Abstract
Introduction: Recurrent urinary tract infections (rUTI) largely contribute to antibiotic use in older adults. Understanding the genetic characteristics of Escherichia coli (E.coli) is needed to identify patients at risk for recurrence. The aim of this study was to obtain a greater understanding of the genetics of E. coli rUTI in nursing home residents. Methods: This is a secondary analysis of a multicenter Dutch nursing home study (PROGRESS). E. coli strains from residents with a suspected UTI and positive urine culture were analyzed using antimicrobial susceptibility testing and whole-genome sequencing (WGS). Same-strain recurrences were identified by single-nucleotide polymorphism (SNP) analysis. Result: In total, 121 E. coli strains were analyzed using WGS, of which 54 belonged to a rUTI episode. One third of E. coli rUTI episodes were caused by the same strain (n = 18, 33.3%). Same-strain recurrence occurred anywhere between 30 and 434 days after the index UTI, caused by sequence types (ST): ST12, ST23, ST73, ST131, ST453, ST538 and ST2522, in seven nursing home residents. In both single UTI and rUTI, antimicrobial resistance rates were low. Conclusion: Recurrent UTI in nursing home residents are caused by same-strain E. coli as well as due to different E. coli strains or other uropathogens. Same-strain recurrence can occur over 400 days after the index UTI, suggesting that some strains have the ability to colonize the bladder or gut for longer periods.
Keywords: recurrent urinary tract infection, nursing homes, antibiotic resistance, whole-genome sequencing
1. Introduction
Urinary tract infections (UTIs) are one of the most common infections among nursing home residents [1] and contribute to a large burden of disease [2], including mental distress [3]. The majority of UTIs are caused by uropathogenic Escherichia coli (UPEC) strains [4,5], which are mostly clonal and belong to the E. coli phylogenetic groups B2 or D. The major pandemic lineages globally found are sequence types (ST): ST69, ST73, ST95, and ST131 [6]. Risk factors for recurrent urinary tract infection (rUTI) in older women are urine incontinence, a history of UTI and nonsecretor status. Treatment of rUTI in older adults contributes enormously to the total use of antibiotics [7,8]. The proportion of recurrence in older women varies from 10 to 44% [9,10].
The genetic characteristics of E. coli colonizing the intestine in relation to rUTI were previously studied among (younger) adults [9,11,12,13]. Chen et al., characterized E. coli strains from stool and urine specimens from women with rUTI, between 18 and 41 years old. Some clonal E. coli populations, determined by multi-locus sequence typing (MLST) and whole-genome-sequencing (WGS) analysis, were found in multiple UTI episodes during a 3-month follow-up period, whereas shifts in dominant E. coli strains between UTI episodes were also described. It was found that uropathogens were simultaneously present in the urine and the intestine according to clonal tracking, implicating a different potential hypothesis for uropathogen persistence in rUTI (e.g., reinfection of urinary tract from an external source or bacterial persistence within urinary tract) [13]. No distinctive variation was found in the core genome of E. coli causing rUTI compared to E. coli in non-rUTI based on single nucleotide polymorphism analyses (SNP-analyses) [11]. The current knowledge about the genetics of E. coli causing rUTI is mostly based on research in adults instead of older adults (aged ≥ 65 years). One study found no distinctive virulence factors in E. coli isolated from rUTI episodes compared to E. coli isolated from the index UTI episode by using restriction fragment length polymorphism (RFLP) in women aged 17 to 82 years in primary care [9].
The empirical antibiotic regimen for rUTI is usually based on previous urine cultures and their susceptibility test results, which may help to predict susceptibility [14], as it is known that rUTIs are frequently caused by the re-introduction of the same index strain from the genitorectal area [15,16]. The persistence of infection from internal bladder colonies is an alternative mechanism for recurrence [12,13,16,17]. Most recurrences have been associated with specific antibiotic resistance traits and sequence types, for example: one study found that recurrences of E. coli causing UTI were caused by the same phylogenetic group and ST131 subclones [18] and another study reported that ST131 was predominantly observed from patients with rUTI [19].
Increasing antimicrobial resistance hampers the effective treatment of UTIs. As rUTI is a major cause of antimicrobial use and risk for antimicrobial resistance (AMR), strategies are needed to identify patients at risk for recurrence combined with understanding bacterial strain compositions responsible for recurrence to target preventive strategies. The aim of this study is to obtain a greater understanding of the genetics behind rUTI caused by E. coli in nursing home residents.
2. Materials and Methods
2.1. Study Design and Study Population
This is a secondary analysis of the PROGRESS study which assessed the diagnostic accuracy of C-reactive protein and procalcitonin, to diagnose UTI, in a Dutch multicenter study in nursing homes [20]. Nursing home residents (=> 65 years old) residing at psychogeriatric, somatic or rehabilitation wards were consecutively enrolled when provided informed consent and a UTI was suspected by the treating physician or nurse between November 2017 and August 2019. Exclusion criteria were a suspected respiratory tract infection, another infection requiring antibiotic treatment, or previous enrolment in the past 30 days.
