Abstract
We demonstrate that a mucoid, alginate-producing strain of Pseudomonas aeruginosa isolated from the lungs of a cystic fibrosis (CF) patient secretes multiple enzymes with nucleoside diphosphate kinase (Ndk), ATPase, adenylate kinase, 5′-nucleotidase, and ATP-modifying enzymatic activities. The secretion is triggered at high cell density and in complex media but is greatly reduced when the mucoid cells are grown in mineral salts media or in presence of 5.0 mM Ca2+ or Mg2+. Interestingly, the secretion is triggered primarily in the mucoid CF isolate of strain 8821M (or in strain FRD1) but not in a nonmucoid laboratory strain, PAO1. The purified secreted Ndk shows 100% match in its N-terminal amino acid sequence with that of purified intracellular Ndk and demonstrates similar enzymatic properties. The N-terminal sequence of the purified ATPase isolated from an ndk knockout mutant shows its identity with that of the heat shock chaperonin Hsp60. During fractionation, the flowthrough fraction from a Mono Q column demonstrates the presence of 5′-nucleotidase, adenylate kinase, and a putative ATP reductase activity. These fractions demonstrate high cytotoxic activities for murine peritoneal primary macrophages which can be further stimulated in the presence of ATP or inhibited by pretreatment of macrophages with oxidized ATP (oATP). The cytotoxicity associated with ATP-induced stimulation is believed to be due to activation of macrophage surface-associated P2Z (P2X7) receptors, which are one of the purinergic receptors responsible for pore formation on macrophage membrane. Blocking of these receptors by pretreatment with oATP blocks ATP-induced macrophage cell death. Thus mucoid P. aeruginosa cells elaborate enzymes that modulate the external ATP levels of macrophages, thereby modulating macrophage cell death through P2Z receptor activation. Evidence for the presence of secreted cytotoxic agents that act independently of P2Z receptor activation is also presented.
Pseudomonas aeruginosa is a major pathogen in the lungs of cystic fibrosis (CF) patients. During the initial infection, the infecting P. aeruginosa cells are usually nonmucoid, but on prolonged infections, the cells turn mucoid due to the production of an exopolysaccharide called alginate (29). Encapsulation of the nonmucoid cells by alginate allows the cells to become resistant to antibiotics as well as to phagocytosis, but exactly how the mucoid cells are better able to evade the host immune system is not clearly understood (12, 21, 25). It is known that during transition to mucoidy in the CF lung, the mucoid cells produce new gene products such as AlgE that are absent in the nonmucoid cells. AlgE is known to be associated with the outer membrane and is believed to act as a nonporin channel for alginate secretion (5, 26). It is also known that transition to mucoidy, which is secretion of alginate, somehow interferes in the efficient secretion of virulence factors such as exotoxin A and elastase, etc. (24, 32), and indeed, in contrast to nonmucoid cells that secrete essentially all of the elastase to the outside medium, mucoid cells have been shown to retain a good part of elastase in the periplasm and to secrete only part of it (16). Since the enzyme nucleoside diphosphate kinase (Ndk) has been shown to be important for alginate synthesis (31) and must be cleaved by elastase to generate a 12-kDa truncated form that allows synthesis of large amounts of GTP for alginate synthesis (3, 4), the role of this enzyme in P. aeruginosa virulence is well established (4). We now report the secretion of this enzyme, as well as other ATP-utilizing enzymes, by mucoid P. aeruginosa which demonstrates a new role of these enzymes in the pathogenicity of mucoid P. aeruginosa in the CF lung.
MATERIALS AND METHODS
Bacterial strains.
P. aeruginosa strains were maintained in Luria-Bertani and Pseudomonas isolation agar (Difco) media. All strains were grown at 37°C in TYE (10 g of tryptone, 5 g of yeast extract per liter) broth.
Composition of modified minimal media to study Ndk and ATPase secretion.
P. aeruginosa 8821M (21) was grown in morpholine propanesulfonic acid (MOPS) minimal medium (MOPSmmI) containing 10 mM MOPS buffer (pH 7.0), 0.1 mM potassium phosphate buffer (pH 7.0), 4 g of succinate per liter, 51 mM (NH4)2SO4, 1 μm of FeSO4, 0.5 mM MgSO4, 2 mM l-histidine, and 0.1 g of yeast extract per liter to an optical density at 600 nm (OD600) of 0.4 to 0.5, harvested, and resuspended at OD600 of 2.0 in MOPSmmII preheated at 37°C. MOPSmmII is MOPSmmI lacking FeSO4 and MgSO4; this medium did not allow further growth of cells but promoted secretion of Ndk and ATPase. Cells suspended in MOPSmmII at an OD600 of 2.0 were treated with water (control) or 1 mg of various eukaryotic proteins (as specified in Fig. 2 and 3, for example) per ml. Supernatant samples were taken at various times during incubation at 37°C and assayed for Ndk and ATPase activities.
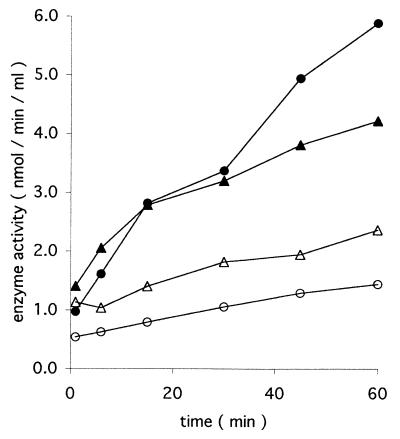
FIG. 2.
Stimulation of secretion of Ndk (circles) and ATPase (triangles) by κ-casein. P. aeruginosa 8821M was grown in minimal medium (MOPSmmI), harvested, and resuspended at an OD600 of 2.0 in MOPSmmII. The suspension was then divided into two parts. One was supplemented with κ-casein at 1 mg/ml (filled symbols), while the other was supplemented with an equal amount of water (open symbols). Supernatant fractions were collected at various times during incubation at 37°C as specified and assayed for Ndk and ATPase activities.
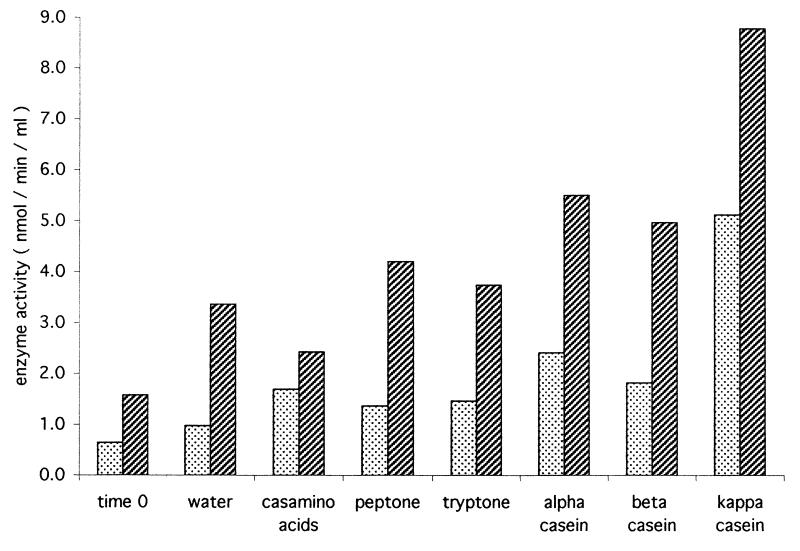
FIG. 3.
Stimulation of secretion of Ndk (stippled bars) and ATPase (hatched bars) in the presence and absence of eukaryotic proteins. P. aeruginosa 8821M was grown in minimal medium (MOPSmmI), harvested, and resuspended at an OD600 of 2.0 in MOPSmmII. A supernatant sample was collected (time zero), and the suspension was then supplemented with water as a control or with per milliliter 1 mg of Casamino Acids, peptone, tryptone, α-, β-, or κ-casein. Supernatant fractions were collected after 60 min of incubation at 37°C and assayed for Ndk and ATPase activities.
Purification of extracellular Ndk from P. aeruginosa 8821M.
