Abstract
We found unique behaviors among platelets within a few minutes of the intravenous injection of lipopolysaccharide (LPS) into mice. Platelets accumulated primarily in the liver at lower doses of LPS, but at higher doses they accumulated largely in the lungs. When the platelets accumulated in these organs were degraded, there was a rapid anaphylactoid shock. The platelet response depended on the strain of mouse and on the source of LPS. Of various LPSs tested, the LPS from the smooth type of Klebsiella O3 (KO3-S LPS) was the most potent at inducing the platelet response and shock. K-76 monocarboxylic acid, an inhibitor of complement C5, effectively prevented the KO3-S LPS-induced degradation (but not accumulation) of platelets and the ensuing rapid shock in BALB/c mice. Moreover, in DBA/2 mice (which are deficient in complement C5), platelets accumulated in the lungs and liver in response to KO3-S LPS but soon returned to the circulation without degradation, and there was no rapid shock. The LPS from the rough type of KO3 induced an accumulation of platelets in the liver and lungs but not a degradation of platelets. On the basis of these results and those reported by other investigators, we propose that in the platelet response to LPS, the lectin pathway to form C3 convertase from C4 and C2 is involved in the rapid accumulation of platelets in the liver and lungs and that the pathway from C5 to C9 is involved in the destruction of platelets and the consequent anaphylactoid shock.
Takada and Galanos (25) found that an intravenous injection of a lipopolysaccharide (LPS) into mice pretreated with a muramyl dipeptide induces rapid anaphylactoid shock that quickly results in death. They further observed that the induction of the shock depends both on the source of the LPS and on the strain of the mouse (25, 26). Recently, we found that in mice not pretreated with muramyl dipeptide, an intravenous injection of an LPS induces a rapid and extensive accumulation of platelets predominantly in the liver and lungs, and we suggested that the degradation of platelets in the lungs may be an important factor in the LPS-induced anaphylactoid shock (9, 23). Moreover, a partial depletion of platelets prevented the induction of shock by LPS (24).
Recently, we noticed that among the four strains of mice that showed no anaphylactoid shock in the experiment reported by Takada et al. (26), two (DBA/2 and AKR) are strains deficient in complement C5 (4, 20). In the present study, we set out to clarify the mechanisms underlying the rapid accumulation of platelets and the ensuing anaphylactoid shock that are both induced by LPS while paying particular attention to the possible role of complement.
MATERIALS AND METHODS
Mice.
BALB/c mice (male; 6 to 7 weeks old) were provided by our university, and C57BL, C3H/HeN, and DBA/2 mice (male; 6 to 7 weeks old) were obtained from SLC Japan (Shizuoka, Japan). All experiments complied with the Guideline for Care and Use of Laboratory Animals in Tohoku University.
LPS and other reagents.
LPS from Escherichia coli O55:B5 (smooth [S] type) prepared by the trichloroacetic acid method (1) was obtained from Difco (Detroit, Mich.). LPS from Salmonella minnesota R 60 (rough [R] type) prepared by the phenol-chloroform-petroleum ether method (10) and LPS from S. minnesota S519 (S type) prepared by the phenol-water method (29) were kindly provided by C. Galanos (Max-Planck-Institut für Immunbiologie, Freiburg, Germany). LPSs from Klebsiella O3 (KO3) strain LEN-1 (S type), KO3 strain LEN-113 (R type), and E. coli K-12 (R type) were prepared by the phenol-water method (29, 32). LPS from Prevotella intermedia ATCC 25611 was prepared by the phenol-chloroform-petroleum ether method as described previously (14). LPS from E. coli O111:B4 (S type) and Salmonella typhimurium (S type) prepared by the phenol-water method were purchased from Sigma Chemical Co. (St. Louis, Mo.). The LPSs were dissolved or dispersed in sterile saline by the use of a vortex mixer and injected intravenously (0.1 ml/10 g of body weight). All experiments were carried out at 26 to 28°C. An anticomplement agent, K-76 monocarboxylic acid (13), was provided by Otsuka Pharmaceutical Co. Ltd. (Tokushima, Japan). This agent was dissolved in saline with the addition of enough NaOH solution to bring the pH to about 7.5.
Determination of the amount of protein in LPS preparations.
The protein was assayed with a Micro BCA protein assay reagent kit (Pierce, Rockford, Ill.); the assay procedures were performed as described by the manufacturer.
Estimation of platelet accumulation and degranulation.
