Abstract
Head and neck squamous cell carcinomas (HNSCCs) belong to a group of diverse tumors, which can be induced by infection with human papillomavirus (HPV) or tobacco and alcohol consumption. The viral etiology of HNSCC relates to better clinical outcomes reflecting a different immune system response. Here, we retrospectively analyzed 97 tissue samples from oral and oropharyngeal carcinomas associated and non-associated with HPV infection using multispectral fluorescent immunohistochemistry. To evaluate the immune cell infiltration in tumor and stroma compartments, we designed four panels of four to five antibodies. We detected more T lymphocytes in the stroma, compared to the tumor parenchyma. In HPV positive (HPV+) in comparison to HPV negative (HPV−) tumors, higher counts of CD3+CD4+, CD3+CD8+, PD1+CD4+, PD1+CD8+ T cells, and ICOS− Treg cells were detected while more ICOS+ Treg cells and CTLA4+CD4+ T cells were observed in HPV− than in HPV+ tumors. The results of the univariate and multivariate analyses confirmed the predominant impact of HPV status on prognosis. More importantly, the number of CD8+PD-1+ T cells was identified as an independent factor, influencing the overall and/or disease-specific survival of patients with oral cavity or oropharyngeal carcinomas.
Keywords: head and neck cancer, PD-1, survival, HPV
1. Introduction
Head and neck squamous cell carcinomas (HNSCCs) are the world’s sixth most common cancer with locations in the oropharynx, hypopharynx, nasopharynx, larynx, oral cavity, and nasal cavity [1]. Historically, most HNSCCs were associated with tobacco and alcohol consumption; however, in recent decades, a growing number of oropharyngeal (OP) and oral cavity (OC) tumors are caused by a persistent infection with high-risk human papillomavirus (HPV) [1,2]. Patients with HPV-positive (HPV+) tumors are younger and, compared to patients with non-virally induced tumors (HPV−), have better prognosis, reflecting their improved treatment response [3,4,5].
One of the factors that can explain the difference in clinical outcomes between patients with different tumor etiology is their immune response. The phenotypes and numbers of tumor-infiltrating lymphocytes (TIL) as one of the components of the tumor microenvironment (TME) were described as a significant prognostic factor in many tumors, including HNSCC [5,6,7,8,9]. Moreover, the level of CD3+ and CD8+ T lymphocytes in a tumor invasive margin in colorectal carcinoma was recently introduced as an immunoscore, which serves as a prognostic factor for colorectal carcinoma patients and is now approved as an addition to the conventional tumor-node-metastasis (TNM) staging [6]. Multispectral fluorescence immunohistochemistry (fIHC) is a powerful tool for a detailed analysis of the TME. This method allows us to assess the phenotype and quantity of cell types in different compartments of the tumor, since, in comparison to flow cytometry, the architecture of the tissue remains preserved. The fIHC is uniquely suited for the study of the interaction between immune and cancer cells in situ.
CD4+ and CD8+ T lymphocytes are important TIL subpopulations in the TME. As CD4+ T lymphocytes include diverse functional subpopulations, their effect on prognosis is difficult to decipher. Many studies do not analyze T lymphocyte spatial location in the TME and HPV status of HNSCC tumors, which modify the observed correlations [10]. CD8+ T cells represent the main anti-tumor TIL population and are considered as a positive prognostic factor in the majority of tumors [10,11,12]. High CD8+ TILs infiltration was also shown to be associated with better survival of patients with OP, hypopharyngeal, and laryngeal SCC [9,13]. The anti-tumor function of T cells in TME may be suppressed by the FOXP3+ regulatory T cells (Treg) activity or by the PD-1 (programmed cell death 1)/PD-L1 (PD-1 ligand) interactions [14]. Moreover, the expression of the inducible co-stimulator (ICOS) receptor on T cells, including Treg, can regulate the checkpoint inhibitors efficacy [15]. In this retrospective study with a 10-year follow-up, we analyzed the HNSCC TME with respect to the presence of an active HPV infection and prognostic significance of the studied TILs.
