Abstract
Vibrio cholerae WO7 (serogroup O1) isolated from patients with diarrhea produces an extracellular toxin despite the absence of ctx, zot, and ace genes from its genome. The toxin elongates Chinese hamster ovary cells, produces fluid accumulation in ligated rabbit ileal loops, and agglutinates freshly isolated rabbit erythrocytes. Maximal production of this toxin (WO7 toxin) was seen in AKI medium with the pH adjusted to 8.5 at 37°C under shaking conditions. We purified this toxin to homogeneity by sequential ammonium sulfate precipitation, affinity chromatography using a fetuin-Sepharose CL-4B column, and gel filtration chromatography, which increased the specific activity of the toxin by 1.6 × 106-fold. The toxin is heat labile and sensitive to proteases and has a subunit structure consisting of two subunits with molecular masses of about 58 and 40 kDa as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Agglutination of GM1-coated sheep erythrocytes by toxin suggests that GM1 might be the physiologic receptor for WO7 toxin on the enterocytes. An immunodiffusion test between the antiserum raised against the purified WO7 toxin and the purified toxin gave a well-defined precipitation band. In the immunoblot assay, two bands were observed in the 58- and 40-kDa region. At the same time, antiserum against WO7 toxin failed to show any cross-reactivity with cholera toxin or Escherichia coli heat-labile toxin (LT1) in an immunodiffusion test or immunoblot assay. The enterotoxic activity of WO7 toxin could be inhibited by antiserum against purified WO7 toxin. Our results indicate that WO7 toxin is structurally and functionally distinct from other cholera toxins and that the enterotoxic activities expressed by WO7 toxin appear to contribute to the pathogenesis of disease associated with V. cholerae O1 strains.
Vibrio cholerae is a normal inhabitant of the aquatic environment (27). However, contaminated water supplies in some areas of the world have enabled some clones of the species to become pathogenic to humans. Previous studies have revealed that V. cholerae has a high level of genetic exchange and a relatively low level of clonality. Random genetic drifts and recombination events have enabled the V. cholerae organisms to survive and perpetuate even in regions of high endemicity.
Until recently, it was thought that clinical manifestations of cholera result primarily from interaction between cholera toxin (CT) and intact intestinal epithelial cells. However, the occurrence of mild to moderate diarrhea in human volunteers fed live oral cholera vaccine strains (11) or genetically engineered mutants (15, 16) incapable of producing whole biologically active CT (19) prompted investigators to search for additional toxins that could contribute to the pathogenesis of cholera. This led to the discovery of new cholera toxin (26), zona occludens toxin (10), accessory cholera enterotoxin (32), and then a heat-stable enterotoxin (29). The role of the toxins other than CT in the pathogenesis of disease due to V. cholerae is largely unknown. These toxins clearly cannot cause cholera gravis; however, toxins other than CT may contribute to diarrhea and may also be responsible for certain symptoms associated with CT-negative strains. Additionally, such toxins may serve as secondary secretogenic mechanisms when conditions for producing CT are not optimal.
Morris et al. (22) first indicated that V. cholerae O1 strains which do not produce CT may be able to cause gastrointestinal disease in humans. In this study, we report that V. cholerae O1 (clinical isolate) produces yet another toxin in the absence of ctx, zot, and ace genes which is antigenically and genetically distinct. The strain V. cholerae WO7 used in this study was an isolate from an outbreak of cholera which occurred in the city of Warrangal located in southern India in the state of Andhra Pradesh. The toxin produced by this strain causes fluid accumulation in the rabbit ileal loop assay (RILA) and elongates Chinese hamster ovary (CHO) cells. We present the method for purifying this toxin of V. cholera WO7 and a characterization of its biological and physicochemical properties.
MATERIALS AND METHODS
Bacterial strains.
A clinical isolate of V. cholerae WO7 was procured from NICED, Calcutta, India. The strains V. cholerae 569B and V. cholerae 0139 were obtained from Central Research Institute, Kasauli, India. The organisms were maintained in Trypticase soy broth without glucose (Difco Laboratories) supplemented with 0.5% sodium chloride plus 5% glycerol at −80°C.
Isolation of genomic DNA.
DNA was isolated from overnight cultures of V. cholerae WO7, V. cholerae 569B, and V. cholerae 0139 and grown in Luria broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl) at 37°C by the method of Mekalanos (21) with minor modifications.
Southern hybridization.
Chromosomal DNA (1 μg) was digested with restriction enzymes as suggested by the manufacturer (Amersham, Hong Kong, China). Digests were fractionated by horizontal electrophoresis (0.7% agarose gel) containing 0.04 M Tris (pH 8.0), 0.04 M sodium acetate, and 0.001 M EDTA. The gel was processed as described in the Hybond instruction manual (Amersham) with a few modifications. The gels were soaked in 0.25 N HCl for 30 min to allow partial depurination and cleavage of larger DNA fragments. The gels were then soaked in 0.5 M sodium hydroxide and 1.5 M sodium chloride for 30 min followed by neutralization in 0.5 M Tris-HCl (pH 7.0) containing 0.3 M NaCl (20× SSC) for 1 h each, and blotting was done with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The nylon membrane was dried and baked for 2 h at 80°C. The ctx, zot, and ace gene probes (28) were labelled with 32P by nick translation (24). The hybridization was carried out in a high-stringency solution (40% [vol/vol] formamide, 5× SSC, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 50 μg of salmon sperm DNA per ml, polyvinylpyrrolidone, 0.05% Ficoll). Radioactive denatured probes (ctx, zot, and ace) were added to the prehybridization buffer. The plasmid pCT5A11 was used as a positive control for the ctx and ace gene probes, and pMZP11 was used as a positive control for the zot gene probe (28). The hybridizations were carried out at 37°C for 20 h typically with 5,000 cpm of probe/ml. Washing was carried out first at 37°C for 45 min in 5× SSC–0.01% SDS and then at room temperature for 15 min in 2× SSC. The blots were exposed to autoradiography at −70°C.
