Abstract
Apicomplexan parasites are the causal agents of different medically important diseases, such as toxoplasmosis, cryptosporidiosis, and malaria. Toxoplasmosis is considered a neglected parasitosis, even though it can cause severe cerebral complications and death in immunocompromised patients, including children and pregnant women. Drugs against Toxoplasma gondii, the etiological agent of toxoplasmosis, are highly toxic and lack efficacy in eradicating tissue cysts, promoting the establishment of latent infection and acute relapsing disease. Cryptosporidiosis has been recognized as the most frequent waterborne parasitosis in US outbreaks; anti-cryptosporidium drug discovery still faces a major obstacle: drugs that can act on the epicellular parasite. Severe malaria is most commonly caused by the progression of infection with Plasmodium falciparum. In recent years, great progress has been made in the field of antimalarial drugs and vaccines, although the resistance of P. falciparum to artemisinin has recently gained a foothold in Africa. As seen, the search for new drugs against these parasites remains a challenge. Peptide-based drugs seem to be attractive alternative therapeutic agents recently recognized by the pharmaceutical industry, as they can kill different infectious agents and modulate the immune response. A review of the experimental effects of bioactive peptides on these parasites follows, along with comments. In addition, some biological and metabolomic generalities of the parasites are reviewed to elucidate peptide mechanisms of action on Apicomplexan targets.
Keywords: Apicomplexan, bioactive peptides, toxoplasmosis, cryptosporidiosis, malaria
1. Introduction
Parasitism is a biological interaction present in nature. Some parasites can cause a severe clinical picture, and others can even cause host death. Millions of people are infected by parasites worldwide, mainly in lower- and middle-income countries. Among the most important human parasites are single-cell protozoan organisms, which are divided into different phyla [1,2]. The protozoan phylum Apicomplexa is a large group of intracellular alveolates; its name is derived from the complex of organelles located at the apical end that allow them to survive in the host cell. Apicomplexan parasites cause important infectious diseases in humans, including malaria, toxoplasmosis, and cryptosporidiosis [3]. Some intestinal coccidian infections and toxoplasmosis are considered by the World Health Organization (WHO), neglecting parasitosis; therefore, they are not a priority for pharmaceuticals to invest in the research of new compounds for their control, and malaria is one of the most dangerous infections that caused approximately 627,000 human deaths in 2020 [4]. Anti-Toxoplasma drugs are highly toxic and ineffective in destroying tissue cysts, and cryptosporidiosis treatments are partially effective mostly in immunocompromised patients. Despite antimalarial drug research on the development of novel treatments, the emergence of strains resistant to first-line drugs is increasing; therefore, new alternatives are necessary [5,6]. Based on this background, a search for active molecules is needed. Drug development against these parasites has been approached from different perspectives, including in silico models, hybrid compound design, bio-guided studies in natural products, and even the use of combined therapies with known antibiotic drugs [7].
An interesting emerging category of active molecules is antimicrobial peptides (AMPs), which are attractive alternative therapeutic agents. Peptides are a diverse group of proteins of 10–100 amino acid residues. They have amphipathic structures, contain up to 50% hydrophobic residues, and possess a net positive charge of +2 to +9 [8]. AMPs are found naturally in tissues and cells from multicellular organisms and play a crucial role in the innate immune response to protect themselves since these organisms do not develop an adaptive immune system such as vertebrates. The interest in these compounds is due to their biochemical features that can interfere with ion channels and structural components of the cell membrane [9,10]. The first AMP was identified in mid-1990 from Drosophila melanogaster; at the time of this writing, at least 5000 AMPs have been reported [11,12].
The applications of AMPs are still under constant investigation, and in the last decade, their interesting antibacterial drug resistance, anticancer, anti-inflammatory, immunomodulatory, and antiparasitic activities have been reported [13,14,15]. However, the clinical application of AMPs has been limited due to the toxicity and stability of these molecules and other drawbacks, such as high production costs compared to conventional antibiotics. Although there are no commercial AMP products to date, we cannot ignore the great potential of AMPs. These molecules offer great alternatives due to their results in in vitro models [16,17].
In this review, we provide an in-depth overview of the main Apicomplexan human parasites and AMPs with antiparasitic activity, as well as their mechanisms of action.
1.1. Toxoplasmosis
This parasitic infection is caused by Toxoplasma gondii, an obligate intracellular distributed worldwide that infects a wide range of homothermic animals, including humans [18,19]. It is recognized as the main public health problem in human and veterinary medicine and is one of the five neglected parasitic infections cited by the WHO. T. gondii sexual reproduction involves species from the Felidae family, including domestic cats [20]. T. gondii affect approximately one-third of the human population, and climate change is increasing its prevalence of infection [21,22]. Epidemiological studies worldwide revealed that the prevalence in pregnant women is approximately 1.1% and could be related to cultural habits, such as eating undercooked meat (one of the main risk factors for T. gondii infection), especially of pork, lamb, or venison [23,24,25]. Humans can also be infected by eating raw shellfish (like oysters, clams, and mussels), by accidental ingestion of oocysts in contaminated soil, or by congenital transmission [25].
The toxoplasmosis incubation period is 10 to 14 days, and 90% of cases are asymptomatic. In symptomatic individuals, lymphadenitis, lymphadenopathy, fever, sore throat, headache, and myalgia have been reported [26]. The presence of hepatosplenomegaly, pulmonary or cardiac symptoms, conjunctivitis, and skin rash were recorded. Clinical manifestations are generally self-limited within 3–4 weeks. In immunocompetent individuals, neurological symptoms rarely occur; in some exceptional cases, moderate cognitive impairment has been reported [26]. In immunocompromised people with toxoplasmosis, parasites have a predilection for immune privilege sites, and extensive cell lesions are present, which can lead to encephalitis, retinochoroiditis, pericarditis, interstitial pneumonia, and Guillain-Barre syndrome. Encephalitis is an important clinical manifestation, especially in patients with AIDS, and congenital infections can lead to death [27].
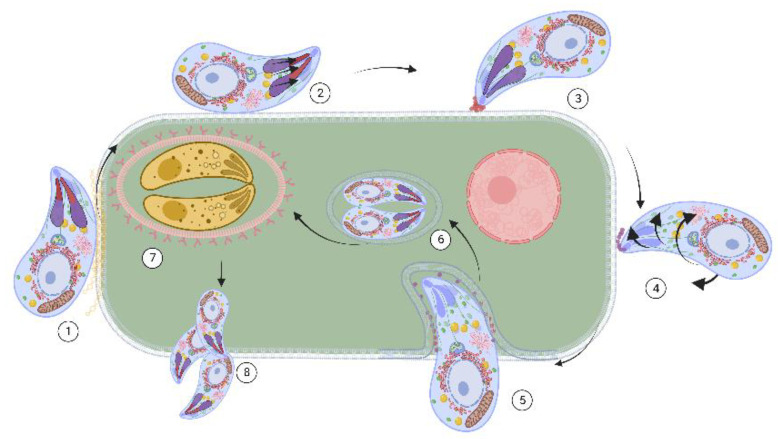
In the biological life cycle of T. gondii, four parasitic forms are involved: tachyzoites, bradyzoites, tissue cysts, and oocysts. Definitive hosts ingest prey infected with tissue cysts, mainly in the skeletal muscle or brain. Due to digestive action, the bradyzoites contained in the tissue cysts invade the enterocytes and, through schizogony replication, differentiate into macro- and microgametes. Subsequently, fertilization takes place, which gives rise to a zygote. This zygote transforms into an immature, noninfectious oocyst that is released into the environment along with the host’s feces. The noninfecting oocyst sporulates and becomes infective, and contaminates water, soil, and food in favorable environmental conditions. Intermediate hosts (i.e., warm-blooded animals, including humans) become infected through the consumption of water and food contaminated with sporulated oocysts or raw or undercooked meat with tissue cysts. Oocysts and tissue cysts release sporozoites and bradyzoites, respectively, and differentiate into tachyzoites within the intestinal epithelium. After replication, the tachyzoites exit the cell, destroying it, and the infection spreads to neighboring cells. The immune response will eliminate most parasites; those that are not removed will become bradyzoites and will form tissue cysts that can remain in the host’s organs and tissues throughout life (chronic infection). In immunocompromised individuals, bradyzoites differentiate back to tachyzoites, causing severe or fatal acute disseminated infection [18,28,29] (Figure 1).
Figure 1.
Active invasion of T. gondii. In Apicomplexan, three types of secretory organelles are observed: micronemes, rhoptries, and dense granules, carrying characteristic proteins. Attachment to host cell membrane via micronemes (MIC) proteins (1). Invasion and moving junction development by secretion of proteins from rhoptries neck (RON) and rhoptires (ROP) (2,3). Internalization via secretion of RON/AMA proteins (4). Parasitophorous vacuole development via granule dense proteins (GRA) (5). Proliferation and tachyzoite asexual replication (6). Increases immune response, interconversion to bradyzoite, and tissue cyst formation (7). Decreases immune response, interconversion to bradyzoites-tachyzoites, and dissemination of the parasite (8). Tachyzoites cause acute infection, leading to severe toxoplasmosis. While several drugs are available against tachyzoites, there is no treatment against tissue cysts, which are responsible for chronic infection. An ideal anti-Toxoplasma drug should be effective against both stages and prevent interconversion. Protein targeting secretory organelles is a matter of interest. Created with BioRender.com under license to publish by Anacleto SJ.