P. aeruginosa 8821M cells (2 liters) were grown at 37°C in Luria broth to an OD600 of around 2.0. The cells were removed by centrifugation, and the supernatant was subjected to precipitation with 70% saturation of ammonium sulfate at 4°C. The precipitate was resuspended in 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2 (TM buffer), dialyzed overnight against the same buffer, and then centrifuged. The supernatant was loaded onto a Blue-Sepharose fast-performance liquid chromatography (FPLC) column (2.6 by 25 cm) equilibrated with TM buffer. The protein was eluted by an increasing gradient (0 to 3 M KCl) in TM buffer, and active fractions were collected (at 2 M KCl) and concentrated by using a Centricon 10 concentrator to 1 ml. This concentrate was loaded onto a cyclic AMP (cAMP)-agarose column (1.2 by 5.4 cm) equilibrated with 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2, 25 mM KCl, and 0.8 mM dithiothreitol (TMD buffer). The column was washed with TMD buffer until all nonbound protein was removed; then, nonspecifically bound proteins were eluted with TMD buffer containing 1 M KCl, and the column was washed with TMD buffer. The elution of protein containing Ndk activity was done with 2 mM cAMP in TMD buffer. The fractions were analyzed for Ndk activity as described previously (30, 31). The active fractions were concentrated and subjected to 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in Tris-tricine running buffer followed by transfer to a polyvinylidene difluoride transfer protein membrane (Schleicher & Schuell) for N-terminal sequencing of the band at 16 kDa. An automated Edman degradation in an AB1 477A protein sequencer (Applied Biosystems) was used to sequence NH2-terminal amino acids of the protein.
Construction of an ndk knock-out mutant in P. aeruginosa 8821M.
Plasmid pSAK1 harboring a 1.2-kb SphI-SstI fragment containing the ndk gene as well as the upstream region (31) was used. The chloramphenicol cassette (27) was amplified by PCR. Plasmid pACYC184 was used as the template, and PCR was performed with specific primers designed to generate RsrII sites at the ends of the chloramphenicol cassette. The 720-bp PCR product was cloned into the pGEMTeasy vector (Promega), and chloramphenicol-resistant colonies were selected. The colonies were tested for the presence of the PCR product by digestion of the plasmid DNA with RsrII. The fragment harboring the chloramphenicol cassette was gel purified. Plasmid pSAK1 was digested with RsrII and gel purified. The RsrII-digested and gel-purified chloramphenicol cassette fragment was then cloned into the RsrII site of plasmid pSAK1. Chloramphenicol-resistant colonies were selected, and the presence of the chloramphenicol cassette was confirmed. Suicide vector pSAK9 DNA containing the inactivated ndk gene was used to electroporate P. aeruginosa 8821M in an IBI electroporator. Putative mutant colonies were selected for chloramphenicol (Cm) resistance and carbenicillin sensitivity. The ndk::Cm strain was confirmed for double crossover event on the chromosome by Southern hybridization. For this purpose, the whole 432-bp ndk gene was used as a probe. The DNA probe for Southern hybridization was internally labeled with [α-32P]dCTP by using the Mega prime DNA labeling system (Amersham) as described by the manufacturer. Southern blotting was performed by capillary transfer of DNA fragments to positively charged nylon membranes (Hybond-N+). Membranes were hybridized with the DNA probe in Rapid-hyb buffer (Amersham) at 65°C and washed under high-stringency conditions. This mutant ndk::Cm strain was tested for absence of intra- and extracellular Ndk activity as described earlier.
Purification of ATPase from the culture filtrate of ndk::Cm mutant of mucoid P. aeruginosa.
The ndk knockout mutant of P. aeruginosa 8821M cells (4 liters) were grown at 37°C in TYE broth to an OD600 of around 1.2. The cells were removed by centrifugation, and the supernatant was concentrated to 50 ml by ultrafiltration with a YM10 Amicon membrane followed by exchanging TYE broth with 5 mM potassium phosphate buffer (pH 7.0). Concentrated supernatant was loaded on a hydroxyapatite column (2.6 by 8.0 cm) equilibrated with the same buffer. The protein with ATPase activity was eluted with 100 mM potassium phosphate (pH 7.0) buffer. The active fractions were pooled and concentrated by ultrafiltration through a YM10 Amicon membrane followed by exchange of the potassium phosphate buffer with 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2, 0.8 mM dithiothreitol, and 25 mM KCl (TMD buffer). Concentrated fractions were loaded onto an ATP-agarose column (0.5 by 8.0 cm) equilibrated with the same buffer. After the sample was loaded, the column was washed with the same buffer followed by the buffer with 1 M KCl instead of 25 mM KCl to elute nonspecifically bound proteins. The column was again washed with the buffer containing 25 mM KCl. The protein with ATPase activity was finally eluted with 2 mM ATP in the same buffer. All fractions collected in the ATP elution step were concentrated with Centricon 10 concentrators followed by exchange of the eluting buffer with 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2 (TM buffer). ATPase activity in the fractions was detected after removal of free ATP during the last step of concentration. The purified protein with ATPase activity was subjected to SDS–12% PAGE followed by transfer to a polyvinylidene difluoride transfer protein membrane for N-terminal sequencing of the predominant band (60 kDa). Automated Edman degradation in an AB1 477A protein sequencer (Applied Biosystems) was used to sequence NH2-terminal amino acids of the protein.
Column chromatography of secreted enzymes and determination of their cytotoxic activities.
The ndk::Cm mutant P. aeruginosa cells (4 liters) were grown at 37°C in TYE broth to an OD600 of around 1.2. The cells were removed by centrifugation, and the supernatant was concentrated to 50 ml by ultrafiltration through a YM10 Amicon membrane followed by exchange of TYE broth with 5 mM potassium phosphate buffer (pH 7.0). Concentrated supernatant was loaded on hydroxyapatite column (26 by 80 mm) equilibrated with the same buffer. The proteins with nucleotidase and adenylate kinase activities giving rise to [32P]ADP from [γ-32P]ATP activity did not bind to the hydroxyapatite column; thus, the active fractions were pooled from flowthrough and concentrated by ultrafiltration through a YM10 Amicon membrane followed by exchange of the potassium Phosphate buffer with TMD buffer. Concentrated fractions were loaded on an ATP-agarose column (Sigma) equilibrated with the same buffer. Similar to that in the hydroxyapatite column, the protein(s) with [32P]ADP-forming activity did not bind to the ATP agarose; thus, the active fractions were pooled from flowthrough and concentrated by ultrafiltration through a YM10 Amicon membrane followed by exchange of the loading buffer with TM buffer. Concentrated fractions were loaded on Mono Q column equilibrated with the same buffer, and active fractions were collected from flowthrough. The flowthrough fractions from the ATP agarose and Mono Q columns were tested for cytotoxicity against murine peritoneal macrophages as described below.
Animal and macrophage cultures.
BALB/c AKR/J mice were obtained from Jackson Laboratory (Bar Harbor, Maine) and maintained in the Biological Laboratory of the University of Illinois at Chicago. Mice were sex matched and used at 6 to 10 week of age. Resident peritoneal macrophages were harvested by lavage of the peritoneal cavities of mice with 5 ml of cold incomplete Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO-BRL, Grand Island, N.Y.). The cells were collected by centrifugation, washed, and resuspended at 0.5 × 106 cells/ml in complete DMEM containing l-glutamine, buffered with 10 mM HEPES, and supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Macrophages were allowed to adhere to tissue culture dishes for 1 h at 37°C in 5% CO2 before gentle rinsing to remove nonadherent cells.
Cytotoxicity assay (LDH Release).
Macrophages were plated in 96-well plates (Becton Dickinson Labware, Lincoln Park, N.J.) at a final concentration of 105 cells/well in 200 μl of complete DMEM and were activated with 50 ng of lipopolysaccharide (LPS) (Sigma Chemical Co., St. Louis, Mo.) per ml for 24 h. LPS-primed cells were washed and incubated for 4 h in presence of 2 mM ATP with or without supernatant samples from strains 8821M and PAO1 or the purified ATP-utilizing enzymes (ATPase, Ndk) or the Mono Q column effluent from strain 8821M. At the end of each incubation, 50 μl of the supernatants was transferred to 96-well plates and lactate dehydrogenase (LDH) activity was determined with CytoTox 96 assay kit (Promega, Madison, Wis.). Triplicate samples were tested for each datum point. Prior to challenge with macrophages, the reaction of ATP-utilizing enzymes with nucleotides was allowed to proceed for 2 to 4 h. In experiments with the P2Z antagonist, periodate oxidized ATP (oATP), macrophages were pretreated with 1 mM oATP for 2 h prior to cytotoxicity assay with ATP- and ATP-utilizing enzymes.
Microscopy.
Macrophages (106 cells/ml) were cultured on 13-mm plastic Thermonax coverslips (Nunc, Naperville, Ill.) within 24-well plates (Becton Dickinson Labware) with a volume of 1 ml/well. After 2 h of adherence, coverslips were washed with warm medium to remove nonadherent cells and incubated at 37°C in 5% CO2. Phase-contrast pictures were taken with an inverted microscope (Nikon DIAPHOT 200) equipped with a ×40 objective.
Statistics.
Data were expressed as the means ± standard deviations wherever triplicate determinants were available. Comparison between these groups was performed by Student’s t test for independent samples. Significance was established at a P value of <0.05.
