different subjects. 44 In longitudinal data analysis, observations of an individual are correlated with one another due to multiple measurements being taken on the same subject at various time points. 36 As well as from clustering measurements taken on individuals who share a common category or characteristic results in correlation. Longitudinal data can be continuous or discrete binary. 36 This study focused on both cases of binary and continuous repeated measurements.
Individual profiles (individual FBS trajectory), the average structure (time‐average response), variance‐covariance structure, and correlation structure were performed using independently and identically distributed error term, and randomly occurring effects, were performed in this study as exploratory data analysis.
Mixed models are useful and versatile tools for analyzing data with complicated covariation patterns. 45 The linear mixed effect model for data from longitudinal measurements approves the intent that the response composition of an individual depends on some attributes of that individual, including: some hidden effects are then included as random variables or as equivalent to the so‐called random effects in the model 46 , 47 The mixed model has two components, namely the fixed effect model or average model and the random model. Depending on the way that variables occur in the study explanatory variables categorized either from fixed effects or random components. 44 It can also be used to calculate data that has different measurement numbers for each individual. Random effect is one effect among a population of effects, but the fixed effect is thoughtful as invariant that we aim to measure. 48
In general, linear mixed models are written as
| (2) |
Where Yi is the response vector for the ith subject, Xi is the nixp fixed between‐subject design matrix, β is the p × 1 vector of fixed effects (averaged by population) assumed common for all subjects, Zi is the random design matrix nixq within subjects (specific design matrix of subjects). bi is b1…, bn independent random component parameters with q‐dimensional vectors and we can express statistically as bi ~ N(0, D), ɛi is the error term of the model with ni row vectors, it is also random, and ɛi ~ N(0, Ʃi), D and Ʃi are variance covariance components. 49
Restricted maximum likelihood estimation (REML) and maximum likelihood estimation (ML) methods were applied to estimate the model parameters. Both the model and variance parameters are included under the maximum likelihood estimation technique. In other words, the terms of the fixed effect and the random effect of the probability (likelihood) function. The coefficients of the model variables treated as fixed and not known quantities when the variance parameters of the fixed effects are estimating the degree of freedom are not taken into account. As a result, the maximum likelihood estimates have lower variances, and are biased.
The estimates of D and Ʃi are constant values of the model coefficients and less sensitive to extreme values than the estimates of maximum likelihood. However, in the REML estimation technique, only the variance parameters are included. In estimating the parameters of the fixed effects in the linear mixed effects model, the degree of freedom that parameterize the random effects is lost. Therefore, the estimation of the variance for random effects was less biased. The log‐likelihood function of maximum likelihood and restricted maximum likelihood function for Ʃi and D are given as below.
| (3) |
Using a Newton‐Raphson algorithm stabilized on the ridge to reduce the above equation twice, we may determine the variance value (V). After determining the variance, the equation used to estimate the variable coefficients in the linear mixed model are obtained as follows:
| (4) |
By simplifying Equation (4) above, one can get the estimate of
Missing data may hide values in an actual estimation, and ignoring missing values leads to biased estimates. Multiple imputations are the methods which are used to handle missing data values. It substitutes all the missing values with five or more acceptable values, which represents the distribution of possibilities. 50 The multiple calculation conclusion consists of three different phases 50 : The first step is to fill the missing data m times to create the absolute data set, then using appropriate methods, analyze the m data set, and then combine the results of the m data set to draw conclusions.
In these investigations, the models were compared to see which model is better suited to the data. Although there are different techniques to select the best‐fitted model, this paper used the likelihood ratio test, the Bayesian information criterion (BIC), and the Akaike information criterion (AIC). A better understanding was also obtained by examining the plot of the standardized residuals versus fitted values. 51 , 52 The normality assumption of the error within the group was diagnosed using the quantile plot of the residuals of covariates. The hypothesis for the statistical tests was a two‐sided test. Data was entered and cleaned using SPSS version 23, and they were exported to R statistical software version 3.6.1 and SAS 9.2 statistical software for further analysis.
