Abstract
Simple Summary
Lifestyle modifications such as diet and exercise are a first-line defense to promote health in individuals with obesity. High-intensity interval exercise has recently gained popularity as a time-effective exercise modality. As such, this work compared the acute exercise induced benefits on vascular health between high-intensity interval exercise and traditional continuous moderate-intensity exercise. We found that both exercise modalities lead to improvements in indicators of vascular health, with some enhancements lasting up to 2 h following exercise. Therefore, high-intensity interval exercise is a time-effective strategy to improve vascular health similarly to traditional continuous moderate-intensity exercise in individuals with obesity.
Abstract
C1q-TNF-related protein-9 (CTRP9) increases endothelial nitric oxide synthase and reduces vasoconstrictors. There is limited information regarding exercise-mediated CTRP9 in obesity. The purpose of this study was to compare high-intensity interval exercise (HIIE) and continuous moderate-intensity exercise (CME) on the CTRP9 response and an indicator of endothelial function (FMD) in obese participants. Sixteen young male participants (9 obese and 7 normal-weight) participated in a counterbalanced and caloric equated experiment: HIIE (30 min, 4 intervals of 4 min at 80–90% of VO2 max with 3 min rest between intervals) and CME (38 min at 50–60% VO2 max). Serum CTRP9 and FMD were measured prior to, immediately following exercise, and 1 h and 2 h into recovery. CTRP9 was significantly increased immediately following acute HIIE and CME in both groups (p = 0.003). There was a greater CME-induced FMD response at 2 h into recovery in obese participants (p = 0.009). A positive correlation between CTRP9 and FMD percent change was observed in response to acute CME when combined with both obese and normal-weight participants (r = 0.589, p = 0.016). The novel results from this study provide a foundation for additional examination of the mechanisms of exercise-mediated CTRP9 on endothelial function in individuals with obesity.
Keywords: high-Intensity interval exercise, endothelial function, flow-mediated dilation, C1q-TNF-related protein-9, obesity
1. Introduction
Obesity is a national epidemic in the United States as its incidence has steadily increased for the last three decades and continues to rise [1]. The metabolic profile associated with obesity is linked to an augmented risk of inflammatory diseases, such as diabetes and cardiovascular disease [2]. The development of these cardiovascular consequences include infiltration of inflammatory leukocytes (e.g., macrophages) to the vessel wall and apoptosis of smooth muscle cells, leading to endothelial dysfunction [3,4]. The subsequent impairment of endothelium-dependent vasodilation or vasomotor function is one of the first subclinical stages in the atherosclerotic process [5].
A method of assessing endothelial function and vasomotor reactivity is flow-mediated dilation (FMD), which stimulates the release of endothelial relaxing factors (e.g., nitric oxide [NO]), resulting in vasodilation [6]. Additionally, obesity-related inflammation and oxidative stress have a destructive effect on the endothelium via down-regulation of vasodilatory signaling pathways [7,8], such as the AMP-activated protein kinase (AMPK) mechanism [9]. Specifically, AMPK promotes endothelial cell NO synthase (eNOS) phosphorylation through direct or indirect protein kinase B (Akt) activation [10]. A recent novel adipocytokine, C1q-TNF-related protein-9 (CTRP9), increases eNOS activation via the AMPK-Akt-eNOS mechanism in human umbilical vein endothelial cells [9]. Moreover, CTRP9 is a paralog of adiponectin that activates AMPK, Akt, and p44/42 MAPK signaling pathways [11]. Importantly, CTRP9 is down-regulated in obese mice [10] and patients with insulin resistance [12,13]. Serum CTRP9 is also inversely correlated with visceral fat in humans [12]. Interestingly, the level of circulating CTRP9 is elevated in obese individuals but significantly decreases following weight loss surgery, suggesting a compensatory role of CTRP9 in obesity [14]. Taken together, these findings may support the important role of CTRP9 as a potential mechanism to attenuate obesity-induced endothelial dysfunction.