RESULTS
Secretion of several ATP-utilizing enzymes including Ndk by mucoid CF isolate of P. aeruginosa 8821M.
Ndk is a highly conserved enzyme that allows maintenance of cellular nucleoside triphosphate (NTP) pools by phosphotransfer from ATP or GTP to any nucleoside diphosphates (NDPs) or their deoxyderivatives to generate NTPs or deoxy-NTPs (dNTPs); Ndk utilizes either ATP, GTP, or any of the other NTPs as a phosphodonor to any NDPs or dNDPs as recipients of the terminal phosphate. As such, this enzyme generates the precursors of cellular RNA and DNA, as well as various signalling NTP molecules that modulate bacterial growth, virulence, and cell signalling (3). We previously reported that in mucoid P. aeruginosa 8830, Ndk exists in two forms: a 16-kDa cytoplasmic form that predominates in the log phase and a 12-kDa truncated, membrane-associated form that predominantly generates GTP through complex formation with various proteins at the stationary phase (4, 30, 31). We recently observed that Ndk, along with an ATPase activity that corresponds to DnaK, are secreted by Mycobacterium bovis BCG to the outside medium (33). We were, therefore, curious to see if Ndk might also be secreted by P. aeruginosa. The results in Fig. 1 clearly show that the supernatant growth medium of the TYE broth-grown P. aeruginosa 8821M, a mucoid CF isolate, shows the presence of both Ndk and ATPase activity (Fig. 1, lanes 2 to 5). This activity is manifested in the production of GTP, CTP, and UTP when the supernatant samples are incubated with [γ-32P]ATP and a mixture of 1 mM each of GDP, CDP, and UDP. In absence of an exogenous supply of GDP, CDP, and UDP, the levels of GTP, CTP, and UTP are greatly reduced (lanes 10 to 13). Commensurate with the reduction of the levels of these NTPs, the level of inorganic orthophosphate (Pi), released from [γ-32P]ATP due to an ATPase action, is greatly increased (compare lanes 10 to 13 with lanes 2 to 5), suggesting that an ATPase activity is also present in the supernatant, and in absence of Ndk activity because of a lack of NDPs, more [γ-32P]ATP becomes available for the ATPase action. It should be noted that when NDPs are limiting, resulting in reduced NTP formation by the supernatant fraction, a major band that runs between UTP and CTP also appears on the thin-layer chromatography (TLC) plates (lanes 10 to 12). This band is composed of ADP. Thus in the absence of NDPs, secreted Ndk activity is minimal, but because of [γ-32P]ATP availability, both the ATPase, resulting in 32Pi release, and some enzymatic activity giving rise to radioactive ADP from [γ-32P]ATP become prominent. The formation of radioactive ADP from [γ-32P]ATP by the secreted enzymes will be discussed later.
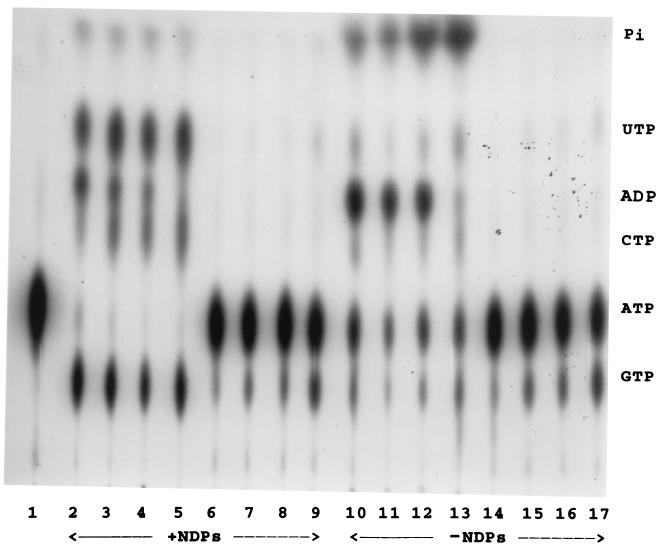
FIG. 1.
Secretion of ATP-utilizing enzymes during growth of mucoid strain 8821M in TYE broth or in mineral salts medium (27). P. aeruginosa 8821M was inoculated into the two media and grown for up to 20 h; at various times, aliquots were withdrawn, centrifuged, and filtered through 0.22 μm-pore-size filters, and the filtrates were assayed for ATP-utilizing activities by using 0.06 μM [γ-32P]ATP in the presence or absence of 1.0 mM each of NDPs (CDP, GDP, and UDP). After a 5- to 10-min incubation, the resultant NTPs were separated by TLC and radioautographed, as described previously (4, 30). Lanes: 1, [γ-32P]ATP control; 2 to 5, growth medium filtrate of strain 8821M grown in TYE broth for 13, 15, 16.5, and 19 h, respectively. Growth up to 10 or 11 h resulting in an OD600 of less than 1.9 showed very little secretion (data not shown). Lanes 6 to 9, growth medium filtrate of strain 8821M grown in mineral salts medium (27) for 13, 15, 16.5, and 19 h, respectively. The assays for lanes 2 to 9 were conducted in presence of NDPs (1 mM each of CDP, GDP, and UDP). Lanes 10 to 13 are the same as lanes 2 to 5, and lanes 14 to 17 are the same as lanes 6 to 9 except the assays were conducted in absence of NDPs. When the assays were conducted in the absence of NDPs, so that very little NTPs were produced by Ndk, a prominent band of ADP appeared between UTP and CTP (lanes 10 to 13).
In order to examine the kinetics of secretion of these enzymes, we grew strain 8821M in TYE broth at 37°C, took samples at various time periods corresponding to early log (3 to 4 h), mid-log (5 to 6 h), and late log to stationary phase (12 h and beyond), filtered them through 0.22-μm-pore-size filters, and examined the filtrates directly for the enzymatic activities, by using [γ-32P]ATP with or without NDPs as substrates. Secretion of the enzymes was observed only at high cell density (OD600 of 1.90) when the cells enter the stationary phase (such as those shown in Fig. 1, lanes 2 to 5). Secretion of these enzymes (ATPase, Ndk, and enzyme(s) giving rise to [32P]ADP from [γ-32P]ATP) was completely abolished when P. aeruginosa 8821M cells were grown in a synthetic mineral medium containing succinate as a sole source of carbon (Fig. 1, lanes 6 to 9). Prolonged growth in the mineral medium, which allowed high cell density corresponding to the TYE broth-grown cells, failed to demonstrate the presence of any of these three enzymes in the supernatant in presence (Fig. 1, lanes 6 to 9) or absence of NDPs (lanes 14 to 17, Fig. 1), suggesting that growth in a complex medium containing tryptone and/or yeast extract is necessary to trigger secretion of these ATP-utilizing enzymes. Note that cells grown in the mineral medium do secrete an enzyme that produces small amounts of GTP either in the presence (Fig. 1, lanes 6 to 9) or in the absence (Fig. 1, lanes 14 to 17) of NDPs, suggesting that a G-protein-like protein, having bound GDP that is released as [γ-32P]GTP in the presence of [γ-32P]ATP, is secreted by the 8821M cells even during growth in the mineral salts medium. It is also possible that a small amount of GDP is secreted in the medium and is then used as a substrate by a secreted kinase.
Nonmucoid P. aeruginosa cells secrete very little Ndk; effect of salts on Ndk secretion.
Since strain 8821M is a mucoid CF isolate, it was of interest to determine if the non-CF laboratory strain PAO1 can also secrete Ndk and the other enzymes. The ability of TYE broth-grown 8821M and strain PAO1 to secrete Ndk to the outside medium was then tested. While mucoid strain 8821M demonstrated the presence of Ndk, the [32P]ADP-forming enzyme, and the ATPase activities, strain PAO1 grown on synthetic medium or TYE broth showed very little secretion of Ndk and [32P]ADP-forming activities (data not shown). In contrast, the nonspecific GTP-producing activity is present in the growth medium of strain PAO1. Strain PAO1 did not show appreciable secretion of either Ndk or [32P]ADP-forming enzyme during the entire growth phase either in TYE broth or in the mineral salts medium. We are currently examining a few other nonmucoid environmental isolates for their ability to secrete the ATP-utilizing enzymes. Curiously, addition of the mineral salts medium to TYE broth facilitated the growth of mucoid strain 8821M but significantly inhibited the secretion of Ndk and other enzymes. Since the mineral salts medium had as its components potassium phosphate buffer, CaCl2, NH4Cl, and MgSO4, we investigated the effects of individual components added to the TYE broth medium on the secretability of Ndk and the other enzymes during early-stationary-phase growth. We determined the effect of 5 mM of each of NaCl, KCl, MgCl2, CaSO4, K2HPO4/KH2PO4 buffer (pH 7.0), and NH4Cl during growth in the TYE medium. None of these salts at 5 mM concentration had any significant inhibitory effect on the growth of the 8821M cells. While addition of 5 mM each of NaCl, KCl, potassium phosphate buffer, or NH4Cl in the TYE growth medium had no significant effect on the level of secretion of Ndk, addition of 5 mM each of either MgCl2 or CaSO4 greatly reduced the secretion of Ndk and [32P]ADP-forming activity. When the assays were conducted in the absence of NDPs, very little UTP or CTP was formed but there was formation of small amounts of GTP compared to those of CTP and UTP in all the lanes (data not shown). Commensurate with those samples that secreted large amounts of Ndk in the absence of added Ca2+ or Mg2+, a large spot corresponding to [32P]ADP that migrates close to CTP was always observed. This suggests that the secretion of Ndk and another enzyme(s) that generates [32P]ADP from [γ-32P]ATP is inhibited either by Mg2+ or by Ca2+ at 5 mM concentrations but not by NH4+, K+, or Na+ at the equivalent concentration. It should be noted that the activity of these enzymes in vitro was not affected by 5 mM concentration of Ca2+ or Mg2+, and indeed 10 mM Mg2+ is routinely added for the assays of Ndk and other enzymes. The secretion of these ATP-utilizing enzymes is not limited to only strain 8821M, since several CF isolates of mucoid strains such as FRD1 (24) and others show similar levels of secretion of these enzymes (data not shown).
