Abstract
Sexually transmitted infections (STIs), such as Chlamydia trachomatis (Ct) infection, have serious consequences for sexual and reproductive health worldwide. Ct is one of the most common sexually transmitted bacterial infections in the world, with approximately 129 million new cases per year. C. trachomatis is an obligate intracellular Gram-negative bacterium. The infection is usually asymptomatic, notwithstanding, it could also be associated with severe sequels and complications, such as chronic pain, infertility, and gynecologic cancers, and thus there is an urgent need to adequately treat these cases in a timely manner. Consequently, beyond its individual effects, the infection also impacts the economy of the countries where it is prevalent, generating a need to consider the hypothesis of implementing Chlamydia Screening Programs, a decision that, although it is expensive to execute, is a necessary investment that unequivocally will bring financial and social long-term advantages worldwide. To detect Ct infection, there are different methodologies available. Nucleic acid amplification tests, with their high sensitivity and specificity, are currently the first-line tests for the detection of Ct. When replaced by other detection methods, there are more false negative tests, leading to underreported cases and a subsequent underestimation of Ct infection’s prevalence. Ct treatment is based on antibiotic prescription, which is highly associated with drug resistance. Therefore, currently, there have been efforts in line with the development of alternative strategies to effectively treat this infection, using a drug repurposing method, as well as a natural treatment approach. In addition, researchers have also made some progress in the Ct vaccine development over the years, despite the fact that it also necessitates more studies in order to finally establish a vaccination plan. In this review, we have focused on the therapeutic options for treating Ct infection, expert recommendations, and major difficulties, while also exploring the possible avenues through which to face this issue, with novel approaches beyond those proposed by the guidelines of Health Organizations.
Keywords: Chlamydia trachomatis, genital infection, treatment guidelines, drug resistance, screening, antibiotics, vaccines, infertility, carcinogenesis
1. Introduction
Sexually transmitted infections (STIs) have a huge impact on communities; they are associated with individuals’ morbidity and mortality, and also with increased public health expenses through their direct effect on fertility, pregnancy process, and carcinogenesis [1,2]. Chlamydial infection is among the most common curable STIs worldwide, caused by Chlamydia trachomatis (Ct) [3]. This bacterium is an obligate intracellular microorganism that preferentially infects epithelial cells, however, it can also infect phagocytes present in the genital tract, such as macrophages [4,5]. It has a life cycle comprised of two distinct forms, the elementary body (EB), which is the infectious form, and the reticulate body (RB), a non-infectious form that is metabolically active [3,6]. The infectious process begins with the contact between the EB and the host cell for the Ct invasion. This contact is established through the major outer membrane protein (MOMP) of the bacterium, triggering molecular pathways that subsequently drive the EB internalization into the host cell [7,8,9]. When in the cytoplasm of the host cell, the bacterium reproductive cycle can start with the EBs conversion into RBs, allowing pathogen replication through binary fission [10]. Finally, RBs differentiate again into the EBs form, to allow their release into the extracellular microenvironment by cell lysis or extrusion [7,11]. Therefore, this immunogenic environment establishment and the biphasic life cycle of Ct seem to facilitate the therapeutic intervention. Nevertheless, Ct has some immune escape evasion mechanisms, interfering with the host’s natural elimination and making infection treatment difficult [12,13,14].
Indeed, the fact that the chlamydial infection is mainly asymptomatic (in more than 60% of men and women), contributes to the frequency of patients being undiagnosed and consequent undertreatment. Consequently, it could be associated with complications and sequels, and the risk of infection transmission rises [15]. Beyond genital infection, it can also cause rectal, oropharyngeal, and ophthalmologic infections [16]. Moreover, an increased risk of co-infection with human papillomavirus (HPV) [17], Neisseria gonorrhoeae [18], and Mycoplasma genitalium has been reported [19].
According to the World Health Organization (WHO) bulletin on Ct infection prevalence, comparing the 2012 and 2016 available numbers, there was a global decrease in the prevalence of these infections [20]. Notwithstanding, the estimated numbers are still concerning: the WHO anticipated 129 million new Ct infections in 2020 [21]. Therefore, the implementation of Chlamydia Screening Programs, testing asymptomatic women and men, could be one of the key strategies to eradicate this infection [22]. The diagnostic tools available are diverse and are associated with different sensitivities and specificities. In detail, Ct can be detected by culture (not recommended due to the lack of sensibility and the consequent higher incidence of false negative results), enzyme-linked immunosorbent assays (ELISAs), direct immunofluorescence assays, and nucleic acid amplification tests (NAATs). NAATs are the most accurate tests, with specificities and sensitivities higher than 98%, for the genital samples (when non-genital specimens are used, these percentages drop) [23,24,25,26]. Briefly, NAATs must be the gold standard for Ct diagnosis, because the other tests are associated with less sensibility and could result in false negative results, increasing the probability of new infections because of these misdiagnosed cases. Furthermore, NAATs’ methodologies bring an advantage; they can be used with non-invasive test specimens, such as urine and self-collected vaginal swabs, which allied with new, rapid point-of-care diagnostic methods, can diagnose the individuals in around 15 min with a higher rate of accuracy, enabling a “test and treat strategy”; this could be a turning point for controlling Ct infections [27,28,29,30]. In line with this, Herbst de Cortina et al. accomplished a systematic review regarding the performance of these Ct diagnostic tools in order to confront all of these methods and understand which one best fits each country/national health system [31].
Herein, we expose the current state-of-the-art and future treatments in Ct infections, with a particular focus on the standard treatment recommendation guidelines for antibiotics use given by distinct organizations and the potential new therapeutical approaches. Additionally, we discuss the possible mechanisms of antibiotic resistance developed by the bacterium, as well as some strategies to overcome this resistance, using novel drugs development, natural compounds, nanoparticles, and other molecules further detailed in this paper (such as cyclic peptomers, cyclic peptomers, peptide-based inhibitors). Finally, we investigate the promising future directions of this health problem control concerning future vaccination strategies.
2. Clinical Presentation of Chlamydial Infection
In the majority of cases, chlamydial infection is asymptomatic [32]. Nevertheless, symptoms could be felt in distinct anatomical regions with different intensities, depending on the bacterium serovar, which is determined based on the specific epitopes of the MOMP encoded by ompA [33,34,35]. Particularly, serovars A, B, Ba, and C are associated with a chronic ophthalmologic disease, designated as trachoma, and blindness; serovars D, Da, E, F, G, Ga, H, I, Ia, J, and K, infect mainly the urogenital tract, resulting in cervicitis in women and urethritis in men and women, or other more complicated outcomes; serovars L1, L2, L2a, and L3, are related with lymphogranuloma venereum (LGV) [6,33,35,36]. The latter are considered the most invasive ones, and if untreated, can lead to rectal fistula or stricture [34]. Indeed, several studies have reported the association between the Ct genotype and the pathogenicity and severity of the infection [36,37,38]. Chen and colleagues have shown that patients with genotype D, the most prevalent in their study, have a low risk of co-infection with other pathogens, as well as a lower association with cervical cancer. On the other hand, genotype F of Ct is mostly associated with bacterial co-infections. Additionally, individuals infected with serovar G share this risk of co-infections. Furthermore, genotype G is associated with mucopurulent cervicitis and cervical dysplasia [39]. Of note, serovar E is commonly associated with co-infection with HPV, a prerequisite for cervical tumorigenesis [40]. Of note, comprehensive studies regarding Ct serovars prevalence revealed distinct geographical distributions, depending on the region studied, the individual’s gender, ethnicity, and sexual orientation [29,36,41,42,43].
Importantly, the authors also indicate that, when not adequately diagnosed and treated, patients may face serious symptoms and consequences, such as pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility, neonatal complications, and other symptoms in different body regions, as shown in Table 1. In fact, persistent Ct infection is also a risk factor for genital tract tumors, demonstrating the urgent need for the screening of asymptomatic sexually active women [36,40,44].
Table 1.
The most common symptoms of Ct infection according to gender, condition, and anatomical region infected [34].
| Genital Tract | Symptoms |
|---|---|
| Uncomplicated infection | |
| Female | Abnormal vaginal discharge; dysuria; post-coital and intermenstrual bleeding |
| Male | Urethral discharge; dysuria; testicular pain |
| Persistent infection | |
| Female | Pelvic inflammatory disease; ectopic pregnancy; salpingitis; tubal factor infertility |
| Male | Epididymitis |
| Non-genital Tract | |
| Rectal infection | Rectal discharge; rectal pain; blood in the stools |
| Oropharyngeal infection | Pharyngitis and mild sore throat |
| Pregnancy complications | Preterm birth and low birth weight |
| Perinatal transmission | Neonatal conjunctivitis and/or nasopharyngeal infection; ocular discharge and swollen eyelids |
3. Current Therapeutic Options
Ct treatment is based on antibiotics prescription. Table 2 presents the drugs recommended by WHO, characterizing their ADME (Absorption, Distribution, Metabolism, and Excretion) profile, mechanism of action, and chemical structure.
