Abstract
Calcium carbonate nanoparticles have been widely used in biomedicine due to their biocompatibility and biodegradability. Recently, calcium carbonate nanoparticles are largely integrated with imaging contrast and therapeutic agents for various imaging and therapeutic approaches. In this review, we first described the advantages and preparation methods of calcium carbonate nanoparticles, then the state-of-the-art progress of calcium carbonate nanoparticles in diagnosis, treatment and theranostics was summarized. Finally, we discussed the challenges and recommendations for future studies of the calcium carbonate nanoparticles.
Keywords: CaCO3 NPs, drug delivery, diagnosis, theranostics
1. Introduction
The rapid development of nanotechnology over the past few decades has led to the approval of multiple nanoparticle (NP)-based drug delivery systems in clinics [1,2]. Furthermore, a large number of NPs are undergoing clinical trials or preclinical studies [3]. These NPs can be roughly divided into organic NPs and inorganic NPs. Among different inorganic materials, calcium carbonate (CaCO3) NPs have gained much attention due to their excellent biocompatibility and biodegradability, as well as easy preparation and pH sensitivity [4]. CaCO3 exists as an amorphous calcium carbonate (ACC) phase, two hydrated metastable phases (calcium carbonate hexahydrate and monohydrocalcite), and three anhydrous crystalline polymorphs (calcite, aragonite, vaterite) [5]. Among them, ACC phase displays the highest solubility and is the precursor of anhydrous crystalline polymorphs, which is easily crystallized in solutions to form polymorphs [6].
By the combination of CaCO3 NPs with imaging contrast agents, different imaging modalities such as fluorescence imaging (FLI), magnetic resonance imaging (MRI) and ultrasound (US) imaging could be realized. By the combination of CaCO3 NPs with drugs, diverse treatments including chemical therapy, gene therapy, photothermal therapy (PTT)/photodynamic therapy (PDT) and immunotherapy could be achieved. Furthermore, by the combination of CaCO3 NPs with both contrast agents and drugs, multimodal theranostics could be reached. Therefore, the development of CaCO3 NPs would contribute to the diagnosis, treatment and theranostics of diseases.
In this review, we will first summarize the advantages and preparation methods of CaCO3 NPs. Then, CaCO3 NP-based biomedical applications will be classified in detail. Finally, we will discuss the challenges and recommendations for future studies of CaCO3 NPs.
2. The Advantages of CaCO3 NPs
2.1. Excellent Biocompatibility/Biodegradability and pH-Sensitive Property
In biological systems, calcium carbonate and calcium phosphate are important components of bones, shells or teeth [7]. Therefore, it is believed that CaCO3-based drug delivery systems have excellent biocompatibility due to their chemical similarity with tissues. Furthermore, some common NPs such as Au, Ag, Se, Cr, TiO2 and ZnO have been demonstrated to improve mutation frequency and reactive oxygen species production, thus, leading to cell apoptosis [8,9]. In contrast, CaCO3 NPs are one of the safest biomaterials because their by-products (only Ca2+ and CO32−) already exist in the blood.
In addition, CaCO3 NPs are stable under normal blood pH (7.4) while decompose quickly in an acidic tumor microenvironment, of which facilitates tumor-targeted delivery [10].
2.2. Ease of Preparation and Surface Modification
The preparation of CaCO3 NPs only needs common salts without organic solvents in most cases, which makes them low-cost [11]. Moreover, the surface of CaCO3 NPs can be modified with targeted moiety, which promotes these CaCO3 NPs to arrive at the target sites [12].
3. The Preparation Methods and Controlled Release of CaCO3 NPs
So far, the commonly used preparation methods of CaCO3 NPs include the precipitation method [13], gas diffusion [14], flame synthesis [15], decomposition of cockle shells [16], biomineralization and so on [17,18]. Among them, solution precipitation, microemulsion and gas diffusion methods have been widely used for CaCO3 NP-based drug delivery systems.
3.1. Solution Precipitation Method
The solution precipitation method is the most established technique for CaCO3 NP preparation, which uses the reaction between the Ca2+ and CO32− aqueous solution. This method could produce large quantities of CaCO3 NPs without a surfactant, thus, reducing the production cost. Because of the mild preparation conditions, many bioactive species, including small molecule drugs, genes and proteins, could load into CaCO3 NPs during the precipitation process [4]. Notably, the synthesis parameters such as pH, temperature, ion concentration, stirring speed, solvent species and additives are often used to control the size, shape and phase of CaCO3 NPs [13].
3.2. Microemulsion Method
As an extension of the precipitation method, the microemulsion methods are widely used for CaCO3 NP preparation and gene encapsulation [19,20]. Microemulsion methods contain the reversed microemulsion (water in oil, W/O) method and double emulsion method. The reversed microemulsion method used the W/O microemulsion droplets as the nano-reactors [19]. First, “calcium microemulsion” and “carbonate microemulsion” were, respectively, prepared through adding the Ca2+ or CO32− aqueous phase into an organic phase. Then, “calcium microemulsion” and “carbonate microemulsion” were mixed to form CaCO3 NPs. Finally, a centrifuge was used to separate the CaCO3 NPs. For example, Huang et al. developed CaCO3 NP loading with the therapeutic peptide by the reversed microemulsion method for lung cancer treatment [21].
