Abstract
Antibiotic resistance has become a severe public threat to human health worldwide. Supplementing antibiotic growth promoters (AGPs) at subtherapeutic levels has been a commonly applied method to improve the production performance of livestock and poultry, but the misuse of antibiotics in animal production plays a major role in the antibiotic resistance crisis and foodborne disease outbreaks. The addition of AGPs to improve production performance in livestock and poultry has been prohibited in some countries, including Europe, the United States and China. Moreover, cross-resistance could result in the development of multidrug resistant bacteria and limit therapeutic options for human and animal health. Therefore, finding alternatives to antibiotics to maintain the efficiency of livestock production and reduce the risk of foodborne disease outbreaks is beneficial to human health and the sustainable development of animal husbandry. Essential oils (EOs) and their individual compounds derived from aromatic plants are becoming increasingly popular as potential antibiotic alternatives for animal production based on their antibacterial properties. This paper reviews recent studies in the application of EOs in animal production for the control of foodborne pathogens, summarizes their molecular modes of action to increase the susceptibility of antibiotic-resistant bacteria, and provides a promising role for the application of nanoencapsulated EOs in animal production to control bacteria and overcome antibiotic resistance.
Keywords: essential oils, antibacterial property, foodborne bacteria, antibiotic resistance, animal production
1. Introduction
Today, the world produces more than three times the quantity of meat as it did 50 years ago. In 2018, meat production was around 340 million tons [1]. By 2050, a 102% increase in the food supply will be necessary to meet the demand [2]. However, in livestock and poultry production, animals usually face various stressors, such as oxidative processes, nutritional imbalances, allergens, pathogenic bacteria, etc., which could result in diarrhea, growth retardation, and high morbidity and mortality. The conventional way to maintain or improve yield in animal products was to use antibiotic growth promoters (AGPs) at subtherapeutic levels. Unfortunately, exposure to AGPs in the early stages of life adversely affects the development of the immune function and intestinal bacteria and ultimately results in increased susceptibility to infections and diseases in livestock [3]. Antibiotic abuse in food-producing animals is a primary reason for the selectivity and diffusion of antibiotic resistance and disease outbreaks induced by resistant foodborne bacteria, which could create foodborne risks to human health [4]. Although the addition of AGPs to diets has been forbidden in some countries to promote the growth performance of food-producing animals, antibiotic resistance is still widespread in the world [5]. As animal production is one of the main sources of antibiotic resistance genes due to the selective pressure that occurs in this environment, the presence of mobile genetic elements in the intestinal bacteria could spread antibiotic resistance [6]. Animal-derived foods contaminated with resistant bacteria can lead to serious infections and diseases that are difficult to treat. Globally, an estimated 2 million people receive treatment for resistant infections each year, with approximately 700,000 deaths and treatment costs exceeding 100,000 million dollars [1]. Such data demonstrate the economic and social consequences of the emergence of resistant foodborne pathogens–an aftereffect of the misuse of antibiotics in animal production. It is necessary to seek novel feed additives for maintaining the efficiency of livestock production and reducing the spread of drug-resistant pathogens, which is beneficial to human health and the sustainable development of animal husbandry.
Essential oils (EOs) are becoming potential antibiotic alternatives due to their natural origin, low toxicity, and free of residues [7]. EOs are a mixture of various volatile compounds extracted from aromatic plants (flowers, fruits, seeds, stems, leaves, etc.) [8]. Lamiaceae are one of the most important families of plant EOs with antibacterial effects, among which oregano, thyme, and rosemary have been widely used in the food industry [9,10,11]. Several in vivo studies indicated that EOs increased Lactobacillus abundance and decreased Escherichia coli or total coliforms in piglets [12,13,14]. These results were similar to those of several studies of poultry supplemented with EOs [15,16,17], suggesting that EOs resulted in some fundamental changes within the gut microbiota, primarily the observed numbers of Lactobacillus species. In addition to the antibacterial property, EOs also exhibit other biological activities, including anti-inflammatory, antioxidant, anti-tumor, and immune-regulating properties [1], suggesting that EOs could improve production performance in animals. Franz et al. [18] and Windisch et al. [19] reviewed that the average improvement in weight gain, feed intake, and feed conversion caused by EOs was 2.0, 0.9, and 3.0% for piglets and 0.5, 1.6, and −2.6% for poultry, respectively. However, the use of aromatic plant-derived EOs in grower–finisher pigs appears unsuccessful. Janz et al. [20] and Yan et al. [21] did not observe any improvement in the growth performance induced by EOs in finisher pigs. The different results may be caused by different digestive physiology, sources of aromatic plant EOs, the quantity used in the diet, and the environmental conditions used in the experiments.
The indiscriminate use of antibiotics in animal production plays an important role in the antibiotic resistance crisis and foodborne disease outbreaks. Since antibiotic resistance remains a major threat to public health, most research has focused on the causes, threats, and management strategies related to human health, leaving aside aspects of animal production. This paper collects the research progress of the use of EOs in animal production to control foodborne pathogens, summarizes their molecular modes of action with respect to antibiotic resistance, and provides a promising role for the application of nanoencapsulated EOs in animal production to more effectively control bacterial infections and overcome antibiotic resistance.
2. Foodborne Pathogenic Bacteria and Antibiotic Resistance
Undoubtedly, the discovery of AGPs is one of the greatest inventions of the 20th century, as they could improve growth performance and combat infectious diseases in livestock. Globally, the total consumption of antimicrobials in animal production, including pigs, chickens, and cattle was 93,309 tons in 2017 and is expected to grow by 11.5% to 104,079 tons by 2030 [22]. With the excessive use and abuse of AGPs or antibiotics used to treat infections in humans and food-producing animals worldwide, there is a greater chance for bacteria to develop complicated resistances against antibiotics [23]. Antibiotic resistance is defined as a natural process of selecting resistant microorganisms that could thrive in the environment and multiply and perpetuate resistance characteristics [24]. Moreover, antibiotic resistance could spread from food-producing animals to humans by eating meat, milk, and their products [25,26]. Therefore, animal-derived foods contaminated with drug-resistant pathogens could result in severe infections and untreatable diseases in humans. If no action is taken, antibiotic resistance is expected to cause more deaths than cancer by 2050, which could be a massive threat to human health [27]. Increased emergence of E. coli infection has been shown to result in higher morbidity and mortality in weaned pigs [28]. According to an estimate, 80 species of bacteria, including E. coli and Salmonella, could pose a severe threat to poultry production [29]. Mastitis, induced by Staphylococcus aureus, Streptococcus agalactiae, Corynebacterium bovis, Streptococcus uberis, Streptococcus dysgalactiae, E. coli, Serratia marcescens and Proteus mirabilis in dairy cows, has been an economic welfare problem for dairy farms [30,31,32]. To date, many studies have reported main antibiotic resistant bacteria from different animal-derived foods in some countries, including E. coli (Table 1), Salmonella spp. (Table 2), Staphylococcus spp. (Table 3), and Listeria spp. (Table 4). These data denote that foodborne antibiotic resistance is a widespread problem worldwide-a result of the abuse of AGPs in animals. To ensure human health and animal food safety, new and multidimensional approaches are needed to control bacterial infections for animal production. Here, we discuss the trend and development of EOs and their individual compounds as alternatives to antibiotics to address antibiotic resistance.
Table 1.
Escherichia coli isolated from animal-derived foods and their resistance to antibiotics.
| Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|
| Australia | Dairy cows | Amoxicillin-clavulanate, ceftiofur, cefoxitin, gentamicin | [33] |
| Brazil | Chicken carcass | Aminoglycosides, colistin, β-lactams, macrolides, quinolones, sulfonamides, tetracyclines, trimethoprim | [34] |
| Cambodia | Broiler carcass and pig carcass | Ampicillin, cefotaxime, ceftazidime, chloramphenicol, ciprofloxacin, streptomycin, tetracyclines, trimethoprim | [35] |
| China | Retail chicken and pork | Ampicillin/sulbactam, aztreonam, cefepime, cefotaxime, ciprofloxacin, colistin, doxycycline, gentamicin, levofloxacin, minocycline, piperacillin/tazobactam constant 4, polymyxin B, tigecycline, trimethoprim/sulfamethoxazole | [36] |
| Denmark | Pig carcass | Ampicillin, chloramphenicol, gentamicin, streptomycin, trimethoprim | [37] |
| Egypt | Raw dromedary camel milk | cefoxitin, erythromycin, novobiocin, piperacillin, rifampicin, rifamycin, streptomycin | [38] |
| Ethiopia | Chicken, goat, and beef meat | Ampicillin, chloramphenicol, erythromycin, gentamycin, streptomycin, tetracyclines, trimethoprim/sulfamethoxazole | [39] |
| Germany | Retail chicken meat | Ampicillin, cephalosporin, ciprofloxacin, nalidixic acid, streptomycin, tetracyclines, trimethoprim | [40] |
| Italy | Pig carcass | Ampicillin, chloramphenicol, gentamicin, streptomycin, tetracyclines, trimethoprim | [37] |
| South Africa | Raw meat | Ampicillin, ceftazidime, streptomycin, sulphafurazole, tetracyclines | [41] |
| Thailand | Broiler carcass and pig carcass | Ampicillin, cefpodoxime, ceftazidime, ciprofloxacin, gentamicin, sulfamethoxazole, tetracyclines, trimethoprim | [35] |
| United States | Dairy cattle | Azithromycin, ciprofloxacin, gamithromycin, tulathromycin | [42] |
| Vietnam | Retail raw foods (chicken, pork, fish, and shrimp) | Chloramphenicol, ciprofloxacin, gentamicin, nalidixic acid, streptomycin, tetracyclines, trimethoprim/sulfamethoxazole | [43] |
Table 2.
Salmonella spp. isolated from animal-derived foods and their resistance to antibiotics.
| Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|
| Brazil | Fresh tilapia fillets | amoxicillin/clavulanic acid, chloramphenicol, sulfonamide, tetracyclines | [44] |
| Cambodia | Retail poultry | Amoxicillin, cefalotin, chloramphenicol, cotrimoxazole, nalidixic acid, streptomycin, sulfonamide, tetracyclines, ticarcillin | [45] |
| China Egypt Iran |
Pork, chicken, duck, and fish | Ampicillin, streptomycin, tetracyclines | [46] |
| Chicken meat | Amoxicillin, ampicillin, erythromycin, nalidixic acid, oxytetracyclines, penicillin, sulfamethoxazole | [47] | |
| Chicken meat | Difloxacin, erythromycin, florfenicol, flumequine, lincomycin/spectinomycin, penicillin, tetracyclines, tiamulin, trimethoprim/sulfamethoxazole, tylosin | [48] | |
| Mexico | Ground beef | amoxicillin-clavulanic acid, carbenicillin, chloramphenicol, tetracyclines, trimethoprim-sulfamethoxazole | [49] |
| Thailand | Retail pork | Ampicillin, streptomycin, tetracyclines | [50] |
| United States | Retail meat | Amoxicillin-clavulanate, ampicillin, cefoxitin, ceftiofur, ceftriaxone, tetracyclines, gentamicin, streptomycin | [51] |
Table 3.
Staphylococcus spp. isolated from animal-derived foods and their resistance to antibiotics.
| Species | Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|---|
| Staphylococcus aureus | Brazil | Cow milk | ampicillin, cefoxitin, ceftiofur, clindamycin, erythromycin, oxacillin, penicillin, streptomycin, teicoplanin | [52] |
| China | Raw cow milk | Clindamycin/norfloxacin, erythromycin, gentamicin, tetracyclines, | [53] | |
| Japan | Raw cow milk | Ampicillin, oxacillin, cefazolin, enrofloxacin, gentamicin, kanamycin | [54] | |
| South Africa | Raw meat | Erythromycin, oxacillin/cefoxitin, penicillin, tetracyclines | [41] | |
| Thailand | Fresh pork | Ampicillin, tetracyclines, vancomycin | [55] | |
| United States | Pork, beef, turkey, and chicken | Clindamycin, dalfopristin/quinupristin, erythromycin, gentamicin, levofloxacin, mupirocin, oxacillin, penicillin, tetracyclines | [56] | |
| Methicillin-resistant S. aureus (MRSA) | Brazil | Cow milk | Ampicillin, erythromycin, oxacillin, penicillin, tetracyclines | [57] |
| China | Bovine milk | Amoxicillin, ampicillin, cefoxitin, ceftiofur, cefuroxime, ciprofloxacin, clarithromycin, clindamycin, penicillin, sulfadiazine sodium | [58] | |
| Denmark | Retail food products (chicken, turkey, and pork) | Macrolides, penicillin, tetracyclines | [59] | |
| Egypt | Retail chicken | Amikacin, amoxicillin, ampicillin, chloramphenicol, ciprofloxacin, cloxacillin, erythromycin, gentamicin, netilmicin, penicillin, rifampicin, streptomycin, sulfamethoxazole-trimethoprim, tetracyclines, vancomycin | [60] | |
| Iran | Raw meat (beef, sheep, and goat) | Amoxicillin-clavulanic acid, ampicillin, azithromycin, ceftriaxone, clindamycin, cotrimoxazole, erythromycin, gatifloxacin, lincomycin, minocycline, oxacillin, penicillin G, tetracyclines | [61] | |
| United States | Pork, beef, turkey, and chicken | Cefoxitin, clindamycin, dalfopristin/quinupristin, erythromycin, gentamicin, levofloxacin, oxacillin, penicillin, tetracyclines | [56] | |
| S. aureus and MRSA | China | Retail yak butter | amoxicillin/clavulanic acid, ampicillin, cefoperazone, cefoxitin, erythromycin, gentamicin, oxacillin, penicillin, sulfamethoxazole, tetracycliness, trimethoprim | [62] |
Table 4.