Table 2.
ADME profile, mechanism of action, and chemical structure of the most common drugs used in the Ct infection treatment [45,46,47,48,49,50,51,52].
| Drug | Chemical Structure | Main Information |
|---|---|---|
| Azithromycin |
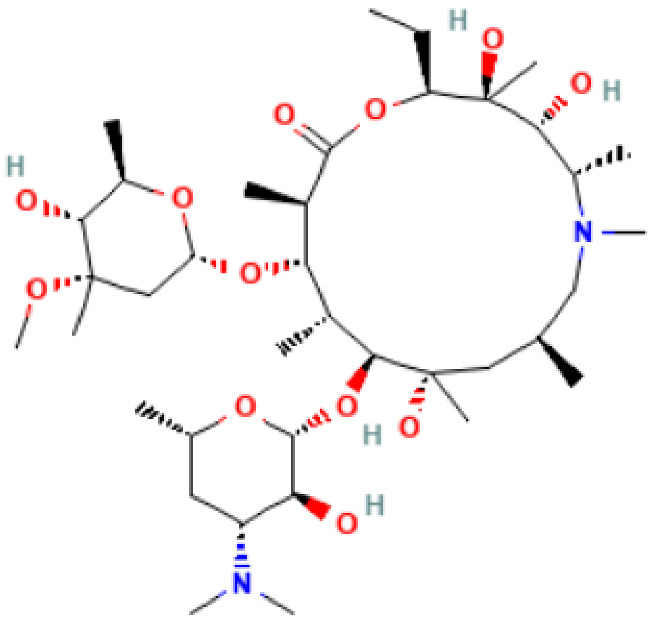
|
Mechanism of action: Inhibition of bacterial protein synthesis by interrupting the transpeptidation/translocation pathway due to its binding to the bacterial 50S ribosomal subunit’s 23S rRNA (ribosomal RNA). Absorption: Following oral administration, peak plasma concentrations occur after 2–3 h. Maximum concentration (Cmax): 0.4 mg.L−1). When administered intravenously, peak plasma concentration is reported to be 3–4 mg.L−1 after 1 h infusion. Distribution: Mostly distributed in the body (except in the brain and cerebrospinal fluid) the volume of distribution (Vd) is about 31.1 L.kg−1. It is well tolerated within cells (phagocytes, e.g.,) allowing high efficacy in Ct infection treatment. Metabolism: Although its metabolic pathway has yet to be explored, it is known that azithromycin undergoes some hepatic metabolism. Route of elimination: Biliary excretion is the major route of elimination; 12.2% of the drug is eliminated in the urine after intravenous (IV) administration and 4.5% when administered orally. Half-life: The elimination half-life (t1/2) is 40–68 h due to extensive tissue retention. |
| Doxycycline |
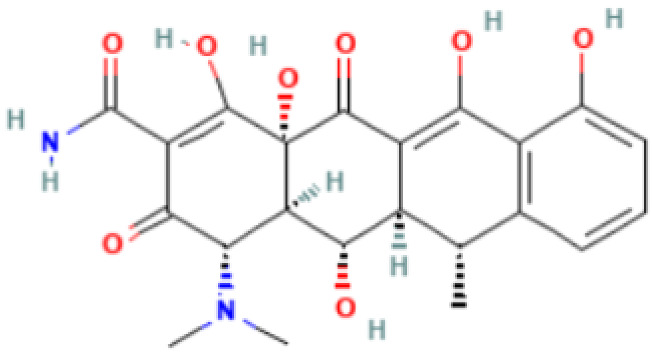
|
Mechanism of action: It prevents aminoacyl-tRNA (aa-tRNA) from binding to the ribosomal site, hence inhibiting bacterial protein synthesis, namely the elongation phase. Absorption: Peak plasma concentration of approximately 3.0–5.0 μg.mL−1 occur 2–3 h after oral administration and 4–10 µg.mL−1 within 30 min after IV dosing. Distribution: Despite the scarcity of data, it is known that the drug is widely distributed in tissues and body fluids, including cerebrospinal fluid. Metabolism: It has not been studied yet. Route of elimination: Most of the drug is excreted through the kidneys, with a small fraction being eliminated in the bile. It can also be excreted in feces. Half-life: 18–22 h. |
| Tetracycline |
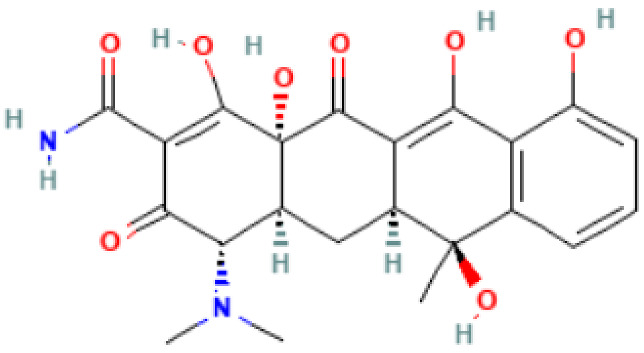
|
Mechanism of action: Inhibition of the ribosome subunits association by binding to the 30S ribosomal subunit via passive diffusion in bacterial membrane porin channels, hence interfering with protein synthesis. Absorption: Following oral administration, peak plasma concentrations of 3–5 μg.mL−1 within 2 h. Intramuscular (IM) administration has low bioavailability (<40%), followed by oral (60–80%) and IV administration (100%). Distribution: Limited information available. This drug’s class of antibiotics has a solubility-dependent distribution in the tissues and body fluids. Metabolism: Not metabolized. Route of elimination: It is excreted in the urine (30%) and feces (20–60%) at high concentrations in its biologically active form. Half-life: 6–12 h. |
| Erythromycin |
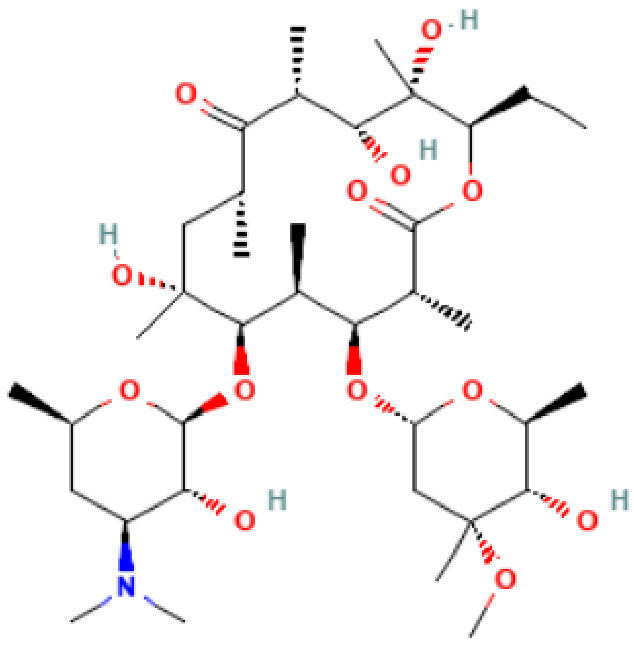
|
Mechanism of action: It inhibits transpeptidation/translocation and the assembly of the 50S ribosomal subunit, preventing bacterial protein synthesis. Absorption: Despite the interindividual heterogeneity in absorption, the peak plasma concentration is reported to be 1.8 μg.L−1 after 1.2 h of an orally administered dose. Its bioavailability ranges from 18–45%. Distribution: Found in most body fluids and accumulated in leucocytes and inflammatory liquid (Vd: 1.5 L.kg−1). This drug is well diffused in meningitis, as the blood-brain barrier (BBB) is easily penetrated (inflamed tissues). Metabolism: It undergoes hepatic first-pass metabolism after an oral dose. CYP3A4 enzyme partially metabolizes it to N-desmethylerythromycin. In acidic conditions, it is also hydrolyzed to anhydro forms (inactive against bacteria). Route of elimination: It is excreted in the bile. After an oral dosage, less than 5% is eliminated in the urine. Half-life: 2.4–3.1 h. |
| Levofloxacin |
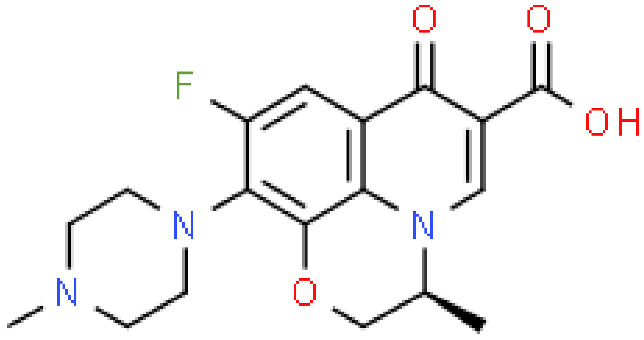
|
Mechanism of action: It prevents normal cell division by acting on the DNA (deoxyribonucleic acid) gyrase and topoisomerase IV, enzymes responsible for avoiding excessive supercoiling of DNA during replication or transcription. Absorption: Peak plasma concentrations of 11.5 µg.mL−1 within 2–3 h following oral administration. Bioavailability is approximately 99%. Distribution: Extensive distribution in body fluids and inflammatory exudates. Vd ranges between 1.09 and 1.26 L.kg−1 after an orally administered dose. Metabolism: Levofloxacin metabolism in humans occurs by demethylation and oxidation originating the metabolites: desmethyl-levofloxacin and levofloxacin-N-oxide. Route of elimination: After oral administration, approximately 87% is excreted in urine and less than 4% in feces. Half-life: 6–8 h. |
| Amoxicillin |
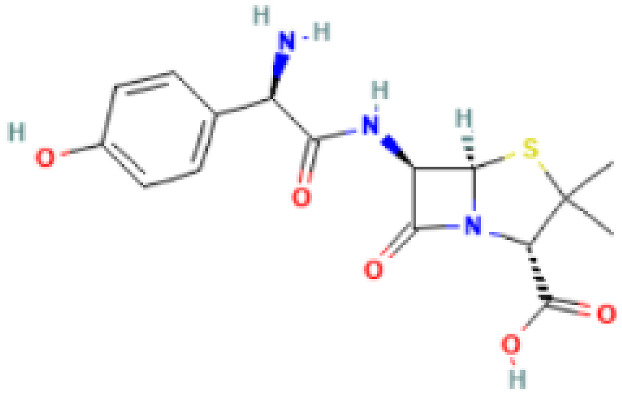
|
Mechanism of action: It inhibits penicillin-binding proteins, which are responsible for glycosyltransferase and transpeptidase reactions that lead to cross-linking of D-alanine and D-aspartic acid in bacterial cell walls. This affects the formation and repair of the cell wall, resulting in cell lysis. Absorption: A 250 mg of oral dose reaches peak plasma concentrations of 3.93 mg.L−1 after 1.31 h. Bioavailability is approximately 60%. Distribution: Distribution into liver, lungs, prostate, muscle, and bone is reported in several studies. Vd has been measured to be 27.7 L. Metabolism: It has several metabolic pathways, from hydroxylation, oxidative deamination to decarboxylation. Route of elimination: 70–78% of the drug is eliminated in the urine. Half-life: 1 h. |
| Chloramphenicol |
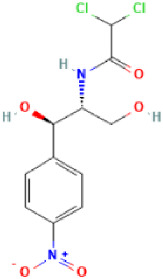
|
Mechanism of action: It binds to the L16 protein of the 50S ribosomal subunit, preventing the transfer of amino acids to growing peptide chains and subsequent protein synthesis. Absorption: Topical application to the eye may also be intraocular and little systemic absorption. Distribution: It has no volume of distribution. Metabolism: It is not metabolized. Route of elimination: Not very clear information. Half-life: 1.5–3.5 h. |
