Abstract
Cancer is a complex disease including approximately 200 different entities that can potentially affect all body tissues. Among the conventional treatments, radiotherapy and chemotherapy are most often applied to different types of cancers. Despite substantial advances in the development of innovative antineoplastic drugs, cancer remains one of the most significant causes of death, worldwide. The principal pitfall of successful cancer treatment is the intrinsic or acquired resistance to therapeutic agents. The development of more effective or synergistic therapeutic approaches to improve patient outcomes and minimize toxicity has become an urgent issue. Inula viscosa is widely distributed throughout Europe, Africa, and Asia. Used as a medicinal plant in different countries, I. viscosa has been characterized for its complex chemical composition in order to identify the bioactive compounds responsible for its biological activities, including anticancer effects. Sesquiterpene lactones (SLs) are natural, biologically active products that have attracted considerable attention due to their biological activities. SLs are alkylating agents that form covalent adducts with free cysteine residues within enzymes and key proteins favoring cancer cell cytotoxicity. They are effective inducers of apoptosis in several cancer cell types through different molecular mechanisms. This review focuses on recent advances in the cytotoxic effects of I. viscosa and SLs in the treatment of neoplastic diseases, with a special emphasis on their proapoptotic molecular mechanisms.
Keywords: Inula viscosa, tomentosin, inuviscolide, intrinsic pathway of apoptosis, extrinsic pathway of apoptosis, ROS production, cytotoxic effect
1. Introduction
Traditional herbal medicines are excellent sources of natural biologically active products with powerful therapeutic effects that can be utilized in the treatment of various diseases, including cancer. Inula viscosa (L.) Aiton. (Dittrichia viscosa L.) is a medicinal perennial plant belonging to the genus Inula and family Asteraceae and is considered one of the most crucial pharmaceutical plants in the Mediterranean Basin [1]. As a medicinal plant in, I. viscosa has been characterized in different countries for its complex chemical composition to identify the bioactive compounds responsible for its biological activities [2]. I. viscosa has long been used in folk medicine owing to its anti-inflammatory [3], anthelmintic, antipyretic, antiseptic, and antiphlogistic activities [4,5], and in the treatment of lung disorders [6] and diabetes [7]. Hernandez et al. isolated and tested several flavanones, such as sakuranetin, 7-O-methylaromadendrin, and 3-acetyl-7-O-methylaromadendrin showing relevant effects on enzymes involved in the inflammatory response [8]. Recently, several studies have explained the biological mechanisms triggering the anti-inflammatory effect of I. viscosa, which are based on the inhibition of COX1, COX2, and iNOS enzymatic activity [9,10]. Kheyar-Kraouche et al. performed a high-performance liquid chromatography associated with electrospray ionization mass spectrometry on the ethanolic extract obtained by I. viscosa leaves growing in Algeria. They identified different phytochemical components, including phenolic acids, flavonoids, lignans, and terpenoids; some of which were recognized for the first time and belong to different subfamilies of compounds [11].
Recently, cytotoxic and anticancer activities of I. viscosa have been demonstrated in various cancer cell lines [12,13,14]. Therefore, elucidating the anticancer properties of this plant could be a relevant strategy to identify new antitumor agents. Different biologically active compounds belonging to several families isolated from I. viscosa were tested alone or in association with cancer cell lines [15,16,17,18], and in vivo experiments showed their strong antineoplastic activity [19,20]. Messaoudi et al. performed a chemical composition analysis of different I. viscosa extracts from three different regions of Morocco, revealing the presence of sesquiterpene lactones (SLs), tomentosin, and inuviscolide as major representative compounds, with tomentosin concentrations ranging from 22% to 64% and inuviscolide concentrations ranging from 0% to 58% in different Moroccan regions [14]. The SL structure consists of 15-carbon terpenoids obtained by the condensation of three isoprene units and a lactone ring [21]. Most sesquiterpenes, but not all, contain the α-methylene-γ-butyrolactone motif, the functional group responsible for their biological effects, and, above all, the antitumor activity [22]. Although they are mainly found in the Asteraceae family, SLs have been identified in several families of flowering plants, including Solanaceae, Araceae, and Cactaceae [23]. As reported by Wang et al., 396 types of sesquiterpenoids with high structural diversity have been isolated and characterized with the Inula (Asteraceae) genus [2].
Recently, several studies have reported the potential anticancer effects of SLs and have shown that tomentosin and inuviscolide exert antiproliferative effects on human cancer cell lines [12,24]. In particular, SLs are alkylating agents that cause DNA damage with consequent activation of ATM/ATR kinase and involvement of CDC2, TP53, survivin, and NF-κB inducing cell cycle arrest and apoptosis activation [12]. Tomentosin and inuviscolide, purified from I. viscosa extracts, characterize previous molecular mechanisms. SLs induce cell cycle arrest in the G2/M phase and appearence of a sub-G0 fraction evocative of apoptotic cell death in melanoma cell lines [12]. Japonicone A, derived from I. japonica, performs antineoplastic activity against Burkitt lymphoma (BL) cells [25]. Additionally, studies by Merghoub et al. identified tomentosin as responsible of antineoplastic effects of I. viscosa on cervical cancer cells [26].
This review summarizes the emerging roles of I. viscosa and its prevalent SLs in the treatment of neoplastic diseases, with special emphasis on their proapoptotic molecular mechanisms.
2. Materials and Methods
The purpose of this review is to provide an overview of the current knowledge regarding the antineoplastic role of I. viscosa and SLs in solid and hematologic cancers. A literature search for original and review articles was performed using electronic databases of Medline (PubMed, PubMed Central) by using the terms ‘Inula viscosa’, ‘tomentosin’, ‘inuviscolide’, ‘cancer’, ‘carcinoma’, ‘apoptosis’. Combinations of these terms were used to screen the mentioned databases for relevant content: title, abstract, and full content, respectively. Only studies written in English were considered for evaluation. Pre-screening and screening selection removed duplicate studies, foreign language studies, irrelevant studies, and studies for which updated research was unavailable. We considered both clinical and experimental studies (in vivo and in vitro).