3. RESULTS AND DISCUSSION
3.1. Results
This study discovered that the FBS level was significantly above the norm at all periods, which is contradicting with the presumption of normality. From Figure 1, the box plot of the actual FBS data showed that the distribution of FBS skewed to the right (high FBS level), especially for minor points. Hence, the transformation of FBS was needed. The outliers are reduced after the right‐side plot of the logarithmic transformation. Therefore, the logarithmically transformed FBS level is better than the actual FBS.
Figure 1.
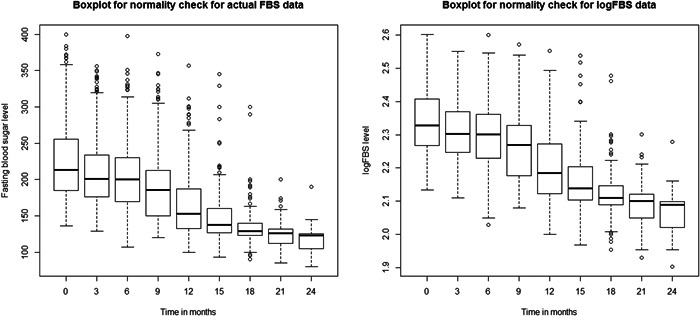
Box plot of fasting blood sugar (FBS) and logarithmic of FBS of diabetes mellitus patients
Figure 2 indicates that the linear time effect decreases and there is no need for other forms of time effects as observed that there was high variability between patients, but there was low variability within patients over time. It is also observed from this graph that there was a fluctuation in the FBS of the patients over time. The variation at the beginning was higher than at the end of the follow‐up time for patients. A linear mixed model was used to effectively fit the data, which have heterogeneity in the intercept and slope of the trajectories.
Figure 2.
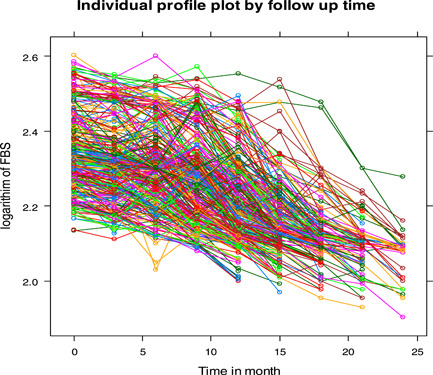
Individual profile for logarithmic transformed fasting blood sugar level of diabetes mellitus patients
Figure 3 reveals the mean profile plot of log FBS level over time. Generally, as one can easily understand from the mean profile plot, the log FBS level of these patients showed linear decreases over the treatment time. The mean log FBS level of the patients seems to decrease and showed a linear pattern over time for all covariates, even if the amount varies from group to group within a covariate.
Figure 3.
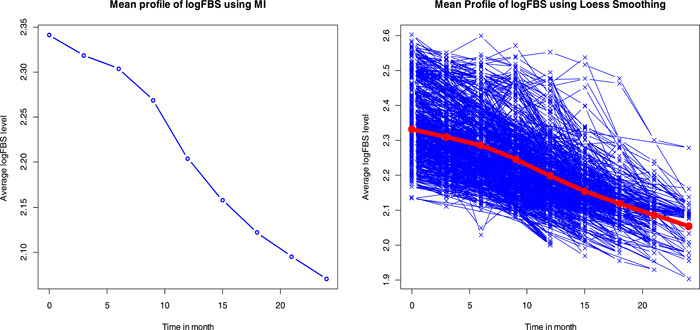
Mean profile plot of logarithm of fasting blood sugar level over time using Loess smoothing and MI
The variance structure for logarithmic FBS showed an irregular pattern over time. As Figure 4 showed, there was high variability at the beginning of the study but low variation between patients at the end of the follow‐up time. The variance of FBS levels of patients fluctuates over follow‐up time.
Figure 4.
