Abstract
Adhesion of Penicillium marneffei conidia to the extracellular matrix protein laminin via a sialic acid-dependent process has previously been demonstrated. This study describes the interaction of P. marneffei conidia with fibronectin and examines the relationship of this process to the recognition of laminin via conidia. Immunofluorescence microscopy demonstrated that fibronectin bound to the surface of conidia and to phialides, but not to hyphae, in a pattern similar to that reported for laminin. Conidia were able to bind to fibronectin immobilized on microtiter plates in a concentration-dependent manner. However, binding to fibronectin (at any given concentration of protein and conidia) was less than that to laminin under equivalent conditions. Soluble fibronectin and antifibronectin antibody inhibited adherence of conidia to fibronectin in the plate adherence assay; soluble laminin also caused pronounced inhibition. Various monosaccharides and several peptides had no effect on adherence to fibronectin. However, N-acetylneuraminic acid abolished adherence to fibronectin, indicating that the interaction was mediated through a sialic acid-dependent process; the latter parallels observations of laminin binding by conidia. Fibronectin binding (and binding of laminin) was considerably reduced by prolonged preincubation of conidia with chymotrypsin, suggesting the protein nature of the binding site. Conidia from older cultures were more adherent to both immobilized fibronectin and laminin than conidia from younger cultures. Ligand affinity binding demonstrated the presence of a 20-kDa protein with the ability to bind both fibronectin and laminin. There would therefore appear to be a common receptor for the binding of fibronectin and laminin on the surface of P. marneffei, and the interaction described here maybe important in mediating attachment of the fungus to host tissue.
Infections caused by the dimorphic fungal pathogen Penicillium marneffei are increasingly common in Southeast Asia, particularly Thailand (3, 19), Hong Kong (21), and southern China (13). The incidence of human infection with P. marneffei was, until relatively recently, very low (5, 11); the recent rise in cases can be attributed almost totally to the arrival of the AIDS pandemic in this geographical area (3, 19). In AIDS patients, infection with P. marneffei presents as a disseminated illness with fever, weight loss, skin lesions, and pancytopenia (18, 22), and it is fatal if untreated.
Infection with P. marneffei is presumed to originate via the inhalation of airborne conidia. The latter are thought to be sufficiently small to reach the alveoli. Virtually nothing is known of the pathogenic mechanisms responsible for the development of infection following conidial inhalation, although recently P. marneffei conidia have been shown to bind laminin via a sialic acid-dependent process (10). Laminin is an important extracellular matrix (ECM) glycoprotein (12) that is present in basement membranes, and it may become exposed after tissue damage. The P. marneffei laminin binding receptor would appear to have some similarities to a laminin binding protein on the surface of Aspergillus fumigatus conidia, which appears to be a sialic acid-specific lectin (1).
Other ECM proteins, such as fibronectin, have been implicated in the attachment of a variety of pathogens to both host tissues and cells (6, 7, 15, 17). Fibronectin is also a glycoprotein that contains sialic acid residues (2, 20), and in this report we describe the interaction between P. marneffei conidia and fibronectin, with particular emphasis on the interrelationship of this interaction with the previously described laminin-conidium interaction.
MATERIALS AND METHODS
Organism and culture conditions.
P. marneffei ATCC 200051 was grown in the mycelial phase on Sabouraud dextrose agar slopes at 30°C, and conidia were obtained from 8-day-old cultures as previously described (10). Conidia were quantified in a hemocytometer. For some experiments, suspensions of conidia were prepared from 1-, 2-, 4-, and 8-day-old cultures of P. marneffei as described elsewhere (10). For experiments involving the immunofluorescent labelling of fibronectin binding sites, suspensions of mycelial scraping were washed three times in sterile PBS before use.
ECM components and peptides.
Fibronectin (from human plasma) was obtained from Sigma Chemical Co. (Poole, Dorset, United Kingdom), as were laminin, derived from the basement membrane of Engelbreth-Holm-Swarm mouse sarcoma, and Arg-Gly-Asp (RGD) and Tyr-Ile-Gly-Ser-Arg (YIGSR) peptides. All reagents were stored as aliquots at −80°C until required.