2. Materials and Methods
2.1. Sample Collection, Processing, and Characterization
Samples of primary HNSCC (ICD-10: C01–C06, C09, C10) and non-malignant tonsillar tissue were provided by the Department of Otorhinolaryngology and Head and Neck Surgery, Motol University Hospital, Prague. They were collected, processed, and characterized in previous studies between 2001–2012 [4,16,17]. Briefly, they were screened for the presence and type of HPV and active viral infection and were classified by a pathologist using the TNM nomenclature as valid at the time of patients’ enrollment (7th edition) [18]. For this study, 97 formalin-fixed and paraffin-embedded (FFPE) samples were selected. According to the presence of active viral infection, they were divided into HPV+ or HPV−. All patients signed an informed consent form and completed a questionnaire about risk factors for HPV infection and HNSCC induction when enrolled in the study approved by the Ethical Committee of the Motol University Hospital in 2001.
2.2. Tissue Sections Preparation and Antibodies Validation
From each FFPE tumor tissue block, 2 μm sections were prepared on Superfrost® Plus microscope slides (VWR, Leuven, Belgium) and confirmed by a pathologist for the presence of tumor tissue. The slides were deparaffinized at 60 °C for two hours, washed in xylene for 3 × 10 min, hydrated with descending grades of ethanol (100%, 96%, and 70%; Penta, Prague, Czech Republic), and fixed for 20 min in neutral buffered formalin, pH 7.2–7.4 (Diapath, Martinengo, Italy). Heat-induced epitope retrieval (HIER) was carried out by using microwave (96–98 °C) in buffer of pH 9.0 (AR9, Tris-EDTA; Zytomed Systems, Berlin, Germany) or pH 6.0 (AR6, citrate buffer; Akoya Biosciences, Menlo Park, CA, USA). After 10 min of blocking using Antibody Diluent/Block (Akoya Biosciences), the slides were incubated with the primary antibodies listed in Table 1 and subsequently stained with the Opal™ 7-Color Manual IHC Kit (Akoya Biosciences), according to the manufacturers’ instructions. Validation of the antibodies was carried out on samples of non-malignant tonsillar and HNSCC tissue. To verify staining specificity, the corresponding isotype controls, as well as no-primary controls, were performed using the appropriate isotype antibody or Antibody Diluent/Block, respectively.
Table 1.
List of antibodies.
| Name | Clone | Manufacturer |
|---|---|---|
| CD3e | SP7 | ThermoFisher Scientific (Waltham, MA, USA) |
| CD4 | EP204 | Zeta Corporation (Arcadia, CA, USA) |
| CD8-α | C8/144B | Santa Cruz Biotechnology (Dallas, TX, USA) |
| FoxP3 | 206D | BioLegend (San Diego, CA, USA) |
| PD-1 | EPR4877(2) | Abcam (Cambridge, United Kingdom) |
| CTLA4 | F-8 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| ICOS | SP98 | Abcam (Cambridge, United Kingdom) |
| VEGF | EP1176Y | Biocare Medical (Pacheco, CA, USA) |
| Ki67 | sc-23900 | Santa Cruz Biotechnology (Dallas, TX, USA) |
| CCR4 | polyclonal | Novus Biological (Centennial, CO, USA) |
| Cytokeratin Pan Type I/II | AE1/AE3 | ThermoFisher Scientific (Waltham, MA, USA) |
2.3. Multispectral fIHC Panel Design and Optimization
We designed four different antibody panels. A staining order for primary antibodies was set with respect to different HIER length requirements and stability of the epitopes after multiple HIER treatments. For matching fluorophores with the primary antibodies, possible colocalization of antibodies in the panel was considered. After each round of staining, the complex of primary and secondary antibodies was removed by HIER using the antigen retrieval (AR) buffer, optimal for the following antibody in the panel. To ensure complete removal of the antibodies complex, stripping quality controls were performed. The final settings for each panel with optimized dilutions of antibodies are specified in Table S1.