The double emulsion method is similar to the reversed microemulsion method [20]. Firstly, W/O “calcium microemulsion” was prepared the same as the reversed microemulsion method. Then, a great deal of aqueous phase (consisting of CO32−) was mixed with “calcium microemulsion” to form the W/O/W double emulsion. CaCO3 NPs were formed through the Ca2+ and CO32− reaction in the W/O/W double emulsion.
In general, through the microemulsion method, the structure, size and crystallinity of CaCO3 NPs could be regulated by optimizing the surfactants, temperature, pH and ion concentration [22].
3.3. Gas Diffusion Method
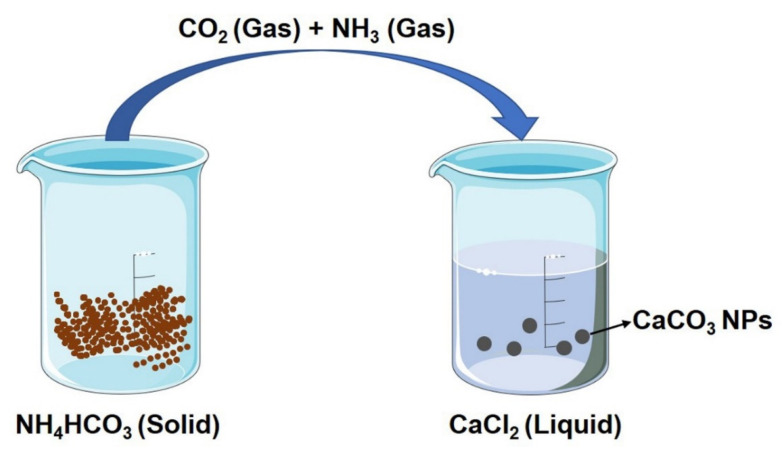
The gas diffusion method is mainly used for preparing ACC loading with small molecule drugs [14]. As shown in Figure 1, CaCl2 was dissolved in ethanol and transferred into a glass bottle. Then, the bottle was left in a desiccator along with another bottle of ammonia bicarbonate. CO2 and NH3 were generated from ammonium bicarbonate, then dissolved in the ethanol solution to form CO32− and NH4+. Under an alkaline condition caused by NH4+, CO32− was reacted with Ca2+ to form ACC. In this method, the size, shape and polymorph of the prepared ACC could be controlled through changing the additives, temperature and Ca2+ concentration [23].
Figure 1.
Illustration of the gas diffusion method. CO2 and NH3 were generated from ammonium bicarbonate, which then dissolved in the ethanol solution and reacted with Ca2+ to form CaCO3 NPs.
3.4. Controlled Release of CaCO3 NPs
CaCO3 NPs could improve the pharmacokinetics of loading drugs through a controlled release, thus, reducing the side effects and enhancing the treatment effect. CaCO3 NPs release the drugs by three ways, including diffusion, carrier dissolution and recrystallization [24]. pH is the key parameter for the controlled release of CaCO3 NPs. Under acidic conditions, free protons react with CO32− to form HCO3−, then dissolve CaCO3 NPs and accelerate the release of loading drugs [25].
4. The Biomedical Applications of CaCO3 NPs
4.1. CaCO3 NPs for Diagnosis
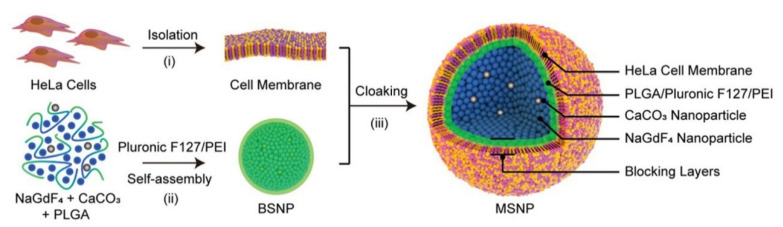
Through combining CaCO3 NPs with fluorophores or paramagnetic elements (such as Mn2+, Gd3+), FLI and MRI could be realized [26,27]. Moreover, CaCO3 NPs themselves can produce CO2 bubbles under acidic conditions, which can then enhance the US imaging signal. For example, Kim et al. prepared CaCO3 NPs for US imaging [28]. After an intravenous injection, the prepared CaCO3 NPs showed a remarkable US contrast enhancement in the tumor tissue. In addition, Yi and co-workers reported membrane-cloaking nanoconjugates comprising NaGdF4 and CaCO3 NPs [27], which displayed more than a 60-fold contrast enhancement compared with Magnevist (commercially used contrast agent) in tumor MRI (Figure 2).
Figure 2.
Schematic illustration of the prepared CaCO3 NPs for MRI. Reprinted with permission. Copyright 2019, Wiley-VCH [27].
4.2. CaCO3 NPs for Treatment
Because of the excellent biocompatibility/biodegradability, pH-sensitive property, ease of preparation and surface modification, CaCO3 NPs have been widely used as carriers for a variety of treatments including chemical therapy [29], gene therapy [21], PTT/PDT [30] and combination therapy [31]. Moreover, CaCO3 NPs themselves could be used as Ca2+ generators which induce immunogenic cell death (ICD) and autophagy to activate immunotherapy [12].
