Abstract
Perinatally, and between menarche and menopause, increased levels of estrogen cause large amounts of glycogen to be deposited in the vaginal epithelium. During these times, the anaerobic metabolism of the glycogen, by the epithelial cells themselves and/or by vaginal flora, causes the vagina to become acidic (pH ∼4). This study was designed to test whether the characteristics of acid production by vaginal flora in vitro can account for vaginal acidity. Eight vaginal Lactobacillus isolates from four species—L. gasseri, L. vaginalis, L. crispatus, and L. jensenii—acidified their growth medium to an asymptotic pH (3.2 to 4.8) that matches the range seen in the Lactobacillus-dominated human vagina (pH 3.6 to 4.5 in most women) (B. Andersch, L. Forssman, K. Lincoln, and P. Torstensson, Gynecol. Obstet. Investig. 21:19–25, 1986; L. Cohen, Br. J. Vener. Dis. 45:241–246, 1969; J. Paavonen, Scand. J. Infect. Dis. Suppl. 40:31–35, 1983; C. Tevi-Bénissan, L. Bélec, M. Lévy, V. Schneider-Fauveau, A. Si Mohamed, M.-C. Hallouin, M. Matta, and G. Grésenguet, Clin. Diagn. Lab. Immunol. 4:367–374, 1997). During exponential growth, all of these Lactobacillus species acidified their growth medium at rates on the order of 106 protons/bacterium/s. Such rates, combined with an estimate of the total number of lactobacilli in the vagina, suggest that vaginal lactobacilli could reacidify the vagina at the rate observed postcoitally following neutralization by the male ejaculate (W. H. Masters and V. E. Johnson, Human sexual response, p. 93, 1966). During bacterial vaginosis (BV), there is a loss of vaginal acidity, and the vaginal pH rises to >4.5. This correlates with a loss of lactobacilli and an overgrowth of diverse bacteria. Three BV-associated bacteria, Gardnerella vaginalis, Prevotella bivia, and Peptostreptococcus anaerobius, acidified their growth medium to an asymptotic pH (4.7 to 6.0) consistent with the characteristic elevated vaginal pH associated with BV. Together, these observations are consistent with vaginal flora, rather than epithelial cells, playing a primary role in creating the acidity of the vagina.
Acidity has long been thought to be one of the protective mechanisms of the vagina. The mild acidity of the healthy vagina (∼pH 4) has been shown to correlate with decreased risk for chlamydia, trichomoniasis (12), urinary tract infections (37), and infection with genital mycoplasma (12) as well as decreased carriage of bacteria in the introitus (38). In contrast, bacterial vaginosis (BV), a common (8) syndrome characterized by an elevated vaginal pH (>4.5) and an overgrowth of a variety of mostly anaerobic bacteria, has been associated with premature birth (8, 30, 36), increased risk of human immunodeficiency virus infection (41), and pelvic inflammatory disease (14).
During the perinatal period and again from menarche to menopause, increased levels of estrogen stimulate the deposition of glycogen in the vaginal epithelium (11, 31). It is during these times that the vagina is most acidic. The vagina is believed to be acidified by the anaerobic metabolism of vaginal glycogen to acidic products, predominantly acetic and lactic acids. However, whether this metabolism is performed by vaginal bacteria (31) and/or epithelial cells (5, 39) has yet to be determined (for a recent review, see reference 33).
The presence of lactobacilli has previously been shown (7, 43) to correlate with a low vaginal pH, but it has been argued that bacteria could not be responsible for vaginal acidification, since during infancy, the vaginal pH of infant girls is low despite a lack of vaginal bacteria (23). More recently, however, it has been shown (26) that by the time of delivery, infants are already colonized by their mother’s microflora. Thus, the strongest argument against bacteria being the primary source for vaginal acidification is the existence of women whose vaginas are acidic but are not colonized by lactobacilli (34). This is inconsistent with the bacterial hypothesis only if lactobacilli are considered the only possible bacterial source of acidification, but other species of bacteria may also play a role. For example, it has recently been demonstrated that Escherichia coli isolates can acidify their growth media in vitro through the production of d-lactate (28).
L. acidophilus was once believed to be the dominant species of Lactobacillus in the vagina, but with more recent techniques, the L. acidophilus group has been subdivided into a number of genospecies. Although some studies still show L. acidophilus (45) as dominant, others have identified L. gasseri (10, 22), L. jensenii (10, 35), L. cellobiosus (40), L. fermentum (10), and L. crispatus (10) as the most abundant species.
Shifts in bacterial flora have long been associated with shifts in vaginal pH (3, 8, 36). During BV, for example, the vaginal pH rises and the vaginal flora shifts from being Lactobacillus dominated (32) to a flora in which Gardnerella vaginalis, Mycoplasma hominis, and anaerobic bacteria (14, 15) predominate. Although this correlation suggests that vaginal acidity is produced by vaginal flora, it is also possible that the shift in flora may alter acid production by the vaginal epithelium.