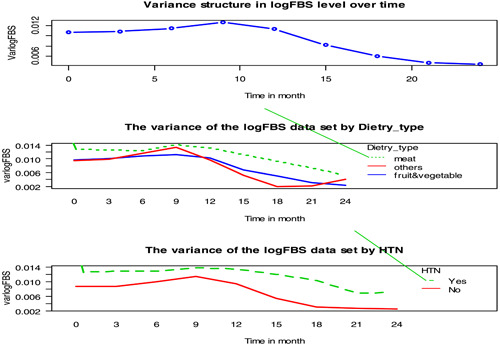
Variance profile plot of log fasting blood sugar level by follow up time for some categorical covariates
The correlation matrix shown in Table 1 revealed a positive correlation between any two repeated measurements and the correlation deemed decreasing over time. Since the off‐diagonal correlation has no constant value over time, it gives clues about the unstructured correlation.
Table 1.
Correlation structure matrix
| logFBS0 | logFBS3 | logFBS6 | logFBS9 | logFBS12 | logFBS15 | logFBS18 | logFBS21 | logFBS24 | |
|---|---|---|---|---|---|---|---|---|---|
| logFBS0 | 1.0000 | ||||||||
| logFBS3 | 0.9303 | 1.0000 | |||||||
| logFBS6 | 0.8051 | 0.8617 | 1.0000 | ||||||
| logFBS9 | 0.8676 | 0.8819 | 0.8118 | 1.0000 | |||||
| logFBS12 | 0.7766 | 0.8294 | 0.8006 | 0.8394 | 1.0000 | ||||
| logFBS15 | 0.6097 | 0.6662 | 0.6484 | 0.7311 | 0.7685 | 1.0000 | |||
| logFBS18 | 0.5911 | 0.6574 | 0.6068 | 0.6885 | 0.7662 | 0.8585 | 1.0000 | ||
| logFBS21 | 0.5516 | 0.5990 | 0.5571 | 0.6154 | 0.6776 | 0.7803 | 0.8870 | 1.0000 | |
| logFBS24 | 0.3817 | 0.4376 | 0.3764 | 0.4048 | 0.5183 | 0.6210 | 0.7434 | 0.8238 | 1.0000 |
Compared to AIC and BIC, the correlation structure with the smaller value was the unstructured correlation structure that best explained the model in the study shown in Table 2. Therefore, the unstructured variance covariance is used in identifying the correlation structure.
Table 2.
Correlation structure checking
| Compound symmetry | Unstructured | AR (1) | |
|---|---|---|---|
| AIC | 5831.52 | 5783.03 | 5912.65 |
| BIC | 5713.93 | 5689.13 | 5782.60 |
Abbreviations: AIC, Akaike information criterion; BIC, Bayesian information criterion.
AIC and the likelihood ratio test were used to evaluate the random intercept, and slope models. As shown in Table 3, the inclusion of the random intercept and the random slope is reasonable. So, in the final model, both random intercept and random slope with linear time effect were considered for this study.
Table 3.
Comparison of random effect model
| Random effects model | AIC | Likelihood ratio‐test |
|---|---|---|
| Model 1 intercept | 5534.4 | 2808.2 |
| Model 2 intercept, time | 5277 | 2677.7 |
From Table 4, the variances of the intercepts and linear effects of time were significantly different from zero. These showed that the values of the logarithmic FBS level at the beginning of the study vary between subjects, and the change in the logarithmic FBS level over time varies between subjects. This indicated that the random intercepts exhibited the highest level of variability. It also showed that the standard deviation of the random intercepts was higher than that of the random slopes, indicating that between‐patient variability is higher than within‐patient variability. The value of the intraclass correlation is the ratio of the between‐patients variance to the total variance presented in Table 5. It tells us that the proportion of the total variance in the log FBS level (68.7%), which is accounted for the variance among patients. The value of the intraclass correlation revealed that the mixed model is appropriate to fit the data.
Table 4.