Immunofluorescence microscopy.
Immunofluorescence microscopy was performed as previously described (10), using suspensions of mycelial scrapings (prepared as described above). Briefly, conidia were resuspended in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) containing fibronectin (at a concentration of 500 μg/ml), incubated for 3 h at 37°C, then washed and resuspended in rabbit antifibronectin antibody (Sigma) diluted 1:10 in PBS, and finally incubated for 1 h at 37°C. Suspensions were then washed and resuspended in fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin antibody (1:20 dilution; Jackson Immunochemicals, West Grove, Pa.) in PBS for 1 h at 37°C. Finally, the suspensions were washed again and examined. Negative controls consisted of suspensions incubated in the absence of fibronectin, suspensions in which the antifibronectin antibody was replaced with either a rabbit antilaminin antibody (Dako Ltd., High Wycombe, United Kingdom) or PBS, and suspensions in which FITC-conjugated goat anti-rabbit immunoglobulin was replaced with PBS.
Adherence assays.
Adherence assays were performed as previously described (4, 10); 96-well microtiter plates (Maxisorp; Nunc A/S, Kamstrup, Denmark) were initially coated with a range of fibronectin and laminin concentrations (from 0.1 through to 500 μg/ml). Subsequent experiments made used of plates coated with either fibronectin or laminin (each at 100 μg/ml). Plates were washed and blocked as described elsewhere (10), and conidia were added as appropriate (100 μl per well at 106 conidia per ml). Nonadherent cells were removed by washing in PBS containing 0.05% Tween 20. Adherent conidia were counted as previously described (4, 10). Control wells were incubated in PBS only in the absence of fibronectin and laminin. In each experiment, 10 microscope fields were counted in each of three wells, and each experiment was performed on three separate occasions with different conidial preparations. Statistical analysis was performed throughout via the Student t test.
Specificity of binding.
The effect of preincubation of conidia (30 min at 37°C) with soluble fibronectin, soluble laminin (both at final concentrations of 500 μg/ml and 1 mg/ml), soluble RGD peptide, and soluble short laminin fragment (YIGSR) (both at concentrations of 1 mg/ml only) on adherence to immobilized fibronectin was also investigated. Soluble bovine serum albumin (BSA; 1 mg/ml) was used throughout as a negative control, and conidia in PBS only were used as a positive control. The ability of rabbit antifibronectin antibody to inhibit adherence was also investigated; the latter (at a final dilution of 1:50) was added to wells coated with immobilized fibronectin, together with P. marneffei conidia, and the adherence assay was performed as described. Rabbit antilaminin antibody (at a final dilution of 1:50) was also used in place of the antifibronectin antibody. Finally, the ability of soluble fibronectin to interfere with the binding of conidia to immobilized laminin was also assessed (conditions as described).
Influence of carbohydrates on the interaction between conidia and immobilized fibronectin.
The following compounds were preincubated (30 min at 37°C) with conidia prior to addition to the standard adherence assay: glucose (200 mM), galactose (200 mM), mannose (200 mM), asialomucin (200 μg/ml), mucin (200 μg/ml), and N-acetylneuraminic acid (NANA; 200 mM) (all values shown are final concentrations; all reagents were from Sigma). All of the above were made up in PBS (pH was adjusted to 7.4 as appropriate), and the positive control consisted of conidia preincubated with PBS only, and negative controls consisted of wells which were not coated in fibronectin.
Influence of enzyme treatment of fibronectin and laminin on the adhesion of conidia.
To determine the effect of proteolysis on the interaction of conidia and immobilized fibronectin and laminin, conidia were preincubated for 1, 4, and 12 h at 37°C in chymotrypsin (Sigma) made up in PBS at a final concentration of 1,000 μg/ml. The reaction was stopped by the addition of 10 μl of a 100 mM phenylmethylsulfonyl fluoride stock solution in methanol. After two further washes in PBS, the chymotrypsin-treated cells were tested in the fibronectin and laminin adherence assay in the standard manner. Controls consisted of conidia preincubated in the presence of PBS only.