Listeria spp. isolated from animal-derived foods and their resistance to antibiotics.
| Species | Country | Sources | Antibiotic Resistance | References |
|---|---|---|---|---|
| Listeria spp. | Iran | Raw milk and traditional dairy products |
Amoxicillin/clavulanic acid, chloramphenicol, penicillin, tetracyclines | [63] |
| Spain | Meat and dairy products | Ciprofloxacin, clindamycin, tetracyclines | [64] | |
| Listeria monocytogenes | China | Pork, fish, sheep casing, chicken, and beef | Chloramphenicol, clindamycin, oxacillin, tetracyclines | [65] |
| Indonesia | Chicken carcass | Ampicillin, erythromycin, penicillin | [66] | |
| Japan | Chicken meat | Cefoxitin, clindamycin, flomoxef, fosfomycin, linezolid, oxacillin | [67] | |
| Poland | Ready-to-eat food (heat-treated sausages and delicatessen), raw meat, raw sausages, and seafood (Fish and shrimp). | Ceftriaxone, ciprofloxacin, clindamycin, gatifloxacin, gentamycin, linezolid, oxacillin, tetracyclines | [68] | |
| Romania | Ready-to-eat food (sausages and ham), minced pork, and cheeses | Benzylpenicillin, ciprofloxacin, clindamycin, fosfomycin, fusidic acid, imipenem, oxacillin, rifampin, tetracyclines, trimethoprim-sulfamethoxazole | [69] | |
| Turkey | Chicken meat and beef | Ampicillin, ceftriaxone, clindamycin, fusidic acid, penicillin | [70] |
3. EOs and Their Individual Compounds Derived from Common Aromatic Plants and Their Antibacterial Actions
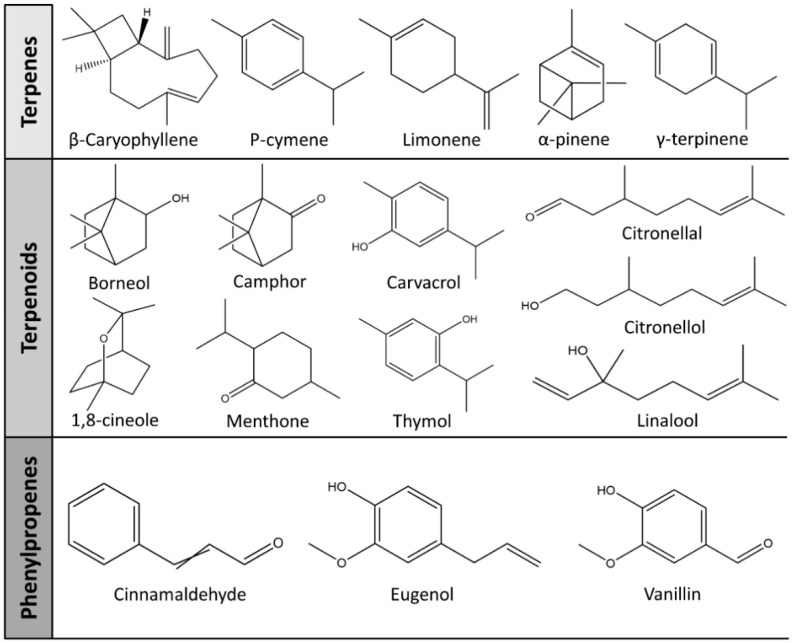
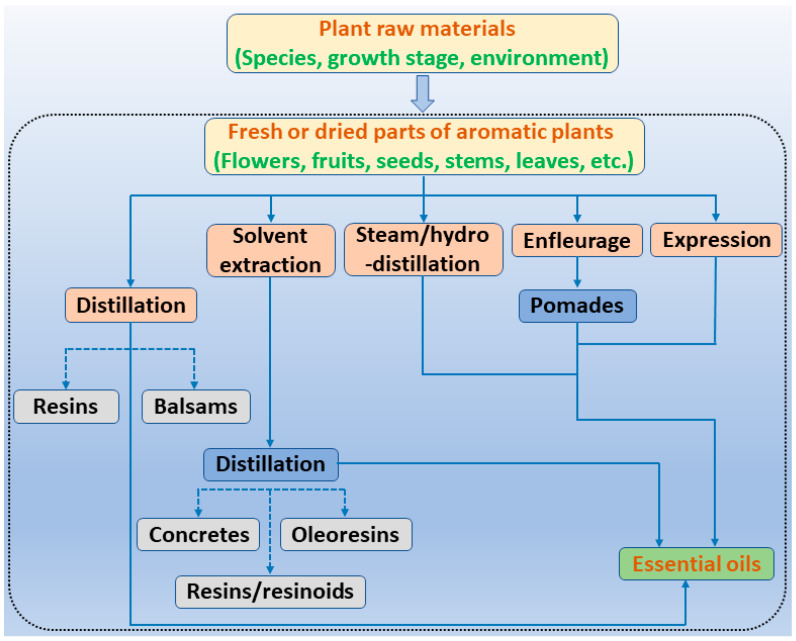
It has been reported that there are 3000 species of aromatic plants widely distributed in European countries along the Mediterranean coast, as well as in China, India, Central Asia, and South America [71], which are mainly concentrated in Apiaceae, Asteraceae, Lamiaceae, Lauraceae, Myrtaceae, Poaceae, Rutaceae, and Zingiberaceae. The most well-known species are from the genera Origanum, Rosmarinus, Thymus, and Ocimum, and all belong to the family Lamiaceae. These species are most commonly used to produce EOs due to the high content of aromatic compounds, where variable chemical compositions are divided into mainly three categories, namely terpenes, terpenoids, and phenylpropenes (Figure 1). The changes in various active ingredients and their content in EOs are mainly related to the raw materials of the plants and the extraction process (Figure 2). In general, two or three active ingredients in EOs have relatively high proportions, ranging from 20% to 70%, which contribute to the primary property of the mixture [8]. The utilization of EOs and their individual compounds in livestock production is considered a promising alternative to antibiotics for bacterial control. In the antibacterial evaluation system, the minimum inhibitory concentration (MIC) is an essential indicator for evaluating the antibacterial properties of EOs and their individual compounds. Briefly, EOs were dissolved at two-fold serial dilutions and the MIC was considered as the lowest concentration of EOs at which no visible bacterial growth was observed. Numerous in vitro studies have reported the antimicrobial properties of EOs and their individual compounds against common pathogens in animal product processing environments, including E. coli, Salmonella spp., Staphylococcus spp., and Listeria spp. Table 5 and Table 6 summarize the antimicrobial activity of EOs and their active components from aromatic plants according to the MIC values.
Figure 1.
Chemical structures of major components in aromatic plant-derived EOs.
Figure 2.
The extraction process of aromatic plant-derived EOs (adapted from [72]).
Table 5.
Essential oils (EOs) derived from aromatic plants and their antibacterial properties according to their minimum inhibitory concentration (MIC) values.
| Family | Latin Name | Part Used | Extraction Method | Location | Main Constituents | Target Bacteria | Doses | MIC | References |
|---|---|---|---|---|---|---|---|---|---|
| Apiaceae | Carum carvi L. | Seeds | Hydro-distillation | Kelibia | γ-terpinene (31.03%), β-pinene (18.77%), p-cymene (17.16) | E. coli, S. aureus, S. Typhimurium, Listeria monocytogenes | - | 0.469 mg/mL (E. coli, L. monocytogenes), 0.117 mg/mL (S. aureus), 0.234 mg/mL (S. Typhimurium) | [73] |
| Coriandrum sativum L. | Seeds | Hydro-distillation | Kelibia | Linalool (76.41), γ-terpinene (5.35%), α-pinene (4.44%) | E. coli, S. aureus, S. Typhimurium, L. monocytogenes | - | 0.938 mg/mL (E. coli, L. monocytogenes, S. Typhimurium), 0.234 mg/mL (S. aureus) | [73] | |
| Foeniculum vulgare Mill. | Seeds | Hydro-distillation | India | trans-anethole (50.4%), methyl chavicol (22.4%), limonene (11.4%) | E. coli, S. Typhimurium | 0.0075–2.0% (v/v) | 0.062% (E. coli), 0.031% (S. Typhimurium) (v/v) | [74] | |
| Asteraceae | Achillea millefolium L. | Inflorescence, leaves, whole aerial parts | Hydro-distillation | India | Borneol (4.7–24.9%), sabinene (4.0–38.9%), germacrene D (1.1–46.6%) | S. aureus, S. epidermidis, Klebsiella pneumoniae | - | 125–500 μg/mL | [75] |
| Helichrysum italicum (Roth) G. Don | Inflo-rescence | Hydro-distillation | Central Europe | Neryl acetate (16.38%), nerol (15.73%), geraniol (6.32%) | E. coli, S. aureus, Pseudomonas aeruginosa | - | 64 mg/mL (E. coli, P. aeruginosa), 1 mg/mL (S. aureus) | [76] | |
| Helichrysum microphyllum subsp. tyrrhenicum | - | Hydro-distillation | Iglesias | γ-curcumene (28.94%), linalool (14.21%), 5-eudesmen-11-ol (9.81%) |
E. coli, S. aureus, P. aeruginosa | 0.063–4 mg/mL | >4 mg/mL (E. coli, P. aeruginosa), 2 mg/mL (S. aureus) | [77] | |
| Lamiaceae | Origanum vulgare L. spp. |
O. vulgare L. ssp. virens |
n-Hexane hydro-distillation | Southern Italy | Carvacrol (63.8%), γ-terpinene (7.4%), p-cymene (6.7%) | E. coli, S. aureus, S. Typhi | 0.8–100 μg/mL | 50 μg/mL (E. coli, S. aureus), 100 μg/mL (S. Typhi) | [78] |
| Rosmarinus officinalis L. | Air-dried leaves | Steam distillation | Taizhou, Zhejiang | 1,8-Cineole (26.54%), α-pinene (20.14%), camphor (12.88%), camphene (11.88%) | S. aureus, S. epidermidis, Bacillus subtilis | 0.2–4% (v/v) | 0.03–1.0% (v/v) | [79] | |
| Thymus vulgaris | Dried leaves | Hydro-distillation | North Yemen | Thymol (51.34%), p-cymene (18.35%), caryophyllene (4.26%), α-pinene (2.95%) | E. coli, S. aureus, B. subtilis, Mycobacterium smegmatis | 0.01–30 mg/mL | 0.075–1.1 mg/mL | [80] | |
| Mentha pulegium L. | Air-dried leaves | Steam distillation | Algerian | Pulegone (70.66%), neo-menthol (11.21%), menthone (2.63%) | E. coli, S. aureus, B. subtilis | 0.3–20 μL/mL | 1.25–10 μL/mL | [81] | |
| Ocimum basilicum L. | Leaves | Steam distillation | Ponta Grossa, Brazil | Linalool (55.2%), 1,8-cineole (8.8%), α-trans-bergamotene (7.0%), eugenol (3.2%) | S. aureus | 2–1024 μg/mL | 1024 μg/mL | [82] | |
| Lavandula x intermedia (lavandin) ‘Grosso’ | Flowers, stems | Steam distillation | Lazio Region, Italy | Linalool (35.8%), 1,8-cineole (19.8%), α-pinene (8.7%) | E. coli, B. cereus | - | 1.87% (E. coli), 0.94% (B. cereus) (v/v) |
[83] | |
| Chenopodium ambrosioides L. | Leaves | Hydro-distillation | Crato, Brazil | α-terpinene (40.73%), p-cymene (21.81%), trans-ascaridol (12.48%) | S. aureus | 0.5–1024 μg/mL | ≥1024 μg/mL | [84] | |
| Lauraceae | Cinnamomum cassia Blume | - | Hydro-distillation | China | Cinnamaldehyde (85.06%) | E. coli, S. aureus, P. aeruginosa, Proteus vulgaris, Enterobacter aerogenes, Vibrio parahaemolyticus, V. cholerae | - | 75–600 μg/mL | [85] |
| Cinnamomum camphora var. linaloofera Fujita | - | - | Guangzhou | Linalool (69.94%), camphor (10.90%), nerolidol (10.92%), safrole (8.24%) | E. coli | - | 0.2 μL/mL | [86] | |
| Litsea cubeba (Lour.) Pers. | - | - | Caussols plateau, France | β-Citral (39.25%), α-citral (30.90%), limonene (8.28%), trans-verbenol (4.18%) | MRSA | 0.125–4 mg/mL | 0.5 mg/mL | [87] | |
| Myrtaceae | Eucalyptus globulus L. | Aerial parts | Hydro-distillation | Takelsa | p-cymene (12.58–37.82%), α-pinene (10.41–13.39%), 1,8-cineole (7.71–13.23%), γ-terpinene (2.94–10.57%) | S. aureus, MRSA, B. cereus | - | 1–4 mg/mL | [88] |
| Syzygium aromaticum | Fresh leaves | Steam distillation | Nitra, Slovakia | Eugenol (82.4%), (E)-caryophyllene (14.0%), α-humulene (1.8%) | S. aureus | 0.2–400 μL/mL | 93.35 μL/mL | [89] | |
| Poaceae | Cymbopogon nardus | Leaves | Cleavenge hydro-distillation | Ceara’, Brazil | Geraniol (33.88%), citronellal (27.55%), citronellol (14.40%), carvone (10.06%) | S. aureus, E. coli | 0.125–8 mg/mL | 0.5 mg/mL (S. aureus), >8 mg/mL (E. coli) | [90] |
| Rutaceae | Citrus limon L. Burm. | Peels | - | Sichuan Province | Limonene (48.48%), β-terpinene (17.08%), 4-carene (8.46%) |
S. mutans | 2.25–9 mg/mL | 4.5 mg/mL | [91] |
| Zingiberaceae | Alpinia pahangensis Ridl. | Rhizomes | Hydro-distillation | Pahang, Peninsular Malaysia | γ-selinene (11.60%), β-pinene (10.87%), (E,E)-farnesyl acetate (8.65%), α-terpineol (6.38%) | S. aureus | 0.039–5 mg/mL | <0.31 mg/mL | [92] |
Table 6.