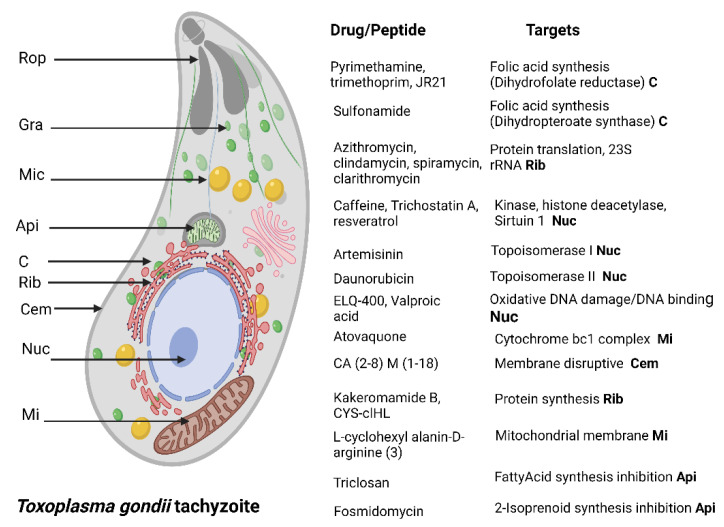
A combination of dihydrofolate reductase inhibitors such as pyrimethamine and trimethoprim, and dihydropteroate synthetase inhibitors (sulfonamides) are currently used as the first-choice treatment for toxoplasmosis; nevertheless, drug-resistant strains have been reported. It is worth mentioning that in the last decade, more than 50 resistant strains were identified and have developed resistance mainly to sulfonamides [30,31]. In addition to this, the presence of adverse effects and the fact that treatments are only effective in the acute phase of infection, turn out necessary to have new alternatives to treatment that are safe, effective, affordable, and active against the tissue cysts. For this reason, the recent emergence of AMPs offers wide potential for the discovery of new anti-Toxoplasma drugs. In Figure 2, drugs that have been tested against Toxoplasma are described.
Figure 2.
T. gondii tachyzoite drug targets. Rop, rohptry. Gra, dense granule. Mic micronemes. Api, apicoplast. C, cytoplasm. Cem, cell membrane. Rib, ribosome. Nuc, nucleus. Mi, mitochondrion. Created with BioRender.com under license to publish by Anacleto SJ.
1.2. Cryptosporidiosis
Cryptosporidium spp. is an important public health problem currently recognized as the main cause of diarrhea in humans and farm animals, causing significant morbidity and mortality worldwide, mainly in children. Approximately 40 species have been described in the Cryptosporidium genus. Two species are the most common, Cryptosporidium hominis and C. parvum, both of which can infect humans. C. parvum also infects cattle [32,33]. In low-income countries, 54% of children have had diarrhea associated with cryptosporidiosis. Children and immunocompromised patients are the most vulnerable groups to Cryptosporidium infections. It is estimated that two million children die worldwide annually, and 7 million cases are associated with morbidity in Asian and African populations [34]. In the last seventeen years, the incidence of Cryptosporidium infection in HIV-positive patients has increased up to 41.3% in Russia [35].
Cryptosporidium incubation period takes a week after the ingestion of infective oocysts. The clinical manifestations include diarrhea, fever, nausea, vomiting, abdominal pain, general malaise, and malnutrition. Chronic diarrhea in HIV patients is recognized as a classical clinical manifestation, and severe dehydration, weight loss, and malnutrition that can lead to death have been observed [36,37].
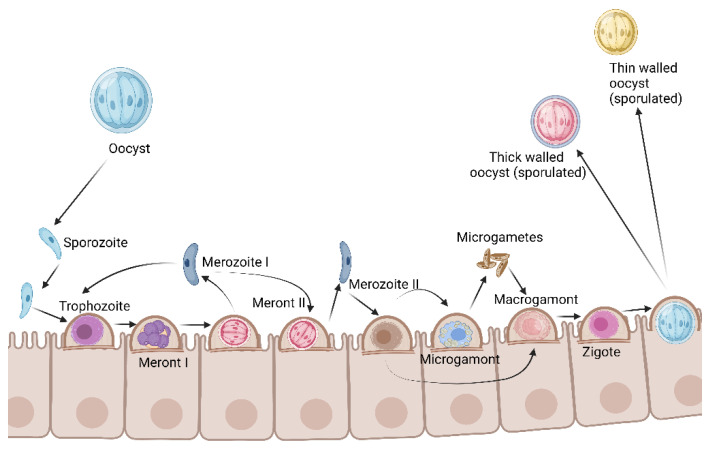
There are different parasitic stages in the life cycle of Cryptosporidium spp.: oocysts, sporozoites, trophozoites, and merozoites. The oocyst is the infective stage and can be consumed in contaminated water or food. Four sporozoites are contained inside each oocyst and are released by digestive processes in the intestinal epithelium. A schizogonic division takes place, resulting in the production of eight merozoites (type I merozoites), which reinvade new cells, and after a period of intracellular growth (type II merozoite), merozoites differentiate into micro and macrogametocytes that lead to fertilization and zygote formation. Mature zygotes develop into infective thin or thick-walled oocysts that are released from enterocytes. Infective thin-walled oocysts are broken in the intestine and lead to reinfections, while infective thick-walled oocysts are released into the environment through feces, contaminating water, soil, and food [38,39,40,41] (Figure 3).
Figure 3.
Cryptosporidium spp. development in the host cell. Anti-cryptosporidial drug development challenges a major problem: the discovery of systemic drugs that can reach epicellular parasites (preventing schizogonic reproduction); and the absorption by patients undergoing diarrhea. Created with BioRender.com under license to publish by Anacleto SJ.
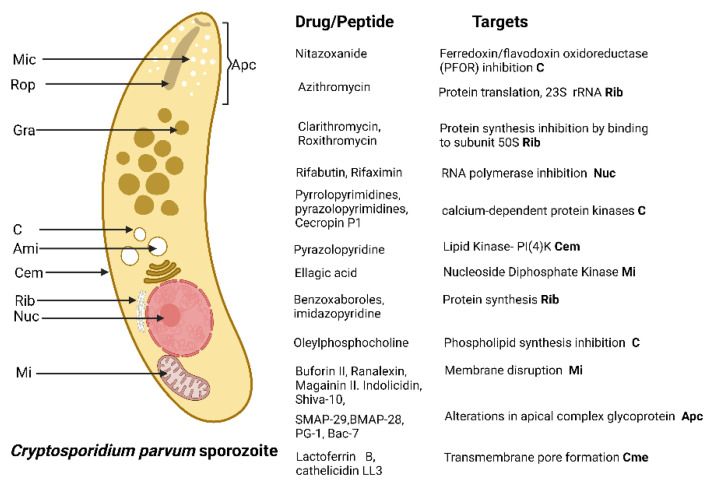
Only nitazoxanide has demonstrated efficacy in human cryptosporidiosis. A number of new targets have been identified for chemotherapy, and progress has been made in developing drugs for these targets (Figure 4).
Figure 4.
Cryptosporidium drug targets. Cryptosporodium lacks many drug targets present in other Apicomplexans because of a simplified metabolism and the absence of de novo nutrient synthetic pathways. Mic micronemes. Rop, rohptry. Gra, dense ganule. Apc, apical complex. C, cytoplasm. Ami, amylopectin granules. Cem, cell membrane. Rib, ribosome. Nuc, nucleus. Mi, mitochondrion. Created with BioRender.com under license to publish by Anacleto SJ.
1.3. Malaria
Malaria is a parasitic disease considered a major public health problem because it causes a great number of morbidity and mortality cases, mostly in tropical and subtropical zones worldwide. In 2020, 241 million malaria cases were reported, and 627,000 deaths occurred, which represented a substantial increase compared to what was reported in 2019 [42]. Malaria is caused by Plasmodium parasites, which are intracellular Parasites transmitted mainly by the bite of female mosquitoes of the genus Anopheles. There are more than 120 Plasmodium species capable of infecting mammals, birds, and reptiles; nevertheless, only five species can infect humans, P. malariae, P. falciparum, P. knowlesi, P. ovale, and P. vivax [43,44].
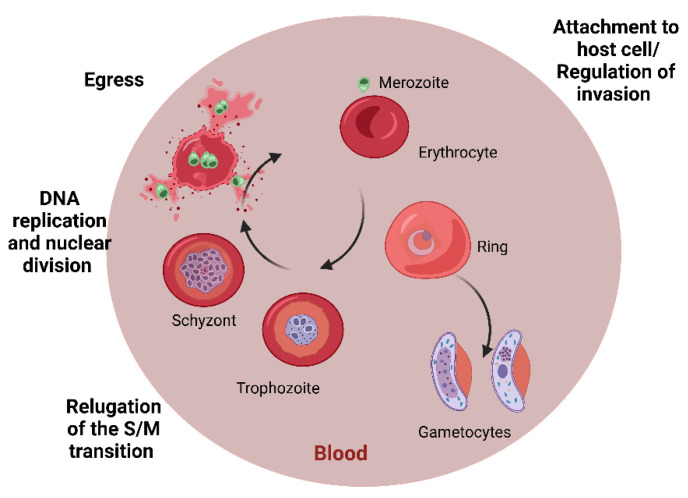
In humans, parasites replicate asexually, while sexual reproduction takes place in Anopheles mosquitoes. Sporozoites injected by the Anopheles mosquito while feeding, reach the liver through the bloodstream and invade hepatocytes forming merozoites [45]. In the liver, P. ovale and P. vivax sporozoites can convert into hypnozoites, which are dormant forms that can relapse months or years later [44]. After liver parasite replication, merozoites are released into the bloodstream, and the intraerythrocytic cycle begins, in which rings, trophozoites, schizonts, merozoites, and gametocytes are developed [43]. Gametocytes are ingested by Anopheles mosquitos, and the cycle begins again. In the midgut of the mosquito, gametocytes develop a zygote, then a mobile ookinete capable of traversing the intestinal wall and forming an oocyst that, when mature, will develop sporozoites that will be released to invade the salivary glands [46,47]. During the intraerythrocytic cycle (Figure 5), the clinical features observed include high fever, chills, headache, myalgias, arthralgias, nausea, vomiting, and diarrhea [48,49]. P. falciparum infections can cause complicated malaria as a consequence of the cytoadherence phenomenon in which infected erythrocytes adhere to the vascular endothelium of different organs, causing cerebral malaria, acute respiratory distress syndrome, acute renal failure, anemia, thrombocytopenia, and placental malaria [48]. The intensity of clinical manifestation during complicated malaria varies according to age and the intensity of transmission, and if not treated promptly, mortality is high [40].