Effect of conidial age on binding to immobilized fibronectin and laminin.
The adherence of conidia from 1-, 2-, 4-, and 8-day-old cultures in the plate adherence assay system was determined (all conidia used at a concentration of 106/ml). Controls and methodology were as detailed previously (10).
Preparation of conidial extracts and ligand affinity binding.
Extracts were prepared exactly according to the method of Penalver et al. (16), using a total of approximately 1010 conidia. Three extracts were produced: a cell-free homogenate, a cell wall preparation made via β-mercaptoethanol extraction, and a cell wall preparation made via sodium dodecyl sulfate (SDS) extraction. Protein contents of samples were determined by the method of Lowry et al. (14). SDS-polyacrylamide gel electrophoresis was carried out as previously described (8, 9), using a 10% resolving gel. Gels were stained with silver stain (Bio-Rad, Hemel Hempstead, United Kingdom) to detect the presence of proteins. Electrophoretic transfer of proteins from polyacrylamide gels to Immobilon-P membranes was carried out as previously described (8, 9). Blotted proteins were assayed for fibronectin and laminin binding by first blocking overnight with 3% BSA in PBS buffer and then incubating for 6 h with agitation in PBS containing human fibronectin and murine laminin (each at 250 μg/ml). Membranes were washed six times in PBS (10 min per wash) and then incubated for 1 h with agitation at 37°C with either rabbit antifibronectin antibody or rabbit antilaminin antibody (both at a dilution of 1:250 in PBS). After a further six washes, blots were incubated with agitation at 37°C for 1 h with peroxidase-labelled goat anti-rabbit immunoglobulins (Sigma) at a dilution of 1:2,500 in PBS. Blots were then developed as previously described (8, 9).
RESULTS
Immunofluorescence microscopy.
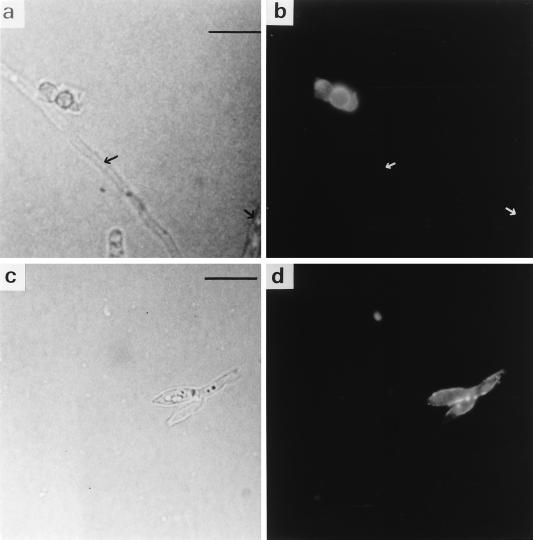
The surface of P. marneffei conidia demonstrated strong immunofluorescence labelling when incubated with fibronectin, antifibronectin antibody, and an appropriate fluorescent probe, indicating a clear interaction between conidia and fibronectin (Fig. 1a and b). Conidial labelling tended to be uniform, with no obvious spatial localization. However, hyphae were nonfluorescent, indicating the absence of an interaction with fibronectin (Fig. 1a and b). Fluorescent reactivity was also seen on the surface of phialides, the bottle-shaped structures which give rise to conidia (Fig. 1c and d). No reactivity was evident when the cells were incubated in the absence of fibronectin (data not shown), demonstrating that fluorescence was dependent on the previous interaction of the cells with fibronectin and its appropriate recognition. None of the other negative controls demonstrated any fluorescence activity.
FIG. 1.
Immunofluorescence identification of the binding of fibronectin. (a and b) Phase-contrast and immunofluorescence microscopy of conidia and hyphae incubated with fibronectin, antifibronectin antibody, and FITC-labelled conjugate. Negatively staining hyphae are arrowed. (c and d) Phase-contrast and immunofluorescence microscopy of phialides incubated as described above. Bars represent 10 μm.