Random parameter estimates for FBS data set
| Groups | Name | Standard deviation | Intraclass corr. |
|---|---|---|---|
| Subject | (Intercept) | 0.105 | |
| Time | 0.004 | 0.687 | |
| Residual | 0.04943 |
Abbreviation: FBS, fasting blood sugar.
Table 5.
Parameter estimates for full linear mixed‐effects model with both random intercept and slope model
| Effect | Estimate | St. error | DF | t Value | p Value |
|---|---|---|---|---|---|
| Intercept | 1.9439499 | 0.018031159 | 1795 | 107.8105 | <0.001** |
| Age | −0.0002012 | 0.000212467 | 292 | −0.94697 | 0.03* |
| Sex male | −0.0025840 | 0.004581226 | 292 | −0.56404 | 0.57 |
| Educational_status primary | −0.0018078 | 0.006890196 | 292 | −0.26238 | 0.79 |
| Educational_status secondary | −0.0055161 | 0.006625390 | 292 | −0.83257 | 0.04* |
| Educational_status tertiary | −0.0058844 | 0.006963855 | 292 | −0.84500 | 0.04* |
| Marital_status married | 0.0121576 | 0.005556352 | 292 | 2.18805 | 0.29 |
| Marital_status divorced | 0.0049914 | 0.007617271 | 292 | 0.65528 | 0.51 |
| Marital_status widowed | 0.0024773 | 0.010917391 | 292 | 0.22692 | 0.82 |
| Residence urban | 0.0048482 | 0.004746779 | 292 | 1.02138 | 0.30 |
| Dietry_type meat | 0.0027650 | 0.004710933 | 292 | 0.58694 | 0.04* |
| Dietry_type others | −0.0029909 | 0.005119327 | 292 | −0.58423 | 0.56 |
| Exercise_activity perform exercise | −0.0036818 | .005100623 | 292 | −0.72184 | 0.47 |
| Drug_type OHA | 0.0018733 | 0.005500709 | 292 | 0.34056 | 0.73 |
| Drug_type both | −0.0069765 | 0.004634013 | 292 | −1.50549 | 0.01* |
| HTN yes | 0.0062799 | 0.005408709 | 292 | 1.16107 | 0.02* |
| Alcohol_status yes | 0.0056387 | 0.004998443 | 292 | 1.12808 | 0.26 |
| DBP | 0.0004749 | 0.000208397 | 292 | 2.27878 | 0.02* |
| BMI | 0.0009951 | 0.000731718 | 1795 | 1.35992 | 0.04* |
| Time | −0.0111267 | 0.002707189 | 1795 | −4.11007 | <0.001** |
| Other_commorbid yes | 0.0043492 | 0.004075806 | 292 | 1.06709 | 0.28 |
Abbreviation: OHA, Oral Hyperglycemia Agents.
Is significant at 1% level of significant and,
Is significant at 5% level of significant.
Figure 5 illustrates how the residual plots were utilized in this study to assess the validity of the model assumptions. The quantile plot shows that the residuals do not exhibit a departure from normality. The “Residual fit” concentrated around zero implies that a linear mixed effect model is well fitted to the FBS data.
Figure 5.
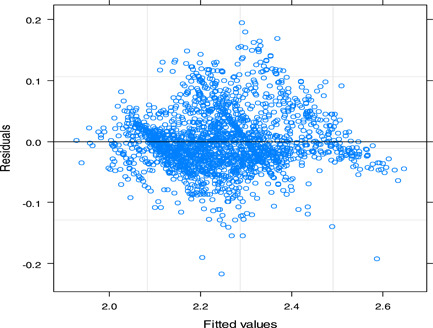
Residuals plot for logarithm of fasting blood sugar level data
After the reasonable exploratory data analysis, the variables were selected in the univariate and fixed effects model using the backward elimination method. Table 5 shows the results of the estimate of restricted maximum probability (likelihood) of a linear mixed effects model.