2.2. Bacterial Isolates and Definitions
Details on urine specimen collection and bacterial isolation procedures were previously described [21]. Briefly, urine specimens obtained from nursing home residents with a suspected UTI were used for semi-quantitative bacterial culture and urine dipstick testing. The urine culture procedure consisted of 10 μL of urine streaked out on two selective agar plates. After overnight incubation at 37 °C, uropathogens were identified using MALDI-TOF mass spectrometry (Microflex; Bruker Daltonik, Billerica, MA, USA). The identified bacterial strains were classified as uropathogen according to the European Consensus Guideline [22] and antibiotic susceptibility testing was performed when ≥104 colony-forming units per milliliter (CFU/mL) growth was found. The recovered E. coli isolates were stored using glycerol peptone 4% at −80 °C until being processed to be used for SNP-analysis (n = 135).
Residents were enrolled when a UTI was suspected according to the treating physician based on clinical signs and symptoms. In this secondary analysis, all residents enrolled with a positive urine culture were considered as having a UTI. Residents with a single UTI episode caused by E. coli during the study period were referred to as single UTI. Residents with multiple E. coli UTIs (at least a 30-day interval between subsequent UTI episodes, to exclude persistent infections) were defined as E. coli rUTI. Same-strain E. coli rUTIs were referred to when the same E. coli strain was observed over time based on whole-genome sequencing and single-nucleotide polymorphism (SNP) analysis.
2.3. Genome Sequencing, Assembly and Typing
Deoxyribonucleic acid (DNA) was extracted using a QIAamp-DNA mini-kit (Qiagen, Germantown, MD, USA). DNA concentration was measured using a Qubit fluorometer (ThermoFisher, Waltham, MA, USA). Specimens were normalized and KAPA HTP Dual indexed library preparation was performed (Roche, Durban, South Africa). After library preparation, specimens were pooled and sequenced on an Illumina NextSeq platform using the P2 300 cycles kit (Illumina, San Diego, CA, USA). Illumina reads were trimmed using Trimmomatic v0.36 (Bolger 2014), after which reads were assembled using the Shovill v1.1.0 wrapper (https://github.com/tseemann/shovill, accessed on 27 January 2022) for SPAdes v3.13.0 [23]. Isolates were excluded if the genome size was outside the range of 4.5–5.6 Mbp, the N50 was lower than 30,000 bp, or the assembly consisted of more than 500 contigs, all assessed using Quast v4.6.3 [24]. Sequence types were inferred using mlst v2.19.0 (https://github.com/tseemann/mlst, accessed on 27 January 2022). fumC-fimH clonotypes were determined using the CHtyper webtool [25], serotypes were predicted in silico using ECtyper v1.0.0 [26], and EzClermont v0.6.3 was used to infer phylogroups [27].
2.4. Analysis of Strain Recurrence by SNP Analysis (Same-Strain rUTI)
Isolates were included in the SNP analysis if multiple isolates of the same sequence type were identified in the dataset. SNP analysis was performed using snippy v4.4.5 (https://github.com/tseemann/snippy, accessed on 27 January 2022), with reference genomes selected per sequence type using reference seeker v1.8.0 [28]. We defined the recurrence of the same E. coli strains (same-strain rUTI) as follows: (i) ≥2 E. coli strains isolated from the same participant; (ii) from different UTI episodes (positive urine culture obtained at different time points with at least 30 days between sampling); (iii) during the study period. E. coli isolates are considered ‘the same strain’ when the SNP differences are 25 or less [29]. A phylogenetic tree of all strains was constructed by mapping sequence reads on the ATCC 25922 reference genome (CP009072.1) as described above.
2.5. Antibiotic Resistance Rates
Antimicrobial susceptibility testing was performed by using theVITEK2 platform (BioMérieux). Results from the most recent E. coli strain in time were analyzed. AMR rates were calculated based on available susceptibility data.
2.6. Descriptive Analysis
Descriptive statistics were reported using Social Sciences software (SPSS) for Windows version 17.0 [30]. Figures and tables were constructed using Microsoft Excel and CANVA [31,32].