Figure 5.
Plasmodium spp. intraerythrocytic cycle. Most antimalarial drugs target the asexual erythrocytic stages (rings, throphozoites, and schyzonts).
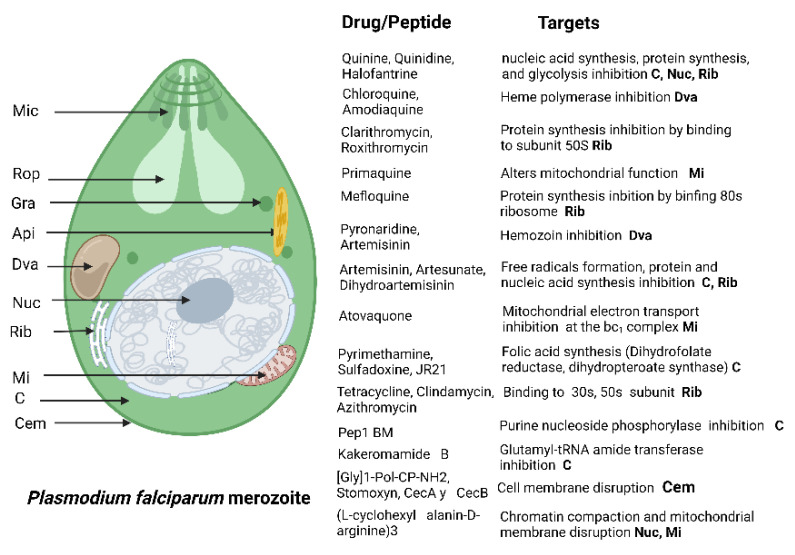
Multiple antimalarial drugs are used, including chloroquine, mefloquine, pyrimethamine, primaquine, and artemisinin derivatives [49] (Figure 6). Unfortunately, it is estimated that malaria morbidity and mortality have increased since 2020 due to the convergence of multiple factors, such as COVID-19 and Ebola outbreaks, natural disasters, and drug resistance, mainly to chloroquine and recently to artemisinin derivatives [44]. Malaria parasites have developed immune evasion strategies. Therefore, it is essential to find new alternatives for malaria control [42,44,50].
Figure 6.
Plasmodium spp. drug targets. Antimalarial drugs such as aryl amino alcohol (chloroquine, mefloquine, primaquine), antifolate compounds (pyrimethamine), and artemisinin derivatives (artesunate, artemether) target the asexual erythrocytic stages of the parasite. Mic micronemes. Rop, rohptry. Gra, dense ganule. Api, apicoplast. Dva, digestive vacuole. Nuc, nucleus. Rib, ribosome. Mi, mitochondrion. C, cytoplasm. Cem, cell membrane. Created with BioRender.com under license to publish by Anacleto SJ.
2. Antimicrobial Peptide Classification
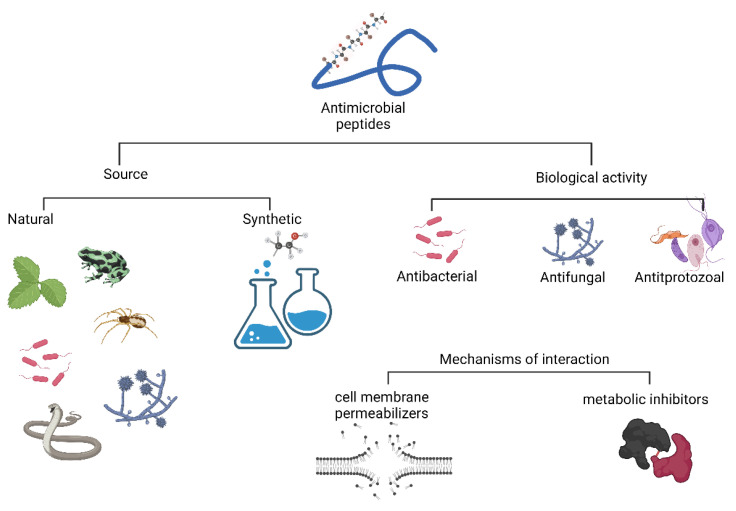
The need to categorize everything that is known has facilitated the management of information in different settings, and chemical structures also have their own classification according to the functional groups present in their chemical structures. However, peptides are made up of a series of amino acids that are present in different functional groups depending on their biological activities. According to various authors, AMPs can be categorized according to different features, such as their charges (cationic, anionic), biological activities (antibacterial, antifungal, antiprotozoal, etc.), mechanisms of action, and even the source from which they were isolated (either from natural sources or synthetically) (Figure 7). A general form of classification is based on their physicochemical characteristics, which can be divided into four main groups: (1) α-helices, (2) β-pleated sheets, (3) those with mixed structures, and (4) those with atypical conformations [51,52,53]. The α helical structure is characterized by coiling on itself through peptide bonds and creating a type of tube. This conformation, in addition to providing amphipathic characteristics, allows it to be easily inserted into the cell membrane, creating channels [54]. β-pleated sheets are structures that fold back on themselves through N-H bonds of amino acids that conform by forming hydrogen bonds with the C=O groups of the opposite amino acids. Mixed structures can be present, within the same chain of amino acids, of the two conformations, both helical and β-pleated sheets. Finally, the atypical structures present forms that do not correspond to those mentioned above [55,56,57].
Figure 7.
Antimicrobial peptides classification and interaction.
3. Mechanisms of Interaction by AMPs
Currently, research on AMPs has constantly been increasing, together with new research techniques such as bio-guided studies, in silico analysis, and synthesis, offering a broad number of peptides that have been described and evaluated in different biological models and clinical phases. To date, according to the Database of the Antimicrobial Activity and Structure of Peptides, 19,398 have been described, 82.5% of which are synthetic, and the rest have been isolated by natural sources, such as animals (75%), bacteria (12%), plants (9%) and fungi (4%) [58]. The knowledge of their mechanisms of action is continually increasing. It is noted that several peptides active against Apicomplexa parasites act directly on components of the cell membrane and extracellular components and the mechanism of surface membranes, mainly because AMPS are cationic and amphipathic molecules [59]. Most AMPs interfere with the correct functioning of the cytoplasmic membrane. With the progress in the discovery of AMPs and the elucidation of their mechanisms of action, researchers managed to understand different pathways by which they interact in both the host and host cells. Once the AMPs enter the cell, they can interact with components of the cytoplasm, altering the electrochemical balance as well as inhibiting metabolic processes essential for the survival of the parasite, altering cellular homeostasis and essential processes for cell replication [60].
AMPs’ mechanisms of action have been categorized into two main groups: those that exert a direct effect on killing cells and those that modulate the immune response. The first group is subdivided into two subgroups, those that kill directly by permeabilizing the cell membrane due to hydrophobic and electrostatic interactions of the peptides, and the second group, those peptides that kill by affecting the internal components of the cell acting as metabolic inhibitors [60,61,62,63].
4. Peptides Active against Apicomplexan Parasites
4.1. Toxoplasma gondii
Regarding AMPs that can modulate the immune response, it has been shown in vivo that HPRP-A1/A2 (amphipathic α-helical peptide) treatment induced a Th1/Tc1 response and elicited proinflammatory cytokines in mice infected with T. gondii; it is the only peptide with this type of mechanism of action in the parasite. These peptides affect the viability of tachyzoites at low concentrations; in addition, their activities against gram-negative and gram-positive bacteria and some pathogenic fungi have been reported [64]. A group of peptides that weaken the cell membrane, CA (2–8) M (1–18), lycosin-Ι, XYP1, XYP2, XYP3, longicin and longicin P4, have been tested in in vitro models against T. gondii. Lycosin-Ι was the most active, with an IC50 of 10 µM. However, other effects on the integrity of the tachyzoites were reported, such as the aggregation of the parasites induced by longicin P4, which in an in vivo model has managed to prolong the survival of mice for up to 11 days compared to the control [64,65,66,67,68,69,70].
Venoms from invertebrates such as spiders, scorpions, amphibians, and some reptiles are composed of different peptides, which in turn act mainly as modulators of ion channels and have been widely investigated in the pharmacological field for different diseases such as cancer and AIDS [71]. Some of these toxins have been evaluated against Toxoplasma [71]; however, peptides responsible for this activity have not been identified, although it is worth continuing with this research to identify the active peptides and elucidate their mechanisms of action. It should be noted that of the venoms and secretions evaluated, those obtained from the spiders Ornitoctonus huwena and Chilobrachys jingzhao were active against T. gondii tachyzoites at 3 µg/mL and increased the survival rate in vivo. There is only one study reporting peptide efficacy against T. gondii tissue cysts. The venom of the scorpion Tityus serrulatus was evaluated, and the Pep 1 peptide decreased the number of cerebral tissue cysts in infected mice, although its mechanism of action is still unknown [72,73,74].