Optimization of growth conditions for production of WO7 toxin.
Different media such as brain heart infusion (BHI) broth, Casamino Acids yeast extract (CAYE) medium (2% Casamino Acids, 0.6% yeast extract, 0.87% K2HPO4, 0.25% NaCl, 0.25% glucose) (pH 8.0 to 8.2), Trypticase soy broth (TSB), and AKI medium (14) (1.5% Bacto Peptone, 0.4% yeast extract, 0.5% NaCl, 0.3% filter-sterilized NaHCO3 [pH 7.4]) were used to determine the optimum medium for production of V. cholerae WO7 toxin.
Standardization of optimum culture conditions for production of WO7 toxin.
Two different culture conditions, a 24-h stationary period and a 6-h stationary period followed by 18 h of shaking (13), were used to determine the optimal conditions for toxin production. V. cholerae WO7 was cultured in a test tube (preculture) for 6 h and subsequently cultured under different conditions in 250-ml flasks containing 100 ml of medium for 18 h. Shaking was performed in an orbital shaker at 120 rpm. For each culture condition, strains were incubated at either 30 or 37°C to determine the optimal temperature for production of WO7 toxin.
Toxin purification.
Unless otherwise stated, all the steps were performed at 4°C. Four 2.8-liter Fernbach flasks, each containing 500 ml of AKI medium with seed culture suspension, were taken (grown at 37°C for 4 h). The culture was incubated for 16 to 18 h at 37°C on a rotary shaker at 100 rpm in the presence of 1 mM EGTA. Culture supernatants were recovered by centrifugation. EDTA was added in a final concentration of 1 mM to culture supernatants (stage 1). Ammonium sulfate was dissolved to a final concentration of 80% saturation. The precipitate was dissolved in 10 ml of phosphate-buffered saline (PBS; pH 7.0), and this preparation was dialyzed against 0.001 M Tris-HCl (pH 7.2) in the presence of 0.01 M EDTA. Any precipitate formed during this stage was discarded, and the supernatant was retained (stage 2).
The crude WO7 toxin from stage 2 was purified by using a cation-exchange MonoS column (Pharmacia). The column was equilibrated with 0.01 M Tris (pH 7.2). Culture supernatants, containing 80 100 mg of protein, equilibrated by dialysis with the same buffer as the column, were applied to the MonoS column. The column was then washed with 0.01 M Tris (pH 7.2) buffer for 10 min. The bound fraction was eluted with the same buffer containing 0.2 M NaCl (pH 7.4) and dialyzed against PBS. Thereafter, it was concentrated and checked for biological activity, GM1 binding properties, and total protein content.
Fractions from stage 2 were further subjected to affinity chromatography on fetuin coupled to a CNBr-activated Sepharose CL-4B column (Pharmacia) previously equilibrated with buffer A (0.05 M Tris, 0.15 M NaCl, 0.03 M CaCl2). Overnight binding was allowed. After the column was washed with buffer A, the toxin was eluted with buffer B (0.05 M Tris, 0.15 M NaCl, 0.04 M sodium citrate) (stage 3). Pooled fractions of stage 3 were dialyzed against 0.01 M Tris (pH 7.2), and the dialysate was concentrated by ultrafiltration through a PM10 membrane (Amicon) and applied to a gel filtration SW300 column (stage 4).
Samples obtained at all the stages were analyzed for toxin activity, GM1 binding activity, total protein content, and homogeneity.
The fetuin-Sepharose CL-4B-eluted material from affinity chromatography was further purified by gel filtration chromatography on a Protein Pak SW300 gel filtration column (8 by 30 cm; Nihon Ltd., Japan Waters) in a fast protein liquid chromatography system (Pharmacia).
Native gel electrophoresis (alkaline) was performed by the method of Davis (7) with a 7.5% gel. Sodium dodecyl sulfate-polyacrylamide gel (12.5%) electrophoresis was carried out by the method of Laemmli (18).
Antiserum preparation.
New Zealand White rabbits (2-kg body weight) were injected subcutaneously with a water-in-oil emulsion containing 250 μg of purified toxin emulsified with 2 volumes of Freund’s complete adjuvant (Sigma). The rabbits were then given a booster injection of 150 μg of the toxin emulsified with Freund’s incomplete adjuvant (Sigma) and two further boosters of WO7 toxin (100 μg) alone administered at a 14-day interval. Three days after the fourth injection, the animal was bled. Serum was collected and stored at −20°C. The reactivity of the serum with purified WO7 toxin was examined by the Ouchterlony immunodiffusion test.
Protein determination.
The protein content was determined by the methods of (i) Lowry et al. (20) and (ii) Bradford (3) and by the (iii) bicinchoninic acid protein assay as described by Redinbaugh and Turby (23).
Toxicity assays: RILA.
Toxin activity was determined by the methods described by De (8). Briefly, animals were fasted for 25 h before they were anesthetised. The abdomen of each animal was opened aseptically, and the small intestine (leaving 15 cm from proximal and distal ends) was divided into 3- to 4-cm-long loops by string ligatures. The toxin preparations (culture supernatants or purified toxin) were injected into alternate loops. The intervening segments acted as uninoculated negative controls. The results were noted after 18 h. The secretory response in each loop was determined in terms of the dilatation index (DI = volume of fluid accumulated in the loop divided by the length of the loop). A DI equal to 1.0 or more was taken as positive for RILA. The statistical significance was calculated by analysis of variance.
Effect of toxin on CHO and Vero cells.