Individual compounds of common EOs and their antibacterial properties according to their MIC values.
| Item | Individual Compounds | Chemical Structures | Target Bacteria | Doses | MIC | References |
|---|---|---|---|---|---|---|
| Terpenes | β-Caryophyllene |
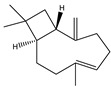
|
E. coli, S. aureus | 0.1–4 mg/mL | >4 mg/mL | [93] |
| Limonene |
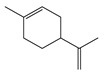
|
E. coli, S. aureus, S. Typhimurium, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL (E. coli, S. aureus, B. cereus), 0.06 mg/mL (S. Typhimurium) | [94] | |
| Terpenoids | Borneol |
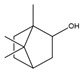
|
E. coli, S. Typhimurium, S. aureus, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL (E. coli), 0.03 mg/mL (S. aureus), 0.12 mg/mL (B. cereus, S. Typhimurium) | [94] |
| Camphor |
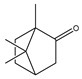
|
E. coli, S. Typhimurium, S. aureus, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL (E. coli, S. Typhimurium, B. cereus), 0.015 mg/mL (S. aureus) | [94] | |
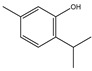
| Carvacrol |
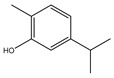
|
E. coli, MRSA, S. mutans, Aggregatibacter actinomycetemcomitans | - | 0.4 mg/mL (E. coli, MRSA, S. mutans), 0.2 mg/mL (A. actinomycetemcomitans) | [95] | |
| E. coli, Salmonella spp., Clostridium perfringens | 0.075–2 mg/mL | >0.6 mg/mL | [96] | |||
| S. aureus | 0.05–3.2 mg/mL | >0.4 mg/mL | [97] | |||
| Citral |
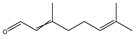
|
E. coli, S. Typhimurium, S. aureus, B. cereus | 0.002–0.25 mg/mL | 0.06 mg/mL (E. coli, S. aureus, B. cereus), 0.07 mg/mL (S. Typhimurium) | [94] | |
| Citronellal |
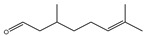
|
E. coli, S. aureus | - | 0.3 mg/mL (E. coli), 0.4 mg/mL (S. aureus) | [98] | |
| Citronellol |
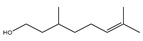
|
E. coli, S. aureus | - | 0.005 mg/mL (E. coli), 0.375 mg/mL (S. aureus) | [98] | |
| Farnesol |
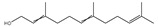
|
Cutibacterium acnes | 0.004–0.576 μmol/mL | 0.14 μmol/mL | [99] | |
| trans-Geraniol |
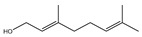
|
E. coli, S. aureus, S. Typhimurium, B. cereus | 0.002–0.25 mg/mL | 0.06 mg/mL (E. coli), 0.03 mg/mL (S. aureus, S. Typhimurium), 0.07 mg/mL (B. cereus) | [94] | |
| Linalool |
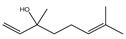
|
E. coli, S. aureus, S. Typhimurium, B. cereus | 0.002–0.25 mg/mL | 0.25 mg/mL | [94] | |
| Menthone |
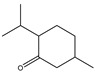
|
K. pneumoniae | - | 224 mg/mL | [100] | |
| Nerolidol |
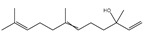
|
S. aureus, K. pneumonia, P. aeruginosa | - | 2 mg/mL (S. aureus), 0.5 mg/mL (K. pneumonia, P. aeruginosa) | [101] | |
| Thymol |
|
E. coli, MRSA, A. actinomycetemcomitans, S. mutans | - | 0.2 mg/mL (E. coli, MRSA, S. mutans), 0.1 mg/mL (A. actinomycetemcomitans) | [95] | |
| E. coli, C. perfringens, Salmonella spp. | 0.075–2 mg/mL | >1.2 μL/mL | [96] | |||
| S. aureus | 0.05–3.2 mg/mL | >0.8 mg/mL | [97] | |||
| Phenylpropanoids | Cinnamaldehyde |
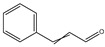
|
E. coli, S. aureus, B. cereus, Yersinia enterocolitica | - | 5 mg/mL (E. coli), 1.875 mg/mL (S. aureus), 2 mg/mL (B. cereus), 5 mg/mL (Yersinia enterocolitica) | [102] |
| E. coli, C. perfringens, Salmonella spp. | 0.075–2 mg/mL | >0.6 μL/mL (E. coli, Salmonella spp.), >0.3 μL/mL (C. perfringens) | [96] | |||
| Eugenol |
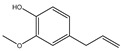
|
E. coli, S. aureus | 0.1–4 mg/mL | 0.4 mg/mL (E. coli), 1.3 mg/mL (S. aureus) | [93] | |
| Campylobacter spp. | - | 0.5 mg/mL | [103] |
The unique antimicrobial effects of plant EOs depend on their active components and are associated with the functional groups and structural arrangement of their active molecules, while different chemical components often have a synergistic antibacterial effect. Among the main components of EOs, phenols and aldehydes have the most potent antimicrobial activity, followed by alcohols, ketones, esters, and hydrocarbons [104]. Previous studies have shown that lipophilicity and the existence of phenolic hydroxyl, methoxy, and olefin bonds play a vital role in the antibacterial ability of active compounds in EOs, because these functional groups could consume proton motive force, affect intracellular pH value, and disrupt the oxidative phosphorylation of bacteria [105]. Many individual components of EOs with the essential functional groups mentioned above, including carvacrol, thymol, cinnamaldehyde, and eugenol have significant bactericidal activity. Therefore, EOs derived from aromatic plants, including oregano, thyme, cinnamon, and clove have potent antibacterial activity due to their high content of these compounds.
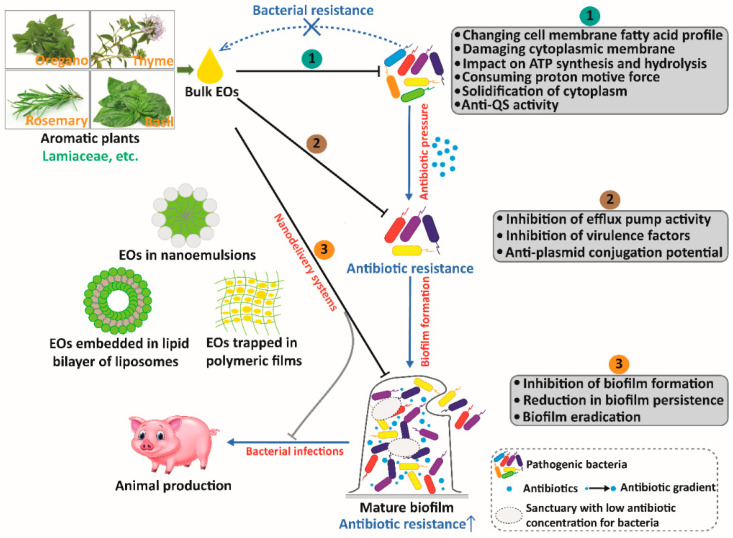
EOs have different actions against bacteria. Most EOs target bacterial cell walls, which also explains that EOs have a better ability to suppress gram-positive bacteria compared to gram-negative bacteria [106,107]. EOs and their components also change the fatty acid profile of the cell membrane, damage the cytoplasmic membrane, consume the proton motive force, reduce the synthesis of adenosine triphosphate (ATP) and increase ATP hydrolysis, and decrease membrane potential [108,109,110,111,112,113]. The hydrophobicity of EOs could increase membrane permeability, which further results in the leakage of bacterial cell content, including potassium ions and genetic materials [114,115]. For example, Origanum compactum EO (mainly carvacrol, thymol, and p-cymene) could alter the integrity of the cell membrane and increase the permeability of the membrane, leading to the leakage of genetic materials in Bacillus subtilis [114]. In some instances, EOs could also alter membrane permeability by disrupting electron transport systems [116]. Some EOs, especially those rich in phenolic compounds, can enter the phospholipid bilayer of cell membranes and interact with membrane proteins to disrupt the normal physiological activities of bacteria [108]. Alterations in membrane permeability and the disruption of molecular and ion transportation leads to imbalances within bacterial cells, which could induce the denaturation of cellular enzymes and proteins, leakage of ions and metabolites, and the solidification of the cytoplasm [117]. ATP is important for bacterial respiration and metabolism, and could be influenced by EOs and their components. For example, cinnamon oil and its active compound, cinnamaldehyde, decrease intracellular ATP levels in Mycobacterium avium subsp. paratuberculosis [118]. Quorum sensing (QS) is an intercellular communication system that allows bacteria to secrete and detect external signaling molecules, which could promote the development of virulence factors and biofilms and the production of secondary metabolites. Gram-positive bacteria use auto-inducing peptides (AIPs) for signaling, whereas gram-negative bacteria use N-acyl-homoserine lactones (AHLs). Terpenes and phenylpropenes such as carvacrol, thymol, eugenol, and cinnamaldehyde have anti-biofilm and anti-QS properties against bacteria [119]. Carvacrol and thymol have been shown to inhibit new and existing biofilms of pathogenic bacteria, such as L. monocytogenes and Pseudomonas aeruginosa [120,121]. Cinnamaldehyde could suppress biofilm formation in pathogenic bacteria, including Staphylococcus epidermidis, L. monocytogenes, and Cronobacter sakazakii [121,122,123]. Eugenol could suppress the production of QS-regulated violacein in Chromobacterium violaceum and virulence factors in P. aeruginosa [124]. Several aromatic plant-derived EO components have specific anti-QS strategies through binding to LuxR-type AHL receptor proteins and LuxI-type AHL synthases, which are present in terpenes and phenylpropenes, including carvacrol, thymol, cinnamaldehyde, and eugenol [125]. Collectively, the antibacterial activity of EOs may not rely on a single mechanism due to the complexity of the active components, so pathogenic bacteria are less likely to develop resistance to EOs.
Given the above antibacterial mechanisms, EOs have the potential to replace AGPs in animal production. Interestingly, several studies have shown that EOs and their components have synergistic effects on controlling resistant bacteria when combined with antibiotics. Origanum vulgare EO (mainly carvacrol, β-caryophyllene, and γ-terpinene) has synergistic effects against multidrug-resistant Acinetobacter baumannii when combined with polymyxin B [126]. Thymus zygis EO (mainly thymol, carvacrol, and p-cymene) has synergistic effects with ciprofloxacin, ampicillin, or vancomycin against S. aureus, and could change the phenotype from antibiotic resistance to antibiotic susceptibility [127]. Carvacrol, thymol, eugenol, and α-pinene have shown synergistic interactions with tetracyclines and gentamicin against pathogenic bacteria, including E. coli, methicillin-resistant S. aureus, and P. aeruginosa [128]. Moreover, carvacrol has synergistic effects on suppressing erythromycin-resistant Group A Streptococci when combined with erythromycin [129]. Cinnamaldehyde synergistically increases the antibiotic susceptibility of E. coli to tetracyclines and erythromycin [130]. Therefore, in addition to being used as antibiotic alternatives to boost animal production, EOs could prevent the development of bacterial resistance, which has important implications for animal production and human health. In the following content, this review summarizes antibiotic resistance genes (ARGs) and the effects of EOs and their components on antibiotic resistance.
4. Antibiotic Resistance Genes and the Impact of EOs and Their Individual Compounds on Antibiotic Resistance
Food-producing animals are a major source of ARGs. ARGs can be transferred to humans primarily through the ingestion of animal-derived foods [131]. Many genes associated with bacterial resistance are present in chromosomes and certain plasmids [132]. Numerous studies reported ARGs of foodborne pathogens, such as E. coli, Salmonella spp., Staphylococcus spp., and Listeria spp. ARGs and their resistance to the AGPs of E. coli, Salmonella spp., Staphylococcus spp., and Listeria spp. are shown in Table 7 [31,35,38,60,133,134,135,136,137,138,139,140,141,142,143,144]. According to the data, different species have their own ARGs. Notably, shared genes between different species may result from interspecific communication of ARGs among bacteria [145].
Table 7.
Summary of antibiotic resistance genes and corresponding resistance phenotype in E. coli, Salmonella spp., Staphylococcus spp., and Listeria spp.
| Species | Resistance Genes and Types of Antibiotics or Antimicrobial Groups | References |
|---|---|---|
| E. coli | blaTEM, blaOXA-1: ampicillin, cefotaxime; cat1, cat2, cmlA: chloramphenicol; sul1, sul2, folP: sulfonamides; tet(A), tet(B): tetracyclines; aphA1, aphA2: kanamycin; aadA1: streptomycin; aac(3)-IV: gentamicin; gyrA (Ser83Leu, Asp87Asn), gyrB (Asp426Asn), parC (Ser80Ile), parE (Leu445His): quinolone; pmrA (Arg81His, Glu106Ala), pmrB (Gly206Arg, Tyr222His): colistin; rpoB (Ile572Phe): rifamycin; 23S: macrolides; 16S rrsB: gentamicin, spectinomycin, tetracyclines; 16S rrsH: spectinomycin | [31,35,38,133,134] |
| Salmonella spp. | blaTEM-1, blaTEM-117, blaTEM-135, blaCTX-M-9, blaCTX-M55, blaCYM-2: β-lactams; gyrA (Ser83Tyr, Asp87Asn), gyrB (Tyr420Cys), parC (Ser80Arg), parE (Ser458Pro): quinolone; pmrB (Val164Met, Arg92Pro): colistin; sul1, sul2, sul3: sulfonamides; tet(A), tet(B), tet(C), tet(G), tet(M), tet(R): tetracyclines; dfrA1, dfrA12, dfrA14: trimethoprim; floR, cmlA1: chloramphenicol; aac(6′)-I, strA, strB, aadA (ant(3”)Ia), aac3-VIa, aph(3′)Ib, aphA (aph(3′)IIa), aac3-IId, aadB (ant(2”)Ia), aac-IVa, aph(4)Ia: aminoglycosides; fosA2: fosfomycin; mph(A), ere(A), mef(B): macrolides; arr2: rifampicin | [135,136,137,138] |
| Staphylococcus spp. | blaZ, mecA, mecC: β-lactams; erm(A), erm(C): erythromycin, clindamycin; mphC, msrA: erythromycin; aacA-aphD: gentamicin; aadD: tobramycin; fusB, fusC: fusidic acid; tet(K), tet(L), tet(M): tetracyclines; fexA: chloramphenicol; fosB: fosfomycin; inuA: lincomycin; vanA: vancomycin; msr(A), mph(C): macrolides, lincosamides, streptogramins | [60,139,140,141] |
| Listeria spp. | blaTEM, blaCTX-M-9: β-lactams; tet(A), tet(B), tet(C), tet(M), tet(O), tet(S): tetracyclines; strA, aadA, aadB, ant6: aminoglycosides; dfrD: trimethoprim; sul1, sul2: sulfonamides; erm(B): macrolides; fos(X), vga(D): lincosamides | [142,143,144] |
As mentioned earlier, EOs and their components have synergistic effects against resistant bacteria when combined with antibiotics, indicating that EOs and their individual compounds may increase antibiotic susceptibility of drug-resistant bacteria. The determinant of multidrug resistance is through the increased expression of the efflux pump genes, which could lead to a decrease in the antibiotic concentration of bacteria and an increase in the MIC values of antibiotics [146]. The efflux pump is a transport protein associated with intercellular communication and biofilm formation, which could protect bacteria by pumping large amounts of AGPs out of cells [147]. Recently, several studies have reported that EOs and their individual compounds could suppress the activities of bacterial efflux pumps [148]. For example, Origanum vulgare EO (pulegone, 1,8-cineole, and borneol) and Thymus daenensis EO (carvacrol, γ-terpinene, and α-terpinene) could inhibit the activity of the PmrA efflux pump at sub-MIC levels against fluoroquinolone-resistant Streptococcus pneumoniae [149]. Satureja khuzistanica EO (mainly thymol and carvacrol) has been shown to reduce the expression of mexY and mexE efflux pump genes in P. aeruginosa [150]. Rosmarinus officinalis EO (mainly α-pinene, γ-sitosterol, and 1,8-cineole) could decrease the expression of the blaTEM efflux pump gene in A. baumannii and P. aeruginosa, and the blaOXA-23 efflux pump gene in A. baumannii [151]. Chenopodium ambrosioides EO (mainly α-terpinene, p-cymene, and ascaridole) and its main constituent, α-terpinene could suppress the activity of the TetK and NorA efflux pumps in S. aureus [152]. Similarly, Salvia fruticosa EO could markedly inhibit the activity of the TetK efflux pump in tetracyclines-resistant S. epidermidis [153]. Piper caldense EO (mainly caryophyllene oxide, δ-cadinene, and spathulenol) has the potential to suppress the MepA, NorA, and QacC efflux pumps of multidrug-resistant S. aureus [154]. The EtBr efflux inhibition analysis has been used to evaluate the inhibitory activity of EOs and their components in efflux pumps. The EtBr efflux inhibition assay showed that Cuminum cyminum EO (mainly cuminic aldehyde, γ-terpinene, α,β-dihydroxyethylbenzene, 2-caren-10-al, and β-pinene) could significantly inhibit the activity of the NorA efflux pump in S. aureus [155]. For the main active components of EOs, carvacrol, thymol, and eugenol could inhibit EtBr efflux through active pumps from E. coli, S. Typhimurium, S. Enteritidis, and S. aureus [156].