2.4. Image Acquiring and Processing
All slides were snapped by using the MantraSnap 1.0.3 software included in the Mantra Quantitative Pathology Workstation (Akoya Biosciences) for DAPI, FITC, Cy3, Texas Red, and Cy5 data acquisition. From each tissue section, five different regions of interest were randomly selected with a 20X objective. Images were analyzed using the InForm 2.4.6. software (Akoya Biosciences). Four separated algorithms for trainable tissue and cell segmentation and cell phenotyping were prepared. The tissue was segmented into the tumor parenchyma, stroma, and background compartments and checked by the pathologist. The algorithms were trained to specifically phenotype the cells according to different expression of markers. The optimization workflow of algorithms is listed in Supplement Figure S1. For determining the phenotype of the cells, a phenotyping confidence cut off of 80% was set. The numbers of positive cells of different phenotypes were counted separately per megapixel (Mpx) for the tumor parenchymal and stromal compartments. We introduced several TIL phenotypes for each panel (Table 2).
Table 2.
Immune cell phenotypes in Panels 1–4.
| Markers | Markers | ||
|---|---|---|---|
| Panel 1 | Th: CD3+CD4+ | Panel 3 | CD3+ |
| Tc: CD3+CD8+ | Ki67+ | ||
| Treg: FOXP3+CD3+CD4+ | VEGF+ | ||
| Panel 2 | CD4+ | Panel 4 | CD4+ |
| PD-1+CD4+ | FOXP3+CD4+ | ||
| CTLA4+CD4+ | ICOS+CD4+ | ||
| CD8+ | ICOS+FOXP3+CD4+ | ||
| PD-1+CD8+ | CCR4+ | ||
| CTLA4+CD8+ | CD4+FOXP3+CCR4+ |
Abbreviations: Th—helper T, Tc—cytotoxic T, Treg—regulatory T, PD-1—programmed cell death 1, CTLA4—cytotoxic T lymphocyte antigen 4, VEGF—vascular endothelial growth factor, ICOS—inducible T cell co-stimulator, CCR4—C-C chemokine receptor 4.
2.5. Statistical Analysis
The numbers of cells of distinct phenotypes were evaluated separately for the parenchyma and the stroma and counted per Mpx for the five regions of interest. Differences between parenchyma and stroma infiltration were evaluated using the sign test, and the Mann–Whitney U test was used to detect differences between the HPV+ and HPV− groups. Patients were grouped based on HPV E6 mRNA detection as HPV+ or HPV−.
The Cox proportional hazards model was applied for the overall survival (OS) and disease-specific survival (DSS) analyses. The following factors were included: age, gender, education (≤12 years, >12 years), smoking history (nonsmoker, ex-smoker, smoker), alcohol use (nondrinker, ex-drinker, drinker), tumor location (oropharyngeal, oral), pathological tumor extension (pT1–4), pathological nodal status (pN0–3), tumor stage (S I, II, III, IV), metastasis (pM1), tumor grade (1–3), and relapse (yes, no). As the samples were collected between 2001 and 2012, the tumors were classified according to the TNM 7 staging system, hence, the p16 positive tumors do not have a separate category. Furthermore, the p16 status (p16+, p16−), HPV status (HPV+, HPV−), and cell numbers of distinct phenotypes per Mpx separately in the parenchyma and the stroma or in total were evaluated. The zero values in the cell numbers were substituted by the 0.1 value as the lowest non-zero value. The models were analyzed using the Bayesian information criterion (BIC). For all the statistical tests, a p value of <0.05 was considered as a significant difference. The statistical analyses were performed using the GraphPad Prism 8.4.2. software (GraphPad Software, San Diego, CA, USA) and R version 4.0.2 (https://www.R-project.org/ accessed on 02 November 2022) [19].
