Abstract
The phenomenon of bacterial antimicrobial resistance (AMR) is a rapidly growing global problem. Overuse and misuse of antibiotics as well as self-prescription are among the most important causes contributing to the growth of antibiotic resistance in humans. This systematic review describes the phenomenon of antibiotics self-medication (ASM) in children. The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist by searching PubMed, Scopus, and Web of Science until July 2022. Published English language studies containing information regarding parents knowledge, attitudes, and behaviors in self-administration of antibiotics in children were included. A total of 702 articles were identified, and 57 were selected. A higher prevalence of ASM among children was found in the Middle-East (34%), Africa (22%), Asia (20%) and South America (17%), while the lowest prevalence was found in Europe (8%). High distance from hospital, and low income, such as having more than one child, are related with an increased risk of ASM in children. Fever and cough can also promote the misuse of antibiotics by parents. A greater attention to the regulation of the sale of antimicrobial drugs can certainly limit the risk of self-medicating behavior.
Keywords: antibiotics, self-medication, children, pre-school, parents, scholar-age
1. Introduction
The phenomenon of bacterial antimicrobial resistance (AMR), which occurs when changes in bacteria cause drugs used to treat infections to become less effective, is a rapidly growing global problem [1]. In 2017, the World Health Organization first published a document with the 12 families of bacteria that, due to their particular antibiotic resistance mechanisms, are considered dangerous to human health [2].
Specifically, each year worldwide, AMR is directly involved in more than 1.27 million deaths and contributes to another 4.95 million deaths [1]. Because of this enormous impact on human health, in 2019 WHO listed AMR as one of the top ten health threats to the global population [3].
Leading international public health organizations have highlighted the need for early action through prevention strategies and countermeasures to limit the spread of multidrug-resistant microorganisms [4,5]. Among the causes investigated, overuse and misuse of antibiotics are among the most important causes contributing to the growth of antibiotic resistance in humans. In particular, misuse of antimicrobial drugs can occur either through poor adherence to therapy or self-medication [6].
Antibiotic self-medication (AMS) refers to the purchase and use of antibiotic drugs without consulting a physician, as well as storing drugs previously used to treat infections in the home in order to more quickly resolve health problems that are considered similar [7]. Numerous studies have shown that the phenomenon of self-medication with antimicrobial drugs is constantly growing, both in adults and children [8,9]. Often, indeed, having used a drug to treat a previous infection can transmit, in parents, the mistaken belief that the antibiotic can also be used to treat similar symptoms in their children [10].
In children, precisely because of their increased susceptibility, upper airway infections (URTIs) are very common [11]. However, most of these infections are caused by viruses [12]. Thus, the use of antibiotic drugs self-administered by parents can lead to misuse, which can have important implications for children’s health (e.g., side effects, allergies) and, on the other hand, promotes the spread of antibiotic resistance [13].
Numerous studies in the literature show that socio-cultural factors, particularly educational level, socioeconomic status, and nationality, are important in influencing self-medication among adolescents [14,15] and adults [16,17]. To our knowledge, however, there are no systematic reviews examining parental self-medication of children. The purpose of this systematic review is to describe the phenomenon of antibiotic administration in children by parents without a physician’s prescription. In addition, through the analysis of parents’ knowledge, attitudes, and behaviors, we analyze how socio-demographic and geographic variables are involved in the phenomenon of self-medication in children.
2. Results
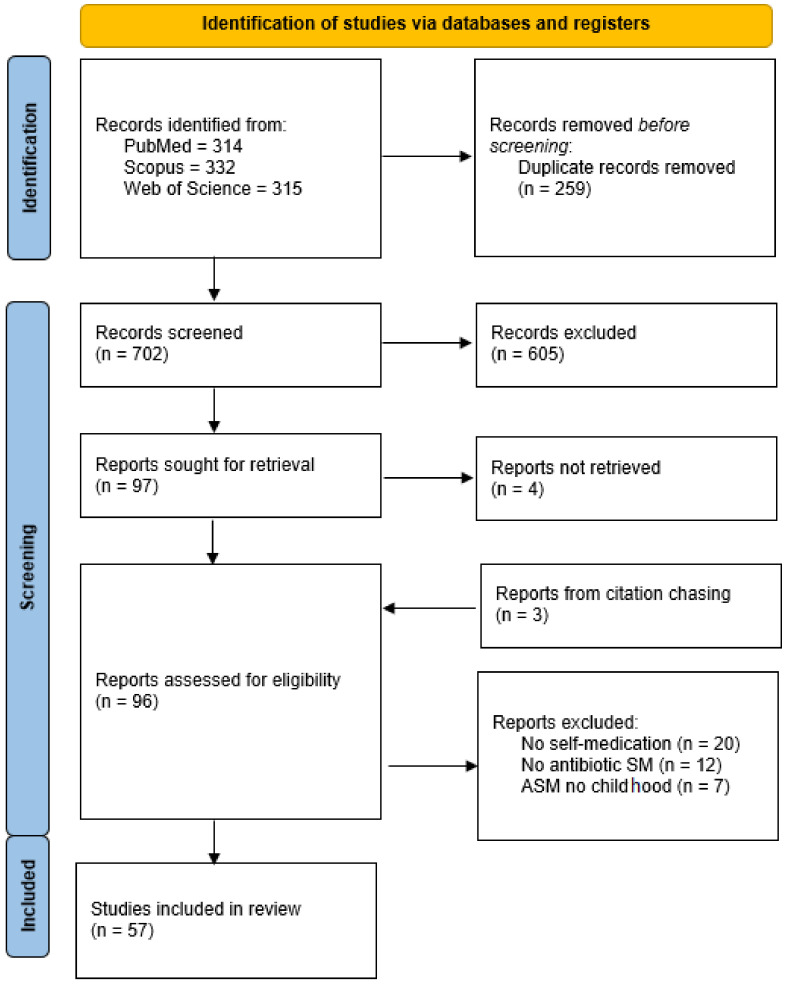
A total of 961 articles were found in PubMed, Scopus, and Web of Science (314, 332, and 315, respectively). Other three article were identified from citation chasing. After the presence of duplicates was assessed a total of 702 articles were screened following the PRISMA Flow Diagram (Figure 1). An overall of 605 studies were excluded based on non-pertinent title or abstract while the remaining ninety seven were full-text read. Among them 43 were excluded due to various reasons: 20 article did not deal with self-medication, 12 did not deal with antibiotics self-medication, 7 deal with antibiotic self-medication but they did not focus on childhood, and 4 were not available.
Figure 1.
Prisma flow chart.
2.1. Study Characteristics
In total, 57 studies were examined, 56 were cross-sectional articles while one was a systematic review. All studies contained information about antibiotic self-medication attitude. The sample size varied consistently with a minimum number of participants of 85 caregiver and a maximum number of 9526. The average of participants was 1471 and the standard deviation was 2075.
Geographically, the studies analyzed were conducted in Asia (n = 19), the Middle East (n = 11), Africa (n = 9), Europe (n = 9), South America (6), U.S.A. (n = 2), and the Caribbean (n = 1). The studies more frequently came from China (n = 11), Nigeria (n = 5), India (n = 4), Brazil (n = 3), and Saudi Arabia (n = 3).