4.2.1. CaCO3 NPs as Carriers for Chemical Therapy
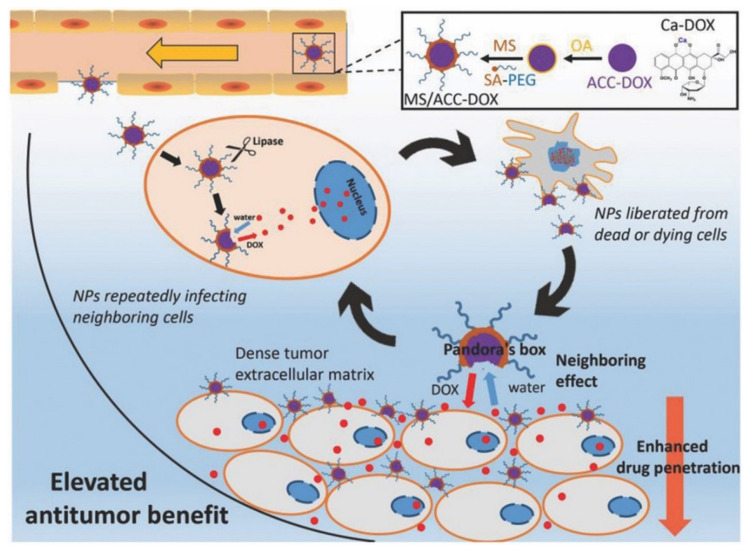
CaCO3 NPs were able to load both hydrophobic and hydrophilic molecules, making them suitable carriers for chemotherapy [9]. For instance, Wang et al. designed monostearin-coated CaCO3 NPs for doxorubicin (DOX) loading [29]. Monostearin coating induced a lipase-triggered DOX release in a lipase-overexpressed tumor site, which improved the drug penetration (Figure 3).
Figure 3.
Illustration of the formation and elevation of the designed CaCO3 NPs. Reprinted with permission. Copyright 2018, Wiley-VCH [29].
4.2.2. CaCO3 NPs as Carriers for Gene Therapy
Gene therapy works by substituting or silencing the defective gene to achieve the therapeutic effect [32]. However, it has been a challenge for nucleic acid delivery due to their negative charge, large size and easy degradation [33]. CaCO3 NPs could bind with nucleic acids, making them promising vehicles for gene therapy [21]. For example, He et al. constructed CaCO3 NPs for vascular endothelial growth factor small interfering RNA (VEGF siRNA) delivery [34]. Both in vitro and in vivo results demonstrated that CaCO3 NPs are a suitable system for siRNA delivery. In another study, Chen et al. synthesized CaCO3 NPs and modified them with polyethyleneimine (PEI), named as PEI-CaCO3 NPs, which could be used for p53 gene adsorption [35]. After transfected, p53-loaded PEI-CaCO3 NPs significantly decreased the proliferation of tumor cells.
4.2.3. CaCO3 NPs as Carriers for PTT/PDT
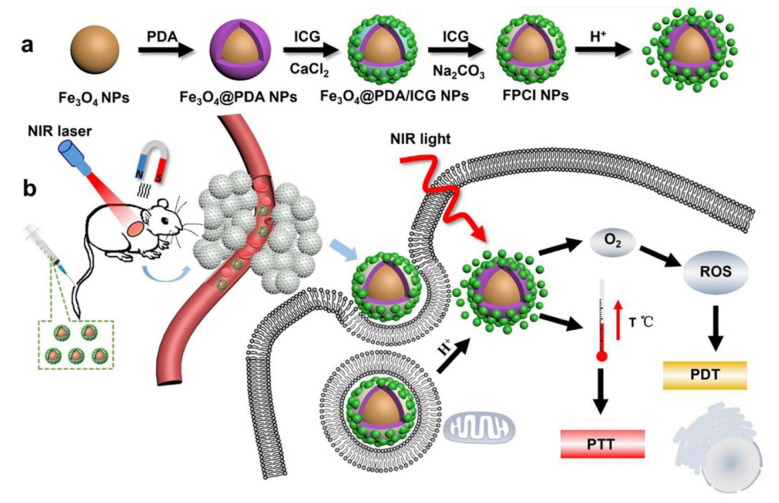
PTT and PDT have become promising strategies for cancer therapy because of the noninvasiveness and specific selectivity [36,37]. Recently, Xue et al. fabricated a nanocomposite consisting of CaCO3, indocyanine green (ICG) and polydopamine (PDA), named as Fe3O4@PDA@CaCO3/ICG (FPCI) NPs, which can achieve the combination of PDA-based PTT and ICG-based PDT (Figure 4) [30].
Figure 4.
Schematic of the preparation process (a) and PTT/PDT treatment (b) of FPCI NPs. Reprinted with permission. Copyright 2018, Elsevier [30].
