Abstract
Chronic immune activation by coinfecting pathogens has been suggested as a cofactor in human immunodeficiency virus (HIV) disease progression, particularly in the setting of developing countries. Here, we used in vivo-infected mononuclear cells to examine the role of the protozoan parasite Leishmania donovani and its major membrane constituent, lipophosphoglycan (LPG), in mediating CD4+ T-lymphocyte activation-induced HIV replication and CD4+ T-cell death. We found that Leishmania antigens upregulated HIV replication in CD8-depleted peripheral blood mononuclear cells from asymptomatic HIV-infected donors compared to unstimulated cells. L. donovani-induced viral replication was associated with cellular proliferation, increased expression of the cellular immune activation markers CD25 and HLA-DR within the CD4+ subpopulation, and enhanced secretion of tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), and IL-6. LPG induced TNF-α secretion in the absence of increased expression of cellular activation markers. Moreover, in a few cases we observed that L. donovani induced HIV replication without significant cellular activation but with cytokine secretion. The rate of apoptosis was accelerated in these latently infected CD4+ T cells primed with Leishmania antigens compared to controls, and TNF-α production appeared to be the central event necessary for this effect. Furthermore, we demonstrate that thalidomide inhibited Leishmania-induced virus replication coupled with abrogated Leishmania-induced TNF-α secretion but not IL-2 or IL-6 production. Furthermore, thalidomide did not affect Leishmania-induced apoptosis. The results suggest that Leishmania and its product, LPG, up-regulate HIV replication in latently infected cells through distinct antigen-specific and non-antigen-specific cellular immune activation mechanisms and that TNF-α secretion is pivotal in this process. The immunomodulatory role of thalidomide raises interest as a potential adjuvant to reduce HIV disease progression in Leishmania-HIV coinfected individuals.
Leishmaniasis, a chronic infection caused by a protozoan parasite belonging to Leishmania species, has emerged as an important potential opportunistic disease among patients with human immunodeficiency virus type 1 (HIV-1) infection (2). Both Leishmania and HIV exert several overlapping effects on immune cells and their effector functions. Though HIV-1 can infect CD4+ T lymphocytes, both pathogens can infect and replicate in a common cell target, namely, the macrophage (21). In addition, the organisms cause T-helper 1 (Th1)/Th2 imbalances (8, 22). Thus, infection of a common cell target by the two different pathogens, including their influences on the other arms of the immune system, has important bidirectional implications. Previously, we showed that HIV-1 inhibits Leishmania-induced lymphocyte proliferation without affecting the parasite-induced production of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) (26) and that the virus increases intracellular multiplication of Leishmania donovani in monocyte-derived cells (27). The above findings and the observation that patients with visceral leishmaniasis (VL) demonstrate elevated levels of serum IL-6 and TNF-α (6), the cytokines implicated in inducing HIV replication in T cells and macrophages (10), led us to hypothesize a role for Leishmania and other parasites in cytokine-induced transactivation of HIV-1 (26). This was then confirmed by Bernier and colleagues, who demonstrated with isolated cell lines that Leishmania and its major surface constituent, lipophosphoglycan (LPG), can induce activation of HIV-1 in in vitro-generated, latently infected monocyte and T-cell lines (4, 5).
CD4+ T lymphocytes are the principal target for HIV-1 (9), contributing to more than 95% of total virus production (18). On the other hand, monocytes/macrophages are responsible for only 1 to 2% of total virus production (18). In vivo and in vitro studies have convincingly demonstrated that HIV replication is associated with activation of the immune system through antigen-specific as well as non-antigen-specific mechanisms (11, 17, 29). The effects of L. donovani and its specific antigenic constituents on activation-induced HIV replication in in vivo-infected mononuclear cells are, however, unknown. In this study, therefore, we specifically addressed whether the protozoan parasite L. donovani and/or its LPG molecule can induce cellular activation and positively modulate HIV-1 replication in vitro in CD8-depleted peripheral blood mononuclear cells (PBMC) from asymptomatic HIV-1-infected subjects. The potential of leishmanial antigens in modulating the viability of CD4+ T cells was also investigated.
MATERIALS AND METHODS
Blood samples.
Peripheral blood samples were obtained from a cohort study group of asymptomatic HIV-1-infected patients (age range, 25 to 40 years; CD4+ T-cell count range, 190 to 490/mm3) from Black-Lion Teaching Hospital (Addis Ababa, Ethiopia). Informed consent was obtained from all participating individuals, and ethics committees of each of the participating institutes approved the study protocol. A detailed history was obtained from each patient, each of whom underwent a complete physical examination for the presence of any opportunistic infections. All patients belonged to stage II of the Centers for Disease Control and Prevention classification system (7), but one had constitutional symptoms (stage IVA). All were negative for antileishmanial antibodies by the leishmanial K39 antigen dipstick method (InSure Rapid Test; InBios International Inc., Seattle, Wash.). Four patients had histories of tuberculosis (completely treated) prior to 2 to 8 years before enrollment. None of the patients received antiretrovirus therapy.