In addition to inhibiting the activities of efflux pumps in bacteria, some EOs and their components could inhibit gene expressions related to virulence factors and have anti-plasmid conjugation potential for bacteria. Cinnamomum camphora EO (mainly linalool, cineole, and sabenene) could inhibit the expressions of QS-regulated virulence genes such as lasA, lasB, pilE3, vioA, vioB, vioC, vioD, vioE, and hmsHNFR in Chromobacterium violaceum [157]. Lemongrass EO (mainly geranial, neral, limonene, and geraniol) could down-regulate the expression of genes related to virulence factors such as hly, inlB, inlC, inlJ, plcA, plcB, and lmo2470 in L. monocytogenes [158]. Carvacrol, an important component of Origanum vulgare EO, has been shown to down-regulate the expression of genes related to virulence factors such as ctxB, hlyA, tcpA, and toxT in Vibrio cholerae [159]. Eugenol could decrease the content of virulence factors, including rhamnolipid and pyocyanin, and inhibit related gene expression such as rhlA in P. aeruginosa [160]. Moreover, the single constituents of Thymus vulgaris EO, including thymol, linalool, R-carvone, eugenol, eucalyptol, S-carvone, and borneol, have anti-plasmid conjugation potential such as decreasing the transfer of plasmid pKM101, which could reduce virulence and spread of resistance in E. coli [161].
Given that animal-derived foods are one of the main sources of ARGs, some EOs and their components could not only replace antibiotics to improve animal performance and gut health, but may also have great potential to alleviate the widespread problem of antibiotic resistance in animal production. However, much research is needed in the future to study and elucidate this strategy.
5. Nanoencapsulated EOs as a Promising Option for Animal Production against Antibiotic Resistance
Most bacteria grow as single planktonic cells or communities within the biofilm. As the main form of bacterial survival, the biofilm is a bacterial community encased in a self-generated extracellular polymer matrix that provides the community with a variety of competitive advantages, including increased resistance to various stress stimuli [162]. Moreover, bacterial biofilms facilitate horizontal gene transfer through the exchange of genome fragments and mobile genetic elements in bacteria, which could contribute to the spread of ARGs [163]. The extreme tolerance of bacterial biofilms to antibiotics is particularly problematic because it makes it more challenging to fight antibiotic-resistant bacteria. Mixed bacterial biofilms have been observed in intestinal diseases, most of which are pathogenic. Biofilm-related pathogens have become a severe problem not only in humans but also in animal production. In host-microbiota interactions, bowel biofilms play a critical role in the pathogenesis of inflammatory bowel disease (IBD) and other infectious diseases in humans [164]. An invasive biofilm, which harbors the opportunistic pathogen Bacteroides fragilis as a crucial species, has been shown to be detected in patients with IBD [165]. Biofilm formations have also been observed in common antibiotic-resistant foodborne bacteria such as E. coli, S. aureus, S. Typhimurium, and L. monocytogenes [166,167]. The SslE protein, secreted by E. coli, degrades intestinal mucins, including MUC2, MUC3, and MUC5AC and accelerates biofilm maturation, which is a significant factor in the infection process of highly virulent species [167]. Enterotoxigenic E. coli, a virulent strain, causes severe infections and diarrheal diseases in animals, including weaned pigs, and is closely related to increased mortality and severe impairments in production [20]. S. Typhimurium could decrease the expressions of Occludin and Claudin-1 and subsequently disrupt the intestinal epithelial barrier in broiler chickens [168]. Peptidoglycan from S. aureus induces intestinal inflammation and disrupts intestinal barrier functions through TLR2-regulated activation of the NF-κB pathway in porcine jejunal epithelial cells [169]. During foodborne infection, L. monocytogenes cross the intestinal mucosal barrier via Listeria adhesion protein, which could break down the epithelial tight junction barrier for bacteria to enter the lamina propria [170]. The loss of a critical protective barrier facilitates the migration of pathogenic bacteria across the epithelial barrier and biofilm information [171]. Furthermore, biofilms provide a favorable environment for pathogens to escape host defenses, further promoting the occurrence and progression of intestinal diseases [172]. Collectively, bowel biofilms dominated by antibiotic-resistant foodborne bacteria are of great significance in the development of intestinal disorders and the transition to the pathogenic microbiota, which could further induce food safety problems of animal origin and harm public health.
Concerns about the potential for antibiotic resistance to transfer from animal intestinal bacteria to humans through the consumption of animal-derived foods calls for alternatives to antibiotics in animal production. This review focuses on the advance of novel antibacterial agents, particularly those effective against the strong resistance of bacterial biofilms. EOs and their individual compounds are effective against resistant bacterial infections and are expected to decrease the selection and spread of AGRs [173]. As the main form of bacterial survival, bacterial biofilm information could increase antibiotic resistance by 10–1000 than their planktonic counterpart, which is the main cause of bacterial resistance [174,175]. However, the lipophilic properties of EOs and their individual compounds generally severely limit their applications due to their low permeability and poor absorption under aqueous biological and non-biological conditions such as biofilm matrices [176]. Therefore, the regular applications of EOs and their individual compounds may be ineffective against resistant bacterial infections because of their poor permeability to the biofilm matrix and extracellular polymer materials. The limitation could be overcome by developing nanoencapsulation methods, including polymeric nanocapsules, nanostructured lipid particles, liposomes and nanoemulsions, and other nanosystems [176]. Biomolecule-based nanoencapsulation can be designed and engineered for antimicrobial agents to surmount current and classical challenges, including the emergence of multidrug-resistant bacteria, the inefficiency and applicability limitations of existing antimicrobial agents, and biofilm formation [177]. Nanoencapsulated EOs and their individual components could make the whole formation water-soluble, allowing nanosystems to easily penetrate water-filled channels and the cavities of bacterial biofilms [178]. In addition, nanodelivery systems could sustain and control EO release at the site of action and mask unpleasant tastes or odors of EOs to minimize unacceptable organoleptic effects [178,179]. Numerous studies have reported different nanocarrier systems with encapsulated common EOs and their individual components, including oregano oil, cinnamon oil, thyme oil, carvacrol, cinnamaldehyde, thymol, and eugenol (Table 8). These in vitro results summarize that nanodelivery systems containing EOs and their components as novel antibacterial agents could suppress biofilm formation and combat bacteria within mature biofilms on biotic and abiotic surfaces, indicating that nanoencapsulated EOs may be a prospective approach for controlling resistant bacterial biofilm-related infections and overcoming existing antibiotic resistance in animal production.
Table 8.
Nanoencapsulated EOs and individual compounds with anti-biofilm activity.
| Essential Oils or Components | Emulsifier/Carrier System | Target Bacteria | Antibacterial Effects | References |
|---|---|---|---|---|
| Carvacrol | Polylactic acid nanoemulsions | E. coli, MRSA, Acinetobacter baumannii | Biofilm eradication | [180] |
| Cinnamon oil | Liposomes (average particle size: 144.3 nm) | MRSA | Biofilm eradication | [181] |
| Cinnamon oil, eucalyptus oil, orange oil | Mesoporous silica nanoparticles | S. aureus, E. coli | Inhibition of biofilm formation | [175] |
| Citral | Nanoemulsions (tween 80) | L. monocytogenes | Inhibition of biofilm formation | [182] |
| Eucalyptus oil | Silica nanoparticles | E. coli | Biofilm eradication | [164] |
| Eugenol | Nanoemulsions (tween 80, medium-chain triglyceride) | P. aeruginosa | Inhibition of biofilm formation | [45] |
| Lemongrass oil | Nanoemulsions (tween 80) | Enterococcus faecalis | Inhibition of biofilm formation | [183] |
| Limonene | Nanoemulsions (tween 80, propylene glycol) | MRSA | Reduction in biofilm persistence | [184] |
| Mandarin oil | Chitosan nanoparticles | S. aureus, E. coli | Inhibition of biofilm formation | [185] |
| Oregano oil | Biological silver nanoparticles | S. aureus | Decrease in cell density and exopolysaccharide matrix | [186] |
| Peppermint oil, cinnamaldehyde | Silica nanocapsules | E. coli, S. aureus, P. aeruginosa | Biofilm eradication | [187] |
| Tea tree oil | Nanostructured lipid carriers (average particle size: 166 nm) | P. aeruginosa | Decrease in adhesion and inhibition of biofilm formation | [188] |
| Thyme oil | Nanoarchaeosomes (made by soybean phosphatidylcholine, total polar archaeolipids and polysorbate 80), nanoliposomes (made by soybean phosphatidylcholine and polysorbate 80) | S. aureus | Biofilm eradication, inhibition of biofilm formation | [189] |
| Thyme oil, thymol | Chitosan nanoemulsions | S. aureus, E. coli | Inhibition of biofilm formation | [190] |
In animal-derived foods, chitosan nanoparticles containing mandarin EO could inhibit biofilm formation and destroy mature biofilms of E. coli and S. aureus, as well as have great potential for pork preservation [185]. Moreover, nanoencapsulated EOs used directly in animal food have been shown to be effective in reducing the rate of foodborne bacterial infections in animals. For in vivo studies, thymol nanoemulsion has been shown to upregulate the gene expression of IgA, MUC2, IL-10, and FABP2, and downregulate the gene expression of the vital virulence gene invA in a broiler chicken after a S. Typhimurium infection [191]. Chitosan nanoencapsulated thyme and cinnamon EOs could more effectively improve breast percentage, increase serum IgM and IgY contents, and improve intestinal Lactobacillus spp. abundances in broiler chickens compared with free EOs [17], with chitosan having its own benefits on growth rate due to its antioxidant and antibacterial properties and increased ileal digestibility of dry matter [192]. Chitosan nanoencapsulated garlic EO enhanced more evaluated parameters, including body weight gain, feed conversion ratio, intestinal MUC2 gene expression, and the Lactobacilli population in broilers compared with free garlic EO [193]. Thyme EO loaded in chitosan nanoparticles could more effectively improve the feed conversion ratio and decrease the number of coliform and total aerobic bacteria in broilers compared to unencapsulated thyme EO [194]. Cumin EO in chitosan nanoparticles could more effectively improve growth performance, MUC2 gene expression and sustain broiler immune responses compared with free-form cumin EO [195]. Together, EOs and plant extracts are mainly encapsulated bioactive substances and phytochemicals used in animal diets, and chitosan was found to be the most effective nanocarrier to load EOs and plant extracts [196]. Nanoparticles and nanocapsules are frequently studied nanocarriers, most of which are processed by the ionotropic/ionic gelation. However, nanofibers, nanohydrogels, and nanoemulsions have not been found yet for their application in feed bioactive substances. These nanocarriers have improved protection, stability, and controlled release of feed bioactive substances, which provides additional nutrition for the growth performance of livestock regardless of the low stability and water solubility of bioactive substances. However, like other emerging technologies, nanocarriers may threaten the health of animals and, ultimately, human consumers. The physicochemical properties of nanocarriers allow them to penetrate the physical barriers of enterocytes and put the animal at risk of gastrointestinal disease. At the same time, we lack a fundamental understanding of the behavior of nanocarriers in the biological system in terms of in vivo distribution at the cellular and organ levels. In addition, one of the biggest obstacles to commercializing nanoencapsulation technology in animal feed is legislation. Despite promising, more quantitative and in vivo studies should be performed before the commercial application of nanoencapsulated EOs as antibiotic alternatives.