The objective of the experiments reported here was to determine, analogous to Koch’s postulates, whether vaginal bacteria when grown in vitro exhibit the characteristics required to account for the observed properties of vaginal acidity in vivo. (i) Can vaginal lactobacillus species grow in the pH range (3.6 to 4.5) found in a healthy vagina? (ii) Can they produce acid at a rate that can account for the rate of vaginal acidification observed after intercourse? (iii) Can organisms associated with BV acidify their media only to the elevated range (pH >4.5) seen in women with the BV, but not to the more acidic pH range of the healthy vagina?
Due to the great disparity in the literature (10, 22, 35, 40, 45) as to what species of lactobacilli are most commonly isolated from women’s vaginas, we used several criteria to select which bacteria we would use for our experiments. Since the presence of H2O2-producing lactobacilli in the vagina has been shown to correlate with a decreased incidence of BV (13, 16) and chlamydia (16) and in vitro such bacteria have been shown to be viricidal to human immunodeficiency virus type 1 (21), six H2O2-positive isolates of two common species, L. jensenii and L. crispatus, were chosen for this study, along with single isolates of two H2O2-negative species, L. gasseri and L. vaginalis. For BV-associated organisms, G. vaginalis and Peptostreptococcus anaerobius were selected because they were found to be the most acid resistant of seven common BV organisms tested (G. vaginalis, P. anaerobius, Prevotella bivia, Mycoplasma hominis, Bacteroides ureolyticus, Mobiluncus curtisii, and Mobiluncus mulieris [18]), and thus were most likely to maximally acidify the growth medium. P. bivia was selected since it has an acid sensitivity more typical of the other common BV-associated bacteria (18).
MATERIALS AND METHODS
Bacterial strains.
We tested three H2O2-positive vaginal isolates from each of the two species L. jensenii and L. crispatus (gift of Sharon Hillier, University of Pittsburgh School of Medicine, Pittsburgh, Pa.). Vaginal isolates of L. vaginalis (ATCC 49540) and L. gasseri (ATCC 9857) and human isolates of G. vaginalis (ATCC 14018), P. bivia (ATCC 29303), and P. anaerobius (ATCC 27337) were obtained from the American Type Culture Collection, Manassas, Va.
Measurement of bacterial growth and acidification by lactobacillus spp.
Freshly thawed aliquots of Lactobacillus stock were inoculated in MRS broth (BBL; Sparks, Md.) and grown overnight at 37°C in 5% CO2–95% air. MRS broth was used at its formulated pH of ∼6, and acidified medium was prepared by titrating nonsterile MRS broth to the target pH with concentrated HCl and then autoclaving the medium. We chose to use MRS broth instead of a chemically defined medium designed to simulate genital secretions (9), since, to allow for bacterial growth, the defined medium is supplemented with metabolites that are not present in the vagina, and as such we did not feel it added sufficient verisimilitude to our study to be worth the decreased viability observed in that medium.
For acidification experiments and experiments examining the effects of acidified medium, for each time point individual tubes were prepared containing 900 μl of acidified medium and 100 μl of bacterial solution—giving a starting concentration of ∼107 bacteria/ml. These tubes were incubated at 37°C in 5% CO2–95% air for between 0 and 200 h. For each time point, a tube was removed from the incubator, 200 μl of medium was placed in a 96-well plate, and the optical density at 600 nm (OD600) was measured (SpectraMaxPro; Molecular Devices, Sunnyvale, Calif.) to determine the approximate bacterial concentration (where 1 OD unit is ∼8 × 108 cells/ml). The pH of the remaining medium was then measured with a pH meter (Beckman Φ11; Wilmington, Del.) by using a calibrated glass electrode (Beckman 39849). A total of six replicates were done for all assays, representing at least two separate experiments.
For experiments to determine whether bacterial acidification was the factor that limited growth, freshly thawed aliquots of L. crispatus, L. gasseri, or L. vaginalis stock were inoculated in MRS broth and grown for 48 h at 37°C in 5% CO2–95% air. Four sets of tubes were prepared for each experiment containing 100 μl of bacterial solution and 1 ml of either MRS (initial pH [pHi] 6), MRS (pHi 4), or medium from the initial incubation sterilized by filtration (0.2-μm-pore-size sterile syringe filters; Valuprep; VWR, West Chester, Pa.) and either neutralized with NaOH to pH ∼6 or left at the pH (∼4) to which the bacteria had acidified it during the initial incubation. Bacterial concentration and pH of the medium were measured as described above at 0, 24, and 48 h. These experiments were performed in triplicate.