Peptides with interesting biological activities have also been detected in marine organisms, as is the case of the conotoxin isolated from Conus californicus that affected tachyzoites in concentrations from 10 nM; of all the peptides investigated, it showed the highest activity [75].
Synthetic peptides represent an important component of known peptides to date, many of which have been identified from natural sources. Of the five synthetic peptides evaluated, Ac2-26 identified in human cells was able to reduce the parasite load from a concentration of 5 µM. (Table 1) [76].
Table 1.
AMPs with in vitro anti-Toxoplasma activity on tachyzoites.
| AMP Name | Type | Source | Evaluated Concentrations |
Cytotoxicity | Activity and Possible Mechanism of Action | IC50 |
|---|---|---|---|---|---|---|
| Frog skin secretion [71] | ND |
Phyllomedusa distincta [Amphibia] Corythomanti greening [Amphibia] |
25 µg/mL and 22 µg/mL respectively |
None in human Fibroblasts |
Inhibits invasion | ND |
| CA (2–8) M(1–18) [65] |
Cecropin/ melittin hybrid peptide |
Synthetic | 5 µM | None in human fibroblasts |
Reduces viability Membrane lytic activity |
ND |
| Ac2-26 peptide mimetic of Annexin A1 [76] | Human peptide | Synthetic | 5 μM | ND | Decreases proliferation rate |
ND |
| Lycosin-Ι [68] | Linear peptide | Lycosa singoriensis [Arachnida] | 20 µM | Cytotoxic at 34.69 µM in human fibroblasts |
Invasion and proliferation inhibition. Cell membrane alteration |
28 and 10.08 μM for intracellular and extracellular tachyzoites, respectively |
| Longicin [69] | Cationic | Haemaphysalis longicornis [Arachnida] | 50 µM | ND | Reduces proliferation. Cell membrane disruption |
ND |
| ND [72] | Venoms | Ornitoctonus huwena Chilobrachys jingzhao [Arachnida] | 12.5 µg/mL | Cytotoxic to Hella cells |
Proliferation and invasion reduction |
ND |
| XYP1 [67] | Cationic | synthesized | 2.5–40 µM | Low cytotoxicity at 20 µM in human fibroblasts |
Inhibition of viability, invasion, and proliferation. Damage to membrane associated proteins (HSP29) |
38.79 µM |
| cal14.1a [75] | Conotoxin |
Conus californicus [Gastropoda] |
10–50 µM | Not detected up to 50 µM in Hep-2 cells | Affects viability and replication by disrupting cell membrane |
ND |
| ND [73] | Venom | Hemiscorpius Lepturus [Arachnida] | 50 µg/mL | CC50 72.46 µg/mL (Vero cells) | Reduces viability and invasion. Probably damaging ion channels and enzymatic activity |
39.06 µg/mL |
| Killer peptide (KP) [77] |
Decapeptide | Synthetic | 25–200 µg/mL | Nontoxic to Vero cells. Genotoxic effects were reported |
Reduces invasion and proliferation. Maybe triggers an apoptosis like cell death |
ND |
| Longicin P4 [70] | ND | Haemaphysalis Longicornis [Arachnida] | 50 µM | Nontoxic up to 25 µM |
Reduces proliferation. Induces aggregation and affects membrane integrity |
ND |
| HPRP-A1/A2 [64] | Cationic peptide |
Synthetic | 10–40 µg/mL | Nontoxic in peritoneal macrophages. |
Reduces viability, adhesion, and invasion |
ND |
| Sub6-B, Pep1, Pep2a and Pep2b [74] | Venom fractions |
Tityus serrulatus [Arachnida] |
100 µg/mL | Nontoxic in peritoneal macrophages |
Reduces invasion and replication. Disruption of cell membrane | ND |
ND: Not Determined.
4.2. Cryptosporidum spp.
AMPs that have been active in in vivo and in vitro evaluations against specific parasitic states of Cryptosporidium spp. are summarized in Table 2. Although human cryptosporidiosis is mainly caused by two species, Cryptosporidium hominis and Cryptosporidium parvum, AMP investigations against this parasite have specifically used C. parvum in both its sporozoite and oocyst forms and through evaluations in cell cultures and in vivo. The use of the meront phase has also been reported to determine the parasite load in these investigations. Approximately 16 cationic peptides have been tested to determine their anti-Cryptosporidium activity; three of them have been evaluated in more than one trial with similar results, and even combined treatments have been carried out to improve activity, as in the case of indolicidin, ranalexin, and magainin II. However, these combinations cannot be effectively compared because the pharmacological parameters of IC50 are not reported, and even in most of these evaluations, only 1 to 3 different concentrations up to 50 mM were evaluated. Evaluating these AMPs at different concentrations to determine their IC50 values, as well as their average cytotoxicity is of great importance to continue their research. Those with the best activity were the Buforin II and Magainin II peptides, which affected approximately 99.8% of the parasites in vitro at a concentration of 10 µg/mL [78,79,80,81,82,83,84,85]. However, the coupling of the peptide octarginine and the antibiotic nitazoxanide showed excellent results, lowering the IC50 value to 2.9 nM compared to the IC50 of nitozoanide alone, which was 197 nM. Of all the peptides evaluated, this combination showed the best results [86].
In in vivo experiments, peptides, such as glucagon-like peptides, in a treatment of 50 μg/kg of weight in calves infected with C. parvum, managed to reduce the symptoms of the infection, and eliminate the release of oocysts in the feces. Other peptides that act by regulating the immune response, SA35, and SA40, were isolated from C. parvum. These peptides were tested in mice infected and immunized with 5 μg of each peptide. Evaluations of the parasite load generate specific IgA antibodies and reductions of up to 96% of all intestinal forms of the parasite (Table 2) [87,88]. To date, the efficacies of none of these peptides have been demonstrated in clinical trials. However, it should not be ignored the biological activities that they present in low concentrations, and the synergistic effects that some reported peptides exert in combination with commercial antibiotics. The search for new alternatives for the treatment of cryptosporidiosis should focus on not only AMPs but also their combination with other active molecules, with the goal of attacking the parasite by different mechanisms of action.
Table 2.
Synthetic AMPs with in vitro anti-Cryptosporidium activity.
| AMP Name | Type | Evaluated Concentrations |
Cytotoxicity | Activity and Possible Mechanisms of Action | |
|---|---|---|---|---|---|
| Buforin II [85] | α-Helical | 20 μM | None in A549 cells. |
Reduces sporozoites viability. Cell membrane Disruption |
|
| Ranalexin [84] | Cationic | 64 µg/mL | Non in A549 cells. |
Sporozoites growth suppression. Cell membrane Disruption |
|
| Ranalexin, Magainin II. Indolicidin [82] |
Cationic, helix and tridecapeptide | 50 mM | Non in A549 cells |
Sporozoites growth suppression. Cell membrane damage by synergic effect between peptide and lipophilic antibiotics |
|
| Shiva-10 [89] | Lytic peptide | 10 µM | ND | Reduces sporozoite viability. Membrane lytic effect |
|
| Cecropin P1, magainin II, ranalexin, and indolicidin [83] |
Cationic peptides | 50 μM | ND | Reduction in the proliferation of schizonts. Inhibition of Na/H and Na/Ca2 exchanges in the cell membrane |
|
| KFFKFFKFF and IKFLKFLKFL [81] | Cationic peptides | 100 µg/mL | ND | Reduction in the viability of sporozoites. Cell membrane disruption |
|
| SMAP-29, BMAP-28, PG-1, Bac-7 [80] | Helical peptides | 100 μg/mL | ND | Strong cytotoxic effect on sporozoites. Alterations in the glycoprotein of the apical complex |
|
| Indolicidin, Magainin II, Ranalexin [79] |
Cathionic peptides | 50 μM | ND | Reduction in merozoites proliferation | |
| Octaarginine-6-FAM-Nitazoanide combination [86] |
Cathionic peptides | 197 nM | No cytotoxic effects in human ileocecal adenocarcinoma cells | Reduction in trophozoites and meronts replication |
|
| Lactoferrin B, cathelicidin LL3, indolicidin, βdefens1in, ß defensin 2. [90] |
Cathionic peptides | 10 µg/mL | Low cytotoxic effect in human colorectal adenocarcinoma cells |
Inhibition of sporozoites attachment and invasion. Transmembrane pore formation |
|
| Buforin II, Magainin II, Lasalocid. [78] |
Cathionic peptides | 10 µg/mL | ND | Reduction in oocysts infectivity. Membrane disruption |
|
ND: Not determined. IC50 values were not established.
4.3. Peptides Active against Plasmodium spp.
Peptides against Plasmodium have multiple mechanisms of action that cause decreases in parasitemia (Table 3), and one of the predominant mechanisms is the interaction of peptides with enzymes causing their inhibition and, consequently damage to the metabolic pathways in which they participate. For example, for the maintenance and processing of genetic material, peptides can inhibit the enzyme purine nucleoside phosphorylase of P. falciparum, and the enzyme dihydrofolate reductase-thymidylate synthase, resulting in the death of the parasite [91,92]. Another example of enzyme inhibition occurs during the erythrocyte cycle, during the digestion of hemoglobin in the digestive vacuole for protein biosynthesis and heme crystallization, a process that is catalyzed by enzymes such as falcispainins that, if their function is inhibited, the parasite cannot obtain the amino acids necessary for protein synthesis and therefore would die; this strategy is used by certain peptides, such as CYS-IHL and CYS-cIHL, that are capable of inhibiting these enzymes [93,94].
Table 3.