The statistical computation of linear mixed model shows the following outputs. For a given patient, maintaining the impact of other continuous covariates as constant over 3 months will reduce the average log FBS level to 0.0111267 mg/dl. After 3 months of treatment, the age of the patients increased and the predicted value of the log FBS level decreased from 0.00002 mg/dl. On the other hand, when the patient's body mass index shows a unit increment, the average logarithm of FBS level increases to 0.0434 mg/dl. Likewise, with an increase in unit DBP, when the effect of other factors remains unchanged, the logarithmic level of FBS increases to 0.0004749 mg/dl. Secondary and higher education levels have reduced average log FBS levels to 0.0058844 and 0.0055161, respectively, compared to uneducated patients (reference groups). Drug type has an undeniable effect on the change of logarithm of FBS levels: patients who take a combination of Oral Hyperglycemia Agents (OHA) and insulin drug lowered their average logarithm of FBS levels by 0.0069765 amount compared to patients who take insulin drug (the reference group), keeping the effect of other covariates constant.
4. DISCUSSION
From the mixed linear model, the study revealed that the age of a patient has a negative correlation with the value of the FBS level (p = 0.03), and this is a similar finding to 28 and contrasts with the study of. 24 Individuals who had a history of hypertension increased FBS levels more than individuals who had no history of hypertension with a p value of 0.02. This result is consistent with the findings of. 21 , 29 Furthermore, the body mass index (p = 0.04) of a patient has a positive association with FBS level, and hence elevated body mass index increases the risk of developing diabetes complications. This is consistent with the studies conducted by. 22 , 28 , 29 , 34 The educational status indicated that the highest educated people (secondary and tertiary levels of education) have a better understanding of disease management and treatment plans with significantly higher changes in FBS level compared to patients without education. The result of the present variable agrees with the study conducted by authors [ 19 , 25 , 28 ].
Patients who frequently use meat have a significantly (p = 0.04) higher increase in the mean change of FBS levels than other types of dietary meals. People who eat primarily meat were exposed to DM disease and could not control FBS to reach FBS due to a lack of mineral content for the protection of the disease. A diet which is high in cereals, vegetables, and fruits is recommended to lower blood sugar levels while diets high in proteins, eggs, and meat raise FBS levels which is linked to diabetes and its complications. This finding is consistent with. 27 , 28 The type of drug (insulin and OHA) has a negative significant (p = 0.01) effect on the change of FBS level patients who take a combination of (insulin + OHA) drug lowers their FBS level.
5. CONCLUSION
In this research, we demonstrated a linear mixed‐effects model (random intercept and random slope) with an unstructured covariance structure for the rate of change in logarithmic level (FBS) experienced by patients over the treatment time. Concerning time, we found that the pattern of an average FBS level revealed a linear decrement over time, confirming with the model that the estimate of time was negative. This study illustrated that 68.7% of the variability in log FBS levels was due to the variability between patients.
Finally, we conclude that time (duration of follow‐up), BMI, diastolic blood pressure, dietary type, education status, age, history of hypertension, and drug type were found to be significant predictors of the FBS level variation over time. According to the results, patients who had no history of hypertension, lower BMI, higher educational attainment, and who used a combination of insulin and OHA drugs were better able to manage and control their FBS levels in their body.
According to the study, maintaining healthy body weight and taking a healthy diet along with lower blood sugar levels are essential to control blood sugar in the body and to prevent long‐term complications. Educated patients can manage their blood sugar levels more than uninformed individuals. Therefore, the government should spread education and educate people about the prevention mechanism of diabetes in communities in the study area.
6. LIMITATIONS
The main limitation of the study was that the data on some predictors such as the patient's income status, side effects from medication, illness, and stress that may have influenced the rate of change in FBS levels were not available due to retrospective nature of the data. The sample was recruited at a hospital where diabetic patients with high self‐awareness and self‐care visit for follow‐up, so it seems to be over the representativeness of the population.