Parasite and LPG preparation.
The isolation, cultivation, and maintenance of the promastigote stage of the parasite L. donovani (Ld 399) have been described in detail previously (26, 27). The LPG used in this study was derived from L. donovani promastigotes and was kindly donated by S. J. Turco (University of Kentucky, Lexington).
CD8-depleted PBMC preparation and cell priming.
Human peripheral blood samples were obtained by venipuncture into heparinized vacutainer tubes. PBMC were obtained from whole blood by sedimentation over Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) by density gradient centrifugation. Cells at the interphase were washed twice in phosphate-buffered saline (PBS). PBMC were depleted of CD8+ T cells by treatment with Dynabeads M-450 CD8 (Dynal, Oslo, Norway) to remove the inhibitory effects on virus replication (11). Briefly, PBMC and Dynabeads were washed in PBS containing 2% human AB+ serum before use, and cells and beads were mixed (at a 1:10 cell-to-bead ratio) and incubated for 30 min on ice with gentle agitation. CD8 depletions were performed on a magnetic particle concentrator (Dynal) for 3 min. The remaining cells were aspirated and then washed in medium. The purity of the cell suspension was assessed by FACScan. Contaminating CD8+ T cells were less than 1%. CD8+ T-cell-depleted fractions were resuspended at 106 cells/ml in 24-well plates in RPMI medium supplemented with 10% heat-inactivated normal human serum (NHS), 1% l-glutamine, and 1% penicillin-streptomycin. The following were then added: stationary-phase L. donovani promastigotes at a 1:1 cell-to-parasite ratio and LPG at a 12.5-μg/ml final concentration. Negative controls were incubated with medium only, and phytohemagglutinin (PHA) was used at a final concentration of 5 μg/ml as a positive control. Preliminary titration and kinetics studies were undertaken in order to determine the appropriate concentrations of the various antigens and mitogens used in the experiments. Plates were incubated at 37°C in 5% CO2 in humidified air. Half of the culture supernatant was collected at indicated time points and was replaced with fresh medium. Harvested supernatants were filtered through a 0.45-μm filter to remove cells and were then stored at −70°C for determinations of cytokines and p24 core antigen levels.
Flow cytometric analysis.
Phenotypic analysis was performed by FACScan (Becton Dickinson) after surface staining with anti-CD4 and anti-CD8 antibodies. Anti-CD45 and anti-CD14 antibodies were used for gating lymphocytes in the forward and side scatter profiles. Furthermore, expression of cellular activation markers was determined by staining with anti-CD25 and anti-HLA-DR antibodies. Isotype antibodies were used as controls. All antibodies were purchased from Becton Dickinson. Phenotypic analysis of PBMC was done at day 0, before and after CD8 depletion, and following culture of CD8-depleted PBMC with or without Leishmania antigens. The proportion of cells expressing activation markers within the population CD4+ T cells was analyzed between days 8 and 10 poststimulation. In each case, more than 20,000 events were accumulated for the samples and analysis was carried out with LYSIS II software (Becton Dickinson).
Proliferation assay.
An aliquot of CD8-depleted PBMC was harvested at day 5 and seeded into 96-well U-bottomed microtiter plates. Cells were pulsed with 0.5 μCi of [3H]thymidine (Amersham, Little Chalfont, United Kingdom) and incubated for 18 h at 37°C in 5% CO2 in air. After harvest, incorporation of [3H]thymidine was measured in a scintillation counter.
Apoptosis assay.
At indicated time points, cell aliquots were stained with 0.2% trypan blue and assessed for viability. The presence of apoptosis was also determined by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) analysis (Boehringer, Mannheim, Germany). Briefly, harvested cells were washed twice in 1% NHS in PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. After being permeabilized for 2 min with 1% Triton X-100 in 1% sodium citrate, cells were incubated for a further 60 min with dUTP-fluorescein. Apoptotic cells were assessed by FACScan. Cellular debris were excluded from analysis by gating by their characteristic light scatter properties; only intact or apoptotic cells were included.
ELISA.
Levels of IL-2, TNF-α, and IL-6 in culture supernatants were quantitated with commercially available enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (R&D Systems, Minneapolis, Minn.). Each culture supernatant was assayed in duplicate. Levels of HIV p24 antigen in culture supernatants were quantitated by enzyme immunoassay (HIVAG-1 monoclonal; Abbott GmbH Diagnostika, Germany).
Statistical analysis.
Data were analyzed with Student’s t test or the Mann-Whitney U test, as appropriate. Differences were considered significant if P was <0.05. Statistical analysis was performed with the SPSS statistical package (SPSS Inc., Chicago, Ill.).
RESULTS
Effects of Leishmania antigens on induction of HIV replication in CD8-depleted PBMC.