2.2. Antibiotics Self-Medication (ASM)
Prevalence of antibiotics self-medication among children was always obtained from surveys. In an Iraqi study, only parents who self-medicated their children were selected [18].
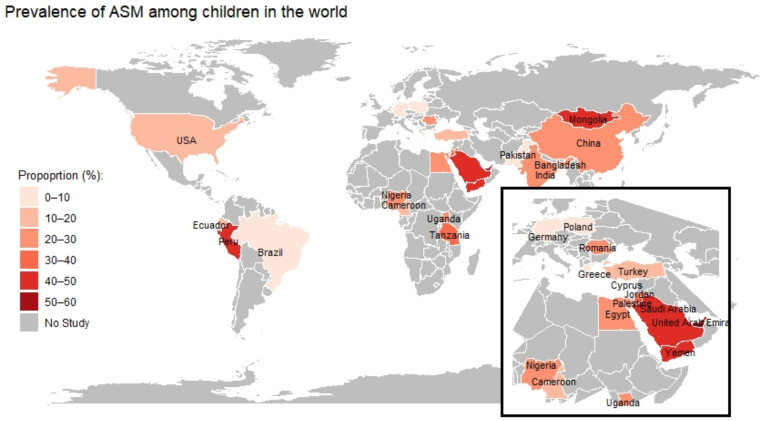
On average, parents who have self-medicated their children or who would have were 24%. The lowest prevalence was found in Greece where 1% of parents admitted to use antibiotics without prescription for their children [19]. On the other hand, the highest prevalence of ASM was found in a Saudi article where Al-Ayed M. S. Z. et al. found a prevalence of parents purchasing antibiotics without prescription of 69% [20] (Figure 2).
Figure 2.
World map showing prevalence of self-medication with antibiotics in children.
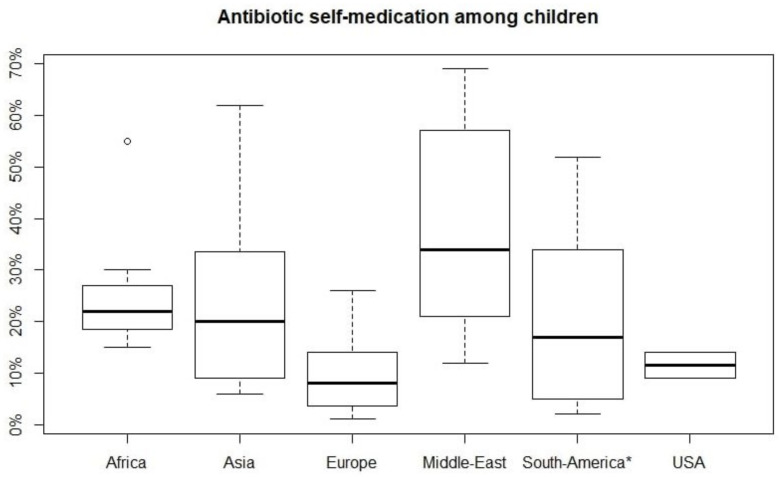
A wide range of prevalence of antibiotics self-medication was found, but generally it was higher in some countries than in others. A box plot was created to analyze the distribution of ASM in the world region from which the studies were conducted (Figure 2). A higher prevalence was found in Middle East (34%), Africa (22%), Asia (20%), and South America (17%), while the lowest prevalence was found in Europe (8%) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] (Figure 3).
Figure 3.
Distribution of prevalence of antibiotics self-medication represented by box plot in world regions. Median (middle black line), confidence region (box), and the maximum non-outlying envelope (whiskers). The circles are the outliers. * Caribbean study was included in South America.
2.3. Parents’ Characteristics and Association with Antibiotic Self-Medication
Many studies highlighted that none or few of the parent’s features are significantly related with antibiotics self-medication. Nevertheless, some articles found that significant association between parents characteristics and ASM.
Parents’ main characteristics such as age, living in a rural area, socioeconomic status, relationship with child, level of instruction and occupation, were often obtained.
Mothers answered 68% of questionnaires or interviews, on average. Generally, age, socioeconomic status, and level of instruction were obtained using categorical variable, while questions about occupation were focused on distinguishing who worked in a medical field.
2.3.1. Parents Relationship with Children
Two studies discovered that the risk of ASM was significantly higher for mothers than fathers [40,46], but other three studies found that the risk of self-medication was higher in fathers than in mothers [47,57,63].
2.3.2. Age of Parents
Only two study found that parent’s age resulted significantly related to self-medication. In the first one, from Tanzania [56], researchers found that parents younger than 40 tended to administer more antibiotics without consulting a physician. On the contrary, in a study from Jordan parents older than 40 were more inclined to self-medicate their children with antibiotics [45].
2.3.3. Socioeconomic Status
Low and middle income were statistically related with a high risk of antibiotics self-medication [21,45,46,65,66] with a maximum odds ratio of 4.44 (1.52 to 18.95) [66], while high economic status reduced the risk of self-medication [57,61] such as having an health insurance [65]. In addiction, Palmer D.A. et al. demonstrated that self-medication risk increased if parents attended a Community Health Center instead of a private center [70]. Conversely, two studies demonstrated that medical insurance increased the risk of antibiotic self-medication in children with an odds ratio of 2.31 and a confidence interval (C.I.) of 1.38–4.02 and of 1.30 (C.I. 1.05–1.61), respectively [27,29].
2.3.4. Educational Level
There are many studies which considered a medium or high level of instruction as a protective factor. For example both in a Brazilian study and in a Chinese study, high-school degree significantly reduced the risk of ASM [57,64]. The same result was observed in a Jordan study were parents who have attended university had a reduced risk of antibiotic self-medication [47]. Moreover, a high level of instruction turned out to be a risk factor for ASM only for mothers in a Chinese study [27] and in a German study (OR 1.37, IC 1.19–1.57) that considered both children and adolescents [69].
2.3.5. Working in a Medical Field
Despite Mukattash, T. L. et al. demonstrated that working in a medical field resulted to be a protective factor against AMS with an odds ratio (OR) < 1 (p < 0.001) [45], two Chinese studies found that this factor was associated with antibiotics self-medication with an odds ratio of 2.74 (1.080–7.077) and an odds ratio of 1.38 (1.14–1.66), respectively [29,65].
2.3.6. Accessibility to Health Services
Distance from hospital and living in a rural area was associated with antibiotic self-medication. In an Ugandan article the risk of self-medication was 3.7 times higher (C.I. 1.86–7.22) for those parents who lived in rural areas [47]. In a Tanzanian article, a distance >30 Km was statistically associated with ASM (OR 1.2; C.I. 1.1–1.3) and a similar result was found in an American and in a Chinese study, respectively [34,56,62].
2.4. Parents’ Knowledge Associated with Antibiotics Self-Medication
2.4.1. Antimicrobial Resistance
On average, 53% of parents, ranging from 11.3% [33] to 90% [71], knew the problem of antimicrobial resistance [20,25,36,37,39,42,45,48,51,53,54,55,58,59,62,67,70,72].
2.4.2. Symptoms Affecting ASM in Children
From 6.4% [35] to 85% [39] of parents thought that antibiotics were indicated to relief symptoms such as fever, cough, runny nose, abdominal pain, or common cold [20,25,32,33,35,37,39,42,48,53,59,62,66,67,70,71,72] and this was associated with antibiotic self-medication in a Saudi study with an odds ratio of 2.17 (C.I. 1.19–3.96) [21].