3. Results
3.1. Patients’ Characterization
From the previously characterized cohort [4,16], 97 patients were chosen according to the HPV E6 expression status and tumor location. The selected group consisted of 78 men (78/97, 80%) and 19 women (19/97, 20%) with a median age of 58 years. The median of follow-up time was 45 months. The patients were divided based on HPV E6 mRNA detection into the HPV+ (45/97, 46%) and HPV− (52/97, 54%) groups. Of the 97 patients, 18 relapsed and 43 died. The patient demographics and clinical characteristics are listed in Table 3.
Table 3.
Patient demographics and clinical characteristics.
| Characteristics | Total | HPV+ | HPV− | |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| No. of patients | 97 (100%) | 45 (46%) | 52 (54%) | |
| Age | Mean age | 57.23 | 57.98 | 56.58 |
| (years) | Median age | 58 | 59 | 56 |
| Gender | female | 19 (20%) | 10 (22%) | 9 (17%) |
| male | 78 (80%) | 35 (78%) | 43 (83%) | |
| Tumor location | oropharynx | 83 (86%) | 45 (100%) | 38 (73%) |
| oral cavity | 14 (14%) | 0 (0%) | 14 (27%) | |
| Education a | >12 years | 33 (34%) | 18 (41%) | 15 (29%) |
| ≤12 years | 63 (66%) | 26 (59%) | 37 (71%) | |
| Smoking | never | 19 (20%) | 15 (33%) | 4 (8%) |
| past | 32 (33%) | 19 (42%) | 13 (25%) | |
| current | 46 (47%) | 11 (25%) | 35 (67%) | |
| Alcohol | never | 20 (21%) | 12 (27%) | 8 (15%) |
| consumption | past | 13 (13%) | 7 (16%) | 6 (12%) |
| current | 64 (66%) | 26 (57%) | 38 (73%) | |
| No. of sex partners | >7 | 41 (46%) | 15 (35%) | 26 (55%) |
| ≤6 | 49 (54%) | 28 (65%) | 21 (45%) | |
| Tumor size | T1 | 17 (18%) | 7 (16%) | 10 (19%) |
| (pT) | T2 | 61 (63%) | 27 (60%) | 34 (65%) |
| T3 | 13 (13%) | 9 (20%) | 4 (8%) | |
| T4 | 6 (6%) | 2 (4%) | 4 (8%) | |
| Nodal status | N0 | 31 (32%) | 9 (20%) | 22 (42%) |
| (pN) | N1 | 16 (16.5%) | 5 (11%) | 11 (21%) |
| N2 | 47 (48.5%) | 28 (62%) | 19 (37%) | |
| N3 | 3 (3%) | 3 (7%) | 0 (0%) | |
| Metastasis | 0 | 97 (100%) | 45 (100%) | 52 (100%) |
| (M) | 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| Tumor stage | I | 5 (5%) | 1 (2%) | 4 (8%) |
| (S) | II | 24 (25%) | 6 (13%) | 18 (35%) |
| III | 16 (16%) | 7 (16%) | 9 (17%) | |
| IV | 52 (54%) | 31 (69%) | 21 (40%) | |
| Tumor grade | 1 | 14 (14%) | 3 (7%) | 11 (21%) |
| 2 | 57 (59%) | 25 (55%) | 32 (62%) | |
| 3 | 26 (27%) | 17 (38%) | 9 (17%) | |
| Relapse | no | 82 (85%) | 41 (91%) | 41 (79%) |
| yes | 15 (15%) | 4 (9%) | 11 (21%) |
a For one patient, data were not available.