Measurement of bacterial growth and acidification by BV organisms.
Freshly thawed aliquots of G. vaginalis stock were grown overnight in basal broth as described previously (29). Individual tubes for each time point were established by adding 0.1 ml of bacterial solution to 9.9 ml of basal broth. Tubes were incubated at 37°C in 5% CO2–95% air. The pH and concentration were measured at each time point as described above.
Freshly thawed aliquots of P. bivia and P. anaerobius were grown overnight in chopped meat carbohydrate medium in Hungate capped tubes (Anaerobe Systems, San Jose, Calif.) which form self-contained anaerobic chambers. Individual tubes for each time point were prepared by adding 0.1 ml of bacterial solution to the 7 ml of medium present in the tubes. To measure bacterial growth, P. bivia was plated on PRAS LKV plates (Anaerobe Systems) and incubated in a sealed BBL GasPak Bag to form an anaerobic environment. P. anaerobius was plated on Brucella laked blood agar plates (Oxyrase, Mansfield, Ohio). Both bacteria were plated by the serial tract dilution method (19), and the pH was measured as described above. All of these BV-associated organisms have a recommended growth pH of ∼7. A total of three replicates were done for all assays, representing at least two separate experiments.
Measurement of cell area and bacterial counts.
Vaginal swabs were obtained from women during the course of regular clinical exams at the Johns Hopkins Student Health Service, under an institutional review board-approved protocol. Swabs were placed in tubes containing 1 ml of sterile saline, and multiple slides were prepared within 4 h of collection. One slide for each woman was immediately fixed with acetone and Giemsa stained (Accustain; Sigma, St. Louis, Mo.). For some women, duplicate slides were also Gram stained.
Slides were examined with bright-field microscopy under a Nikon Eclipse E800 (Image Systems, Inc., Columbia, Md.) microscope with a ×60 objective, and images were acquired with a Princeton Instruments charge-coupled device camera (CCD-1317-K/1; Princeton Instruments; Trenton, N.J.). IP Lab Spectrum (Signal Analytics Corporation, Vienna, Va.) image analysis software was used to measure the surface area and major and minor axes of representative cells.
In women with apparently normal flora (women with diagnosed BV were excluded), the number of adherent large rods per epithelial cell (top surface only) was counted. This was done either by direct microscopic observation or by analysis of a captured image.
Statistics.
All statistical analysis was performed with Origin 4.1 (Microcal Software, Inc., Northampton, Mass.). Individual statistical tests are labeled on each figure. All growth curves are means ± standard deviations. The asymptotic pH for each bacterial species was calculated by fitting the averaged acidification curves to a first-order exponential decay and taking the y intercept, using a χ2 test to indicate the goodness of fit. The acid production rate was calculated from the mean change in pH over the first 8 h (except where otherwise noted), with data normalized for the average concentration of bacteria present during that interval.
RESULTS
Effect of acidification of medium on growth.
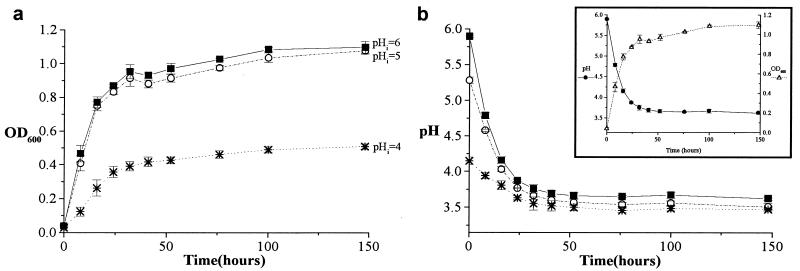
Table 1 shows the extent of growth of the four strains of lactobacilli when they were grown in either standard growth medium (pHinitial 6) or in growth medium acidified with HCl to pHi 5 or 4. As the pH of the growth medium was reduced to 4, the bacterial species showed various degrees of pH-dependent growth inhibition (Fig. 1a); however, even at pH 4, the strains of lactobacilli remained viable and still exhibited significant growth.
TABLE 1.
Extent of growth by Lactobacillus cultures started at various pHis
| Species | ΔOD600 for Lactobacillus cultures started ata:
|
||
|---|---|---|---|
| 6.2>pHi>5.7 | 5.3>pHi>5.0 | 4.2>pHi>4.0 | |
| L. crispatus | |||
| I | 1.09 (0.03) | 1.10 (0.04) | 0.36 (0.07) |
| II | 1.19 (0.04) | 1.02 (0.20) | 0.86 (0.02) |
| III | 1.25 (0.02) | 0.98 (0.06) | 0.77 (0.20) |
| L. gasseri | 1.08 (0.01) | 1.04 (0.02) | 0.50 (0.02) |
| L. jensenii | |||
| I | 0.52 (0.03) | 0.30 (0.03) | 0.06 (0.002) |
| II | 0.76 (0.72) | 0.13 (0.02) | 0.08 (0.01) |
| III | 1.44 (0.04) | 0.43 (0.04) | 0.20 (0.01) |
| L. vaginalis | 0.78 (0.04) | 0.88 (0.02) | 0.97 (0.02) |
One OD600 unit is ∼8 × 108 bacteria. Values are means; values in parentheses are standard deviations. All experiments started at ∼107 bacteria/ml.