3. Results
3.1. Inula viscosa: Antineoplastic Activities and Molecular Mechanisms
Cytotoxic screening models represent crucial preliminary data for analyzing the antineoplastic properties of selected plant extracts. Early tests were cell-based assays performed on established cell lines in which the toxic effects of plant extracts or isolated compounds could be measured. Conventional antitumor agents display significant cytotoxic activities in cell culture systems [27].
Benbacer et al., with the purpose of developing new anticancer drugs against cervical cancer, applied the human cervical carcinoma SiHa and HeLa cell lines as a model system to screen the anticancer effects of plants from traditional Moroccan medicine. In particular, they demonstrated that I. viscosa hexane extract showed pronounced cytotoxic effects against both cervical cancer cell lines, inducing dose-dependent cell growth inhibition by stimulating apoptosis, which is related to the decrease in mitochondrial membrane potential (ΔΨm) and increase in intracellular reactive oxygen species (ROS) production. Thus, I. viscosa extracts showed significant cytotoxic effects against cervical cancer cell lines through the inhibition of proliferation and induction of apoptosis involving a mitochondria-mediated signaling pathway by pro-caspase activation, BCL-2 expression, and PARP cleavage [13]. The same group demonstrated that I. viscosa extracts target the telomerase machinery and induce apoptosis in human cervical carcinoma cell lines (SiHa and HeLa) and that the molecular mechanism underlying I. viscosa extract-induced apoptosis includes a caspase-3 mediated signaling pathway [28].
An interesting study by Messaoudi et al. showed the cytotoxic activity of ethyl acetate and ethanolic I. viscosa extracts harvested from three different regions of Morocco (Taouante, Sefrou, and Imouzzer) in two breast cancer cell lines, MCF7, an estrogen receptor-positive cell line, and MDAMB-231, an estrogen receptor-negative cell line. These two I. viscosa extracts showed different toxicity on breast cancer cells, suggesting that the different cytotoxic activity can be an integral effect of the combination of three major compounds, tomentosin, inuviscolide, and isocostic acid which are present in variable concentrations in plants from different regions. Furthermore, the reduced toxicity exerted by the two extracts on MCF-7 cells when compared with MDA-MB-231 cells suggests that heterogeneous susceptibility could be dependent on the activation of different signaling pathways [14]. In addition, they demonstrated that ethanolic and ethyl acetate extracts of I. viscosa from Taounate, Imouzzer, and Sefrou had different rates of polyphenols associated with different antioxidant activities [29]. Further studies have demonstrated the selective cytotoxic effects of I. viscosa on MCF-7 cells [30,31].
Bar-Shalom et al. examined the possible therapeutic effects of I. viscosa aqueous extract on colon cancer cell growth in vitro and tumor growth in vivo, using a xenograft mouse model. In vitro experiments revealed that exposure of colorectal cancer cells to I. viscosa extract significantly reduced cell viability in a dose- and time-dependent manner. Interestingly, the analysis of the molecular mechanisms underlying the I. viscosa effect showed the activation of caspase-9 in HCT116 well-differentiated cells and of caspases-8 and -9 in colo320 poorly-differentiated cells. These findings suggest that I. viscosa extract induces apoptosis through the intrinsic mitochondrial pathway in well-differentiated cells, and through both the intrinsic and extrinsic pathways in poorly-differentiated cells. In vivo studies revealed that treatment with I. viscosa extract inhibited tumor growth in mice transplanted with MC38 cells, showing a strong reduction in the weight and volume of neoplastic lesions. Interestingly, no side effects such as weight loss, behavioral changes, ruffled fur, or changes in kidney and liver function were observed, suggesting the absence of toxicity from I. viscosa [32].
I. viscosa collected from an uncontaminated area of the National Park on Asinara Island, Sardinia, revealed powerful anti-lymphoma activity. Specifically, Raji cells treated with increasing concentrations of I. viscosa ethanolic extract demonstrated a dose- and time-dependent decrease in cell viability, displaying a reduction in cell proliferation obtained by the induction of cell cycle arrest in the G2/M phase, and a dose-dependent increase in cell apoptosis. A gene expression analysis of signal transduction and apoptotic pathway players involved in B-lymphocyte functions showed that the molecular mechanisms involved in I. viscosa anticancer activity were characterized by the downregulation of genes involved in cell cycle and proliferation (c-MYC, CCND1), as well as in the inhibition of cell apoptosis (BCL2, BCL2L1, BCL11A) [33].
An interesting study evaluated the cytotoxic and anticancer effects of I. viscosa methanol and aqueous extracts on the malignant melanoma cell lines A2058 and MeWo, and normal fibroblasts. Cytotoxicity, apoptosis induction, and migration suppression were strongly induced in malignant melanoma cell lines by I. viscosa methanol extracts compared to the aqueous extracts [34], confirming that the solvent used in the extraction steps can influence the content and biological activity of the extract [28,30]. For the first time, an epigenetic mechanism underlying the anticancer activity of I. viscosa has been demonstrated. Specifically, I. viscosa methanol extract promotes the downregulation of miRNAs related to epithelial-mesenchymal transition and poor prognosis in malignant melanoma, such as miR-191 and miR-193, while favoring the overexpression of miR-579 and miR-524, which mainly repress the MAPK signaling pathway in malignant melanoma [34].