| Povidone-iodine |
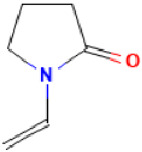
|
Mechanism of action: It is a complex that gradually releases free iodine at the application site. Free iodine penetrates the cell wall, resulting in disruption of protein and nucleic acid structure and synthesis. Absorption: Topical application; it is not absorbed. Distribution: It has no volume of distribution. Metabolism: It is not metabolized. Route of elimination: It is not eliminated. Half-life: Not applicable. |
| Silver Nitrate |
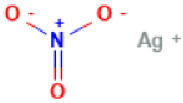
|
Pharmacokinetics information is not available. |
Azithromycin is a semisynthetic molecule with antibacterial activity, which is derived from erythromycin [53]. It is a prescribed antibiotic, approved by the Food Drug Administration (FDA) for the treatment of a wide variety of bacterial infections, including Ct. In fact, the WHO recommends azithromycin, as well as doxycycline, as first-line drugs for the treatment of Ct. This molecule can be administered orally, locally (ophthalmological solution), and occasionally, parenterally, depending on the clinical indication. It is highly stable at low pH, increasing its concentration in tissues for long periods of time, compared to erythromycin. The antibiotic activity of azithromycin guarantees that bacterial growth is blocked due to its affinity for the bacterial ribosomes. Specifically, this molecule can infiltrate into the intracellular milieu, binding to the 23S rRNA of the 50S ribosomal subunit of the Ct and inhibiting the assembly of the 50S ribosomal subunit and the translocation step of protein synthesis [54]. Thus, the process of protein synthesis (mRNA, messenger ribonucleic acid, translation) is impeded [55]. Additionally, azithromycin has an immunomodulatory effect that controls the inflammatory process. Drug delivery is mainly at the inflamed tissues, as well as penetrating the phagocytes (leukocytes, monocytes, macrophages, and fibroblasts) allowing it to be effective against Ct [4,5,54].
Doxycycline is part of the class of tetracycline antibiotics, which show biological activity against bacteria through the inhibition of protein synthesis by binding to the 16S rRNA section of the ribosome, inhibiting the binding of tRNA to the 30S bacterial ribosomal subunit. Its administration could be oral or parenteral, depending on the clinical indication. Moreover, there is a reported hepatotoxicity of this drug use [56]. Importantly, following the International Union Against Sexually Transmitted Infections (IUSTI) guidelines, azithromycin is being used as the first-line therapy for this infection in addition to doxycycline. Despite this choice, doxycycline is associated with higher efficacy, and Centers for Disease Control and Prevention (CDC) guidelines recommend a doxycycline regimen as the first treatment, with some exceptions, as detailed further in this section [57,58,59,60]. Reveneau et al. proposed that the different forms of the bacterium, RB and EB, could be responsible for the differences between the drug’s efficacies. In detail, they argue that azithromycin has a better efficacy against the EB form, responsible for persistent infections, whereas doxycycline is more appropriate against the RB form, present in acute infections [60,61].
Erythromycin is an antibiotic of the macrolide class. Its anti-bacterial activity is similar to that of azithromycin [62]. Despite both drugs having the same efficacy against Ct infection, the advantage of erythromycin is its low cost. Nevertheless, it is less safe to use on pregnant women [63].
Tetracycline is a broad-spectrum antibiotic that acts by inhibiting the cell translation process by binding to the 30S bacterium ribosomal subunit. In addition, it can interfere with the cytoplasmic membrane of Ct, affecting the leakage of intracellular content into the extracellular medium. Clinicians must consider that this molecule can cause adverse effects in asthmatic patients [64].
Levofloxacin is a fluoroquinolone antibiotic that can be administered orally. In terms of biological action, its bactericidal activity is through interference with the DNA replication by binding to the key enzyme’s DNA gyrase and topoisomerase IV. Rarely, it has been associated with liver injury [65].
Amoxicillin is an antibiotic whose mode of action is through the inhibition of cell wall biosynthesis, leading to bacterial lysis. This pharmacological compost formulation can be used orally or parenterally. In cases of overdose, individuals can develop hematuria, oliguria, abdominal pain, acute renal failure, vomiting, diarrhea, rash, hyperactivity, and drowsiness [66,67]. In pregnant women, amoxicillin was shown to be a better option than azithromycin in terms of side effects [68]. Moreover, it was verified that it has high efficacy in Ct infection treatment [69].
Tetracycline and povidone iodine are part of the first-line Ct treatment, according to the WHO guidelines. Tetracycline hydrochloride is a semi-synthetical naphthacene antibiotic that inhibits protein synthesis through different mechanisms, including: blocking of the A site of bacteria ribosomes, interruption of the elongation process, inhibition of oligosaccharide side chains attached to glycoproteins, and misreading of the genetic code [70]. In turn, povidone iodine (water-based solution) is an anti-septic agent that can be used locally for ocular prophylaxis immediately after birth [34,71]. Alternatively, silver nitrate is used, which destroys harmful microorganisms or inhibits their activity [72], or chloramphenicol eye solutions, which is a broad-spectrum antibiotic can also be used [73]. Furthermore, it is important to know that depending on the type of Ct infection and the patient’s condition, clinicians must choose an adequate therapeutic strategy in each case. Table 3 synthesized the current therapy strategies recommended based on WHO, IUSTI, and CDC guidelines to effectively treat each type of Ct infection [20,57,58].