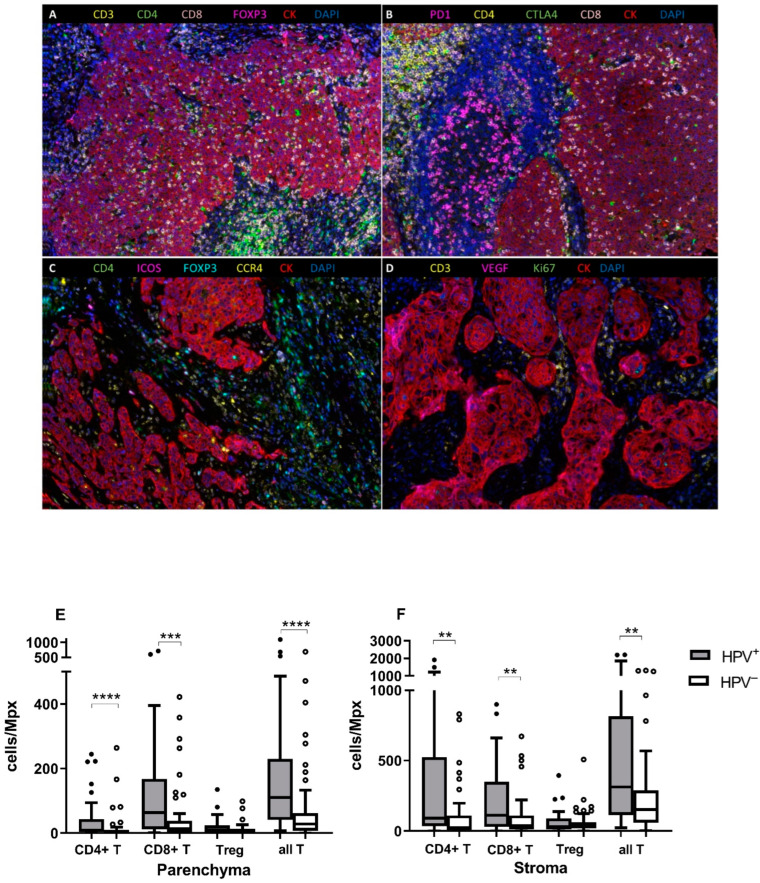
3.2. HPV+ Tumors Are More Infiltrated by T lymphocytes
We analyzed 97 FFPE samples with four antibody panels using multispectral fIHC (Figure 1A–D). For panel 2, three samples were excluded due to poor quality of staining. Regardless of the tumor status, the higher infiltration with CD3+CD4+ (Th), CD3+CD8+ (Tc), and Treg cells was detected in the stroma, compared to the parenchyma (p < 0.0001 for all cell populations, data not shown). For all measured populations, we observed a highly or moderately positive correlation of TIL counts/Mpx between the parenchyma and the stroma compartments (Table S2). Next, we analyzed the data with respect to tumor etiology. Significantly higher infiltration by all T cells (sum of Th, Tc, and Treg cells), Th cells, and Tc cells was detected both in the parenchyma and the stroma of HPV+ tumors (all T cells p < 0.0001 for parenchyma, p = 0.0014 for stroma; Th p < 0.0001 for parenchyma, p = 0.0017 for stroma; Tc p = 0.0003 for parenchyma, p = 0.0093 for stroma). The Treg counts did not statistically differ between the two tumor compartments neither in the HPV+ nor in HPV− groups. However, we observed statistically non-significantly higher Treg counts in HPV+ patients (Figure 1E,F).
Figure 1.
Representative multispectral fIHC staining of FFPE tissue samples with four antibody panels, 20X magnification. (A) CD3 (yellow), CD4 (green), CD8 (pink), FOXP3 (magenta), CK (red), and DAPI (blue) staining. (B) PD-1 (magenta), CD4 (yellow), CTLA4 (green), CD8 (pink), CK (red), and DAPI (blue) staining. (C) CD4 (green), ICOS (magenta), FOXP3 (cyan), CCR4 (yellow), CK (red), and DAPI (blue) staining. (D) CD3 (yellow), VEGF (magenta), Ki67 (green), CK (red), and DAPI (blue) staining. T lymphocytes infiltration in the parenchyma and the stroma compartments according to tumor etiology. (E) The numbers of CD3+CD4+, CD3+CD8+, all CD3+ T lymphocytes, and Treg cells per Mpx were compared between the HPV+ and HPV− groups in the parenchyma and (F) the stroma compartments. Significantly higher infiltration of CD3+CD4+, CD3+CD8+, and all CD3+ T lymphocytes was observed in the HPV+ groups in both compartments. The observed difference for Treg cells was not statistically significant. ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, Mann–Whitney U test.