2.4.3. Antibiotics to Treat Viruses
Over 60%, ranging from 17.9% [35] to 92% [32], of parents believed that antibiotics were useful to treat disease typically provoked by viruses [20,25,32,35,37,38,39,42,45,48,51,53,54,55,58,59,62,67,70,71,72]. In a Caribbean study, it was demonstrated that parents with low knowledge about antibiotics (Caregivers’ Antibiotic Knowledge Score (AKS)<12) had an higher risk to self-medicate their children [51].
2.4.4. Ability to Recognize Antibiotics
From 32.5% [20] to 80% [33] of parents recognized common antibiotics such as amoxicillin [20,33,42,66,72]. The ability to recognize antibiotics was also related with ASM by Lin L. et al. in a Chinese study where parents with a medium or high ability to recognize antibiotics tended to self-medicate their children: OR 1.55 (C.I. 1.14–2.11), OR 1.73 (C.I. 1.31–2.29), respectively [41].
2.5. Parents’ Attitude Associated with Antibiotics Self-Medication
2.5.1. Management of Leftover Antibiotics
On average, about 40%, ranging from 12% [47] to 80.5% [65], of parents interviewed believed that leftover antibiotics used to treat their children or an other member of their family in the past could be used to treat their children [21,37,45,47,50,51,53,54,55,61,62,66,72]. This belief was statistically associated with higher risk of ASM with an odds ratio of 3.01 (C.I. 1.77–5.37) [21].
2.5.2. Experience with Antibiotics
Some studies demonstrated that parents who had some previous experience with antibiotics tended to self-medicate their children with those drugs [18,22,55]. In a Mongolian study, mothers who had already medicated children with antibiotics, had an increased risk of ASM with an odds ratio of 6.3 (C.I. 3.8–10.5) [59]. Moreover it was demonstrated that being used to self-medication was statistically associated to an increased risk of antibiotic self-medication of children [36] as well as using an high number of antibiotics in the last year [37].
2.5.3. Relationship with Physician
Requesting more antibiotics to physician augmented the risk of ASM with an odds ratio of 3.22 (C.I. 1.20–8.63) [60]. In addiction, low confidence with physicians increased the risk of antibiotic self-medication [58].
Finally, Yu M. et al. found that parents who did not know that antibiotics are not over-the-counter drugs, but must be prescribed by a physician, tended to self-medicate their children [62]. A similar observation was found by Chang, J. et al. who highlighted that knowing prescription-only regulation for sales of antibiotics at community pharmacies was a protective factor against ASM in children with an OR of 0.77 (0.66–0.91) [29].
2.6. Children Features Associated with Antibiotic Self-Medication
Children characteristics were often considered as possible factors affecting self-medication. Some of the studies considered infant and preschool (0–5 years) [22,23,29,33,39,46,47,56,59,66] or scholar-aged children (6–12 years) [31,49] while all other surveys did not specify the age of the children or included parents whose children were under eighteen [18,19,20,21,24,25,26,27,28,30,32,34,35,36,37,38,40,41,42,43,44,45,48,50,51,52,53,54,55,57,58,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74].
A German survey considering behaviors of children from 0 to 17 was also included [69].
2.6.1. Age of Children
Age of children was associated with antibiotic self-medication in a Peruvian study (OR 1.3, C.I. 1.1–1.4) and in a Chinese study where the OR related to age of child was 1.15, CI 1.04–1.27 [33,62]. Moreover, Yuan, J. et al. found a similar relationship between age of children and ASM for two age ranges: children whose age was between 3 and 5 years had an odds ratio of 1.82 (C.I. 1.15–3.02), while for those was age was between 6 and 11 odds ratio augmented to 2.19 (C.I. 1.40–3.60) [63].
2.6.2. Number of Children
Having more children was a factor that augmented the risk of antibiotics self-medication in several studies. A Chinese and a Jordan study found a significant relationship with having more than one child and antibiotic self-medication; the Chinese study reported an odds ratio of 2.17 (C.I. 1.48–3.18) [47,62]. Moreover, a Saudi study found a greater risk of antibiotic self-medication in those parents with more than two children with an odds ratio of 1.68 (C.I. 0.99–2.85) [21].
2.6.3. Children Symptoms
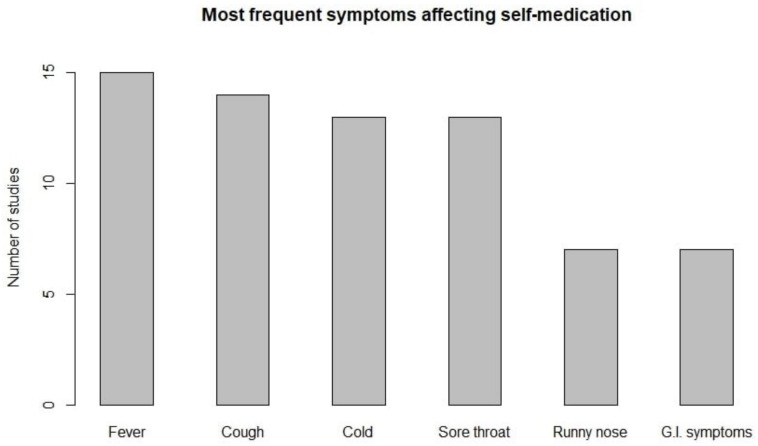
Other child factors taken into account and influencing antibiotics self-medication were. Fever, cough, common cold, and sore throat were the most frequent symptoms, followed by runny nose and gastrointestinal symptoms (Figure 4).
Figure 4.
Frequency of symptoms affecting self-medication among parents: Fever (n = 15), cough (n = 14), cold (n = 13), sore throat (n = 13), runny nose (n = 7), and G.I. symptoms (n = 7).
On average, about an half of parents who practiced ASM administered an antibiotic to relief common cold or cough, while this percentage reduced to 40% for fever and sore throat [18,19,22,26,27,30,31,34,40,41,42,43,46,47,50,51,54,55,56,58,59,60,61,64,69,70,71,74].
Moreover, a study highlighted that there were symptoms causing a greater risk of self-medication. Fever appeared to be a risk factor for self-medication in a Chinese study with an odds ratio of 1.89 (C.I. 1.58–2.26) [41]. Cough was statistically related to ASM in an Ugandan study where the odds ration was 3.54 (C.I. 1.55–8.06) [47]. In this study, diarrhea was also a condition affecting self-medication, with an odds ratio of 8.00 (C.I. 3.31–19.30). Moreover, Zhu, Y. et al. found that runny nose turned out to be a possible risk factor [66].
2.6.4. Children Health Status Perception
Pfaffenbach G. et al. associated the practice of self-medication with personal health state perception. In particular, despite the fact that this study also focused on adolescents, it was demonstrated that the children or adolescents who considered their health status low tended to practice more self-medication [69]. A similar result was found in two Chinese studies: the first one highlighted that parents who considered their children’s health status “good” or “very good” had low risk of ASM (OR 0.48, C.I. 0.40–0.57) [29], while the second one found that medium/high severity of children auto-diagnosed disease OR 1.76 (C.I. 1.40–2.23) [41].
