Abstract
We performed this cohort study to test whether further analysis of intrathecal inflammation can be omitted if the free light chain kappa (FLCκ) quotient is within the reference range in the corresponding quotient diagram. FLCκ concentrations were measured in serum and cerebrospinal fluid (CSF) samples. The intrathecal fraction (IF) of FLCκ was calculated in relation to the hyperbolic reference range. 679 patient samples were used as a discovery cohort (DC). The sensitivity and negative predictive value (NPV) of the FLCκ-IF for the detection of an intrathecal humoral immune response (CSF-specific OCB and/or IF IgG/A/M > 0%) was determined. Based on these data, a diagnostic algorithm was developed and prospectively validated in an independent validation cohort (VC, n = 278). The sensitivity of the FLCκ-IF was 98% in the DC and 97% in the VC with a corresponding NPV of 99%. The use of the FLCκ-IF as a first line analysis would have reduced the Ig and OCB analysis by 62% in the DC and 74% in the VC. The absence of a FLCκ-IF predicts the absence of a humoral intrathecal immune response with a very high NPV of 99%. Thus, integration of our proposed algorithm into routine CSF laboratory analysis could help to reduce analytical efforts.
Keywords: cerebrospinal fluid, free light chain kappa, immunoglobulin, laboratory workflow, protein analytic
1. Introduction
The detection of an intrathecal humoral immune response is an important diagnostic aspect in many neurological diseases of the central nervous system (CNS). One method to represent this is the quantitative protein analysis of immunoglobulins. It is usually performed using quotient diagrams in which the cerebrospinal fluid (CSF) and serum quotient of immunoglobulins (IgG, IgA, IgM) is related to the CSF and serum albumin quotient. These calculations are necessary to distinguish the fractions of proteins that enter the CSF from blood by passive diffusion from those produced intrathecally [1]. Another approach is the qualitative analysis of IgG by isoelectric focusing and immunoblotting, the determination of the presence of oligoclonal bands (OCB) in CSF [2]. The free light chains kappa (FLCκ) in CSF as biomarker for intrathecal inflammation has recently gained renewed interest. At least in Multiple Sclerosis (MS), measurement of FLCκ is superior to quantitative Ig analysis in terms of sensitivity for detection of intrathecal inflammation. In comparison with OCB analysis, the measurement of FLCκ provides the advantage of comparable sensitivity, the possibility of a fully automated measurement, and objective as well as quantitative value interpretation rather than the qualitative and therefore subjective OCB interpretation [3,4,5,6,7,8,9]. Its diagnostic specificity though is clearly inferior. OCB provide information about intrathecal IgG synthesis, FLCκ synthesis can also be seen in an intrathecal IgA- and IgM-synthesis [10].
Despite a broad variety of publications about the use of FLCκ in CSF in the diagnostics of MS and other neurological diseases, integration into the diagnostic routine in CSF laboratories has not yet been undertaken. We hypothesized that in the absence of intrathecal FLCκ synthesis, a qualitative or quantitative Ig synthesis (hereafter referred to as an intrathecal humoral immune response) should also not be detectable. Therefore, the main objective of this work was to evaluate a laboratory workflow that integrates the presence of intrathecal FLCκ synthesis as a highly sensitive marker with a strong negative predictive value for an intrathecal humoral immune response. This has the potential to minimize the qualitative and quantitative determination of Ig in laboratory routine CSF diagnostics.
2. Materials and Methods
2.1. Standard Protocol Approvals and Patient Consent
The retrospective part of the study was based on the votum of the local ethics research committee (III UV 39/03 and BB019/18; University Medicine Greifswald, Greifswald, Germany). For the prospective part of the study, consecutive CSF and serum samples were collected between 3 January 2019 and 28 May 2019, based on the votum of the local ethics research committee (BB117/18; University Medicine Greifswald, Greifswald, Germany). Written informed consent was obtained from all participants.
2.2. Patient Samples and Cohorts
All samples were acquired from patients of the Department of Neurology, University Medicine Greifswald (Greifswald, Germany). For this cohort study, a two-step study design was used: First, a discovery cohort (n = 679) was established to determine sensitivity and negative predictive value of the FLCκ-IF to predict an intrathecal humoral immune response. Based on these data, a laboratory workflow integrating the FLCκ as a first line analysis for detection of an intrathecal humoral immune response was created. The discovery cohort consisted of patient samples which had been acquired prospectively between 2015 and 2017, as well as samples collected between 2008 and 2016, which were stored at −80 °C. This novel workflow was then prospectively evaluated with an independent validation cohort (n = 278), which has been acquired consecutively between 3 January 2019 and 28 May 2019.