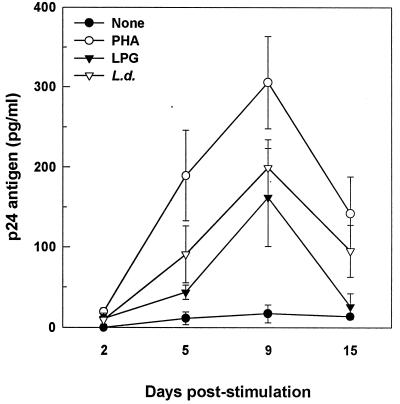
To ascertain whether Leishmania and/or LPG can modulate HIV replication in CD8-depleted PBMC, HIV-1 p24 core protein levels were measured at 2, 5, 9, and 15 days following initiation of culture. Kinetics studies showed that there was no significant difference in expression of p24 antigen on day 2, but there was a significant increase between days 5 and 9 in culture supernatants of cells primed with either LPG (3.8- and 9.5-fold increase [P < 0.05]) or Leishmania (7.9- and 11.7-fold increase [P < 0.05]) compared to the unstimulated group (Fig. 1). The peak expression of p24 antigen occurred at day 9 poststimulation.
FIG. 1.
Effects of Leishmania antigens on HIV replication in CD8-depleted PBMC. CD8-depleted PBMC were incubated in the presence or absence of PHA (5 μg/ml) (used as a positive control), LPG (12.5 μg/ml), or L. donovani (L.d.) promastigotes (at a 1:1 cell-to-parasite ratio). Half of the culture supernatants were harvested at the indicated time points and were replenished with complete RPMI medium. Levels of p24 antigen in culture supernatants were measured by ELISA. Leishmania- and LPG-treated CD8-depleted PBMC cultures had significantly higher p24 antigen levels between days 5 and 9 than did negative controls (P < 0.05). Data are expressed as means ± SEM from seven individual donors.
Effects of Leishmania antigens on cellular immune activation and apoptosis.
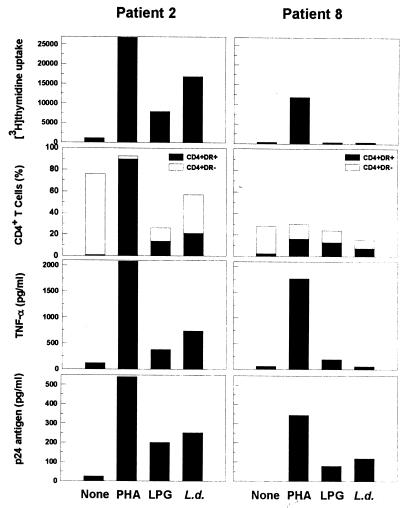
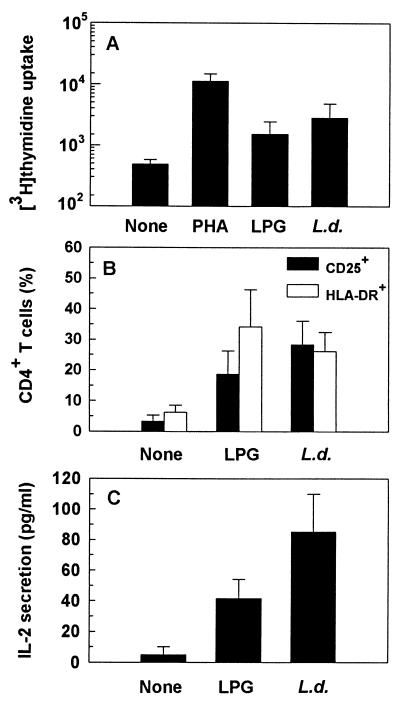
Efficient induction of HIV replication is initiated through cellular immune activation (24). Thus, we next examined whether Leishmania- and LPG-induced viral replication was associated with cellular immune activation. To address this, we used antigen-induced cellular proliferation, flow cytometric analyses of the cellular activation markers CD25 and HLA-DR, and induction of IL-2 secretion. Whereas 71% of HIV-negative individuals respond to Leishmania antigens by lymphoproliferation (26), only 2 of 8 (25%) and 4 of 8 (50%) asymptomatic HIV-positive patients responded (as defined by a stimulation index of ≥2.5) to LPG and Leishmania promastigote antigens, respectively (Table 1). An increase in cellular proliferation of 3.7- to 7.0-fold and 2.5- to 14.9-fold in response to LPG and Leishmania, respectively, was noted in responding patients depending on the donor (Fig. 2). All but one responded (as defined by an stimulation index of ≥5) to PHA (P = 0.001). Overall, though there was no significant difference in the lymphoproliferative responses of PBMC stimulated with LPG (P = 0.328), we observed that there was a significant increase in cellular proliferation of cells stimulated with Leishmania (P = 0.038) compared to unstimulated cultures (Fig. 3A). Consistent with cellular proliferation, there was a parallel increase in expansion of the CD25+ and HLA-DR+ population within the CD4+ T-cell subpopulation (Fig. 2 and 3B). Leishmania-induced HIV replication was also correlated with significant induction of IL-2 secretion (P = 0.01) (Fig. 3C).