AMPs with in vitro anti-Malarial activity.
| AMP Name | Type | Source | Evaluated Concentration |
Cytotoxicity | Activity and Possible Mechanisms of Action |
IC50 |
|---|---|---|---|---|---|---|
| Pep1 BM [91] | ND | Synthetic | 20 µL | ND | Inhibition of purine nucleoside phosphorylase in P. falciparum rings | 16.14 μg/mL |
| JR21 [92] | ND | Synthetic | 10 µM | ND | Dihydrofolate reductase- thymidylate synthase inhibition in P. falciparum rings |
3.87 µM |
| CYS-IHL [94] | Linear | Synthetic | 69.91 µM | Noncytotoxic in human liver carcinoma cell. | Hemoglobinase activity inhibition in late P. falciparum Trophozoites |
27.55 µM |
| Kakeromamide B [95] |
Cyclic |
Moorea producens [Cyanobacteria] |
11 µM | Noncytotoxic in HEK293T and HepG2 cells | Reduction in proliferation of P. falciparum sexual blood-stages and P. berghei liver-stage. High affinity to actin, sortilin and subunit A of glutamyl-tRNA amide transferase |
8.9 µM |
| [Gly]1-Pol-CP-NH2 [96] | ND | Synthetic derived from Pol-CP-NH2 |
6.25 µM | Cytotoxic in human mammary adenocarcinoma, Hep G2, SHSY-5Y, and SK-mel-147 |
Cell membrane disruption in P. falciparum sporozoites | ND |
| Crotamine [97,98] | Cationic |
Crotalusdurissusterrificus [Lepidosauria] |
20 µM | No hemolytic activity in human erythrocytes |
Peptide–membrane interactions and H+ homeostasis disruption in P. falciparum asexual blood stages | 1.87 µM |
| (L-cyclohexyl alanin-D- arginine) 3 [99] |
ND | Synthetic | 59.16 ng/mL | No cytotoxic effects in human erythrocytes and leukocytes |
Chromatin compaction and mitochondrial membrane disruption in P. falciparum asexual blood stages |
8.94 ng/mL |
| rR8-JR21 [92] | ND | Synthetic | 13.22 | ND | Dihydrofolate reductase-thymidylate synthase inhibition in P. falciparum ring stages | 1.53 µM |
| LZ1 [100] | Linear peptide | Synthetic derived fromcathelicidin-BF |
25 µM and 4 mg/kg | ND | Blockade of ATP production by selective inhibition of pyruvate kinase activity in P. falciparum blood stages. | 3.045 µM |
| Mtk-1 y Mtk-2 [101] | Rich in proline | Drosophila melanogaster [Insecta] | 50 µM | Hemolytic activity in pig and mouse (CD1) erythrocytes |
Cell membrane disruption in P. falciparum asexual blood stages | ND |
| Stomoxyn [101] | ND |
Lucilia sericata [Insecta] |
50 µM | Hemolytic activity in highest concentrations in pig and mouse (CD1) erythrocytes |
Cell membrane disruption in P. falciparum asexual blood stages | ND |
| CecA y CecB [101] | Linear cations |
Galleria mellonella [Insecta] |
50 µM | Hemolytic activity in highest concentrations in pig and mouse (CD1) erythrocytes |
Cell membrane disruption in P. falciparum asexual blood stages. | ND |
| [Arg]3-VmCT1-NH2, [Arg]7-VmCT1-NH2 [102] | Synthetic | 5 µM/L | Lower Cytotoxic effects in MCF-7 human breast epithelial cells, CC50 20 and 18 µM/L | Cell membrane disruption in P. gallinaceum sporozoites | 0.57, 0.51 µM/L | |
| VmCT1-NH2 [102] |
Vaejovis mexicanus [Arachnida] |
5 µM/L | CC50 8.3 µM/L in MCF-7 human breast epithelial cells | Cell membrane disruption in P. gallinaceum sporozoites | 0.49 µM/L |
ND: not determined.
Peptide–membrane interactions and H+ homeostasis disruption in P. falciparum asexual blood stages
Other targets of peptides are proteins and membranes, which, if damaged, can modify the morphology of the parasite; however, not all peptides have parasiticidal effect, and some only stop the development of Plasmodium spp., which is reflected in the slowed kinetics of the life cycle [92,94,95,96,97,98].
In addition to reducing parasitemia, some peptides are capable of modifying the immune response in the host by reducing the overproduction of proinflammatory cytokines and, as a consequence, modulating damage to organs that are severely affected, such as the liver [99].
Nevertheless, more information is needed to elucidate the mechanisms of action of antimicrobial peptides against Plasmodium spp.
5. Concluding Remarks and Future Research Directions
Antimicrobial peptides have been described in many species, including fungi, plants, insects, and humans (allowing access to an endless number of possible peptides with diverse biological activities), and are currently presented as a therapeutic solution to control different pathogenic microorganisms. Microorganisms that cause diseases in humans are constantly evolving, which represents a challenge in the pursuit of effective treatments against these pathogens. Some characteristics that make peptides attractive as potential drugs are that they have been evolving for almost the same amount of time as the species that produce them, and their effects on the control of microorganisms are very remarkable. Some peptides are being used in experimental phases, and others are already marketed, e.g., peptides against fungal agents such as Candida albicans, Cryptococcus neoformans, and Fusarium oxysporum. Some peptides have been developed for topical application against human papilloma virus, and others have been developed against protozoa and nematodes, gram-negative bacteria, tumors, and as neuroprotectors.
Endogenous bioactive peptides can be produced in different cell types, such as neural cells, immune cells, or glands, while exogenous peptides can be obtained from nutrients, insects, nematodes, or marine organisms. Cecropin is one of the most explored insect peptides that can destroy cell membranes and inhibit proline uptake.
Unlike other parasites, Apicomplexans have complex life cycles comprised of different stages characterized by rapid replication, which enables adaptation to drug treatment. The Apicomplexa invasion process involves secretory organelles housing proteins that allow host-cell entrance and the development of an intracellular compartment in which the parasites reproduce asexually. As intracellular organisms, their nutritional needs rely on biosynthetic pathways or salvaging metabolites from their host [103]. Apicomplexa drug targets include calcium-dependent protein kinases, mitochondrial electron transport chain, proteins secretion pathways, type II fatty acid synthesis, DNA synthesis and replication, and, DNA expression, among others [104]. Most of the peptides reviewed in this text produce the disruption of parasite cell membranes, in contrast to conventional chemotherapeutic drugs, which act on precise targets such as DNA or specific enzymes. Nonetheless, plasma membrane disruption, produces fast depolarization triggering protein and DNA/RNA inhibition synthesis, which can lead to parasite death. Some peptides are rich in amino acids, such as tryptophan and lysine, that might have an effect on anionic biological membranes, producing pores, which allow peptides to distribute into internal membranes and organelles [64].
Unlike Apicomplexan, hemoflagellate protozoa, such as Trypanosoma and Leishmania, have less complex life cycles. Various research groups have been dedicated to the discovery and structural elucidation of novel peptides against these parasites since the early 90s. Extracellular forms (promastigote and trypomastigote) are the most common stages used for the screening of peptides’ activity [105]. The antiprotozoal activity is supposed to occur by membrane disruption, apoptosis, or by immunomodulatory responses. In vivo assessments are considerably underexplored, due to their rapid degradation by endogenous proteases [105]. It seems that peptide-based antiprotozoal drug development, presents several challenges related to the complex life cycles. Therefore, computational models and tools for the prediction of peptide activity are urgently needed. However, peptides have some advantages over traditional drugs, such as slower emergence of resistance [106].
There are some issues to consider while scaling up peptide design. Peptides have various limitations that could hinder their anti-Apicomplexa therapeutic use. They have unfavorable plasma stability, are unable to cross the cell membrane to target intracellular targets, degrade easily, and have poor penetration of the intestinal mucosa; thus, it can be assumed that they are not good candidates to treat intracellular parasites. [107]. Nonetheless, the results obtained so far show that they can be a good alternative to control these parasites. It must be taken into consideration, that novel peptides must easily reach intracellular targets with little or no toxicity to mammalian cells. To improve these disadvantages, encapsulation into a micro- or nanoparticle, can be achieved, as well as in silico sequence-based prediction of cell-penetrating and toxicity. Penetratin-like peptides bind to glycosaminoglycans at the cell surface. Natural DNA-binding peptides can be the source for designing cell-penetrating peptides, such as those rich in lysine, or arginine [107].
Although there are currently some pharmacological alternatives for the control of Apicomplexan parasites, these are sometimes inefficient, especially due to resistance mechanisms and severe side effects, and they do not act against all parasite stages and sometimes restrict access to some intracellular locations. Based upon the abovementioned results, it seems that synthetic peptides, as well as those derived from natural sources, could be promising alternatives for the treatment of infectious diseases. It is necessary to develop new anti-Apicomplexan compounds combining drug research pathways, such as in silico rational drug design and bio-guided natural substance studies, to identify new molecules that might be able to act directly in the parasites or indirectly by activating the host immune system.
As reported in the literature, peptides show a broad antimicrobial spectrum; therefore, it would be recommended to explore their synergistic ability in combination with those drugs in which resistance is reported, their capacity to decrease or increase the adverse effects of currently used drugs, and their distribution in the parasite and in the host cell. Genetic engineering or chemical modification of these peptides to improve their functional properties would also be recommended. There is a high potential for the use of antimicrobial peptides, and more research in this field can lead to promising results that can have considerable effects on the control of human Apicomplexan parasites.