Table 3.
| Type of Ct Infection | Treatment Options |
|---|---|
| Uncomplicated genital chlamydia |
Doxycycline 100 mg orally twice a day for 7 days Azithromycin 1 g orally as a single dose |
| Tetracycline 500 mg orally four times a day for 7 days | |
| Erythromycin 500 mg orally four times a day for 7 days | |
| Levofloxacin 500 mg orally once daily for 7 days | |
| Anorectal chlamydial infection |
Doxycycline 100 mg orally twice a day for 7 days over |
| Azithromycin 1 g orally as a single dose | |
| Genital chlamydial infection in pregnant women |
Azithromycin 1 g orally as a single dose |
| Amoxicillin 500 mg orally three times a day for 7 days | |
| Erythromycin 500 mg orally four times a day for 7 days | |
| Lymphogranuloma venereum (LGV) |
Doxycycline 100 mg orally twice daily for 21 days Azithromycin 1 g orally, weekly for 3 weeks Erythromycin 500 mg orally four times a day for 21 days |
| Ophthalmia neonatorum | |
| Conjunctivitis | Azithromycin 20 mg/kg/day orally, one dose daily for 3 days |
| Erythromycin 50 mg/kg/day orally, in four divided doses daily for 14 days | |
| Ocular prophylaxis | Tetracycline hydrochloride 1% eye ointment |
| Erythromycin 0.5% eye ointment | |
| Povidone iodine 2.5% solution | |
| Silver nitrate 1% solution | |
| Chloramphenicol 1% eye ointment |
Generally, in cases of uncomplicated genital infection, the guidelines highlight that doxycycline is the treatment with higher efficacy and it must be used as first-line therapy, the others are alternative options to use in case of drug contra-indication, resistance, or other reasons [57]. For patients with anorectal infection, they recommend doxycycline orally twice daily for 7 days. The WHO guidelines suggest pregnant women should use azithromycin over erythromycin or amoxicillin. Of note, doxycycline and levofloxacin are contraindicated in pregnancy. For LGV, the recommended treatment is doxycycline; in case of contraindication, azithromycin may be considered. For ophthalmia neonatorum, particularly in conjunctivitis, the use of azithromycin over erythromycin is recommended. Importantly, guidelines recommend topical ocular prophylaxis as an infection prevention measure for all neonates. There are several options for topical application to both eyes immediately after birth [20].
It must be highlighted that treatment options, in some countries, are based on associated costs, rather than on biological behavior, therefore, adverse events may occur [20]. In order to avoid the adverse outcomes of Ct treatment, the therapeutic agents’ properties must be explored, as well as the host infection establishment.
Studies regarding the pharmacological interventions for Ct infection, comparing efficacy and safety of the drugs, are still few and were mainly developed with pregnant women patients, thus potentially biased and not generalizable.
4. Treatment Failure and Novel Approaches
The main reasons for treatment failure are poor compliance with treatment, the test of the cure performed too early, and the fact that the partner(s) of the infected ones are not informed and subsequently, not treated, thus they could infect others and re-infect the partner(s) [74]. Additionally, this lack of therapy efficacy can occur due to antibiotic resistance, triggered by gene mutations in the bacteria, or persistence, which occurs in the case that the bacteria are not efficiently eliminated due to their natural features becoming tolerant to the drug [75]. Concerning Ct antibiotic resistance, an in vitro study, including a country with the greatest consumption of azithromycin in Europe, Croatia, did not find azithromycin and doxycycline resistance in the 24 studied samples of urogenital isolates of Ct infection [76]. Nevertheless, an experimental study in the UK, comparing azithromycin with doxycycline, demonstrated a higher treatment failure rate of azithromycin in non-genital infections [77]. Multidrug-resistant Ct serovars may be one of the reasons for azithromycin treatment inefficiency. In detail, some in vitro studies report that point mutations in the ribosomal protein of the bacterium genotype L are responsible for azithromycin resistance [60]. In addition, there is in vitro evidence demonstrating that prior exposure to penicillin could lead to Ct azithromycin resistance [78]. Mestrovic et al. have reported that azithromycin resistance, in vitro, could be raised through mutations in Ct 23S rRNA genes [79]. In addition, tetracycline resistance is developed by tet(M) gene mutations [80]. Benamri and colleagues described the fact that fluoroquinolones resistance could be developed via gyrA, parC, and ygeD gene mutations [81].
Antibiotherapy persistence of Ct occurs due to the life cycle of this bacterium [82]. As previously detailed, the Ct life cycle has two distinct forms, with RB being the one that can go through growth arrest in stress cell conditions [6,11,83]. In line with this, several factors can contribute to this pathogen phase persistence, as reported by Mpiga and Ravaoarinoro [84]. Specifically, there is evidence that cytokine, tumor necrosis factor (TNF–α), and interferon gamma (IFN–γ) have an influence in the persistent stage [85,86]; as well as the bacterium growth in non-permissive cells [87]; nutrient limitation [88]; additionally, some antibiotics, such as penicillin, ofloxacin, and ciprofloxacin can interfere with the Ct differentiation stage [78,89,90]. Of note, it must be highlighted that this persistence state phase or heterotolerance is difficult to surpass due to the difficulty in measuring it [75].
Therefore, based on the evidence, there is a need for the development of novel drugs in order to successfully combat Ct infection [10]. Some authors have investigated the role of Corallopyronin A, an antimicrobial compound synthesized by Corallococcus coralloides. It acts specifically by binding to a domain of the bacterial DNA-dependent RNA polymerase, inhibiting the growth of Ct [91]. Furthermore, Shima et al. have demonstrated promising outcomes, suggesting it as a future alternative for Ct therapy [91,92]. Additionally, a nanoparticle designated PDGFR-β siRNA-PEI-PLGA-PEG NP, developed by Yang et al., successfully reduces vaginal Ct infection through autophagy induction in human cells, concomitantly with the knock-down of a gene coding of an important surface binding protein of Ct, platelet-derived growth factor receptor beta (PDGFR-β) [93]. Recently, Núñez-Otero and his team, have developed and uncovered the second-generation 2-pyridone amide (KSK213) role in Ct infection control, with reduced toxicity for humans without disturbing the commensal flora. This molecule has revealed its effects in the transcription inhibition of crucial genes responsible for the differentiation from EB to RB, which could be a key control phase of Ct infection [94]. Additionally, there have been efforts to develop natural anti-chlamydial treatments based on extracts. Hamarsheh et al. have investigated in vitro the effect of Artemisia inculta Delile extract, which was shown to effectively inhibit Ct infection in HeLa cells [95].
Since 2020, as the antibiotic resistance issue has remained critical, some authors developed studies with potential non-antibiotic weapons. Lam and colleagues have published findings regarding cyclic peptomers as inhibitors of Gram-negative bacteria, and they suggest using 4EpDN cyclic peptomer as a prophylactic treatment against Chlamydia trachomatis due to the strong inhibitor effect that they found in the type III secretion system (T3SS), a virulence factor of the bacteria [96]. Additionally, Hwang et al., optimized peptide-based inhibitors (2-Pyridone-based analogs) in order to better target HtrA serine protease in Ct, an enzyme essential to several bacterial vital functions, which seems to be a promising strategy [97]. Finally, Kazakova and colleagues have defended the need for further research to investigate the promising role ofC-ring oxygen and nitrogen erythrodiol derivatives against Ct infections [98].
Interestingly, drug repurposing, a strategy commonly investigated for cancer treatment, has also been explored in this field [99,100]. Specifically, Itoh et al. have reported the potential role of bortezomib, an anticancer drug, to treat Ct infections by apoptosis induction [100]. Notwithstanding all these new strategies for treating Ct infection, further comprehensive studies are needed in order to improve the translation of these research results into clinical practice.