Adherence of conidia to various concentrations of fibronectin and laminin.
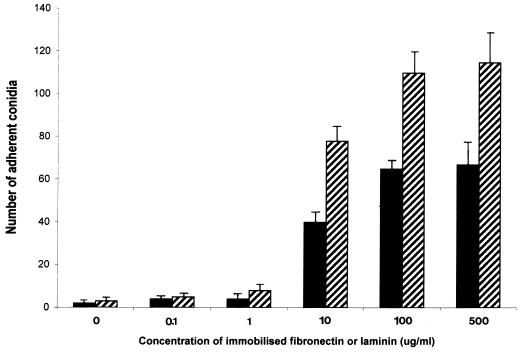
When conidia were incubated with immobilized fibronectin and laminin at increasing concentrations ranging from 0.1 to 500 μg/ml, conidial counts demonstrated that adherence increased progressively and then reached a plateau at a concentration of 100 μg/ml in both cases (Fig. 2). Attachment of conidia to fibronectin was less than their attachment to laminin at all concentrations of immobilized proteins used. Between 5 and 10% of the 105 conidia added to the wells remained attached to the plates after washing. Controls in which no fibronectin or laminin was present on the bottom of wells demonstrated little or no attachment (in all subsequent data, these control values have been subtracted from experimental values and are not shown).
FIG. 2.
Attachment of P. marneffei conidia to immobilized fibronectin (solid bars) and laminin (hatched bars) at a range of protein concentrations (0.1 to 500 μg/ml). Conidia concentration was constant at 105 per well. Results, expressed as the number of adherent conidia for 10 fields, are the means of triplicate counts performed three times (with standard deviations included) and are shown in the same manner in Fig. 3 to 6.
Specificity of binding of conidia to fibronectin.
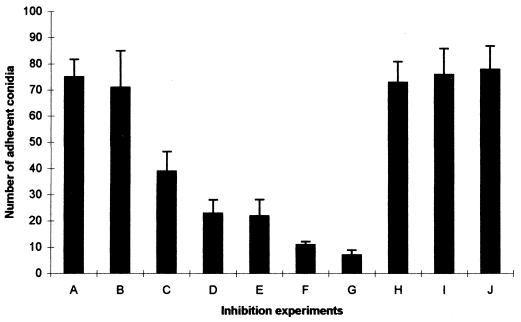
Soluble fibronectin had a clear inhibitory effect on the adherence of conidia to immobilized fibronectin; this effect was greatest at the highest concentration of soluble fibronectin used (Fig. 3, experiments C and D). Soluble laminin also had an inhibitory effect, which appeared more pronounced than that of fibronectin (Fig. 3, experiments E and F). Antifibronectin antibody almost completely abolished conidial adherence to bound fibronectin (Fig. 3, experiment G), whereas antilaminin antibody had no measurable effect (Fig. 3, experiment H). The short peptides (RGD and YIGSR) had no detectable effect on the interaction between conidia and fibronectin. In separate experiments, soluble fibronectin (at a concentration of 1 mg/ml) was demonstrated to cause statistically significant (P < 0.05) inhibition of conidia binding to immobilized laminin (data not shown).
FIG. 3.
Inhibition of attachment of P. marneffei conidia to immobilized fibronectin by soluble ligand and specific antibody. Wells were coated with a 100-μg/ml fibronectin solution, and conidia were allowed to adhere after preincubation in PBS (A), BSA (1 mg/ml) (B), soluble fibronectin (500 μg/ml [C] and 1 mg/ml [D]), soluble laminin (500 μg) (E), and (1 mg/ml) (F). Conidia were also coincubated in the presence of antifibronectin antibody (1:50) (G), antilaminin antibody (1:50) (H), RGD peptide (I), and YIGSR peptide (J). The values in the presence of soluble fibronectin, laminin, and antifibronectin antibody were significantly different from the value with PBS (P < 0.05).
Influence of carbohydrates on adherence of conidia to fibronectin.