6. Conclusions
Although dietary supplementation with AGPs at sub-therapeutic levels is an effective way to improve performance and prevent bacterial infections in animals, the abuse of AGPs could induce public risks, environmental contamination, and the diffusion of antibiotic-resistant bacteria. The prohibition of AGPs in feed is associated with many challenges in animal production, such as poor growth performance and severe intestinal diseases. Due to their powerful antibacterial properties, EOs and their individual compounds have emerged as novel antibiotic alternatives to combat bacterial infections. The successful application of EOs and their individual compounds is based on whether our understanding of how EOs work is based on sufficient research. As shown in Figure 3, this article reviews foodborne pathogenic bacteria and antibiotic resistance, and the impacts of EOs and their individual compounds on foodborne pathogenic bacteria and ARGs. In addition, nanotechnology provides a promising tool for the delivery of EOs and their individual compounds to the gut and for enhancing the effectiveness of EOs and their components in animal production. It should be noted that evidence for a link between EOs and antibiotic-resistant foodborne bacteria in animal production is incomplete, as in vitro studies could not directly demonstrate the impact of EOs and their individual compounds on alleviating antibiotic resistance in livestock production. Therefore, it is necessary to establish and strengthen extensive cooperation between academic research and the livestock industry, to meet the needs of experimental research, and to clarify the precise application mode and benefits of EOs and their individual compounds in animal production in a timely manner.
Figure 3.
Summary of the potential of aromatic plant-derived EOs as antibacterial agents in animal production against antibiotic resistance.
Author Contributions
L.Z. wrote the manuscript. F.G., J.G., H.L., F.X., H.B., X.P. and L.S. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data is contained within the article.
Conflicts of Interest
We declare that we have no conflict of interest.
Funding Statement
This research was supported by grants from the National Key R&D Program of China (2019YFD1002701) and the Key Research Program of the Chinese Academy of Sciences (Grant NO. KFZD-SW-113).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evangelista A.G., Corrêa J.A.F., Pinto A.C.S.M., Luciano F.B. The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance—A review. Crit. Rev. Food Sci. Nutr. 2022;62:5267–5283. doi: 10.1080/10408398.2021.1883548. [DOI] [PubMed] [Google Scholar]
- 2.Fukase E., Martin W. Economic growth, convergence, and world food demand and supply. World Dev. 2020;132:104954. doi: 10.1016/j.worlddev.2020.104954. [DOI] [Google Scholar]
- 3.Schokker D., Zhang J., Vastenhouw S.A., Heilig H.G., Smidt H., Rebel J.M., Smits M.A. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS ONE. 2015;10:e0116523. doi: 10.1371/journal.pone.0116523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roskam J.L., Lansink A.G.J.M.O., Saatkamp H.W. The technical and economic impact of veterinary interventions aimed at reducing antimicrobial use on broiler farms. Poult. Sci. 2019;98:6644–6658. doi: 10.3382/ps/pez517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudi K., Zhao L. Grand challenges in understanding gut microbes. Front. Microbiol. 2021;12:752829. doi: 10.3389/fmicb.2021.752829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagbø M., Ravi A., Angell I.L., Sunde M., Ludvigsen J., Diep D.B., Foley S.L., Vento M., Collado M.C., Perez-Martinez G., et al. Experimental support for multidrug resistance transfer potential in the preterm infant gut microbiota. Pediatr. Res. 2020;88:57–65. doi: 10.1038/s41390-019-0491-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhai H., Liu H., Wang S., Wu J., Kluenter A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018;4:179–186. doi: 10.1016/j.aninu.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G., Wu S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021;12:72. doi: 10.1186/s40104-021-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong X., Wang X., Zhou N., Li J., Liu J., Yue J., Hao X., Gan M., Lin P., Shang X. Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 2021;344:128674. doi: 10.1016/j.foodchem.2020.128674. [DOI] [PubMed] [Google Scholar]
- 10.Hao Y., Kang J., Yang R., Li H., Cui H., Bai H., Tsitsilin A., Li J., Shi L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022;374:131629. doi: 10.1016/j.foodchem.2021.131629. [DOI] [PubMed] [Google Scholar]
- 11.Snoussi A., Chouaibi M., Koubaier H.B.H., Bouzouita N. Encapsulation of Tunisian thyme essential oil in O/W nanoemulsions: Application for meat preservation. Meat Sci. 2022;188:108785. doi: 10.1016/j.meatsci.2022.108785. [DOI] [PubMed] [Google Scholar]
- 12.Li P., Piao X., Ru Y., Han X., Xue L., Zhang H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 2012;25:1617–1626. doi: 10.5713/ajas.2012.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng Z., Xu X., Zhang Q., Li P., Zhao P., Li Q., Liu J., Piao X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015;86:279–285. doi: 10.1111/asj.12277. [DOI] [PubMed] [Google Scholar]
- 14.Wei H.K., Xue H.X., Zhou Z.X., Peng J. A carvacrol-thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal. 2017;11:193–201. doi: 10.1017/S1751731116001397. [DOI] [PubMed] [Google Scholar]
- 15.Tiihonen K., Kettunen H., Bento M.H., Saarinen M., Lahtinen S., Ouwehand A.C., Schulze H., Rautonen N. The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 2010;51:381–392. doi: 10.1080/00071668.2010.496446. [DOI] [PubMed] [Google Scholar]
- 16.Cetin E., Yibar A., Yesilbag D., Cetin I., Cengiz S.S. The effect of volatile oil mixtures on the performance and ilio-caecal microflora of broiler chickens. Br. Poult. Sci. 2016;57:780–787. doi: 10.1080/00071668.2016.1214682. [DOI] [PubMed] [Google Scholar]
- 17.Nouri A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br. Poult. Sci. 2019;60:530–538. doi: 10.1080/00071668.2019.1622078. [DOI] [PubMed] [Google Scholar]
- 18.Franz C., Baser K., Windisch W. Essential oils and aromatic plants in animal feeding–a European perspective. A review. Flavour Frag. J. 2010;25:327–340. doi: 10.1002/ffj.1967. [DOI] [Google Scholar]
- 19.Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- 20.Janz J.A., Morel P.C., Wilkinson B.H., Purchas R.W. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007;75:350–355. doi: 10.1016/j.meatsci.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Yan L., Wang J.P., Kim H.J., Meng Q.W., Ao X., Hong S.M., Kim I.H. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower-finisher pigs. Livest. Sci. 2010;128:115–122. doi: 10.1016/j.livsci.2009.11.008. [DOI] [Google Scholar]
- 22.Tiseo K., Huber L., Gilbert M., Robinson T.P., Van Boeckel T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics. 2020;9:918. doi: 10.3390/antibiotics9120918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abushaheen M.A., Muzaheed , Fatani A.J., Alosaimi M., Mansy W., George M., Acharya S., Rathod S., Divakar D.D., Jhugroo C., et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020;66:100971. doi: 10.1016/j.disamonth.2020.100971. [DOI] [PubMed] [Google Scholar]
- 24.Woolhouse M.E., Ward M.J. Sources of antimicrobial resistance. Science. 2013;341:1460–1461. doi: 10.1126/science.1243444. [DOI] [PubMed] [Google Scholar]
- 25.Rahman M., Fliss I., Biron E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics. 2022;11:766. doi: 10.3390/antibiotics11060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch B.J., Hungate B.A., Price L.B. Food-animal production and the spread of antibiotic resistance: The role of ecology. Front. Ecol. Environ. 2017;15:309–318. doi: 10.1002/fee.1505. [DOI] [Google Scholar]
- 27.de Kraker M.E., Stewardson A.J., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13:e1002184. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y., Lahaye L., He Z., Zhang J., Yang C., Piao X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+) Anim. Nutr. 2020;6:269–277. doi: 10.1016/j.aninu.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao H., Cheng G., Iqbal Z., Ai X., Hussain H.I., Huang L., Dai M., Wang Y., Liu Z., Yuan Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heikkilä A.M., Nousiainen J.I., Pyörälä S. Costs of clinical mastitis with special reference to premature culling. J. Dairy Sci. 2012;95:139–150. doi: 10.3168/jds.2011-4321. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Z., Palma-Vera S., Buggiotti L., Salavati M., Becker F., Werling D., Wathes D.C., GplusE Consortium Transcriptomic analysis of circulating leukocytes obtained during the recovery from clinical mastitis caused by Escherichia coli in Holstein dairy cows. Animals. 2022;12:2146. doi: 10.3390/ani12162146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomanić D., Božin B., Kladar N., Stanojević J., Čabarkapa I., Stilinović N., Apić J., Božić D.D., Kovačević Z. Environmental bovine mastitis pathogens: Prevalence, antimicrobial susceptibility, and sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. essential oils. Antibiotics. 2022;11:1077. doi: 10.3390/antibiotics11081077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludbey P.A., Sahibzada S., Annandale C.H., Robertson I.D., Waichigo F.K., Tufail M.S., Valenzuela J.L., Aleri J.W. A pilot study on bacterial isolates associated with purulent vaginal discharge in dairy cows in the south-west region of Western Australia. Aust. Vet. J. 2022;100:205–212. doi: 10.1111/avj.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasconcelos P.C., Leite E.L., Araújo W.J., Silva N., Saraiva M., Filho L.S., Neto O.C.F., Givisiez P., Oliveira C. Draft genome sequence of mcr-1-mediated colistin-resistant Escherichia coli ST359 from chicken carcasses in Northeastern Brazil. J. Glob. Antimicrob. Resist. 2020;23:135–136. doi: 10.1016/j.jgar.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Trongjit S., Angkittitrakul S., Chuanchuen R. Occurrence and molecular characteristics of antimicrobial resistance of Escherichia coli from broilers, pigs and meat products in Thailand and Cambodia provinces. Microbiol. Immunol. 2016;60:575–585. doi: 10.1111/1348-0421.12407. [DOI] [PubMed] [Google Scholar]
- 36.Li H., Liu Y., Yang L., Wu X., Wu Y., Shao B. Prevalence of Escherichia coli and antibiotic resistance in animal-derived food samples—Six districts, Beijing, China, 2020. China CDC Wkly. 2021;3:999–1004. doi: 10.46234/ccdcw2021.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Österberg J., Wingstrand A., Jensen A.N., Kerouanton A., Cibin V., Barco L., Denis M., Aabo S., Bengtsson B. Antibiotic resistance in Escherichia coli from pigs in organic and conventional farming in four European countries. PLoS ONE. 2016;11:e0157049. doi: 10.1371/journal.pone.0157049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeed E., Amer A.A.E., Keshta H.G., Hafez E.E., Sultan R.M.S., Khalifa E. Prevalence, antibiotic sensitivity profile, and phylogenetic analysis of Escherichia coli isolated from raw dromedary camel milk in Matrouh Governorate, Egypt. J. Adv. Vet. Anim. Res. 2022;9:138–143. doi: 10.5455/javar.2022.i578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messele Y.E., Abdi R.D., Yalew S.T., Tegegne D.T., Emeru B.A., Werid G.M. Molecular determination of antimicrobial resistance in Escherichia coli isolated from raw meat in Addis Ababa and Bishoftu, Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2017;16:55. doi: 10.1186/s12941-017-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Honert M.S., Gouws P.A., Hoffman L.C. A preliminary study: Antibiotic resistance of Escherichia coli and Staphylococcus aureus from the meat and feces of various South African wildlife species. Food Sci. Anim. Resour. 2021;41:135–144. doi: 10.5851/kosfa.2020.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor E.A., Ossa-Trujillo C., Vinasco J., Jordan E.R., Buitrago J.A.G., Hagevoort R., Norman K.N., Lawhon S.D., Piñeiro J.M., Levent G., et al. Use of critically important antimicrobial classes early in life may adversely impact bacterial resistance profiles during adult years: Potential co-selection for plasmid-borne fluoroquinolone and macrolide resistance via extended-spectrum beta-lactam use in dairy cattle. Lett. Appl. Microbiol. 2021;72:220–224. doi: 10.1111/lam.13419. [DOI] [PubMed] [Google Scholar]
- 43.Le P.Q., Awasthi S.P., Hatanaka N., Hinenoya A., Hassan J., Ombarak R.A., Iguchi A., Tran N., Dao K., Vien M.Q., et al. Prevalence of mobile colistin resistance (mcr) genes in extended-spectrum β-lactamase-producing Escherichia coli isolated from retail raw foods in Nha Trang, Vietnam. Int. J. Food Microbiol. 2021;346:109164. doi: 10.1016/j.ijfoodmicro.2021.109164. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira A., Pavelquesi S., Monteiro E., Rodrigues L., Silva C., Silva I., Orsi D.C. Prevalence and antimicrobial resistance of Salmonella spp. in aquacultured Nile Tilapia (Oreochromis niloticus) commercialized in Federal District, Brazil. Foodborne Pathog. Dis. 2021;18:778–783. doi: 10.1089/fpd.2021.0010. [DOI] [PubMed] [Google Scholar]
- 45.Lay K.S., Vuthy Y., Song P., Phol K., Sarthou J.L. Prevalence, numbers and antimicrobial susceptibilities of Salmonella serovars and Campylobacter spp. in retail poultry in Phnom Penh, Cambodia. J. Vet. Med Sci. 2011;73:325–329. doi: 10.1292/jvms.10-0373. [DOI] [PubMed] [Google Scholar]