Modulation of Ndk and other enzyme secretion by casein.
The ability of mucoid strain 8821M to secrete Ndk, ATPase, and other enzymes after growth in TYE broth but its inability to secrete these enzymes after growth in a synthetic mineral medium raised the question of whether the lack of secretion during growth in the synthetic medium is due solely to the presence of Ca2+ and Mg2+ in the synthetic medium which are inhibitory to the secretion process or whether the secretion is facilitated in the presence of proteins such as the tryptic digest of casein or proteins present in yeast extract which are components of TYE broth. In order to keep the secretion process active, we eliminated the Ca2+ and reduced the Mg2+ concentration to 0.5 mM in a modified MOPS minimal medium (MOPSmmI). Growth for 16 h under such conditions led to an OD600 of about 0.75, but again very little secretion was observed. When such cells were harvested and resuspended in MOPSmmII lacking Mg2+ at an OD600 of about 2.0, secretion of both Ndk and ATPase was detected within 60 min but only when kappa casein was present (Fig. 2). In order to see if different forms of casein, including acid hydrolyzed casein (Casamino Acids), promote secretion equally, we measured the levels of secreted Ndk and ATPase during a 60-min incubation of strain 8821M cells grown in MOPSmmI, harvested, resuspended in MOPSmmII, to an OD600 of 2.0, and incubated with water, Casamino Acids, peptone, tryptone, and α, β, and κ forms of casein (Fig. 3). It is interesting that the κ form of casein is most active in promoting Ndk and ATPase secretion than is the α or the β form or the hydrolyzed forms of casein. Estimation of the secreted and intracellular Ndk levels under such conditions demonstrated that about 70% of the total Ndk is secreted in presence of κ-casein. These data clearly indicate that eukaryotic proteins such as casein promote secretion of the ATP-utilizing enzymes, similar to our previous observations on the enhanced secretion of Ndk and ATPase by M. bovis BCG in the presence of various eukaryotic proteins (33). The enhanced secretion of ATP-utilizing enzymes in the presence of eukaryotic proteins may be a signal that P. aeruginosa has entered the host cell and needs a mechanism to evade host defense. Secretion of the enzymes in synthetic medium deficient in Ca2+ and Mg2+ but requiring the presence of eukaryotic proteins also demonstrates that the enzymes are not unstable or rendered inactive in synthetic media.
Properties of purified secreted Ndk.
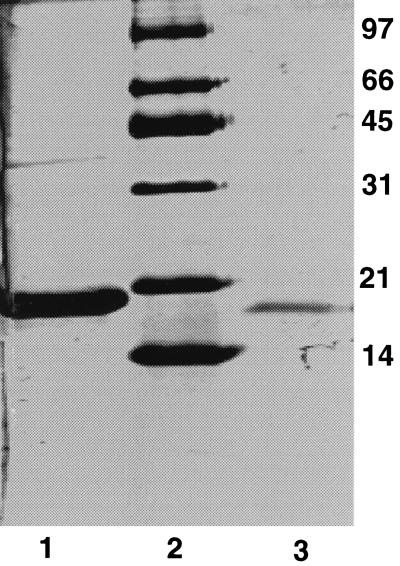
The secreted Ndk was purified from 2 liters of the growth medium of strain 8821M as described in Materials and Methods. A purified secreted Ndk preparation was run on an SDS-PAGE gel along with a purified preparation of 16-kDa cytoplasmic Ndk tagged with six histidine residues. The secreted Ndk ran somewhat faster (Fig. 4, lane 3) than the His-tagged cytoplasmic Ndk (Fig. 4, lane 1), presumably because the His-tagged Ndk has a higher molecular mass. The N-terminal amino acid sequence of 5 amino acids of the secreted Ndk showed an exact match with that of the intracellular Ndk previously reported (31), confirming the nature of the enzyme. To evaluate any putative differences with regard to substrate specificity, both the purified intracellular Ndk and the secreted Ndk were assayed in the presence of 50 μM, 100 μM, 500 μM, and 1 mM concentrations of NDPs (CDP, GDP, and UDP). There were no obvious differences between these two forms of the enzyme (data not shown).
FIG. 4.
Purification of secreted Ndk from culture filtrate of P. aeruginosa 8821M grown in TYE broth. The details of the purification procedure are given in Materials and Methods. Lane 1, purified His-tagged cytoplasmic Ndk; lane 2, molecular weight markers (in thousands); lane 3, purified secreted Ndk.
Construction of an ndk knockout mutant.
We previously showed that a regulatory mutant, algR2 algH, had essentially no Ndk activity but could grow well because of the presence of pyruvate kinase (PK), which generated all the NTPs needed for growth (31). Since regulatory mutations may be global in nature and affect various functions, we attempted to construct an ndk knockout mutant by an insertional gene replacement technique as previously described for the algR2 gene (27). The procedure is described in detail in Materials and Methods. The insertional inactivation of the ndk gene by a chloramphenicol resistance cassette was verified by the absence of Ndk enzymatic activity, as well as by Southern hybridization of the EcoRI-digested chromosomal DNA of P. aeruginosa 8821M with the ndk gene as probe. In the genomic digest of the parent strain 8821M, a 6.0-kb EcoRI fragment lighted up with the ndk gene as a probe, while in the insertion mutant, two bands with sizes of 4.0 kb and 2.7 kb lighted up, corresponding to the presence of an EcoRI site on the 700-bp Cmr gene cassette (data not shown).
Purification of secreted ATPase activity.
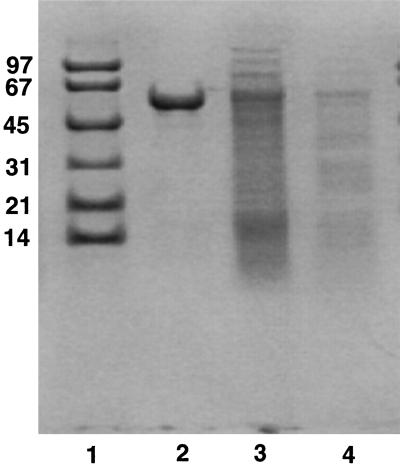
Since the Ndk and ATPase activities appeared to be closely associated, presumably as a complex, we decided to use the ndk knockout mutant for the isolation and characterization of the secreted ATPase. The ATPase activity was purified through successive chromatography on hydroxyapatite and ATP-agarose columns, as described in Materials and Methods. The eluate from the ATP-agarose column showed strong ATPase activity and the presence of a major band with a size of about 60 kDa by SDS-PAGE (Fig. 5, lane 2). The N-terminal amino acid sequence of this 60-kDa protein band (AAKEVKF), as well as the sequence of an internal fragment (LQIALTGG), showed 100% match with that of Hsp60, a molecular chaperonin. It is interesting to note that while Hsp60 is a cytoplasmic protein in Escherichia coli, it is surface exposed in virulent strains of Legionella pneumophila and is released into the newly formed and mature phagosome during engulfment of the bacteria by macrophages (10, 11). Thus P. aeruginosa Hsp60 behaves like L. pneumophila Hsp60 and crossreacts with an L. pneumophila anti-Hsp60 antibody.
FIG. 5.
Purification of ATPase as revealed by SDS-PAGE. The various steps of ATPase purification are described in Materials and Methods. Lane 1, low molecular mass standard protein samples with sizes in kilodaltons indicated on the left; lane 2, 2 mM ATP eluate from the ATP-agarose column showing a predominant single band; lane 3, eluate from hydroxyapatite column; lane 4, concentrated supernatant sample.