Moderate intensity exercise has been shown to reduce inflammation [15], while high-intensity interval exercise (HIIE) improves endothelial function in cardiac patients more than continuous moderate-intensity exercise (CME) [16,17]. A greater improvement in aerobic capacity, as evidenced by increased peak oxygen consumption was observed in patients with coronary artery disease following ten weeks of HIIE training when compared to CME [16]. In comparison of CME training, HIIE training reduced resting blood pressure and arterial stiffness as an indication of improved vascular function in hypertensive patients [18]. A meta-analysis concluded that HIIE training elicits a greater improvement of FMD compared to CME training [19]. Regarding the time course of FMD response, an elevation was observed up to two hours into recovery following acute HIIE, whereas CME remained unchanged in healthy adolescents [20]. Moreover, acute high-intensity continuous exercise showed a significant increase in FMD in normal-weight but not obese participants, with no difference observed in response to acute moderate-intensity continuous exercise [21]. Acute HIIE has been demonstrated to increase CTRP9 immediately following exercise in healthy young participants [22]. However, the exact mechanisms regarding obesity-mediated FMD and CTRP9 responses with either HIIE or CME remains to be elucidated. Therefore, the primary purpose of this study was to utilize acute HIIE as a time-effective exercise modality to understand CTRP9 response and its possible relationship with an indicator of endothelial function (FMD) when compared to CME in obese vs. normal-weight participants. We hypothesized that HIIE would be as effective as CME at increasing CTRP9 and FMD following exercise.
2. Methods
2.1. Participants
Sixteen (9 obese and 7 normal-weight) relatively healthy young male participants participated in this study. Participants with a body mass index (BMI) above 30 kg/m2 were classified as obese, and those with a BMI between 18.5 and 24.9 kg/m2 were classified as normal-weight. All participants completed an informed consent form, a medical history questionnaire, and 7-day physical activity record prior to data collection. The study was approved by the Florida Atlantic University’s Institutional Review Board and were performed according to the Declaration of Helsinki, with written informed consent from each participant.
Participants were excluded from the study if they had any known or suspected cardiovascular, metabolic, rheumatologic, or other inflammatory disease. Participants were also excluded from the study if they were taking any medication or supplements, users of tobacco products (cigarettes, cigars, chewing tobacco, vapors), or if they consumed an average of more than ten alcoholic beverages per week. These exclusion criteria were determined using the health history questionnaire. Participants fasted overnight for at least eight hours and abstained from alcohol, caffeine intake, and intense physical activity for at least 24 h prior to each lab visit.
2.2. Experimental Protocol
All participants performed three exercise protocols, with a minimum of one week separating each session. Participants started each study visit at the Exercise Biochemistry Laboratory between 7:00–7:30 a.m. During the first visit, following completion of the informed consent form, medical history questionnaire, and 7-day physical activity questionnaire, height and weight was measured (SECA 769, Chino, CA, USA), we also measured each participant’s hip and waist circumference to determine waist-to-hip ratio. Additionally, after 20 min of sitting, resting heart rate was recorded using a heart rate monitor (Polar T31, Polar Electro, Kempele, Finland) and blood pressure was assessed using a sphygmomanometer (752M-Mobile Series, American Diagnostic Corporation, Hauppauge, NY, USA). Next, blood sampling was performed by a trained phlebotomist (B.G.F) using standard aseptic techniques. A closed IV catheter system (BD Nexiva 20 GA, REF 383516, Franklin Lakes, NJ, USA) was inserted in the superficial vein of the upper arm in the antecubital space. Approximately 30 mL of blood were collected into specific collection tubes for subsequent analysis. Prior to the beginning of the exercise protocol, participants rested supine for 20 min before the assessment of their resting (baseline) FMD measurement using an ultrasound (Phillips iU22, Foster City, CA, USA).
Participants completed a graded exercise test using a treadmill (Norditrack X11i) designed to assess maximal oxygen consumption (VO2 max) measured by open-circuit spirometry (ParvoMedics Metabolic Measurement System (ParvoMedics, Sandy, UT, USA)) and maximal heart rate (HRmax). The maximal exercise protocol started with a three-minute warm up at 60% age-predicated maximal heart rate (HRmax), followed by an increase in speed until 80% HRmax. Subsequently, the grade was increased by 2% every two minutes until attainment of VO2 max. The validation of VO2 max was verified by either the primary criterion of a plateau in VO2 or 2 of the 3 secondary criteria are achieved. The secondary criteria used were (1) reaching predicted maximal heart rate, (2) achieving a respiratory exchange ratio of >1.15, and (3) reporting a rating of perceived exertion (with the 15-point Borg Scale) of 19 or 20. Importantly, this perceived exertion scale was used to measure the perception of stress each minute during exercise. If participants reported a difficulty to maintain exercise intensity, then the exercise testing was terminated.