FIG. 1.
Growth and acidification as a function of time for L. gasseri. (a) Bacterial growth (shown as OD600) was inhibited as pHi was decreased. (b) The L. gasseri bacteria acidified their growth medium to a similar asymptotic pH regardless of pHi. The inset shows how acidification (●) parallels growth (▵) (pHi 6). All points are means ± standard deviations.
Asymptotic pH.
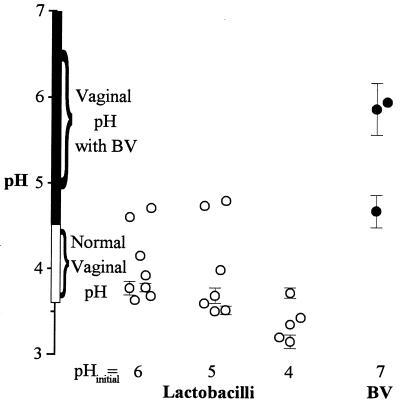
As shown by the representative plot (Fig. 1b and inset), over the time course of these experiments, the rate at which the bacteria acidified their growth medium paralleled their rate of growth, and an asymptotic pH was approached as the bacteria neared terminal growth. The asymptotic pH is the estimated final pH of the medium that would have occurred had the experiment been allowed to continue indefinitely. All of these Lactobacillus species acidified their medium to an asymptotic pH of 3.2 to 4.8, a range comparable to that seen in the healthy vagina (pH 3.6 to 4.5 for most women) (1, 5, 31, 42) (Table 2 and Fig. 2). Upon examination of growth and acidification curves, all species except for L. vaginalis appeared to experience acid-limited growth. This was tested in L. vaginalis, L. crispatus, and L. gasseri, by comparing their growth in fresh medium at pHis 6 and 4 to their growth in conditioned medium (medium in which the bacteria had been grown to their asymptotic pH with terminal growth) which had either been neutralized with NaOH (to pH ∼6) or left at the pH to which it had been acidified by the bacteria (pH ∼4) during their initial incubation. L. crispatus and L. gasseri exhibited significant growth in neutralized conditioned medium and fresh pHi 6 MRS broth, but not in unneutralized conditioned medium or fresh pHi 4 MRS broth. L. vaginalis could not grow in the conditioned medium, regardless of neutralization, but grew well in fresh medium at either pH. This suggests that, within the pH range observed here, acidification was the limiting factor for growth of L. crispatus and L. gasseri, but not for L. vaginalis.
TABLE 2.
Asymptotic pH of growth medium after acidification by vaginal lactobacillus cultures as a function of pHi
| Species | Asymptotic pH for Lactobacillus culturesa started atb:
|
||
|---|---|---|---|
| 6.2>pHi>5.7 | 5.3>pHi>5.0 | 4.2>pHi>4.0 | |
| L. crispatus | |||
| I | 3.69 ± 0.04 | 3.53 ± 0.05 | 3.74 ± 0.06 |
| II | 3.79 ± 0.05 | 3.61 ± 0.04 | 3.22 ± 0.04 |
| III | 3.78 ± 0.08c | 3.70 ± 0.09c | 3.17 ± 0.08 |
| L. gasseri | 3.64 ± 0.01 | 3.52 ± 0.02 | 3.45 ± 0.01 |
| L. jensenii | |||
| Id | 4.72 ± 0.04 | 4.81 ± 0.02 | No growth |
| II | 4.61 ± 0.02 | No growth | No growth |
| III | 3.93 ± 0.01 | 4.75 ± 0.01 | No growth |
| L. vaginalis | 4.16 ± 0.04 | 4.00 ± 0.04 | 3.37 ± 0.03 |
Initial concentration, ∼107 bacteria/ml.
Curves fit to 1st-order exponential decay.
χ2, >0.01.
This species showed a ΔOD600 of <0.2 when started in this pH range.
FIG. 2.
Asymptotic pHs observed during growth of Lactobacillus spp. (○) in vitro correlate with normal vaginal pH regardless of the pHi of the growth medium. The asymptotic pHs observed during the growth of BV organisms (●) fall within the vaginal pH range seen in women with BV—these bacteria do not grow well, if at all, at a lower pHi. All points are means ± standard deviations. Error bars are not shown where the standard deviation is smaller than the symbol.