- 46.Yang X., Huang J., Wu Q., Zhang J., Liu S., Guo W., Cai S., Yu S. Prevalence, Antimicrobial resistance and genetic diversity of Salmonella isolated from retail ready-to-eat foods in China. Food Control. 2016;60:50–56. doi: 10.1016/j.foodcont.2015.07.019. [DOI] [Google Scholar]
- 47.Abd-Elghany S.M., Sallam K.I., Abd-Elkhalek A., Tamura T. Occurrence, genetic characterization and antimicrobial resistance of Salmonella isolated from chicken meat and giblets. Epidemiol. Infect. 2015;143:997–1003. doi: 10.1017/S0950268814001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir R., Salari S., Najimi M., Rashki A. Determination of frequency, multiple antibiotic resistance index and resistotype of Salmonella spp. in chicken meat collected from southeast of Iran. Vet. Med. Sci. 2022;8:229–236. doi: 10.1002/vms3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgado-Suárez E.J., Palós-Guitérrez T., Ruíz-López F.A., Pérez C.F.H., Ballesteros-Nova N.E., Soberanis-Ramos O., Méndez-Medina R.D., Allard M.W., Rubio-Lozano M.S. Genomic surveillance of antimicrobial resistance shows cattle and poultry are a moderate source of multi-drug resistant non-typhoidal Salmonella in Mexico. PLoS ONE. 2021;16:e0243681. doi: 10.1371/journal.pone.0243681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patchanee P., Tansiricharoenkul K., Buawiratlert T., Wiratsudakul A., Angchokchatchawal K., Yamsakul P., Yano T., Boonkhot P., Rojanasatien S., Tadee P. Salmonella in pork retail outlets and dissemination of its pulsotypes through pig production chain in Chiang Mai and surrounding areas, Thailand. Prev. Vet. Med. 2016;130:99–105. doi: 10.1016/j.prevetmed.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 51.McDermott P.F., Tyson G.H., Kabera C., Chen Y., Li C., Folster J.P., Ayers S.L., Lam C., Tate H.P., Zhao S. Whole-genome sequencing for detecting antimicrobial resistance in nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016;60:5515–5520. doi: 10.1128/AAC.01030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroning I.S., Iglesias M.A., Mendonça K.S., Lopes G.V., Silva W.P. Presence of classical enterotoxin genes, agr typing, antimicrobial resistance, and genetic diversity of Staphylococcus aureus from milk of cows with mastitis in southern Brazil. J. Food Prot. 2018;81:738–742. doi: 10.4315/0362-028X.JFP-17-436. [DOI] [PubMed] [Google Scholar]
- 53.Liao G., Wu Z., Lv J., Ren Q., Chen W. Investigation of clonal diversity, virulence genes, and antibiotic resistance of Staphylococcus aureus recovered from raw cow milk in southern Xinjiang, China. Folia Microbiol. 2022;67:245–252. doi: 10.1007/s12223-021-00924-7. [DOI] [PubMed] [Google Scholar]
- 54.Thongratsakul S., Usui M., Higuchi H., Takahashi T., Sato T., Poolkhet C., Tamura Y. Prevalence and characterization of Staphylococcus aureus isolated in raw milk from cows in Hokkaido, Japan. Trop. Anim. Health Prod. 2020;52:1631–1637. doi: 10.1007/s11250-019-02169-6. [DOI] [PubMed] [Google Scholar]
- 55.Kanungpean D., Takai S., Kakuda T. Contamination and antimicrobial susceptibility testing of Staphylococcus aureus isolated from pork in fresh markets, Nongchok district, Thailand. Vet. Med. Int. 2021;2021:6646846. doi: 10.1155/2021/6646846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge B., Mukherjee S., Hsu C.H., Davis J.A., Tran T., Yang Q., Abbott J.W., Ayers S.L., Young S.R., Crarey E.T., et al. MRSA and multidrug-resistant Staphylococcus aureus in U.S. retail meats, 2010–2011. Food Microbiol. 2017;62:289–297. doi: 10.1016/j.fm.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira C.J., Tiao N., de Sousa F.G., de Moura J.F., Filho L.S., Gebreyes W.A. Methicillin-resistant Staphylococcus aureus from Brazilian dairy farms and identification of novel sequence types. Zoonoses Public Health. 2016;63:97–105. doi: 10.1111/zph.12209. [DOI] [PubMed] [Google Scholar]
- 58.Yi Y., Su L., Li B., Li S., Zhang B., Su Y. Analysis of the genetic diversity in methicillin-resistant Staphylococcus aureus isolates from bovine subclinical mastitis case in Xinjiang, China. Foodborne Pathog. Dis. 2018;15:568–575. doi: 10.1089/fpd.2018.2424. [DOI] [PubMed] [Google Scholar]
- 59.Li H., Andersen P.S., Stegger M., Sieber R.N., Ingmer H., Staubrand N., Dalsgaard A., Leisner J.J. Antimicrobial resistance and virulence gene profiles of methicillin-resistant and -susceptible Staphylococcus aureus from food products in Denmark. Front. Microbiol. 2019;10:2681. doi: 10.3389/fmicb.2019.02681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sallam K.I., Abd-Elghany S.M., Elhadidy M., Tamura T. Molecular characterization and antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus in retail chicken. J. Food Prot. 2015;78:1879–1884. doi: 10.4315/0362-028X.JFP-15-150. [DOI] [PubMed] [Google Scholar]
- 61.Hasanpour Dehkordi A., Khaji L., Sakhaei Shahreza M.H., Mashak Z., Safarpoor Dehkordi F., Safaee Y., Hosseinzadeh A., Alavi I., Ghasemi E., Rabiei-Faradonbeh M. One-year prevalence of antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus recovered from raw meat. Trop. Biomed. 2017;34:396–404. [PubMed] [Google Scholar]
- 62.Zhang P., Liu X., Zhang J., Fu X., Wan Y., Pan H., Wu C., Wang X. Prevalence and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from retail yak butter in Tibet, China. J. Dairy Sci. 2021;104:9596–9606. doi: 10.3168/jds.2020-19604. [DOI] [PubMed] [Google Scholar]
- 63.Akrami-Mohajeri F., Derakhshan Z., Ferrante M., Hamidiyan N., Soleymani M., Conti G.O., Tafti R.D. The prevalence and antimicrobial resistance of Listeria spp in raw milk and traditional dairy products delivered in Yazd, central Iran (2016) Food Chem. Toxicol. 2018;114:141–144. doi: 10.1016/j.fct.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Escolar C., Gómez D., García M.D.C.R., Conchello P., Herrera A. Antimicrobial resistance profiles of Listeria monocytogenes and Listeria innocua isolated from ready-to-eat products of animal origin in Spain. Foodborne Pathog. Dis. 2017;14:357–363. doi: 10.1089/fpd.2016.2248. [DOI] [PubMed] [Google Scholar]
- 65.Shen J., Zhang G., Yang J., Zhao L., Jiang Y., Guo D., Wang X., Zhi S., Xu X., Dong Q., et al. Prevalence, antibiotic resistance, and molecular epidemiology of Listeria monocytogenes isolated from imported foods in China during 2018 to 2020. Int. J. Food Microbiol. 2022;382:109916. doi: 10.1016/j.ijfoodmicro.2022.109916. [DOI] [PubMed] [Google Scholar]
- 66.Sugiri Y.D., Gölz G., Meeyam T., Baumann M.P., Kleer J., Chaisowwong W., Alter T. Prevalence and antimicrobial susceptibility of Listeria monocytogenes on chicken carcasses in Bandung, Indonesia. J. Food Prot. 2014;77:1407–1410. doi: 10.4315/0362-028X.JFP-13-453. [DOI] [PubMed] [Google Scholar]
- 67.Maung A.T., Mohammadi T.N., Nakashima S., Liu P., Masuda Y., Honjoh K.I., Miyamoto T. Antimicrobial resistance profiles of Listeria monocytogenes isolated from chicken meat in Fukuoka, Japan. Int. J. Food Microbiol. 2019;304:49–57. doi: 10.1016/j.ijfoodmicro.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 68.Sosnowski M., Lachtara B., Wieczorek K., Osek J. Antimicrobial resistance and genotypic characteristics of Listeria monocytogenes isolated from food in Poland. Int. J. Food Microbiol. 2019;289:1–6. doi: 10.1016/j.ijfoodmicro.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 69.Tîrziu E., Herman V., Nichita I., Morar A., Imre M., Ban-Cucerzan A., Bucur I., Tîrziu A., Mateiu-Petrec O.C., Imre K. Diversity and antibiotic resistance profiles of Listeria monocytogenes serogroups in different food products from the Transylvania region of central Romania. J. Food Prot. 2022;85:54–59. doi: 10.4315/JFP-21-172. [DOI] [PubMed] [Google Scholar]
- 70.Arslan S., Baytur S. Prevalence and antimicrobial resistance of Listeria species and subtyping and virulence factors of Listeria monocytogenes from retail meat. J. Food Saf. 2019;39:e12578. doi: 10.1111/jfs.12578. [DOI] [Google Scholar]
- 71.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils--a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 72.El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A.M., Elnesr S.S., El-Hack M.E.A. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hajlaoui H., Arraouadi S., Noumi E., Aouadi K., Adnan M., Khan M.A., Kadri A., Snoussi M. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules. 2021;26:3625. doi: 10.3390/molecules26123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bisht D.S., Menon K.R.K., Singhal M.K. Comparative antimicrobial activity of essential oils of Cuminum cyminum L. and Foeniculum vulgare Mill. seeds against Salmonella typhimurium and Escherichia coli. J. Essent. Oil Bear. Plants. 2014;17:617–622. doi: 10.1080/0972060X.2014.956675. [DOI] [Google Scholar]
- 75.Verma R.S., Joshi N., Padalia R.C., Goswami P., Singh V.R., Chauhan A., Verma S.K., Iqbal H., Verma R.K., Chanda D., et al. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind. Crop. Prod. 2017;104:144–155. doi: 10.1016/j.indcrop.2017.04.046. [DOI] [Google Scholar]
- 76.Węglarz Z., Kosakowska O., Pióro-Jabrucka E., Przybył J.L., Gniewosz M., Kraśniewska K., Szyndel M.S., Costa R., Bączek K.B. Antioxidant and antibacterial activity of Helichrysum italicum (Roth) G. Don. from central Europe. Pharmaceuticals. 2022;15:735. doi: 10.3390/ph15060735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juliano C., Marchetti M., Campagna P., Usai M. Antimicrobial activity and chemical composition of essential oil from Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso collected in South-West Sardinia. Saudi J. Biol. Sci. 2019;26:897–905. doi: 10.1016/j.sjbs.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Falco E., Roscigno G., Landolfi S., Scandolera E., Senatore F. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind. Crop. Prod. 2014;62:242–249. doi: 10.1016/j.indcrop.2014.08.037. [DOI] [Google Scholar]
- 79.Jiang Y., Wu N., Fu Y.J., Wang W., Luo M., Zhao C.J., Zu Y.G., Liu X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011;32:63–68. doi: 10.1016/j.etap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 80.Al Maqtari M.A.A., Alghalibi S.M., Alhamzy E.H. Chemical composition and antimicrobial activity of essential oil of Thymus vulgaris from Yemen. Turk. J. Biochem. 2011;36:342–349. [Google Scholar]
- 81.Abdelli M., Moghrani H., Aboun A., Maachi R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crop. Prod. 2016;94:197–205. doi: 10.1016/j.indcrop.2016.08.042. [DOI] [Google Scholar]
- 82.Silva V.A., da Sousa J.P., de Luna Freire Pessôa H., de Freitas A.F.R., Coutinho H.D.M., Alves L.B.N., Lima E.O. Ocimum basilicum: Antibacterial activity and association study with antibiotics against bacteria of clinical importance. Pharm. Biol. 2016;54:863–867. doi: 10.3109/13880209.2015.1088551. [DOI] [PubMed] [Google Scholar]
- 83.Garzoli S., Petralito S., Ovidi E., Turchetti G., Masci V.L., Tiezzi A., Trillia J., Cesa S., Casadei M.A., Giacomello P., et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crop Prod. 2020;145:112068. doi: 10.1016/j.indcrop.2019.112068. [DOI] [Google Scholar]
- 84.de Morais Oliveira-Tintino C.D., Tintino S.R., Limaverde P.W., Figueredo F.G., Campina F.F., da Cunha F., da Costa R., Pereira P.S., Lima L.F., de Matos Y., et al. Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018;262:72–77. doi: 10.1016/j.foodchem.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 85.Ooi L.S., Li Y., Kam S.L., Wang H., Wong E.Y., Ooi V.E. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am. J. Chin. Med. 2006;34:511–522. doi: 10.1142/S0192415X06004041. [DOI] [PubMed] [Google Scholar]
- 86.Wu K., Lin Y., Chai X., Duan X., Zhao X., Chun C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019;7:2546–2555. doi: 10.1002/fsn3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu W., Li C.Z., Dai J.M., Cui H.Y., Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA) Ind. Crop. Prod. 2019;130:34–41. doi: 10.1016/j.indcrop.2018.12.078. [DOI] [Google Scholar]
- 88.Salem N., Kefi S., Tabben O., Ayed A., Jallouli S., Feres N., Hammami M., Khammassi S., Hrigua I., Nefisi S., et al. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crop. Prod. 2018;124:115–125. doi: 10.1016/j.indcrop.2018.07.051. [DOI] [Google Scholar]
- 89.Kačániová M., Galovičová L., Borotová P., Valková V., Ďúranová H., Kowalczewski P.Ł., Said-Al Ahl H., Hikal W.M., Vukic M., Savitskaya T., et al. Chemical composition, in vitro and in situ antimicrobial and antibiofilm activities of Syzygium aromaticum (Clove) essential oil. Plants. 2021;10:2185. doi: 10.3390/plants10102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pontes E., Melo H.M., Nogueira J., Firmino N., de Carvalho M.G., Júnior F.C., Cavalcante T. Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2018;28:633–639. doi: 10.1007/s10068-018-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Y., Chen S., Zhang C., Liu Y., Ma L., Zhang X. Effects of sub-minimum inhibitory concentrations of lemon essential oil on the acid tolerance and biofilm formation of Streptococcus mutans. Arch. Oral Biol. 2018;87:235–241. doi: 10.1016/j.archoralbio.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 92.Awang K., Ibrahim H., Syamsir D.R., Mohtar M., Ali R.M., Ali N.A.M. Chemical constituents and antimicrobial activity of the leaf and rhizome oils of Alpinia pahangensis Ridl., an endemic wild ginger from peninsular Malaysia. Chem. Biodivers. 2011;8:668–673. doi: 10.1002/cbdv.201000225. [DOI] [PubMed] [Google Scholar]