The ability of the toxin to alter the morphology of CHO or Vero cells was assayed in 96-well flat-bottom tissue culture plates (Nunc Intermed, Roskilde, Denmark) with either CHO or Vero cells. CHO cells were grown in Dulbecco modified Eagle medium (Gibco) supplemented with 10% horse serum (Gibco), penicillin G, and streptomycin sulfate (Sigma) and incubated at 37°C in an atmosphere of humidified 5% CO2–95% air (Heraeus, Hanau, Germany). Vero cells were grown under similar conditions but in Eagle’s modified minimal essential medium (Gibco) containing 10% horse serum. After confluent growth of the cells in a 25-cm2 flask, the cells were washed twice with Hank’s balanced salt solution (Gibco) and treated with 0.025% trypsin–EDTA (Sigma) solution. Approximately 103 trypsinized cells per well were dispersed in 96-well plates and overlaid with either neat or diluted culture supernatants. Thereafter, the plates were incubated for 24 h in a CO2 incubator and then examined with a phase-contrast inverted microscope (Leica) to observe morphological changes.
Hemagglutination assays.
Twofold serial dilutions of 25-μl samples in PBS (pH 7.4) were mixed with 25 μl of a rabbit erythrocyte suspension in 96-well (Costar) U-bottom plates. The mixture was incubated at 25°C for 1 h, and agglutination was monitored visually.
The inhibitory effect of different monosaccharides, namely, fetuin, asialofetuin, N-acetylneuraminic acid, mellibiose, d-glucose, α-methyl-d-mannoside, galactose, Gluc-NAc, and methyl-β-d-galactose, on agglutination by toxin was done as previously described (2).
Monoganglioside (GM1) was coupled to sheep erythrocytes by the method of Tayot and Tardy (30). To a 10% suspension of washed sheep erythrocytes was added an equal volume of 0.5% glutaraldehyde solution in PBS (0.001 M sodium phosphate, 0.14 M NaCl [pH 7.2]). After 30 min, the suspension was washed in the abovementioned buffer, glycine was then added to a final concentration of 0.05 M, and the pH was adjusted to 11.0. This overnight treatment neutralized the aldehyde groups and thus prevented further covalent associations. The sheep erythrocytes were then washed in 0.1 M NaOH. To a 10% suspension of these alkaline cells, GM1 gangoliside was then added to a final concentration of 250 nmol/ml. After 6 h of incubation at 45°C, the sensitized erythrocytes were washed in the abovementioned phosphate buffer and the erythrocyte concentration was adjusted to 1%. The hemagglutination tests were performed in 96-well microtiter plates. Serial dilutions of toxin preparations in PBS were mixed with equal volumes (50 μl) of a 0.25% suspension of GM1-coated erythrocytes. After incubation for 1 h, the tray was centrifuged at 1,000 × g for 1 min and tilted to an angle of 60°C, after which erythrocyte sedimentation patterns were inspected visually.
Hemolysin assay.
A hemolysin assay was performed with 1% rabbit erythrocytes as described by Rowe and Welch (25). Culture supernatants, ammonium sulfate-purified toxin, affinity-purified toxin, or gel filtration-purified toxin was serially diluted with PBS (pH 7.4) and incubated with equal volumes of 1% rabbit erythrocytes or PBS at room temperature for 1 h and then centrifuged at 800 × g for 10 min to remove unlysed cells. Released hemoglobin was assayed spectrophotometrically at 540 nm. Complete hemolysis (100%) was defined as the absorbance of the same number of erythrocytes lysed by water or detergent.
Characterization of WO7 toxin.
The following experiments were performed to further characterize WO7 toxin. (i) Proteolytic treatments. One hundred micrograms of toxin was incubated for 1 h at 37°C with trypsin pepsin and chymotrypsin (25 to 150 μg/ml; Sigma) and before performing RILA for WO7 toxin. The enterotoxin activity was then determined relative to that of controls incubated with same volume of PBS.
(ii) Heat sensitivity.
To assess the heat stability of WO7 toxin, samples of purified WO7 toxin were heated at 60, 70, 80, and 100°C for 10 min. The remaining toxin activity was then assessed in RILA and compared to that of untreated controls.
(iii) Neutralization assay.
Neutralization of biological activity was performed by mixing undiluted anti-WO7 toxin serum and purified WO7 toxin (100 μg/ml). The mixture was incubated for 2 h at 37°C, and the RILA was performed as described above.
Amino acid sequencing of WO7 toxin.
The N termini of the 58- and 40-kDa bands were sequenced by the method of Tous et al. (31). Briefly, purified WO7 toxin preparations (4 μg) were boiled in 1× loading buffer and applied to the wells of duplicate SDS–12.5% acrylamide gels by the method of Laemmli (18). Electrophoresis was carried out until the bromophenol blue dye migrated to the bottom of the gel. Polyvinylidene difluoride membranes were cut to the size of the gel and wetted with 100% methanol. Both polyvinylidene difluoride membranes and the gel were soaked in CAPS [3-(cyclohexylamino)-propanesulfonic acid] buffer for 30 min. The gels were electroblotted in the CAPS transfer buffer. Electroblotting was performed at 100 V for 45 min. Each membrane was stained with 0.5% Coomassie brilliant blue in 10% acetic acid–50% methanol and destained. The bands corresponding to WO7 toxin were excised from the membrane with a scalpel and stored in separate tubes. The membrane slices containing each of the subunits of WO7 toxin were placed in the reaction cartridge of an Applied Biosystems model 476 A-gas phase sequencer and subjected to 15 cycles of Edman degradation as described in the manufacturer’s instructions.
RESULTS
V. cholerae WO7 did not hybridize with the ctx, zot, or ace gene probes (Fig. 1). V. cholerae 569B and V. cholerae 0139 were used as controls. Serologically, V. cholerae WO7 was found to agglutinate O1 antisera and Inaba antisera.
FIG. 1.