Three bacteria associated with BV, G. vaginalis, P. anaerobius, and P. bivia, were all cultured at pH ∼7, which is the optimal growth pH for these species. The range of asymptotic pHs (4.7 to 6.0) (Table 3) was significantly less acidic than that seen after acidification by lactobacilli, even though G. vaginalis and P. anaerobius are the most acid resistant of eight common BV-associated bacteria (18).
TABLE 3.
Asymptotic pH of growth medium after acidification by cultures of BV bacteria
| Species | Asymptotic pH for bacterial culturesa
|
||
|---|---|---|---|
| pHi | pHfinal | No. of bacteria (CFU)/mlinitial | |
| G. vaginalis | 6.58 ± 0.01 | 4.70 ± 0.19 | 4.8 × 106 |
| P. bivia | 6.98 ± 0.03 | 5.89 ± 0.30 | 1 × 106 |
| P. anaerobis | 6.92 ± 0.01 | 5.97 ± 0.04 | 1.7 × 107 |
Curves fit to 1st-order exponential decay. χ2, <0.01.
Rate of acidification.
Since only total concentration (OD), and not viability, was assessed during the measurement of bacterial growth, the acidification rates per bacterium were determined during the period of most rapid growth (in the first 8 h of incubation, except where otherwise noted), because most bacteria present could be presumed to be viable during rapid growth. In all eight Lactobacillus spp., each bacterium produced protons at the rate of 106/s (protons per second per bacterium) when growth was started at a pHi of >5.0 (Table 4). In all species where it could be determined, as bacteria were grown in medium formulated with pHi close to the asymptotic pH for that isolate, the rate of growth decreased, whereas the rate of acid production per bacterium, if anything, increased.
TABLE 4.
Rate of acid production by Lactobacillus spp.
| Species | Acid production (106 proton/s/bacterium) by Lactobacillus cultures started ata:
|
||
|---|---|---|---|
| 6.2>pHi>5.7 | 5.3>pHi>5.0 | 4.2>pHi>4.0 | |
| L. crispatus | |||
| I | 0.6 | 1.2 | 25b |
| II | 0.6 | 1.2 | 7.7b |
| III | 0.3 | 1.4b | 7.1b |
| L. gasseri | 1 | 2.6 | 15 |
| L. jensenii | |||
| Ic | 0.8 | 1.1 | No growth |
| II | Not determined | ||
| III | 1.6 | 1.2 | No growth |
| L. vaginalis | 0.6 | 1.0 | 2.7 |
All experiments started at ∼107 bacterium/ml. Results were calculated during the 0- to 8-h period unless otherwise noted.
Calculated during the 8- to 16-h period.
This species showed a ΔOD600 of <0.2 when started in this pH range.
Rate of vaginal acidification.
The rate of acid production in the vagina has not been directly observed, but Masters and Johnson (27) demonstrated that the alkaline buffering action of the ejaculate abolishes vaginal acidity for several hours after intercourse and that the reacidification rate of the vagina after intercourse is ∼0.5 pH units/h. Since the buffer capacity of semen dominates the buffer capacity of the vagina after intercourse, it is possible to estimate the rate of acid production in the vagina by equating it to the amount needed to acidify an average ejaculate (3.3 ml at pH 7.6 [4]) at the rate observed postcoitally in the vagina. The buffer capacity of semen is 40 mM/pH (44) (i.e., it takes 40 mM/liter of HCl to lower the pH 1 unit). This is approximately 1.3 times the buffer capacity of MRS broth (titration data not shown). Therefore, acid production by lactobacilli would acidify semen at about 0.75 the rate at which it would acidify a similar volume of MRS broth. MRS broth acidified by bacteria does, in fact, acidify semen to an extent consistent with both fluids having comparable buffer capacities (data not shown). At concentrations of ∼108 bacteria/ml, the lactobacilli in these experiments acidified MRS broth at rates of 0.75 to 1 pH units/h, and as such would acidify a typical ejaculate of semen (∼3 ml) at rates of 0.56 to 0.75 pH units/h. This implies there must be ≳108 lactobacilli in the vagina to acidify it at the rate observed after intercourse.
Estimation of the number of lactobacilli in the vagina.