During the second and third visits, participants were randomly allocated to participate in either HIIE or CME following a previously validated treadmill protocol [16,23]. The HIIE consisted of 30 min of exercise, including a 5 min warm-up period of 50–60% VO2 max (65–75% HRmax), 4 intervals of 4 min at an intensity that elicits 80–90% VO2 max (85–95% HRmax). Between the intervals the participants walked for 3 min at 50–60% VO2 max (65–75% HRmax), with no active recovery following the last high-intensity bout. To achieve an isocaloric protocol between the exercise protocols, CME consisted of 38 min at 50–60% VO2 max (65–75% of HRmax), which equated total oxygen consumption (VO2) across time of exercise as previously described [16]. Blood collection and FMD measurements for both visits 2 and 3, followed the same protocol as the first visit.
2.3. Blood Sampling
During each blood draw, 30 mL of the blood were collected with 5 mL into a serum separation tube (SST) for serum protein analysis and centrifuged for 10 min at 1300× g at room temperature. The serum was collected and stored in aliquots at −80 °C for subsequent analysis by enzyme-linked immunosorbent assays (ELISA). Serum CTRP9 was analyzed using (Cloud-Clone Corp., Houston, TX, USA).
2.4. FMD Measurement
The FMD measurement and protocol followed previously established guidelines [24]. The brachial artery was identified and imaged longitudinally using a Phillips L9-3 broadband 9.0 MHz vascular probe on the medial upper arm 2–10 cm above the antecubital fossa near the level of the heart. Landmarks were identified to ensure the same location for all repeated FMD measurements. Additionally, with-in each visit the probe was outlined to ensure identical placement for each time-point. Diameter and blood flow velocity were recorded in Digital Imaging and Communications in Medicine (DICOM) format using a duplex mode of ultrasound that allows simultaneous B-mode imaging for diameter measurements and Doppler for blood velocity (shear rate) using a Philips iU22 ultrasound. The insonation angle was set to 60° and gate width was adjusted for an accurate measurement of blood velocity as described by Harris et al., 2010.
Participants laid supine for 20 min prior to baseline FMD measurements, following acclimatization baseline measurements were recorded for 1 min. A blood pressure cuff (WelchAllyn 406920 series) was placed two centimeters distal to the antecubital fold and was then inflated to ≤250 mmHg for 5 min. Prior to the cuff release, measurements were recorded for 20 s, following release post-occlusion measurements were recorded for 3 min.
Validated software (Medical Imaging Applications, LLC., Coralville, IA, USA) was used for offline analysis of the recorded images for brachial diameter (mm) and blood velocity (shear rate). ECG gating was used for consistent cardiac cycles (end diastole) for brachial artery diameter measurements. FMD (%) was quantified as the peak diameter observed post-occlusion and reported as percent change from the average 1 min baseline diameter.
2.5. Statistical Analyses
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS version 22.0). Normality of the data was confirmed with a Shapiro–Wilk test. Differences between obese and normal-weight groups in baseline variables were computed by independent t tests. A 2 (group) × 2 (treatment) × 4 (time points: Pre, Post, R1 [1 h into recovery], and R2 [two hours into recovery]) repeated measures analyses of variance (ANOVA) were utilized to examine the effect of acute aerobic exercise on serum levels of CTRP9 and FMD. Bonferroni post hoc analysis was utilized for pairwise comparisons. The. Greenhouse-Geisser correction of degrees of freedom was used when sphericity assumptions were violated. Pearson product-moment correlations were used to examine the relationship of CTRP9 with FMD. A post hoc power analysis was conducted using the program G*Power (version 3.1.9.2) for primary outcome measures. Based on the effect size ranging from 0.54 to 0.72 with an α-level of 0.05 for CTRP9 and FMD in response to both HIIE and CME, the overall sample size of 16 participants in this study achieved an adequate power (>80%). Statistical significance was defined as a p-value ≤ 0.05.
3. Results
3.1. Anthropometric Measurements of the Study Participants
As shown in Table 1, the analysis revealed a significant difference in age, weight, BMI, relative VO2 max, waist circumference, hip circumference, waist-to-hip ratio, resting systolic blood pressure, and diastolic blood pressure between obese and normal-weight participants.
Table 1.