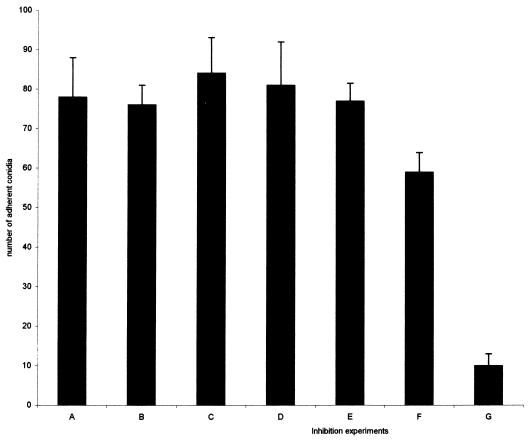
None of the monosaccharides (Fig. 4, experiments B to D) had any influence on the adherence of conidia to fibronectin, and asialomucin (experiment E) also had no effect. However, mucin caused some reduction in adherence, while sialic acid almost completely inhibited adherence (experiments F and G, P < 0.05).
FIG. 4.
Inhibition of attachment of P. marneffei conidia to immobilized fibronectin by sialic acid. Wells were coated with a 100-μg/ml fibronectin solution, and conidia were allowed to adhere after preincubation in PBS (A), galactose (B), mannose (C), glucose (D), asialomucin (E), mucin (F), and sialic acid (G). The value in the presence of sialic acid was significantly different from the value with PBS (P < 0.01).
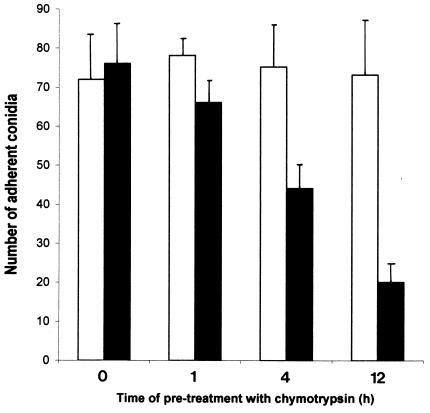
Effect of pretreatment of conidia with chymotrypsin on adherence to fibronectin and laminin.
Pretreatment of conidia with chymotrypsin for 12 h resulted in a pronounced decline in adherence to both bound fibronectin and laminin (Fig. 5, P < 0.05; laminin data not shown). This fall was less marked with shorter periods of pretreatment (Fig. 5). The magnitude of decline in adherence with time of pretreatment was similar in the case of fibronectin and laminin. Conidia which were not pretreated demonstrated no fall in adherence.
FIG. 5.
Effect on adherence of P. marneffei conidia to immobilized fibronectin after preincubation with chymotrypsin (at 1,000 μg/ml) with time. Open bars, controls not incubated with chymotrypsin; solid bars, samples incubated with chymotrypsin and bound to fibronectin.
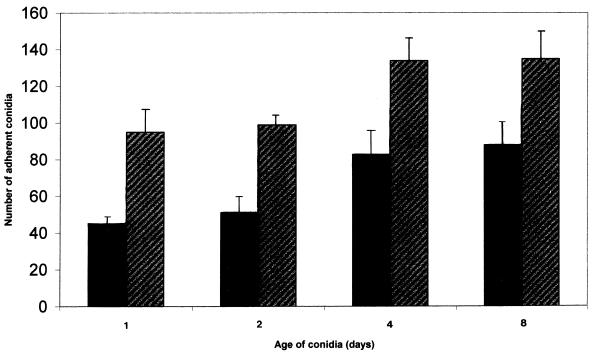
Effect of conidial age on the binding of conidia to fibronectin and laminin.
Conidia from older (day 4 and day 8) cultures showed an increase in adherence to both fibronectin and laminin compared to conidia from day 1 and day 2 cultures (Fig. 6, P < 0.05). Throughout time of culture, adherence of conidia to fibronectin remained less than adherence to laminin.
FIG. 6.
Effect of ageing of P. marneffei conidia on adherence to fibronectin (solid bars) and to laminin (hatched bars).