4.2.4. CaCO3 NPs as Ca2+ Generators for Immunotherapy
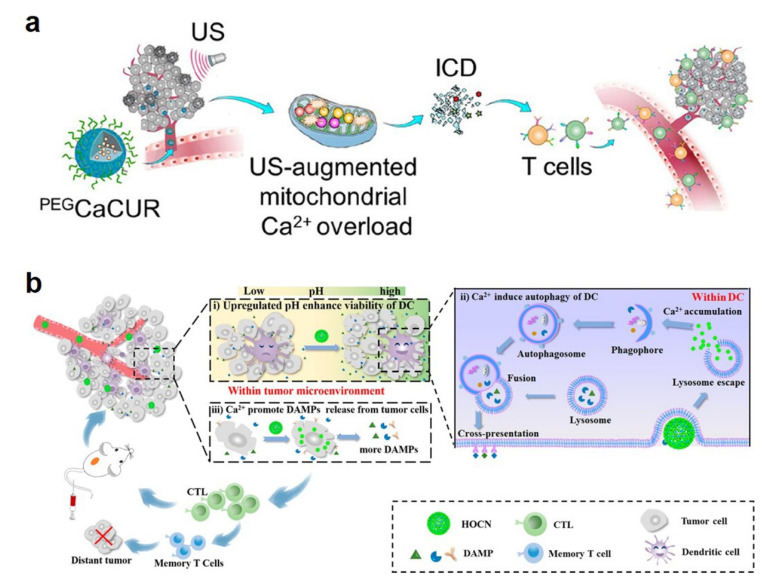
Immunotherapy works by activating the immune system for searching and destroying cancer cells [38]. CaCO3 NPs can be used not only as carriers for immunotherapy drugs themselves, but also could increase Ca2+ concentration, thus, inducing immunogenic cell death (ICD) and autophagy [39,40]. Most recently, Zheng et al. prepared polyethylene glycol (PEG)-decorated CaCO3 NP loading with curcumin (namely, PEGCaCUR) [39]. PEGCaCUR NPs can serve as a Ca2+ nanomodulator to induce Ca2+ overload, thus, enhancing the ICD effect and eventually inhibiting tumor growth and migration (Figure 5a). In another study, An et al. designed ovalbumin (OVA)-loaded CaCO3 (OVA@CaCO3) NPs as a Ca2+ nanogenerator to destroy the autophagy inhibition condition in dendritic cells, promote the damage-associated molecular patterns (DAMPs) and release and upregulate the pH of the tumor microenvironment (Figure 5b) [40].
Figure 5.
(a) Schematic illustration of PEGCaCUR NPs-based immunotherapy. Reprinted with permission. Copyright 2021, American Chemical Society [39]. (b) Schematic diagram of OVA@CaCO3 NP-mediated immunotherapy. Reprinted with permission. Copyright 2020, American Chemical Society [40].
4.2.5. CaCO3 NP-Based Combination Therapy
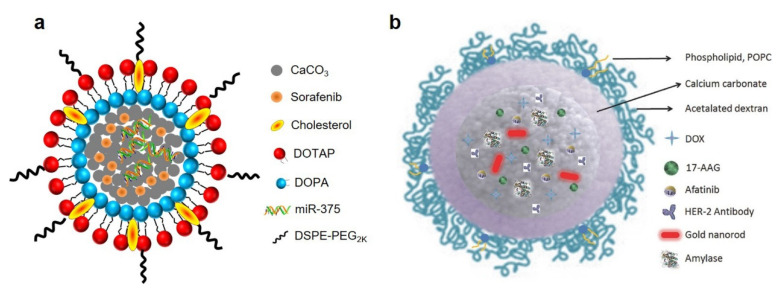
Combination therapy is able to notably decrease multidrug resistance and increase efficiency [41]. CaCO3 NPs are commonly used for the co-delivery of chemotherapeutics and gene drugs, which realized the combination of chemotherapy and gene therapy [31]. For example, Xiang’s group designed a lipid-coated CaCO3 NPs for the co-delivery of sorafenib and miR-375 (miR-375/Sf-LCC NPs, Figure 6a) [42]. Both in vitro and in vivo results proved that miR-375/Sf-LCC NPs are promising carriers for combination therapy. In another study, Kong et al. developed gold nanorods@CaCO3 NPs coated with dextran and phospholipid for the incorporation of different molecules, including DOX, 17-(allylamino)-17-demethoxygeldanamycin, afatinib and amylase (Figure 6b) [43]. This platform has great potential for the combination of PTT and chemotherapy.
Figure 6.
(a) Schematic representation of miR-375/Sf-LCC NPs. Reprinted with permission. Copyright 2018, Elsevier [42]. (b) Schematic representation of gold nanorods@CaCO3 NPs. Reprinted with permission. Copyright 2016, Wiley-VCH [43].
4.3. CaCO3 NPs for Theranostic
The therapeutic effect could be significantly improved through the rational design of novel theranostic platforms with both imaging and treatment functions [44]. CaCO3 NPs have shown potential in both diagnosis and therapy, which encourages researchers to design theranostic CaCO3 NPs for achieving imaging-guided treatment [4]. Specifically, CaCO3 NP-based theranostic platforms can be classified as three types according to the imaging mode, including US imaging-guided therapy [45], FLI-guided therapy [46] and MRI-guided therapy [47].