Characteristics of Participants.
| Variable | Normal-Weight n = 7 |
Obese n = 9 |
p-Value |
|---|---|---|---|
| Age (years) | 23 ± 2 | 28 ± 5 | 0.019 |
| Height (m) | 1.79 ± 0.04 | 1.78 ± 0.06 | 0.815 |
| Weight (kg) | 72 ± 10 | 116 ± 18 | <0.001 |
| Body Mass Index (kg/m2) | 22 ± 2 | 36 ± 4 | <0.001 |
| Waist (cm) | 80 ± 7 | 113 ± 16 | <0.001 |
| Hip (cm) | 96 ± 5 | 118 ± 8 | <0.001 |
| WHR (a.u.) | 0.84 ± 0.06 | 0.95 ± 0.09 | 0.009 |
| VO2 max (mL/kg/min) | 51 ± 5 | 37 ± 6 | <0.001 |
| Resting Heart Rate (bpm) | 67 ± 10 | 73 ± 5 | 0.148 |
| rSBP (mmHg) | 116 ± 7 | 138 ± 12 | <0.001 |
| rDBP (mmHg) | 72 ± 5 | 85 ± 8 | <0.001 |
Data are represented as mean ± SD. WHR = waist-to-hip ratio; VO2 max = maximal oxygen consumption; rSBP = resting systolic blood pressure; rDBP = resting diastolic blood pressure.
3.2. Measurement of Serum CTRP9 and Flow-Mediated Dilation
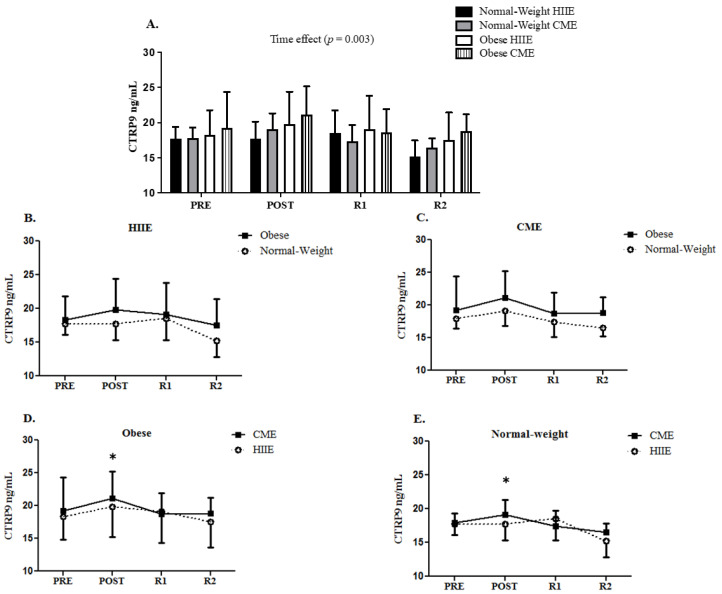
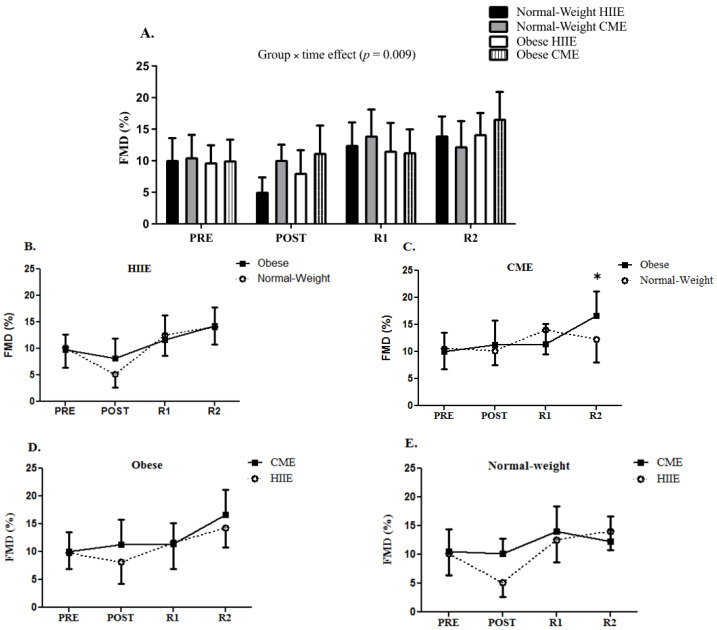
At baseline, no difference was observed in CTRP9 and FMD between obese and normal-weight participants. However, the obese participants had greater brachial artery diameters than the normal-weight participants at baseline for CME (4.5 mm vs. 3.8 mm, p = 0.021) but not HIIE (4.3 mm vs. 3.9 mm, p = 0.064). Repeated measures ANOVA demonstrated a significant time effect for CTRP9 immediately following acute HIIE and CME in both groups (F [3, 42] = 5.435, p = 0.003) (see Figure 1). Furthermore, a group by time interaction for FMD was observed (F [3, 42] = 4.346, p = 0.009), with a greater CME-induced FMD response at two hours into recovery in obese participants than normal-weight participants (see Figure 2).
Figure 1.