(A) Southern blot hybridization of XbaI-digested V. cholerae WO7, 569B, and O139 chromosomal DNA with the ctx probe. Lanes: 1, PCT5A11 (positive control); 2, 569B; 3, 0139; 4, WO7. Positions of λ HindIII markers run on same gel are indicated at the left, at 23.11, 9.41, 6.55, 4.36, 2.32, and 2.02 kb (top to bottom). (B) Southern blot hybridization of XbaI/BamHI-digested V. cholerae WO7, 569B, and O139 chromosomal DNA with the zot probe. Lanes: 1, PMZP11 (positive control); 2, 569B; 3, 0139; 4, WO7. Positions of λ HindIII markers run on same gel are indicated at the left, at 23.11, 9.14, 2.32, and 2.02 kb (top to bottom). (C) Southern blot hybridization of PstI-digested V. cholerae WO7, 569B, and O139 chromosomal DNA with ace probe. Lanes: 1, PCT5A11 (positive control); 2, 569B; 3, 0139; 4, WO7. Positions of λ HindIII markers run on the same gel are indicated at the left, at 23.11, 9.14, 2.32, and 2.02 kb (top to bottom).
Toxicity of the strain.
V. cholerae WO7 was checked for its toxin-producing capacity by performing a RILA after injecting (i) normal saline, (ii) cholera toxin (2 μg), (iii) V. cholerae WO7 (106 organisms/ml of normal saline), or (iv) purified WO7 toxin. The bacterial suspension containing 106 organisms/ml induced a significantly higher (P < 0.01) fluid accumulation response (DI = 1.25 ± 0.04) in ligated ileal loop than the negative control (normal saline) (DI = 0.01 ± 0.02). Purified cholera toxin (2 μg) was used as the positive control (DI = 1.5 ± 0.08) (Fig. 2C). The culture supernatants when added to CHO and Vero cell cultures resulted in elongation of CHO cells and rounding up of Vero cells after 18 h of incubation at 37°C (Fig. 2A and B).
FIG. 2.
Toxic effects of WO7 toxin manifested as elongation in CHO cells (A), rounding up in Vero cell lines (B), and fluid accumulation in RILA (C) after injecting normal saline (a), CT (2 μg) (b), V. cholerae WO7 (106 organisms/ml of normal saline) (c), and purified WO7 toxin (d).
Optimum conditions for toxin production by V. cholerae WO7.
A variety of media and different conditions, including different incubation temperatures, were assessed to determine the optimum production of the WO7 toxin. The amount of toxin produced was measured in terms of DI in RILA (Table 1). When AKI medium with the pH adjusted to pH 8.5 was used, the DIs were 1.5 ± 0.8, 1.6 ± 0.22, and 1.20 ± 0.14, respectively, in 24-h stationary, 4-h stationary, and 18-h shaking conditions, respectively, at 37°C and with shaking at 30°C. From these observations, it was clear that AKI medium optimally supported WO7 toxin production. The optimum temperature for production of WO7 toxin was found to be 37°C (Table 1).
TABLE 1.
Optimization of culture conditions for toxin production by V. cholerae WO7
| Medium | Culture conditions | DI
|
|
|---|---|---|---|
| 30°C | 37°C | ||
| BHI | Stationary, 24 h | 0.40 ± 0.08 | 0.59 ± 0.10 |
| Stationary, 4 h; with shaking, 16 to 18 h | 0.44 ± 0.06 | 0.64 ± 0.02 | |
| TSB | Stationary, 24 h | 0.9 ± 0.04 | 1.20 ± 0.08 |
| Stationary, 4 h; with shaking, 16 to 18 h | 1.10 ± 0.09 | 1.34 ± 0.05 | |
| CAYE | Stationary, 24 h | 0.33 ± 0.11 | 0.56 ± 0.07 |
| Stationary, 4 h; with shaking, 16 to 18 h | 0.42 ± 0.04 | 0.42 ± 0.12 | |
| AKI | Stationary, 24 h | 1.15 ± 0.15 | 1.50 ± 0.18 |
| Stationary, 4 h; with shaking, 16 to 18 h | 1.20 ± 0.14 | 1.60 ± 0.22 | |
Purification of WO7 toxin.
The 80% ammonium sulfate fraction gave maximum fluid accumulation (DI = 1.92 ± 0.04) in RILA. The WO7 toxin in the supernatant of strain WO7 was concentrated by almost 35-fold by ammonium sulfate precipitation and applied to a cation-exchange MonoS (Pharmacia) column. The elution pattern from this column shows two peaks (Fig. 3). All the toxin activity was found to be associated with the bound fraction. The specific activity at all the steps was assessed by determining the GM1 binding activity. The specific activity of WO7 toxin purified on a cation-exchange column was very high, but the final recovery rate after cation-exchange chromatography was only 3.2%. The yield of WO7 toxin after cation-exchange chromatography was poor, and an alternative strategy was devised to purify WO7 toxin.
FIG. 3.
Elution profile of MonoS column for WO7 toxin. Elution was achieved with 0.01 M Tris buffer (pH 7.2) for 10 min followed by the same buffer containing 0.2 M NaCl for 10 min at 0.5 ml/min. The purified WO7 toxin eluted from the last peak at 0.2 M (100%) NaCl concentration in 14.7 min.
The partially purified WO7 toxin obtained by cation-exchange chromatography agglutinated freshly isolated rabbit erythrocytes, which was enhanced by the presence of Ca2+ ions and inhibited by sialic acid and fetuin.