4.3.1. US Imaging-Guided Therapy
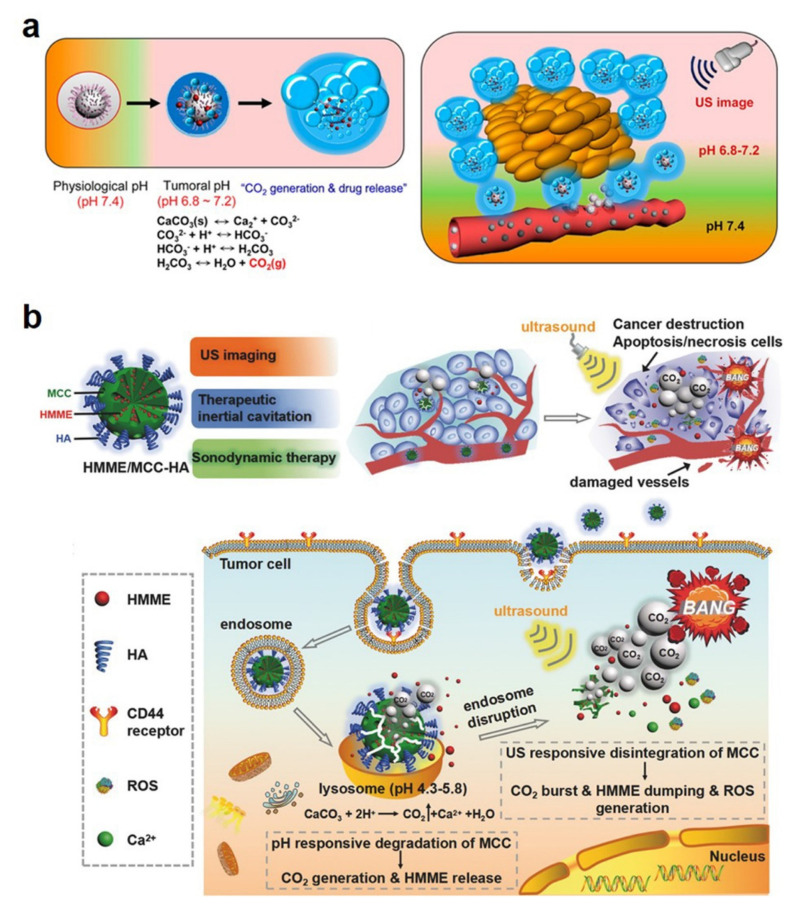
CaCO3 NPs can generate CO2 bubbles and display potential as a US contrast agent in the acidic tumor microenvironment. As a typical paradigm, Min et al. developed DOX-loaded CaCO3 NPs that express US imaging and chemotherapy for cancer theranostics (Figure 7a) [45]. These NPs displayed a strong echogenic signal and long echo persistence, as well as a simultaneous DOX release at the tumor site, which exhibited efficient antitumor effects guided by US imaging. Recently, Feng and co-workers reported CaCO3 NP loading with hematoporphyrin monomethyl ether (HMME, a sonosensitizer) [48]. Under US irradiation, generated CO2 bubbles could lead to cavitation-mediated necrosis and be used as US contrast agents. Meanwhile, HMME can produce reactive oxygen species for sonodynamic therapy (Figure 7b). These nanoplatforms provided the US imaging-guided cavitation/sonodynamic combined therapy, which highlighted the possibility of cancer theranostics.
Figure 7.
(a) Bubble generation and drug release of the designed CaCO3 NPs. Reprinted with permission. Copyright 2015, American Chemical Society [45]. (b) Schematic illustration of the US imaging-guided cavitation/sonodynamic combined therapy. Reprinted with permission. Copyright 2017, Wiley-VCH [48].
4.3.2. FLI-Guided Therapy
CaCO3 NPs could be constructed as FLI-guided therapy nanoplatforms through the co-delivery of FLI contrast and therapeutic agents. For example, Huang et al. designed a theranostic CaCO3 NP encapsulation with DOX and fluorescence contrast agent indocyanine green (ICG) for chemotherapy and fluorescence/US dual-mode imaging [46]. The prepared CaCO3 NPs showed a satisfactory treatment effect guided by dual-mode imaging, which demonstrated a promising strategy for dual-mode theranostics.
4.3.3. MRI-Guided Therapy
CaCO3 NPs can also load with MRI contrast and therapeutic agents for realizing MRI-guided therapy. For instance, Gorin’s group prepared a CaCO3 NP-capsuling paramagnetic element (Fe3O4) and DOX, which could be used for an MRI/photoacoustic imaging-guided precise drug release [47].
5. The Challenges and Recommendations for Future Studies of CaCO3 NPs
Although CaCO3 NPs have been widely investigated for diverse biomedical applications including diagnosis, treatment and theranostics due to their excellent biocompatibility/biodegradability and pH-sensitive property, as well as their ease of preparation and modifications, there are still several challenges that need to be addressed for clinical translation.
First, long-term potential risks of CaCO3 NPs need to be noticed [49,50]. Although calcium is an essential element in humans, overloaded calcium could induce thrombosis, hypercalcemia and other potential dangers [51]. Furthermore, the most recent studies only evaluated the short-term toxicity of mice through describing organ damage and immune responses after a CaCO3 NP injection, which was obviously insufficient for a biosafety evaluation. Thus, for the clinical translation of CaCO3 NPs, it is necessary to systematically assess the long-term effects of CaCO3 NPs from rodent models to mammalian models [4].