The response of serum CTRP9 following HIIE vs. CME in normal-weight and obese participants (panel (A)). The CTRP9 response to HIIE in normal-weight and obese participants (panel (B)). The CTRP9 response to CME in normal-weight and obese participants (panel (C)). The comparison of CTRP9 response to CME vs. HIIE in obese participants (panel (D)). The comparison of CTRP9 response to CME vs. HIIE in normal-weight participants (panel (E)). Data are presented as means ± SD. * Time effect vs. PRE.
Figure 2.
The FMD response to HIIE vs. CME in normal-weight and obese participants (panel (A)). The FMD response to HIIE in normal-weight and obese participants (panel (B)). The FMD response to CME in normal-weight and obese participants (panel (C)). The comparison of FMD response to CME vs. HIIE in obese participants (panel (D)). The comparison of FMD response to CME vs. HIIE in normal-weight participants (panel (E)). Data are presented as means ± SD. * Group × time effect.
3.3. Correlations between CTRP9 and FMD
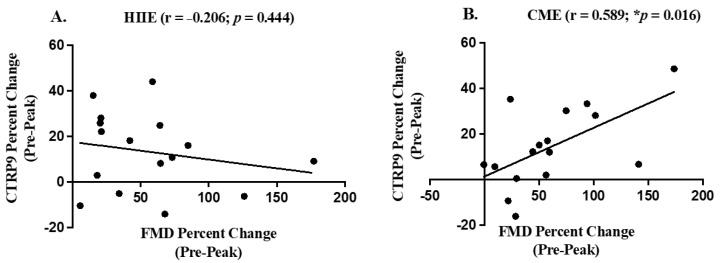
When combined with both obese and normal-weight participants, our analyses did not observe a significant correlation between CTRP9 and FMD at baseline. Moreover, a positive Pearson correlation in percent change (baseline to peak value) between CTRP9 and FMD was found following acute CME (r = 0.589, p = 0.016) (see Figure 3B). However, this relationship between CTRP9 and FMD following HIIE failed to exist (r = −0.206, p = 0.444) (see Figure 3A). Finally, when the relationship of CTRP9 with FMD was analyzed in either obese or normal-weight group alone, our analyses did not show any significant results.
Figure 3.
The relationship between CTRP9 percent change (pre-peak value) and FMD percent change (pre-peak value) using Pearson correlations in response to HIIE (panel (A)) and CME (panel (B)). * Indicates significant Pearson correlation.
4. Discussion
We examined the effect of acute HIIE vs. CME on serum CTRP9 and brachial artery FMD responses in obese and normal-weight participants. Our results demonstrated that obese participants elicited a similar elevation in serum CTRP9 immediately following both acute HIIE and CME compared to normal-weight participants. Furthermore, a greater elevation in FMD was only found at 2 h into recovery following acute CME in obese participants when compared to normal-weight participants. These findings support the use of CME in this population as an effective modality to improve cardiovascular health, as evidenced by increased FMD in our participants with obesity.
While this study did not observe a difference at the baseline level of CTRP9 between obese and normal-weight participants, the literature regarding CTRP9 and obesity remains debated. Specifically, research has previously shown that the circulating level of CTRP9 was lower following a high-fat diet in obese mice when compared to lean mice [25]. While other research that utilized a CTRP9 knockout mice model demonstrated that mice became obese even when fed normal chow [26]. In humans, the level of serum CTRP9 has been shown to inversely relate to visceral fat [12]. In contrast, another study found serum levels of CTRP9 were higher in patients with obesity-associated comorbidities (e.g., diabetes, hypertension, hypercholesterolemia) than healthy normal-weight individuals, but following weight-loss surgery there was decrease in CTRP9 [14]. While lower serum CTRP9 levels in metabolically unhealthy individuals were observed [12], participants in our study did not have a history of metabolic syndrome or diseases.
To the best of our knowledge, this study is the first to examine how obesity might influence the release of circulating CTRP9 in response to acute exercise (HIIE or CME), although no difference was found between obese and normal-weight groups. In agreement with our findings, previous research demonstrated an elevation in CTRP9 following acute HIIE in normal-weight participants [22]. However, the literature has previously reported that CTRP9 plays a compensatory role in obesity and arterial stiffness [14,27]. Specifically, CTRP9 promotes vasodilation through eNOS activation via the AMPK-Akt-eNOS mechanism in human umbilical vein endothelial cells [9]. The expression of increased CTRP9 in pulmonary epithelial cells enhanced the activation of eNOS and reduced the release of vasoconstricting factor, such as endothelin-1 [28]. Furthermore, CTRP9 can attenuate cytokine-induced vascular inflammation in endothelial cells via AMPK activation, including a reduction in TNF-α induced activation of inflammatory transcription factor (NF-kB) and adhesion molecules (ICAM-1 and VCAM-1) [27]. Thus, further studies are warranted to discover the effect of exercise training (either HIIE or CME) to gain a better understanding of how CTRP9 might play a potential compensatory role in the improvement of cardiovascular health in obesity-associated metabolic complication, such as insulin resistance [12,14]. As HIIE is a time-effective strategy to reduce body fat percentage in adults with obesity [29] and has been demonstrated to be more enjoyable than CME [30].