Indeed, the more effective way to control and eradicate Ct infection is through a vaccination plan that must comprise the individuals before they became sexually active, to maximize immunity, reducing Ct prevalence, and consequently, eradicating the infection. However, Ct vaccine development has proven to be a challenge throughout the years [101]. Brunham and Rappuoli have made assertive conclusions about the barriers to vaccine development, defending the position that there are currently no scientific impediments to this purpose, highlighting recent advances in modern medicine as positive for progress, but also showing that other non-scientific barriers to progress, which do not prioritize this research, have been a negative influence on vaccine advancement. They also propose that the secret to vaccine success is the involvement of four different sectors: the public sector, the clinical sector, industry, and discovery, all working in the same direction [102]. In detail, researchers have been developing different types of vaccines, among these, (1) first-generation Ct vaccines, with an associated biological risk due to their bacterial inoculation origin, (2) the second-generation vaccines, which were designed only using subunits of the bacterium, and the (3) third-generation, more modern than the others, using the pathogen’s DNA [101,103]. Firstly, a first-generation vaccine was evaluated in 1960 to treat trachoma; however, even when adjuvants were used to increase the immune response, immunogenicity was not induced at a sufficient level; re-infections still occurred. Additionally, it was associated with increased inflammation, resulting in the worsening of inflammatory diseases [7,104,105,106,107]. Later, second-generation vaccines were assembled, using subunits of the pathogen as their expressed surface antigens (MOMP) in order to be more effective and safer. Interestingly, this generation of vaccines is already capable of promoting cellular and humoral immunity [108]. Researchers developed oral vaccines using this strategy and tested them in non-human primates and mice. Human clinical trials followed, demonstrating immunoglobulin G (IgG) and immunoglobulin A (IgA) stimulation by the vaccine, as well as other molecules associated with immunity stimulation (IFN-γ). Importantly, these studies have proved the capacity of these vaccines to stimulate antigen-specific immune responses in humans [7,109,110,111]. However, researchers report that it provides limited protection against Ct infection [7,111]. Therefore, the third generation of vaccines was developed using DNA techniques and, in some cases, plasmid vectors carrying the foreign gene of interest. These are more cost-effective and more adequate at triggering humoral and cell-mediated immune responses [7,101]. However, there are several disadvantages associated with this type of vaccine, including the possibility of genome integration and the risk of anti-DNA antibody development [112]. In addition, as Vasilevsky et al. described, even more vaccine approaches have been developed, yet, despite massive efforts, vaccine effectiveness is still not at the levels needed, thus some researchers are focusing their attention on computational strategies [112]. Currently, the efforts are in line with the advances in genomics and bioinformatic tools, in a multi-omics landscape, allowing for an in silico vaccine design that now requires in vitro validation [113,114,115]. Recently, some authors developed a method to create new candidate vaccines, using the biosoftware AllerTOP (Bioinformatics tool for allergenicity prediction. Available online: https://www.ddg-pharmfac.net/AllerTOP/ (accessed on 15 November 2022)) [116]. They studied the predicted epitopes of lymphocytes T and B that could stimulate long-lasting immunity against Ct and concluded that a chimeric peptide will be more efficient. The novel therapeutic epitope vaccine candidates, known as “LSWEMELAY”, “LSNTEGYRY”, “TSDLGQMEY”, “FIDLLQAIY” and “FSNNFSDIY”, described by Shiragannavar and colleagues, must be validated experimentally in order to complement the in silico studies to conclude whether the vaccine is efficacious and provides long-term immunity stimulation for translational application [113,115]. In addition, as defended by the authors, a vaccine combining multi-epitopes must be studied because it could be more promising due to the distinct interactions that it could have with the human leukocyte antigen (HLA) molecules [115]. This thesis is also defended by Aslam et al., who developed a study concerning in silico multi-epitope-based vaccine (MEBV) development, concomitantly with an adjuvant (Cholera toxin subunit B) coupled to increase the immune system response because the MEBV itself cannot trigger enough immunogenicity. The authors tested the physio-chemical properties, antigenicity, immunogenicity, allergenicity, secondary structure, solubility, and other important features of this vaccine, using bioinformatic tools, a cost-effective method for the vaccine design, and have concluded that this prototype can successfully stimulate the humor and cell immune responses against Ct. Thus, a forward step is required to test the tolerance, safety, and effectiveness of this MEBV in vitro in future experimental trials in order to approve an effective vaccine [113].
5. Conclusions
Ct infection is one of the most common sexually transmitted infections worldwide that could be associated with serious health problems in the genital tract as well as perinatal morbidity of fetuses, even when it runs an asymptomatic course. Therefore, a need for screening measures arises in order to adequately treat the infection according to the guidelines. Ct infection treatments are based on antibiotics prescriptions. Nevertheless, there is a risk of drug resistance and re-infection. Therefore, it is urgent to achieve progress in the development of therapeutic weapons against Ct infection. Indeed, in the future, the key to Ct control must focus on public health intervention through populational screening of asymptomatic individuals to avoid infection transmission and adequately treat patients in a timely matter. Concomitant with this strategy to eradicate the infection worldwide should be the administration of an effective vaccine.
In conclusion, this review highlights the need for a public health intervention with Ct screening to better treat this infection which could have serious complications for human health. Moreover, we reinforce the necessity for further laboratory studies regarding vaccine development and the MEBV approach in order to prove the effectiveness of in silico studies and consequently, allow for immunization of future populations, which will only be possible by combining efforts to study potential vaccine candidates, study safety and efficacy within the population, and accelerating cost-effective vaccine manufacture and implementation in order to eradicate this health problem.
Acknowledgments
R.R. acknowledges FCT for funding her PhD grant (2022.11755.BDANA). N.V. would also like to thank the support from FCT and FEDER (European Union), award number IF/00092/2014/CP1255/CT0004, and CHAIR in Onco-Innovation from FMUP.
Author Contributions
Conceptualization, N.V.; methodology, R.R. and N.V.; validation, N.V.; formal analysis, R.R., L.M., C.S. and N.V.; investigation, R.R., L.M., P.V.-B., C.S. and N.V.; resources, N.V.; data curation, R.R.; writing—original draft preparation, R.R., L.M.; writing—review and editing, P.V.-B., C.S. and N.V.; visualization P.V.-B., C.S. and N.V.; supervision, N.V.; project administration, N.V.; funding acquisition, N.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financed by FEDER—Fundo Europeu de Desenvolimento Regional through the COMPETE 2020—Operational Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia, in a framework of the projects in CINTESIS, R&D Unit (reference UIDB/4255/2020), and within the scope of the project “RISE—LA/P/0053/2020. This work was also financed from FCT and FEDER (European Union), award number IF/00092/2014/CP1255/CT0004 and CHAIR in Onco-Innovation at FMUP.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jayes D., Merrick R., Pulford C., Buitendam E., Mohammed H., Saunders J. What is the role of sexual health services in the delivery of primary prevention of sexually transmitted infections. A narrative review. Sex. Health. 2022;19:319–328. doi: 10.1071/SH22047. [DOI] [PubMed] [Google Scholar]
- 2.Starnbach M.N., Roan N.R. Conquering sexually transmitted diseases. Nat. Rev. Immunol. 2008;8:313–317. doi: 10.1038/nri2272. [DOI] [PubMed] [Google Scholar]
- 3.Mohseni M., Sung S., Takov V. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2022. Chlamydia. [Google Scholar]