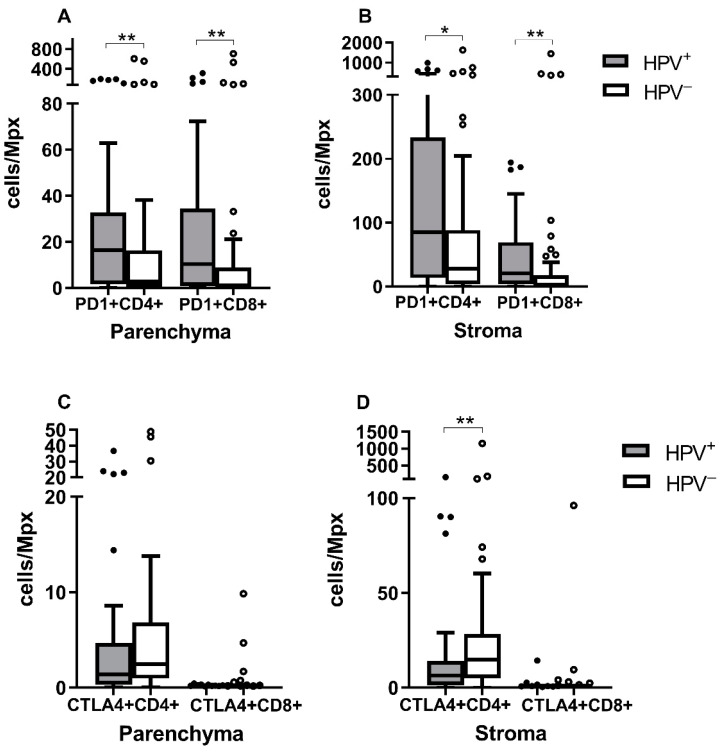
3.3. PD-1+CD4+ and PD-1+CD8+ Levels Are Higher in HPV+ Tumors
To determine the functional status of CD4+ and CD8+ TILs, we detected the expression of PD-1 and CTLA4 proteins. As shown in Figure 2A,B, significantly higher quantities of PD-1+CD4+ and PD-1+CD8+ T cells were observed both in the parenchyma and the stroma of HPV+ tumors than in HPV− tumors (PD-1+CD4+ p = 0.0092 for parenchyma, p = 0.0125 for stroma; PD-1+CD8+ p = 0.0039 for parenchyma, p = 0.0055 for stroma). Increased levels of CTLA4+CD4+ T cells were detected in the stroma and parenchyma of HPV− tumors, but the difference was statistically significant only for the stroma (p = 0.0021). No difference was observed in the CTLA4+CD8+ T cell numbers between the two groups in either the parenchyma or the stroma (Figure 2C,D).
Figure 2.
PD-1+ or CTLA4+ T lymphocyte infiltration in different compartments of the tumors of different etiology. The numbers of CD4+ and CD8+ T lymphocytes expressing the PD-1 molecule in (A) the parenchyma and (B) the stroma, and the numbers of CD4+ and CD8+ T lymphocytes producing the CTLA4 molecule in (C) the parenchyma and (D) the stroma were compared between groups. PD-1 expression was significantly higher in both compartments of HPV+ tumors, while the CTLA4 production was significantly higher only in the stroma of HPV− tumors. * p ≤ 0.05, ** p ≤ 0.01, Mann–Whitney U test.