Vaginal lavages are the standard method of measuring the number of bacteria in the vagina and indicate that between 107 and 109 lactobacilli (2, 6, 24) can be removed from the vagina by vaginal lavage. However, lavage data must significantly underestimate the total number of bacteria present in the vagina, since lavage samples only contain the organisms that are shed from the vaginal epithelium, and many lactobacilli remain in the vagina after a lavage. Therefore, to estimate another lower bound for the total number of lactobacilli in the vagina, we counted the average number of large rods per shed vaginal epithelial cell in our vaginal swab samples and determined the approximate number of epithelial cells required to cover the vaginal surface. The surface area of the vaginal epithelium can be estimated by multiplying its length at full extension, ∼12 cm (27), by the circumference of the vaginal vault—an upper bound for which can be approximated as the circumference of an infant’s head, ∼30 cm (diameter, ∼10 cm, depending on presentation [25]). This yields an area of ∼360 cm2, which is about 1.5 times the surface area of an erect penis (200 cm2) (17 [see also reference 20]). We found that the average area of a shed vaginal epithelial cell was ∼1,500 μm2 (range, 900 to 2,500 μm2). Therefore covering the surface of the vagina with a monolayer of cells requires ∼1.2 × 107 epithelial cells. Since there were an average of 30 large bacilli adherent to each cell, this implies there are at least ∼4 × 108 bacilli in the vagina, most of which are probably lactobacilli. Visual inspection of the swabs showed that the majority of large bacilli in the samples did not adhere to the epithelial cells, so our best estimate is that there are ∼108 to 109 lactobacilli in the healthy vagina. This estimated number is both large enough to account for and consistent with the rate of acid production in the vagina after intercourse.
DISCUSSION
Regardless of initial pH and concentration of bacteria, the lactobacilli in this study all stopped growing and acidifying when they reached an asymptotic pH in the range of 3.2 to 4.8. This range is comparable to that seen in vivo in the vagina. Furthermore, three bacterial species commonly associated with BV were found to acidify their medium to a significantly higher asymptotic pH than the lactobacilli—pHs 4.7 to 5.9—a range comparable to the higher vaginal pH found in the vaginas of women with BV. This suggests that lactobacilli are not only acidophilic, but that they create an acidic environment that can inhibit the growth of other organisms.
For all Lactobacillus species examined, except L. vaginalis, the lack of dependence of extent of growth on bacterial concentration suggested that depletion of metabolites and/or buildup of waste products, other than acids, was not a limiting factor. When this was explicitly tested with L. crispatus and L. gasseri, we found that these bacteria could resume growth in their conditioned medium if it was neutralized to a higher pH, showing that acidity alone was the limiting factor in their growth. In contrast, L. vaginalis, which was not pH sensitive in the range we tested, did not resume growth in neutralized conditioned medium if these bacteria had been grown in that medium to an asymptotic level. This suggests that in the growth medium used here and within the range of physiological pH testable in that medium, L. vaginalis reaches its terminal growth due to the depletion of a metabolite or the buildup of a toxic waste product other than acidity. Due to difficulties with precipitation of the medium, L. vaginalis could not be tested in MRS broth formulated at pH <3.5.
Acid production rates during rapid growth were similar for all of the Lactobacillus species studied, approximately 106 protons per second per bacterium between pH 5 and 6. The acid production appeared to increase as the growth rate slowed, when the bacteria were grown nearer to their asymptotic pH. A possible explanation for this increase in acid production per bacterium is that the bacteria may have to devote a larger fraction of metabolic energy to pumping protons out of their cytoplasm. This would lead them to produce even more acid waste products while decreasing their ability to replicate.
Our observations in vitro demonstrate that on the order of 108 lactobacilli are required to produce acid at a rate comparable to the acid production rate of the vagina observed in vivo after intercourse. The Lactobacillus content of vaginal lavages (6) together with our observations of the number/unit area of bacteria adherent to shed epithelial cells indicate that there are at least this many lactobacilli present in the healthy vagina.
Our results are consistent with, and do not rule out, the hypothesis that the acidity of the vagina is predominantly produced and regulated by bacteria. A crucial further test of this hypothesis will be direct observation of pH regulation by the estrogen-stimulated vaginal epithelium in the absence of bacterial metabolism. Also, further experiments are planned to examine the ratio of vaginal lactic acid produced by epithelial cells to that produced by bacteria.
ACKNOWLEDGMENTS
We express our sincere appreciation to Sharon Hillier and May Antonio of the University of Pittsburgh School of Medicine for providing the L. jensenii and L. crispatus isolates used in these studies. We also thank Linda Rhodes and Kathy Slone of the Johns Hopkins Student Health Service for assistance with clinical samples.
This work was supported in part by NIH training grant T32-GM-0-7231.