- 4.Lausen M., Christiansen G., Bouet Guldbæk Poulsen T., Birkelund S. Immunobiology of monocytes and macrophages during Chlamydia trachomatis infection. Microbes Infect. 2019;21:73–84. doi: 10.1016/j.micinf.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Tietzel I., Quayle A.J., Carabeo R.A. Alternatively Activated Macrophages Are Host Cells for Chlamydia trachomatis and Reverse Anti-chlamydial Classically Activated Macrophages. Front. Microbiol. 2019;10:919. doi: 10.3389/fmicb.2019.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkin S.S., Minis E., Athanasiou A., Leizer J., Linhares I.M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017;24:e00203-17. doi: 10.1128/CVI.00203-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray S.M., McKay P.F. Chlamydia trachomatis: Cell biology, immunology and vaccination. Vaccine. 2021;39:2965–2975. doi: 10.1016/j.vaccine.2021.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Poston T.B., Darville T. Chlamydia trachomatis: Protective Adaptive Responses and Prospects for a Vaccine. Curr. Top Microbiol. Immunol. 2018;412:217–237. doi: 10.1007/82_2016_6. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb S.L., Brunham R.C., Byrne G.I., Martin D.H., Xu F., Berman S.M. Introduction: The natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. J. Infect. Dis. 2010;201((Suppl. 2)):S85–S87. doi: 10.1086/652392. [DOI] [PubMed] [Google Scholar]
- 10.Ni M., Xu S., Liu Z., Xue Y., Xie W., Yang S., Liu L., Bao X. Inhibitory Activity of Pyrroloisoxazolidine Derivatives against Chlamydia trachomatis. BioMed Res. Int. 2021;2021:8889247. doi: 10.1155/2021/8889247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues R., Sousa C., Vale N. Chlamydia trachomatis as a Current Health Problem: Challenges and Opportunities. Diagnostics. 2022;12:1795. doi: 10.3390/diagnostics12081795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields K.A., Hackstadt T. The Chlamydial Inclusion: Escape from the Endocytic Pathway. Annu. Rev. Cell Dev. Biol. 2002;18:221–245. doi: 10.1146/annurev.cellbio.18.012502.105845. [DOI] [PubMed] [Google Scholar]
- 13.Hogan R.J., Mathews S.A., Mukhopadhyay S., Summersgill J.T., Timms P. Chlamydial Persistence: Beyond the Biphasic Paradigm. Infect. Immun. 2004;72:1843–1855. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastidas R.J., Elwell C.A., Engel J.N., Valdivia R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013;3:a010256. doi: 10.1101/cshperspect.a010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huai P., Li F., Chu T., Liu D., Liu J., Zhang F. Prevalence of genital Chlamydia trachomatis infection in the general population: A meta-analysis. BMC Infect. Dis. 2020;20:589. doi: 10.1186/s12879-020-05307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan P.A., Robinette A., Montgomery M., Almonte A., Cu-Uvin S., Lonks J.R., Chapin K.C., Kojic E.M., Hardy E.J. Extragenital Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A Review of the Literature. Infect. Dis. Obstet. Gynecol. 2016;2016:5758387. doi: 10.1155/2016/5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escarcega-Tame M.A., López-Hurtado M., Escobedo-Guerra M.R., Reyes-Maldonado E., Castro-Escarpulli G., Guerra-Infante F.M. Co-infection between genotypes of the human papillomavirus and Chlamydia trachomatis in Mexican women. Int. J. STD AIDS. 2020;31:1255–1262. doi: 10.1177/0956462420947587. [DOI] [PubMed] [Google Scholar]
- 18.Lim R.B.T., Wong M.L., Cook A.R., Brun C., Chan R.K.W., Sen P., Chio M. Determinants of Chlamydia, Gonorrhea, and Coinfection in Heterosexual Adolescents Attending the National Public Sexually Transmitted Infection Clinic in Singapore. Sex. Transm. Dis. 2015;42:450–456. doi: 10.1097/OLQ.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 19.Harrison S.A., Olson K., Ratliff A.E., Xiao L., Van Der Pol B., Waites K.B., Geisler W.M. Mycoplasma genitalium Coinfection in Women With Chlamydia trachomatis Infection. Sex. Transm. Dis. 2019;46:e101–e104. doi: 10.1097/OLQ.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowley J., Vander Hoorn S., Korenromp E., Low N., Unemo M., Abu-Raddad L.J., Chico R.M., Smolak A., Newman L., Gottlieb S., et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019;97:548–562. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Sexually Transmitted Infections (STIs) 2021. [(accessed on 14 July 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)
- 22.Land J., Van Bergen J., Morre S., Postma M. Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum. Reprod. Updat. 2009;16:189–204. doi: 10.1093/humupd/dmp035. [DOI] [PubMed] [Google Scholar]
- 23.Neinstein L.S., Rabinovitz S. Detection of Chlamydia trachomatis. A study of the direct immunofluorescence technique and a review diagnostic limitation. J. Adolesc. Health Care. 1989;10:10–15. doi: 10.1016/0197-0070(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 24.de Haro-Cruz M.J., Guadarrama-Macedo S.I., López-Hurtado M., Escobedo-Guerra M.R., Guerra-Infante F.M. Obtaining an ELISA test based on a recombinant protein of Chlamydia trachomatis. Int. Microbiol. 2019;22:471–478. doi: 10.1007/s10123-019-00074-4. [DOI] [PubMed] [Google Scholar]
- 25.Brook G. The performance of non-NAAT point-of-care (POC) tests and rapid NAAT tests for chlamydia and gonorrhoea infections. An assessment of currently available assays. Sex. Transm. Infect. 2015;91:539–544. doi: 10.1136/sextrans-2014-051997. [DOI] [PubMed] [Google Scholar]
- 26.Novak D.P., Lindholm L., Jonsson M., Karlsson R.B. A Swedish cost-effectiveness analysis of community-based Chlamydia trachomatis PCR testing of postal urine specimens obtained at home. Scand. J. Public Health. 2004;32:324–332. doi: 10.1080/14034940410026282. [DOI] [PubMed] [Google Scholar]
- 27.Adamson P.C., Loeffelholz M.J., Klausner J.D. Point-of-Care Testing for Sexually Transmitted Infections: A Review of Recent Developments. Arch. Pathol. Lab. Med. 2020;144:1344–1351. doi: 10.5858/arpa.2020-0118-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding-Esch E., Fuller S., Chow S.-L., Nori A., Harrison M., Parker M., Piepenburg O., Forrest M., Brooks D., Patel R., et al. Diagnostic accuracy of a prototype rapid chlamydia and gonorrhoea recombinase polymerase amplification assay: A multicentre cross-sectional preclinical evaluation. Clin. Microbiol. Infect. 2018;25:380.e1–380.e7. doi: 10.1016/j.cmi.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sylvan S.P.E., Von Krogh G., Tiveljung A., Siwerth B.-M., Henriksson L., Norén L., Asp A.-K., Grillner L. Screening and Genotyping of Genital Chlamydia trachomatis in Urine Specimens From Male and Female Clients of Youth-Health Centers in Stockholm County. Sex. Transm. Dis. 2002;29:379–386. doi: 10.1097/00007435-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Parra-Sánchez M., Palomares J.C., Bernal S., González M.T., Sivianes N., Pérez L., Pueyo I., Martín-Mazuelos E. Evaluation of the cobas 4800 CT/NG Test for detecting Chlamydia trachomatis and Neisseria gonorrhoeae DNA in urogenital swabs and urine specimens. Diagn. Microbiol. Infect. Dis. 2012;74:338–342. doi: 10.1016/j.diagmicrobio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Herbst de Cortina S., Bristow C.C., Joseph Davey D., Klausner J.D. ASystematic Review of Point of Care Testing for Chlamydia trachomatis, Neisseriagonorrhoeae, and Trichomonas vaginalis. Infect. Dis. Obstet. Gynecol. 2016;2016:4386127. doi: 10.1155/2016/4386127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frej-Mądrzak M., Gryboś A., Gryboś M., Teryks-Wołyniec D., Jama-Kmiecik A., Sarowska J., Choroszy-Król I. PCR diagnostics of Chlamydia trachomatis in asymptomatic infection by women. Ginekol. Pol. 2018;89:115–119. doi: 10.5603/GP.a2018.0020. [DOI] [PubMed] [Google Scholar]
- 33.Lesiak-Markowicz I., Schötta A.-M., Stockinger H., Stanek G., Markowicz M. Chlamydia trachomatis serovars in urogenital and ocular samples collected 2014–2017 from Austrian patients. Sci. Rep. 2019;9:18327. doi: 10.1038/s41598-019-54886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . WHO Guidelines for the Treatment of Chlamydia Trachomatis. WHO; Geneva, Switzerland: 2016. [(accessed on 16 July 2022)]. p. 56. Available online: https://www.who.int/publications/i/item/978-92-4-154971-4. [PubMed] [Google Scholar]
- 35.Morré S.A., Rozendaal L., van Valkengoed I.G., Boeke A.J., van Voorst Vader P.C., Schirm J., de Blok S., van Den Hoek J.A., van Doornum G.J., Meijer C.J., et al. Urogenital Chlamydia trachomatis serovars in men and women with a symptomatic or asymptomatic infection: An association with clinical manifestations? J. Clin. Microbiol. 2000;38:2292–2296. doi: 10.1128/JCM.38.6.2292-2296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Chen J., Yang L., Jiang Y., Li L., Yi W., Lan L., Zhang L. Distribution of Chlamydia Trachomatis Genotypes in Infective Diseases of the Female Lower Genital Tract. Med. Sci. Monit. 2017;23:4477–4481. doi: 10.12659/MSM.902756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borges V., Cordeiro D., Salas A.I., Lodhia Z., Correia C., Isidro J., Fernandes C., Rodrigues A.M., Azevedo J., Alves J., et al. Chlamydia trachomatis: When the virulence-associated genome backbone imports a prevalence-associated major antigen signature. Microb. Genom. 2019;5:e000313. doi: 10.1099/mgen.0.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelsamed H., Peters J., Byrne G.I. Genetic variation in Chlamydia trachomatis and their hosts: Impact on disease severity and tissue tropism. Future Microbiol. 2013;8:1129–1146. doi: 10.2217/fmb.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balasubramaniam S.D., Balakrishnan V., Oon C.E., Kaur G. Key Molecular Events in Cervical Cancer Development. Medicina. 2019;55:384. doi: 10.3390/medicina55070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva J., Cerqueira F., Medeiros R. Chlamydia trachomatis infection: Implications for HPV status and cervical cancer. Arch. Gynecol. Obstet. 2013;289:715–723. doi: 10.1007/s00404-013-3122-3. [DOI] [PubMed] [Google Scholar]