TABLE 1.
Correlation between CD4 count and lymphoproliferation in vitro in response to Leishmania antigens in HIV-positive asymptomatic patients
| Patient | CD4+ cellsa/mm3 | [3H]thymidine uptake (cpm)b
|
|||
|---|---|---|---|---|---|
| None | PHA | LPG | L. donovani | ||
| 1 | 190 | 330 | 9,545 | 309 | 410 |
| 2 | 352 | 1,126 | 26,801 | 7,888 | 16,827 |
| 3 | 281 | 385 | 5,560 | 315 | 589 |
| 4 | 208 | 448 | 1,911 | 524 | 1,120 |
| 5 | 490 | 456 | 26,834 | 1,008 | 1,592 |
| 6 | 321 | 252 | 1,904 | 924 | 748 |
| 7 | 206 | 474 | 5,286 | 779 | 554 |
| 8 | 234 | 388 | 11,847 | 393 | 401 |
Phenotypic analysis of whole blood at baseline and before CD8+ T-cell depletion.
CD8-depleted PBMC were cultured in the presence or absence of PHA (5 μg/ml) (used as a positive control), LPG (12.5 μg/ml), or L. donovani (at a 1:1 cell-to-parasite ratio). An aliquot of cells was harvested at day 5, pulsed with [3H]thymidine for 18 h, and counted. Results are expressed as mean counts per minute of triplicate cultures. Increases in proliferative responses (test/background) by ≥2.5-fold for antigens and ≥5-fold for PHA were considered positive.
FIG. 2.
Effects of Leishmania antigens on cellular activation and virus induction in an individual with a strong Leishmania-specific response (left panels) compared to an individual with a poor response (right panels). Methods are as described in the legends to Fig. 1, 3, 4, and 5.
FIG. 3.
Effects of Leishmania antigens on cellular immune activation. For determination of cellular proliferation (A), an aliquot of cells was harvested at day 5, pulsed with [3H]thymidine for 18 h, and counted. Results are expressed as mean counts per minute ± SEM of triplicate cultures from eight independent experiments. For determination of cellular activation (B), an aliquot of cells was harvested between days 8 and 10 and assessed by FACScan for expression of CD25 or HLA-DR immune activation markers. IL-2 secretion (C) was measured by ELISA in culture supernatants harvested after 24 h of stimulation. Data in panels B and C represent mean values (± SEM) from four experiments done independently.
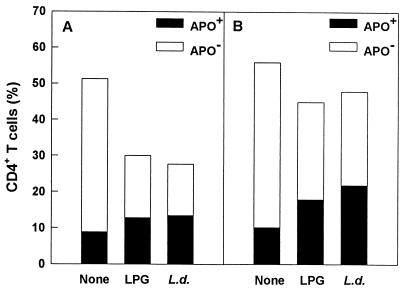
Because immune-activated cells are more prone to undergo apoptosis than are nonactivated cells and activation-induced cell death occurs in CD4+ T-cell subpopulations from HIV-infected individuals (12), we next examined whether cellular activation correlated with induction of cell death. Cellular loss was higher within the subpopulation of activated cells primed with Leishmania antigens than in unstimulated cultures, and, consistent with cell loss, Leishmania antigens induced significant CD4+ T-cell death by apoptosis compared to unstimulated cultures (Fig. 4A).
FIG. 4.
Effects of Leishmania antigens on cell loss and apoptosis (APO) in CD4+ T cells (A) and effects of thalidomide on Leishmania-induced apoptosis (B). Cells were stimulated with LPG or L. donovani (L.d.) or were left untreated for 6 days in vitro. In some cultures, cells were also treated with thalidomide. CD4+ T-cell loss was determined by FACScan. LPG or L. donovani activation-induced apoptosis was determined by FACScan analysis by the TUNEL method. The data shown were obtained from an individual and are representative of three similar experiments.
Effects of Leishmania antigens on cytokine secretion.