REFERENCES
- 1.Andersch B, Forssman L, Lincoln K, Torstensson P. Treatment of bacterial vaginosis with an acid cream: a comparison between the effect of lactate-gel and metronidazole. Gynecol Obstet Investig. 1986;21:19–25. doi: 10.1159/000298923. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett J G, Onderdonk A B, Drude E, Goldstein C, Anderka M, Alpert S, McCormack W M. Quantitative bacteriology of the vaginal flora. J Infect Dis. 1977;136:271–277. doi: 10.1093/infdis/136.2.271. [DOI] [PubMed] [Google Scholar]
- 3.Caillouette J C, Sharp C F, Zimmerman G J, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270–1275. doi: 10.1016/s0002-9378(97)70345-4. [DOI] [PubMed] [Google Scholar]
- 4.Ciba-Geigy Corporation. Geigy scientific tables. 1. Units of measurement, body fluids, composition of the body, nutrition. West Caldwell, N.J: Medical Education Division, Ciba-Geigy Corporation; 1981. pp. 185–186. [Google Scholar]
- 5.Cohen L. Influence of pH on vaginal discharges. Br J Vener Dis. 1969;45:241–246. doi: 10.1136/sti.45.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook R L, Tannock G W, Meech R J. The normal microflora of the vagina. Proc Univ Otago Med Sch. 1984;62:72–74. [Google Scholar]
- 7.Drake S M, Evans B A, Gerken A. Vaginal pH and microflora related to yeast infections and treatment. Br J Vener Dis. 1980;56:107–110. doi: 10.1136/sti.56.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eschenbach D A. History and review of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:441–445. doi: 10.1016/0002-9378(93)90337-i. [DOI] [PubMed] [Google Scholar]
- 9.Geshnizgani A M, Onderdonk A B. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol. 1992;30:1323–1326. doi: 10.1128/jcm.30.5.1323-1326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giorgi A, Torriani S, Dellaglio F, Bo G, Stola E, Bernuzzi L. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica. 1987;10:377–384. [PubMed] [Google Scholar]
- 11.Gregoire A T, Kandil O, Ledger W J. The glycogen content of human vaginal epithelial tissue. Fertil Steril. 1971;22:64–68. doi: 10.1016/s0015-0282(16)37989-4. [DOI] [PubMed] [Google Scholar]
- 12.Hanna N F, Taylor-Robinson D, Kalodiki-Karamanoli M, Harris J R, McFadyen I R. The relation between vaginal pH and the microbiological status in vaginitis. Br J Obstet Gynaecol. 1985;92:1267–1271. doi: 10.1111/j.1471-0528.1985.tb04874.x. [DOI] [PubMed] [Google Scholar]
- 13.Hawes S E, Hillier S L, Benedetti J, Stevens C E, Koutsky L A, Wolner-Hanssen P, Holmes K K. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 14.Hillier S L, Kiviat N B, Hawes S E, Hasselquist M B, Hanssen P W, Eschenbach D A, Holmes K K. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol. 1996;175:435–441. doi: 10.1016/s0002-9378(96)70158-8. [DOI] [PubMed] [Google Scholar]
- 15.Hillier S L, Krohn M A, Rabe L K, Klebanoff S J, Eschenbach D A. The normal vaginal flora, peroxide-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16(Suppl.4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 16.Hillier S L, Krohn M A, Klebanoff S J, Eschenbach D A. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstet Gynecol. 1992;79:369–373. doi: 10.1097/00006250-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Jaimeson P J, Gebhard P H. Penis size increase between flaccid and erect states: an analysis of the Kinsey data. J Sex Res. 1988;24:177–183. doi: 10.1080/00224498809551408. [DOI] [PubMed] [Google Scholar]
- 18.Jansen A M. Bacterial vaginosis. Masters thesis. Baltimore, Md: Johns Hopkins School of Hygiene and Public Health; 1998. [Google Scholar]
- 19.Jett B D, Hatter K L, Huycke M M, Gilmore M S. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 20.Katz D F, Henderson M H, Owen D H, Plenys A M, Walmer D K. What is needed to advance vaginal formulation technology? In: Rencher W F, editor. Vaginal Microbicide Formulations Workshop. Philadelphia, Pa: Lippincott-Raven Publishers; 1998. pp. 90–99. [Google Scholar]
- 21.Klebanoff S J, Coombs R W. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991;174:289–292. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachlak N, Ageron E, Zampatti O, Michel G, Grimont P A. Composition of the Lactobacillus acidophilus complex isolated from vaginal flora. New Microbiol. 1996;19:123–132. [PubMed] [Google Scholar]
- 23.Larsen B. Vaginal flora in health and disease. Clin Obstet Gynecol. 1993;36:107–121. doi: 10.1097/00003081-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Larsen B, Glask R P. Vaginal microbial flora: practical and theoretic relevance. Obstet Gynecol. 1980;55:100S–113S. doi: 10.1097/00006250-198003001-00032. [DOI] [PubMed] [Google Scholar]