- 41.Sabbatucci M., Salfa M.C., Regine V., Pezzotti P., Suligoi B. Estimated burden of Chlamydia trachomatis female infection and consequent severe pelvic inflammatory disease, Italy, 2005–2016. Ann. Dell’istituto Super. Di Sanità. 2019;55:217–223. doi: 10.4415/ANN_19_03_04. [DOI] [PubMed] [Google Scholar]
- 42.Borrego M.J., Gomes J.P., Lefebvre J.F., Eb F., Orfila J., Catry M.A. Genotyping of Portuguese Chlamydia trachomatis urogenital isolates. Sex. Transm. Infect. 1997;73:561–563. doi: 10.1136/sti.73.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casillas-Vega N., Morfín-Otero R., García S., Llaca-Díaz J., Rodríguez-Noriega E., Camacho-Ortiz A., Merced Ayala-Castellanos M.d.l., Maldonado-Garza H.J., Ancer-Rodríguez J., Gallegos-Ávila G., et al. Frequency and genotypes of Chlamydia trachomatis in patients attending the obstetrics and gynecology clinics in Jalisco, Mexico and correlation with sociodemographic, behavioral, and biological factors. BMC Women’s Health. 2017;17:83. doi: 10.1186/s12905-017-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie X., Yang M., Ding Y., Chen J. Microbial infection, inflammation and epithelial ovarian cancer. Oncol. Lett. 2017;14:1911–1919. doi: 10.3892/ol.2017.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DrugBank. [(accessed on 8 December 2021)]. Available online: https://go.drugbank.com/
- 46.Bakheit A.H.H., Al-Hadiya B.M.H., Abd-Elgalil A.A. Chapter One—Azithromycin. Profiles Drug Subst. Excip. Relat. Methodol. 2014;39:1–40. doi: 10.1016/B978-0-12-800173-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 47.Parnham M.J., Haber V.E., Giamarellos-Bourboulis E.J., Perletti G., Verleden G.M., Vos R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Klein N.C., Cunha B.A. Tetracyclines. Med. Clin. N. Am. 1995;79:789–801. doi: 10.1016/S0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- 49.Agwuh K.N., MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006;58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 50.Amsden G.W. Erythromycin, clarithromycin, and azithromycin: Are the differences real? Clin. Ther. 1996;18:56–72. doi: 10.1016/S0149-2918(96)80179-2. [DOI] [PubMed] [Google Scholar]
- 51.Lamp K.C., Bailey E.M., Rybak M.J. Ofloxacin Clinical Pharmacokinetics. Clin. Pharmacokinet. 1992;22:32–46. doi: 10.2165/00003088-199222010-00004. [DOI] [PubMed] [Google Scholar]
- 52.Huttner A., Bielicki J., Clements M.N., Frimodt-Møller N., Muller A.E., Paccaud J.P., Mouton J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020;26:871–879. doi: 10.1016/j.cmi.2019.11.028. [DOI] [PubMed] [Google Scholar]
- 53.Bakheit A.H., Al-Hadiya B.M., Abd-Elgalil A.A. Azithromycin. Profiles Drug Subst. Excip. Relat. Methodol. 2014;39:1–40. doi: 10.1016/B978-0-12-800173-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 54.PubChem . PubChem Compound Summary for CID 447043, Zithromax. PubChem, National Center for Biotechnology Information; 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Zithromax. [Google Scholar]
- 55.PubChem . PubChem Pathway Summary for Pathway—PathBank. Volume SMP0000247. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Azithromycin Action Pathway. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Azithromycin. [Google Scholar]
- 56.PubChem . PubChem Compound Summary for CID 54671203, Doxycycline. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Doxycycline. [Google Scholar]
- 57.Workowski K.A., Bachmann L.H., Chan P.A., Johnston C.M., Muzny C.A., Park I., Reno H., Zenilman J.M., Bolan G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR. Recomm. Rep. 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanjouw E., Ouburg S., de Vries H., Stary A., Radcliffe K., Unemo M. 2015 European guideline on the management of Chlamydia trachomatis infections. Int. J. STD AIDS. 2016;27:333–348. doi: 10.1177/0956462415618837. [DOI] [PubMed] [Google Scholar]
- 59.Pitt R.A., Alexander S., Horner P.J., Ison C.A. Presentation of clinically suspected persistent chlamydial infection: A case series. Int. J. STD AIDS. 2013;24:469–475. doi: 10.1177/0956462412472815. [DOI] [PubMed] [Google Scholar]
- 60.Manavi K., Hettiarachchi N., Hodson J. Comparison of doxycycline with azithromycin in treatment of pharyngeal chlamydia infection. Int. J. STD AIDS. 2016;27:1303–1308. doi: 10.1177/0956462415614723. [DOI] [PubMed] [Google Scholar]
- 61.Reveneau N., Crane D.D., Fischer E., Caldwell H.D. Bactericidal activity of first-choice antibiotics against gamma interferon-induced persistent infection of human epithelial cells by Chlamydia trachomatis. Antimicrob. Agents Chemother. 2005;49:1787–1793. doi: 10.1128/AAC.49.5.1787-1793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.PubChem . PubChem Compound Summary for CID 12560, Erythromycin. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Erythromycin. [Google Scholar]
- 63.Pitsouni E., Iavazzo C., Athanasiou S., Falagas M.E. Single-dose azithromycin versus erythromycin or amoxicillin for Chlamydia trachomatis infection during pregnancy: A meta-analysis of randomised controlled trials. Int. J. Antimicrob. Agents. 2007;30:213–221. doi: 10.1016/j.ijantimicag.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 64.PubChem . PubChem Compound Summary for CID 54675776, Tetracycline. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetracycline. [Google Scholar]
- 65.PubChem . PubChem Compound Summary for CID 149096, Levofloxacin. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Levofloxacin. [Google Scholar]
- 66.PubChem . PubChem Compound Summary for CID 33613, Amoxicillin. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amoxicillin. [Google Scholar]
- 67.Belko J., Urueta G., Emre U. Amoxicillin overdose manifested by hematuria and acute renal failure. Pediatr. Infect. Dis. J. 1995;14:917–918. [PubMed] [Google Scholar]
- 68.Kacmar J., Cheh E., Montagno A., Peipert J.F. A Randomized Trial of Azithromycin Versus Amoxicillin for the Treatment of Chlamydia trachomatis in pregnancy. Infect. Dis. Obstet. Gynecol. 2001;9:197–202. doi: 10.1155/S1064744901000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahangdale L., Guerry S., Bauer H.M., Packel L., Rhew M., Baxter R., Chow J., Bolan G. An Observational Cohort Study of Chlamydia trachomatis Treatment in Pregnancy. Sex. Transm. Dis. 2006;33:106–110. doi: 10.1097/01.olq.0000187226.32145.ea. [DOI] [PubMed] [Google Scholar]
- 70.PubChem . PubChem Compound Summary for CID 54704426, Tetracycline Hydrochloride. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetracycline-HCl. [Google Scholar]
- 71.PubChem . PubChem Compound Summary for CID 410087, Povidone-Iodine. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Povidone-iodine. [Google Scholar]
- 72.PubChem . PubChem Compound Summary for CID 24470, Silver Nitrate. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Silver-Nitrate. [Google Scholar]
- 73.PubChem . PubChem Compound Summary for CID 5959, Chloramphenicol. PubChem, National Center for Biotechnology Information; Bethesda, MD, USA: 2004. [(accessed on 15 November 2022)]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chloramphenicol. [Google Scholar]
- 74.Sherrard J., Jensen J.S. Chlamydia treatment failure after repeat courses of azithromycin and doxycycline. Int. J. STD AIDS. 2019;30:1025–1027. doi: 10.1177/0956462419857303. [DOI] [PubMed] [Google Scholar]
- 75.Huemer M., Shambat S.M., Brugger S.D., Zinkernagel A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020;21:e51034. doi: 10.15252/embr.202051034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ljubin-Sternak S., Mestrovic T., Vilibic-Cavlek T., Mlinaric-Galinovic G., Sviben M., Markotic A., Skerk V. In vitro susceptibility of urogenital Chlamydia trachomatis strains in a country with high azithromycin consumption rate. Folia Microbiol. Praha. 2013;58:361–365. doi: 10.1007/s12223-012-0218-2. [DOI] [PubMed] [Google Scholar]
- 77.Hathorn E., Opie C., Goold P. What is the appropriate treatment for the management of rectal Chlamydia trachomatis in men and women? Sex. Transm. Infect. 2012;88:352. doi: 10.1136/sextrans-2011-050466. [DOI] [PubMed] [Google Scholar]
- 78.Wyrick P.B., Knight S.T. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 2004;54:79–85. doi: 10.1093/jac/dkh283. [DOI] [PubMed] [Google Scholar]
- 79.Mestrovic T., Ljubin-Sternak S. Molecular mechanisms of Chlamydia trachomatis resistance to antimicrobial drugs. Front. Biosci. 2018;23:656–670. doi: 10.2741/4611. [DOI] [PubMed] [Google Scholar]