3. Results
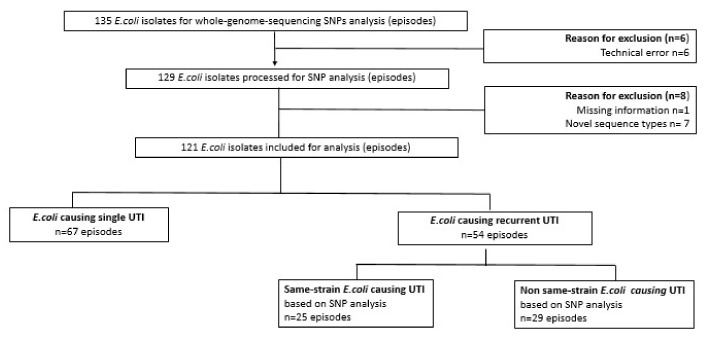
The present study is based on a dataset from a multicenter nursing home study (PROGRESS) with the broader aim to improve UTI diagnosis [21]. The dataset consisted of 298 suspected UTI episodes based on clinical signs and symptoms (208 unique nursing home residents): 149 single suspected UTI episodes (71.6%) and 59 nursing home residents with suspected rUTIs (n = 149 episodes, 28.3%). Of these 149 suspected rUTIs, 69 episodes (39 unique nursing home residents) were urine-culture-positive for growing E. coli, further referred to as E. coli rUTI (46.3%). Due to missing isolates and/or technical errors, 54 out of 69 E. coli episodes (78.3%) were included for SNP analysis. Another 14 strains were excluded due to technical errors/missing information (n = 7), or novel sequence types observed from SNP analysis (n = 7). Of the remaining 121 E. coli isolates, about half were single UTIs (n = 67, 55.4%), the remaining were rUTIs (n = 54, 44.6%); see Figure 1.
Figure 1.
Overview of Escherichia coli (E. coli) isolates analyzed by whole-genome sequencing. E. coli causing single urinary tract infection (UTI) was defined as a single UTI episode with ≥104 CFU/mL growth of E. coli in the urine culture; E. coli causing recurrent UTI (rUTI) was defined as unique nursing home residents with ≥2 UTI episodes with ≥104 CFU/mL growth of E. coli in the urine culture at ≥30 days’ interval between subsequent episodes; same-strain E. coli causing UTI was defined as the same E. coli strain observed over different time points based on whole-genome sequencing and single-nucleotide-polymorphism (SNP) analysis; non-same-strain E. coli was defined by a subgroup of nursing home residents with E. coli causing rUTI but not same-strain based on SNP analysis.
3.1. E. coli Sequence Types in Nursing Home Residents with Single Versus Recurrent UTI
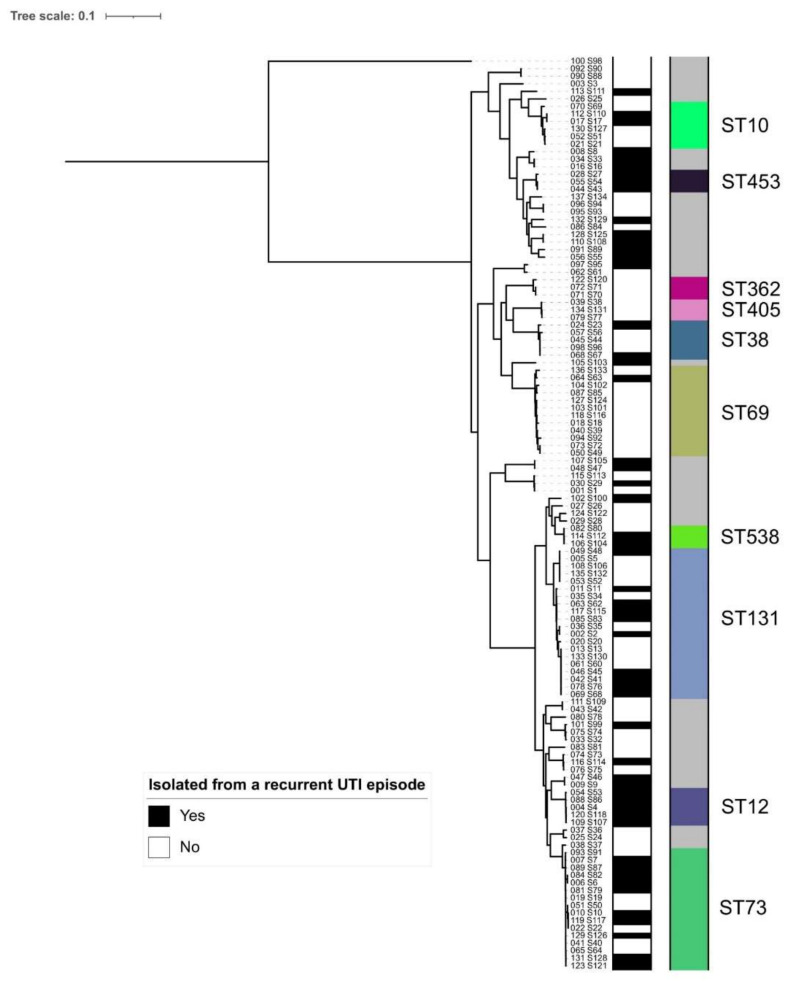
Overall, the most frequently isolated E. coli sequence types were ST131 (n = 20, 16.5%), ST73 (n = 16, 13.2%), ST69 (n = 12, 9.9%), ST10 (n = 6, 5.0%), ST12 (n = 5, 4.1%), ST38 (n = 5, 4.1%), ST362 (n = 3, 2.5%), ST405 (n = 3, 2.5%), ST453 (n = 3, 2.5%) and ST538 (n = 3, 2.5%). These ten sequence types represented more than 60% of all included E. coli isolates.