Second, the present preparation processes of CaCO3 NPs are instable, which easily leads to large particles [9]. Thus, it is necessary to design precise methods for size control, components, and surface modifications, sequentially achieving a large-scale production of CaCO3 NPs.
Third, the drug release kinetics from CaCO3 NPs is difficult to predict. Although the pH-sensitive property of CaCO3 NPs has been widely studied, their release under a normal pH has not been evaluated in detail.
6. Conclusions
In summary, CaCO3 NPs have great potential in biomedical applications due to their excellent properties, such as biocompatibility/biodegradability, pH-sensitivity, ease of preparation and surface modifications. Although much has been carried out, more efforts are still needed to solve the above challenges. We believe that more efficient CaCO3 NPs will be developed as safe carriers for the diagnosis, treatment and theranostics of diseases.
Author Contributions
Conceptualization, P.Z. and Y.L.; writing—original draft preparation, P.Z.; writing—review and editing, Y.T., J.Y., X.H. and Y.L.; funding acquisition, P.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors disclose no conflict.
Funding Statement
This research was funded by Natural Science Foundation of China grants grant (No. 82102080), Natural Science Foundation of Hubei Province in China (2021 CFB336).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.d.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou X., Zaks T., Langer R., Dong Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021;6:1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson K.K., Koshy P., Yang J.-L., Sorrell C.C. Preclinical Cancer Theranostics—From Nanomaterials to Clinic: The Missing Link. Adv. Funct. Mater. 2021;31:2104199. doi: 10.1002/adfm.202104199. [DOI] [Google Scholar]
- 4.Qi C., Lin J., Fu L.-H., Huang P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018;47:357–403. doi: 10.1039/C6CS00746E. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright J.H.E., Checa A.G., Gale J.D., Gebauer D., Sainz-Díaz C.I. Calcium Carbonate Polyamorphism and Its Role in Biomineralization: How Many Amorphous Calcium Carbonates Are There? Angew. Chem. Int. Ed. 2012;51:11960–11970. doi: 10.1002/anie.201203125. [DOI] [PubMed] [Google Scholar]
- 6.Wolf S.E., Leiterer J., Kappl M., Emmerling F., Tremel W. Early Homogenous Amorphous Precursor Stages of Calcium Carbonate and Subsequent Crystal Growth in Levitated Droplets. J. Am. Chem. Soc. 2008;130:12342–12347. doi: 10.1021/ja800984y. [DOI] [PubMed] [Google Scholar]
- 7.Palmer L.C., Newcomb C.J., Kaltz S.R., Spoerke E.D., Stupp S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired By Bone and Enamel. Chem. Rev. 2008;108:4754–4783. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song B., Zhou T., Liu J., Shao L. Involvement of Programmed Cell Death in Neurotoxicity of Metallic Nanoparticles: Recent Advances and Future Perspectives. Nanoscale Res. Lett. 2016;11:484. doi: 10.1186/s11671-016-1704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S., Verma A., Teja B.V., Pandey G., Mittapelly N., Trivedi R., Mishra P.R. An insight into functionalized calcium based inorganic nanomaterials in biomedicine: Trends and transitions. Colloids Surf. B Biointerfaces. 2015;133:120–139. doi: 10.1016/j.colsurfb.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Bian Y., Xiao X., Liu B., Ding B., Cheng Z., Ma P.a., Lin J. Tumor Microenvironment-Responsive Cu/CaCO3-Based Nanoregulator for Mitochondrial Homeostasis Disruption-Enhanced Chemodynamic/Sonodynamic Therapy. Small. 2022;18:2204047. doi: 10.1002/smll.202204047. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C., Chen T., Wu C., Zhu G., Qiu L., Cui C., Hou W., Tan W. Aptamer CaCO3 Nanostructures: A Facile, pH-Responsive, Specific Platform for Targeted Anticancer Theranostics. Chem. Asian J. 2015;10:166–171. doi: 10.1002/asia.201403115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai S., Lan Y., Fu S., Cheng H., Lu Z., Liu G. Connecting Calcium-Based Nanomaterials and Cancer: From Diagnosis to Therapy. Nano-Micro Lett. 2022;14:145. doi: 10.1007/s40820-022-00894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno Y., Futagawa H., Takagi Y., Ueno A., Mizushima Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J. Control. Release. 2005;103:93–98. doi: 10.1016/j.jconrel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Luo Z., Li M., Qu Q., Ma X., Yu S.-H., Zhao Y. A Preloaded Amorphous Calcium Carbonate/Doxorubicin@Silica Nanoreactor for pH-Responsive Delivery of an Anticancer Drug. Angew. Chem. Int. Ed. 2015;54:919–922. doi: 10.1002/anie.201408510. [DOI] [PubMed] [Google Scholar]