The ubiquitin–proteasome system plays a key role in intracellular proteolysis, particularly in the degradation of abnormal proteins. In fact, it is directly involved in the regulation of most biological processes, such as cell cycle, apoptosis, muscle differentiation, and immune response [35]. Many studies have revealed that proteasome levels can be used as biomarkers for various types of cancer [36,37,38]. Recently, Yaagoubi et al. investigated the antitumor effects and proteasome inhibition capacity of I. viscosa extract in a mouse model of DMBA/croton oil-induced skin carcinoma. Animals received treatment with the extract before and after the induction of skin carcinogenesis, showing that I. viscosa extract inhibited the development of papilloma in mice. Furthermore, ingestion of I. viscosa extract delayed the formation of cutaneous papillomas in animals and simultaneously decreased the size and number of papillomas. A structure–activity study showed that I. viscosa extract contains bioactive molecules with much greater inhibition of the subunits of the proteasome, as well as a decrease in the concentration of proteasome and its catalytic activity in serum and intracellularly when compared to chemically synthesized inhibitors, thus emerging as a new candidate for targeted therapy against skin carcinoma. Specifically, molecular docking analysis revealed that tomentosin, inuviscolide and isocostic acid compounds obtained from I. viscosa extract were stabilized in the pocket of the 20S proteasome β5 receptor subunits by various interactions, mirroring the same mechanisms exerted by carfilzomib, a potent second-generation proteasome inhibitor with significant anti-myeloma activity [39]. I. viscosa extracts also increased cell cycle arrest and cell death in the glioblastoma LN229 cell line, characterized by a TP53 mutation, compared to U87MG cells with wild-type TP53. SW620 cells were more sensitive to I. viscosa extracts, suggesting that they may contain molecules with high therapeutic potential against MDR cell lines. PC-3 are androgen-insensitive and apoptosis-resistant prostate cancer cells, on which I. viscosa extracts induce growth inhibition, cell cycle arrest and apoptosis, supporting the idea that active compounds effectively targeting extrinsic and intrinsic apoptosis pathways are present in the plant [40]. Table 1 summarizes the biological and molecular effects induced by I. viscosa treatment with in vitro and in vivo models.
Table 1.
Summary of biological and molecular effects induced by Inula viscosa treatment with in vitro and in vivo system models.
| Type of Cancer | Treatment | Model | Biological and Molecular Effects | Ref. |
|---|---|---|---|---|
| Cervical cancer | Hexane extract from 15.6 to 500 μg/mL for 48 or 72 h | SiHa and HeLa cell lines | Cell growth inhibition and apoptosis induction. Decrease ΔΨm and intracellular ROS production | [13] |
| Cervical cancer | Hexane and methanol extract from 5 to 80 μg/mL for 72 h | SiHa and HeLa cell lines | Cell growth inhibition and apoptosis induction. Inhibition of telomerase activity and induction of telomere shortening | [28] |
| Breast cancer | Ethyl acetate and ethanolic extract from 15.6 to 500 μg/mL for 72 h | MCF-7 and MDA-MB231 cell lines | Cytotoxic effect | [14] |
| Colorectal cancer | Aqueous extract from 100 to 300 μg/mL for 24–72 h | HCT116 and colo320 cell lines | Reduction in cell viability. Apoptosis induction through the intrinsic mitochondrial pathway in well-differentiated cells and through both, the intrinsic and extrinsic pathways in poorly differentiated cells | [32] |
| Aqueous extract 150 or 300 mg/kg | C57BL/6 mice transplanted with MC38 cells | Inhibition of tumor growth | ||
| Burkitt lymphoma | Ethanolic extract: 5, 10, 20, 30, 40, 60, and 80 mg/mL for 24 and 48 h | Raji cell line | Cell cycle arrest in the G2/M phase, decreased cell viability and increased cell apoptosis. Downregulation of genes involved in cell cycle and proliferation (c-MYC, CCND1) and in the inhibition of cell apoptosis (BCL2, BCL2L1, BCL11A) | [33] |
| Malignant melanoma | Aqueous and methanolic extracts from 10 µg/mL to 140 µg/mL for 24–72 h | A2058 and MeWo cell lines | Antiproliferative effect by induction of apoptosis and cell cycle arrest, suppression of cell migration. Deregulation of oncogenic and oncosuppressive miRNAs | [34] |
| Skin carcinogenesis | Ethanolic extract 100 μL for 4 days | Swiss albino mice treated with DMBA/croton oil | Inhibition of the development of papilloma. I. viscosa extract induces inhibition on the subunits of the proteasome, as well as decrease in the concentration of proteasome and its catalytic activity in serum and intracellularly. | [39] |
3.2. Sesquiterpene Lactones Tomentosin and Inuviscolide: Antineoplastic Activities and Molecular Mechanisms
Different biologically active compounds, belonging to several families, isolated from I. viscosa were tested alone or in association in cancer cell lines [15,16,17,18] or within in vivo experiments [19,20], showing strong antineoplastic activity. SLs represent one of the most abundant and globally distributed groups of plant-derived bioactive compounds and have been identified as worthwhile therapeutic agents against various types of cancer [21,22].
In SLs, it is assumed that the electrophilic ab-unsaturated carbonyl structures, such as α-methylene-γ-lactone, represent bioactive functional groups interacting in a Michael-type addition with the nucleophilic sites of biological molecules. SLs are thought to inhibit tumor cell proliferation by selective alkylation of cysteine sulfhydryl groups in growth-regulatory biological macromolecules, such as key enzymes that control cell division by restoring the ability of tumor cells to undergo apoptosis [41]. In addition, DNA alkylation is a potential molecular cytotoxicity mechanism of SLs [42]. SLs are potentially selective toward neoplastic and cancer stem cells by targeting specific signaling pathways, making them lead compounds in cancer clinical trials [43]. Although the specific molecular targets and mechanisms of the antitumor activity of SLs in vitro and in vivo have not yet been explained, different studies have reported that SLs, tomentosin and inuviscolide, exert antiproliferative and proapoptotic effects on various human cancer cell lines, then trying to identify the underlying molecular mechanisms.