Author Contributions
Conceptualization: N.R.-F.; investigation: J.A.-S. and B.C.-T.; resources: T.d.J.L.-P. and M.R.-L.; writing—original draft preparation: all authors; writing—review and editing: all authors; visualization: all authors; supervision: all authors; project administration: N.R.-F.; funding acquisition: N.R.-F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Dirección General de Asuntos del Personal Académico (DGAPA) Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAIIT)-Universidad Nacional Autónoma de México (UNAM) proyect IN200721.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cummings R.D., Hokke C.H., Haslam S.M. Essentials of Glycobiology. 4th ed. Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2022. Parasitic infections. [Google Scholar]
- 2.Memariani H., Memariani M. Melittin as a promising anti-protozoan peptide: Current knowledge and future prospects. AMB Express. 2021;11:69. doi: 10.1186/s13568-021-01229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding C.R., Frischknecht F. The Riveting Cellular Structures of Apicomplexan Parasites. Trends Parasitol. 2020;36:979–991. doi: 10.1016/j.pt.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Chan K., Tusting L.S., Bottomley C., Saito K., Djouaka R., Lines J. Malaria transmission and prevalence in rice-growing versus non-rice-growing villages in Africa: A systematic review and meta-analysis. Lancet Planet. Health. 2022;6:e257–e269. doi: 10.1016/S2542-5196(21)00349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shammaa A.M., Powell T.G., Benmerzouga I. Adverse outcomes associated with the treatment of Toxoplasma infections. Sci. Rep. 2021;11:1035. doi: 10.1038/s41598-020-80569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargala G. Drug treatment and novel drug target against Cryptosporidium. Parasite. 2008;15:275–281. doi: 10.1051/parasite/2008153275. [DOI] [PubMed] [Google Scholar]
- 7.Alven S., Aderibigbe B. Combination Therapy Strategies for the Treatment of Malaria. Molecules. 2019;24:3601. doi: 10.3390/molecules24193601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y., Hao W., Wang X., Ouyang J., Deng X., Yu H., Wang Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022;42:1377–1422. doi: 10.1002/med.21879. [DOI] [PubMed] [Google Scholar]
- 9.Erdem Büyükkiraz M., Kesmen Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2022;132:1573–1596. doi: 10.1111/jam.15314. [DOI] [PubMed] [Google Scholar]
- 10.Mahlapuu M., Håkansson J., Ringstad L., Björn C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso P., Glossop H., Meikle T.G., Aburto-Medina A., Conn C.E., Sarojini V., Valery C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021;13:35–69. doi: 10.1007/s12551-021-00784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaitre B., Nicolas E., Michaut L., Reichhart J.-M., Hoffmann J.A. The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 13.Kardani K., Bolhassani A. Antimicrobial/anticancer peptides: Bioactive molecules and therapeutic agents. Immunotherapy. 2021;13:669–684. doi: 10.2217/imt-2020-0312. [DOI] [PubMed] [Google Scholar]
- 14.Guryanova S.V., Ovchinnikova T.V. Immunomodulatory and allergenic properties of antimicrobial peptides. Int. J. Mol. Sci. 2022;23:2499. doi: 10.3390/ijms23052499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogrado K., Adisakwattana P., Reamtong O. Antimicrobial peptides: On future antiprotozoal and anthelminthic applications. Acta. Trop. 2022;235:106665. doi: 10.1016/j.actatropica.2022.106665. [DOI] [PubMed] [Google Scholar]
- 16.Fry D.E. Antimicrobial peptides. Surg. Infect. 2018;19:804–811. doi: 10.1089/sur.2018.194. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C., Yang M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics. 2022;11:349. doi: 10.3390/antibiotics11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguirre A.A., Longcore T., Barbieri M., Dabritz H., Hill D., Klein P.N., Lepczyk C., Lilly E.L., McLeod R., Milcarsky J., et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth. 2019;16:378–390. doi: 10.1007/s10393-019-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cossu G., Preti A., Gyppaz D., Gureje O., Carta M.G. Association between toxoplasmosis and bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2022;153:284–291. doi: 10.1016/j.jpsychires.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Hajimohammadi B., Ahmadian S., Firoozi Z., Askari M., Mohammadi M., Eslami G., Askari V., Loni E., Barzegar-Bafrouei R., Boozhmehrani M.J. A Meta-Analysis of the Prevalence of Toxoplasmosis in Livestock and Poultry Worldwide. EcoHealth. 2022;19:55–74. doi: 10.1007/s10393-022-01575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Barros R.A.M., Torrecilhas A.C., Marciano M.A.M., Mazuz M.L., Pereira-Chioccola V.L., Fux B. Toxoplasmosis in Human and Animals Around the World. Diagnosis and Perspectives in the One Health Approach. Acta Trop. 2022;231:106432. doi: 10.1016/j.actatropica.2022.106432. [DOI] [PubMed] [Google Scholar]
- 22.Molan A., Nosaka K., Hunter M., Wang W. Global status of Toxoplasma gondii infection: Systematic review and prevalence snapshots. Trop. Biomed. 2019;36:898–925. [PubMed] [Google Scholar]
- 23.Robinson E., de Valk H., Villena I., Le Strat Y., Tourdjman M. National perinatal survey demonstrates a decreasing seroprevalence of Toxoplasma gondii infection among pregnant women in France, 1995 to 2016: Impact for screening policy. Eurosurveillance. 2021;26:1900710. doi: 10.2807/1560-7917.ES.2021.26.5.1900710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostami A., Riahi S.M., Contopoulos-Ioannidis D.G., Gamble H.R., Fakhri Y., Shiadeh M.N., Foroutan M., Behniafar H., Taghipour A., Maldonado Y.A., et al. Acute Toxoplasma infection in pregnant women worldwide: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2019;13:e0007807. doi: 10.1371/journal.pntd.0007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.López-Fabal F., Gómez-Garcés J.L. Marcadores serológicos de gestantes españolas e inmigrantes en un área del sur de Madrid durante el periodo 2007–2010. Rev. Esp. Quimioter. 2013;26:108–111. [PubMed] [Google Scholar]
- 26.Dubey J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors. 2021;14:263. doi: 10.1186/s13071-021-04769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLeod R., Cohen W., Dovgin S., Finkelstein L., Boyer K.M. Toxoplasma Gondii. Elsevier; Amsterdam, The Netherlands: 2020. Human toxoplasma infection; pp. 117–227. [Google Scholar]
- 28.Attias M., Teixeira D.E., Benchimol M., Vommaro R.C., Crepaldi P.H., De Souza W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors. 2020;13:588. doi: 10.1186/s13071-020-04445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubey J.P. Toxoplasma Gondii. Elsevier; Amsterdam, The Netherlands: 2020. The history and life cycle of Toxoplasma gondii; pp. 1–19. [Google Scholar]
- 30.Montazeri M., Mehrzadi S., Sharif M., Sarvi S., Tanzifi A., Aghayan S.A., Daryani A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018;9:2587. doi: 10.3389/fmicb.2018.02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunay Ildiko R., Gajurel K., Dhakal R., Liesenfeld O., Montoya Jose G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018;31:e00057-17. doi: 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adkins P.R.F. Cryptosporidiosis. Vet. Clin. N. Am. Food Anim. 2022;38:121–131. doi: 10.1016/j.cvfa.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y., Ryan U.M., Xiao L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Korpe P.S., Valencia C., Haque R., Mahfuz M., McGrath M., Houpt E., Kosek M., McCormick B.J.J., Penataro Yori P., Babji S., et al. Epidemiology and Risk Factors for Cryptosporidiosis in Children From 8 Low-income Sites: Results From the MAL-ED Study. Clin. Infect. Dis. 2018;67:1660–1669. doi: 10.1093/cid/ciy355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadpour E., Safarpour H., Xiao L., Zarean M., Hatam-Nahavandi K., Barac A., Picot S., Rahimi M.T., Rubino S., Mahami-Oskouei M., et al. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite. 2020;27:27. doi: 10.1051/parasite/2020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan U., Hill K., Deere D. Review of generic screening level assumptions for quantitative microbial risk assessment (QMRA) for estimating public health risks from Australian drinking water sources contaminated with Cryptosporidium by recreational activities. Water. Res. 2022;220:118659. doi: 10.1016/j.watres.2022.118659. [DOI] [PubMed] [Google Scholar]
- 37.Urrea-Quezada A., González-Díaz M., Villegas-Gómez I., Durazo M., Hernández J., Xiao L., Valenzuela O. Clinical manifestations of cryptosporidiosis and identification of a new Cryptosporidium subtype in patients from Sonora, Mexico. J. Pediatr. Infect. Dis. 2018;37:e136–e138. doi: 10.1097/INF.0000000000001762. [DOI] [PubMed] [Google Scholar]
- 38.Guérin A., Striepen B. The Biology of the Intestinal Intracellular Parasite Cryptosporidium. Cell Host Microbe. 2020;28:509–515. doi: 10.1016/j.chom.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 39.English E.D., Guérin A., Tandel J., Striepen B. Live imaging of the Cryptosporidium parvum life cycle reveals direct development of male and female gametes from type I meronts. PLoS Biol. 2022;20:e3001604. doi: 10.1371/journal.pbio.3001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tandel J., English E.D., Sateriale A., Gullicksrud J.A., Beiting D.P., Sullivan M.C., Pinkston B., Striepen B. Life cycle progression and sexual development of the Apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 2019;4:2226–2236. doi: 10.1038/s41564-019-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borowski H., Thompson R.C.A., Armstrong T., Clode P.L. Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system. Parasitology. 2010;137:13–26. doi: 10.1017/S0031182009990837. [DOI] [PubMed] [Google Scholar]
- 42.WHO World Malaria Report 2021. [(accessed on 26 September 2022)]; Available online: https://www.who.int/publications/i/item/9789240040496.