- 93.García-Salinas S., Elizondo-Castillo H., Arruebo M., Mendoza G., Irusta S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules. 2018;23:1399. doi: 10.3390/molecules23061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guimarães A.C., Meireles L.M., Lemos M.F., Guimarães M., Endringer D.C., Fronza M., Scherer R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24:471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang T.H., Hsia S.M., Wu C.H., Ko S.Y., Chen M.Y., Shih Y.H., Shieh T.M., Chuang L.C., Wu C.Y. Evaluation of the antibacterial potential of liquid and vapor phase phenolic essential oil compounds against oral microorganisms. PLoS ONE. 2016;11:e0163147. doi: 10.1371/journal.pone.0163147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gómez-García M., Sol C., de Nova P., Puyalto M., Mesas L., Puente H., Mencía-Ares Ó., Miranda R., Argüello H., Rubio P., et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porcine Health Manag. 2019;5:32. doi: 10.1186/s40813-019-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rúa J., Del Valle P., de Arriaga D., Fernández-Álvarez L., García-Armesto M.R. Combination of carvacrol and thymol: Antimicrobial activity against Staphylococcus aureus and antioxidant activity. Foodborne Pathog. Dis. 2019;16:622–629. doi: 10.1089/fpd.2018.2594. [DOI] [PubMed] [Google Scholar]
- 98.Lopez-Romero J.C., González-Ríos H., Borges A., Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid. Based Complement. Alternat. Med. 2015;2015:795435. doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu G.X., Wang Y.W., Wu C.S., Lin Y.H., Hung C.H., Huang H.H., Kuo S.M. Therapeutic efficacy of sesquiterpene farnesol in treatment of Cutibacterium acnes-induced dermal disorders. Molecules. 2021;26:5723. doi: 10.3390/molecules26185723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wiatkowski P., Sienkiewicz M., Pruss A., Łopusiewicz Ł., Arszyńska N., Wojciechowska-Koszko I., Kilanowicz A., Kot B., Dołęgowska B. Antibacterial and anti-biofilm activities of essential oil compounds against new delhi metallo-β-lactamase-1-producing uropathogenic Klebsiella pneumoniae strains. Antibiotics. 2022;11:147. doi: 10.3390/antibiotics11020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Moura D.F., Rocha T.A., Barros D.D.M., da Silva M.M., Santana M.D.S., Neta B.M., Cavalcanti I.M.F., Martins R.D., da Silva M.V. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol. Arch. Microbiol. 2021;203:4303–4311. doi: 10.1007/s00203-021-02377-5. [DOI] [PubMed] [Google Scholar]
- 102.Siddiqua S., Anusha B.A., Ashwini L.S., Negi P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2015;52:5834–5841. doi: 10.1007/s13197-014-1642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hassan W.H., Abdel-Ghany A.E., Afifi S.I., Sedik S.H. Genotypic characterization of Campylobacter species isolated from livestock and poultry and evaluation of some herbal oil antimicrobial effect against selected Campylobacter species. Adv. Anim. Vet. Sci. 2019;7:1083–1092. doi: 10.17582/journal.aavs/2019/7.12.1083.1092. [DOI] [Google Scholar]
- 104.Marinelli L., Di Stefano A., Cacciatore I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018;17:903–921. doi: 10.1007/s11101-018-9569-x. [DOI] [Google Scholar]
- 105.Zhang D., Gan R.Y., Zhang J.R., Farha A.K., Li H.B., Zhu F., Wang X.H., Corke H. Antivirulence properties and related mechanisms of spice essential oils: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020;19:1018–1055. doi: 10.1111/1541-4337.12549. [DOI] [PubMed] [Google Scholar]
- 106.Seow Y.X., Yeo C.R., Chung H.L., Yuk H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014;54:625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- 107.Yap P.S., Yiap B.C., Ping H.C., Lim S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014;8:6–14. doi: 10.2174/1874285801408010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Juven B.J., Kanner J., Schved F., Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994;76:626–631. doi: 10.1111/j.1365-2672.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 109.Ultee A., Bennik M.H., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ultee A., Kets E.P., Alberda M., Hoekstra F.A., Smid E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000;174:233–238. doi: 10.1007/s002030000199. [DOI] [PubMed] [Google Scholar]
- 111.Ultee A., Smid E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001;64:373–378. doi: 10.1016/S0168-1605(00)00480-3. [DOI] [PubMed] [Google Scholar]
- 112.Elmi A., Prosperi A., Zannoni A., Bertocchi M., Scorpio D.G., Forni M., Foni E., Bacci M.L., Ventrella D. Antimicrobial capabilities of non-spermicidal concentrations of tea tree (Melaleuca alternifolia) and rosemary (Rosmarinus officinalis) essential oils on the liquid phase of refrigerated swine seminal doses. Res. Vet. Sci. 2019;127:76–81. doi: 10.1016/j.rvsc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 113.Rao J., Chen B., McClements D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019;10:365–387. doi: 10.1146/annurev-food-032818-121727. [DOI] [PubMed] [Google Scholar]
- 114.Bouyahya A., Abrini J., Dakka N., Bakri Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019;9:301–311. doi: 10.1016/j.jpha.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aljaafari M.N., AlAli A.O., Baqais L., Alqubaisy M., AlAli M., Molouki A., Ong-Abdullah J., Abushelaibi A., Lai K.S., Lim S.E. An overview of the potential therapeutic applications of essential oils. Molecules. 2021;26:628. doi: 10.3390/molecules26030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tassou C., Koutsoumanis K., Nychas G.J.E. Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res. Int. 2000;33:273–280. doi: 10.1016/S0963-9969(00)00047-8. [DOI] [Google Scholar]
- 117.Burt S.A., Reinders R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765X.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 118.Nowotarska S.W., Nowotarski K., Grant I.R., Elliott C.T., Friedman M., Situ C. Mechanisms of antimicrobial action of cinnamon and oregano oils, cinnamaldehyde, carvacrol, 2,5-dihydroxybenzaldehyde, and 2-hydroxy-5-methoxybenzaldehyde against Mycobacterium avium subsp. paratuberculosis (Map). Foods. 2017;6:72. doi: 10.3390/foods6090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ta C.A., Arnason J.T. Mini review of phytochemicals and plant taxa with activity as microbial biofilm and quorum sensing inhibitors. Molecules. 2015;21:E29. doi: 10.3390/molecules21010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soumya E.A., Saad I.K., Hassan L., Ghizlane Z., Hind M., Adnane R. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011;5:3229–3232. [Google Scholar]
- 121.Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013;36:79–89. doi: 10.1016/j.fm.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Amalaradjou M.A., Venkitanarayanan K. Effect of trans-cinnamaldehyde on inhibition and inactivation of Cronobacter sakazakii biofilm on abiotic surfaces. J. Food Prot. 2011;74:200–208. doi: 10.4315/0362-028X.JFP-10-296. [DOI] [PubMed] [Google Scholar]
- 123.Sharma G., Raturi K., Dang S., Gupta S., Gabrani R. Combinatorial antimicrobial effect of curcumin with selected phytochemicals on Staphylococcus epidermidis. J. Asian Nat. Prod. Res. 2014;16:535–541. doi: 10.1080/10286020.2014.911289. [DOI] [PubMed] [Google Scholar]
- 124.Zhou L., Zheng H., Tang Y., Yu W., Gong Q. Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol. Lett. 2013;35:631–637. doi: 10.1007/s10529-012-1126-x. [DOI] [PubMed] [Google Scholar]
- 125.Deryabin D., Galadzhieva A., Kosyan D., Duskaev G. Plant-derived inhibitors of AHL-mediated quorum sensing in bacteria: Modes of action. Int. J. Mol. Sci. 2019;20:5588. doi: 10.3390/ijms20225588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amaral S.C., Pruski B.B., de Freitas S.B., Allend S.O., Ferreira M., Moreira C., Jr., Pereira D., Junior A., Hartwig D.D. Origanum vulgare essential oil: Antibacterial activities and synergistic effect with polymyxin B against multidrug-resistant Acinetobacter baumannii. Mol. Biol. Rep. 2020;47:9615–9625. doi: 10.1007/s11033-020-05989-0. [DOI] [PubMed] [Google Scholar]
- 127.Coimbra A., Miguel S., Ribeiro M., Coutinho P., Silva L., Duarte A.P., Ferreira S. Thymus zygis essential oil: Phytochemical characterization, bioactivity evaluation and synergistic effect with antibiotics against Staphylococcus aureus. Antibiotics. 2022;11:146. doi: 10.3390/antibiotics11020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Özel Y., Yılmaz U., Ünlü M., Ünlü G.V. Antibacterial activity and synergistic interaction of various essential oil components and antibiotics. Mikrobiyol. Bul. 2022;56:95–102. doi: 10.5578/mb.20229908. [DOI] [PubMed] [Google Scholar]
- 129.Magi G., Marini E., Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015;6:165. doi: 10.3389/fmicb.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Visvalingam J., Palaniappan K., Holley R.A. In vitro enhancement of antibiotic susceptibility of drug resistant Escherichia coli by cinnamaldehyde. Food Control. 2017;79:288–291. doi: 10.1016/j.foodcont.2017.04.011. [DOI] [Google Scholar]
- 131.Rodrigues I.D.A., Ferrari R.G., Panzenhagen P.H.N., Mano S.B., Conte-Junior C.A. Antimicrobial resistance genes in bacteria from animal-based foods. Adv. Appl. Microbiol. 2020;112:143–183. doi: 10.1016/bs.aambs.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 132.Rozwandowicz M., Brouwer M., Fischer J., Wagenaar J.A., Gonzalez-Zorn B., Guerra B., Mevius D.J., Hordijk J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018;73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 133.Nawaz M., Sung K., Kweon O., Khan S., Nawaz S., Steele R. Characterisation of novel mutations involved in quinolone resistance in Escherichia coli isolated from imported shrimp. Int. J. Antimicrob. Agents. 2015;45:471–476. doi: 10.1016/j.ijantimicag.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 134.Quesada A., Porrero M.C., Téllez S., Palomo G., García M., Domínguez L. Polymorphism of genes encoding PmrAB in colistin-resistant strains of Escherichia coli and Salmonella enterica isolated from poultry and swine. J. Antimicrob. Chemother. 2015;70:71–74. doi: 10.1093/jac/dku320. [DOI] [PubMed] [Google Scholar]
- 135.Ahmed H.A., El-Hofy F.I., Shafik S.M., Abdelrahman M.A., Elsaid G.A. Characterization of virulence-associated genes, antimicrobial resistance genes, and class 1 integrons in Salmonella enterica serovar Typhimurium isolates from chicken meat and humans in Egypt. Foodborne Pathog. Dis. 2016;13:281–288. doi: 10.1089/fpd.2015.2097. [DOI] [PubMed] [Google Scholar]
- 136.Neuert S., Nair S., Day M.R., Doumith M., Ashton P.M., Mellor K.C., Jenkins C., Hopkins K.L., Woodford N., de Pinna E., et al. Prediction of phenotypic antimicrobial resistance profiles from whole genome sequences of non-typhoidal Salmonella enterica. Front. Microbiol. 2018;9:592. doi: 10.3389/fmicb.2018.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McMillan E.A., Gupta S.K., Williams L.E., Jové T., Hiott L.M., Woodley T.A., Barrett J.B., Jackson C.R., Wasilenko J.L., Simmons M., et al. Antimicrobial resistance genes, cassettes, and plasmids present in Salmonella enterica associated with United States food animals. Front. Microbiol. 2019;10:832. doi: 10.3389/fmicb.2019.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lauteri C., Maggio F., Serio A., Festino A.R., Paparella A., Vergara A. Overcoming multidrug resistance in Salmonella spp. isolates obtained from the swine food chain by using essential oils: An in vitro study. Front. Microbiol. 2022;12:808286. doi: 10.3389/fmicb.2021.808286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nobrega D.B., Naushad S., Naqvi S.A., Condas L., Saini V., Kastelic J.P., Luby C., De Buck J., Barkema H.W. Prevalence and genetic basis of antimicrobial resistance in non-aureus Staphylococci isolated from Canadian Dairy herds. Front. Microbiol. 2018;9:256. doi: 10.3389/fmicb.2018.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu S., Huang J., Wu Q., Zhang J., Zhang F., Yang X., Wu H., Zeng H., Chen M., Ding Y., et al. Staphylococcus aureus isolated from retail meat and meat products in China: Incidence, antibiotic resistance and genetic diversity. Front. Microbiol. 2018;9:2767. doi: 10.3389/fmicb.2018.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qu Y., Zhao H., Nobrega D.B., Cobo E.R., Han B., Zhao Z., Li S., Li M., Barkema H.W., Gao J. Molecular epidemiology and distribution of antimicrobial resistance genes of Staphylococcus species isolated from Chinese dairy cows with clinical mastitis. J. Dairy Sci. 2019;102:1571–1583. doi: 10.3168/jds.2018-15136. [DOI] [PubMed] [Google Scholar]
- 142.Urban-Chmiel R., Marek A., Stępień-Pyśniak D., Wieczorek K., Dec M., Nowaczek A., Osek J. Antibiotic resistance in bacteria-A review. Antibiotics. 2022;11:1079. doi: 10.3390/antibiotics11081079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Srinivasan S., Nam H.M., Nguyen L.T., Tamilselvam B., Murinda S.E., Oliver S.P. Prevalence of antimicrobial resistance genes in Listeria monocytogenes isolated from dairy farms. Foodborne Pathog. Dis. 2005;2:201–211. doi: 10.1089/fpd.2005.2.201. [DOI] [PubMed] [Google Scholar]
- 144.Kayode A., Semerjian L., Osaili T., Olapade O., Okoh A. Occurrence of multidrug-resistant Listeria monocytogenes in environmental waters: A menace of environmental and public health concern. Front. Environ. Sci. 2021;12:373. doi: 10.3389/fenvs.2021.737435. [DOI] [Google Scholar]
- 145.Yin Z., Zhou X., Kang J., Pei F., Du R., Ye Z., Ding H., Ping W., Ge J. Intraspecific and interspecific quorum sensing of bacterial community affects the fate of antibiotic resistance genes during chicken manure composting under penicillin G stress. Bioresour. Technol. 2022;347:126372. doi: 10.1016/j.biortech.2021.126372. [DOI] [PubMed] [Google Scholar]