Rozenblat et al. purified the SLs, tomentosin and inuviscolide, from I. viscosa leaves and analyzed their anticancer effectiveness against human melanoma cell lines with the aim of developing new agents for melanoma treatment, taking into consideration the aggressiveness and chemotherapeutic resistance of this tumor. Tomentosin and inuviscolide reduced cell viability in three different human melanoma cell lines by favoring cell cycle arrest at the G2/M phase, associated with an increase in the sub-G0 fraction indicative of apoptotic cell death, as demonstrated by changes in membrane phospholipids, mitochondrial membrane potential, and activation of caspase-3. The molecular mechanism of SL-mediated G2/M arrest and apoptosis in melanoma cell lines suggests that SLs are possible alkylating agents that might cause DNA damage, which activates the kinase ATM/ATR. This activation is followed by early phosphorylation of TP53 (Ser15) and CDC2 (Thr14 and Tyr15) providing early G2/M arrest. The activation of TP53 transactivates p21waf1, which also reduces the protein concentration of CDC2/Cyclin B1, favoring the elongation of G2/M arrest, and ultimately resulting in apoptosis [12]. To better understand the mechanisms responsible for apoptotic death, the authors detected the effects of SLs on survival protein, such as the survivin that favor the acquisition of chemoresistance of neoplastic cells by its anti-apoptotic potency based on the inhibition of the effector caspase 3 and 7 [44]. In human melanoma cell lines, the decreased levels of survivin (by both SLs) and the p65/RelA subunit of NF-κB (only by inuviscolide) suggest an apoptotic induction. The induction of apoptosis by tomentosin and inuviscolide in aggressive human melanoma cell lines has high pharmacological value, implying that SLs could be potentially developed as novel agents for melanoma treatment [12].
To explain possible mechanisms underlying tomentosin-induced apoptosis, Merghoub et al. studied the effects of tomentosin on telomere lengthening by hybridization with a telomeric C-rich probe (21C) under non-denaturing conditions, which drastically induced telomere G-overhang shortening in human cervical cell lines. This mechanism was validated on JW10 cells in which tomentosin induced a significant anti-proliferative effect acting on hTERT expression, while it exhibited a low cytotoxic effect on Wi38 fibroblast cells, a primary cell culture without telomerase expression and activity [26]. Furthermore, the molecular mechanism underlying tomentosin-induced apoptosis in human cervical cell lines involves a mitochondria-mediated signaling pathway. In fact, tomentosin obtained from the aerial parts of I. viscosa causes a reduction in the mitochondrial membrane potential and an increase in ROS levels in human cervical cell lines. It leads to the downregulation of pro-caspase-3 protein, cleavage of PARP, and enhanced caspase-3 activity associated with BCL2 downregulation in tomentosin-treated SiHa and HeLa cells [26].
ROS are commonly produced by mitochondrial oxidative metabolism in response to cellular stress [45]. They are vital chemical messengers that play essential roles in cell growth and proliferation [46]. Paradoxically, the pro-oxidant activity of phytochemicals has been described as a critical mechanism underlying their anticancer effects [47]. In fact, celastrol causes G2/M cell cycle arrest, autophagy, and apoptosis through the ROS/JNK pathway in human osteosarcoma cells [48], and phenyl arsine oxide induces apoptosis in HepG2 cells via ROS-dependent signaling pathways [49]. Lee et al. analyzed the role of intracellular ROS in tomentosin-induced apoptosis in an osteosarcoma cell line and demonstrated that the tomentosin-induced apoptosis can be inhibited through the suppression of ROS production by N-acetyl-cysteine. Moreover, a decrease in peroxiredoxin-1, an antioxidant enzyme that reduces the levels of hydrogen peroxide and alkyl hydroperoxides, has been shown after treatment with tomentosin. These results support the hypothesis that tomentosin-induced apoptosis is associated with intracellular ROS production. By analyzing the molecular mechanisms, the authors demonstrated that tomentosin-induced ROS upregulate FOXO3 and p27 expression, thus suggesting that FOXO3 upregulation controls G2/M phase cell cycle arrest through p27 overexpression after tomentosin treatment in an osteosarcoma cell line [24]. A similar molecular mechanism was identified by Yu et al., who investigated the role of tomentosin in hepatocellular carcinoma cell lines (HepG2 and Huh7), in which tomentosin induced G2/M phase cell cycle arrest through p27 overexpression regulated by the upregulation of FOXO3. In addition, cell cycle arrest and apoptosis induction are boosted by the overexpression and phosphorylation of TP53 and activation of the ERK signaling pathway [50].
A recent study validated the hypothesis that tomentosin-induced oxidative stress is involved in apoptosis via the mitochondria-mediated signaling pathway in gastric carcinoma cell lines. The molecular mechanisms that explain the antiproliferative effect of tomentosin are based on the reduction in PCNA and cyclin D1, which are able to control cell growth. The induction of apoptosis is related to BAX overexpression, BCL-2 downregulation, and inhibition of inflammation, as revealed by the downregulation of IL-6, TNF-α, IL-1β, and IL-8 which modulate cell growth proteins in gastric cancer cells [51].
Recently, the antineoplastic activity of SLs has also been evaluated in hematologic tumors such as LB, multiple myeloma (MM), and leukemia cancer cell lines. Virdis et al. demonstrated that tomentosin exerts strong antitumor activity on human BL (Raji cell line; [52]) and MM (RPMI 8226 cell line; [53]), mediated by inhibition of cell proliferation and induction of apoptosis. Apoptosis was induced by activating both the death receptor and mitochondrial pathways in Raji cells. Gene expression profiling analysis was performed to assess differentially expressed genes contributing to tomentosin activity in BL and MM. Seventy-five genes deregulated by tomentosin were identified in BL. Downregulated genes are enriched in the immune system, PI3K/AKT, and JAK/STAT pathways, which assist proliferation and growth. Notably, different deregulated genes identified in tomentosin-treated BL cells are prevalent in molecular pathways known to lead to cellular death, downregulation of anti-apoptotic genes such as BCL2A1 and CDKN1A, and upregulation of the proapoptotic PMAIP1 gene [52]. In total, 126 genes deregulated by tomentosin were identified in MM. In total, 126 genes deregulated by tomentosin were identified in MM. Protein–protein interaction network analysis revealed that the tomentosin treatment of MM produced the downregulation of genes involved in pathways implied in immune system processes and in cellular neoplastic processes, such as growth, proliferation, migration, invasion, and apoptosis. Furthermore, tomentosin causes endoplasmic reticulum stress via upregulation of the ATF4 and DDIT3 genes, suggesting that tomentosin treatment the activation of the protective unfolded protein response signaling might induce cell apoptosis. Functional connection analysis executed by the connectivity map tool indicated that tomentosin acts as the heat shock protein inhibitors on MM cells [53].