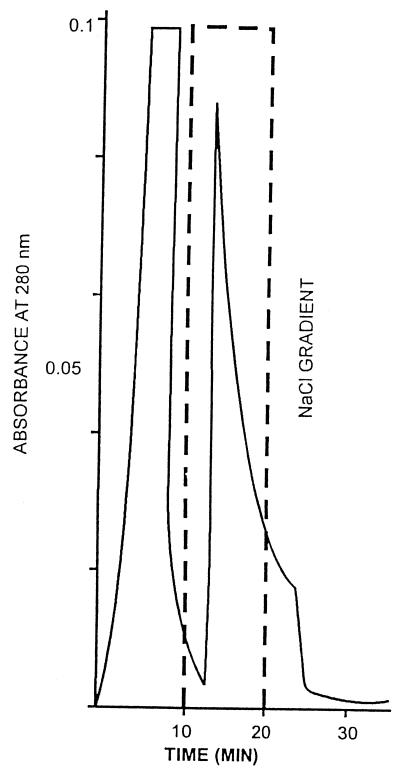
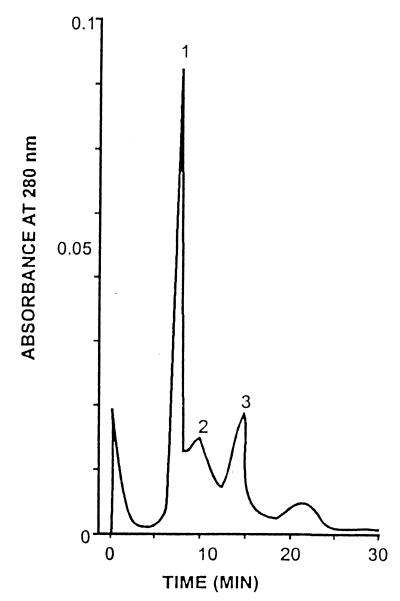
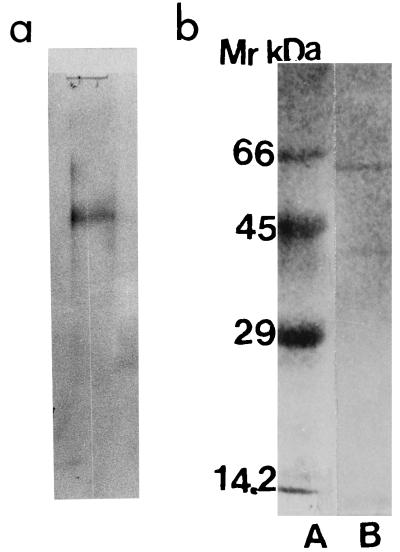
Based on these observations, an affinity fetuin-Sepharose CL-4B column was designed in order to purify WO7-toxin. The ammonium sulfate-precipitated fraction (80%) was applied to the affinity column. The elution profile from this column is shown in Fig. 4. The toxin activity was found to be associated with the bound proteins eluted in the presence of sodium citrate (0.04 M). The recovery at all steps of purification was determined by its ability to agglutinate erythrocytes coated with GM1. Toxin was seen to have lectin-like activity which was inhibited by fetuin and sialic acid. Since the sialic acid moeity is present in GM1, it is presumed that GM1 might be the receptor for the new toxin. Table 2 summarizes the typical data on purification of WO7 toxin; from these data it is clear that the specific activity of WO7 toxin gradually increased with the decline in the amount of protein, suggesting 140-fold purification of the WO7 toxin. The recovery rate was about 25% after affinity chromatography. The homogeneity of 0.5 μg of purified WO7 toxin obtained from affinity chromatography was analyzed on a gel filtration SW300 column (Pharmacia) In the gel filtration SW300 column, the toxin eluted just before blue dextran, which is suggestive of it being a very high-molecular-weight protein (Fig. 5). The WO7 toxin that eluted from the gel filtration column, when analyzed by denaturing SDS–12.5% PAGE, gave two bands of 58 and 40 kDa and a single band when analyzed by native PAGE (Fig. 6). Since the toxin fraction elutes before blue dextran in the gel filtration column, it perhaps occurs as a highly aggregated form so the molecular size of the holotoxin could not be determined.
FIG. 4.
Elution profile of the affinity column. Twenty milliliters of dialyzed ammonium sulfate fraction (80%) of WO7 toxin was applied to a fetuin-Sepharose CL-4B column (2 by 10 cm). After washing the column with buffer A until the absorbance of effluent at 280 nm was less than 0.02, 30 test tubes, each containing a 1.5-ml fraction of the eluate, were eluted with buffer B at room temperature. Absorbance was monitored at 280 nm for the protein.
TABLE 2.
Summary of purification of the toxin produced by V. cholerae WO7 using cation-exchange chromatography
| Sample | Total vol (ml) | Total protein (mg) | GMI binding (titer/ml) | Sp act (titer/mg) | Fold purification | Recovery (%) |
|---|---|---|---|---|---|---|
| Culture supernatant | 2,000 | 7,600 | 640 | 1.7 × 102 | 1 | 100 |
| Ammonium sulfate precipitation | 30 | 110 | 20,480 | 6.7 × 103 | 33.7 | 48.8 |
| Purified toxin (MonoS column) | 1 | 0.980 | 40,960 | 4.2 × 104 | 248.2 | 3.2 |
FIG. 5.
Elution profile of Protein Pak SW300 gel filtration column. The affinity chromatography-eluted fraction was applied to the column (8 by 30 cm) previously equilibrated with 0.01 M Tris (pH 7.2) buffer. Three separate peaks (1, 2, and 3) were eluted at retention times of 6.8, 9.8, and 12.4 min, respectively.
FIG. 6.
Migration of purified WO7 toxin obtained after gel filtration chromatography on PAGE (a) and SDS-PAGE (12.5% acrylamide) (b, lane B) and migration of marker proteins of known molecular mass (66, 45, 29, and 14.2 kDa (b, lane A).
Immunological characterization.
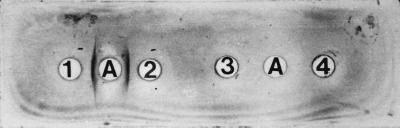
Polyclonal antibody raised in rabbit against purified WO7 toxin gave a well-defined precipitation band against the WO7 toxin in an immunodiffusion test but did not react with CT or E. coli heat-labile toxin (LT1) (Fig. 7). When polyclonal antisera was used in an immunoblot assay with the WO7 toxin, E. coli toxin (LT), and CT, two well-defined bands were observed corresponding to 58 and 40 kDa with the WO7 toxin antibody but no cross-reactivity with LT or CT was demonstrated (Fig. 8). The inhibition experiment revealed that incubation with polyclonal antisera completely inhibited the biological activity of the toxin in the RILA.
FIG. 7.
Reactivity of antiserum raised against WO7 toxin (well A) with WO7 toxin (wells 1 and 2) and with CT (well 3) and LT1 toxin (well 4) in the Ouchterlony immunodiffusion test.