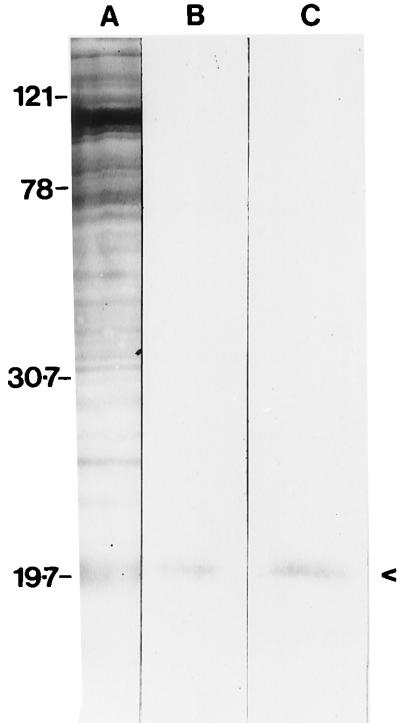
Identification of cell component that binds fibronectin and laminin.
On silver-stained SDS–10% polyacrylamide gels, proteins were detectable only in the cell extracts (Fig. 7, lane A). There was no detectable protein in either the cell wall β-mercaptoethanol extract or the cell wall SDS extract (data not shown). Binding of fibronectin was seen with a component with a relative molecular mass of 20 kDa; laminin bound to a component with apparently the same molecular mass (Fig. 7). There was an absence of bands when incubation with either soluble fibronectin or laminin was omitted (with detector antibodies used as normal). Addition of either antifibronectin or antilaminin antibodies to the equivalent reaction mixture blocked binding of the respective ligand to the separated proteins on the membrane (data not shown).
FIG. 7.
Identification by ligand affinity blotting of proteins from P. marneffei conidia which bind fibronectin and laminin. Lanes: A, whole conidial homogenate stained with silver stain; B, immunoblotting of lane A with fibronectin, antifibronectin antibody, and peroxidase conjugate; C, immunoblotting of lane A with laminin, antilaminin antibody, and peroxidase conjugate. Relative molecular masses are shown on the left in kilodaltons.
DISCUSSION
Immunofluorescence labelling clearly demonstrated the presence of fibronectin binding sites on the surface of P. marneffei conidia. These binding sites are also present on the surface of phialides, the bottle-shaped structures which give rise to conidia, although they appear to be absent from the surface of hypae in general. This distribution of fibronectin binding sites appears to be identical to that previously described for laminin binding sites in this pathogen (10). As previously noted (10), the observed absence of hyphal labelling appears analogous to the absence of ECM protein receptors on the surface of A. fumigatus mycelia (6). The presence of fibronectin binding sites on the surface of conidia was confirmed by the plate adherence assay, which demonstrated that conidia could bind immobilized fibronectin: the number of bound conidia increased with increasing protein concentration and then reached a plateau. This pattern was essentially the same as that for binding of conidia to laminin, with the proviso that more conidia bound to any given quantity of laminin than to the equivalent fibronectin concentration.
In fact, the pronounced similarities in the fibronectin and laminin binding processes under various conditions strongly suggests that they are mediated through the same receptor molecule. Thus, the pattern of fluorescence labelling, the profile of increased binding as conidia age (a phenomenon common to ECM receptors in A. fumigatus [1]), the effect of chymotrypsin, the ability of soluble laminin to block adherence of conidia to immobilized fibronectin (and vice versa), and the shared inhibitory effect of NANA (see below) are all suggestive of a shared receptor. However, the most convincing evidence that there is a single receptor comes from ligand affinity studies which reveal the presence of a 20-kDa receptor that binds both fibronectin and laminin. The existence in A. fumigatus of receptors capable of recognizing more than one ligand has been hypothesized (1); the latter provides a model for the adherence of P. marneffei conidia to various ECM components. The greater numbers of adherent conidia binding to a given quantity of immobilized laminin compared to fibronectin might suggest that the common receptor has a greater affinity for laminin. However, this question can be fully addressed only via receptor-ligand affinity studies using purified receptor. It is unclear at this time whether the 20-kDa species which binds fibronectin and laminin represents the intact receptor or some fragment thereof. Its apparently anomalous distribution (present in homogenate, absent in cell wall extracts) may have resulted from its lower relative concentration in the cell wall, which is below the detection threshold of the assay. No attempt was made to identify the receptor in culture media, as it is unlikely that detectable amounts would be present.