- 43.Su X.-z., Lane K.D., Xia L., Sá J.M., Wellems T.E. Plasmodium Genomics and Genetics: New Insights into Malaria Pathogenesis, Drug Resistance, Epidemiology, and Evolution. Clin. Microbiol. Rev. 2019;32:e00019-19. doi: 10.1128/CMR.00019-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashley E.A., Pyae Phyo A., Woodrow C.J. Malaria. Lancet. 2018;391:1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 45.Sinnis P., Zavala F. The skin: Where malaria infection and the host immune response begin. Semin. Immunopathol. 2012;34:787–792. doi: 10.1007/s00281-012-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drahansky M. Liveness Detection in Biometrics. [(accessed on 26 September 2022)]. Available online: https://www.intechopen.com/chapters/17746.
- 47.Abugri J., Ayariga J., Sunwiale S.S., Wezena C.A., Gyamfi J.A., Adu-Frimpong M., Agongo G., Dongdem J.T., Abugri D., Dinko B. Targeting the Plasmodium falciparum proteome and organelles for potential antimalarial drug candidates. Heliyon. 2022;8:e10390. doi: 10.1016/j.heliyon.2022.e10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trampuz A., Jereb M., Muzlovic I., Prabhu R.M. Clinical review: Severe malaria. Crit. Care Med. 2003;7:315. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schofield L., Grau G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 50.Siddiqui F.A., Liang X., Cui L. Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook. Int. J. Parasitol. Drugs Drug Resist. 2021;16:102–118. doi: 10.1016/j.ijpddr.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernández-Aristizábal, Antimicrobial Peptides with Antibacterial Activity against Vancomycin-Resistant Staphylococcus aureus Strains: Classification, Structures, and Mechanisms of Action. Int. J. Mol. Sci. 2021;22:7927. doi: 10.3390/ijms22157927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luong H.X., Thanh T.T., Tran T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020;260:118407. doi: 10.1016/j.lfs.2020.118407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lima A.M., Azevedo M.I.G., Sousa L.M., Oliveira N.S., Andrade C.R., Freitas C.D.T., Souza P.F.N. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022;214:10–21. doi: 10.1016/j.ijbiomac.2022.06.043. [DOI] [PubMed] [Google Scholar]
- 54.Böhmová E., Machová D., Pechar M., Pola R., Venclíková K., Janoušková O., Etrych T. Cell-Penetrating peptides: A useful tool for the delivery of various cargoes into cells. Physiol. Res. 2018;67:S267–S279. doi: 10.33549/physiolres.933975. [DOI] [PubMed] [Google Scholar]
- 55.Huan Y., Kong Q., Mou H., Yi H. Antimicrobial peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020;11:2559. doi: 10.3389/fmicb.2020.582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H.-T., Lee C.-C., Yang J.-R., Lai J.Z.C., Chang K.Y. A large-scale structural classification of antimicrobial peptides. Biomed. Res. Int. 2015;2015:475062. doi: 10.1155/2015/475062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hafeez A., Jiant X., Bergen P., Zhu Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021;22:11691. doi: 10.3390/ijms222111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirtskhalava M., Amstrong A.A., Grigolava M., Chubinidze M., Alimbarashvili E., Vishnepolsky B., Gabrielian A., Rosenthal A., Hurt D.E., Tartakovsky M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021;49:D288–D297. doi: 10.1093/nar/gkaa991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straub K.W., Cheng S.J., Sohn C.S., Bradley P.J. Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell. Microbiol. 2009;11:590–603. doi: 10.1111/j.1462-5822.2008.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabiá Júnior E.F., Menezes L.F.S., de Araújo I.F.S., Schwartz E.F. Natural occurrence in venomous arthropods of antimicrobial peptides active against protozoan parasites. Toxins. 2019;11:563. doi: 10.3390/toxins11100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 62.Kumar P., Kizhakkedathu J., Straus S. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules. 2018;8:4. doi: 10.3390/biom8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raheem N., Straus S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol. 2019;10:2866. doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu R., Ni Y., Song J., Xu Z., Qiu J., Wang L., Zhu Y., Huang Y., Ji M., Chen Y. Research on the effect and mechanism of antimicrobial peptides HPRP-A1/A2 work against Toxoplasma gondii infection. Parasite Immunol. 2019;41:e12619. doi: 10.1111/pim.12619. [DOI] [PubMed] [Google Scholar]
- 65.Seeber F. An enzyme-release assay for the assessment of the lytic activities of complement or antimicrobial peptides on extracellular Toxoplasma gondii. J. Microbiol. Methods. 2000;39:189–196. doi: 10.1016/S0167-7012(99)00117-7. [DOI] [PubMed] [Google Scholar]
- 66.Shin I.S., Seo C.S., Lee M.Y., Ha H.K., Huh J.I., Shin H.K. In vitro and in vivo evaluation of the genotoxicity of Gumiganghwal-tang, a traditional herbal prescription. J. Ethnopharmacol. 2012;141:350–356. doi: 10.1016/j.jep.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Tang Y., Tang X., Wu M., Hou S., Liu X., Li J., Deng M., Huang S., Jiang L. Anti-Toxoplasma gondii Effects of a Novel Spider Peptide XYP1 In Vitro and In Vivo. Biomedicines. 2021;9:934. doi: 10.3390/biomedicines9080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang Y., Hou S., Li X., Wu M., Ma B., Wang Z., Jiang J., Deng M., Duan Z., Tang X., et al. Anti-Parasitic effect on Toxoplasma gondii induced by a spider peptide lycosin-I. Exp. Parasitol. 2019;198:17–25. doi: 10.1016/j.exppara.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka T., Maeda H., Galay R.L., Boldbattar D., Umemiya-Shirafuji R., Suzuki H., Xuan X., Tsuji N., Fujisaki K. Tick longicin implicated in the arthropod transmission of Toxoplasma gondii. J. Vet. Sci. Technol. 2012;3:3633–3640. doi: 10.4172/2157-7579.1000112. [DOI] [Google Scholar]
- 70.Tanaka T., Maeda H., Matsuo T., Boldbattar D., Umemiya-Shirafuji R., Kume A., Suzuki H., Xuan X., Tsuji N., Fujisaki K. Parasiticidal activity of Haemaphysalis longicornis longicin P4 peptide against Toxoplasma gondii. Peptides. 2012;34:242–250. doi: 10.1016/j.peptides.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 71.Gustavo Tempone A., de Souza Carvalho Melhem M., Oliveira Prado F., Motoie G., Mitsuyoshi Hiramoto R., Maria Antoniazzi M., Fernando Baptista Haddad C., Jared C. Amphibian secretions for drug discovery studies: A search for new antiparasitic and antifungal compounds. Lett. Drug Des. Discov. 2007;4:67–73. doi: 10.2174/157018007778992856. [DOI] [Google Scholar]
- 72.Hou S., Liu Y., Tang Y., Wu M., Guan J., Li X., Wang Z., Jiang J., Deng M., Duan Z. Anti-Toxoplasma gondii effect of two spider venoms in vitro and in vivo. Toxicon. 2019;166:9–14. doi: 10.1016/j.toxicon.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Khaleghi Rostamkolaie L., Hamidinejat H., Razi Jalali M.H., Jafari H., Najafzadeh Varzi H., Seifi Abadshapouri M.R. In vitro therapeutic effect of Hemiscorpius lepturus venom on tachyzoites of Toxoplasma gondii. J. Parasit. Dis. 2019;43:472–478. doi: 10.1007/s12639-019-01113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Assis D.R.R., Pimentel P.M.d.O., Dos Reis P.V.M., Rabelo R.A.N., Vitor R.W.A., Cordeiro M.d.N., Felicori L.F., Olórtegui C.D.C., Resende J.M., Teixeira M.M. Tityus Serrulatus (Scorpion): From the Crude Venom to the Construction of Synthetic Peptides and Their Possible Therapeutic Application Against Toxoplasma gondii Infection. Front. Cell. Infect. Microbiol. 2021;11:706618. doi: 10.3389/fcimb.2021.706618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De León-Nava M.A., Romero-Núñez E., Luna-Nophal A., Bernáldez-Sarabia J., Sánchez-Campos L.N., Licea-Navarro A.F., Morales-Montor J., Muñiz-Hernández S. In vitro effect of the synthetic cal14.1a conotoxin, derived from Conus californicus, on the human parasite Toxoplasma gondii. Mar. Drugs. 2016;14:66. doi: 10.3390/md14040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Oliveira Cardoso M.F., Moreli J.B., Gomes A.O., de Freitas Zanon C., Silva A.E., Paulesu L.R., Ietta F., Mineo J.R., Ferro E.A., Oliani S.M. Annexin A1 peptide is able to induce an anti-parasitic effect in human placental explants infected by Toxoplasma gondii. Microb. Pathog. 2018;123:153–161. doi: 10.1016/j.micpath.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Giovati L., Santinoli C., Mangia C., Vismarra A., Belletti S., ’Adda T., Fumarola C., Ciociola T., Bacci C., Magliani W. Novel activity of a synthetic decapeptide against Toxoplasma gondii tachyzoites. Front. Microbiol. 2018;9:753. doi: 10.3389/fmicb.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giacometti A., Cirioni O., Del Prete M.S., Barchiesi F., Scalise G. Short-term exposure to membrane-active antibiotics inhibits Cryptosporidium parvum infection in cell culture. Antimicrob. Agents Chemother. 2000;44:3473–3475. doi: 10.1128/AAC.44.12.3473-3475.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giacometti A., Cirioni O., Barchiesi F., Caselli F., Scalise G. In vitro activity of polycationic peptides against Cryptosporidium parvum, Pneumocystis carinii and yeast clinical isolates. J. Antimicrob. Chemother. 1999;44:403–406. doi: 10.1093/jac/44.3.403. [DOI] [PubMed] [Google Scholar]