Platelets contain a large amount of 5-hydroxytryptamine (5HT; serotonin) in their granules, and free 5HT in blood is rapidly cleared from the circulation (30). Therefore, as described in our previous papers, by measuring the changes that occur in the amount of 5HT in the blood and in tissues such as lung, liver, and spleen, it is possible to assess the translocation of platelets from the circulation to the tissues (7–9, 19, 23, 24). 5HT in blood and tissues was determined as described in these papers. Briefly, mice were decapitated and blood (3 or 4 drops) was collected in preweighed test tubes containing 3 ml of 0.4 M HClO4, 0.1% cysteine-HCl, and 2 mM EDTA-2Na. After being weighed, the tube was cooled in an ice bath. The lungs, livers, and spleens of the mice were rapidly removed and kept in a jar with dry ice until use. Determination of 5HT levels in the blood was done on the day the blood was collected, because the 5HT in blood collected in this way is unstable. 5HT levels in the tissues were determined within 1 week of collection. After 5HT had been separated by column chromatography, it was measured fluorometrically as previously described (7).
Scoring of rapid shock induced by LPS.
The incidence and the score given to the severity of the rapid shock and the subsequent mortality were recorded within 30 min of the injection of LPS. The scoring of the shock was as follows: 0, no symptoms of shock; 1, staggering; 2, crawling and prostration; 3, prostration and weak convulsions; 4, prostration and strong convulsions.
Statistical analysis.
Experimental values for 5HT are given as the mean ± standard deviation (SD). The statistical significance of differences was assessed by using Dunnett’s multiple comparison test after analysis of variance; P values of less than 0.05 were considered to indicate significance.
RESULTS
Induction of rapid anaphylactoid shock by LPSs in BALB/c mice.
The abilities of LPSs from various sources to induce rapid shock in BALB/c mice are shown in Table 1. KO3-S LPS was the most potent of the LPSs tested. This LPS was lethal at 0.5 mg/kg. In contrast, KO3-R LPS did not produce rapid shock even at 8 mg/kg. E. coli O55:B5-S LPS, too, induced rapid shock at 0.5 mg/kg, but it was not lethal. The amounts of contaminating protein in LPS preparations used in this study are also shown in Table 1. We cannot explain the action of KO3-S LPS by its contaminating protein, because KO3-R LPS, with a dose 16 times or more that of KO3-S LPS, was entirely inactive at inducing rapid shock.
TABLE 1.
Abilities of various LPSs to induce shock in BALB/c mice
| Source of LPS | Protein (μg/mg of LPS preparation) | Dose (mg/kg) | Shocka
|
||
|---|---|---|---|---|---|
| Incidence | Score | Mortality | |||
| KO3-S | 21 | 0.5 | 4/4 | 4 | 3/4 |
| 1 | 4/4 | 4 | 4/4 | ||
| KO3-R | 14 | 4 | 0/5 | 0 | 0/5 |
| 8 | 0/5 | 0 | 0/5 | ||
| E. coli O55:B5-S | 118 | 0.5 | 5/5 | 2 | 0/5 |
| 1 | 5/5 | 4 | 3/5 | ||
| E. coli O111:B4-S | 34 | 4 | 0/5 | 0 | 0/5 |
| E. coli K-12-R | 76 | 4 | 5/9 | 1 | 0/9 |
| S. minnesota-S | 20 | 8 | 4/4 | 4 | 1/4 |
| S. minnesota-R | 15 | 8 | 0/4 | 0 | 0/4 |
| S. typhimurium-S | 7.4 | 8 | 0/4 | 0 | 0/4 |
| P. intermediab | 5.2 | 2 | 4/4 | 2 | 0/4 |
| 4 | 5/5 | 4 | 2/5 | ||
Incidence and mortality figures are shown as number/total.
Lacking typical O polysaccharide composed of repeating sugar units (14).
Dependence of LPS-induced rapid shock on the strain of mice.
As shown in Table 2, KO3-S LPS induced rapid shock in both BALB/c and C57BL/6 mice. E. coli O55:B5-S LPS induced rapid shock only in BALB/c mice. In contrast, P. intermedia LPS induced rapid shock in BALB/c, C57BL/6, and C3H/HeN mice. Interestingly, P. intermedia LPS induced shock most strongly in C3H/HeN mice, although C3H/HeN mice proved to be resistant to the other two LPSs. None of the LPSs produced rapid shock in DBA/2 mice, which are complement C5 deficient.
TABLE 2.