FIG. 8.

Immunoblots showing immunoreactivity of WO7 toxin antisera with WO7 toxin (lane A), CT (lane B), and LT1 (lane C).
Biological activity.
The biological activities of purified enterotoxin and various fractions obtained are shown in Table 3. In its crude state (culture supernatants), WO7 toxin evoked fluid accumulation, with a DI of 1.7 ± 0.11. Upon purification, the enterotoxic activity of WO7 toxin progressively increased but fluid secretion was not hemorrhagic in nature. However, in comparison to CT, which was used as a control, four times less WO7 toxin was required to evoke a positive fluid accumulation ratio (Table 4). The secretory effects of purified WO7 toxin were completely blocked by prior incubation of WO7 toxin for 60 min at 37°C with anti-WO7 toxin serum raised in a rabbit.
TABLE 3.
Biological activities of the various fractions obtained during purification of WO7 toxin
| Sample | Amt of protein or no. of cells | DI |
|---|---|---|
| V. cholerae WO7 | 106 organisms | 1.68 ± 0.07 |
| Culture supernatants of V. cholerae WO7 | 2.1 mg/ml | 1.7 ± 0.11 |
| Ammonium sulfate-precipitated fraction (80%) | 3.3 mg/ml | 1.92 ± 0.04 |
| WO7 toxin fraction after affinity chromatography | 0.8 mg/ml | 2.01 + 0.08 |
| Purified WO7 toxin | 0.16 mg/ml | 2.3 + 0.06 |
TABLE 4.
Biological activity of V. cholerae WO7 toxin versus CT
| Toxin | Protein (μg/ml) | DI |
|---|---|---|
| None (PBS) | 0 | |
| CT | 2 | 1.5 ± 0.22 |
| WO7 toxin | 10 | 2.21 ± 0.08 |
| 5 | 2.0 ± 0.14 | |
| 2 | 1.95 ± 0.06 | |
| 1 | 1.8 ± 0.04 | |
| 0.5 | 1.4 ± 0.12 | |
| 0.2 | 1.0 ± 0.08 | |
| 0.1 | 0.7 ± 0.26 | |
| 0.05 | 0.3 ± 0.07 |
Physicochemical properties of purified WO7 toxin.
The WO7 toxin retained its biological activity on heating at 60°C for 10 min but did not retain its activity at 70°C and above for 10 min. Treatment with pepsin, trypsin, and chymotrypsin resulted in a complete loss of enterotoxin activity of WO7 toxin. The WO7 toxin agglutinated freshly isolated rabbit erythrocytes, and the titer was found to be 1:10,240. The agglutination was found to be enhanced twofold in the presence of 5 mM Ca2+ ions but inhibited by sialic acid (15 μg/ml) and fetuin (20 μg/ml), which suggested lectin-like activity. The toxin also agglutinated GM1-coated sheep erythrocytes.
Amino acid sequencing.
The N-terminal amino acid sequence for the 58-kDa band was found to be Asp-Gly-Ile-Asn-Gln-Tyr/Val-Gly-Asp-Lys-Ala-Gly-Gly/Lys-Thr-Val-Tyr. The N-terminal sequence for the 40-kDa band was found to be Asp/Gly-Gly/Ile-Asn-Leu-Gly-Arg-Gly/Lys.
The amino acid homology search showed that the sequences were unique and did not show homology with any of the earlier known V. cholerae toxin sequences.
DISCUSSION
V. cholerae O1 isolates produce a variety of extracellular products which have been implicated in the pathogenesis of the disease. Hence, it was important to initially determine that the toxin produced by V. cholerae WO7, which was examined in this study, was distinct from those already reported. It is known that in addition to CT, V. cholerae produces Zot and Ace toxins (10, 32). V. cholerae WO7 DNA failed to hybridize with probes specific for the ctx, zot, and ace genes, thereby suggesting that V. cholerae WO7 produced a toxin that is distinct from CT, Zot, and Ace toxins, even though two faint bands appeared in lane 4 of Fig. 1A. The band seen at the 23-kb region is due to the incomplete digestion of genomic DNA in the particular experiment reported. One of the two faint bands which appeared in the WO7 lane comigrated with this partial digestion band, while the other one appeared at the molecular size region of 11 kb. The 1.8-kb ctx gene probe spans part of the RS1 element (28); we believe that the faint signals which appeared after overexposure of the film could be due to hybridization of the RS1 part of the gene probe with possible RS1-like sequences which are known to exist in many strains of V. cholerae (28), or they could be due to a sequence present in the WO7 genome which bears minimal homology with a part of ctx, although this aspect has not been vigorously examined. Therefore, we decided to purify and characterize the toxin produced by V. cholerae WO7 for the following reasons. (i) WO7 toxin is distinct from existing V. cholerae toxins and hemolysin. (ii) WO7 toxin-producing V. cholerae 01 strains are isolated from patients with cholera-like illness. (iii) The enterotoxin activity is rapid, dramatic, and pronounced and appears to be related to the pathogenesis of cholera.
In this regard, the first step was to optimize conditions for WO7 toxin production. For this, V. cholerae WO7 was grown in different media, at different temperatures, and under different conditions (static, shaking, or both). It was found that V. cholerae WO7 produced WO7 toxin optimally in TSB and AKI medium at 37°C (4 h of stationary and 18 h of shaking conditions). For further purification procedures, AKI medium was utilized because it supports production of only WO7 toxin and not the hemolysin (data not shown). In order to improve the yield and rule out the action of metalloproteases on the toxin, V. cholerae WO7 was cultured in the presence of 1 mM EGTA. The stepwise ammonium sulfate precipitation of culture supernatants revealed the toxin actively to be associated with an 80% fraction. Here we would like to mention that CT is recovered in a 98% fraction from the culture supernatants of V. cholerae 569B.