As has been previously described with regard to the adherence of P. marneffei conidia to laminin (10), various monosaccharides demonstrated no inhibitory effect on the binding to immobilized fibronectin. However, in contrast, there was complete inhibition of adherence to fibronectin when conidia were preincubated with NANA. Bovine submaxillary mucin, which is rich in terminal sialic acid residues, also demonstrated some inhibition of adherence. Asialomucin (the desialylated form of mucin) was unable to cause inhibition. Fibronectin is known to expose terminal sialic acid residues at the end of oligosaccharide chains (2, 20), in much the same way that laminin does (1, 12). The sialic acid recognition system described in this report maybe directly analogous to that recently elucidated for laminin binding by A. fumigatus conidia (1), although no data implicating this latter receptor in any interaction with fibronectin have yet been produced. Indeed, in A. fumigatus a fibronectin binding mechanism which is inhibited by the peptide RGD (6) has already been described, although this does not rule out the presence of an additional a sialic acid-mediated fibronectin recognition system in A. fumigatus.
The susceptibility of the fibronectin (and laminin) binding molecule to chymotrypsin indicates that it is protein in nature, and it can therefore be tentatively classified as a lectin. In fact, compared to the ECM binding proteins recognized in A. fumigatus (6, 16), the P. marneffei receptor is relatively resistant to proteolysis; extended incubation at a high chymotrypsin concentration is required for a sizable inhibition of receptor-ligand interaction.
The attachment of P. marneffei conidia to the bronchioalveolar epithelium has a hypothetical role to play in the establishment of initial infection, and it is possible that the interactions described here play some part in this process. Thus, adherence would enable conidia to avoid entrapment by respiratory tract mucus, with subsequent removal by the action of ciliary cells. However, while the existence of a conidial receptor which recognizes a ligand (sialic acid) common to at least two important ECM proteins is of interest, considerably more data are required to actually implicate this process in pathogenesis. Accordingly, we are presently attempting to purify this receptor as a first step in its full characterization.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust and by the Special Trustees of Guys Hospital.
REFERENCES
- 1.Bouchara J P, Sanchez M, Chevailler A, Marot-Leblond A, Lissitzky J C, Tronchin G, Chabasse D. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect Immun. 1997;65:2717–2724. doi: 10.1128/iai.65.7.2717-2724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carsons S, Lavietes B B, Slomiany A, Diamond H S, Berkowitz E. Carbohydrate heterogeneity of fibronectins. Synovial fluid fibronectin resembles the form secreted by cultured synoviocytes but differs from the plasma form. J Clin Investig. 1987;80:1342–1349. doi: 10.1172/JCI113211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiewchanvit S, Mahanupab P, Hirunsri P, Vanittanakom N. Cutaneous manifestations of disseminated Penicillium marneffei mycosis in five HIV-infected patients. Mycoses. 1991;34:245–249. doi: 10.1111/j.1439-0507.1991.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 4.Coulot P, Bouchara J P, Renier G, Annaix V, Planchenault C, Tronchin G, Chabasse D. Specific interaction of Aspergillus fumigatus with fibrinogen and its role in cell adhesion. Infect Immun. 1994;62:2169–2177. doi: 10.1128/iai.62.6.2169-2177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Z L, Ribas J L, Gibson D W, Connor D H. Infections caused by Penicillium marneffei in China and Southeast Asia. Review of eighteen cases and report of four more Chinese cases. Rev Infect Dis. 1988;10:640–652. doi: 10.1093/clinids/10.3.640. [DOI] [PubMed] [Google Scholar]