Nature of the enzyme(s) that generates [32P]ADP from [γ-32P]ATP.
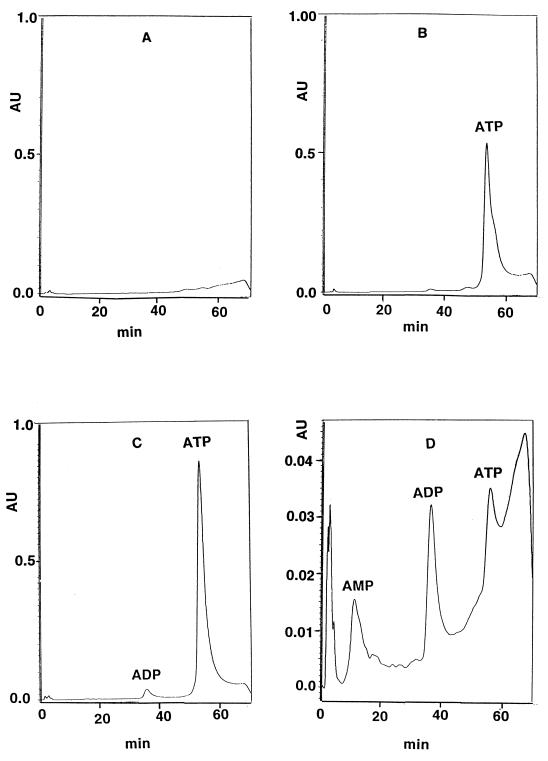
One of the intriguing observations during the assay of Ndk or ATPase activity was the formation of a radioactive band, particularly in the absence of NDPs so that Ndk was without its substrates and the [γ-32P]ATP was available for other reactions, that migrated at the same position as ADP (Fig. 1, lanes 10 to 12). Both Ndk and ATPase remove the terminal phosphate of [γ-32P]ATP, either to NDPs to generate NTPs or to release 32Pi. Thus [32P]ADP cannot be generated from [γ-32P]ATP by these enzymes. In order to see if there is one or more enzymes that somehow generate [32P]ADP from [γ-32P]ATP, we grew 4 liters of the ndk knockout mutant in TYE broth, separated the supernatant from the cells by high-speed centrifugation, and concentrated it by ultrafiltration through a YM10 Amicon membrane. The concentrated supernatant was loaded on a hydroxyapatite column. The ADP-generating enzymatic activity did not bind to the column and was present in the flowthrough fraction. This fraction was further concentrated and loaded onto an ATP-agarose column equilibrated with TMD buffer (50 mM Tris · HCl buffer (pH 7.5), 10 mM MgCl2, 0.8 mM dithiothreitol, 25 mM KCl). While the ATPase activity was bound to this column and removed, the [32P]ADP-generating activity was present in the flowthrough fraction. This fraction was further concentrated by ultrafiltration through a YM10 Amicon membrane and loaded onto an ADP-agarose column equilibrated with TMD buffer. The [32P]ADP generating activity was again found in the flowthrough fraction. This fraction was further concentrated and loaded onto a Mono Q column. Again, the enzyme activity did not bind to the column and came out in the flowthrough fraction. SDS-PAGE of this fraction showed several bands on Coomassie blue staining (data not shown), suggesting the presence of several proteins. This fraction, termed Mono Q flowthrough, was then treated with nonradioactive 5 mM ATP or 5 mM ADP and the nature of the products formed was characterized by high-pressure liquid chromatography (HPLC). The results shown in Fig. 6C demonstrate the formation of small amounts of ADP when the Mono Q flowthrough (Fig. 6A) and ATP (Fig. 6B) were mixed together. To further characterize this reaction, the Mono Q flowthrough fraction in TM buffer was treated with 5 mM ADP. ADP alone showed a retention time of about 37 min without any other major peak (data not shown); however, when 5 mM ADP was treated with the Mono Q column flowthrough fraction, clear peaks corresponding to the formation of AMP and ATP were observed (Fig. 6D). No such changes were seen when 5 mM AMP alone was treated with the Mono Q fraction under such conditions. Thus the enzymatic activities present in the Mono Q flowthrough fraction allow formation of ADP from ATP and also the formation of AMP and ATP from ADP. Another interesting activity is that when ATP is treated with the Mono Q flowthrough fraction, its absorbance goes up significantly (compare the ATP peaks in Fig. 6B and 6C). Preliminary experiments using HPLC-mass spectrometry with the ATP peak shown in Fig. 6C demonstrated fragments with masses higher than that of ATP by 2 (H) atoms, as if the ATP were being reduced. The nature of any putative ATP reductase is conjectural at present, and the enzyme needs to be purified and more rigorously studied.
FIG. 6.
HPLC analysis of reaction mixture containing Mono Q column flowthrough fraction with or without 5 mM ATP or ADP. HPLC was performed with a strong anion-exchange. Protein-Pak Q-8HR column (Waters) in a gradient of 7 mM KH2PO4 (pH 3.8) and 0.5 M KH2PO4 (pH 4.5) at a rate 0.5 ml/min. (A) Mono Q column effluent in TM buffer alone; (B) ATP (5 mM) alone in TM buffer; (C) reaction mixture containing Mono Q column effluent fraction and ATP (5 mM) in TM buffer; (D) reaction mixture containing Mono Q column effluent fraction and ADP (5 mM) in TM buffer.
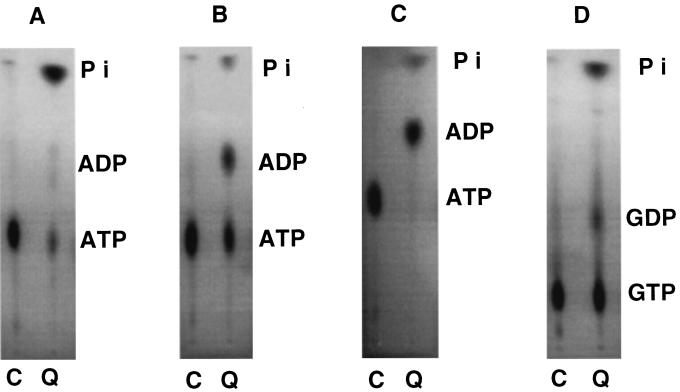
To understand how [32P]ADP is formed from [γ-32P]ATP or how AMP and ATP are formed from ADP, we incubated [γ-32P]ATP with the Mono Q column flowthrough fraction in the absence and in the presence of nonradioactive ADP or AMP. To have a better understanding of the reaction products, we also incubated the Mono Q column flowthrough fraction with [α-32P]GTP. The Mono Q column effluent generates 32Pi from both [γ-32P]ATP (Fig. 7A, lane Q) and [α-32P]GTP (Fig. 7D, lane Q), suggesting the presence of 5′-nucleotidase (phosphatase) activity. The Mono Q column effluent could also generate 32Pi from [α-32P]ATP or [α-32P]CTP. When nonradioactive ADP or AMP is added to the mixture of [γ-32P]ATP and the Mono Q column flowthrough fraction, clear bands of radioactive ADP could be seen (Fig. 7B and C, lanes Q). Thus it appears that radioactive [32P]ADP is generated by a combined action of 5′-nucleotidase, which generates nonradioactive ADP and AMP from [γ-32P]ATP, and adenylate kinase (myokinase) where the nonradioactive AMP is phosphorylated by the adenylate kinase by terminal phosphotransfer from [γ-32P]ATP, generating 32P-ADP. Myokinase (ATP:AMP phosphotransferase), also known as adenylate kinase, is a well-known enzyme that converts two molecules of ADP to one each of AMP and ATP, and vice versa (34). These enzymes are present as ectoenzymes on the outer membrane of many mammalian cells to regulate the level of external purines (34). One consequence of the secretion of similar enzymes by pathogens may be to disrupt this regulation as part of a takeover of the host defense.
FIG. 7.
Enzymatic analysis of Mono Q column effluent (lanes Q) compared to control (lanes C) without additions. (A) Lane C (control), 5 μl of TM buffer with [γ-32P]ATP (0.15 μl) and TM buffer (4.85 μl) used instead of sample; lane Q, 5 μl of Mono Q flowthrough fraction with 0.15 μl [γ-32P]ATP and 4.85 μl TM buffer. (B) [γ-32P]ATP and ADP used as substrates in reaction mixture containing 5 μl of buffer alone (lane C) or Mono Q effluent (lane Q) containing 0.15 μl of [γ-32P]ATP, 0.1 μl of 10 mM ADP, and 4.75 μl of TM buffer. (C) [γ-32P]ATP and AMP used as substrates in reaction mixture containing 5 μl of TM buffer (C) or Mono Q flowthrough fraction (lane Q) containing 0.15 μl of [γ-32P]ATP, 0.1 μl of 10 mM AMP, and 4.75 μl of TM buffer. (D) [α-32P]GTP used as a substrate in reaction mixture containing 5 μl of the TM buffer (C) or Mono Q flowthrough fraction (lane Q) containing 0.15 μl of [α-32P]GTP and 4.85 μl of TM buffer.