- 25.Llewellyn-Jones . Fundamentals of obstetrics and gynaecology. 1. Obstetrics. London, United Kingdom: Faber and Faber, Ltd.; 1969. p. 86. [Google Scholar]
- 26.Mandar R, Mikelsar M. Transmission of the mother’s microflora to the newborn at birth. Biol Neonate. 1996;69:30–35. doi: 10.1159/000244275. [DOI] [PubMed] [Google Scholar]
- 27.Masters W H, Johnson V E. Human sexual response. Boston, Mass: Bantam Books; 1966. p. 93. [Google Scholar]
- 28.McCabe K, Mann M D, Bowie M E. d-Lactate production and [14C]succinic acid uptake by adherent and nonadherent Escherichia coli. Infect Immun. 1998;66:907–911. doi: 10.1128/iai.66.3.907-911.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean N W, McGroarty J A. Growth inhibition of metronidazole-susceptible and metronidazole-resistant strains of Gardnerella vaginalis by lactobacilli in vitro. Appl Environ Microbiol. 1996;62:1089–1092. doi: 10.1128/aem.62.3.1089-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minkoff H, Grunebaum A, Feldman J, Cummings M, McCormack W M. Relationship of vaginal pH and Papanicolaou smear results to vaginal flora and pregnancy outcome. Int J Gynaecol Obstet. 1987;25:17–23. doi: 10.1016/0020-7292(87)90179-2. [DOI] [PubMed] [Google Scholar]
- 31.Paavonen J. Physiology and ecology of the vagina. Scand J Infect Dis Suppl. 1983;40:31–35. [PubMed] [Google Scholar]
- 32.Parent D. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol—a placebo-controlled multicentric clinical trial. Arzneim-Forsch Drug Res. 1996;46:68–73. [PubMed] [Google Scholar]
- 33.Pybus V, Onderdonk A B. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1999;1:285–292. doi: 10.1016/s1286-4579(99)80024-0. [DOI] [PubMed] [Google Scholar]
- 34.Redondo-Lopez V, Cook R L, Sobel J D. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 35.Reid G, McGroarty J A, Tomeczek L, Bruce A W. Identification and plasmid profiles of Lactobacillus species from the vagina of 100 healthy women. FEMS Immunol Med Microbiol. 1996;15:23–26. doi: 10.1111/j.1574-695X.1996.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 36.Sagawa T, Negishi H, Kishida T, Yamada H, Fujimoto S. Vaginal and cervical pH in bacterial vaginosis and cervicitis during pregnancy. Hokkaido Igaku Zasshi. 1995;70:839–846. [PubMed] [Google Scholar]
- 37.Stamey T A, Kaufman M F. Studies of introital colonization in women with recurrent urinary infections. II. A comparison of growth in normal vaginal fluid of common versus uncommon serogroups of Escherichia coli. J Urol. 1975;114:264–267. doi: 10.1016/s0022-5347(17)67004-6. [DOI] [PubMed] [Google Scholar]
- 38.Stamey T A, Timothy M M. Studies of introital colonization in women with recurrent urinary infections. I. The role of vaginal pH. J Urol. 1975;114:261–263. doi: 10.1016/s0022-5347(17)67003-4. [DOI] [PubMed] [Google Scholar]
- 39.Stamey T A, Timothy M M. Studies of introital colonization in women with recurrent urinary infections. III. Vaginal glycogen concentrations. J Urol. 1975;114:268–270. doi: 10.1016/s0022-5347(17)67005-8. [DOI] [PubMed] [Google Scholar]
- 40.Steyn P L, Holzapfel W H. Identity and antibiotic sensitivity of human vaginal lactic acid bacteria from the population of northern Namibia. S-Afr Tydskr Wet. 1991;87:68–69. [Google Scholar]
- 41.Taha T E, Dallabetta G A, Hoover D R, Chiphangwi J D, Mtimavalye L A, Liomba G N, Kumwenda N I, Miotti P G. Trends of HIV-1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi. AIDS. 1998;12:197–203. doi: 10.1097/00002030-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Tevi-Bénissan C, Bélec L, Lévy M, Schneider-Fauveau V, Si Mohamed A, Hallouin M-C, Matta M, Grésenguet G. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin Diagn Lab Immunol. 1997;4:367–374. doi: 10.1128/cdli.4.3.367-374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinstien L, Howard J H. The effect of estrogenic hormone on the H-ion concentration and the bacterial content of the human vagina with special reference to the Doderlein bacillus. Am J Obstet Gynecol. 1939;37:698–703. [Google Scholar]
- 44.Wolters-Everhardt E, Dony J M J, Doesburg W H, De Pont J-J H H M. Buffering capacity of human semen. Fertil Steril. 1986;46:114–119. doi: 10.1016/s0015-0282(16)49468-9. [DOI] [PubMed] [Google Scholar]
- 45.Wylie J G, Henderson A. Identity and glycogen-fermenting ability of lactobacilli isolated from the vagina of pregnant women. J Med Microbiol. 1969;2:363–366. doi: 10.1099/00222615-2-3-363. [DOI] [PubMed] [Google Scholar]