Furthermore, Yang et al. showed that tomentosin stimulates intracellular ROS production, causing mitochondria-centered death in leukemia MOLT-4 cells, revealing significant cytotoxicity. Tomentosin induces apoptosis in MOLT-4 cells by suppression of NF-κB and proinflammatory cytokines, as revealed by the complete blocking of anti-apoptotic proteins (cyclin D1 and BcL-2) and activation of proapoptotic proteins (caspase-3 and BAX) via the mTOR/PI3K/AKT pathway [54]. These findings suggest that tomentosin could be considered a potential natural product with limited toxicity and relevant antitumor activity among the therapeutic options available for onco-hematologic patients.
Recently, Güçlü et al. analyzed the anticancer effects of tomentosin on PANC-1 and MIA PaCa-2 human pancreatic cancer cells on which the treatment induces suppression of proliferation, migration, invasion, and colony formation capacity. Tomentosin increases apoptosis rate and ROS production and decreases mitochondrial membrane potential apoptosis in pancreatic cancer cells. At a molecular level, tomentosin induces overexpression of apoptosis-related genes, such as CASP8, PPARG, FAS, FADD, TNF, and TNFR1 genes for extrinsic pathway and BAX, BCL2, CASP3, CASP7, CASP9, and CYCS genes for intrinsic pathway and increases caspase-3 and caspase-9 protein levels [55].
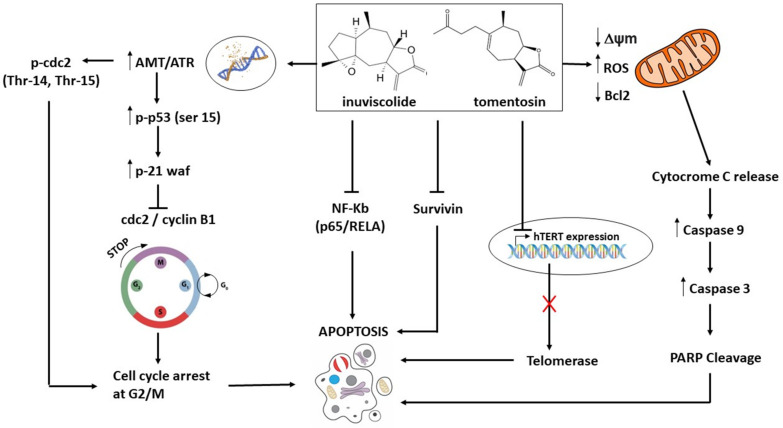
Table 2 summarizes the biological and molecular effects induced by SLs treatment within in vitro system models. Figure 1 describes the prevalent proapoptotic molecular mechanisms induced by SLs treatment in human tumors.
Table 2.
Summary of the biological and molecular effects of sesquiterpene lactones treatment with in vitro system models.
| Type of Cancer | Treatment | Model | Biological and Molecular Effects | Ref. |
|---|---|---|---|---|
| Melanoma | Tomentosin and Inuviscolide 9–36 mM for 24 h | SK-28, 624 mel, and 1363 mel cell lines | Cell cycle arrest at the G2/M phase and apoptosis. Activation of ATM/R followed by phosphorylation of TP53 and CDC2 and p21waf1 overexpression. Decrease of Survivin and of NF-kB | [12] |
| Cervical cancer | Tomentosin 0–100 mM for 24, 48, 72, and 96 h | HeLa and SiHa cell lines | Cell cycle arrest and apoptosis. Increased ROS and decrease in mitochondrial membrane potential. Telomeric G-overhang shortening | [26] |
| Osteosarcoma | Tomentosin 0, 10, 20, and 40 µM for 24 and 48 h | MG-63 cell line | Decreased cell viability and migration, apoptosis, cell cycle arrest. Increase of ROS induces FOXO3 and p27 overexpression. Decrease of peroxiredoxin-1 | [24] |
| Gastric cancer | Tomentosin from 5 to 30 μM for 24 h | AGS cell line | Apoptosis via mitochondria-mediated signaling pathway induced by increase of ROS. PCNA and Cyclin D1 downregulation. BAX overexpression and BCL-2 downregulation. Inhibition of inflammation | [51] |
| Hepatocellular carcinoma | Tomentosin 0,10, 20 and 40 μM for 24 and 48 h | HepG2 and Huh7 cell lines | G2/M phase cell cycle arrest through p27 overexpression regulated by upregulation of FOXO3 | [50] |
| Leukemia | Tomentosin 0–25 μM for up to 48 h | MOLT-4 cell line | Apoptosis via mitochondria-mediated signaling pathway induced by increase of ROS. Suppression of NF-κB and proinflammatory cytokines. mTOR/PI3K/AKT pathway activation | [54] |
| Burkitt lymphoma | Tomentosin 50, 25, 12.5, 6.25, 3.125, 1.56 and 0.75 μM for 24 h | Raji cell line | Cell proliferation inhibition and cell apoptosis induction. Induction of apoptosis by upregulation of the PERK/eIF2a/ATF4/DDIT3 pathway | [52] |
| Multiple myeloma | Tomentosin 50, 25, 12.5, 6.25, 3.125, 1.56 and 0.75 μM for 24 h | RPMI-8226 cell line | Cell proliferation inhibition and cell apoptosis induction. Downregulation of anti-apoptotic genes such as BCL2A1 and CDKN1A and upregulation of the proapoptotic PMAIP1 gene | [53] |
Figure 1.
Molecular mechanisms of apoptosis induced by tomentosin and inuviscolide in neoplastic cells.