2.7. Source of Information
Many studies have investigated parents sources of information about the correct use of antibiotics.
2.7.1. Physicians
The main source of information were physicians. In fact, in three European studies [71,72,73] the percentage of parents whose main source of information was physician ranged between 80% and 90%. This result was similar in a Chinese study where physicians were the main source of information for 70% of the sample [62], while in a Pakistani study this percentage was around 90% [26]. By the way, sometimes physicians were not the main source of information. For example, in a Nigerian study, just one third of parents obtained information about the correct use of antibiotics from doctors [50] and in a Pakistani study, family, friends, and pharmacists were a greater source of information about the correct use of antibiotics [41].
2.7.2. Pharmacists
The second most important source of information was a pharmacist. Especially, in some studies, a great percentage of parents search for information in a pharmacy [40,58]. In a European study, the amount of parents who received information about the use of antibiotics by a pharmacist was 15%. The same result was seen in an Indian study. On the contrary many articles reported percentage of information by pharmacist from 24% to 63% [25,35,43,62,65,73]. In addiction, in a Peruvian study, the odds ratio of self-medication was 3.0 for parents who received information in pharmacy with a significant confidence interval (1.9–4.6) [22].
2.7.3. Other Source of Information
Other source of information reported were: television and mass media [43,72], drug leaflet [37,73], family and friends [43,50,65], and the advice from a third-party was significantly related to antibiotics self-medication in a Cameroonian study (p < 0.05) [35]. Finally, a Saudi article highlighted that around 33% of parents search for information on the Internet, which was an important source of information in a Greek study too (37%) [25,72].
2.8. Source of Antibiotics
Most of these studies reporting information regarding the source of antibiotics described two main ways by which parents obtained antibiotics, which are discussed as follows.
2.8.1. Purchasing Antibiotics without Prescription
This was a worldwide practice found in various articles from Africa, Asia, and the Middle East, but also in Europe (Macedonia) [18,20,36,38,45,47,55,56,66,68,74] and it was significantly related to the practice of self-medication in a Chinese study with an odds ratio of 1.15 (C.I. 1.01–1.30) [27].
2.8.2. Leftover Antibiotics from Previous Prescribed Treatment
This practice was found less frequently and, in general, it was a marginal source of antibiotics: 4% in a Macedonian study, 12% in an Egyptian study, 14.7% in a Emirate study, and 15.7% in a Chinese study [36,38,54,66]. There was also an exception represented by an Indian article where the percentage of parents who used leftover antibiotics for their children self-medication was over one third [68].
Zhu Y. et al. [66] described an other practice in Yiwu, a city of about 2 million people situated in the central Zhejiang Province of China: about one third of parents who self-medicated their children tend to stock antibiotics at home.
3. Materials and Methods
3.1. Search Strategy and Selection Criteria
The systematic review was conducted following the PRISMA checklist by searching PubMed, Scopus, and Web of Science until July 2022, no limit was set as to the year of publication or study location. It was registered with the Open Science Framework (OSF).
The review was conducted according to the PRISMA guidelines that ensure transparency, accuracy, and complete reporting of systematic reviews [75]. Two researcher conducted the initial screening of title and abstract, evaluated independently all the screened full-text article and finally extracted the data to conduct semi-quantitative analysis and quality analysis of studies.
Three databases were searched: PubMed, Scopus, Web Of Science. Search terms included both MeSH terms and free text (keywords, synonyms, and word variations), connected with Boolean operators. Specifically, we applied “OR” in each group of keywords and MeSH terms to identify the areas of interest and “AND” operator to combine each group. Strings used for each database are available as Supplementary Materials.
3.2. Eligibility Criteria
All types of studies designs were included if they met the following inclusion criteria:
Study was available in English language.
Study contained information about parental attitude to antibiotics self-medication.
Study focused on infant, pre-school, or scholar-age children.
Studies that dealt with antibiotics self-medication in both children and adolescents were also included.
3.3. Definitions
According to National Library of Medicine:
Self-medication consists in the self administration of medication not prescribed by a physician or in a manner not directed by a physician.
Antibiotics or anti-bacterial agents are defined as substances that inhibit the growth or reproduction of bacteria.
Children are divided by age in: infant (less than 2), pre-school (between 2 and 5), and school-age (between 6 and 12).
3.4. Data Extraction and Management
The screening of search results was performed using the web-based, open-access platform Rayyan (https://www.rayyan.ai/, accessed on 25 July 2022). Data were independently extracted by two authors into pre-defined and labeled columns in an Excel spreadsheet. Data extracted include the nation, population, socio-demographic and socio-economic characteristics of the parents, and their knowledge, attitudes, and behaviors related to self-administering antibiotics to their children. Proportion of attitude or practice of antibiotic self-medication was always obtained. Bar chart, box plot, and map were obtained processing data on R software [76].
4. Discussion
The phenomenon of antimicrobial resistance is a rapidly progressing problem worldwide. In recent years, major public health agencies have highlighted the urgency of monitoring and investigating this phenomenon to counter the spread of antibiotic-resistant microorganisms [1,2,3,4,5]. The misuse of these drugs, as well as self-medication, are the main causes of this phenomenon. In particular, self-medication in children, precisely because of their greater susceptibility to airway infections, can play a key role in combating the spread of AMR. Understanding the socio-cultural, geographical, and economic variables that may influence parents’ behavior regarding self-medication of their children is essential for planning future health education interventions aimed at contain the overuse and misuse of these drugs.
In this systematic review, which includes studies from all over the world, significant differences regarding ASM emerged. In particular, it was observed that in some geographic areas, the practice of antibiotic self-medication in children is frequent. One of the causes that may partly justify these differences on a macro-regional level is the different possibility of purchasing antibiotics without a prescription [77,78,79,80].
The regions showing high rates of antibiotics bought without a prescription seem to overlap geographically with the same ones that showed a higher prevalence of antibiotic self-medication in children. In recent years, many countries have introduced stricter regulations on the sale of antibiotics, but the prevalence of drugs sold without a prescription remains high. This is mainly due to non-compliance by community pharmacies with existing laws. Despite the severe punishments provided, these interventions do not seem to be decisive [78]. Therefore, in these contexts, community pharmacists trained in antibiotic stewardship could play a significant role in ensuring rational use of antibiotics [80].
In addition to the legislative aspect, the common cultural denominator, at the level of the general population and public health policies, may play a key role in explaining this phenomenon. In particular, this can be partially appreciated by comparing the sales of antibiotics without prescription in some macro-regions with the prevalence of self-medication in children. For example, in some European countries, it is possible to buy antibiotics without a prescription, although it is not legal, but the prevalence of ASM in children is low (Figure 3).
This is probably related to a higher average level of education and more developed health and welfare policies. As shown in our study, a high level of parental education is a potential protective factor. Children of these parents have a lower risk of receiving nonprescription antibiotics (Table 1). In addition, another aspect that influences attitudes toward antibiotic self-medication in children is the accessibility of healthcare. In particular, physical distance from health care services in rural areas may cause under-utilization of these services and encourage self-medication behaviors such as storing previously used drugs. Furthermore, considering the non-universality of most health care systems, parents’ difficulty in bearing the cost of medical consultation may be a risk factor for self-medication of antibiotics in children (Table 1). To overcome these difficulties, many parents reported asking pharmacists for information about antibiotic use.