Physiological significance of the secretion of ATP-utilizing enzymes by mucoid P. aeruginosa.
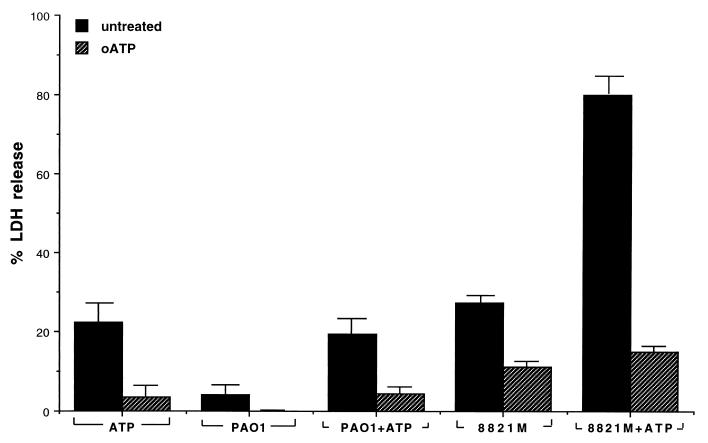
The secretion of the ATP-utilizing enzymes by the mucoid CF isolate of strain 8821M, but the absence of secretion by the nonmucoid PAO1 strain, raised the question of whether such secretion may be physiologically important for strain 8821M to survive in the CF lung environment for long periods of time. It is known that early infections in the CF lung are due to nonmucoid P. aeruginosa, which is normally cleared well by the immune system. As the nonmucoids increasingly become mucoid in the CF lung, they also become persistent and are cleared inefficiently. While the alginate capsule is believed to contribute to this mechanism of protection from phagocytosis (2, 28), it is equally possible that other cellular processes, similar to the alginate biosynthetic machinery, that additionally contribute to the survival mechanism of the mucoid cells are activated. The secretion of ATP-utilizing enzymes could very well be such a mechanism, since ATP is a vital constituent of all cells, and it has become apparent recently that mammalian cells extrude ATP to the extracellular fluid in order to carry out various functions that require ATP (1, 13). Many cellular functions that are mediated by external ATP require the presence of specific receptors for ATP, called the P2 purinergic receptors (7). Among P2 receptors, there are six classes, P2D, P2T, P2U, P2X, P2Y, and P2Z. P2Y and P2Z receptors are present on the surface of macrophages that are the first line of defense against infection by bacterial pathogens. We therefore considered it likely that ATP-utilizing enzymes secreted by a pathogen such as mucoid P. aeruginosa may be targeted towards macrophage P2Z receptors to modulate macrophage activity. Indeed, macrophage surface-associated P2Z receptors are known to be involved in macrophage cell death, and presumably in phagosome-lysosome fusion, when they are activated in presence of mM concentrations of external ATP (6, 18). To examine whether secretion of ATP-utilizing enzymes by mucoid 8821M cells may have any effect on macrophage cell death, we determined the extent of macrophage cell death in the presence of external ATP (2 mM) as well as in the absence or presence of supernatant growth medium harboring the secreted enzymes of the mucoid 8821M cells. As a control, we also used the supernatant growth medium of strain PAO1 which does not secrete the ATP-utilizing enzymes. The treatment of the macrophages with 2 mM ATP alone for 2 h led to about 23% macrophage cell death (Fig. 8, column ATP), as has been reported by other groups (8, 18, 33). The supernatants of strain PAO1 and mucoid strain 8821M showed some cytotoxic activity (about 4 and 28%, respectively), while a mixture of PAO1 supernatant and 2 mM ATP allowed a higher level of macrophage cell death (about 20% [Fig. 8]). It is, however, no higher than that noted with 2 mM ATP alone (P = 0.2), suggesting that the PAO1 supernatant does not modulate ATP-induced P2Z receptor-mediated macrophage cell death. In contrast, an equivalent amount of the 8821M supernatant in presence of 2 mM ATP accelerated the extent of macrophage cell death (78%; P = 2.9 × 10−5), suggesting that the growth medium of 8821M contains factors that enhance macrophage cell death in the presence of external ATP. However, no significant difference was observed in the ATP-mediated effect on macrophages isolated from two different strains of mice (AKR/J and BALB/c), which are susceptible and resistant to infection with P. aeruginosa, respectively.
FIG. 8.
Effect of growth medium supernatants of P. aeruginosa 8821M and PAO1 on ATP-mediated killing of macrophages pretreated with or without oATP. Macrophages were prepared, plated, and stimulated with LPS as described in Materials and Methods. The cells were treated with oATP (1 mM) for 2 h as indicated. After this incubation, ATP (2 mM) and eightfold-concentrated supernatants (50 μl) of P. aeruginosa 8821M and PAO1 grown to an OD600 of 1.9 were added to cultures. Supernatants without ATP were also included to assess external ATP-independent cytotoxic effects of the supernatants. Cultures were incubated for 4 h, and LDH release was determined as described previously (33). Data presented represent the average ranges for triplicate determinations. Similar results were obtained in two independent experiments.
A characteristic feature of macrophage P2Z receptors is that the agonist profile is very distinct, i.e., benzoyl benzoyl ATP and ATP are the most potent agonists and oxidized ATP is inhibitory, while other nucleotides, such as GTP and UTP, etc., have no significant effect (6, 18). It has been shown that oATP is an irreversible inhibitor of the macrophage P2Z receptors (22) so that pretreatment of macrophages with oATP blocks the subsequent activation of the P2Z receptors by external ATP (9). oATP-treated macrophages show a significant reduction in killing in the presence of ATP (P = 0.002) or PAO1 plus ATP (P = 0.003) (Fig. 8). Interestingly, while the oATP-treated macrophages show reduced killing on subsequent treatment with ATP or ATP plus PAO1 supernatant, oATP-treated macrophages show a certain amount of cell death (14% compared to 78%) on treatment with 8821M supernatant plus ATP, suggesting that while the bulk of the enhanced macrophage killing by the 8821M supernatant is due to P2Z receptor activation, a small amount of the macrophage killing may be mediated by a P2Z receptor-independent process.
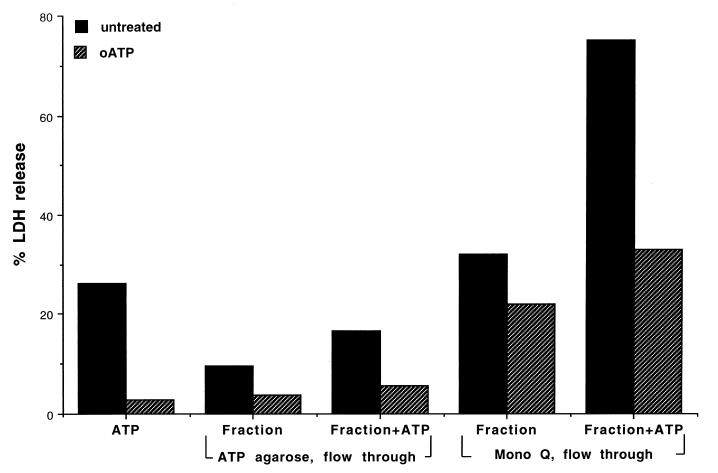
In order to examine whether the ATP-utilizing enzymes of the 8821M supernatant are involved in the modulation of macrophage cell death, we also examined the relative cytotoxicity of the various column effluents during fractionation and purification of the ATP utilizing enzymes from the 8821M growth medium supernatant. The relative cytotoxicities of the two flowthrough fractions (2 μg of protein each) from the ATP agarose and Mono Q columns with untreated and oATP-pretreated macrophages are shown in Fig. 9. The Mono Q flowthrough fraction is relatively enriched with cytotoxic factors as the sample itself shows 30% macrophage cell death compared to less than 10% of the ATP-agarose flow through fraction. The addition of ATP (2 mM) greatly stimulated the macrophage killing (about 75%). This cytotoxicity, mediated by the Mono Q column flowthrough fraction (as well as the less severe cytotoxicity shown by the ATP agarose column flowthrough fraction) is significantly reduced when the macrophages are pretreated with oATP, suggesting that a major part of the cytotoxicity involved activation of the P2Z receptors. There is, however, a residual cytotoxicity in the Mono Q column flowthrough fraction that appears to be independent of the P2Z receptor-mediated macrophage cell death.