4. Conclusions and Future Prospects
Despite the advances in the development of innovative antineoplastic drugs, cancer remains one of the most important causes of death worldwide. The principal pitfall of successful cancer treatment is the intrinsic or acquired resistance to therapeutic agents. Among the conventional treatment options, chemotherapy is most often applied to different types of cancer. Several chemotherapeutic molecules induce cell cycle arrest but not apoptosis, permitting neoplastic cells to repair their damaged DNA and therefore potentially limit treatment effectiveness [26]. These approaches can be associated with variable response rates and potentially severe side effects, which may impair quality of life and often favor cancer progression. Thus, the development of more effective and/or synergistic anticancer agents that can overcome resistance and are not associated with severe side effects has become an extremely important issue. Recently, drugs belonging to the glycolytic inhibitor category have attracted considerable attention due to their capacity of inhibiting aerobic glycolysis which represents an attractive strategy to specifically kill tumor cells. Interestingly, small molecule alkylating agents have shown great potential, such as 3-bromopyruvate as a promising antitumor drug by blocking tumor energy metabolism [56], as well as lonidamine that may be very promising as a sensitizer of tumors to chemotherapeutic agents and physical therapies [57].
This review describes recent advances in our understanding of the potential anticancer activity of I. viscosa and its natural biologically active compounds, such as tomentosin and inuviscolide. Moreover, it provides a detailed description of the molecular mechanisms of action in the context of in vitro experiments. Interestingly, cytotoxic effects against human cancer cell lines induced by I. viscosa and SLs are characterized by cell growth inhibition and apoptosis induction, related to the decrease in mitochondrial membrane potential and increase in intracellular ROS production. Various molecular mechanisms are responsible for the activation of apoptosis. In particular, cell treatment induces activation of pathways known to lead to cellular death, downregulation of anti-apoptotic genes such as BCL2A1 and CDKN1A, and upregulation of the proapoptotic PMAIP1 gene. In addition, SLs can target multiple signaling pathways, such as NF-κB, PIK/AKT/mTOR, and MAPK, which control apoptosis, growth, proliferation, migration, and invasion. In vivo tests within preclinical animal studies and subsequently in the context of controlled clinical trials should also be encouraged to shed further light on the exact molecular mechanisms by which tomentosin induces pharmacological effects in human cancer and to assess possible side effects. These preliminary steps could allow the development of potential natural products with limited toxicity and relevant antitumor activity, making them available among the therapeutic armamentarium offered to patients affected by solid and hematologic tumors.
Author Contributions
Conceptualization, M.R.D.M. and R.M.; writing the manuscript, M.R.D.M.; collection of information and data curation, G.P., P.V., G.G., C.A., G.L. and D.C.; supervision and review, M.R.M., C.F. and L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partly supported by grants from Fondazione di Sardegna 2022–2023, Italy, and “Fondo di Ateneo per la Ricerca 2020” from the University of Sassari, Italy. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Çelik T.A., Aslantürk Ö.S. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J. Biomed. Biotechnol. 2010;2010:189252. doi: 10.1155/2010/189252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G.W., Qin J.J., Cheng X.R., Shen Y.H., Shan L., Jin H.Z., Zhang W.D. Inula sesquiterpenoids: Structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs. 2014;23:317–345. doi: 10.1517/13543784.2014.868882. [DOI] [PubMed] [Google Scholar]
- 3.Barbetti P., Chiappini I., Fardella G., Menghini A. A New Eudesmane Acid from Dittrichia (Inula) viscosa. Planta Med. 1985;51:471. doi: 10.1055/s-2007-969564. [DOI] [PubMed] [Google Scholar]
- 4.Lauro L., Rolih C. Observations and research on an extract of Inula viscosa Ait. Boll. Soc. Ital. Biol Sper. 1990;66:829–834. [PubMed] [Google Scholar]
- 5.Lev E., Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000;72:191–205. doi: 10.1016/S0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 6.Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009;123:45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Yaniv Z., Dafni A., Friedman J., Palevitch D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987;19:145–151. doi: 10.1016/0378-8741(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 8.Hernández V., Recio M.C., Máñez S., Giner R.M., Ríos J.L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007;81:480–488. doi: 10.1016/j.lfs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Máñez S., Hernández V., Giner R.M., Ríos J.L., Recio M.d.C. Inhibition of pro-inflammatory enzymes by inuviscolide, a sesquiterpene lactone from Inula viscosa. Fitoterapia. 2007;78:329–331. doi: 10.1016/j.fitote.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Khan A.L., Hussain J., Hamayun M., Gilani S.A., Ahmad S., Rehman G., Kim Y.H., Kang S.M., Lee I.J. Secondary Metabolites from Inula britannica L. and Their Biological Activities. Molecules. 2010;15:1562. doi: 10.3390/molecules15031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheyar-Kraouche N., da Silva A.B., Serra A.T., Bedjou F., Bronze M.R. Characterization by liquid chromatography-mass spectrometry and antioxidant activity of an ethanolic extract of Inula viscosa leaves. J. Pharm. Biomed. Anal. 2018;156:297–306. doi: 10.1016/j.jpba.2018.04.047. [DOI] [PubMed] [Google Scholar]