The measurement of FMD provides prognostic information to possibly exceed the assessment of traditional risk factors for cardiovascular events [31], although there is a debate regarding the normalization for FMD stimulus (shear rate) [6]. Our laboratory has recently demonstrated that the normalization of FMD (using FMD [%]/shear rate [area under the curve]) did not change the results for the acute response to aerobic exercise using a subset of the participants from this study [32]. As such, the presentation of FMD (%) was utilized in the current study as it is clinically relevant [33]. Importantly, obesity has been associated with the impairment of FMD [34]; however, the present study did not observe any baseline difference with normal-weight individuals in agreement with the findings by Hallmark et al., 2014. This discrepancy might be due to the utilization of different age groups: older adults by Davison and colleagues vs. young adults in the current study. Interestingly, our results demonstrated a significant interaction for group by time effect in FMD, with a greater CME-induced response in obese than normal-weight participants, which remained elevated two hours into recovery. These findings may support the use of acute CME to improve endothelial function in individuals with obesity [35]. Specifically, enhanced endothelial function in response to acute CME may be a result of the exercise-mediated shear stress on the endothelium to enhance NO bioavailability [35]. While this study is the first to examine the impact of obesity on FMD response following acute HIIE vs. CME, acute HIIE has been demonstrated to improve FMD equally when compared to CME in patients with coronary artery disease [36]. However, Currie et al. 2012 reported a greater total workload in acute CME than HIIE, whereas this study equated total work (caloric expenditure) between exercise conditions (HIIE and CME) in both groups, which may potentially explain the variance in this finding. Finally, a significant positive relationship in percent change (baseline to peak value) between CTRP9 and FMD was found following acute CME, providing evidence of enhanced endothelial function in both groups. However, additional investigation is needed to further verify the relationship of exercise-mediated CTRP9 and endothelial function in older adults with obesity. Additionally, the impact of sex on these findings should be investigated as only males were included in this study. The use of CTRP9 could potentially predict the effectiveness of exercise treatments to prevent or delay obesity-associated cardiovascular events.
This study is not without limitations. For example, there was an age difference between groups, with the obese participants being slightly older than normal-weight participants. Additionally, our findings are limited to young healthy males, which may not be representative of females and or the general population. Lastly, we did not directly measure adiposity, as we classified our participants for obesity using body mass index and waist-to-hip ratio. Future research should utilize a larger sample size with both males and females and directly measure adiposity, to confirm our findings regarding endothelial function and CTRP9 in response to HIIE and CME in participants with obesity.
5. Conclusions
In conclusion, this study demonstrated that participants with obesity exhibited a similar CTRP9 response following both acute HIIE and CME compared to participants of normal-weight. Furthermore, a greater FMD elevation was observed in obese participants in response to CME when compared to normal-weight participants. While HIIE is a time-effective strategy to improve metabolic health and inflammation [37,38], the novel results from this study provide a foundation for additional examination of the mechanisms of exercise-mediated CTRP9 on endothelial function in individuals with obesity.
Acknowledgments
The authors would like to thank the participants that volunteered their time to guarantee the accomplishment of this study.