ST69 was more frequently isolated among individuals with a single UTI compared to individuals with rUTI (16.4% versus 1.9%) whereas ST73 and ST12 were more often found among individuals with rUTI (18.5% versus 9.0% for ST73; 9.3% versus 0.0% for ST12); see Table 1 and Figure 2.
Table 1.
Frequencies of E. coli sequence types isolated from Dutch nursing home residents. Detailed typing is listed in Table S1.
| Sequence Type | Overall (n = 121) | Recurrent UTI (n = 54) | Non-Recurrent UTI (n = 67) |
|---|---|---|---|
| Number of episodes (%) | |||
| 131 | 20 (16.5) | 10 (18.5) | 10 (14.9) |
| 73 | 16 (13.2) | 10 (18.5) | 6 (9.0) |
| 69 | 12 (9.9) | 1 (1.9) | 11 (16.4) |
| 10 | 6 (5.0) | 2 (3.7) | 4 (6.0) |
| 12 | 5 (4.1) | 5 (9.3) | 0 (0.0) |
| 38 | 5 (4.1) | 2 (3.7) | 3 (4.5) |
| 362 | 3 (2.5) | 0 (0.0) | 3 (4.5) |
| 405 | 3 (2.5) | 0 (0.0) | 3 (4.5) |
| 453 | 3 (2.5) | 3 (5.6) | 0 (0.0) |
| 538 | 3 (2.5) | 2 (3.7) | 1 (1.5) |
| Other * | 45 (37.2) | 19 (35.1) | 26 (38.7) |
* other sequence types identified: ST2, ST14, ST23. ST58, ST59, ST88, ST93, ST104, ST127, ST141, ST345, ST349, ST357, ST362, ST404, ST415, ST428, ST550, ST636, ST646, ST648, ST847, ST998, ST1236, ST1300, ST1444, ST1771, ST1844, ST2015, ST2017, ST2280, ST2522, ST2914, ST3236, ST6467, ST7092.
Figure 2.
Phylogenetic tree inferred from SNPs from a whole-genome alignment for all sequenced E. coli strains. The left bar indicates whether an isolate was sampled from a rUTI episode (black: yes; white: no) and right bar indicates the ten most common sequence types, as listed in Table 1.
3.2. Relatedness of E. coli Isolates Based on SNP Analysis (Same-Strain Recurrence) from Seven Nursing Home Residents
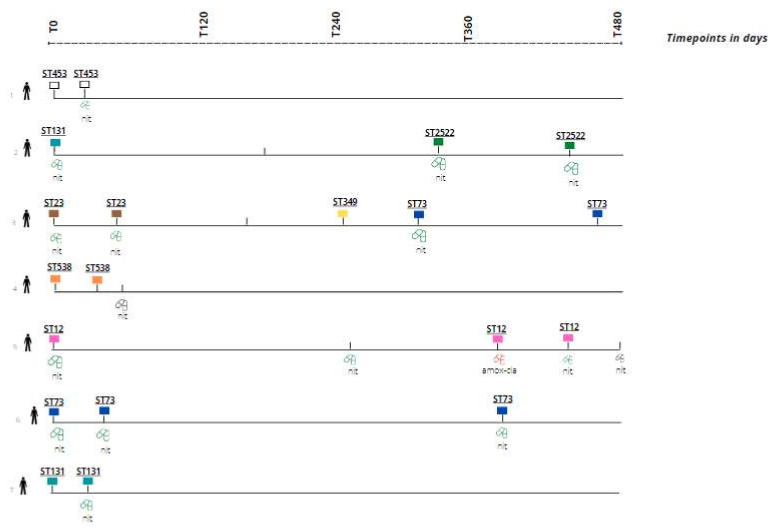
Of the 54 E. coli rUTI episodes, 18 episodes (seven unique nursing home residents) were identified with at least two episodes caused by the same-strain E. coli rUTI based on SNP analysis (33.3%); see Figure 3 (the same color reflects the same strain). When plotted in a phylogenetic tree, most UTI episodes (recurrent and non-recurrent) belonged to phylogroups B2 and D of E. coli (see Supplementary Table S1). We could not observe a clear association between recurrent UTI and phylogenetic placement.
Figure 3.
Overview of nursing home residents (7 unique individuals) with recurrent UTI caused by same-strain E. coli identified based on SNP analysis. Box color represents E. coli sequence type identified; the same color reflects the same strain. STxxx represents sequence type. No box indicates: no urine culture result/isolate available. Green pill indicates a susceptible E. coli strain for antibiotic treatment prescribed; red pill indicates resistant E. coli strains for antibiotic treatment prescribed. Black pill indicates antibiotic treatment with lacking susceptibility testing results. amox/cla = amoxicillin–clavulanic acid; nit = nitrofurantoin; ST = sequence type; T0 = day of the index UT.