Table 1.
Parents characteristics, knowledge, and attitude associated with self-medication.
| Features Associated with ASM | Risk of ASM 1 | Citation |
|---|---|---|
| Child relationship: Mother | OR 0.30 (0.09–0.96) | Nyeko et al. [47] |
| OR 0.83 (0.74-0.94) | Sun et al. [57] | |
| Child relationship: Father | OR 0.74 (0.4–1.3) | Abdulaziz H et al. [21] |
| OR 0.53 (0.3–0.96) | Zhu et al. [66] | |
| OR 1.27 (1.1–1.5) | Yuan et al. [63] | |
| High distance from hospital/Rural Area | OR 3.70 (1.9–7.2) | Nyeko et al. [47] |
| OR 1.20 (1.1–1.3) | Simon and Kazaura [56] | |
| OR 1.60 (1.1–2.4) | Yu et al. [62] | |
| Low/middle Economic Status | OR 4.44 (1.5–19.0) | Zhu et al. [66] |
| OR 3.60 (1.3–9.7) | Zhou et al. [65] | |
| OR 2.00 (1.1–3.8) | Abdulaziz H et al. [21] | |
| High Economic Status | OR 0.66 (0.5–1) | Sun et al. [57] |
| Having medical insurance | OR 0.36 (0.1–1) | Zhou et al. [65] |
| OR 2.31 (1.4–4.0) | Bi et al. [27] | |
| OR 1.30 (1.1–1.6) | Chang et al. [29] | |
| High level of instruction | OR 0.71 (0.5–1) | Sun et al. [57] |
| OR 0.34 (0.2–0.5) | Zhang et al. [64] | |
| Working in medical field | OR 1.38 (1.1–1.7) | Chang et al. [29] |
| OR 2.74 (1.1–7.1) | Zhou et al. [65] | |
| Medium ability to recognize antibiotics | OR 1.55 (1.1–2.1) | Lin et al. [41] |
| High ability to recognize antibiotics | OR 1.73 (1.3–2.3) | Lin et al. [41] |
| Tendency toward self-medication | OR 6.30 (3.8–10.5) | Togoobaatar et al. [59] |
| Requesting antibiotics to physician | OR 3.22 (1.2-8.6) | Xu et al. [60] |
| Knowing antibiotics should be prescribed | OR 0.77 (0.7–0.9) | Chang et al. [29] |
1 Only significant odds ratios, with their confidence interval were inserted in the table.
Having more children could increase the risk of self-medication (Table 2). Rather than an independent risk factor, this may be the result of multiple factors that are enhanced by having more than one child. As just mentioned, access to facilities is not always guaranteed, whether due to physical distance or economic status. This is more important in case parents have more than one child. In addition, since previous experience in antibiotic use is a risk factor for ASM (Table 1), having more children can promote this behavior. This is because those parents with two or more children are more likely to have administered an antibiotic in the past than those with only one child. The probability of having leftover antibiotics in pediatric formulation at home is higher too.
Table 2.
Children’s characteristics associated with self-medication.
| Features Associated with ASM | Risk of ASM 1 | Citation |
|---|---|---|
| Age of children | OR 1.30 (1.1–1.4) | Ecker et al. [33] |
| OR 1.15 (1.1–1.3) | Yu et al. [62] | |
| Having more than one child | OR 2.17 (1.5–3.2) | Yu et al. [62] |
| OR 1.68 (1.0–2.9) | Abdulaziz H et al. [21] | |
| Children poor health status | OR 2.10 (1.75–2.5) 2 | Chang et al. [29] |
| OR 1.76 (1.40–2.23) | Lin et al. [41] | |
| Fever | OR 1.89 (1.6–2.3) | Lin et al. [41] |
| Cough | OR 3.54 (1.6–8.1) | Nyeko et al. [47] |
| Diarrhea | OR 8.00 (3.3–19.3) | Nyeko et al. [47] |
| Runny Nose | OR 1.86 (1.13–3.19) | Zhu et al. [66] |
1 Only significant odds ratios (OR), with their confidence interval (CI) were inserted in the table; 2 The reciprocal odds ratio was calculated.
The age of the children seems to influence parental behavior, as an increase in age would increase the risk of self-medication. This could be linked to a lower perception of the risk of side effects in scholar-age children (Table 2). On the contrary, parental age appears not to be a risk factor for ASM as only two studies showed statistical significance, but the results were diametrically opposed.
Considering the symptoms that most frequently promote self-medication in children, such as fever, cough, and sore throat, these symptoms are often caused by viruses and do not represent an indication for the use of antibiotic therapy. Nevertheless, they are among the symptoms that most frequently elicit parents to practice ASM in children (Table 2). As mentioned earlier, parental education plays a key role in this context. In fact, it was seen that among parents who had appropriate knowledge about the use of antibiotics, the prevalence of self-medication was lower. Although there are limited data, this does not appear to be true for health-care workers. In fact, in this category there is a higher prevalence of parents administering antibiotics without a prescription to their children (Table 1). On the other hand, a lower comprehension of the risks associated with self-medication remained among parents who were able to recognize antibiotics or had previous experience handling antibiotics (Table 1). This highlights that, among the measures to combat self-medication in children, in particular and anti-microbial resistance in general, it is important to involve parents through education and training programs. In fact, is fundamental to note that parents’ knowledge about the proper use of antibiotics was found to be generally low. For example, only 50% of parents on average were unaware of the problem of AMR. Good practices on the correct use of antibiotics could be spread through television and the mass media that many parents reported to be important source of information. Conversely, the acquisition of information through family and friends should be discouraged as it has been correlated with an increase in bad practice in the use of antibiotics.
The main limitation of this review is the poor representation of some macro-regions or continents. We cannot exclude the possibility that studies were conducted mainly where the problem of inappropriate antibiotic use is most felt (publication bias). In addition, the extracted data are mainly derived from surveys. Moreover, there are no studies correlating the type of antibiotic or pharmaceutical form with self-medication practice in children. For these reasons, further studies are needed to better understand the extent of the problem globally.
In conclusion, although antibiotic self-medication among children is a global phenomenon, influenced by a number of geographical, cultural, and economic factors, there is an urgent need to promote a worldwide health strategy.
Specifically, greater attention to the regulation of the sale of antimicrobial drugs can partially limit the risk of self-medicating behavior. The introduction of health education programs, specifically aimed at parents and pharmacists, can at the same time, improve understanding of the risks associated with self-medication.
Lastly, constant monitoring of these phenomena, and raising stakeholders’ awareness of the practices that lead to antibiotic resistance, may favor a more careful use of these drugs in the near future.