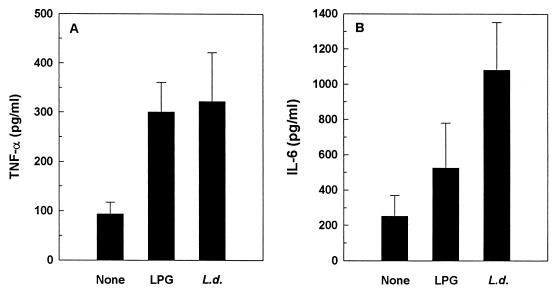
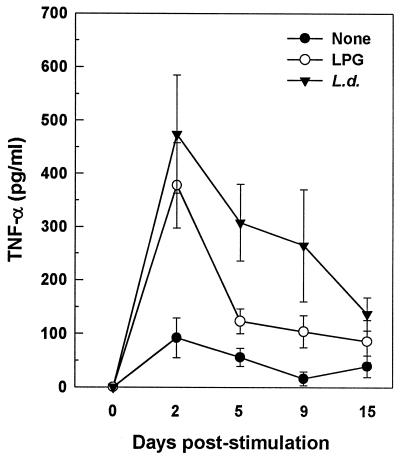
Leishmania and LPG are known to be potent inducers of several cytokines, including TNF-α and IL-6 (4, 6, 26). Furthermore, HIV-1 replication can be enhanced by these cytokines (10, 11). Thus, we next examined whether the effects of Leishmania and LPG on HIV replication were mediated through induction of these cytokines. As illustrated in Fig. 5, Leishmania- and LPG-treated CD8-depleted PBMC cultures secreted higher levels of TNF-α than did unstimulated cultures. Although Leishmania induced secretion of IL-6 (P = 0.019), no significant changes in IL-6 secretion were detected in cultures stimulated with LPG (P = 0.351). Moreover, constitutive secretions of TNF-α and IL-6 were noted in unstimulated cultures (93.5 ± 23.9 and 252 ± 117 pg/ml, respectively [means ± standard errors of the means {SEM}]). Kinetics studies of TNF-α production revealed that peak production occurred at day 2 poststimulation, though significant secretion (P < 0.05) was noted from days 2 to 9 and 2 to 15 for LPG- and L. donovani-primed cultures, respectively (Fig. 6). Of note is that peak production of TNF-α secretion preceded that of virus production (Fig. 1). The possibility that the effects of Leishmania and LPG on TNF-α and IL-6 production were due to contaminating endotoxin was ruled out, since the addition of polymyxin B (25 μg/ml) to the leishmanial antigens before culture did not affect cytokine secretion (data not shown).
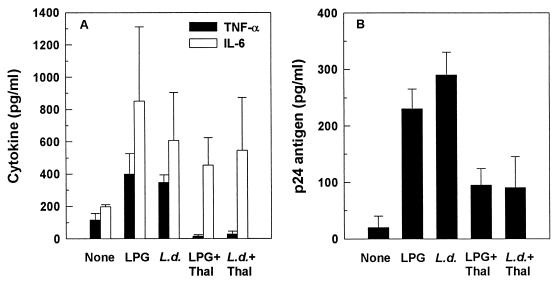
FIG. 5.
Effects of Leishmania antigens on cytokine induction. Culture supernatants collected after 48 h were analyzed for the presence of TNF-α (A) and IL-6 (B) by ELISA. L. donovani (L.d.), but not LPG, induced significant TNF-α and IL-6 secretion (P < 0.05). PHA-stimulated cultures, used as positive controls, resulted in 19-fold (P < 0.001) and 6-fold (P < 0.005) increases over negative controls in TNF-α and IL-6 production, respectively (data not shown). Data are means ± SEM from eight (A) or six (B) independent experiments.
FIG. 6.
Kinetics of TNF-α production. Culture supernatants were harvested at the indicated time points, and TNF-α levels were determined by ELISA. LPG enhanced TNF-α secretion significantly (P < 0.05) at days 2, 5, and 9, and L. donovani (L.d.) increased TNF-α secretion significantly (P < 0.05) between days 2 and 15. Data are means ± SEM of five independent experiments.
Effect of thalidomide on Leishmania antigen-induced cytokine secretion and HIV replication.
Thalidomide is an immunomodulatory compound that has been shown to selectively inhibit TNF-α production by lipopolysaccharide-stimulated monocytes (23). It has also been shown to inhibit HIV replication in vitro (16). The role of thalidomide against Leishmania antigen-mediated cytokine secretion was, therefore, analyzed by determining TNF-α, IL-6, and IL-2 levels by ELISA in culture supernatants of CD8-depleted PBMC. Thalidomide (used at a final concentration of 10 μg/ml) inhibited Leishmania antigen-induced secretion of TNF-α (P = 0.038), with no significant effect on IL-2 or IL-6 production (Fig. 7A and 8) and cellular proliferation (data not shown). Moreover, thalidomide inhibited virus replication induced by Leishmania antigens (Fig. 7B). To ascertain that the inhibitory effects of thalidomide did not simply represent killing of cells by the drug, the viability of the cells was assessed by trypan blue. Viability of cells was not affected by treatment with thalidomide (data not shown), nor was there CD4+ T-cell loss, as assessed by FACScan (Fig. 4B).
FIG. 7.
Effects of thalidomide (Thal) on Leishmania-induced cytokine production (A) and HIV replication (B). The effect of thalidomide on Leishmania antigen-mediated TNF-α and IL-6 production was assessed by ELISA at 48 h in culture supernatants of CD8-depleted PBMC. Levels of TNF-α, but not IL-6, in culture supernatants of cells treated with Leishmania antigen plus thalidomide were significantly lower than the levels in supernatants of cells treated with Leishmania antigen only. Virus replication was monitored by measuring p24 antigen levels in culture supernatants at day 9. Data are expressed as means ± SEM from three independent experiments. L.d., L. donovani.