Some patient samples of the discovery- and validation cohort have already been reported in the context of other studies with a different focus [5,10,11,12,13].
Patient samples of the discovery and validation cohort presenting signs of either CSF specific OCB and/or an IF of IgG, A, or M > 0% were considered to have an intrathecal humoral immune response.
Blood admixture to the CSF can occur due to intrathecal haemorrhage or artificially due traumatic lumbar puncture. Such blood admixture can mimic intrathecal Ig synthesis in the absence of intrathecal inflammation. Ig synthesis due to blood admixture was diagnosed in patient samples presenting the following findings: Erythrocyte count (EC) > 500/µL and QIgM > Qlim (IgM) and if present QIgA > Qlim (IgA) and QIgG > Qlim (IgG) but IF IgM > IF IgA > IF IgG [13]. The resulting Ig synthesis in the corresponding quotient diagram was therefore not considered due to an intrathecal humoral immune response.
Characteristics of the discovery and validation cohort including CSF results are presented in Table 1.
Table 1.
Continuous data are expressed as median (first; third quartiles); nominal data are given as percentages, Ig-synthesis are given as quantity and percent. CSF cerebrospinal fluid, OCB oligoclonal bands, CC cell count (leukocytes), Ig immunoglobulin, QIgG/A/M immunoglobulin G/A/M quotient, QAlb albumin quotient, FLCκ free light chains kappa.
| Discovery Cohort (n = 679) | Validation Cohort (n = 278) | |
|---|---|---|
| Age (y) | 52 (36; 65) | 56 (41; 69) |
| QAlb | 6.4 (4.7; 9.1) | 6.7 (5.1; 9.4) |
| QIgG | 3.6 (2.5; 5.1) | 3.1 (2.3; 4.5) |
| QIgA | 1.9 (1.2; 2.9) | 1.7 (1.2; 2.7) |
| QIgM | 0.4 (0.2; 0.8) | 0.3 (0.2; 0.7) |
| One-class Ig immune response | ||
| IgG-synthesis; n (%) | 71 (10) | 12 (4) |
| IgA-synthesis; n (%) | 10 (1.4) | 0 |
| IgM-synthesis; n (%) | 19 (2.7) | 1 (0.3) |
| Two-class Ig immune response | ||
| IgG/M-synthesis; n (%) | 31 (4.5) | 6 (2) |
| IgA/M-synthesis; n (%) | 12 (1.8) | 1 (0.3) |
| IgG/A-synthesis; n (%) | 9 (1.3) | 1 (0.3) |
| Three-class Ig immune response | 4 (0.05) | 2 (0.7) |
| Artificial blood Contamination, n (%) | 18 (2.3) | 1 (0.3) |
| CSF specific OCB; n (%) | 204 (30) | 31 (11) |
| CC/µL | 1 (1; 4) | 1 (1; 2) |
| FLCκ IF > 0%; n (%) | 302 (44) | 67 (24) |
| FLCκ serum (mg/L) | 13.1 (10.2; 18) | 13.1 (9.81; 17.97) |
| FLCκ CSF (mg/L) | 0.363 (0.189; 1.63) | 0.23 (0.12; 0.48) |
| QFLCκ | 21.5 (12.04; 111.38) | 15.21 (10.29; 26.28) |
2.3. Laboratory Analyses
Laboratory analyses were performed in the interdisciplinary CSF laboratory of the University Medicine Greifswald as described previously [12].
As a qualitative method to detect intrathecal IgG synthesis, the analysis of OCB was done by isoelectric focusing with a semiautomatic agarose electrophoresis system (Hydragel 9 CSF, Sebia Hydrasys 2Scan, Sebia GmbG, Fulda, Germany).
FLCκ in sera and CSF were measured by nephelometry with the N Latex FLC kappa kit (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany), using monoclonal antibodies on the BN Prospec analyzer. CSF pre-dilution was set to 1:2, and serum pre-dilution was set to 1:100. The lower limit of quantification of the assay was 0.034 mg/L.
One major concept in CSF diagnostics is to distinguish the serum fraction of proteins from intrathecally synthesized proteins (IgG, IgA, IgM or FLCκ). Therefore, the interpretation of Ig and FLCκ quotients in quotient diagrams is a well-established method [3,10]. In these quotient diagrams, immunoglobulin and FLCκ CSF and serum quotients (QIgG, QIgA, QIgM, QFLCκ; y-axis) are plotted in reference to the albumin CSF and serum quotient (QAlb; x-axis).