Dependence of rapid shock on source of LPS and strain of mice
| LPS source | Dose (mg/kg) | Shocka
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BALB/c
|
C57BL/6
|
C3H/HeN
|
DBA/2
|
||||||||||
| I | S | M | I | S | M | I | S | M | I | S | M | ||
| KO3-S | 2 | 4/4 | 4 | 4/4 | 7/7 | 4 | 4/7 | 0/4 | 0 | 0/4 | 0/5 | 0 | 0/5 |
| 4 | 0/4 | 0 | 0/4 | 0/4 | 0 | 0/4 | |||||||
| E. coli O55:B5-S | 2 | 5/5 | 4 | 5/5 | 0/5 | 0 | 0/5 | 0/5 | 0 | 0/5 | 0/5 | 0 | 0/5 |
| 4 | 0/4 | 0 | 0/4 | 0/4 | 0 | 0/4 | 0/4 | 0 | 0/4 | ||||
| P. intermedia | 2 | 4/4 | 2 | 0/4 | 5/5 | 2 | 0/5 | 5/5 | 4 | 0/5 | 0/5 | 0 | 0/5 |
| 4 | 5/5 | 4 | 2/5 | 4/4 | 4 | 1/4 | 5/5 | 4 | 2/5 | 0/4 | 0 | 0/4 | |
I, incidence; S, shock; M, mortality. Incidence and mortality are shown as number/total.
Time course of 5HT and platelet responses to the LPSs from S and R types of KO3.
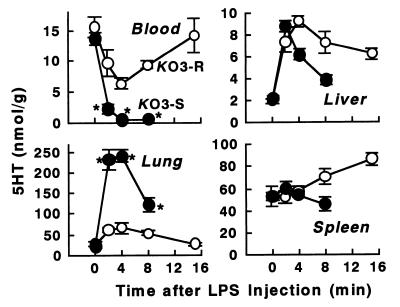
As shown in Fig. 1, KO3-S LPS at 2 mg/kg induced marked and rapid changes in 5HT in the blood, lungs, and liver such that 5HT disappeared almost completely from the circulation within 4 min of the injection of KO3-S LPS and 5HT accumulated in the lungs. The amounts of 5HT accumulated in the lungs and liver at 4 min corresponded to 70 to 80 and 10 to 20% of the 5HT lost from the blood, respectively (calculated as described in our previous papers [9, 23]). The elevated level of 5HT in the lungs and liver had declined at 8 min without a recovery in the 5HT level in the blood. At this dose of KO3-S LPS, severe shock occurred at 4 to 8 min and most mice had died within 20 min of the injection. We have previously shown by electron microscopy that in such shocked mice there is a severe degradation of platelets in the lungs (9, 23). By comparison with the response to KO3-S LPS, the changes in 5HT in the blood and lungs induced by KO3-R LPS were mild, and the elevated 5HT levels in the lungs and liver declined in parallel with the recovery of the decreased 5HT level in the blood. With KO3-R LPS, the decreased 5HT level in the blood returned to almost exactly its initial value, indicating that there was no significant degradation of platelets.
FIG. 1.
Time course of changes in 5HT and platelet levels induced by the LPSs of KO3-S and KO3-R in BALB/c mice. After injection of each LPS at a dose of 2 mg/kg, blood and tissues were taken at the indicated times. Each value is the mean ± SD from four mice. ∗, P <0.01 versus the KO3-R group.
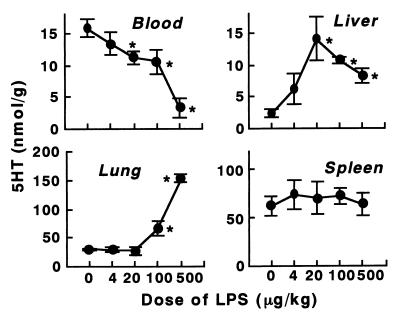
Dose dependence of the 5HT and platelet responses to KO3-S LPS.
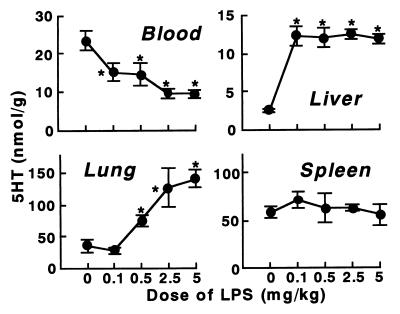
At lower doses of KO3-S LPS, 5HT and platelets accumulated predominantly in the liver (Fig. 2). At 20 μg of this LPS/kg the accumulation in the liver reached a maximum. At this dose, there was no increase in 5HT or platelets in the lungs. At higher doses of this LPS, large amounts of 5HT and platelets accumulated, predominantly in the lungs. In contrast, the 5HT level in the liver decreased at higher doses, suggesting a degradation of platelets (Fig. 2).
FIG. 2.
Dose dependence of 5HT and platelet responses to KO3-S LPS in BALB/c mice. The indicated doses of LPS were injected into mice, and blood and tissues were taken 4 min after the injection. Each value is the mean ± SD from four mice. ∗, P <0.01 versus dose 0.