AUTHOR CONTRIBUTIONS
Abdulmenan Mohammed Abdulahi: Conceptualization; data curation; formal analysis; methodology; visualization; writing – original draft; writing – review & editing. Aragaw Eshetie Aguade: Investigation; methodology; supervision; validation; writing – review & editing. Hunachew Kibret Yohannis: Formal analysis; investigation; supervision; validation; visualization; writing – original draft; writing – review & editing. All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All methods are carried out by relevant guidelines and regulations of the journal. An ethical letter was taken from the University of Gondar, College of Natural and Computational Sciences ethical committee with ref no. CNCS/10 628/19/05/2020 and the committee had approved the study. Adama Hospital Medical college's ethical review board waived the intended consent of individual patients on 28/09/2012 because we used secondary data from hospital patient medical charts.
TRANSPARENCY STATEMENT
The lead author Hunachew Kibret Yohannis affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors are grateful to the Adama Hospital Medical College for providing us with the required data for our work.
Abdulahi AM, Aguade AE, Yohannis HK. Longitudinal modeling of fasting blood sugar with diabetes: a case study of Adama Hospital Medical College, Ethiopia. Health Sci Rep. 2022;5:e951. 10.1002/hsr2.951
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are not publicly available because the data were used for another analysis, but are available from the first author on reasonable request. The guarantor had full access to all data in this study and takes complete responsibility for the integrity of the data and the precision of the data analysis.
REFERENCES
- 1. World Health Organization . Noncommunicable diseases country profiles. 2018.
- 2. Nazir MA, AlGhamdi L, AlKadi M, AlBeajan N, AlRashoudi L, AlHussan M. The burden of diabetes, its oral complications and their prevention and management. Open Access Maced J Med Sci. 2018;6:1545‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarigan TE, Purwaningsih E, Abdullah M, et al. Estimating the incidence of prediabetes and type 2 diabetes among taxi drivers in Indonesia. J Nat Sci Biol Med. 2019;10(3):150‐153. [Google Scholar]
- 4. Al‐Mofarji ST, Hussien H, Mohamed N, Hantoosh S, Abass M, Ali A. The association between gastric bacterial infection and low level of vitamin D among patients with type 2 diabetes mellitus. Baghdad J Biochem Appl Biol Sci. 2021;2(1):39‐47. [Google Scholar]
- 5. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 6. Park HC, Lee YK, Cho A, et al. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS One. 2019;14(7):e0220506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006.
- 8. Petersmann A, Müller‐Wieland D, Müller UA, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127(S 01):1. [DOI] [PubMed] [Google Scholar]
- 9. Roglic G. WHO Global report on diabetes: a summary. Int J Noncommun Dis. 2016;1:3. [Google Scholar]
- 10. Megerssa YC, Gebre MW, Birru SK, Goshu AR, Tesfaye DY. Prevalence of undiagnosed diabetes mellitus and its risk factors in selected institutions at Bishoftu Town, East Shoa. Ethiopia J Diabetes Metab . 2013;S12 (008):1‐7. 10.4172/2155-6156.S12-008 [DOI] [Google Scholar]
- 11. Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107842. [DOI] [PubMed] [Google Scholar]
- 12. Federation ID. IDF diabetes atlas ninth. Dunia: IDF . 2019;9:168. [Google Scholar]
- 13. Dereje N, Earsido A, Temam L, Abebe A. Prevalence and associated factors of diabetes mellitus in Hosanna town, Southern Ethiopia. Ann Glob Health. 2020;86(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahmoudi A. Effects of self care planning on reduction of A1C hemoglobin in adults with diabetes mellitus. Med Sci. 2006;16:171‐176. [Google Scholar]
- 15. Belete R, Ataro Z, Abdu A, Sheleme M. Global prevalence of metabolic syndrome among patients with Type 1 diabetes mellitus: a systematic review and meta‐analysis. Diabetol Metab Syndr. 2021;13(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray CE, Coleman CM. Impact of diabetes mellitus on bone health. Int J Mol Sci. 2019;20(19):4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmitt VH, Hobohm L, Münzel T, Wenzel P, Gori T, Keller K. Impact of diabetes mellitus on mortality rates and outcomes in myocardial infarction. Diabetes Metab. 2021;47(4):101211. [DOI] [PubMed] [Google Scholar]