Genotypical results were not available for three episodes. Recurrence occurred anywhere between 30 and 434 days after the index UTI (see Figure 3). The same-strain rUTI occurred late in resident 5 (377 days after index UTI episode) and resident 6 (399 days after index UTI episode). The signs and symptoms in rUTIs with the same strain were sometimes similar, but in other same-strain rUTIs, different signs and symptoms were observed. The initial presenting symptoms improved during follow-up (day 5 or 10) for most episodes (n = 16, 88.9%).
Overall, seven different E. coli sequence types (ST2522, ST23, ST453, ST538, ST12, ST73 and ST131) were identified with less than 25 SNPs difference between E. coli isolates within an individual nursing home resident, which indicates recurrence by the same index strain. In one nursing home resident (see Figure 3, line 3), two rUTIs were identified: the first rUTI episodes caused by ST23, and the subsequent rUTI episodes caused by ST73. The majority of same-strain E. coli UTI episodes were treated with antibiotics (80%, n = 16) and all but one were treated with nitrofurantoin (93.8%, n = 15). The duration of treatment is unknown, but the national UTI guideline for frail elderly recommends a treatment duration of 5 days [33]. There were no nitrofurantoin-resistant E. coli isolates found among the same-stain E.coli causing UTI. The overall nitrofurantoin resistance for E. coli was low (4.1% in the total dataset, 6/148, Supplementary Table S2) and there were no UTIs caused by (intrinsically) nitrofurantoin-resistant uropathogen (e.g., Proteus spp.) after previous nitrofurantoin antibiotic treatment of the index UTI episode.
3.3. Antibiotic Resistance in E. coli Causing rUTI
The antimicrobial resistance rates in E. coli rUTI (both same-strain and non-same-strain based on SNP analysis) (n = 49 episodes from both same-strain and non-same-strain E. coli with susceptibility data available) were: 44.1% for amoxicillin, 42.1% for amoxicillin–clavulanic acid, 13.1% for ciprofloxacin, 10,7% for sulfamethoxazole–trimethoprim, 2,6% for fosfomycin, and 2.6% resistance against nitrofurantoin. The overall antibiotic resistance rates are listed in Table S2.
4. Discussion
In this secondary analysis from a multicenter study conducted in nursing home residents, we focused on rUTIs caused by E. coli. We showed that rUTI episodes were caused by other E. coli strains (sequence types) and E. coli strains unique (≤25 SNPs difference) to the index strain (same-strain) determined by whole-genome sequencing. The time between the index UTI and subsequent UTI episodes caused by the same E. coli strain varied substantially between nursing home residents. Interestingly, the longest time between an index UTI episode and rUTI episode was 434 days. It is known that a shorter timeframe between two UTI episodes increases the likelihood of an infection by the same strain [9,34,35]. Our findings on same-strain recurrence after >400 days suggest that E. coli is able to either colonize the bladder for very long periods or recolonize the bladder through the re-introduction of E. coli strain from the gut, which is currently not well understood. It remains unknown why some residents suffer from recurrences by the same strain, while others are re-infected with a new strain. It could be hypothesized that treatment with antimicrobials with tissue penetration (unlike nitrofurantoin) eliminates the causing E. coli strain from both the bladder and the genitorectal area during treatment, which may lead to subsequent infections with other strains, instead of same-strain recurrences. We could not support this hypothesis as most residents were treated with nitrofurantoin and only a few nursing home residents were treated with antimicrobials allowing tissue penetration.
As uropathogenic E. coli acts as a reservoir for the development and mobilization of novel resistance genes or combinations of resistance genes in the gut and at infected extraintestinal body locations [36], it is necessary to consider gut–bladder transmission of these strains as a pathway for UTIs. Unfortunately, we did not collect any stool samples and were therefore unable to study gut–bladder transmission.
The sequence types ST131 and ST73 were most commonly found which is in line with previous data [6,36] and we found comparable E. coli sequence types in nursing home residents with a single UTI and rUTI (see Supplementary File Table S1) [11].
Overall AMR rates were low for the five most commonly prescribed antimicrobial agents (amoxicillin–clavulanic acid, sulfamethoxazole–trimethoprim, ciprofloxacin, fosfomycin and nitrofurantoin) and the most identified E. coli strains were susceptible to nitrofurantoin in both rUTI and single-episode UTIs such as previously described in rUTI [37,38].