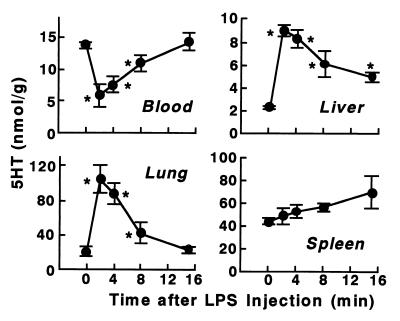
Effect of a C5 inhibitor on 5HT and platelets and on the rapid shock induced by KO3-S LPS in BALB/c mice.
Hong et al. (13) have reported that K-76 inhibits the activity of C mainly at the C5 step, although at high concentrations it also causes some inhibition of other steps. They also suggested that this agent combines specifically with free C5 molecules and forms an inactive complex or it causes the irreversible structural alteration of C5. An intraperitoneal injection of K-76 1 h before injection of a lethal dose of KO3-S LPS (1 mg/kg) largely prevented the degradation of platelets in the lungs and liver (i.e., most of the platelets were returned to the circulation without degradation) (Fig. 3). In this experiment, rapid shock was largely prevented: its incidence, severity score, and lethality were 2 of 4, 0 to 1, and 0 of 4, respectively, compared to 4 of 4, 4, and 4 of 4 in the absence of K-76 (Table 1).
FIG. 3.
Effects of K-76, an inhibitor of complement C5, on 5HT and platelet responses to KO3-S LPS in BALB/c mice. LPS (1 mg/kg) was injected 1 h after an intraperitoneal injection of K-76 (100 mg/kg). Blood and tissues were taken 5 and 15 min after the injection of the LPS. K-76 itself did not alter the levels of 5HT (data not shown). Each value is the mean ± SD from four mice.
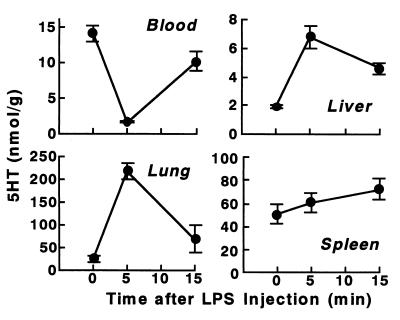
Effects of KO3-S LPS in DBA/2 mice.
Figure 4 shows the effect of KO3-S LPS at 2 mg/kg in DBA/2 mice. In BALB/c mice, this dose is lethal and induces a severe accumulation and degradation of platelets (Fig. 1). However, in DBA/2 mice, the decreased level of 5HT in the blood and the increased level in the lungs soon returned to almost exactly their initial levels (Fig. 4), a pattern very similar to that induced by KO3-R LPS in BALB/c mice (Fig. 1), indicating that there was no degradation of platelets. Indeed, in this experiment, KO3-S LPS did not produce rapid shock. Interestingly, a marked accumulation of 5HT and platelets in the liver, as seen in BALB/c mice at lower doses of KO3-S LPS (Fig. 2), occurred in DBA/2 mice, too (Fig. 5). However, in DBA/2 mice there was no decrease in the elevated level of hepatic 5HT at higher doses, indicating that there was no degradation of platelets. As in our previous studies, a similar accumulation of 5HT and platelets in the liver at lower doses of the LPSs from E. coli and P. intermedia was shown in BALB/c and C3H/HeN mice (9, 23).
FIG. 4.
5HT and platelet responses to KO3-S LPS in DBA/2 mice. Blood and tissues were taken at the indicated times after the injection of LPS (2 mg/kg). Each value is the mean ± SD from four mice. ∗, P <0.01 versus time 0.
FIG. 5.
Dose dependence of 5HT and platelet responses to KO3-S LPS in DBA/2 mice. The indicated doses of LPS were injected into mice, and blood and tissues were taken 4 min after the injection. Each value is the mean ± SD from four mice. It should be noted that the 5HT and platelet responses in the liver reached their maximum at 0.1 mg/kg and, at this dose, there was no response in the lungs. ∗, P <0.01 versus dose 0.
Accumulation of 5HT and platelets in the spleen.
Instead of a rapid elevation of the type that was seen in the lungs and liver following injection of either one of the KO3 LPSs in both BALB/c and DNA/2 mice, the spleen showed a more delayed elevation of 5HT (Fig. 1, 3, and 4). In fact, the level in the spleen was rising while the levels in the lungs and liver were falling. A similar phenomenon has been observed in BALB/c mice given E. coli O55:B5-S LPS (see Fig. 1 in reference 23) and in C3H/HeN mice given P. intermedia LPS (see Fig. 1 in reference 9). These results suggest that some of the platelets returned to the circulation from the lungs and liver may have been taken up by the spleen.