- 18. Papakonstantinou E, Kontogianni MD, Mitrou P, et al. Effects of 6 vs 3 eucaloric meal patterns on glycaemic control and satiety in people with impaired glucose tolerance or overt type 2 diabetes: a randomized trial. Diabetes Metab. 2018;44(3):226‐34. [DOI] [PubMed] [Google Scholar]
- 19. Kocak MZ, Aktas G, Erkus E, Duman TT, SAVLİ H. Analysis of the type 2 diabetic patients followed in a university clinic. Konuralp Medical Journal. 2018;10(2):198‐202. [Google Scholar]
- 20. Kidanie BB, Alem G, Zeleke H, Gedfew M, Edemealem A, Andualem A. Determinants of diabetic complication among adult diabetic patients in Debre Markos referral hospital, northwest Ethiopia, 2018: unmatched case control study. Diabetes Metab Syndr Obes. 2020;13:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aynalem SB, Zeleke AJ. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 years and above in Mizan‐Aman town, Southwest Ethiopia, 2016: a cross sectional study. Int J Endocrinol. 2018;2018:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aktas G, Kocak MZ, Bilgin S, Atak BM, Duman TT, Kurtkulagi O. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098‐1102. [DOI] [PubMed] [Google Scholar]
- 23. Kotwas A, Karakiewicz B, Zabielska P, Wieder‐Huszla S, Jurczak A. Epidemiological factors for type 2 diabetes mellitus: evidence from the Global Burden of Disease. Arch Public Health. 2021;79(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al Mansour MA. The prevalence and risk factors of type 2 diabetes mellitus (DMT2) in a semi‐urban Saudi population. Int J Environ Res Public Health. 2020;17(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alemayehu AM, Dagne H, Dagnew B. Knowledge and associated factors towards diabetes mellitus among adult non‐diabetic community members of Gondar city, Ethiopia 2019. PLoS One. 2020;15(3):e0230880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ketema Moges W. Gaussian longitudinal analysis of progression of diabetes mellitus patients using fasting blood sugar level: a case of Debre Berhan Referral Hospital, Ethiopia. Am j theor appl stat. 2018;7(1):21‐28. [Google Scholar]
- 27. Panagiotakos DB, Tzima N, Pitsavos C, et al. The relationship between dietary habits, blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes; the ATTICA study. Rev Diabet Stud. 2005;2(4):208‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asrat AA, Melkamu AZ. A longitudinal data analysis on risk factors for developing type‐2 diabetes mellitus at the University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia. J Public Health Epidemiol. 2018;10(6):171‐82. [Google Scholar]
- 29. Sanjeevaiah A, Sushmitha A, Srikanth T. Prevalence of diabetes mellitus and its risk factors. IAIM. 2019;6(3):319‐324. [Google Scholar]
- 30. Asiimwe D, Mauti GO, Kiconco R. Prevalence and risk factors associated with type 2 diabetes in elderly patients aged 45‐80 years at Kanungu District. J Diabetes Res. 2020;2020:5152146. 10.1155/2020/5152146 [DOI] [Google Scholar]
- 31. Tusa BS, Geremew BM, Tefera MA. Heath related quality of life and associated factors among adults with and without diabetes in Adama city East Shewa, Ethiopia 2019; using generalized structural equation modeling. Health Qual Life Outcomes. 2020;18(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tusa BS, Weldesenbet AB, Gemada AT, Merga BT, Regassa LD. Heath related quality of life and associated factors among diabetes patients in Sub‐Saharan countries: a systemic review and meta‐analysis. Health Qual Life Outcomes. 2021;19(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saijo Y, Okada H, Hamaguchi M, et al. The risk factors for development of type 2 diabetes: panasonic cohort study 4. Int J Environ Res Public Health. 2022;19(1):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeru MA, Tesfa E, Mitiku AA, Seyoum A, Bokoro TA. Prevalence and risk factors of type‐2 diabetes mellitus in Ethiopia: systematic review and meta‐analysis. Sci Rep. 2021;11(1):21733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adampah T, Nawumbeni DN, Nyadanu SD, Polishuk R. Mixed‐effects model for longitudinal study of type‐2‐diabetes. 2015.