- 15.Huber M., Stark W.J., Loher S., Maciejewski M., Krumeich F., Baiker A. Flame synthesis of calcium carbonate nanoparticles. Chem. Commun. 2005:648–650. doi: 10.1039/b411725e. [DOI] [PubMed] [Google Scholar]
- 16.Islam K.N., Zuki A.B.Z., Ali M.E., Hussein M.Z.B., Noordin M.M., Loqman M.Y., Wahid H., Hakim M.A., Hamid S.B.A. Facile synthesis of calcium carbonate nanoparticles from cockle shells. J. Nanomater. 2012;2012:2. doi: 10.1155/2012/534010. [DOI] [Google Scholar]
- 17.Shafiu Kamba A., Ismail M., Tengku Ibrahim T.A., Zakaria Z.A.B. A pH-Sensitive, Biobased Calcium Carbonate Aragonite Nanocrystal as a Novel Anticancer Delivery System. BioMed Res. Int. 2013;2013:587451. doi: 10.1155/2013/587451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maleki Dizaj S., Barzegar-Jalali M., Zarrintan M.H., Adibkia K., Lotfipour F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015;12:1649–1660. doi: 10.1517/17425247.2015.1049530. [DOI] [PubMed] [Google Scholar]
- 19.Li M., Mann S. Emergent Nanostructures: Water-Induced Mesoscale Transformation of Surfactant-Stabilized Amorphous Calcium Carbonate Nanoparticles in Reverse Microemulsions. Adv. Funct. Mater. 2002;12:773–779. doi: 10.1002/adfm.200290006. [DOI] [Google Scholar]
- 20.Wu G.X., Ding J., Xue J.M. Synthesis of calcium carbonate capsules in water-in-oil-in-water double emulsions. J. Mater. Res. 2008;23:140–149. doi: 10.1557/JMR.2008.0017. [DOI] [Google Scholar]
- 21.Kim S.K., Foote M.B., Huang L. Targeted delivery of EV peptide to tumor cell cytoplasm using lipid coated calcium carbonate nanoparticles. Cancer Lett. 2013;334:311–318. doi: 10.1016/j.canlet.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barhoum A., Rahier H., Abou-Zaied R.E., Rehan M., Dufour T., Hill G., Dufresne A. Effect of Cationic and Anionic Surfactants on the Application of Calcium Carbonate Nanoparticles in Paper Coating. ACS Appl. Mater. Interfaces. 2014;6:2734–2744. doi: 10.1021/am405278j. [DOI] [PubMed] [Google Scholar]
- 23.Boyjoo Y., Pareek V.K., Liu J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A. 2014;2:14270–14288. doi: 10.1039/C4TA02070G. [DOI] [Google Scholar]
- 24.Ferreira A.M., Vikulina A.S., Volodkin D. CaCO(3) crystals as versatile carriers for controlled delivery of antimicrobials. J. Control. Release Off. J. Control. Release Soc. 2020;328:470–489. doi: 10.1016/j.jconrel.2020.08.061. [DOI] [PubMed] [Google Scholar]
- 25.Vikulina A., Voronin D., Fakhrullin R., Vinokurov V., Volodkin D. Naturally derived nano- and micro-drug delivery vehicles: Halloysite, vaterite and nanocellulose. New J. Chem. 2020;44:5638–5655. doi: 10.1039/C9NJ06470B. [DOI] [Google Scholar]
- 26.Begum G., Reddy T.N., Kumar K.P., Dhevendar K., Singh S., Amarnath M., Misra S., Rangari V.K., Rana R.K. In Situ Strategy to Encapsulate Antibiotics in a Bioinspired CaCO3 Structure Enabling pH-Sensitive Drug Release Apt for Therapeutic and Imaging Applications. ACS Appl. Mater. Interfaces. 2016;8:22056–22063. doi: 10.1021/acsami.6b07177. [DOI] [PubMed] [Google Scholar]
- 27.Yi Z., Luo Z., Barth N.D., Meng X., Liu H., Bu W., All A., Vendrell M., Liu X. In Vivo Tumor Visualization through MRI Off-On Switching of NaGdF4–CaCO3 Nanoconjugates. Adv. Mater. 2019;31:1901851. doi: 10.1002/adma.201901851. [DOI] [PubMed] [Google Scholar]
- 28.Kim M., Lee J.H., Kim S.E., Kang S.S., Tae G. Nanosized Ultrasound Enhanced-Contrast Agent for in Vivo Tumor Imaging via Intravenous Injection. ACS Appl. Mater. Interfaces. 2016;8:8409–8418. doi: 10.1021/acsami.6b02115. [DOI] [PubMed] [Google Scholar]
- 29.Wang C., Chen S., Wang Y., Liu X., Hu F., Sun J., Yuan H. Lipase-Triggered Water-Responsive “Pandora’s Box” for Cancer Therapy: Toward Induced Neighboring Effect and Enhanced Drug Penetration. Adv. Mater. 2018;30:1706407. doi: 10.1002/adma.201706407. [DOI] [PubMed] [Google Scholar]
- 30.Xue P., Hou M., Sun L., Li Q., Zhang L., Xu Z., Kang Y. Calcium-carbonate packaging magnetic polydopamine nanoparticles loaded with indocyanine green for near-infrared induced photothermal/photodynamic therapy. Acta Biomater. 2018;81:242–255. doi: 10.1016/j.actbio.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Zhao P., Wu S., Cheng Y., You J., Chen Y., Li M., He C., Zhang X., Yang T., Lu Y., et al. MiR-375 delivered by lipid-coated doxorubicin-calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomed. Nanotechnol. Biol. Med. 2017;13:2507–2516. doi: 10.1016/j.nano.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Mendell J.R., Al-Zaidy S.A., Rodino-Klapac L.R., Goodspeed K., Gray S.J., Kay C.N., Boye S.L., Boye S.E., George L.A., Salabarria S., et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021;29:464–488. doi: 10.1016/j.ymthe.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R., Santa Chalarca C.F., Bockman M.R., Bruggen C.V., Grimme C.J., Dalal R.J., Hanson M.G., Hexum J.K., Reineke T.M. Polymeric Delivery of Therapeutic Nucleic Acids. Chem. Rev. 2021;121:11527–11652. doi: 10.1021/acs.chemrev.0c00997. [DOI] [PubMed] [Google Scholar]