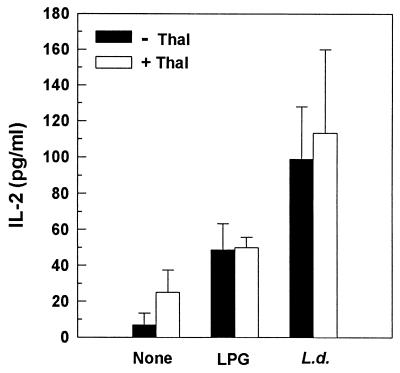
FIG. 8.
Effect of thalidomide (Thal) on Leishmania-induced IL-2 secretion. IL-2 production was assessed by ELISA at 24 h in culture supernatants. Levels of IL-2 in culture supernatants of cells treated with Leishmania antigen plus thalidomide were not significantly different (P > 0.05) from the levels in cells treated with Leishmania antigens only. L.d., L. donovani.
DISCUSSION
With the advent of HIV infection, leishmaniasis has been recognized with increasing frequency, in patients with AIDS. In some southern European countries, up to 70% of all adult cases of VL are related to HIV/AIDS, up to 9% of all AIDS patients suffer from newly acquired or reactivated VL, and mortality in AIDS-associated VL is significantly higher than in AIDS patients without VL (2). The data suggest that VL occurring concurrently with HIV/AIDS plays a significant role in accelerating the course of HIV disease progression.
Chronic immune activation by coinfecting pathogens has been suggested as a cofactor in the pathogenesis of HIV disease progression, especially in the setting of developing countries (3). Bernier and colleagues demonstrated with isolated cell lines that Leishmania and its surface constituent, LPG, can induce HIV replication in latently infected monocytes or T cells in vitro (4, 5). They did not show, however, the need for antigen presentation by macrophages to T cells to lead to virus replication. Host response to Leishmania often requires CD4+ T lymphocytes (22) and, during major histocompatibility complex class II-restricted antigen presentation of Leishmania by macrophages to CD4+ T cells latently infected with HIV, may result in cellular processes that eventually activate virus expression. Indeed, predominant HIV replication occurs under conditions that favor continuous perturbation of CD4+ T cells (24), underscoring the potential importance of Leishmania-specific activation of the immune system in HIV disease progression. In addition, it is worth noting that induction of HIV replication in an antigen-specific manner has been observed to recall antigens (11, 17). In the present study, we used CD8-depleted PBMC (11) from Leishmania antibody-negative donors latently infected with HIV in vivo to demonstrate that whole Leishmania promastigote antigen and LPG up-regulate virus replication through a process correlated with increased TNF-α secretion. That cells from individuals with no prior exposure to Leishmania respond to leishmanial antigens has been previously described (1). However, this enhanced HIV replication in whole Leishmania promastigote cultures was in the presence of generalized cellular activation processes, including enhanced expression of HLA-DR and CD25, IL-2 secretion coupled with cell proliferation, and TNF-α and IL-6 secretion. In contrast, in LPG-stimulated cells only limited evidence of cell activation, such as IL-2 and TNF-α secretion, was associated with enhanced virus replication. We demonstrate that both Leishmania antigens tested accelerated cell death by apoptosis in the activated CD4+ T-cell population. As VL is associated with chronic immune activation (6), and as activated T cells are prone to apoptosis (12), it is highly likely that the increased susceptibility of T cells from patients with HIV to cell death by apoptosis following stimulation with leishmanial antigens might be due to increased cellular immune activation mechanisms.
It has been suggested that cellular activation is a common mechanism whereby infection with pathogenic microorganisms leads to increased HIV replication, and many other studies have addressed such a mechanism (11, 17, 29). We show in this study that Leishmania-derived antigens can induce enhanced HIV replication even in the absence of profound cellular activation. The Leishmania parasite may modulate HIV replication through non-antigen-specific mechanisms by virtue of its ability to induce cytokines that are known to affect positively HIV gene expression (10). In this respect, the cytokine TNF-α, induced by Leishmania, appears to play a major role in up-regulating HIV-1 replication. Although TNF-α has been shown to mediate host protection against Leishmania (14), its uncontrolled production may result in pathology. Previous studies have shown that Leishmania-induced HIV expression in monocytes is mediated through TNF-α (4). In the present study, we demonstrate that TNF-α secretion by CD8-depleted PBMC from HIV-positive patients is increased following stimulation with whole Leishmania promastigote antigen even in those few patients where there is no significant induction of cellular immune activation, which is similar to what we have shown for HIV-negative individuals (26). In addition, LPG stimulation without cellular activation other than IL-2 and TNF-α secretion led to enhanced virus replication. The kinetics of TNF-α secretion preceded the enhanced HIV replication. These findings have led us to suggest that TNF-α is the single most important molecule whose production is required for this effect. Furthermore, it is worth noting that we have observed constitutive secretion of low levels of TNF-α by unstimulated cells; this has been suggested to be responsible for a low level of virus replication (4). The notion that Leishmania may induce HIV replication in a non-antigen-specific manner is also supported by the finding that direct stimulation of latently infected CD4+ T-cell lines by LPG can result in virus induction without the need for antigen presentation by macrophages (5), as reported for virus induced by malaria (29). However, the fact that whole Leishmania promastigote antigen induces the secretion of other cytokines, in particular IL-6, suggests that another mechanism(s) may augment this process. Given that IL-6 also activates HIV through induction of NF-κB (20), NF-IL-6 (30), and post-transcriptional mechanisms (10), however, it is highly likely that Leishmania may readily induce virus replication through both transcriptional and posttranscriptional activation mechanisms. In general, different mechanisms may work in concert to amplify viral expression (28).