DISCUSSION
Our recent (9, 23) and present experiments show that intravenous injection of LPS into mice induces a rapid response in the platelets. This response has the following unique characteristics. (i) Platelets accumulate primarily in the liver at lower doses of LPS, but at higher doses they accumulate largely in the lungs. (ii) The accumulation of platelets occurs within a few minutes of the injection of LPS. The platelet response depends on both (iii) the strain of mouse studied and (iv) the source or preparation of the LPS (and possibly on the structure of the LPS). (v) The platelets accumulated in the liver and lungs soon return to the circulation or are degraded, and the complement system seems to be involved in this degradation. (vi) The degradation of platelets in the lungs and liver, provided it is of sufficient extent, induces a rapid anaphylactoid shock. We discuss these points in the following paragraphs.
Dependence on the strain of mice.
At present, we cannot explain why C3H/HeN mice are resistant to KO3-S LPS and E. coli O55:B5-S LPS but sensitive to P. intermedia LPS and why only BALB/c mice are sensitive to E. coli O55:B55:B5-S LPS. In the present study, therefore, we decided to use BALB/c mice and LPSs from KO3, because BALB/c mice responded to all the LPSs mentioned above (Table 2) and KO3-S LPS was the most potent at inducing rapid shock in this strain of mice (Table 1).
Dependence on the structure of LPS.
In typical gram-negative bacteria, wild-type strains form S-type colonies. The polysaccharide region of the LPS of S-type bacteria is made up of an O antigen region (built from repeating units of three to eight sugars) and a core region. Mutant bacteria lacking the O antigen region form R-type colonies. The present results obtained with the LPSs from the S and R types of both KO3 and S. minnesota (Table 1) suggest that the abilities of these LPSs to induce the platelet response depend on the structure of the polysaccharide region, especially on the presence or absence of the O antigen moiety. However, it is possible that the structures of other regions may also contribute to the activity, because E. coli K-12-R LPS was more potent than E. coli O111-B4-S LPS (Table 1).
Return of platelets from lungs and liver to blood.
The amount of 5HT in the lungs and liver is determined by the number of platelets and the extent of their degradation. KO3-R LPS injected into BALB/c mice (Fig. 1) and KO3-S LPS injected into DBA/2 mice (Fig. 4) induced only a transient decrease in 5HT in the blood, and the elevated 5HT levels in the lungs and liver declined in parallel with the recovery of the decreased 5HT level in the blood. These results indicate that the accumulation of platelets in these organs does not necessarily lead to their degradation and that when they are not degraded, they are returned to the circulation.
Degradation of platelets and shock.
In all the experiments in the present study, there was a close relationship between the degradation of platelets and the production of shock. When the decreased level of 5HT and platelets in the blood returned roughly to its initial level within the study period, shock was not produced. These results indicate that it is the degradation of platelets in the lungs and liver that leads to the rapid shock and that accumulation of platelets in these organs (without degradation) does not itself induce shock.
Rapidity of response.
Many actions of LPS are thought to be mediated through the production of cytokines. However, we suspect that a substantial production of cytokines could not be produced within the space of a few minutes after the injection of LPS. On this basis, it is unlikely that cytokines are responsible for the rapid accumulation and degradation of platelets induced by LPS.
Degradation of platelets in the lungs and liver and possible involvement of the complement system.
In our previous studies of BALB/c and C3H/HeN mice (9, 23), we showed by electron microscopy that there is a degradation of the platelets that accumulate in the lungs following LPS injection. DBA/2 mice have been shown to be deficient in complement C5 (4, 20). In this strain of mice, the response of 5HT and platelets to KO3-S LPS (Fig. 4) was similar in extent to the response to KO3-R LPS in BALB/c mice (Fig. 1), and the decreased level of 5HT in the blood soon returned to its initial level. Moreover, K-76COOH, a C5 inhibitor (13), largely prevented both the LPS-induced degradation of platelets (Fig. 3) and the rapid shock in BALB/c mice. LPSs possessing a mannose homopolymer in the O antigen polysaccharide region (for example, KO3-S LPS) have been shown to activate the complement system much more strongly than LPSs possessing other polysaccharides (15, 21, 31). In our study, KO3-R LPS, which lacks a mannose homopolymer, induced neither a significant degradation of platelets nor rapid shock (Fig. 1 and Table 1). Using human serum, Paeng et al. (21) compared the abilities of various LPSs to activate complement in vitro, and showed that the order of potencies was as follows: KO3 LEN 1 > E. coli K-12 > E. coli O111. This order is consistent with their relative abilities to induce shock (Table 1). Taking these results together, we conclude that the complement system is involved in the degradation of the platelets that accumulate in the lungs and liver under the influence of LPS.