- 34.He X.W., Liu T., Chen Y.X., Cheng D.J., Li X.R., Xiao Y., Feng Y.L. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther. 2008;15:193–202. doi: 10.1038/sj.cgt.7701122. [DOI] [PubMed] [Google Scholar]
- 35.Chen C., Han H., Yang W., Ren X., Kong X. Polyethyleneimine-modified calcium carbonate nanoparticles for p53 gene delivery. Regen. Biomater. 2016;3:57–63. doi: 10.1093/rb/rbv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X., El-Sayed I.H., Qian W., El-Sayed M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 37.Dolmans D.E.J.G.J., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 38.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng P., Ding B., Jiang Z., Xu W., Li G., Ding J., Chen X. Ultrasound-Augmented Mitochondrial Calcium Ion Overload by Calcium Nanomodulator to Induce Immunogenic Cell Death. Nano Lett. 2021;21:2088–2093. doi: 10.1021/acs.nanolett.0c04778. [DOI] [PubMed] [Google Scholar]
- 40.An J., Zhang K., Wang B., Wu S., Wang Y., Zhang H., Zhang Z., Liu J., Shi J. Nanoenabled Disruption of Multiple Barriers in Antigen Cross-Presentation of Dendritic Cells via Calcium Interference for Enhanced Chemo-Immunotherapy. ACS Nano. 2020;14:7639–7650. doi: 10.1021/acsnano.0c03881. [DOI] [PubMed] [Google Scholar]
- 41.Cheng A.L., Hsu C., Chan S.L., Choo S.P., Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020;72:307–319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Zhao P., Li M., Wang Y., Chen Y., He C., Zhang X., Yang T., Lu Y., You J., Lee R.J., et al. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater. 2018;72:248–255. doi: 10.1016/j.actbio.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Kong F., Zhang H., Zhang X., Liu D., Chen D., Zhang W., Zhang L., Santos H.A., Hai M. Biodegradable Photothermal and pH Responsive Calcium Carbonate@Phospholipid@Acetalated Dextran Hybrid Platform for Advancing Biomedical Applications. Adv. Funct. Mater. 2016;26:6158–6169. doi: 10.1002/adfm.201602715. [DOI] [Google Scholar]
- 44.Lammers T., Aime S., Hennink W.E., Storm G., Kiessling F. Theranostic Nanomedicine. Acc. Chem. Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 45.Min K.H., Min H.S., Lee H.J., Park D.J., Yhee J.Y., Kim K., Kwon I.C., Jeong S.Y., Silvestre O.F., Chen X., et al. pH-Controlled Gas-Generating Mineralized Nanoparticles: A Theranostic Agent for Ultrasound Imaging and Therapy of Cancers. ACS Nano. 2015;9:134–145. doi: 10.1021/nn506210a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H., Zhang W., Liu Z., Guo H., Zhang P. Smart responsive-calcium carbonate nanoparticles for dual-model cancer imaging and treatment. Ultrasonics. 2020;108:106198. doi: 10.1016/j.ultras.2020.106198. [DOI] [PubMed] [Google Scholar]
- 47.Vavaev E.S., Novoselova M., Shchelkunov N.M., German S., Komlev A.S., Mokrousov M.D., Zelepukin I.V., Burov A.M., Khlebtsov B.N., Lyubin E.V., et al. CaCO3 Nanoparticles Coated with Alternating Layers of Poly-L-Arginine Hydrochloride and Fe3O4 Nanoparticles as Navigable Drug Carriers and Hyperthermia Agents. ACS Appl. Nano Mater. 2022;5:2994–3006. doi: 10.1021/acsanm.2c00338. [DOI] [Google Scholar]
- 48.Feng Q., Zhang W., Yang X., Li Y., Hao Y., Zhang H., Hou L., Zhang Z. pH/Ultrasound Dual-Responsive Gas Generator for Ultrasound Imaging-Guided Therapeutic Inertial Cavitation and Sonodynamic Therapy. Adv. Healthc. Mater. 2018;7:1700957. doi: 10.1002/adhm.201700957. [DOI] [PubMed] [Google Scholar]
- 49.Bai S., Zhang Y., Li D., Shi X., Lin G., Liu G. Gain an advantage from both sides: Smart size-shrinkable drug delivery nanosystems for high accumulation and deep penetration. Nano Today. 2021;36:101038. doi: 10.1016/j.nantod.2020.101038. [DOI] [Google Scholar]
- 50.Wang X., Zhong X., Li J., Liu Z., Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 2021;50:8669–8742. doi: 10.1039/D0CS00461H. [DOI] [PubMed] [Google Scholar]
- 51.Tinawi M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus. 2021;13:e12420. doi: 10.7759/cureus.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]