The hyperbolic reference range, as well as the intrathecal fraction of Ig and FLCκ was calculated according to the formulas described by Reiber et al. [1,2,3]:
| Q_lim (IgG) = 0.93 × √((QAlb)2 + 6 × 10−6) − 1.7 × 10−3 |
| Q_lim (IgA) = 0.77 × √((QAlb)2 + 23 × 10−6) − 3.1 × 10−3 |
| Q_lim (IgM) = 0.67 × √((QAlb)2 + 120 × 10−6) − 7.1 × 10−3 |
| Q_lim (FLCκ) = 3.27 × √((QAlb)2 + 33 × 10−6) − 8.2 × 10−3 |
| Ig/FLCκ-IF = [1 − Qlim(Ig/ FLCκ)/QIg/FLCκ] × 100 [%]. |
The upper line (Qlim) of the reference range discriminates between the blood derived- and intratecally synthesized- Ig and FLCκ fraction.
2.4. Statistical Analysis
Data from the discovery cohort were used to establish an evaluation algorithm by estimating the sensitivity and the negative-predictive value of the presence of an intrathecal fraction of FLCκ for the prediction of an intrathecal humoral immune response as defined above.
RStudio (R version 3.5.1 2 July 2018) was used for statistical and graphical processing of the data.
3. Results
3.1. Establishing a New Diagnostic Workflow Integrating FLCκ-IF as a First Line Analysis for an Intrathecal Humoral Immune Response
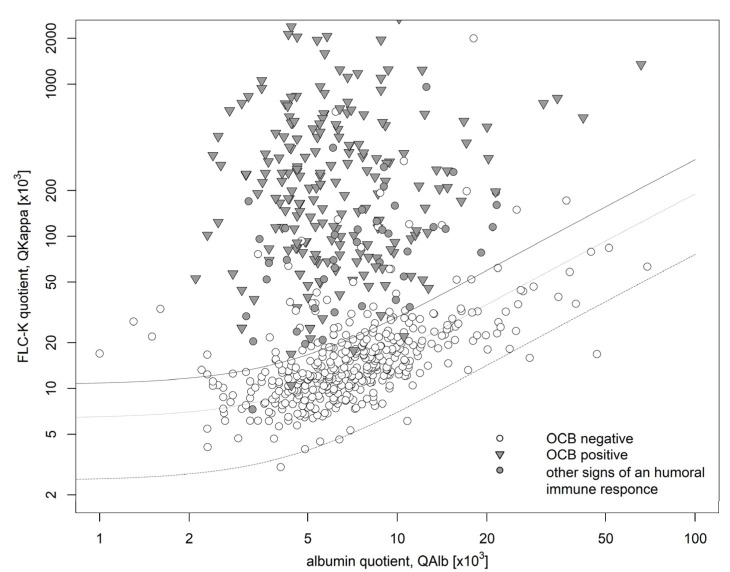
A total of 679 patient samples were identified to form the discovery cohort (Table 1, Figure 1). Signs of an intrathecal humoral immune response as defined above were detected in 245 of these samples. Of those, 241 had an intrathecal synthesis of FLCκ, accounting for 98% of the samples with an intrathecal humoral immune response. The remaining 4 (~2%) had no evidence of an intrathecal synthesis. Of these 4 patient samples, 3 presented CSF specific OCB and had a FLCκ serum value exceeding the reference range (according to manufacturer’s specification: 6.7–22.4 mg/L) (patient1: 57.7 mg/L, patient2: 66.9 mg/L, patient3: 57.1 mg/L). One patient sample though had a borderline IgM synthesis (IF IgM: 10%) that could not be explained by blood contamination or higher serum FLCκ values. 434 patient samples (64%) did not show signs of an intrathecal humoral immune response as defined above. Of these, 60 patient samples had an intrathecal synthesis of FLCκ (9%).
Figure 1.
Data of the discovery cohort in a double logarithmic FLCκ quotient diagram. The bold line shows Qlim, the dashed line shows the Qmean. The bottom dashed line shows the Qlow. 302 samples (44%) showed an intrathecal FLCκ synthesis (IF FLCκ > 0) of which 60 patient samples showed no other signs of a humoral immune response (negative OCB, IF IgG/IF IgA/IF IgM < 0). 245 patient samples presented signs of an intrathecal inflammation (positive OCB ( ) or Ig synthesis in the corresponding quotient diagram (
) or Ig synthesis in the corresponding quotient diagram ( )) of which 4 lacked an intrathecal FLCκ synthesis (IF FLCκ < 0). FLCκ: free light chains kappa, Q: quotient, Alb: albumin.
)) of which 4 lacked an intrathecal FLCκ synthesis (IF FLCκ < 0). FLCκ: free light chains kappa, Q: quotient, Alb: albumin.