Abbreviations
| AMPK | AMP-activated protein kinase |
| eNOS | Endothelial cell nitric oxide synthase |
| ANOVA | Analyses of variance |
| FMD | Flow-mediated dilation |
| BMI | Body mass index |
| HIIE | High-intensity interval exercise |
| CME | Continuous moderate-intensity exercise |
| NO | Nitric oxide |
| CTRP9 | C1q-TNF-related protein-9 |
| RPE | Rating of perceived exertion |
| EDTA | Ethylenediaminetetraacetic acid |
| SST | serum separation tube |
| ELISA | Enzyme-linked immunosorbent assay |
| VO2 max | Maximal oxygen consumption |
Author Contributions
Conceptualization, B.G.F. and C.-J.H.; Methodology, B.G.F., P.J.F., K.M.D., G.S.P. and A.A.R.; Investigation, B.G.F.; Data curation, B.G.F.; Writing—original draft, B.G.F.; Writing—review & editing, B.G.F., R.S.G., M.C.Z., M.W., P.J.F., K.M.D., G.S.P., A.A.R. and C.-J.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Florida Atlantic University’s Institutional Review Board (#891706-3) and were performed according to the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1–8. [PubMed] [Google Scholar]
- 2.Powell-Wiley T.M., Poirier P., Burke L.E., Després J.-P., Gordon-Larsen P., Lavie C.J., Lear S.A., Ndumele C.E., Neeland I.J., Sanders P., et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha V.Z., Libby P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 4.Bennett M.R., Sinha S., Owens G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimbrone M.A., García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thijssen D.H.J., Bruno R.M., Van Mil A.C.C.M., Holder S.M., Faita F., Greyling A., Zock P.L., Taddei S., Deanfield J.E., Luscher T., et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019;40:2534–2547. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 7.Meyer A.A., Kundt G., Steiner M., Schuff-Werner P., Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: The impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 8.Karolina D., Silambarasan M., Armugam A., Yeyaseelan K. MicroRNA and Endothelial Dysfunction in relation to Obesity and Type2 Diabetes. J. Mol. Genet. Med. 2014;1:1747–1862. [Google Scholar]
- 9.Zheng Q., Yuan Y., Yi W., Lau W.B., Wang Y., Wang X., Sun Y., Lopez B.L., Christopher T.A., Peterson J.M. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2011;31:2616–2623. doi: 10.1161/ATVBAHA.111.231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uemura Y., Shibata R., Ohashi K., Enomoto T., Kambara T., Yamamoto T., Ogura Y., Yuasa D., Joki Y., Matsuo K. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. doi: 10.1096/fj.12-213744. [DOI] [PubMed] [Google Scholar]
- 11.Wong G.W., Krawczyk S.A., Kitidis-Mitrokostas C., Ge G., Spooner E., Hug C., Gimeno R., Lodish H.F. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241–258. doi: 10.1096/fj.08-114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang Y., Oh S.W., Park S., Park C. Association of serum C1q/TNF-Related Protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. Int. J. Obes. 2014;38:1207–1212. doi: 10.1038/ijo.2013.242. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Hang T., Cheng X.-M., Li D.-M., Zhang Q.-G., Wang L.-J., Peng Y.-P., Gong J.-B. Associations of C1q/TNF-related protein-9 levels in serum and epicardial adipose tissue with coronary atherosclerosis in humans. BioMed Res. Int. 2015;2015:971683. doi: 10.1155/2015/971683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf R.M., Steele K.E., Peterson L.A., Zeng X., Jaffe A.E., Schweitzer M.A., Magnuson T.H., Wong G.W. C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery. J. Clin. Endocrinol. Metab. 2016;101:2211–2217. doi: 10.1210/jc.2016-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringseis R., Eder K., Mooren F.C., Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc. Immunol. Rev. 2015;21:58–68. [PubMed] [Google Scholar]
- 16.Rognmo Ø., Hetland E., Helgerud J., Hoff J., Slørdahl S.A. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 2004;11:216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 17.Anderson L., Thompson D.R., Oldridge N., Zwisler A.-D., Rees K., Martin N., Taylor R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.cd001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimaraes G.V., Ciolac E.G., Carvalho V.O., D’Avila V.M., Bortolotto L.A., Bocchi E.A. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens. Res. 2010;33:627–632. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- 19.Ramos J.S., Dalleck L.C., Tjonna A.E., Beetham K.S., Coombes J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015;45:679. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 20.Bond B., Hind S., Williams C.A., Barker A.R. The Acute Effect of Exercise Intensity on Vascular Function in Adolescents. Med. Sci. Sports Exerc. 2015;47:2628–2635. doi: 10.1249/MSS.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 21.Hallmark R., Patrie J.T., Liu Z., Gaesser G.A., Barrett E.J., Weltman A. The effect of exercise intensity on endothelial function in physically inactive lean and obese adults. PLoS ONE. 2014;9:e85450. doi: 10.1371/journal.pone.0085450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kon M., Ebi Y., Nakagaki K. Effects of a single bout of high-intensity interval exercise on C1q/TNF-related proteins. Appl. Physiol. Nutr. Metab. 2019;44:47–51. doi: 10.1139/apnm-2018-0355. [DOI] [PubMed] [Google Scholar]