- 36. Diggle P, Diggle PJ, Heagerty P, Liang KY, Heagerty PJ, Zeger S. Analysis of Longitudinal Data. Oxford University Press; 2002. [Google Scholar]
- 37. Aniley TT, Debusho LK, Nigusie ZM, Yimer WK, Yimer BB. A semi‐parametric mixed models for longitudinally measured fasting blood sugar level of adult diabetic patients. BMC Med Res Methodol. 2019;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Islam MR. Association between socio‐demographic factors and blood sugar levels in type 2 diabetes mellitus patients in Bangladesh. Int J Diabetes Mellit. 2017;7:151‐159. [Google Scholar]
- 39. Taylor SE, Lobel M. Social comparison activity under threat: downward evaluation and upward contacts. Psychol Rev. 1989;96:569‐575. [DOI] [PubMed] [Google Scholar]
- 40. Ikezaki A, Hosoda H, Ito K, et al. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI‐1, but not with leptin, in obese children and adolescents. Diabetes. 2002;51:3408‐3411. [DOI] [PubMed] [Google Scholar]
- 41. Baltazar JC, Ancheta CA, Aban IB, Fernando RE, Baquilod MM. Prevalence and correlates of diabetes mellitus and impaired glucose tolerance among adults in Luzon, Philippines. Diabetes Res Clin Pract. 2004;64:107‐115. [DOI] [PubMed] [Google Scholar]
- 42. Miller CK, Edwards L, Kissling G, Sanville L. Nutrition education improves metabolic outcomes among older adults with diabetes mellitus: results from a randomized controlled trial. Prev Med. 2002;34:252‐259. [DOI] [PubMed] [Google Scholar]
- 43. Alemu F. Prevalence of diabetes mellitus disease and its association with level of education among adult patients attending at Dilla Referral Hospital, Ethiopia. J Diabetes Metab. 2015;6:1‐5. [Google Scholar]
- 44. Laird NM, Ware JH. Random‐effects models for longitudinal data. Biometrics. 1982;38:963‐974. [PubMed] [Google Scholar]
- 45. Guo W. Functional data analysis in longitudinal settings using smoothing splines. Stat Methods Med Res. 2004;13:49‐62. [DOI] [PubMed] [Google Scholar]
- 46. Der G, Everitt BS. Statistical Analysis Of Medical Data Using SAS. CRC Press; 2005. [Google Scholar]
- 47. McArdle JJ. Latent curve modeling of longitudinal growth data (2012).
- 48. Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G. Advances in longitudinal data analysis: an historical perspective. In: Fitzmaurice GM, Davidian M, Verbeke G, Molenberghs G, eds. Longitudinal Data Analysis. 1st ed. Chapman and Hall/CRC; 2008:17‐42. 10.1201/9781420011579-9 [DOI] [Google Scholar]
- 49. Hallahan C. Longitudinal data analysis with discrete and continuous responses using proc mixed. 2003. http://www.cpcug.org/user/sigstat/PowerPointSlides
- 50. Rubin DB. Causal inference without counterfactuals: comment. J Am Stat Assoc. 2000;95:435‐438. [Google Scholar]
- 51. Zhang D, Davidian M. Linear mixed models with flexible distributions of random effects for longitudinal data. Biometrics. 2001;57:795‐802. [DOI] [PubMed] [Google Scholar]
- 52. Lange N, Ryan L. Assessing normality in random effects models. The Ann Stat. 1989;57(3):795‐802. 10.1111/j.0006-341x.2001.00795.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are not publicly available because the data were used for another analysis, but are available from the first author on reasonable request. The guarantor had full access to all data in this study and takes complete responsibility for the integrity of the data and the precision of the data analysis.