Abbreviations
The following abbreviations are used in this manuscript:
| AMR | Anti Microbial Resistance |
| ASM | Antibiotic Self-Medication |
| CI | Confidence Interval |
| OR | Odds Ratio |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11111583/s1, search strings.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray C.J., Ikuta K.S., Sharara F., Swetschinski L., Aguilar G.R., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrivastava S.R., Shrivastava P.S., Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018;32:76. doi: 10.4103/jms.jms_25_17. [DOI] [Google Scholar]
- 3.Thangaraju P., Venkatesan S. WHO Ten threats to global health in 2019: Antimicrobial resistance. Cukurova Med. J. 2019;44:1150–1151. [Google Scholar]
- 4.World Health Organization . Antimicrobial Resistance Surveillance in Europe 2022–2020 Data. World Health Organization; Geneva, Switzerland: 2022. [Google Scholar]
- 5.World Health Organization . Monitoring and Evaluation of the Global Action Plan on Antimicrobial Resistance: Framework and Recommended Indicators. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 6.Kardas P., Devine S., Golembesky A., Roberts C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int. J. Antimicrob. Agents. 2005;26:106–113. doi: 10.1016/j.ijantimicag.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Wise R. Antimicrobial resistance: Priorities for action. J. Antimicrob. Chemother. 2002;49:585–586. doi: 10.1093/jac/49.4.585. [DOI] [PubMed] [Google Scholar]
- 8.Knopf H., Wolf I.K., Sarganas G., Zhuang W., Rascher W., Neubert A. Off-label medicine use in children and adolescents: Results of a population-based study in Germany. BMC Public Health. 2013;13:631. doi: 10.1186/1471-2458-13-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schillie S.F., Shehab N., Thomas K.E., Budnitz D.S. Medication overdoses leading to emergency department visits among children. Am. J. Prev. Med. 2009;37:181–187. doi: 10.1016/j.amepre.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Currie J., Lin W., Zhang W. Patient knowledge and antibiotic abuse: Evidence from an audit study in China. J. Health Econ. 2011;30:933–949. doi: 10.1016/j.jhealeco.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Eckel N., Sarganas G., Wolf I.K., Knopf H. Pharmacoepidemiology of common colds and upper respiratory tract infections in children and adolescents in Germany. BMC Pharmacol. Toxicol. 2014;15:44. doi: 10.1186/2050-6511-15-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyquist A.C., Gonzales R., Steiner J.F., Sande M.A. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279:875–877. doi: 10.1001/jama.279.11.875. [DOI] [PubMed] [Google Scholar]
- 13.Goossens H. Antibiotic consumption and link to resistance. Clin. Microbiol. Infect. 2009;15:12–15. doi: 10.1111/j.1469-0691.2009.02725.x. [DOI] [PubMed] [Google Scholar]
- 14.Shehnaz S.I., Agarwal A.K., Khan N. A systematic review of self-medication practices among adolescents. J. Adolesc. Health. 2014;55:467–483. doi: 10.1016/j.jadohealth.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Gualano M.R., Bert F., Passi S., Stillo M., Galis V., Manzoli L., Siliquini R. Use of self-medication among adolescents: A systematic review and meta-analysis. Eur. J. Public Health. 2015;25:444–450. doi: 10.1093/eurpub/cku207. [DOI] [PubMed] [Google Scholar]
- 16.Torres N.F., Chibi B., Kuupiel D., Solomon V.P., Mashamba-Thompson T.P., Middleton L.E. The use of non-prescribed antibiotics; prevalence estimates in low-and-middle-income countries. A systematic review and meta-analysis. Arch. Public Health. 2021;79:2. doi: 10.1186/s13690-020-00517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lescure D., Paget J., Schellevis F., Van Dijk L. Determinants of self-medication with antibiotics in European and Anglo-Saxon countries: A systematic review of the literature. Front. Public Health. 2018;6:370. doi: 10.3389/fpubh.2018.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jasim A. Parental self medication of antibiotics for children in Baghdad city. Int. J. Pharm. Pharm. Sci. 2014;6:485–489. [Google Scholar]
- 19.Maltezou H.C., Dedoukou X., Asimaki H., Kontou I., Ioannidou L., Mitromara K., Theodoridou K., Katerelos P., Theodoridou M. Consumption of antibiotics by children in Greece: A cross-sectional study. Int. J. Pediatr. Adolesc. Med. 2017;4:108–111. doi: 10.1016/j.ijpam.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Ayed M.S.Z. Parents’ knowledge, attitudes and practices on antibiotic use by children. Saudi J. Med. Med. Sci. 2019;7:93. doi: 10.4103/sjmms.sjmms_171_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdulaziz H.A., Haytham A.S., Faiza N.A., Abdulaziz U.J., Rowayda M.M., Amna R.S., Abdulaziz B.S. Socio-demographic determinants of antibiotic misuse in children. A survey from the central region of Saudi Arabia. Saudi Med. J. 2013;34:832–840. [PubMed] [Google Scholar]
- 22.Ochoa T.J., Balmaceda M.P., Elias R., Navarro R., Watanabe T., Moran F., Paredes J.L., Reategui A., Bardellini M. Knowledge, attitudes and practices of parents towards antibiotic use in rural communities in Peru: A cross-sectional multicentre study. BMC Public Health. 2022;22:459. doi: 10.1186/s12889-022-12855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afolabi B., Brieger W., Salako L. Management of childhood febrile illness prior to clinic attendance in urban Nigeria. J. Health Popul. Nutr. 2004;2:46–51. [PubMed] [Google Scholar]
- 24.Afolabi O.A., Ehalaiye B.F., Fadare J.O., Abdur-Rahman A.B., Ehalaiye D.N. Survey of ototopical self medication among patients attending ENT and family medicine departments in a Nigerian hospital. Eur. J. Gen. Pract. 2011;17:167–170. doi: 10.3109/13814788.2011.565323. [DOI] [PubMed] [Google Scholar]
- 25.Al-Shawi M.M., Darwish M.A., Wahab M.M.A., Al-Shamlan N.A. Misconceptions of parents about antibiotic use in upper respiratory tract infections: A survey in primary schools of the Eastern province, KSA. J. Fam. Community Med. 2018;25:5. doi: 10.4103/jfcm.JFCM_46_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solangi M.A., Ali M., Mushtaq D., Zaid M., Riaz M., Nasir A. Parent-based self-medication in Pakistani children: A qualitative cross-sectional survey. Bangladesh J. Med. Sci. 2016;15:33–38. doi: 10.3329/bjms.v15i1.22055. [DOI] [Google Scholar]
- 27.Bi P., Tong S., Parton K.A. Family self-medication and antibiotics abuse for children and juveniles in a Chinese city. Soc. Sci. Med. 2000;50:1445–1450. doi: 10.1016/S0277-9536(99)00304-4. [DOI] [PubMed] [Google Scholar]
- 28.Cantarero-Arévalo L., Hallas M.P., Kaae S. Parental knowledge of antibiotic use in children with respiratory infections: A systematic review. Int. J. Pharm. Pract. 2017;25:31–49. doi: 10.1111/ijpp.12337. [DOI] [PubMed] [Google Scholar]