The use of the IF FLCκ as a primary selection marker in this cohort would have resulted in a sensitivity of 98%, a specificity of 86%, a negative predictive value of 98% and a positive predictive value of 81% (see Table 2).
Table 2.
FLCκ free light chains kappa, IF intrathecal fraction, OCB oligoclonal bands, Ig immunoglobulin.
| Evidence of Intrathecal Humoral Immune Response (OCB and/or IF IgG/A/M > 0%) | No Evidence of Intrathecal Humoral Immune Response (OCB and IF IgG/A/M < 0%) | |||
|---|---|---|---|---|
| Discovery cohort | FLCκ-IF > 0% | 244 | 60 | PPV: 80% |
| FLCκ-IF < 0% | 4 | 374 | NPV: 99% | |
| Sensitivity: 98 % | Specificity: 86 % | |||
| Validation cohort | FLCκ-IF > 0% | 32 | 36 | PPV: 47% |
| FLCκ-IF < 0% | 1 | 211 | NPV: 99% | |
| Sensitivity: 97 % | Specificity: 85 % | |||
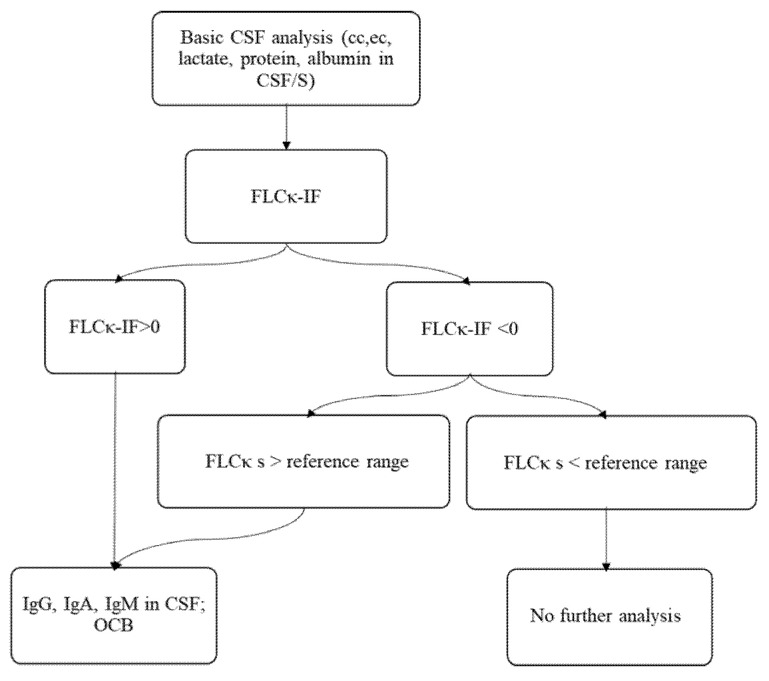
The results from the discovery cohort analyses suggest that there is a high probability of an absent intrathecal humoral immune response in the absence of FLCκ synthesis. Therefore, we established a new laboratory workflow (Figure 2) using the FLCκ-IF as a first line analysis for the presence of an intrathecal humoral immune response. As shown in the discovery cohort, sensitivity to detect an intrathecal inflammation is reduced in patients with pathologically elevated FLCκ serum concentrations. Therefore, patient samples in which the FLCκ serum concentrations exceeded the reference range [10] (9) were excluded in an initial step in the proposed workflow (Figure 2).
Figure 2.
The suggested algorithm for the integration of the FLCκ-IF into the CSF laboratory workflow. After determination of cell count, erythrocyte count, lactate, and total protein, FLCκ is measured in CSF and serum and the quotient is evaluated in relation to the hyperbolic reference range. If the FLCκ-IF is >0, further analysis of immunoglobulin and OCB should be performed. If the FLCκ-IF is <0 and the serum concentration of FLCκ is normal, further analysis of Ig and OCB can be omitted. EC: erythrocyte count; OCB oligoclonal bands, Ig immunoglobulin, CSF cerebrospinal fluid, FLCκ free light chains kappa, cc cell count.