- 80.Giacometti A., Cirioni O., Del Prete M.S., Skerlavaj B., Circo R., Zanetti M., Scalise G. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J. Antimicrob. Chemother. 2003;51:843–847. doi: 10.1093/jac/dkg149. [DOI] [PubMed] [Google Scholar]
- 81.Giacometti A., Cirioni O., Kamysz W., Kasprzykowski F., Barchiesi F., Del Prete M.S., Maćkiewicz Z., Scalise G. In vitro effect of short-term exposure to two synthetic peptides, alone or in combination with clarithromycin or rifabutin, on Cryptosporidium parvum infectivity. Peptides. 2002;23:1015–1018. doi: 10.1016/S0196-9781(02)00026-8. [DOI] [PubMed] [Google Scholar]
- 82.Giacometti A., Cirioni O., Barchiesi F., Fortuna M., Scalise G. In vitro anticryptosporidial activity of ranalexin alone and in combination with other peptides and with hydrophobic antibiotics. Eur. J. Clin. Microbiol. 1999;18:827–829. doi: 10.1007/s100960050410. [DOI] [PubMed] [Google Scholar]
- 83.Giacometti A., Cirioni O., Barchiesi F., Ancarani F., Scalise G. In vitro anti-cryptosporidial activity of cationic peptides alone and in combination with inhibitors of ion transport systems. J. Antimicrob. Chemother. 2000;45:651–654. doi: 10.1093/jac/45.5.651. [DOI] [PubMed] [Google Scholar]
- 84.Giacometti A., Cirioni O., Barchiesi F., Scalise G. Anticryptosporidial activity of ranalexin, lasalocid and azithromycin alone and in combination in cell lines. J. Antimicrob. Chemother. 2000;45:375–377. doi: 10.1093/jac/45.3.375. [DOI] [PubMed] [Google Scholar]
- 85.Giacometti A., Cirioni O., Del Prete M.S., Barchiesi F., Fineo A., Scalise G. Activity of buforin II alone and in combination with azithromycin and minocycline against Cryptosporidium parvum in cell culture. J. Antimicrob. Chemother. 2001;47:97–99. doi: 10.1093/jac/47.1.97. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen-Ho-Bao T., Ambe L.A., Berberich M., Hermosilla C., Taubert A., Daugschies A., Kamena F. Octaarginine Improves the Efficacy of Nitazoxanide against Cryptosporidium parvum. Pathogens. 2022;11:653. doi: 10.3390/pathogens11060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tosini F., Ludovisi A., Tonanzi D., Amati M., Cherchi S., Pozio E., Gómez-Morales M.A. Delivery of SA35 and SA40 peptides in mice enhances humoral and cellular immune responses and confers protection against Cryptosporidium parvum infection. Parasites Vectors. 2019;12:233. doi: 10.1186/s13071-019-3486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kessler M., Connor E., Lehnert M. Volatile organic compounds in the strongly fragrant fern genus Melpomene (Polypodiaceae) Plant. Biol. 2015;17:430–436. doi: 10.1111/plb.12252. [DOI] [PubMed] [Google Scholar]
- 89.Arrowood M.J., Jaynes J.M., Healey M.C. In vitro activities of lytic peptides against the sporozoites of Cryptosporidium parvum. Antimicrob. Agents Chemother. 1991;35:224–227. doi: 10.1128/AAC.35.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carryn S., Schaefer D.A., Imboden M., Homan E.J., Bremel R.D., Riggs M.W. Phospholipases and cationic peptides inhibit Cryptosporidium parvum sporozoite infectivity by parasiticidal and non-parasiticidal mechanisms. J. Parasitol. 2012;98:199–204. doi: 10.1645/GE-2822.1. [DOI] [PubMed] [Google Scholar]
- 91.Martins G.G., de Jesus Holanda R., Alfonso J., Gómez Garay A.F., dos Santos A.P.d.A., de Lima A.M., Francisco A.F., Garcia Teles C.B., Zanchi F.B., Soares A.M. Identification of a peptide derived from a Bothrops moojeni metalloprotease with in vitro inhibitory action on the Plasmodium falciparum purine nucleoside phosphorylase enzyme (PfPNP) Biochimie. 2019;162:97–106. doi: 10.1016/j.biochi.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 92.Chaianantakul N., Sungkapong T., Supatip J., Kingsang P., Kamlaithong S., Suwanakitti N. Antimalarial effect of cell penetrating peptides derived from the junctional region of Plasmodium falciparum dihydrofolate reductase-thymidylate synthase. Peptides. 2020;131:170372. doi: 10.1016/j.peptides.2020.170372. [DOI] [PubMed] [Google Scholar]
- 93.Teixeira C., Gomes J.R., Gomes P. Falcipains, Plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr. Med. Chem. 2011;18:1555–1572. doi: 10.2174/092986711795328328. [DOI] [PubMed] [Google Scholar]
- 94.Mishra M., Singh V., Tellis M.B., Joshi R.S., Pandey K.C., Singh S. Cyclic peptide engineered from phytocystatin inhibitory hairpin loop as an effective modulator of falcipains and potent antimalarial. J. Biomol. Struct. Dyn. 2022;40:3642–3654. doi: 10.1080/07391102.2020.1848629. [DOI] [PubMed] [Google Scholar]
- 95.Sweeney-Jones A.M., Gagaring K., Antonova-Koch J., Zhou H., Mojib N., Soapi K., Skolnick J., McNamara C.W., Kubanek J. Antimalarial Peptide and Polyketide Natural Products from the Fijian Marine Cyanobacterium Moorea producens. Mar. Drugs. 2020;18:167. doi: 10.3390/md18030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torres M.D.T., Silva A.F., Andrade G.P., Pedron C.N., Cerchiaro G., Ribeiro A.O., Oliveira V.X., Jr., de la Fuente-Nunez C. The wasp venom antimicrobial peptide polybia-CP and its synthetic derivatives display antiplasmodial and anticancer properties. Bioeng. Transl. Med. 2020;5:e10167. doi: 10.1002/btm2.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El Chamy Maluf S., Hayashi M.A.F., Campeiro J.D., Oliveira E.B., Gazarini M.L., Carmona A.K. South American rattlesnake cationic polypeptide crotamine trafficking dynamic in Plasmodium falciparum-infected erythrocytes: Pharmacological inhibitors, parasite cycle and incubation time influences in uptake. Toxicon. 2022;208:47–52. doi: 10.1016/j.toxicon.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 98.El Chamy Maluf S., Dal Mas C., Oliveira E.B., Melo P.M., Carmona A.K., Gazarini M.L., Hayashi M.A.F. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides. 2016;78:11–16. doi: 10.1016/j.peptides.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Somsri S., Mungthin M., Klubthawee N., Adisakwattana P., Hanpithakpong W., Aunpad R. A Mitochondria-Penetrating Peptide Exerts Potent Anti-Plasmodium Activity and Localizes at Parasites’ Mitochondria. Antibiotics. 2021;10:1560. doi: 10.3390/antibiotics10121560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang Y., He X., Zhang P., Shen C., Mwangi J., Xu C., Mo G., Lai R., Zhang Z. In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin. Toxins. 2019;11:379. doi: 10.3390/toxins11070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tonk M., Pierrot C., Cabezas-Cruz A., Rahnamaeian M., Khalife J., Vilcinskas A. The Drosophila melanogaster antimicrobial peptides Mtk-1 and Mtk-2 are active against the malarial parasite Plasmodium falciparum. Parasitol. Res. 2019;118:1993–1998. doi: 10.1007/s00436-019-06305-x. [DOI] [PubMed] [Google Scholar]
- 102.Pedron C.N., Silva A.F., Torres M.D.T., de Oliveira C.S., Andrade G.P., Cerchiaro G., Pinhal M.A.S., de la Fuente-Nunez C., Oliveira V.X., Jr. Net charge tuning modulates the antiplasmodial and anticancer properties of peptides derived from scorpion venom. J. Pept. Sci. 2021;27:e3296. doi: 10.1002/psc.3296. [DOI] [PubMed] [Google Scholar]
- 103.Rangel G.W., Llinás M. Re-Envisioning Anti-Apicomplexan Parasite Drug Discovery Approaches. Front. Cell. Infect. Microbiol. 2021;11:691121. doi: 10.3389/fcimb.2021.691121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kloehn J., Harding C.R., Soldati-Favre D. Supply and demand—Heme synthesis, salvage and utilization by Apicomplexa. FEBS Lett. 2021;288:382–404. doi: 10.1111/febs.15445. [DOI] [PubMed] [Google Scholar]
- 105.Robles-Loaiza A.A., Pinos-Tamayo E.A., Mendes B., Teixeira C., Alves C., Gomes P., Almeida J.R. Peptides to Tackle Leishmaniasis: Current Status and Future Directions. Int. J. Mol. Sci. 2021;22:4400. doi: 10.3390/ijms22094400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Apostolopoulos V., Bojarska J., Chai T.-T., Elnagdy S., Kaczmarek K., Matsoukas J., New R., Parang K., Lopez O.P., Parhiz H., et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules. 2021;26:430. doi: 10.3390/molecules26020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Juretić D. Designed Multifunctional Peptides for Intracellular Targets. Antibiotics. 2022;11:1196. doi: 10.3390/antibiotics11091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.