- 12.Rozenblat S., Grossman S., Bergman M., Gottlieb H., Cohen Y., Dovrat S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 2008;75:369–382. doi: 10.1016/j.bcp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Benbacer L., Merghoub N., El Btaouri H., Gmouh S., Attaleb M., Morjani H., Amzazi S., El Mzibri M. Topics on Cervical Cancer with an Advocacy for Prevention. IntechOpen; London, UK: 2012. Antiproliferative Effect and Induction of Apoptosis by Inula viscosa L. and Retama monosperma L. Extracts in Human Cervical Cancer Cells. [DOI] [Google Scholar]
- 14.Messaoudi M., Chahmi N., El-Mzibri M., Gmouh S., Amzazi S., Benbacer L., El-Hassouni M. Cytotoxic Effect and Chemical Composition of Inula viscosa from Three Different Regions of Morocco. Eur. J. Med. Plants. 2016;16:1–9. doi: 10.9734/EJMP/2016/28340. [DOI] [Google Scholar]
- 15.Jafari N., Zargar S.J., Delnavazi M.-R., Yassa N. Cell Cycle Arrest and Apoptosis Induction of Phloroacetophenone Glycosides and Caffeoylquinic Acid Derivatives in Gastric Adenocarcinoma (AGS) Cells. Anticancer Agents Med. Chem. 2018;18:610–616. doi: 10.2174/1871520618666171219121449. [DOI] [PubMed] [Google Scholar]
- 16.Shafabakhsh R., Asemi Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019;12:55. doi: 10.1186/s13048-019-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo P.C., Liu H.F., Chao J.I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004;279:55875–55885. doi: 10.1074/jbc.M407985200. [DOI] [PubMed] [Google Scholar]
- 18.Ong C.S., Tran E., Nguyen T.T.T., Ong C.K., Lee S.K., Lee J.J., Ng C.P., Leong C., Huynh H. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol. Rep. 2004;11:727–733. doi: 10.3892/or.11.3.727. [DOI] [PubMed] [Google Scholar]
- 19.Sharmila G., Bhat F.A., Arunkumar R., Elumalai P., Raja Singh P., Senthilkumar K., Arunakaran J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014;33:718–726. doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Li Q., Ren F.Q., Yang C.L., Zhou L.M., Liu Y.Y., Xiao J., Zhu L., Wang Z.G. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac. J. Cancer Prev. 2015;16:3035–3042. doi: 10.7314/APJCP.2015.16.7.3035. [DOI] [PubMed] [Google Scholar]
- 21.Xiang P., Guo X., Han Y.Y., Gao J.M., Tang J.J. Cytotoxic and Pro-apoptotic Activities of Sesquiterpene Lactones from Inula britannica. Nat. Prod. Commun. 2016;11:7–10. doi: 10.1177/1934578X1601100103. [DOI] [PubMed] [Google Scholar]
- 22.Quintana J., Estévez F. Recent Advances on Cytotoxic Sesquiterpene Lactones. Curr. Pharm. Des. 2019;24:4355–4361. doi: 10.2174/1381612825666190119114323. [DOI] [PubMed] [Google Scholar]
- 23.Chadwick M., Trewin H., Gawthrop F., Wagstaff C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013;14:12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C.M., Lee J., Nam M.J., Choi Y.S., Park S.H. Tomentosin displays anti-carcinogenic effect in human osteosarcoma MG-63 cells via the induction of intracellular reactive oxygen species. Int. J. Mol. Sci. 2019;20:1508. doi: 10.3390/ijms20061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Yang X., Liu Y., Gong N., Yao W., Chen P., Qin J., Jin H., Li J., Chu R., et al. Japonicone A Suppresses Growth of Burkitt Lymphoma Cells through Its Effect on NF-κB. Clin. Cancer Res. 2013;19:2917–2928. doi: 10.1158/1078-0432.CCR-12-3258. [DOI] [PubMed] [Google Scholar]
- 26.Merghoub N., El Btaouri H., Benbacer L., Gmouh S., Trentesaux C., Brassart B., Attaleb M., Madoulet C., Wenner T., Amzazi S., et al. Tomentosin Induces Telomere Shortening and Caspase-Dependant Apoptosis in Cervical Cancer Cells. J. Cell. Biochem. 2017;118:1689–1698. doi: 10.1002/jcb.25826. [DOI] [PubMed] [Google Scholar]
- 27.Cardellina J.H., Fuller R.W., Gamble W.R., Westergaard C., Boswell J., Munro M.H.G., Currens M., Boyd M.R. Bioassay Methods in Natural Product Research and Drug Development. Springer; Berlin/Heidelberg, Germany: 1999. Evolving Strategies for the Selection, Dereplication and Prioritization of Antitumor and HIV-Inhibitory Natural Products Extracts; pp. 25–35. [DOI] [Google Scholar]
- 28.Merghoub N., El Btaouri H., Benbacer L., Gmouh S., Trentesaux C., Brassart B., Terryn C., Attaleb M., Madoulet C., Benjouad A., et al. Inula viscosa extracts induces telomere shortening and apoptosis in cancer cells and overcome drug resistance. Nutr. Cancer. 2016;68:131–143. doi: 10.1080/01635581.2016.1115105. [DOI] [PubMed] [Google Scholar]
- 29.Chahmi N., Anissi J., Jennan S., Farah A., Sendide K., El Hassouni M. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac. J. Trop. Biomed. 2015;5:228–233. doi: 10.1016/S2221-1691(15)30010-1. [DOI] [Google Scholar]
- 30.Talib W.H., Mahasneh A.M. Antiproliferative Activity of Plant Extracts Used against Cancer in Traditional Medicine. Sci. Pharm. 2010;78:33–46. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hepokur C., Budak Y., Karayel H.B., Selvì B., Yaylim İ. Investigation of Cytotoxic Effects of Inula viscosa Extract. Cumhur. Sci. J. 2019;40:578–582. doi: 10.17776/csj.437993. [DOI] [Google Scholar]
- 32.Bar-Shalom R., Bergman M., Grossman S., Azzam N., Sharvit L., Fares F. Inula viscosa Extract Inhibits Growth of Colorectal Cancer Cells in vitro and in vivo Through Induction of Apoptosis. Front. Oncol. 2019;9:227. doi: 10.3389/fonc.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virdis P., Migheli R., Galleri G., Fancello S., Cadoni M.P.L., Pintore G., Petretto G.L., Marchesi I., Fiorentino F.P., Di Francesco A., et al. Antiproliferative and proapoptotic effects of Inula viscosa extract on Burkitt lymphoma cell line. Tumor Biol. 2020;42:1010428319901061. doi: 10.1177/1010428319901061. [DOI] [PubMed] [Google Scholar]