Initially we tried to purify the WO7 toxin by utilizing ion-exchange chromatography with a cation-exchange MonoS column (Pharmacia). Although the specific activity of the toxin preparation obtained after ion-exchange chromatography was very high, the recovery of the total protein was far from satisfactory.
The WO7 toxin, in crude and partially purified preparations, agglutinated freshly isolated rabbit erythrocytes. This agglutination was enhanced by the presence of Ca2+ ions and was inhibited by sialic acid and fetuin, suggesting the lectin-like activity of this toxin. Consequently, we devised a fetuin-Sepharose CL-4B column to purify WO7 toxin. The purified fraction of WO7 toxin recovered from this column had a very high specific activity and the recovery rate was almost 25%. Further purification on the gel filtration SW300 column resulted in progressive increases in the specific activity and recovery rate after this step was almost 13%. The enterotoxin activity of WO7 toxin was found to be heat labile, and the purified toxin was quite unstable, with a rapid decline in activity even at 4°C. Further, sensitivity to chymotrypsin, trypsin, and pepsin treatments indicated that the active component was protein in nature. These observations were also suggestive of a possible involvement of amino acids like arginine, lysine, tryptophane, phenylalanine, and tyrosine in the toxicity of the WO7 toxin. It has been previously documented that arginine, lysine, and tryptophan residues are part of the receptor binding domain of CT (9). On SDS-PAGE, WO7 toxin showed two subunits of 58 and 40 kDa each. Interestingly, progressive purification of the WO7 toxin revealed an increase in enterotoxin activity at all successive steps.
The enterotoxin activity of WO7 toxin was found to be 10-fold greater than that of CT. Immune rabbit serum to the purified WO7 toxin was used in immunoblots, and antiserum was determined to be highly specific for WO7 toxin and was capable of neutralizing biological activity in RILA. However, the WO7 toxin failed to react to either CT or E. coli LT in the immunoblot system. Interestingly, the receptor for WO7 toxin on cells appears to be similar to that of CT, since WO7 toxin agglutinated GM1-coated erythrocytes. WO7 toxin was found to resemble CT since WO7 toxin elongated CHO cells, which is a characteristic feature of CT. However, in immunoblots, WO7 toxin antisera did not cross-react with CT, which is in agreement with previous studies which have shown that number of toxins. LTIIa and LTIIb of E. coli show remarkable structural similarity to CT and LT1 but do not cross-react with anti-CT. Aeromonas hydrophila also produces a toxin (molecular size, 44 kDa) which elongates CHO cells and causes fluid accumulation in rabbit ligated ileal loops but does not cross-react with antibodies to CT (6). A similar observation was made for crude preparations of the CHO cell-elongating toxin produced by various strains of Salmonella enteritidis (1). Studies with enterotoxin produced by Salmonella typhimurium (4, 5) established that the toxin does not require a strong homology to CT to elongate CHO cells and cause fluid accumulation in animal models. Although WO7 toxin resembled CT in some of its properties, the N-terminal amino acid sequences of both the subunits failed to show homology with any of the known cholera toxins, thereby suggesting that WO7 toxin is unique to the V. cholerae species.
A unique observation in this study was the ability of the WO7 toxin to agglutinate erythrocytes and its ability to bind complex sugars and not simple sugars. The agglutination was enhanced in the presence of Ca2+. It is perhaps due to this lectin-like property of WO7 toxin that the toxin exists as a very-high-molecular-weight moiety and is virtually excluded in the SW300 gel filtration column. The aggregation was not reversed by common protein-dissociating agents such as urea, guanidine hydrochloride, Triton X-100, or deoxycholate. However, since the toxin was not sedimented at 100,000 × g for 1 h, it did not appear to be particulate in nature. The majority of bacterial lectins, e.g., fimbrial HA or adhesins consisting of hundreds of pilin subunits (12) or toxins with multiple copies of sugar binding subunits (9), possess highly oligomeric structures. They are therefore capable of binding to multiple sugar receptors on glycoconjugates, leading to effective compensation for their intrinsic low affinity for sugars. Hence, it was speculated that oligomerization, apart from stabilizing the quarternary structure of the native protein, may be a functional necessity for many bacterial lectins.
The ability of serogroup O1 strain V. cholerae WO7 to produce a toxin 10 times more potent than CT, in the absence of ctx, zot, and ace genes, is a matter of concern, and a better understanding of the genesis of this strain is needed. V. cholerae is a species commonly found in marine and estuarine environments (27) and is closely associated with prawns and oysters. Karaolis et al. (17) proposed that pathogenic forms have arisen independently from environmental forms or as part of the ability to survive better.
V. cholera has also been proposed to have a higher level of genetic exchange and a lower level of clonality than species such as Salmonella enterica and E. coli (17). Nucleotide sequence analysis of the asd gene of 45 strains of V. cholerae has shown that the classical, El Tor biotypes and the U.S. Gulf Coast isolate have evolved independently from environmental, nontoxigenic, non-O1 organisms (17). All these observations have led us to conclude that V. cholerae WO7 could be a nontoxigenic strain of environmental origin that has acquired a gene from the environment by virtue of which it elaborates a toxin which was alien to V. cholerae species earlier.
Very recently, it has been shown that structural genes for CT are encoded by a filamentous bacteriophage (CTX φ) which is related to coliphage. This phage can infect existing live attenuated V. cholerae vaccine strains and reverse their toxigenicity, thereby suggesting horizontal gene transfer that may depend on in vivo gene expression (33). Our data have led us to conclude that WO7 toxin, because of its enterotoxic and cytotoxic activities, has a role to play in the pathogenesis of V. cholerae O1 infections. This, coupled with the proven antecedence of association of cytotoxic enterotoxins with diarrhea, suggests that WO7 toxin may constitute a covert virulence element in a whole cascade of events which enables the organism to precipitate the disease. At this point, however, we are unable to comment on the existence of this gene in the other V. cholerae O1 or non-O1 species. Isolates from diverse geographic areas should be analyzed to establish the frequency of occurrence of WO7 toxin-producing V. cholerae O1 isolates.