- 29.Chang J., Lv B., Zhu S., Yu J., Zhang Y., Ye D., Aziz M.M., Yang C., Fang Y. Non-prescription use of antibiotics among children in urban China: A cross-sectional survey of knowledge, attitudes, and practices. Expert Rev. Anti-Infect. Ther. 2018;16:163–172. doi: 10.1080/14787210.2018.1425616. [DOI] [PubMed] [Google Scholar]
- 30.Cruz M.J., Dourado L.F., Bodevan E.C., Andrade R.A., Santos D.F. Medication use among children 0–14 years old: Population baseline study. J. Pediatr. 2014;90:608–615. doi: 10.1016/j.jped.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Santos D.B.d., Barreto M.L., Coelho H.L.L. Use of prescribed and non-prescribed medications among children living in poor areas in the city of Salvador, Bahia State, Brazil. Cad. Saúde Pública. 2011;90:2032–2040. doi: 10.1590/S0102-311X2011001000016. [DOI] [PubMed] [Google Scholar]
- 32.Ecker L., Ochoa T.J., Ruiz J., Vargas M., Del Valle L.J. Caretakers knowledge, attitude and practices about antibiotics use in childrens in a setting where antibiotics are available without medical prescription. Am. J. Trop. Med. Hyg. 2010;83:278. [Google Scholar]
- 33.Ecker L., Ochoa T.J., Vargas M., Del Valle L.J., Ruiz J. Factors affecting caregivers’ use of antibiotics available without a prescription in Peru. Pediatrics. 2013;131:e1771–e1779. doi: 10.1542/peds.2012-1970. [DOI] [PubMed] [Google Scholar]
- 34.Edwards D.J., Richman P.B., Bradley K., Eskin B., Mandell M. Parental use and misuse of antibiotics: Are there differences in urban vs. suburban settings? Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2002;9:22–26. doi: 10.1197/aemj.9.1.22. [DOI] [PubMed] [Google Scholar]
- 35.Elong Ekambi G.A., Okalla Ebongue C., Penda I.C., Nnanga Nga E., Mpondo Mpondo E., Eboumbou Moukoko C.E. Knowledge, practices and attitudes on antibiotics use in Cameroon: Self-medication and prescription survey among children, adolescents and adults in private pharmacies. PLoS ONE. 2019;14:e0212875. doi: 10.1371/journal.pone.0212875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Hawy R.M., Ashmawy M.I., Kamal M.M., Khamis H.A., El-Hamed N.M.A., Eladely G.I., Abdo M.H., Hashem Y., Ramadan M., Hamdy D.A. Studying the knowledge, attitude and practice of antibiotic misuse among Alexandria population. Eur. J. Hosp. Pharm. 2017;24:349–354. doi: 10.1136/ejhpharm-2016-001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gungor A., Cakir B., Yalcin H., Cakir H., Karauzun A. Evaluation of parents’ attitudes and behaviors related to the use of antibiotics in children. Turk. J. Pediatr. Dis. 2019;13:203–207. [Google Scholar]
- 38.Ivanovska V., Angelovska B., Van Dijk L., Zdravkovska M., Leufkens H.G., Mantel-Teeuwisse A.K. Change in parental knowledge, attitudes and practice of antibiotic use after a national intervention programme. Eur. J. Public Health. 2018;28:724–729. doi: 10.1093/eurpub/ckx240. [DOI] [PubMed] [Google Scholar]
- 39.Ivanovska V., Zdravkovska M., Bosevska G., Angelovska B. Antibiotics for upper respiratory infections: Public knowledge, beliefs and self-medication in the Republic of Macedonia. Contrib. Sec. Biol. Med. Sci. 2013;34:60–70. [PubMed] [Google Scholar]
- 40.Kadam Y.R., Pimple A.N., Dhumale G.B., Gore A.D., Patil S.A. Parental Use of Antibiotics as Self Medication to Their School Going Children: A Cross Sectional Study. J. Krishna Inst. Med Sci. (JKIMSU) 2018;7:16–24. [Google Scholar]
- 41.Lin L., Harbarth S., Hargreaves J.R., Zhou X., Li L. Large-scale survey of parental antibiotic use for paediatric upper respiratory tract infections in China: Implications for stewardship programmes and national policy. Int. J. Antimicrob. Agents. 2021;57:106302. doi: 10.1016/j.ijantimicag.2021.106302. [DOI] [PubMed] [Google Scholar]
- 42.Lin L., Harbarth S., Wang X., Zhou X. Survey of parental use of antimicrobial drugs for common childhood infections, China. Emerg. Infect. Dis. 2020;26:1517. doi: 10.3201/eid2607.190631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metlo M., Ubed-Ur-Rehman M.A., Dayo A., Ahmed Z., Arain M.I., Parveen R., Memon A. Evaluation of Sources of Information and Reasons for Self-Medication by Educated Parents Among Their Children in District Khairpur, Pakistan. Lat. Am. J. Pharm. 2019;38:110–115. [Google Scholar]
- 44.Mohanna M. Self-medication with antibiotic in children in Sana’a City, Yemen. Oman Med. J. 2010;25:41. doi: 10.5001/omj.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukattash T.L., Alkhatatbeh M.J., Andrawos S., Jarab A.S., AbuFarha R.K., Nusair M.B. Parental self-medication of antibiotics for children in Jordan. J. Pharm. Health Serv. Res. 2020;11:75–80. doi: 10.1111/jphs.12331. [DOI] [Google Scholar]
- 46.Nazir S., Goel K., Mittal A., Singh J., Goel R., Rashid A. Parent induced self-medication among under five children: An observational cross sectional study. TAF Prev. Med. Bull. 2015;14:81–86. doi: 10.5455/pmb.1-1381246690. [DOI] [Google Scholar]
- 47.Nyeko R., Otim F., Obiya E.M., Abala C. Pre-hospital exposures to antibiotics among children presenting with fever in northern Uganda: A facility-based cross-sectional study. BMC Pediatr. 2022;22:322. doi: 10.1186/s12887-022-03375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okide C., Grey-Ekejiuba O., Ubaka C., Schellack N., Okonta M. Parents’ Knowledge, Attitudes and Use of Antibiotics in Upper Respiratory Infections in Nigerian Children. Afr. J. Biomed. Res. 2020;23:213–220. [Google Scholar]
- 49.Oshikoya K., Njokanma O., Bello J., Ayorinde E. Family self-medication for children in an urban area of Nigeria. Paediatr. Perinat. Drug Ther. 2007;8:124. doi: 10.1185/146300907X199966. [DOI] [Google Scholar]
- 50.Oshikoya K. The Use of Prescribed and Non-Prescribed Drugs in Infants in Lagos, Nigeria “KA Oshikoya,“OF. Njokanma,” JA Bello and” EO Ayorinde. J. Med. Sci. 2008;8:111–117. [Google Scholar]
- 51.Parimi N., Pereira L.M.P., Prabhakar P. Caregivers’ practices, knowledge and beliefs of antibiotics in paediatric upper respiratorytract infections in Trinidad and Tobago: A cross-sectional study. BMC Fam. Pract. 2004;5:28. doi: 10.1186/1471-2296-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peker E., Sahin E.M., Topaloğlu N., Uludağ A., Ağaoğlu H., Güngör S. Knowledge, attitude and behavior of mothers related to acute respiratory infections. Minerva Pediatr. 2014;68:114–120. [PubMed] [Google Scholar]
- 53.Revathi B., Pandurangan K.K. A Cross Sectional Survey of Knowledge, Attitude and Practice of Antibiotic Use for Children in Chennai among Mothers. J. Pharm. Res. Int. 2020;32:103–112. doi: 10.9734/jpri/2020/v32i2030734. [DOI] [Google Scholar]
- 54.Salama R.A., Bader K.N., Rahmen A.S., Hashmi F. Parents Knowledge, attitude and practice of antibiotic use for upper respiratory tract infections in children: A cross-sectional study in Ras Al khaimah, United Arab Emirates. Epidemiol. Biostat. Public Health. 2018;15 doi: 10.2427/12969. [DOI] [Google Scholar]