3.2. Part II: Evaluation of the Laboratory Workflow in a Prospective Validation Cohort
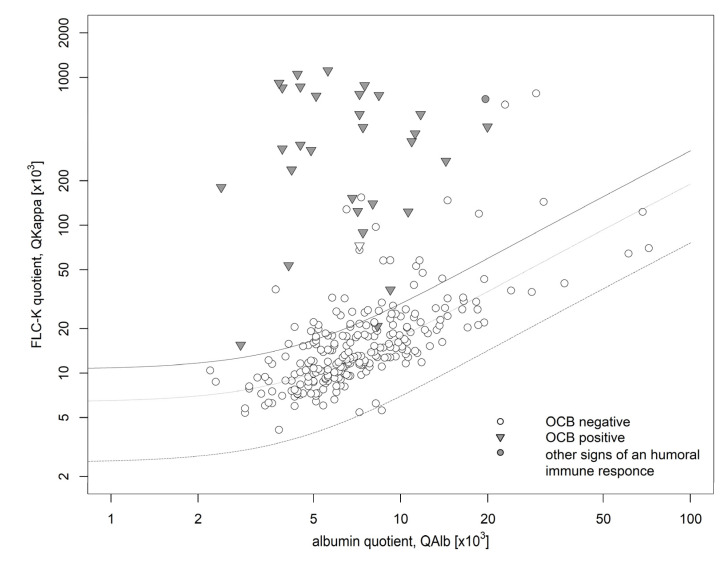
The validation cohort comprised 278 patient samples (Table 1). Of these, 32 (12%) showed an intrathecal humoral immune response as defined in the methods section. Of those, only one patient sample lacked an intrathecal FLCκ synthesis. The diagnosis of intrathecal inflammation in this patient who suffered from an epileptic seizure was based on the presence of two isolated CSF bands in isoelectric focusing. The corresponding CSF profile as well as MRI showed no signs of intrathecal inflammation. Of the prospective cohort 246 patient samples (88%) did not show an intrathecal humoral immune response as defined above. Of these, 36 had an intrathecal synthesis of FLCκ (13%).
All together, these results yielded an excellent sensitivity of 97% and a negative predictive value of 99.5% to identify an intrathecal humoral immune response by using the FLCκ-IF as first line parameter (Figure 3, Table 2).
Figure 3.
Data of the validation cohort in a double logarithmic FLCκ quotient diagram. The bold line shows Qlim, the dashed line shows the Qmean. The bottom dashed line shows the Qlow. 67 (27%) patient samples presented with an intrathecal FLCκ synthesis (IF FLCκ> 0) of which 36 patient samples showed no other signs of a humoral immune response (negative OCB, IF IgG/IF IgA/IF IgM < 0). 32 patient samples presented signs of an intrathecal inflammation (positive OCB ( ) or Ig synthesis in the corresponding quotient diagram (
) or Ig synthesis in the corresponding quotient diagram ( )) of which one lacked an intrathecal FLCκ synthesis (QFLCκ > QLim (FLCκ)). FLCκ free light chains kappa, Q quotient, Alb albumin.
)) of which one lacked an intrathecal FLCκ synthesis (QFLCκ > QLim (FLCκ)). FLCκ free light chains kappa, Q quotient, Alb albumin.
If the FLCκ-IF would have been used in this cohort according to the suggested workflow, the Ig and OCB analyses would have been reduced by 208 patient samples (or 74% of all Ig and OCB analyses). Using the suggested workflow also increases the pre-test probability of a positive OCB result and thus increases the percentage of positive test results.
4. Discussion
While a large body of work has been reported to establish the FLCκ in CSF as a diagnostic biomarker, the final translating step from research into clinical routine is still missing. This is at least partially caused by insufficient agreement on the best method of interpretation and cut-off values [3]. As some studies suggest, the FLCκ index would be sufficient to apply FLCκ analysis in MS patients [14]. In other diseases, evaluation of FLCκ in quotient diagrams is far more accurate and less prone to false negative findings at higher or lower albumin quotient levels. Since a relevant proportion of neurological diseases are associated with an elevation of the albumin quotient [15], evaluation of the intrathecal fraction of FLCκ based on the hyperbolic reference range should be the method of choice for disease-independent evaluation [3,10,11,12,13,16].
Based on previous studies, we hypothesized that in the absence of intrathecal FLCκ synthesis, a humoral intrathecal immune response as defined above should also not be detectable [5,12,13,17,18]. This led us to the idea of introducing the presence of an intrathecal synthesis of FLCκ as a first line analysis for an intrathecal humoral immune response into routine CSF laboratory diagnostics. Our hypothesis is based on the pathophysiological foundation that FLCκ are only produced during the generation and assembly of Ig [17,18]. We have previously demonstrated that the FLCκ index can predict CSF specific OCB with a high sensitivity [5,12]. Furthermore, we could show that this also applies to intrathecal IgA and IgM synthesis. In contrast to Ig quotient diagrams, the intrathecal synthesis of FLCκ as determined in the quotient diagram is robust against blood contamination. This simplifies the differentiation between artificial Ig synthesis caused by blood contamination and inflammatory causes [13]. Based on this, we also decided to evaluate the patient samples with blood admixture as non-inflammatory. An elevated Q FLCκ in these samples would be an expression of a masked intrathecal humoral immune response.