- 146.Mahizan N.A., Yang S.K., Moo C.L., Song A.A., Chong C.M., Chong C.W., Abushelaibi A., Lim S.E., Lai K.S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moo C.L., Yang S.K., Yusoff K., Ajat M., Thomas W., Abushelaibi A., Lim S.H., Lai K.S. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr. Drug Discov. Technol. 2020;17:430–447. doi: 10.2174/1570163816666190304122219. [DOI] [PubMed] [Google Scholar]
- 148.Mikulášová M., Chovanová R., Vaverková Š. Synergism between antibiotics and plant extracts or essential oils with efflux pump inhibitory activity in coping with multidrug-resistant Staphylococci. Phytochem. Rev. 2016;15:651–662. doi: 10.1007/s11101-016-9458-0. [DOI] [Google Scholar]
- 149.Ghafari O., Sharifi A., Ahmadi A., Fasaei B.N. Antibacterial and anti-PmrA activity of plant essential oils against fluoroquinolone-resistant Streptococcus pneumoniae clinical isolates. Lett. Appl. Microbiol. 2018;67:564–569. doi: 10.1111/lam.13050. [DOI] [PubMed] [Google Scholar]
- 150.Islamieh D.I., Goudarzi H., Khaledi A., Afshar D., Esmaeili D. Reduced efflux pumps expression of Pseudomonas eeruginosa with Satureja khuzistanica essential oil. Iran. J. Med. Sci. 2020;45:463–468. doi: 10.30476/ijms.2019.72675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Saviuc C., Gheorghe I., Coban S., Drumea V., Chifiriuc M.C., Otilia B., Banu O., Bezirtzoglou E., Laz V. Rosmarinus officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR Strains. Rom. Biotech. Lett. 2016;21:11796–11804. [Google Scholar]
- 152.Limaverde P.W., Campina F.F., da Cunha F., Crispim F.D., Figueredo F.G., Lima L.F., Oliveira-Tintino C.D.D.M., de Matos Y., Morais-Braga M., Menezes I., et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017;109:957–961. doi: 10.1016/j.fct.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 153.Chovanová R., Mezovská J., Vaverková Š., Mikulášová M. The inhibition the Tet(K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three Salvia species. Lett. Appl. Microbiol. 2015;61:58–62. doi: 10.1111/lam.12424. [DOI] [PubMed] [Google Scholar]
- 154.Leal A., Bezerra C.F., Confortin C., da Silva L.E., Marinho E.M., Marinho M.M., Vasconcelos M.A., da Silva T.G., Marinho E.S., Teixeira A., et al. Chemical composition and potentiating action of Norfloxacin mediated by the essential oil of Piper caldense C.D.C. against Staphylococcus aureus strains overexpressing efflux pump genes. Arch. Microbiol. 2021;203:4727–4736. doi: 10.1007/s00203-021-02393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sharifi A., Mohammadzadeh A., Salehi T.Z., Mahmoodi P., Nourian A. Cuminum cyminum L. essential oil: A promising antibacterial and antivirulence agent against multidrug-resistant Staphylococcus aureus. Front. Microbiol. 2021;12:667833. doi: 10.3389/fmicb.2021.667833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miladi H., Zmantar T., Kouidhi B., Chaabouni Y., Mahdouani K., Bakhrouf A., Chaieb K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017;104:56–63. doi: 10.1016/j.micpath.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 157.Wang W., Li D., Huang X., Yang H., Qiu Z., Zou L., Liang Q., Shi Y., Wu Y., Wu S., et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules. 2019;24:3792. doi: 10.3390/molecules24203792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hadjilouka A., Mavrogiannis G., Mallouchos A., Paramithiotis S., Mataragas M., Drosinos E.H. Effect of lemongrass essential oil on Listeria monocytogenes gene expression. LWT. 2017;77:510–516. doi: 10.1016/j.lwt.2016.11.080. [DOI] [Google Scholar]
- 159.Das S., Chourashi R., Mukherjee P., Kundu S., Koley H., Dutta M., Mukhopadhyay A.K., Okamoto K., Chatterjee N.S. Inhibition of growth and virulence of Vibrio cholerae by carvacrol, an essential oil component of Origanum spp. J. Appl. Microbiol. 2021;131:1147–1161. doi: 10.1111/jam.15022. [DOI] [PubMed] [Google Scholar]
- 160.Lou Z., Letsididi K.S., Yu F., Pei Z., Wang H., Letsididi R. Inhibitive effect of eugenol and its nanoemulsion on quorum sensing-mediated virulence factors and biofilm formation by Pseudomonas aeruginosa. J. Food Prot. 2019;82:379–389. doi: 10.4315/0362-028X.JFP-18-196. [DOI] [PubMed] [Google Scholar]
- 161.Skalicka-Woźniak K., Walasek M., Aljarba T.M., Stapleton P., Gibbons S., Xiao J., Łuszczki J.J. The anticonvulsant and anti-plasmid conjugation potential of Thymus vulgaris chemistry: An in vivo murine and in vitro study. Food Chem. Toxicol. 2018;120:472–478. doi: 10.1016/j.fct.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 162.Otto M. Physical stress and bacterial colonization. FEMS Microbiol. Rev. 2014;38:1250–1270. doi: 10.1111/1574-6976.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Balcázar J.L., Subirats J., Borrego C.M. The role of biofilms as environmental reservoirs of antibiotic resistance. Front. Microbiol. 2015;6:1216. doi: 10.3389/fmicb.2015.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tytgat H.L.P., Nobrega F.L., van der Oost J., de Vos W.M. Bowel biofilms: Tipping points between a healthy and compromised gut? Trends Microbiol. 2019;27:17–25. doi: 10.1016/j.tim.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 165.Swidsinski A., Weber J., Loening-Baucke V., Hale L.P., Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harrell J.E., Hahn M.M., D’Souza S.J., Vasicek E.M., Sandala J.L., Gunn J.S., McLachlan J.B. Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract. Front. Cell. Infect. Microbiol. 2021;10:624622. doi: 10.3389/fcimb.2020.624622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Corsini P.M., Wang S., Rehman S., Fenn K., Sagar A., Sirovica S., Cleaver L., Edwards-Gayle C., Mastroianni G., Dorgan B., et al. Molecular and cellular insight into Escherichia coli SslE and its role during biofilm maturation. NPJ Biofilms Microbiomes. 2022;8:9. doi: 10.1038/s41522-022-00272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang B., Shao Y., Liu D., Yin P., Guo Y., Yuan J. Zinc prevents Salmonella enterica serovar Typhimurium-induced loss of intestinal mucosal barrier function in broiler chickens. Avian. Pathol. 2012;41:361–367. doi: 10.1080/03079457.2012.692155. [DOI] [PubMed] [Google Scholar]
- 169.Li Q., Yu C., Chen Y., Liu S., Azevedo P., Gong J., O K., Yang C. Citral alleviates peptidoglycan-induced inflammation and disruption of barrier functions in porcine intestinal epithelial cells. J. Cell Physiol. 2022;237:1768–1779. doi: 10.1002/jcp.30640. [DOI] [PubMed] [Google Scholar]
- 170.Drolia R., Tenguria S., Durkes A.C., Turner J.R., Bhunia A.K. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe. 2018;23:470–484.e7. doi: 10.1016/j.chom.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johansson M.E., Gustafsson J.K., Holmén-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjövall H., et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hoarau G., Mukherjee P.K., Gower-Rousseau C., Hager C., Chandra J., Retuerto M.A., Neut C., Vermeire S., Clemente J., Colombel J.F., et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial crohn’s disease. mBio. 2016;7:e01250-16. doi: 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Balaure P.C., Boarca B., Popescu R.C., Savu D., Trusca R., Vasile B.Ș., Grumezescu A.M., Holban A.M., Bolocan A., Andronescu E. Bioactive mesoporous silica nanostructures with anti-microbial and anti-biofilm properties. Int. J. Pharm. 2017;531:35–46. doi: 10.1016/j.ijpharm.2017.08.062. [DOI] [PubMed] [Google Scholar]
- 174.Dohare S., Dubey S.D., Kalia M., Verma P., Pandey H., Singh N.K., Agarwal V. Anti-biofilm activity of Eucalyptus globulus oil encapsulated silica nanoparticles against E. coli biofilm. Int. J. Pharm. Sci. Res. 2014;5:5013–5018. [Google Scholar]
- 175.Ju X., Li J., Zhu M., Lu Z., Lv F., Zhu X., Bie X. Effect of the luxS gene on biofilm formation and antibiotic resistance by Salmonella serovar Dublin. Food Res. Int. 2018;107:385–393. doi: 10.1016/j.foodres.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 176.Reichling J. Anti-biofilm and virulence factor-reducing activities of essential oils and oil components as a possible option for bacterial infection control. Planta Med. 2020;86:520–537. doi: 10.1055/a-1147-4671. [DOI] [PubMed] [Google Scholar]
- 177.Vidallon M.L.P., Teo B.M. Recent developments in biomolecule-based nanoencapsulation systems for antimicrobial delivery and biofilm disruption. Chem. Commun. 2020;56:13907–13917. doi: 10.1039/D0CC05880G. [DOI] [PubMed] [Google Scholar]
- 178.Bilia A.R., Guccione C., Isacchi B., Righeschi C., Firenzuoli F., Bergonzi M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014;2014:651593. doi: 10.1155/2014/651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 179.Bazana M.T., Codevilla C.F., de Menezes C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019;26:47–56. doi: 10.1016/j.cofs.2019.03.005. [DOI] [Google Scholar]
- 180.Oz Y., Nabawy A., Fedeli S., Gupta A., Huang R., Sanyal A., Rotello V.M. Biodegradable poly (lactic acid) stabilized nanoemulsions for the treatment of multidrug-resistant bacterial biofilms. ACS Appl. Mater. Interfaces. 2021;13:40325–40331. doi: 10.1021/acsami.1c11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cui H., Li W., Li C., Vittayapadung S., Lin L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling. 2016;32:215–225. doi: 10.1080/08927014.2015.1134516. [DOI] [PubMed] [Google Scholar]
- 182.Prakash A., Vadivel V. Citral and linalool nanoemulsions: Impact of synergism and ripening inhibitors on the stability and antibacterial activity against Listeria monocytogenes. J. Food Sci. Technol. 2020;57:1495–1504. doi: 10.1007/s13197-019-04185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marinković J., Nikolić B., Marković T., Radunović M., Ilić J., Bošković M., Ćirić A., Marković D. Cymbopogon citratus essential oil: An active principle of nanoemulsion against Enterococcus faecalis root canal biofilm. Future Microbiol. 2021;16:907–918. doi: 10.2217/fmb-2021-0081. [DOI] [PubMed] [Google Scholar]
- 184.Mehanna M.M., Mneimneh A.T., El Jalil K.A. Levofloxacin-loaded naturally occurring monoterpene-based nanoemulgel: A feasible efficient system to circumvent MRSA ocular infections. Drug Dev. Ind. Pharm. 2020;46:1787–1799. doi: 10.1080/03639045.2020.1821048. [DOI] [PubMed] [Google Scholar]
- 185.Song X., Wang L., Liu T., Liu Y., Wu X., Liu L. Mandarin (Citrus reticulata L.) essential oil incorporated into chitosan nanoparticles: Characterization, anti-biofilm properties and application in pork preservation. Int. J. Biol. Macromol. 2021;185:620–628. doi: 10.1016/j.ijbiomac.2021.06.195. [DOI] [PubMed] [Google Scholar]
- 186.Scandorieiro S., de Camargo L.C., Lancheros C.A., Yamada-Ogatta S.F., Nakamura C.V., de Oliveira A.G., Andrade C.G., Duran N., Nakazato G., Kobayashi R.K. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016;7:760. doi: 10.3389/fmicb.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Duncan B., Li X., Landis R.F., Kim S.T., Gupta A., Wang L.S., Ramanathan R., Tang R., Boerth J.A., Rotello V.M. Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano. 2015;9:7775–7782. doi: 10.1021/acsnano.5b01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Comin V.M., Lopes L.Q., Quatrin P.M., de Souza M.E., Bonez P.C., Pintos F.G., Raffin R.P., Vaucher R., Martinez D.S., Santos R.C. Influence of Melaleuca alternifolia oil nanoparticles on aspects of Pseudomonas aeruginosa biofilm. Microb. Pathog. 2016;93:120–125. doi: 10.1016/j.micpath.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 189.Perez A.P., Perez N., Lozano C., Altube M.J., de Farias M.A., Portugal R.V., Buzzola F., Morilla M.J., Romero E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicles. Phytomedicine. 2019;57:339–351. doi: 10.1016/j.phymed.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 190.Liu T., Liu L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020;162:1509–1515. doi: 10.1016/j.ijbiomac.2020.07.207. [DOI] [PubMed] [Google Scholar]
- 191.Ibrahim D., Abdelfattah-Hassan A., Badawi M., Ismail T.A., Bendary M.M., Abdelaziz A.M., Mosbah R.A., Mohamed D.I., Arisha A.H., El-Hamid M. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021;11:7742. doi: 10.1038/s41598-021-86990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lim H.S., Paik I.K., Sohn T., Kim W.Y. Effects of supplementary copper chelates in the form of methionine, chitosan and yeast on the performance of broilers. Asian-Australas. J. Anim. Sci. 2006;19:1322–1327. doi: 10.5713/ajas.2006.1322. [DOI] [Google Scholar]
- 193.Amiri N., Afsharmanesh M., Salarmoini M., Meimandipour A., Hosseini S.A., Ebrahimnejad H. Nanoencapsulation (in vitro and in vivo) as an efficient technology to boost the potential of garlic essential oil as alternatives for antibiotics in broiler nutrition. Animal. 2021;15:100022. doi: 10.1016/j.animal.2020.100022. [DOI] [PubMed] [Google Scholar]
- 194.Hosseini S.A., Meimandipour A. Feeding broilers with thyme essential oil loaded in chitosan nanoparticles: An efficient strategy for successful delivery. Br. Poult. Sci. 2018;59:669–678. doi: 10.1080/00071668.2018.1521511. [DOI] [PubMed] [Google Scholar]
- 195.Amiri N., Afsharmanesh M., Salarmoini M., Meimandipour A., Hosseini S.A., Ebrahimnejad H. Effects of nanoencapsulated cumin essential oil as an alternative to the antibiotic growth promoter in broiler diets. J. Appl. Poult. Res. 2020;29:875–885. doi: 10.1016/j.japr.2020.08.004. [DOI] [Google Scholar]
- 196.Siddiqui S.A., Bahmid N.A., Taha A., Abdel-Moneim A.E., Shehata A.M., Tan C., Kharazmi M.S., Li Y., Assadpour E., Castro-Muñoz R., et al. Bioactive-loaded nanodelivery systems for the feed and drugs of livestock; purposes, techniques and applications. Adv. Colloid Interface Sci. 2022;308:102772. doi: 10.1016/j.cis.2022.102772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data is contained within the article.