Selective accumulation of platelets in the liver and lungs, depending on the dose of LPS.
KO3-S LPS strongly binds to a mannose-binding protein (MBP) (15). MBP is related structurally and functionally to the first component of the classical complement pathway, C1q (12, 18, 27). MBP is believed to circulate in a complexed form with a proteinase (called MBP-associated serine proteinase, MASP) corresponding to C1r and C1s. MBPs have a collagen-like domain and a lectin domain. The collagen-like domain is involved in the activation of complements, and the lectin domain is the binding site for polysaccharides or LPS. In the classical pathway of the complement system, the antigen-antibody-C1 complex activates C4 and C2 to form C3 convertase (the assembly of the activated C4 and C2). The LPS-MBP-MASP complex can also activate C4 and C2, and this pathway is now called the lectin pathway (12, 18, 26). The receptors for MBP, which are identical to those for C1q, are present on many cell types, including platelets, endothelial cells, most leukocytes, fibroblasts, and specialized epithelial cells (12). Therefore, it seems likely that MBP might be involved in the KO3-S LPS-induced accumulation and degradation of platelets that occurs in the liver and lungs. The different dose-dependent response of platelets seen in the liver and lungs might be consistent with the presence of receptors with different affinities for the LPS-MBP-MASP complexes.
Comparison with platelet responses to LPS in other species.
Rapid falls in platelet counts and their partial or complete recovery to initial levels also occur in other species given intravenous injections of endotoxin. As early as 1960 and 1961, Davis et al. (5, 6) found that simultaneously with the rapid fall in platelets in rabbits or dogs there is an equally rapid rise in the plasma 5HT level. Later (1972 to 1974), the rapid platelet fall was shown to depend on the complement system in cats (17), rabbits (2), guinea pigs (16), and dogs (3). In C3-C9-depleted rabbits and guinea pigs (treated with cobra venom factor) and in C4-deficient guinea pigs, the rapid drop in platelets was not observed (2, 16).
Hypothesis for the mechanisms underlying the platelet response to LPS.
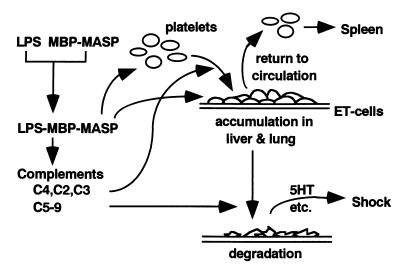
Although the studies by other investigators mentioned above did not examine the accumulation of platelets, their results, taken together with our present results, support the idea that the pathway to form C3 convertase from C4 and C2, in which C4a and C3a are also produced, is involved in the LPS-induced rapid accumulation of platelets in the lungs and liver and that the pathway from C5 to C9, in which C5a and a complex of C5-C9 (called the membrane-attack complex) are formed, is involved in the destruction of platelets in these organs. Our tentative hypothesis is summarized in Fig. 6.
FIG. 6.
Hypothetical pathway for the LPS-induced accumulation and degradation of platelets in the lungs and liver. In this tentative scheme, LPS is assumed to bind to MBP-MASP complex and a consequent complex stimulates MBP receptors on platelets and vascular endothelial (ET) cells in the liver and lungs. The LPS-MBP-MASP complex also activates the complement system. The pathway to form C3 convertase from C4 and C2 may be responsible for the accumulation of platelets in the liver and lungs. The pathway from C5 to C9 may be involved in the destruction of platelets, and the O antigen polysaccharide region of KO3-S LPS may be important in this step. The degradation of platelets in the liver and lungs and the release of their contents, including serotonin (5HT), lead rapidly to shock. When the complement system is not activated, or if its activation is insufficient, the platelets accumulated in the lungs and liver return to the circulation without degradation or with only slight damage. The platelets that suffer slight damage may be removed from the circulation by the spleen.
It is of interest that KO3-S LPS is a very potent inducer of the platelet response. To our knowledge, it is the most potent LPS at inducing lethal shock. This finding, together with those described above, might provide a clue leading to the clarification of the mechanism underlying the pathogenic activities of Klebsiella, especially of Klebsiella pneumoniae, and thus to a better understanding of the origins and development of pulmonary diseases.