Considering the results of our discovery cohort, the intrathecal fraction of FLCκ seemed suitable as first line analysis for the presence of an intrathecal humoral immune response, as only a small proportion of false negative samples were found (0.5%; NPV: 99.5%). Of these, 66% were explicable by elevated serum FLCκ levels above the serum reference range. This is in line with the concept of quotient diagrams that excessive serum concentrations of any protein of interest reduces the sensitivity to detect intrathecal synthesis of this protein (see Appendix A, Table S1, Figure S1). Therefore, the sensitivity of the intrathecal fraction of FLCκ to detect intrathecal inflammation will be reduced in patient samples in which FLCκ serum concentrations exceed the upper reference range. We have therefore integrated the elevated serum FLCκ values into the proposed laboratory workflow used in our prospective confirmation cohort (see Figure 2). In case of elevated serum FLCκ levels, the analysis of IgG, IgA, and IgM in quotient diagrams and analysis of OCB are strongly recommended.
Only one patient sample in the discovery cohort showed an intrathecal IgM synthesis in the quotient diagram which could not be explained by an artificial blood contamination, while lacking an intrathecal fraction of FLCκ. We suspect that inacurracies in measurement of IgM due to its low concentration in CSF and its high serum gradient [19], may be the reason for this.
Appling the newly developed workflow to the prospective validation cohort resulted in an excellent sensitivity of 98% with a negative predictive value of 99.5%, confirming the findings of the discovery cohort. One patient in the validation cohort showed CSF specific borderline OCB results but no intrathecal FLCκ synthesis. Some authors suggest that two CSF bands do not reliably indicate an intrathecal IgG-synthesis [20].
Diagnostic laboratory workflows especially for the diagnosis of MS with a sequential determination of FLCκ and OCB have already been suggested [16,21,22]. Sanz Diaz et al. suggested the use of the FLCκ CSF concentration for a preselection, followed by the FLCκ index, which might be appropriate in case of a suspected MS [21]. However, using CSF FLCκ concentrations alone is too imprecise when applying workflows to a more unselective patient population. This does not consider the individual blood-CSF barrier function as well as the influence of serum FLCκ values, as described above.
Our data demonstrate the benefits of including the intrathecal fraction of FLCκ as a key parameter into the laboratory workflow: In our cohorts, 62% of the Ig and OCB analysis in the discovery cohort and 74% in the validation cohort would not have been required. Thus, the implementation of this workflow would have a considerable cost-, resources- and personnel-saving effect. In addition, using the suggested workflow with a high positive predictive value for positive OCB results will avoid unnecessary OCB analysis. Certainly, if clinical findings, other laboratory parameters such as conspicuous CSF cytology, or imaging suggest an intrathecal inflammation despite a lack of intrathecal FLCκ synthesis, it will be reasonable to perform Ig quotient diagrams and OCB. Nevertheless, the use of this workflow can simplify the diagnostic pathway to exclude intrathecal humoral immune responses due to the high negative predictive value.
The monocentric study design is a limitation of our study. On the other hand, we used a large cohort to establish the workflow. If a general diagnostic pathway is recommended, it is important that it also yields robust results for different assays. Especially OCB (e.g., silver staining vs. immunoblotting) and FLCκ determination on different platforms warrant further investigation. Noteworthy is also that the discovery cohort does not reflect the general neurological patient population due to the selectivity of the previous studies that were utilized in this cohort.
5. Conclusions
The presence of an intrathecal fraction of FLCκ shows an excellent sensitivity to identify an intrathecal Ig synthesis. The integration of the FLCκ-IF as a screening parameter into the routine procedures of CSF laboratories can simplify CSF laboratory evaluation and analytical effort. In addition, this will increase the pre-test probability of a positive OCB result and thus increase the percentage of positive test results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom12111690/s1, Figure S1: Data of the hypothetic patient in a double logarithmic diagram in case of normaland increased serum values as well as with- or without intrathecal FLCκ synthesis. Table S1: CSF-serum values as well as quotient and resulting IF of the hypothetic patient in case of normal- and increased FLCκ serum values as well as with- or without intrathecal FLCκ synthesis.