- 6.Gaur N K, Klotz S A. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect Immun. 1997;65:5289–5294. doi: 10.1128/iai.65.12.5289-5294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil M L, Penalver M C, Lopez-Ribot J L, O’Connor J E, Martinez J P. Binding of extracellular matrix proteins to Aspergillus fumigatus conidia. Infect Immun. 1996;64:5239–5247. doi: 10.1128/iai.64.12.5239-5247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton A J, Bartholomew M A, Figueroa J, Fenelon L E, Hay R J. Production of species-specific murine monoclonal antibodies against Cryptococcus neoformans which recognize a noncapsular exoantigen. J Clin Microbiol. 1991;29:980–984. doi: 10.1128/jcm.29.5.980-984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton A J, Jeavons L, Hobby P, Hay R J. A 34- to 38-kilodalton Cryptococcus neoformans glycoprotein produced as an exoantigen bearing a glycosylated species-specific epitope. Infect Immun. 1992;60:143–149. doi: 10.1128/iai.60.1.143-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton A J, Jeavons L, Youngchim S, Vanittanakom N, Hay R J. Sialic acid-dependent recognition of laminin by Penicillium marneffei conidia. Infect Immun. 1998;66:6024–6026. doi: 10.1128/iai.66.12.6024-6026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayanetra J P, Nitiyanant P, Ajello L, Padhye A A, Lolekha S, Atichartakarn V, Vathesatogit P, Sathaphatayavongs B, Prajaktam R. Penicillium marneffei in Thailand: report of five human cases. Am J Trop Med Hyg. 1984;33:637–644. doi: 10.4269/ajtmh.1984.33.637. [DOI] [PubMed] [Google Scholar]
- 12.Knibbs R N, Perini F, Goldstein I J. Structure of the major concanavalin A reactive oligosaccharides of the extracellular matrix component laminin. Biochemistry. 1989;28:6379–6392. doi: 10.1021/bi00441a034. [DOI] [PubMed] [Google Scholar]
- 13.Li J S, Pan L Q, Wu S X, Su S X, Su S B, Shan L Y. Disseminated penicilliosis marneffei in China. Report of three cases. Chin Med J. 1991;104:247–251. [PubMed] [Google Scholar]
- 14.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Ozeri V, Rosenshine L, Mosher D F, Fassler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1997;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 16.Penalver M C, O’Connor J E, Martinez J P, Gil M L. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect Immun. 1996;64:1146–1153. doi: 10.1128/iai.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha C L, Fischetti V A. Identification and characterization of a new protein from Streptococcus pyogenes having homology with fibronectin and fibrinogen binding proteins. Adv Exp Med Biol. 1997;418:737–739. doi: 10.1007/978-1-4899-1825-3_173. [DOI] [PubMed] [Google Scholar]
- 18.Sirisanthana V, Sirisanthana T. Disseminated Penicillium marneffei infection in human immunodeficiency virus-infected children. Pedriatr Infect Dis. 1995;14:935–940. doi: 10.1097/00006454-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Supparatpinyo K, Kwamwan C, Baosoung V, Nelson K E, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 20.Takamoto M, Endo T, Isemura M, Kochibe N, Kobata A. Structure of asparagine-linked oligosaccharide of human placental fibronectin. J Biochem. 1989;105:742–750. doi: 10.1093/oxfordjournals.jbchem.a122738. [DOI] [PubMed] [Google Scholar]
- 21.Tsang D N C, Li P K C, Tsui M S, Lau Y T, Ma K F, Yeoh E K. Penicillium marneffei: another pathogen to consider in patients infected with human immunodeficiency virus. Rev Infect Dis. 1991;13:766–777. doi: 10.1093/clinids/13.4.766-a. [DOI] [PubMed] [Google Scholar]
- 22.Tsui W M S, Ma K F, Tsang D N C. Disseminated Penicillium marneffei infection in HIV-infected subject. Histopathology. 1992;20:287–293. doi: 10.1111/j.1365-2559.1992.tb00985.x. [DOI] [PubMed] [Google Scholar]