It has been shown that CD14 acts as a receptor that binds LPS, triggering inflammatory responses, such as the production of cytokines (28). CD14-deficient mice are resistant to endotoxin shock (11). However, as described above, it is unlikely that cytokines are responsible for the rapid response of platelets to LPS, and it is not known that CD14-mediated actions of LPS are markedly dependent on the strain of mice. Moreover, as shown by Takada et al. (26), P. intermedia LPS can induce anaphylactoid shock even in LPS-resistant C3H/HeJ mice, which were recently shown to carry a mutation in the Toll-like receptor-4 gene, leading to defective LPS signaling (22). Therefore, it seems unlikely that CD14 is required for anaphylactoid shock.
Finally, although the primary role of platelets is believed to be in hemostasis, our findings might suggest a new role of platelets in innate immunity: platelets, without the help of cytokines, may have a role in preventing the entry of bacteria from the circulation into extravascular tissues in the liver and lungs. However, we still cannot explain (i) why KO3-R LPS induces platelet accumulation but not degradation (i.e., whether MBP is involved in the action of this LPS), (ii) why platelets accumulate selectively in the liver or lungs, depending on the dose of LPS, or (iii) why the platelet response to LPS depends on the strain of mice.
ACKNOWLEDGMENT
This work was supported in part by a grant for scientific research from the Ministry of Education of Japan (no. 10877302).
REFERENCES
- 1.Boivin A, Mesrobeanu I, Mesrobeanu L. Technique pour la préparation des polysaccharide microbiens spécifiques. C R Soc Biol. 1933;113:490–492. [Google Scholar]
- 2.Brown D L, Lachmann P J. The behaviour of complement and platelets in lethal endotoxin shock in rabbits. Int Arch Allergy Appl Immunol. 1973;45:193–205. doi: 10.1159/000231028. [DOI] [PubMed] [Google Scholar]
- 3.Carner R, Chater B V, Brown D L. The role of complement in endotoxin shock and disseminated intravascular coagulation: experimental observations in the dog. Br J Haematol. 1974;28:393–401. doi: 10.1111/j.1365-2141.1974.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 4.Cinader B, Dubiski S, Wardlaw A C. Distribution, inheritance, and properties of an antigen, MuB1, and its relation to hemolytic complement. J Exp Med. 1964;120:897–924. doi: 10.1084/jem.120.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis P B, Meeker W R, Jr, Bailey W L. Serotonin release by bacterial endotoxin. Proc Soc Exp Biol Med. 1961;108:774–776. doi: 10.3181/00379727-108-27063. [DOI] [PubMed] [Google Scholar]
- 6.Davis P B, Meeker W R, Jr, McQuarrie D G. Immediate effects of intravenous endotoxin on serotonin concentrations and blood platelets. Circ Res. 1960;8:234–239. doi: 10.1161/01.res.8.1.234. [DOI] [PubMed] [Google Scholar]
- 7.Endo Y, Nakamura M. The effect of lipopolysaccharide, interleukin-1 and tumor necrosis factor on the hepatic accumulation of 5-hydroxytryptamine and platelets in the mouse. Br J Pharmacol. 1992;105:613–619. doi: 10.1111/j.1476-5381.1992.tb09028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo Y, Nakamura M. Active translocation of platelets into sinusoidal and Disse spaces in the liver in response to lipopolysaccharide, interleukin-1 and tumor necrosis factor. Gen Pharmacol. 1993;24:1039–1053. doi: 10.1016/0306-3623(93)90348-2. [DOI] [PubMed] [Google Scholar]
- 9.Endo Y, Shibazaki M, Nakamura M, Takada H. Contrasting effects of lipopolysaccharides (endotoxins) from oral black-pigmented bacteria and Enterobacteriaceae on platelets, a major source of serotonin, and on histamine-forming enzyme in mice. J Infect Dis. 1997;175:1404–1412. doi: 10.1086/516473. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Lüderitz O, Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Haziot A, Ferrero E, Köntgen F, Hijiya N, Yamamoto S, Silver J, Stewart C L, Goyert S M. Resistance to endotoxin-shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 12.Holmskov U, Malhotra R, Sim R B, Jensenius J C. Collectins: collagenous C type lectins of innate immune defence system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Hong K, Kinoshita T, Miyazaki W, Izawa T, Inoue K. An anticomplementary agent, K-76 monocarboxylic acid: its site and mechanism of inhibition of the complement activation cascade. J Immunol. 1979;122:2418–2423. [PubMed] [Google Scholar]