Appendix A
Due to the usage of CSF and serum quotients in CSF analysis, an increased FLCκ serum value caused by, e.g., plasma cell-proliferative disorders [23] can influence the sensitivity of the quotient diagram. According to Fick’s laws, as described by Reiber et al. [2,3] the following applies: If the serum value rises, the CSF value follows and adjusts proportionately. This entails the re-balancing of the CSF-Serum quotient. The now increased CSF and serum levels have the effect that an additional intrathecal FLCκ synthesis results in a lower relative increase of the CSF level and thus in a lower quotient. This can be seen in the following theoretical example:
Patient 1-A (Supplemental, Table S1; Figure S1, A) presents with FLCκ CSF/serum values corresponding to a QFLCκ of 7.1. A hypothetical 3-fold increase in serum FLCκ value over a longer period also results in a QFLCκ of 7.1 (Supplemental, Patient 1-B, Table S1; Figure S1, B). In case of an intrathecal FLCκ synthesis with an increase of the FLCκ CSF values from 0.13 mg/dL to 0.5 mg/dL, the QFLCκ increases from 7.1 to 27.32 and an IF of 7.3% (Supplemental, Patient 1-D, Table S1, Figure S1, D). If the same amount of FLCκ is produced in Patient 1-B the CSF FLCκ value increases from 0,39 mg/dL to 0.76 mg/dL (Supplemental, Patient 1-C, Table S1; Figure S1, C) resulting in a QFLCκ of 13.84 and a negative IF. The increased serum levels have the effect that Patient 1-B has to produce 200% more intrathecal FLCK to present an equal IF as Patient 1-D.
Author Contributions
Designed and conceptualised the study, M.J.H., A.D. and M.S.; writing—original draft preparation, M.J.H. and M.S.; writing—review and editing, M.J.H., A.D. and M.S.; revised the manuscript for intellectual content, M.R.A., M.N., K.B. and A.P.; data curation, M.J.H., M.R.A. and M.S.; data analyses and visualization, M.J.H.; supervision, A.D., M.N., A.P. and M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
III UV 39/03, BB117/18 and BB019/18, University Medicine of Greifswald, Germany.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.
Conflicts of Interest
M.J.H., M.R.A., K.B., A.D., M.S., declare no conflict of interest.; M.N., reports non-financial support by Siemens Healthineers, The Binding Site, Becton Dickinson, DZHK (German Centre for Cardiovascular Research, Partner Site Greifswald, University Medicine, Greifswald, Germany), DGKL (German federation of clinical chemistry and laboratory medicine), German federal medical association, Roche diagnostics Germany GmbH. He also received personal fees by Boehringer Ingelheim and Becton Dickinson; Grant by DZHK, LVL technologies, Bruker Biospin, Abbott, Radiometer, Tosoh and IDS Immunodiagnostic Systems Deutschland GmbH; A.P., reports non-financial support and received personal fees by Radiometer, Nova Biomedical and Roche.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reiber H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003;21:79–96. [PubMed] [Google Scholar]
- 2.Reiber H. Flow rate of cerebrospinal fluid (CSF)—A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 1994;122:189–203. doi: 10.1016/0022-510X(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 3.Reiber H., Zeman D., Kusnierova P., Mundwiler E., Bernasconi L. Diagnostic relevance of free light chains in cerebrospinal fluid-The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta. 2019;497:153–162. doi: 10.1016/j.cca.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Ramsden D.B. Multiple sclerosis: Assay of free immunoglobulin light chains. Ann. Clin. Biochem. 2017;54:5–13. doi: 10.1177/0004563216652175. [DOI] [PubMed] [Google Scholar]
- 5.Susse M., Hannich M., Petersmann A., Zylla S., Pietzner M., Nauck M., Dressel A. Kappa free light chains in cerebrospinal fluid to identify patients with oligoclonal bands. Eur. J. Neurol. 2018;25:1134–1139. doi: 10.1111/ene.13667. [DOI] [PubMed] [Google Scholar]
- 6.Senel M., Mojib-Yezdani F., Braisch U., Bachhuber F., Lewerenz J., Ludolph A.C., Otto M., Tumani H. CSF Free Light Chains as a Marker of Intrathecal Immunoglobulin Synthesis in Multiple Sclerosis: A Blood-CSF Barrier Related Evaluation in a Large Cohort. Front. Immunol. 2019;10:641. doi: 10.3389/fimmu.2019.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez M.S., Rojas J.I., Lorenzon M.V., Sanchez F., Patrucco L., Miguez J., Azcona C., Sorroche P., Cristiano E. Validation of CSF free light chain in diagnosis and prognosis of multiple sclerosis and clinically isolated syndrome: Prospective cohort study in Buenos Aires. J. Neurol. 2019;266:112–118. doi: 10.1007/s00415-018-9106-2. [DOI] [PubMed] [Google Scholar]