Nitrofurantoin is the recommended empirical antibiotic therapy for uncomplicated UTIs in the Netherlands and was therefore relatively frequently prescribed. The low nitrofurantoin prevalence and absence of selection of (intrinsically) nitrofurantoin-resistant uropathogens in time suggest that nitrofurantoin susceptibility isolated from an index urine culture might predict nitrofurantoin susceptibility for future UTI episodes in this setting. However, this hypothesis should be tested using a sufficient sample size to minimize bias introduced by selection or low numbers included.
To the best of our knowledge, this is the first study evaluating E. coli sequence types in relation to rUTI in nursing home residents. Due to the study design of the primary study (PROGRESS study), nursing home residents were eligible to enroll multiple times which enabled comparison of UTI episodes within residents including residents living at psychogeriatric wards. This group is less frequently studied due to logistic challenges and ethical concerns, but are a particular risk group for rUTI. In addition, we enrolled both residents who were treated with antibiotics and those who were not treated, which closely mimics clinical practice. Moreover, using molecular techniques to identify sequence types gives more in-depth information on E. coli strain composition compared to commonly used phylogenetic analysis by PCR. This created a unique opportunity to study the occurrence and patterns of E. coli causing rUTI over time.
However, there are some limitations in this study. First, misclassification bias (differential misclassification) could have been introduced when a subsequent UTI episode was not included due to various reasons such as: UTIs prior the start of the study, referral or death of the nursing home resident during the study period, or the nursing home resident not applying for enrolment. This was caused by the secondary analysis while the data were collected for a different purpose. This may have led to an overestimation of single UTI episodes in our study. For this reason, we are unable to make any firm statements about differences between nursing home residents with rUTI and nursing home residents with single UTI.
Second, a subset of available E. coli isolates was sequenced, while one third of the (recurrent) UTI episodes were caused by other uropathogens (e.g., Klebsiella spp., Proteus spp. and Aerococcus spp.). Therefore, we cannot make any conclusions about rUTIs caused by other uropathogens. Third, from all isolated E. coli strains, a single colony was used for SNP analysis. There could be some variation within the host or the existence of different E. coli clones [39] which may lead to an underestimation of species diversity.
Although many studies focus on unraveling the mechanism behind rUTI, the landscape remains unclear. Future studies should target specific genetic characteristics (for, e.g., virulence factors) which may help in predicting recurrence and helps to target preventive strategies. Preventive intervention strategies such as immunoprophylaxis could be informed by the genetic information of recurrent strains, and may reduce the burden of disease caused by pathogenic and resistant E. coli strains [40].
5. Conclusions
Recurrent UTIs in nursing home residents are caused by same-strain E. coli as well as due to different E. coli strains or other uropathogens. Recurrence by same-strain E. coli occurs after several months up to over 400 days after the index UTI episode, which suggest that some strains have the ability to colonize the bladder for very long periods or recolonize the bladder by the re-introduction of the E. coli strain from the gut.
Acknowledgments
We would like to thank all laboratory technicians (from the Molecular department of the Medical Microbiology and Infection Prevention) involved for their contribution in preparing samples for molecular analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11111638/s1, Table S1: Overview sequence types; Table S2: Antibiotic resistance rates based on E.coli isolates from PROGRESS study.
Author Contributions
Conceptualization, S.H., C.S. and S.D.K.; Data curation, S.H., B.v.d.P. and S.D.K.; Formal analysis, S.H.; Funding acquisition, C.S.; Investigation, S.H.; Methodology, S.H., B.v.d.P. and S.D.K.; Project administration, S.H., C.S. and S.D.K.; Resources, S.H. and S.D.K.; Software, S.H. and B.v.d.P.; Supervision, S.D.K.; Visualization, S.H.; Writing—original draft, S.H.; Writing—review and editing, B.v.d.P., R.v.H. and S.D.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable to this study. The PROGRESS study was conducted according to the guidelines of the Declaration of Helsinki. The study protocol was approved by the Medical Ethical Committee of Amsterdam UMC location VUmc with reference number 2017.350 and National Central Committee on Research involving Human Subjects with reference number NL62067.029.17.
Informed Consent Statement
Not applicable to this study. Informed consent was obtained from all subjects involved in the PROGRESS study.