- 34.Colak D.K., Egeli U., Eryilmaz I.E., Aybastier O., Malyer H., Cecener G., Tunca B. The Anticancer Effect of Inula viscosa Methanol Extract by miRNAs’ Re-regulation: An in vitro Study on Human Malignant Melanoma Cells. Nutr. Cancer. 2021;74:211–224. doi: 10.1080/01635581.2020.1869791. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Schwesinger C. The ubiquitin–proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 2019;15:393–411. doi: 10.1038/s41581-019-0148-1. [DOI] [PubMed] [Google Scholar]
- 36.Koper-Lenkiewicz O.M., Kamińska J., Reszeć J., Dymicka-Piekarska V., Ostrowska H., Karpińska M., Matowicka-Karna J., Tylicka M. Elevated plasma 20S proteasome chymotrypsin-like activity is correlated with IL-8 levels and associated with an increased risk of death in glial brain tumor patients. PLoS ONE. 2020;15:e0238406. doi: 10.1371/journal.pone.0238406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voutsadakis I.A. Proteasome expression and activity in cancer and cancer stem cells. Tumor Biol. 2017;39:1010428317692248. doi: 10.1177/1010428317692248. [DOI] [PubMed] [Google Scholar]
- 38.Berryman K., Buhimschi C.S., Zhao G., Axe M., Locke M., Buhimschi I.A. Proteasome Levels and Activity in Pregnancies Complicated by Severe Preeclampsia and Hemolysis, Elevated Liver Enzymes, and Thrombocytopenia (HELLP) Syndrome. Hypertens. 2019;73:1308–1318. doi: 10.1161/HYPERTENSIONAHA.118.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Yaagoubi O.M., Lahmadi A., Bouyahya A., Filali H., Samaki H., El Antri S., Aboudkhil S. Antitumor effect of Inula viscosa extracts on DMBA-induced skin carcinoma are mediated by proteasome inhibition. BioMed Res. Int. 2021;2021:6687589. doi: 10.1155/2021/6687589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belayachi L., Aceves-Luquero C., Merghoub N., Bakri Y., de Mattos S.F., Amzazi S., Villalonga P. Screening of North African Medicinal Plant Extracts for Cytotoxic Activity Against Tumor Cell Lines. Eur. J. Med. Plants. 2013;3:310–332. doi: 10.9734/EJMP/2013/3403. [DOI] [Google Scholar]
- 41.Schmidt T.J. Toxic activities of sesquiterpene lactones: Structural and biochemical aspects. Curr. Org. Chem. 1999;3:577–608. [Google Scholar]
- 42.Woynarowski J.M., Konopa J. Inhibition of DNA Biosynthesis in HeLa Cells by Cytotoxic and Antitumor Sesquiterpene Lactones. Mol. Pharmacol. 1981;19:97–102. [PubMed] [Google Scholar]
- 43.Ghantous A., Gali-Muhtasib H., Vuorela H., Saliba N.A., Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Grossman D., McNiff J.M., Li F., Altieri D.C. Expression and targeting of the apoptosis inhibitor, survivin, in human melanoma. J. Investig. Dermatol. 1999;113:1076–1081. doi: 10.1046/j.1523-1747.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 45.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia G., Wang Q., Wang R., Deng D., Xue L., Shao N., Zhang Y., Xia X., Zhi F., Yang Y. Tubeimoside-1 induces glioma apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome C/Caspase-3 pathway. OncoTargets Ther. 2015;8:303. doi: 10.2147/OTT.S76063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galati G., O’Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 48.Li H.Y., Zhang J., Sun L.L., Li B.H., Gao H.L., Xie T., Zhang N., Ye Z.M. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015;6:e1604. doi: 10.1038/cddis.2014.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang P., Zhang Y.H., Zheng X.W., Liu Y.J., Zhang H., Fang L., Zhang Y.W., Yang C., Islam K., Wang C., et al. Phenylarsine oxide (PAO) induces apoptosis in HepG2 cells via ROS-mediated mitochondria and ER-stress dependent signaling pathways. Metallomics. 2017;9:1756–1764. doi: 10.1039/C7MT00179G. [DOI] [PubMed] [Google Scholar]
- 50.Yu S.H., Lee C.M., Ha S.H., Lee J., Jang K.Y., Park S.H. Induction of cell cycle arrest and apoptosis by tomentosin in hepatocellular carcinoma HepG2 and Huh7 cells. Hum. Exp. Toxicol. 2021;40:231–244. doi: 10.1177/0960327120943935. [DOI] [PubMed] [Google Scholar]
- 51.Yang H., Zhao H., Dong X., Yang Z., Chang W. Tomentosin induces apoptotic pathway by blocking inflammatory mediators via modulation of cell proteins in AGS gastric cancer cell line. J. Biochem. Mol. Toxicol. 2020;34:e22501. doi: 10.1002/jbt.22501. [DOI] [PubMed] [Google Scholar]
- 52.Virdis P., Marchesi I., Fiorentino F.P., Migheli R., Sanna L., Bordoni V., Pintore G., Galleri G., Muroni M.R., Bagella L., et al. Tomentosin a sesquiterpene lactone induces antiproliferative and proapoptotic effects in human Burkitt lymphoma by deregulation of anti- and pro-apoptotic genes. Life. 2021;11:1128. doi: 10.3390/life11111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virdis P., Migheli R., Bordoni V., Fiorentino F.P., Sanna L., Marchesi I., Pintore G., Galleri G., Muroni M.R., Bagella L., et al. Clarifying the molecular mechanism of tomentosin-induced antiproliferative and proapoptotic effects in human multiple myeloma via gene expression profile and genetic interaction network analysis. Int. J. Mol. Med. 2021;48:213. doi: 10.3892/ijmm.2021.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L., Xie J., Almoallim H.S., Alharbi S.A., Chen Y. Tomentosin inhibits cell proliferation and induces apoptosis in MOLT-4 leukemia cancer cells through the inhibition of mTOR/PI3K/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2021;35:e22719. doi: 10.1002/jbt.22719. [DOI] [PubMed] [Google Scholar]
- 55.Güçlü E., Çınar Ayan İ., Dursun H.G., Vural H. Tomentosin induces apoptosis in pancreatic cancer cells through increasing reactive oxygen species and decreasing mitochondrial membrane potential. Toxicol. In Vitro. 2022;84:105458. doi: 10.1016/j.tiv.2022.105458. [DOI] [PubMed] [Google Scholar]
- 56.Fan T., Sun G., Sun X., Zhao L., Zhong R., Peng Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers. 2019;11:317. doi: 10.3390/cancers11030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y., Sun G., Sun X., Li F., Zhao L., Zhong R., Peng Y. The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers. 2020;12:3332. doi: 10.3390/cancers12113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.