- 55.Sharif S.I., Masalmeh B.E., Awad H., Osama A., Abdulmqasood Y.A., Bugaighis L.M. Parents’ knowledge and attitude to self-medication of children with antibiotics. Arch. Pharm. Pract. 2015;6:71. doi: 10.4103/2045-080X.166592. [DOI] [Google Scholar]
- 56.Simon B., Kazaura M. Prevalence and factors associated with parents self-medicating under-fives with antibiotics in Bagamoyo District Council, Tanzania: A cross-sectional study. Patient Prefer. Adherence. 2020;14:1445. doi: 10.2147/PPA.S263517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun C., Hu Y.J., Wang X., Lu J., Lin L., Zhou X. Influence of leftover antibiotics on self-medication with antibiotics for children: A cross-sectional study from three Chinese provinces. BMJ Open. 2019;9:e033679. doi: 10.1136/bmjopen-2019-033679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szenborn L., Maciaga P., Dul A., Bortnowska K., Jasonek J. Antibiotic therapy in children–Knowledge and behavior of parents. Pediatr. Pol. 2017;92:699–704. doi: 10.1016/j.pepo.2017.08.001. [DOI] [Google Scholar]
- 59.Togoobaatar G., Ikeda N., Ali M., Sonomjamts M., Dashdemberel S., Mori R., Shibuya K. Survey of non-prescribed use of antibiotics for children in an urban community in Mongolia. Bull. World Health Organ. 2010;88:930–936. doi: 10.2471/BLT.10.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J., Wang X., Sun K.S., Lin L., Zhou X. Parental self-medication with antibiotics for children promotes antibiotic over-prescribing in clinical settings in China. Antimicrob. Resist. Infect. Control. 2020;9:150. doi: 10.1186/s13756-020-00811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y., Lu J., Sun C., Wang X., Hu Y.J., Zhou X. A cross-sectional study of antibiotic misuse among Chinese children in developed and less developed provinces. J. Infect. Dev. Ctries. 2020;14:129–137. doi: 10.3855/jidc.11938. [DOI] [PubMed] [Google Scholar]
- 62.Yu M., Zhao G., Stålsby Lundborg C., Zhu Y., Zhao Q., Xu B. Knowledge, attitudes, and practices of parents in rural China on the use of antibiotics in children: A cross-sectional study. BMC Infect. Dis. 2014;14:112. doi: 10.1186/1471-2334-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan J., Du W., Li Z., Deng Q., Ma G. Prevalence and Risk Factors of Self-Medication Among the Pediatric Population in China: A National Survey. Front. Public Health. 2021;9:770709. doi: 10.3389/fpubh.2021.770709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L., Mendoza R., Costa M.M., Ottoni E.J., Bertaco A.S., Santos J.C., D’avila N.E., Faria C.S., Zenobini E.C., Gomesa A. Antibiotic use in community-based pediatric outpatients in southern region of Brazil. J. Trop. Pediatr. 2005;51:304–309. doi: 10.1093/tropej/fmi022. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Z., Zhao D., Zhang H., Shen C., Cao D., Liu G., Zhu L., Fang Y. Understanding parental self-medication with antibiotics among parents of different nationalities: A cross-sectional study. Glob. Health Res. Policy. 2021;6:42. doi: 10.1186/s41256-021-00226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y., Tang X., Yan R., Shao Z., Zhou Y., Deng X., Luo S., He H. Non-prescription antibiotic use for cough among Chinese children under 5 years of age: A community-based cross-sectional study. BMJ Open. 2021;11:e051372. doi: 10.1136/bmjopen-2021-051372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zyoud S.H., Abu Taha A., Araj K.F., Abahri I.A., Sawalha A.F., Sweileh W.M., Awang R., Al-Jabi S.W. Parental knowledge, attitudes and practices regarding antibiotic use for acute upper respiratory tract infections in children: A cross-sectional study in Palestine. BMC Pediatr. 2015;15:176. doi: 10.1186/s12887-015-0494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakraborty K., Chakraborty A., Devi S., Devi J. Family self medication in children attending a tertiary care hospital in Northeast India. Int. J. Pharm. Sci. Res. 2012;3:4899. [Google Scholar]
- 69.Pfaffenbach G., Tourinho F.S., Bucaretchi F. Self-medication among children and adolescents. Curr. Drug Saf. 2010;5:324–328. doi: 10.2174/157488610792246028. [DOI] [PubMed] [Google Scholar]
- 70.Palmer D.A., Bauchner H. Parents’ and physicians’ views on antibiotics. Pediatrics. 1997;99:e6. doi: 10.1542/peds.99.6.e6. [DOI] [PubMed] [Google Scholar]
- 71.Rousounidis A., Papaevangelou V., Hadjipanayis A., Panagakou S., Theodoridou M., Syrogiannopoulos G., Hadjichristodoulou C. Descriptive study on parents’ knowledge, attitudes and practices on antibiotic use and misuse in children with upper respiratory tract infections in Cyprus. Int. J. Environ. Res. Public Health. 2011;8:3246–3262. doi: 10.3390/ijerph8083246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panagakou S.G., Spyridis N., Papaevangelou V., Theodoridou K.M., Goutziana G.P., Theodoridou M.N., Syrogiannopoulos G.A., Hadjichristodoulou C.S. Antibiotic use for upper respiratory tract infections in children: A cross-sectional survey of knowledge, attitudes, and practices (KAP) of parents in Greece. BMC Pediatr. 2011;11:60. doi: 10.1186/1471-2431-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cristescu C., Negreș S., Suciu M., Voicu A., Buda V., Suciu L., Proks M., Voicu M. Study regarding the parents’ use of self–medication among children under 12 years old. Farmacia. 2018;66:811–819. doi: 10.31925/farmacia.2018.5.10. [DOI] [Google Scholar]
- 74.Penda C.I., Moukoko E.C.E., Youmba J.F.N., Mpondo E.M. Characterization of pharmaceutical medication without a medical prescription in children before hospitalization in a resource-limited setting, Cameroon. Pan Afr. Med. J. 2018;30:302. doi: 10.11604/pamj.2018.30.302.16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:1–11. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2018. [Google Scholar]
- 77.Batista A.D., Rodrigues D.A., Figueiras A., Zapata-Cachafeiro M., Roque F., Herdeiro M.T. Antibiotic dispensation without a prescription worldwide: A systematic review. Antibiotics. 2020;9:786. doi: 10.3390/antibiotics9110786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belachew S.A., Hall L., Selvey L.A. Non-prescription dispensing of antibiotic agents among community drug retail outlets in Sub-Saharan African countries: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2021;10:13. doi: 10.1186/s13756-020-00880-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang J., Ye D., Lv B., Jiang M., Zhu S., Yan K., Tian Y., Fang Y. Sale of antibiotics without a prescription at community pharmacies in urban China: A multicentre cross-sectional survey. J. Antimicrob. Chemother. 2017;72:1235–1242. doi: 10.1093/jac/dkw519. [DOI] [PubMed] [Google Scholar]
- 80.Auta A., Hadi M.A., Oga E., Adewuyi E.O., Abdu-Aguye S.N., Adeloye D., Strickland-Hodge B., Morgan D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019;78:8–18. doi: 10.1016/j.jinf.2018.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Material.