FIG. 9.
Cytotoxic effects of ATP and the various column effluents (ATP agarose and Mono Q) in the presence or absence of external ATP on macrophage cell death as measured by LDH release. Macrophages were cultured, and one set of macrophage cultures was treated with oATP while the other was not. Enzyme fractions from the culture filtrate of mucoid P. aeruginosa 8821M at various stages of purification (ATP agarose or Mono Q flowthrough fraction, 20 μg of protein/ml) were tested for cytotoxic effects. LPS-stimulated macrophages either pretreated with oATP or not pretreated were treated with fractions in the presence and absence of ATP (2 mM).
To understand what might account for enhanced toxicity of the Mono Q column flowthrough fraction, we tested the effect of purified ATPase and Ndk, as these two enzymes were absent in the Mono Q column effluent. While purified ATPase and Ndk had low cytotoxic activity, the presence of these enzymes reduced further the cytotoxicity (P = 3.5 × 10−5 and 0.001 for ATP and Ndk, respectively) associated with ATP-induced P2Z receptor activation and the consequent loss of macrophage viability (data not shown). The reduced macrophage killing is presumably because of the hydrolysis and sequestration of ATP from the P2Z receptors. Thus some secreted enzymes (ATPase and Ndk) reduce P2Z receptor-mediated macrophage death, while others present in the Mono Q column flowthrough fractions enhance macrophage cell death. It is not known whether secretion of these two types of enzymes might be differentially regulated in the host cell.
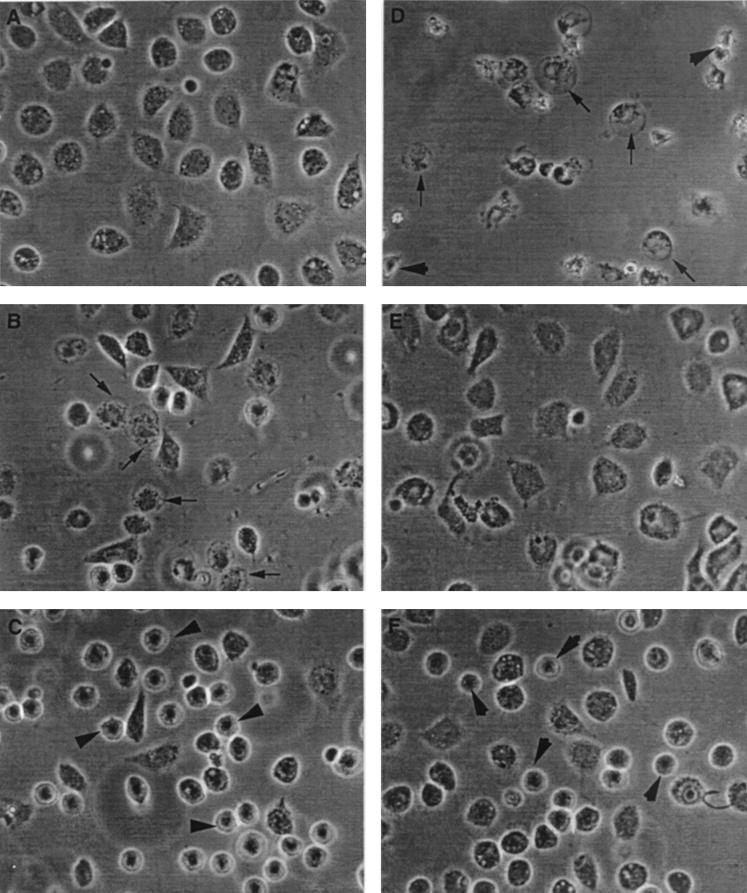
Since the Mono Q column flowthrough fraction shows the presence of cytotoxic agents that act through P2Z receptor-dependent and -independent pathways, it was of interest to examine whether this difference in the mode of action of the secreted cytotoxic agents could be reflected in the morphological changes occurring in the macrophages during their exposure to such agents. Normally growing macrophages acquire irregular shapes, [Fig. 10A]. In presence of 2 mM ATP, which activates the P2Z receptors leading to macrophage cell death, many macrophages are seen to undergo swelling, membrane blebbing, and vacuolization (indicated by arrows in Fig. 10B). This effect is similar to that previously observed (22). In contrast, when macrophages were exposed to the Mono Q column flowthrough fraction, many assumed a rounded shape with nuclear condensation (Fig. 10C, indicated by arrows). When the macrophages were exposed to both the Mono Q column effluent and 2 mM ATP, drastic morphological changes, including rounded macrophages with nuclear condensation and fragmentation, seemed to occur (Fig. 10D). These ATP-induced morphological changes were substantially prevented when the macrophages were pretreated with oATP (Fig. 10E). In contrast, oATP-treated macrophages exposed to the Mono Q column flowthrough fraction plus 2 mM ATP showed the presence of many round-shaped macrophages with nuclear condensation (Fig. 10F), reminiscent of macrophages treated with the Mono Q column effluent alone (Fig. 10C). Thus this rounded morphology appears to be due to the cytotoxic agent which operates via the P2Z receptor-independent pathway.
FIG. 10.
Changes in the morphology of LPS-primed macrophages after treatment with ATP and various ATP-utilizing enzymes. Macrophages were either pretreated with oATP or not pretreated. Macrophages were plated, treated with LPS as described in the legend to Fig. 8, and incubated with ATP (2 mM) and/or Mono Q effluents harboring various ATP-utilizing enzymes. (A) Control, untreated macrophages; (B) macrophages treated with 2 mM ATP; (C) macrophages treated with Mono Q column flowthrough fraction; (D) macrophages treated with Mono Q column flowthrough fraction plus 2 mM ATP; (E) macrophages pretreated with oATP (1 mM) for 2 h before addition of 2 mM ATP; (F) macrophages pretreated with oATP (1 mM) for 2 h before addition of 2 mM ATP plus Mono Q column flowthrough fraction. Various morphological forms are indicated by arrows and arrowheads.
DISCUSSION
The secretion of Ndk as well as several other ATP-utilizing enzymes by the CF isolate but not by the non-CF isolate of P. aeruginosa is reminiscent of the secretion of Ndk and ATPase by M. bovis BCG. We have previously reported (33) that M. bovis BCG secretes Ndk and ATPase (Dnak) activities during growth in Middlebrook 7H9 medium, but this secretion is greatly reduced during growth in synthetic Sauton medium unless eukaryotic proteins such as bovine serum albumin or ovalbumin are present. In a somewhat analogous situation, mucoid P. aeruginosa cells appear to secrete these enzymes during growth in TYE broth, which contains a tryptic digest of casein and extracts of yeast, but not during growth in a synthetic medium. Indeed, the addition of the synthetic medium or just 5.0 mM Ca2+ or Mg2+ to the TYE medium significantly inhibited ATP-utilizing enzyme secretion without any effect on growth, suggesting that certain divalent cations negatively affect the secretion machinery. Nevertheless, such effects of electrolytes, whether mediated through an alteration of the outer membrane protein profile or integrity or through some other means, strongly indicate that the enzymes are present in the outside medium due to active secretion and not due to cell lysis. This conclusion is reinforced by the observation that 8821M cells grown in synthetic media in the absence of Ca2+ and Mg2+ secrete Ndk and ATPase within 60 min but only in presence of casein (Fig. 2 and 3). Thus the secretory apparatus seems to be specifically activated by some eukaryotic proteins. It is interesting in this context that while Ndk in mammalian cells has previously been reported to be either cytosolic or membrane associated (17), a recent report describes the presence of ecto-Ndk in the mammalian cell surface exposed to the outside medium (19). Similarly, both mammalian adenylate kinase and 5′-nucleotidase are believed to be ectoenzymes and located on the external part of the membranes (20, 23).
The presence of a number of ATP-utilizing and -modifying enzymes, such as 5′-nucleotidase, adenylate kinase, and a putative enzyme that allows formation of a reduced form of ATP, in the Mono Q column effluent of the mucoid P. aeruginosa growth medium and the associated cytotoxicity of this effluent suggest a common link between these two. The cytotoxicity mediated through the P2Z receptors may involve formation of modified (reduced) ATP which could be a better agonist for the P2Z receptor activation than ATP itself, thereby enhancing macrophage cell death. Macrophages are known to efflux ATP to the outside on stimulation with bacterial cell wall LPS (8, 9), and this ATP could be acted on by the bacterial secreted enzymes to generate a more potent agonist for P2Z receptor activation. The ability of the Mono Q effluent fraction to release 32Pi from [α-32P]ATP or [α-32P]GTP suggests that adenosine is formed from ATP. Similarly, formation of AMP has been demonstrated from ATP because of the presence of 5′-nucleotidase. Adenosine and AMP activate P1 receptors while ATP and ADP activate P2 receptors. Thus the bacterial enzymes may modulate host cell and macrophage functions through activation of additional receptors. The nature of the cytotoxic agent that operates through the P2Z-independent pathway is unknown, but its availability during chromatographic fractionation should allow us to characterize it biochemically. It should be noted that other pathogens such as Shigella or Salmonella induce apoptosis in infected macrophages through secretion of invasins, such as IpaB or SipB, which bind to caspase-1 leading to its activation which then induces apoptosis (14, 15). It remains to be seen whether similar cytotoxic agents in the Mono Q column effluent induce macrophage cell death through the P2Z-independent pathway.