- 23.Tyldum G.A., Schjerve I.E., Tjønna A.E., Kirkeby-Garstad I., Stølen T.O., Richardson R.S., Wisløff U. Endothelial dysfunction induced by post-prandial lipemia: Complete protection afforded by high-intensity aerobic interval exercise. J. Am. Coll. Cardiol. 2009;53:200–206. doi: 10.1016/j.jacc.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris R.A., Nishiyama S.K., Wray D.W., Richardson R.S. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson J.M., Wei Z., Seldin M.M., Byerly M.S., Aja S., Wong G.W. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013;305:R522–R533. doi: 10.1152/ajpregu.00110.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Z., Lei X., Petersen P.S., Aja S., Wong G.W. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am. J. Physiol.-Endocrinol. Metab. 2014;306:E779–E790. doi: 10.1152/ajpendo.00593.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung C.H., Lee M.J., Kang Y.M., La Lee Y., Seol S.M., Yoon H.K., Kang S.-W., Lee W.J., Park J.-Y. C1q/TNF-related protein-9 inhibits cytokine-induced vascular inflammation and leukocyte adhesiveness via AMP-activated protein kinase activation in endothelial cells. Mol. Cell. Endocrinol. 2016;419:235–243. doi: 10.1016/j.mce.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Geng X., Wang H., Cheng G., Xu S. CTRP9 Ameliorates Pulmonary Arterial Hypertension Through Attenuating Inflammation and Improving Endothelial Cell Survival and Function. J. Cardiovasc. Pharmacol. 2016;67:394–401. doi: 10.1097/FJC.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 29.Türk Y., Theel W., Kasteleyn M.J., Franssen F.M.E., Hiemstra P.S., Rudolphus A., Taube C., Braunstahl G.J. High intensity training in obesity: A Meta-analysis. Obes. Sci. Pr. 2017;3:258–271. doi: 10.1002/osp4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinrich K.M., Patel P.M., O’Neal J.L., Heinrich B.S. High-intensity compared to moderate-intensity training for exercise initiation, enjoyment, adherence, and intentions: An intervention study. BMC Public Health. 2014;14:789. doi: 10.1186/1471-2458-14-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green D.J., Jones H., Thijssen D., Cable N., Atkinson G. Flow-mediated dilation and cardiovascular event prediction. Hypertension. 2011;57:363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 32.Slusher A.L., Fico B.G., Dodge K.M., Garten R.S., Ferrandi P.J., Rodriguez A.A., Pena G., Huang C.-J. Impact of acute high-intensity interval exercise on plasma pentraxin 3 and endothelial function in obese individuals—A pilot study. Eur. J. Appl. Physiol. 2021;121:1567–1577. doi: 10.1007/s00421-021-04632-5. [DOI] [PubMed] [Google Scholar]
- 33.Maruhashi T., Kajikawa M., Kishimoto S., Hashimoto H., Takaeko Y., Yamaji T., Harada T., Han Y., Aibara Y., Mohamad Yusoff F., et al. Diagnostic Criteria of Flow-Mediated Vasodilation for Normal Endothelial Function and Nitroglycerin-Induced Vasodilation for Normal Vascular Smooth Muscle Function of the Brachial Artery. J. Am. Heart Assoc. 2020;9:e013915. doi: 10.1161/JAHA.119.013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davison K., Bircher S., Hill A., Coates A.M., Howe P.R., Buckley J.D. Relationships between obesity, cardiorespiratory fitness, and cardiovascular function. J. Obes. 2011;2010:191253. doi: 10.1155/2010/191253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W., Zeng J., Yin J., Zhang F., Wu H., Yan S., Wang S. Both flow-mediated vasodilation procedures and acute exercise improve endothelial function in obese young men. Eur. J. Appl. Physiol. 2010;108:727–732. doi: 10.1007/s00421-009-1283-3. [DOI] [PubMed] [Google Scholar]
- 36.Currie K.D., McKelvie R.S., MacDonald M.J. Flow-mediated dilation is acutely improved after high-intensity interval exercise. Med. Sci. Sports Exerc. 2012;44:2057–2064. doi: 10.1249/MSS.0b013e318260ff92. [DOI] [PubMed] [Google Scholar]
- 37.Munk P.S., Breland U.M., Aukrust P., Ueland T., Kvaløy J.T., Larsen A.I. High intensity interval training reduces systemic inflammation in post-PCI patients. Eur. J. Cardiovasc. Prev. Rehabil. 2011;18:850–857. doi: 10.1177/1741826710397600. [DOI] [PubMed] [Google Scholar]
- 38.Shiraev T., Barclay G. Evidence based exercise: Clinical benefits of high intensity interval training. Aust. Fam. Physician. 2012;41:960. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.