Data Availability Statement
The data in this study are available from the corresponding author upon request.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The PROGRESS study was funded by The Netherlands Organization for Health Research and Development (ZonMW) grant no 541001003. ZonMW, Laan van Nieuw Oost Indië 334, 2593 CE Den Haag, The Netherlands.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rowe T.A., Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect. Dis. Clin. North Am. 2014;28:75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamm W.E., Norrby S.R. Urinary tract infections: Disease panorama and challenges. J. Infect. Dis. 2001;183((Suppl. S1)):S1–S4. doi: 10.1086/318850. [DOI] [PubMed] [Google Scholar]
- 3.Wagenlehner F., Wullt B., Ballarini S., Zingg D., Naber K.G. Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT) Expert. Rev. Pharm. Outcomes Res. 2018;18:107–117. doi: 10.1080/14737167.2017.1359543. [DOI] [PubMed] [Google Scholar]
- 4.Terlizzi M.E., Gribaudo G., Maffei M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley L.W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014;20:380–390. doi: 10.1111/1469-0691.12646. [DOI] [PubMed] [Google Scholar]
- 7.Mody L., Juthani-Mehta M. Urinary tract infections in older women: A clinical review. JAMA. 2014;311:844–854. doi: 10.1001/jama.2014.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raz R., Gennesin Y., Wasser J., Stoler Z., Rosenfeld S., Rottensterich E., Stamm W.E. Recurrent urinary tract infections in postmenopausal women. Clin. Infect. Dis. 2000;30:152–156. doi: 10.1086/313596. [DOI] [PubMed] [Google Scholar]
- 9.Ikähelmo R., Siitonen A., Heiskanen T., Kärkkäinen U., Kuosmanen P., Lipponen P., Mäkelä P.H. Recurrence of urinary tract infection in a primary care setting: Analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer P.L., O’Reilly M. Recurrent urinary tract infection in the female. Curr. Opin. Obstet. Gynecol. 2002;14:537–543. doi: 10.1097/00001703-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen K., Stegger M., Kiil K., Lilje B., Ejrnæs K., Leihof R., Skjøt-Rasmussen L., Godfrey P., Monsen T., Ferry S., et al. Escherichia coli Causing Recurrent Urinary Tract Infections: Comparison to Non-Recurrent Isolates and Genomic Adaptation in Recurrent Infections. Microorganisms. 2021;9:1416. doi: 10.3390/microorganisms9071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S.L., Wu M., Henderson J.P., Hooton T.M., Hibbing M.E., Hultgren S.J., Gordon J.I. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci. Transl. Med. 2013;5:184ra60. doi: 10.1126/scitranslmed.3005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thänert R., Reske K.A., Hink T., Wallace M.A., Wang B., Schwartz D., Seiler S., Cass C., Burnham C.-A.D., Dubberke E.R., et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio. 2019;10:e01977-19. doi: 10.1128/mBio.01977-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentine-King M.T., Zoorob R., Germanos G., Salemi J., Gupta K., Grigoryan L. Prior cultures predict subsequent susceptibility in patients with recurrent urinary tract infections. Antimicrob. Steward. Heal. Epidemiol. 2022;2:s67. doi: 10.1017/ash.2022.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worby C.J., Olson B.S., Dodson K.W., Earl A.M., Hultgren S.J. Establishing the role of the gut microbiota in susceptibility to recurrent urinary tract infections. J. Clin. Investig. 2022;132 doi: 10.1172/JCI158497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo T.A., Stapleton A., Wenderoth S., Hooton T.M., Stamm W.E. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 1995;172:440–445. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 17.Forde B.M., Roberts L.W., Phan M.-D., Peters K.M., Fleming B.A., Russell C.W., Lenherr S.M., Myers J.B., Barker A.P., Fisher M.A., et al. Population dynamics of an Escherichia coli ST131 lineage during recurrent urinary tract infection. Nat. Commun. 2019;10:3643. doi: 10.1038/s41467-019-11571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindblom A., Kiszakiewicz C., Kristiansson E., Yazdanshenas S., Kamenska N., Karami N., Åhrén C. The impact of the ST131 clone on recurrent ESBL-producing E. coli urinary tract infection: A prospective comparative study. Sci. Rep. 2022;12:10048. doi: 10.1038/s41598-022-14177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D., Reid C.J., Kudinha T., Jarocki V.M., Djordjevic S.P. Genomic analysis of trimethoprim-resistant extraintestinal pathogenic Escherichia coli and recurrent urinary tract infections. Microb. Genom. 2020;6:e000475. doi: 10.1099/mgen.0.000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuil S.D., Hidad S., Fischer J.C., Harting J., Hertogh C.M.P.M., Prins J.M., de Jong M.D., van Leth F., Schneeberger C. Sensitivity of C-Reactive Protein and Procalcitonin Measured by Point-of-Care Tests to Diagnose Urinary Tract Infections in Nursing Home Residents: A Cross-Sectional Study. Clin. Infect. Dis. 2021;73:e3867–e3875. doi: 10.1093/cid/ciaa1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuil S.D., Hidad S., Fischer J.C., Harting J., Hertogh C.M., Prins J.M., van Leth F., de Jong M.D., Schneeberger C. Sensitivity of point-of-care testing C reactive protein and procalcitonin to diagnose urinary tract infections in Dutch nursing homes: PROGRESS study protocol. BMJ Open. 2019;9:e031269. doi: 10.1136/bmjopen-2019-031269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspevall O., Hallander H., Gant V., Kouri T. European guidelines for urinalysis: A collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin. Microbiol. Infect. 2001;7:173–178. doi: 10.1046/j.1198-743x.2001.00237.x. [DOI] [PubMed] [Google Scholar]