TNF-α-up-regulated HIV gene expression is mediated through induction of NF-κB (13). Release of TNF-α triggered by Leishmania may then function in an autocrine or paracrine manner to induce HIV gene expression in latently infected CD4+ T cells and macrophages (4, 28). Thalidomide is an immunomodulatory compound that has been shown to selectively inhibit production of TNF-α production by lipopolysaccharide-stimulated monocytes (23). It also inhibits HIV replication in vitro (16). In the present study, we demonstrate that thalidomide inhibits Leishmania antigen-induced secretion of TNF-α but not that of IL-2 or IL-6. In addition, the drug abrogated Leishmania-induced virus replication. Our results are consistent with earlier reports that thalidomide inhibits lipoarabinomannan-induced upregulation of HIV expression (19). Specific inhibition of TNF-α production by thalidomide coupled with the other findings suggests that this cytokine may play the major role in HIV replication induced by Leishmania antigens. Moreover, thalidomide inhibited TNF-α-dependent Leishmania-induced HIV replication without affecting cellular activation, including IL-2 secretion. These findings suggest that the compound can reduce virus replication without interfering with the Th1 response essential for control of both Leishmania and HIV (8, 22).
Progression to AIDS among HIV-infected subjects has been linked to HIV replication (15). Continuous perturbation of the immune system, therefore, in the context of HIV-1 infection undoubtedly contributes, in an antigen-specific or non-antigen-specific manner, to a heightened state of HIV-1 replication. The results corroborate our recent findings that active VL increases HIV replication in vivo, as evidenced by an increase in plasma viral load in HIV-coinfected patients who fail antileishmanial chemotherapy (unpublished data). Chemoprophylactic regimens for Leishmania in HIV-coinfected patients may prevent reactivation of VL and probably replication of HIV. Moreover, by reducing the number of circulating Leishmania-infected monocytes, it may also decrease secondary cases arising from increased transmission. Both leishmaniasis and HIV are associated with severe cachexia, which has been linked to inappropriate secretion of cytokines, in particular TNF-α (6, 10). Thus, the role of thalidomide in modulating immune activation and altering HIV replication (16, 23) raises the possibility that such a drug is of potential use as an adjuvant for decreasing the accelerated rate of HIV disease progression in patients with concurrent leishmaniasis. Indeed, thalidomide has been proven to be important in tuberculosis-HIV coinfection (25) and warrants the conducting of trials in VL-HIV coinfection.
ACKNOWLEDGMENTS
This work was supported by grants from the Armauer Hansen Research Institute (Addis Ababa, Ethiopia) and the Swedish International Development Agency (SIDA/SAREC). D.W. was a recipient of the African Research Fellowship of the Armauer Hansen Research Institute.
We thank our blood donors, without whom this study would not have been possible. We also thank Nega Berhe, Institute of Pathobiology, Addis Ababa University (Ethiopia), for providing Leishmania parasites, and S. J. Turco, Department of Biochemistry, University of Kentucky Medical Center, Lexington, for providing LPG.