Finally, the ability of the two CF mucoid strains (8821M and FRD1) but the inability of the non-CF strain PAO1 to secrete Ndk, ATPase, 5′-nucleotidase, adenylate, and kinase, etc., raises the important question of whether the genes for the secretory apparatus, perhaps encoding one or more outer membrane proteins, are activated in the CF lung, similar to the alginate gene activation (29). It is known that outer membrane proteins such as AlgE are found specifically in the mucoid cells but are absent in the nonmucoid cells (26). Thus mutational studies of known alginate genes, looking for secretion defects in mutants with mutations in alginate structural and regulatory genes, would be of interest. Of course, the genes involved in encoding the secretory apparatus do not have to be involved in alginate synthesis but may be independently activated in presence of a common signal present in the CF lung. It is interesting that a mucosal pathogen, such as P. aeruginosa, that does not need to grow inside the macrophages elaborates cytotoxic agents that kill the macrophages. In contrast, another respiratory tract pathogen such as M. bovis, which prevents phagosome-lysosome fusion and needs live macrophages for growth, elaborates only Ndk and ATPase that sequester the ATP from the P2Z receptors, thereby preventing macrophage cell death (33). It would be interesting to see whether other intracellular pathogens that use macrophages for growth such as Salmonella and Legionella (10, 14) secrete only ATPase and Ndk types of enzymes while mucosal pathogens such as Vibrio cholerae elaborate all the other cytotoxic enzymes contributing to enhanced macrophage killing. Further characterization of the secretion apparatus, particularly the outer membrane components of the secretion system, as well as the secreted cytotoxic agents would be important as targets for vaccine and drug development, since they appear to be important weapons in the arsenal of the pathogen.
ACKNOWLEDGMENTS
O.Z. and N.M. contributed equally to this work.
This work was supported by Public Health science grant AI 16790-18 (to A.M.C.) and HHS DK 44972 (to B.S.P.) from the National Institutes of Health.
We thank Bob Lee at the Protein Research Laboratory, University of Illinois at Chicago, for the N-terminal amino acid analysis of the secreted proteins and Paul Hoffman of Dalhousie University for L. pneumophila anti-Hsp60 antibody.
REFERENCES
- 1.Al-AwQati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995;269:805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]
- 2.Bayer A S, Speert D P, Park S, Tu J, Witt M, Nast C C, Norman D C. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect Immun. 1991;59:302–308. doi: 10.1128/iai.59.1.302-308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarty A M. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol. 1998;28:875–882. doi: 10.1046/j.1365-2958.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 4.Chopade B A, Shankar S, Sundin G W, Mukhopadhyay S, Chakrabarty A M. Characterization of membrane-associated Pseudomonas aeruginosa Ras-like protein, Pra, a GTP-binding protein that forms complexes with truncated nucleoside diphosphate kinase and pyruvate kinase to modulate GTP synthesis. J Bacteriol. 1997;179:2181–2188. doi: 10.1128/jb.179.7.2181-2188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu L, May T B, Chakrabarty A M, Misra T K. Nucleotide sequence and expression of the algE gene involved in alginate biosynthesis by Pseudomonas aeruginosa. Gene. 1991;107:1–10. doi: 10.1016/0378-1119(91)90290-r. [DOI] [PubMed] [Google Scholar]
- 6.Di Virgilio F. The P2Z purino receptor: an intriguing role in immunity inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 7.Dubyak G R, El-Motassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari D, Chiozzi P, Falzoni S, Susino M D, Melchiorri L, Baricordi O R, Di Virgilio F. Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 9.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garduno R A, Faulkner G, Trevors M A, Vats N, Hoffman P S. Immunolocalization of Hsp60 in Legionella pneumophila. J Bacteriol. 1998;180:505–513. doi: 10.1128/jb.180.3.505-513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garduno R A, Garduno E, Hoffman P S. Surface-associated Hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model. Infect Immun. 1998;66:4602–4610. doi: 10.1128/iai.66.10.4602-4610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti G. ATP transport and ABC proteins. Chem Biol. 1996;3:703–706. doi: 10.1016/s1074-5521(96)90244-6. [DOI] [PubMed] [Google Scholar]
- 14.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavel R A, Yuan J, Sansonetti P J, Zychlinski A. Shigella-induced apoptosis is dependent on caspase 1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 16.Kamath S, Kapatral V, Chakrabarty A M. Cellular function of elastase in Pseudomonas aeruginosa: role in the cleavage of nucleoside diphosphate kinase and in alginate synthesis. Mol Microbiol. 1998;30:933–942. doi: 10.1046/j.1365-2958.1998.01121.x. [DOI] [PubMed] [Google Scholar]
- 17.Kimura N, Shimada N. Membrane-associated nucleoside diphosphate kinase from rat liver: purification, characterization, and comparison with cytosolic enzyme. J Biol Chem. 1998;263:4647–4653. [PubMed] [Google Scholar]
- 18.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingan S, Kumararatne D S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 19.Lazarowski E R, Homolya L, Boucher R C, Harden T K. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem. 1997;272:20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- 20.Lee K S, Schubert P, Reddington M, Kreutzberg G W. The distribution of adenosine Al receptors and 5′-nucleotidase in the hippocampal formation of several mammalian species. J Comp Neurol. 1986;246:427–434. doi: 10.1002/cne.902460402. [DOI] [PubMed] [Google Scholar]
- 21.May T B, Shinabarger D, Maharaj R, Kato J, Chu L, DeVault J D, Roychoudhury S, Zielinski N A, Berry A, Rothmel R K, Misra T K, Chakrabarty A M. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections in cystic fibrosis patients. Clin Microbiol Rev. 1991;4:191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP: an irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 23.Nagy A K, Shuster T A, Delgado-Escueta A V. Rat brain synaptosomal ATP:AMP phosphotransferase activity. J Neurochem. 1989;53:1166–1172. doi: 10.1111/j.1471-4159.1989.tb07410.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohman D E, Chakrabarty A M. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect Immun. 1982;37:662–669. doi: 10.1128/iai.37.2.662-669.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pier G B. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 26.Rehm B H A, Boheim G, Tommassen J, Winkler U K. Overexpression of algE in Escherichia coli: subcellular localization, purification, and ion channel properties. J Bacteriol. 1994;176:5639–5647. doi: 10.1128/jb.176.18.5639-5647.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlictman D, Kavanaugh-Black A, Shankar S, Chakrabarty A M. Energy metabolism and alginate biosynthesis in Pseudomonas aeruginosa: role of the tricarboxylic acid cycle. J Bacteriol. 1994;176:6023–6029. doi: 10.1128/jb.176.19.6023-6029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzmann S, Boring J T. Antiphagocytic effect of slime from a mucoid strain of Pseudomonas aeruginosa. Infect Immun. 1971;3:762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankar S, Ye R W, Schlictman D, Chakrabarty A M. Exopolysaccharide alginate synthesis in Pseudomonas aeruginosa: enzymology and regulation of gene expression. Adv Enzymol. 1995;70:221–255. doi: 10.1002/9780470123164.ch4. [DOI] [PubMed] [Google Scholar]
- 30.Shankar S, Kamath S, Chakrabarty A M. Two forms of the nucleoside diphosphate kinase of Pseudomonas aeruginosa 8830: altered specificity of nucleoside triphosphate synthesis by the cell membrane-associated form of the truncated enzyme. J Bacteriol. 1996;178:1777–1781. doi: 10.1128/jb.178.7.1777-1781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundin G W, Shankar S, Chugani S A, Chopade B, Kavanaugh-Black A, Chakrabarty A M. Nucleoside diphosphate kinase from Pseudomonas aeruginosa: characterization of the gene and its role in cellular growth and exopolysaccharide alginate synthesis. Mol Microbiol. 1996;20:965–979. doi: 10.1111/j.1365-2958.1996.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 32.Woods D E, Sokol P A, Bryan L E, Storey D G, Mattingly S J, Vogel H J, Ceri H. In vivo regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis. 1991;163:143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]
- 33.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty A M. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;31:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996;39:337–352. [Google Scholar]