- 14.Iki K, Kawahara K, Sawamura S, Arakaki R, Sakuta T, Sugiyama A, Tamura H, Sueda T, Hamada S, Takada H. A novel component different from endotoxin extracted from Prevotella intermedia ATCC 25611 activates lymphoid cells from C3H/HeJ mice and gingival fibroblasts from humans. Infect Immun. 1997;65:4531–4538. doi: 10.1128/iai.65.11.4531-4538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang G Z, Sugiyama T, Kato Y, Koide N, Yokochi T. Binding of mannose-binding protein to Klebsiella 03 lipopolysaccharide possessing the mannose homopolysaccharide as the O-specific polysaccharide and its relation to complement activation. Infect Immun. 1995;63:2537–2540. doi: 10.1128/iai.63.7.2537-2540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane M A, May J E, Frank M M. Interactions of the classical and alternate complement pathway with endotoxin lipopolysaccharide: effect on platelets and blood coagulation. J Clin Investig. 1973;52:370–376. doi: 10.1172/JCI107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitzmiller J L, Lucas W E, Yelenosky P F. The role of complement in feline endotoxin shock. Am J Obstet Gynecol. 1972;112:414–421. doi: 10.1016/0002-9378(72)90488-7. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra R, Lu J, Holmskov U, Sim R B. Collectins, collectin receptors and the lectin pathway of complement activation. Clin Exp Immunol. 1994;97(Suppl. 2):4–9. doi: 10.1111/j.1365-2249.1994.tb06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura M, Shibazaki M, Nitta Y, Endo Y. Translocation of platelets into Disse spaces and their entry into hepatocytes in response to lipopolysaccharides, interleukin-1 and tumor necrosis factor: the role of Kupffer cells. J Hepatol. 1998;28:991–999. doi: 10.1016/s0168-8278(98)80348-6. [DOI] [PubMed] [Google Scholar]
- 20.Ooi M, Colten H R. Genetic defect in secretion of complement C5 in mice. Nature. 1979;282:207–208. doi: 10.1038/282207a0. [DOI] [PubMed] [Google Scholar]
- 21.Paeng N, Kido N, Schmidt G, Sugiyama T, Kato Y, Koide N, Yokochi T. Augmented immunological activities of recombinant lipopolysaccharide possessing the mannose homopolymer as the O-specific polysaccharide. Infect Immun. 1996;64:305–309. doi: 10.1128/iai.64.1.305-309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutation in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 23.Shibazaki M, Nakamura M, Endo Y. Biphasic, organ-specific, and strain-specific accumulation of platelets induced in mice by a lipopolysaccharide from Escherichia coli and its possible involvement in shock. Infect Immun. 1996;64:5290–5294. doi: 10.1128/iai.64.12.5290-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibazaki M, Nakamura M, Nitta Y, Endo Y. Displacement of platelets from blood to spleen following intravenous injection of liposomes encapsulating dichloromethylene bisphosphonate. Immunopharmacology. 1998;39:1–7. doi: 10.1016/s0162-3109(97)00092-1. [DOI] [PubMed] [Google Scholar]
- 25.Takada H, Galanos C. Enhancement of endotoxin lethality and generation of anaphylactoid reactions by lipopolysaccharides in muramyl-dipeptide-treated mice. Infect Immun. 1987;55:409–413. doi: 10.1128/iai.55.2.409-413.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada H, Hirai H, Fujiwara T, Koga T, Ogawa T, Hamada H. Bacteroides lipopolysaccharides (LPS) induce anaphylactoid and lethal reactions in LPS-responsive and -nonresponsive mice primed with muramyl dipeptide. J Infect Dis. 1990;162:428–434. doi: 10.1093/infdis/162.2.428. [DOI] [PubMed] [Google Scholar]
- 27.Turner M W. Mannose binding protein. Biochem Soc Trans. 1994;22:1993–1995. doi: 10.1042/bst0220088. [DOI] [PubMed] [Google Scholar]
- 28.Urevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 29.Westphal O, Jann K. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 30.Wiggins R C, Glatfelter A, Campbell A M, Kunkel R G, Ulevitch R J. Acute hypotension due to platelet serotonin-induced chemoreflexes after intravenous injection of dextran sulfate in the rabbit. Circ Res. 1985;57:262–277. doi: 10.1161/01.res.57.2.262. [DOI] [PubMed] [Google Scholar]
- 31.Yokochi T, Inoue Y, Kimura T, Kato N. Strong interaction of lipopolysaccharides possessing the mannose homopolysaccharides with complement and its relation to adjuvant action. J Immunol. 1990;144:3106–3110. [PubMed] [Google Scholar]
- 32.Yokochi T, Inoue Y, Yokoo J, Kimura Y, Kato N. Retention of bacterial lipopolysaccharide at the site of subcutaneous injection. Infect Immun. 1989;57:1786–1791. doi: 10.1128/iai.57.6.1786-1791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]