- 8.Gurtner K.M., Shosha E., Bryant S.C., Andreguetto B.D., Murray D.L., Pittock S.J., Willrich M.A.V. CSF free light chain identification of demyelinating disease: Comparison with oligoclonal banding and other CSF indexes. Clin. Chem. Lab. Med. 2018;56:1071–1080. doi: 10.1515/cclm-2017-0901. [DOI] [PubMed] [Google Scholar]
- 9.Konen F.F., Schwenkenbecher P., Jendretzky K.F., Gingele S., Witte T., Suhs K.W., Grothe M., Hannich M.J., Susse M., Skripuletz T. Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain. Sci. 2022;12:475. doi: 10.3390/brainsci12040475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Susse M., Reiber H., Grothe M., Petersmann A., Nauck M., Dressel A., Hannich M.J. Free light chain kappa and the polyspecific immune response in MS and CIS-Application of the hyperbolic reference range for most reliable data interpretation. J. Neuroimmunol. 2020;346:577287. doi: 10.1016/j.jneuroim.2020.577287. [DOI] [PubMed] [Google Scholar]
- 11.Susse M., Feistner F., Grothe M., Nauck M., Dressel A., Hannich M.J. Free light chains kappa can differentiate between myelitis and noninflammatory myelopathy. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:5. doi: 10.1212/NXI.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Susse M., Feistner F., Holbe C., Grothe M., Nauck M., Dressel A., Hannich M.J. Diagnostic value of kappa free light chains in patients with one isolated band in isoelectric focusing. Clin. Chim. Acta. 2020;507:205–209. doi: 10.1016/j.cca.2020.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Hannich M.J., Dressel A., Budde K., Petersmann A., Nauck M., Susse M. Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern. Cells. 2021;10:616. doi: 10.3390/cells10030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstein I., Rasch S., Axelsson M., Novakova L., Blennow K., Zetterberg H., Lycke J. Kappa free light chain index as a diagnostic biomarker in multiple sclerosis: A real-world investigation. J. Neurochem. 2021;159:618–628. doi: 10.1111/jnc.15500. [DOI] [PubMed] [Google Scholar]
- 15.Brettschneider J., Claus A., Kassubek J., Tumani H. Isolated blood-cerebrospinal fluid barrier dysfunction: Prevalence and associated diseases. J. Neurol. 2005;252:1067–1073. doi: 10.1007/s00415-005-0817-9. [DOI] [PubMed] [Google Scholar]
- 16.Schwenkenbecher P., Konen F.F., Wurster U., Witte T., Gingele S., Suhs K.W., Stangel M., Skripuletz T. Reiber’s Diagram for Kappa Free Light Chains: The New Standard for Assessing Intrathecal Synthesis? Diagnostics. 2019;9:194. doi: 10.3390/diagnostics9040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link H. Immunoglobulin G and low molecular weight proteins in human cerebrospinal fluid. Chemical and immunological characterisation with special reference to multiple sclerosis. Acta Neurol. Scand. 1967;43((Suppl. 28)):21–136. [PubMed] [Google Scholar]
- 18.Nakano T., Matsui M., Inoue I., Awata T., Katayama S., Murakoshi T. Free immunoglobulin light chain: Its biology and implications in diseases. Clin. Chim Acta. 2011;412:843–849. doi: 10.1016/j.cca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Reiber H. Knowledge-base for interpretation of cerebrospinal fluid data patterns. Essentials in neurology and psychiatry. Arq. Neuropsiquiatr. 2016;74:501–512. doi: 10.1590/0004-282x20160066. [DOI] [PubMed] [Google Scholar]
- 20.Hegen H., Zinganell A., Auer M., Deisenhammer F. The clinical significance of single or double bands in cerebrospinal fluid isoelectric focusing. A retrospective study and systematic review. PLoS ONE. 2019;14:e0215410. doi: 10.1371/journal.pone.0215410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz Diaz C.T., de Las Heras Florez S., Carretero Perez M., Hernandez Perez M.A., Martin Garcia V. Evaluation of Kappa Index as a Tool in the Diagnosis of Multiple Sclerosis: Implementation in Routine Screening Procedure. Front. Neurol. 2021;12:676527. doi: 10.3389/fneur.2021.676527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespi I., Sulas M.G., Mora R., Naldi P., Vecchio D., Comi C., Cantello R., Bellomo G. Combined use of Kappa Free Light Chain Index and Isoelectrofocusing of Cerebro-Spinal Fluid in Diagnosing Multiple Sclerosis: Performances and Costs. Clin. Lab. 2017;63:551–559. doi: 10.7754/Clin.Lab.2016.160930. [DOI] [PubMed] [Google Scholar]
- 23.Jenner E. Serum free light chains in clinical laboratory diagnostics. Clin. Chim. Acta. 2014;427:15–20. doi: 10.1016/j.cca.2013.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.