REFERENCES
- 1.Baloda S D, Faris A, Krovacek K, Wadstrom T. Cytotoxic enterotoxins and cytotoxic factors produced by Salmonella enteritidis and Salmonella typhimurium. Toxicon. 1983;21:785–796. doi: 10.1016/0041-0101(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee K K, Ghosh A N, Dutta-Roy K, Pal S C, Ghosh A C. Purification and characterization of novel hemagglutinin from Vibrio cholerae. Infect Immun. 1990;58:3698–3705. doi: 10.1128/iai.58.11.3698-3705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilising principles of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Chary P, Prasad R, Chopra A K, Peterson J W. Location of enterotoxin gene from S. typhimurium and characterisation of the gene products. FEMS Microbiol Lett. 1993;111:87–92. doi: 10.1111/j.1574-6968.1993.tb06366.x. [DOI] [PubMed] [Google Scholar]
- 5.Chopra A K, Peterson J W, Chary P, Prasad R. Molecular characterisation of an enterotoxin from S. typhimurium. Microb Pathog. 1994;16:85–98. doi: 10.1006/mpat.1994.1010. [DOI] [PubMed] [Google Scholar]
- 6.Chopra A K, Houston C W. Purification and partial characteristion of cytotoxic enterotoxin produced by Aeromonas hydrophila. Can J Microbiol. 1989;35:719–727. doi: 10.1139/m89-117. [DOI] [PubMed] [Google Scholar]
- 7.Davis B J. Disc electrophoresis. II. Methods and applications to human serum proteins. Ann N Y Acad Sci. 1964;121:407–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 8.De S N. Enterotoxicity of bacteria free culture filterates of V. cholera. Nature (London) 1959;183:1533–1534. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- 9.Eidels L, Proia R L, Hart D. Membrane receptors for bacterial toxins. Microbiol Rev. 1983;47:596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A, Baudry B, Pumpkin D W, Wasserman S S, Tale B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda T, Finkelstein R A. Purification and characterization of hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979;26:1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hultgren S J, Normark S, Abraham S N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- 13.Iwanaga M, Yamamoto K, Higa N, Ichnose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by V. cholera O1 ElTor. Microbiol Immunol. 1986;30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 14.Iwanaga M, Yamamoto K. New medium for production of cholera toxin by Vibrio cholerae O1 biotype El Tor. J Clin Microbiol. 1985;22:405–408. doi: 10.1128/jcm.22.3.405-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaper J B, Lockman H, Baldini M M, Levine M M. A recombinant live oral cholera vaccine. Biol Technol. 1984;2:345–349. [Google Scholar]
- 16.Kaper J B, Lockman H, Baldini M M, Levine M M. Recombinant nontoxinogenic V. cholerae strains as attenuated cholera vaccine candidates. Nature (London) 1984;308:655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- 17.Karaolis D K R, Lan R, Reeves P R. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental nontoxigenic, non-O1 Vibrio cholerae. J Bacteriol. 1995;177:3191–3198. doi: 10.1128/jb.177.11.3191-3198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Levine M M, Kaper J B, Herrington D, Ketley J, Morris J G, Clemens M L, Black R E, Tall B, Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry O H, Rosebrough N J, Farr A L, Randall R L. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:205–275. [PubMed] [Google Scholar]
- 21.Mekalanos J J. Duplication and amplification of toxin genes in V. cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 22.Morris J G, Takeda T, Tall B P, Lasonsky G A, Bhattacharya S K, Forrest B D, Kay B A, Nishibuchi M. Experimental non group 1 V. cholerae gastroenteritis in human. J Clin Invest. 1990;85:697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redinbaugh M G, Turby R B. Adaptation of bicinchoninic acid protein assay for use with microtitre plates and sucrose gradient fractions. Anal Biochem. 1986;153:267–269. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 24.Rigby P W J, Dieckman M, Rhodes C, Berg P. Labelling doxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977;113:237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- 25.Rowe E G, Welch R A. Assays of hemolytic toxins. Methods Enzymol. 1994;235:657–666. doi: 10.1016/0076-6879(94)35179-1. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal S C, Alam K, Neogi P K B, Huq M I, Al-Mahmud K A. A new cholera toxin. Lancet. 1983;i:1337. doi: 10.1016/s0140-6736(83)92449-2. [DOI] [PubMed] [Google Scholar]
- 27.Sakazaki R. Bacteriology of Vibrio and related organisms. In: Barua D, Greenough W B, editors. Cholera. New York, N.Y: Plenum; 1992. pp. 37–54. [Google Scholar]
- 28.Sharma C, Nair G B, Mukhopadhyay A K, Bhattacharya S K, Ghosh R K, Ghosh A. Molecular characterization of V. cholerae O1 biotype Eltor strains isolated between 1992–1995 in Calcutta, India: evidence for emergence of new clone of the Eltor biotype. J Infect Dis. 1997;175:1134–1141. doi: 10.1086/516453. [DOI] [PubMed] [Google Scholar]
- 29.Takeda T, Peina Y, Ogawa A, Dohi S, Abe H, Nair G B, Pal S C. Detection of heat stable enterotoxin in cholera toxin gene-positive strain of V. cholera O1. FEMS Microbiol Lett. 1991;80:23–28. doi: 10.1016/0378-1097(91)90203-m. [DOI] [PubMed] [Google Scholar]
- 30.Tayot J L, Tardy M. Adv. Exp Med Biol. 1980;125:471–478. doi: 10.1007/978-1-4684-7844-0_41. [DOI] [PubMed] [Google Scholar]
- 31.Tous G I, Fausnaugh J L, Akinyosoye O, Lockland H, Winder-Cash P, Victoria F J, Stein S. Aminoacid analysis on polyvinylidene difluoride membranes. Anal Biochem. 1989;170:50–55. doi: 10.1016/0003-2697(89)90198-x. [DOI] [PubMed] [Google Scholar]
- 32.Trucksis M, Galan J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of V. cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]