- 80.Scurtu L.G., Jinga V., Simionescu O. Fascinating Molecular and Immune Escape Mechanisms in the Treatment of STIs (Syphilis, Gonorrhea, Chlamydia, and Herpes Simplex) Int. J. Mol. Sci. 2022;23:3550. doi: 10.3390/ijms23073550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benamri I., Azzouzi M., Sanak K., Moussa A., Radouani F. An overview of genes and mutations associated with Chlamydiae species’ resistance to antibiotics. Ann. Clin. Microbiol. Antimicrob. 2021;20:59. doi: 10.1186/s12941-021-00465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wyrick P.B. Chlamydia trachomatis Persistence In Vitro: An Overview. J. Infect. Dis. 2010;201:88–95. doi: 10.1086/652394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panzetta M.E., Valdivia R.H., Saka H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018;9:3101. doi: 10.3389/fmicb.2018.03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mpiga P., Ravaoarinoro M. Chlamydia trachomatis persistence: An update. Microbiol. Res. 2006;161:9–19. doi: 10.1016/j.micres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Beatty W.L., Morrison R.P., Byrne G.I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect. Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shemer-Avni Y., Wallach D., Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect. Immun. 1988;56:2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hanada H., Ikeda-Dantsuji Y., Naito M., Nagayama A. Infection of human fibroblast-like synovial cells with Chlamydia trachomatis results in persistent infection and interleukin-6 production. Microb. Pathog. 2003;34:57–63. doi: 10.1016/S0882-4010(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 88.Clements J.D., Harper A., Pogson C.I., Jones M.L., Pearce J.H. Chlamydial Development Is Adversely Affected by Minor Changes in Amino Acid Supply, Blood Plasma Amino Acid Levels, and Glucose Deprivation. Infect. Immun. 2000;68:1457–1464. doi: 10.1128/iai.68.3.1457-1464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dreses-Werringloer U., Padubrin I., Jürgens-Saathoff B., Hudson A.P., Zeidler H., Köhler L. Persistence of Chlamydia trachomatis Is Induced by Ciprofloxacin and Ofloxacin In Vitro. Antimicrob. Agents Chemother. 2000;44:3288–3297. doi: 10.1128/AAC.44.12.3288-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnson F.W.A., Hobson D. The effect of penicillin on genital strains of Chlamydia trachomatis in tissue culture. J. Antimicrob. Chemother. 1977;3:49–56. doi: 10.1093/jac/3.1.49. [DOI] [PubMed] [Google Scholar]
- 91.Shima K., Ledig S., Loeper N., Schiefer A., Pfarr K., Hoerauf A., Graspeuntner S., Rupp J. Effective inhibition of rifampicin-resistant Chlamydia trachomatis by the novel DNA-dependent RNA polymerase inhibitor corallopyronin A. Int. J. Antimicrob. Agents. 2018;52:523–524. doi: 10.1016/j.ijantimicag.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 92.Loeper N., Graspeuntner S., Ledig S., Kaufhold I., Hoellen F., Schiefer A., Henrichfreise B., Pfarr K., Hoerauf A., Shima K., et al. Elaborations on Corallopyronin A as a Novel Treatment Strategy Against Genital Chlamydial Infections. Front. Microbiol. 2019;10:943. doi: 10.3389/fmicb.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang S., Traore Y., Jimenez C., Ho E.A. Autophagy induction and PDGFR-β knockdown by siRNA-encapsulated nanoparticles reduce chlamydia trachomatis infection. Sci. Rep. 2019;9:1306. doi: 10.1038/s41598-018-36601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Núñez-Otero C., Bahnan W., Vielfort K., Silver J., Singh P., Elbir H., Almqvist F., Bergström S., Gylfe Å. A 2-Pyridone Amide Inhibitor of Transcriptional Activity in Chlamydia trachomatis. Antimicrob. Agents Chemother. 2021;65:e01826-20. doi: 10.1128/AAC.01826-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hamarsheh O., Amro A., Al-Zeer M. In Vitro Antibacterial Activity of Selected Palestinian Medicinal Plants against Chlamydia trachomatis. Microbiol. Res. 2021;12:656–662. doi: 10.3390/microbiolres12030047. [DOI] [Google Scholar]
- 96.Lam H.N., Lau T., Lentz A., Sherry J., Cabrera-Cortez A., Hug K., Lalljie A., Engel J., Lokey R.S., Auerbuch V. Developing Cyclic Peptomers as Broad-Spectrum Type III Secretion System Inhibitors in Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2021;65:e01690-20. doi: 10.1128/AAC.01690-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hwang J., Strange N., Phillips M.J., Krause A.L., Heywood A., Gamble A.B., Huston W.M., Tyndall J.D. Optimization of peptide-based inhibitors targeting the HtrA serine protease in Chlamydia: Design, synthesis and biological evaluation of pyridone-based and N-Capping group-modified analogues. Eur. J. Med. Chem. 2021;224:113692. doi: 10.1016/j.ejmech.2021.113692. [DOI] [PubMed] [Google Scholar]
- 98.Kazakova O., Rubanik L., Lobov A., Poleshchuk N., Baikova I., Kapustina Y., Petrova A., Korzun T., Lopatina T., Fedorova A., et al. Synthesis of erythrodiol C-ring derivatives and their activity against Chlamydia trachomatis. Steroids. 2021;175:108912. doi: 10.1016/j.steroids.2021.108912. [DOI] [PubMed] [Google Scholar]
- 99.Rodrigues R., Duarte D., Vale N. Drug Repurposing in Cancer Therapy: Influence of Patient’s Genetic Background in Breast Cancer Treatment. Int. J. Mol. Sci. 2022;23:4280. doi: 10.3390/ijms23084280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Itoh R., Kurihara Y., Yoshimura M., Hiromatsu K. Bortezomib Eliminates Persistent Chlamydia trachomatis Infection through Rapid and Specific Host Cell Apoptosis. Int. J. Mol. Sci. 2022;23:7434. doi: 10.3390/ijms23137434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schautteet K., De Clercq E., Vanrompay D. Chlamydia trachomatis Vaccine Research through the Years. Infect. Dis. Obstet. Gynecol. 2011;2011:1–9. doi: 10.1155/2011/963513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brunham R.C., Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31:1892–1897. doi: 10.1016/j.vaccine.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ababneh M., Ghazal E., khalifeh M. Immunogenicity of an Adenoviral Vector Vaccine (rAd-MOMP-CpG) against Chlamydia Trachomatis. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.02570. [DOI] [Google Scholar]
- 104.Poston T.B., Gottlieb S.L., Darville T. Status of vaccine research and development of vaccines for Chlamydia trachomatis infection. Vaccine. 2019;37:7289–7294. doi: 10.1016/j.vaccine.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 105.Bell S.D., Nichols R.L., Haddad N.A. The immunology of the trachoma agent with a preliminary report on field trials on vaccine. Investig. Ophthalmol. 1963;2:471–481. [PubMed] [Google Scholar]
- 106.Sowa S., Sowa J., Collier L.H., Blyth W.A. Trachoma vaccine field trials in The Gambia. Epidemiol. Infect. 1969;67:699–717. doi: 10.1017/S0022172400042157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang S.-p., Thomas Grayston J., Russell Alexander E. Trachoma Vaccine Studies in Monkeys. Am. J. Ophthalmol. 1967;63:1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- 108.Maza L.M.d.l., Zhong G., Brunham R.C., Papasian C.J. Update on Chlamydia trachomatis Vaccinology. Clin. Vaccine Immunol. 2017;24:e00543-16. doi: 10.1128/CVI.00543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillips S., Quigley B.L., Timms P. Seventy Years of Chlamydia Vaccine Research—Limitations of the Past and Directions for the Future. Front. Microbiol. 2019;10:70. doi: 10.3389/fmicb.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pal S., Theodor I., Peterson E.M., Maza L.M.d.l. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect. Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor H., Whittum-Hudson J., Schachter J., Caldwell H.D., Prendergast R.A. Oral immunization with chlamydial major outer membrane protein (MOMP) Investig. Ophthalmol. Vis. Sci. 1988;29:1847–1853. [PubMed] [Google Scholar]
- 112.Vasilevsky S., Greub G., Nardelli-Haefliger D., Baud D. Genital Chlamydia trachomatis: Understanding the Roles of Innate and Adaptive Immunity in Vaccine Research. Clin. Microbiol. Rev. 2014;27:346–370. doi: 10.1128/CMR.00105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aslam S., Ahmad S., Noor F., Ashfaq U.A., Shahid F., Rehman A., Tahir Ul Qamar M., Alatawi E.A., Alshabrmi F.M., Allemailem K.S. Designing a Multi-Epitope Vaccine against Chlamydia trachomatis by Employing Integrated Core Proteomics, Immuno-Informatics and In Silico Approaches. Biology. 2021;10:997. doi: 10.3390/biology10100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aslam M., Shehroz M., Ali F., Zia A., Pervaiz S., Shah M., Hussain Z., Nishan U., Zaman A., Afridi S.G., et al. Chlamydia trachomatis core genome data mining for promising novel drug targets and chimeric vaccine candidate’s identification. Comput. Biol. Med. 2021;136:104701. doi: 10.1016/j.compbiomed.2021.104701. [DOI] [PubMed] [Google Scholar]
- 115.Shiragannavar S., Madagi S., Hosakeri J., Barot V. In silico vaccine design against Chlamydia trachomatis infection. Netw. Model. Anal. Heal. Inform. Bioinform. 2020;9:1–13. doi: 10.1007/s13721-020-00243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dimitrov I., Flower D.R., Doytchinova I. AllerTOP—A server for in silico prediction of allergens. BMC Bioinform. 2013;14((Suppl. 6)):S4. doi: 10.1186/1471-2105-14-S6-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