- 23.Prjibelski A., Antipov D., Meleshko D., Lapidus A., Korobeynikov A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020;70:e102. doi: 10.1002/cpbi.102. [DOI] [PubMed] [Google Scholar]
- 24.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roer L., Johannesen T.B., Hansen F., Stegger M., Tchesnokova V., Sokurenko E., Garibay N., Allesøe R., Thomsen M.C.F., Lund O., et al. CHTyper, a Web Tool for Subtyping of Extraintestinal Pathogenic Escherichia coli Based on the fumC and fimH Alleles. J. Clin. Microbiol. 2018;56:e00063-18. doi: 10.1128/JCM.00063-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bessonov K., Laing C., Robertson J., Yong I., Ziebell K., Gannon V.P.J., Nichani A., Arya G., Nash J.H.E., Christianson S. ECTyper: In silico Escherichia coli serotype and species prediction from raw and assembled whole-genome sequence data. Microb. Genom. 2021;7:000728. doi: 10.1099/mgen.0.000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters N.R., Abram F., Brennan F., Holmes A., Pritchard L. Easy phylotyping of Escherichia coli via the EzClermont web app and command-line tool. Access Microbiol. 2020;2:acmi000143. doi: 10.1099/acmi.0.000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwengers O., Hain T., Chakraborty T., Goesmann A. ReferenceSeeker: Rapid determination of appropriate reference genomes. J. Open Source Softw. 2020;5:1994. doi: 10.21105/joss.01994. [DOI] [Google Scholar]
- 29.Gorrie C.L., Da Silva A.G., Ingle D.J., Higgs C., Seemann T., Stinear T.P., Williamson D.A., Kwong J.C., Grayson M.L., Sherry N.L., et al. Key parameters for genomics-based real-time detection and tracking of multidrug-resistant bacteria: A systematic analysis. Lancet Microbe. 2021;2:e575–e583. doi: 10.1016/S2666-5247(21)00149-X. [DOI] [PubMed] [Google Scholar]
- 30.StataCorp . Stata Statistical Software: Release 17. StatCorp LLC.; College Station, TX, USA: 2021. [Google Scholar]
- 31.Microsoft Corporation Microsoft Excel [Internet] 2016. [(accessed on 29 June 2022)]. Available online: https://office.microsoft.com/excel.
- 32.CANVA Gratis Ontwerptool. [(accessed on 20 August 2022)]. Available online: www.canva.nl.
- 33.Verenso Richtlijn Urineweginfecties Bij Kwetsbare Ouderen, Dutch National Guideline Urinary Tract Infection in Frail Older Adults 2018. [(accessed on 3 October 2022)]. Available online: https://www.verenso.nl/richtlijnen-en-praktijkvoering/richtlijnendatabase/urineweginfecties.
- 34.Brauner A., Jacobson S.H., Kühn I. Urinary Escherichia coli causing recurrent infections--a prospective follow-up of biochemical phenotypes. Clin. Nephrol. 1992;38:318–323. [PubMed] [Google Scholar]
- 35.Foxman B. Recurring urinary tract infection: Incidence and risk factors. Am. J. Public Health. 1990;80:331–333. doi: 10.2105/AJPH.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manges A.R., Geum H.M., Guo A., Edens T.J., Fibke C.D., Pitout J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019;32:e00135-18. doi: 10.1128/CMR.00135-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haaijman J., Stobberingh E.E., van Buul L.W., Hertogh C., Horninge H. Urine cultures in a long-term care facility (LTCF): Time for improvement. BMC Geriatr. 2018;18:221. doi: 10.1186/s12877-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hisano M., Bruschini H., Nicodemo A.C., Gomes C.M., Lucon M., Srougi M. The Bacterial Spectrum and Antimicrobial Susceptibility in Female Recurrent Urinary Tract Infection: How Different They Are From Sporadic Single Episodes? Urology. 2015;86:492–497. doi: 10.1016/j.urology.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 39.Stegger M., Leihof R.F., Baig S., Sieber R.N., Thingholm K.R., Marvig R.L., Frimodt-Møller N., Nielsen K.L. A snapshot of diversity: Intraclonal variation of Escherichia coli clones as commensals and pathogens. Int. J. Med. Microbiol. 2020;310:151401. doi: 10.1016/j.ijmm.2020.151401. [DOI] [PubMed] [Google Scholar]
- 40.Lorenzo-Gómez M.F., Padilla-Fernández B., Flores-Fraile J., Valverde-Martínez S., González-Casado I., Hernández J.-M.D.D., Sánchez-Escudero A., Arroyo M.-J.V., Martínez-Huélamo M., Criado F.H., et al. Impact of whole-cell bacterial immunoprophylaxis in the management of recurrent urinary tract infections in the frail elderly. Vaccine. 2021;39:6308–6314. doi: 10.1016/j.vaccine.2021.08.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available from the corresponding author upon request.