REFERENCES
- 1.Akuffo H, Maasho K, Howe R. Natural and acquired resistance to Leishmania: cellular activation by Leishmania aethiopica of mononuclear cells from unexposed individuals is through the stimulation of natural killer (NK) cells. Clin Exp Immunol. 1993;94:516–521. doi: 10.1111/j.1365-2249.1993.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Canavante C, Gutierrez-Solar B, Jimenez M, Laguna F, Lopez-Valez R, Molina R, Moreno J. Leishmania and HIV co-infection: the first 10 years. Clin Microbiol Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentwich Z, Kalinovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 4.Bernier R, Turco S J, Olivier M, Tremblay M. Activation of human immunodeficiency virus type 1 in monocytoid cells by the protozoan parasite Leishmania donovani. J Virol. 1995;69:7282–7285. doi: 10.1128/jvi.69.11.7282-7285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernier R, Barbeau B, Tremblay M J, Olivier M. The lipophosphoglycan of Leishmania donovani up-regulates HIV-1 transcription in T cells through the nuclear factor-κB elements. J Immunol. 1998;160:2881–2888. [PubMed] [Google Scholar]
- 6.Cenini P, Berhe N, Hailu A, McGinnes K, Frommel D. Mononuclear cell subpopulations and cytokine levels in human visceral leishmaniasis before and after chemotherapy. J Infect Dis. 1993;168:986–993. doi: 10.1093/infdis/168.4.986. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1992;41:1–17. [PubMed] [Google Scholar]
- 8.Clerici M, Shearer G M. The Th1-Th2 hypothesis of HIV-1 infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 9.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–766. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 10.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 11.Goletti G, Weissman D, Jackson R W, Graham N M H, Vlahov D, Klein R S, Munsiff S S, Ortona L, Cauda R, Fauci A S. Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- 12.Gougeon M L, Lecoeur H, Dulioust A, Enouf M G, Crouvoisier M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 13.Griffin G E, Leung K, Folks T M, Kunkel S, Nabel G J. Activation of HIV gene expression during monocyte differentiation by induction of NF-κB. Nature. 1989;339:70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- 14.Liew F W, Li Y, Millott S. Tumor necrosis factor-α synergizes with interferon-γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 15.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 16.Moreira A L, Corral L G, Ye W, Johnson B, Stirling D, Muller G W, Freedman V H, Kaplan G. Thalidomide and thalidomide analogs reduce HIV-1 replication in human macrophages in vitro. AIDS Res Hum Retroviruses. 1997;13:857–863. doi: 10.1089/aid.1997.13.857. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski M A, Stanley S K, Justement J S, Gantt K, Golletti D, Fauci A S. Increased in vitro tetanus-induced production of HIV-1 following in vivo immunization of HIV-1 infected individuals with tetanus toxoid. AIDS Res Hum Retroviruses. 1997;13:473–480. doi: 10.1089/aid.1997.13.473. [DOI] [PubMed] [Google Scholar]
- 18.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 19.Peterson P K, Gekker G, Bornemann M, Chatterjee D, Chao C C. Thalidomide inhibits lipoarabinomannan-induced upregulation of human immunodeficiency virus expression. Antimicrob Agents Chemother. 1995;39:2807–2809. doi: 10.1128/aac.39.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poli G, Bressler P, Kinter A, Duh E, Timmer W C, Rabson A, Justement J S, Stanley S, Fauci A S. Interleukin-6 induces HIV expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed S G, da Silva J S, Ho J L, Koehler J K, Russo D M, Pihl D L, Coombs R W. Cytokine activation of human macrophages infected with HIV-1 to inhibit intracellular protozoa. J Acquir Immune Defic Syndr. 1992;5:666–675. [PubMed] [Google Scholar]
- 22.Reiner S L, Locksley R M. Cytokines in the differentiation of Th1/Th2 CD4+ subsets in leishmaniasis. J Cell Biochem. 1993;53:323–328. doi: 10.1002/jcb.240530409. [DOI] [PubMed] [Google Scholar]
- 23.Sampaio E P, Sarno E N, Galilly R, Cohn Z A, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor-alpha production by monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T-cell activation and proviral integration. EMBO J. 1990;9:1151–1160. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tramontana J M, Utaipat U, Molloy A, Akarasewi P, Burroughs M, Makonkawkeyoon S, Johnson B, Klausner J D, Rom W, Kaplan G. Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med. 1995;1:384–397. [PMC free article] [PubMed] [Google Scholar]
- 26.Wolday D, Akuffo H, Britton S, Hathaway A, Sander B. HIV-1 inhibits Leishmania-induced cell proliferation, but not TNF-alpha and IL-6 production. Scand J Immunol. 1994;39:380–386. doi: 10.1111/j.1365-3083.1994.tb03389.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolday D, Akuffo H, Fessehaye G, Valantine A, Britton S. Killed and live HIV-1 increases the intracellular multiplication of Leishmania donovani in monocyte-derived cells. Scand J Infect Dis. 1998;30:29–34. doi: 10.1080/003655498750002268. [DOI] [PubMed] [Google Scholar]
- 28.Wolday D, Berhe N, Akuffo H, Britton S. Leishmania-HIV interaction: immunopathogenic mechanisms. Parasitol Today. 1999;15:182–187. doi: 10.1016/s0169-4758(99)01431-3. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L, Sherry M, Rudolph D L, Lal R B, Lal A A. Plasmodium falciparum antigen-induced HIV-1 replication is mediated through induction of TNF-α. J Infect Dis. 1998;177:437–445. doi: 10.1086/514212. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Nakata K, Weiden M, Rom W N. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Investig. 1995;95:2324–2331. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]