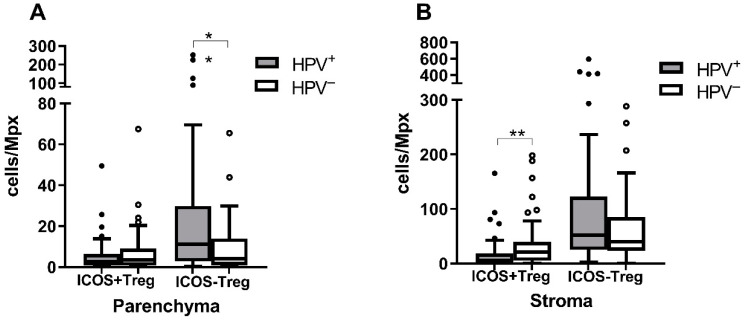
3.4. ICOS+ Treg Are More Abundant in HPV− Tumors
As we did not observe differences in Treg numbers between the HPV+ and HPV− groups, we compared the levels of the Treg subpopulation, producing the costimulatory molecule ICOS (ICOS+FOXP3+CD4+). The ICOS+ Treg subpopulation was more abundant in the HPV− than in the HPV+ tumors (p = 0.0034 for the stroma, p = 0.4334 for the parenchyma) while the ICOS− Treg (ICOS−FOXP3+CD4+) numbers were higher in the HPV+ tumors (p = 0.2158 for the stroma, p = 0.0044 for the parenchyma) (Figure 3).
Figure 3.
Comparison of the ICOS+ and ICOS− Treg levels in the HPV+ and HPV− tumors. The numbers of Treg cells with or without ICOS production per Mpx were compared in (A) the parenchyma and (B) the stroma of the tumors of different etiology. Higher ICOS+ Treg levels were observed in both the parenchyma and stroma of the HPV− tumors, with the difference being significant for the stroma. Higher ICOS− Treg numbers were detected again in both the parenchyma and stroma of HPV+ tumors, with the difference being significant for the parenchyma. * p ≤ 0.05, ** p ≤ 0.01, Mann–Whitney U test.
3.5. The Infiltration Level of T Cell Subpopulations Is Prognostic for OS and DSS
To evaluate the influence of TIL infiltration on prognosis, we used Cox models. We fitted 38 models for OS and DSS analyses. Models with and without HPV status and with the evaluation of the parenchyma and stroma separately or combined were tested. The models with the best BIC score are listed in Table 4, and the remaining models are shown in Table S3. In the models with HPV involvement and parenchyma/stroma distinction for OS (BIC = 308.96), the HPV RNA positivity appeared to be the strongest predictive factor for OS, and the lower tumor stage and higher number of parenchymal PD-1+CD8+ T cells related to better OS (Table 4). The best model for DSS (BIC = 169.16) includes a lower CD4+/Treg ratio, which was associated with better survival, along with the positive HPV RNA status and a higher number of stromal PD-1+CD8+ T cells (Table 4).
Table 4.
Hazard ratio (HR) values for cell populations from the best models influencing overall survival (OS) and disease-specific survival (DSS).
| Models Including HPV Status, IHC Markers Evaluated with Respect to the Stromal or Parenchymal Location. | |||
|---|---|---|---|
| OS | DSS | ||
| BIC = 308.96 | HR (p value) | BIC = 169.16 | HR (p value) |
| HPV RNA+ | 0.26 (0.0003) | HPV RNA+ | 0.15 (0.0011) |
| Increasing tumor stage | 1.55 (0.0179) | CD3+CD4+/Treg ratio | 3.18 (0.0016) |
| Parenchymal PD1+CD8+ T | 0.53 (0.0003) | Stromal PD1+CD8+ T | 0.44 (0.0007) |
| Models including HPV status, the IHC markers counted regardless of location. | |||
| OS | DSS | ||
| BIC = 307.31 | HR (p value) | BIC = 171.50 | HR (p value) |
| HPV RNA+ | 0.26 (0.0004) | HPV RNA+ | 0.22 (0.0057) |
| Increasing tumor stage | 1.57 (0.0160) | CD3+ T | 2.79 (0.0094) |
| PD1+CD8+ T | 0.52 (0.0001) | PD1+CD8+ T | 0.36 (0.0001) |
| Models without HPV status inclusion, the IHC markers evaluated with respect to the stromal or parenchymal location. | |||
| OS | DSS | ||
| BIC = 313.13 | HR (p value) | BIC = 174.57 | HR (p value) |
| Smoking (No) | 0.37 (0.0055) | Increasing age | 0.93 (0.0032) |
| Increasing tumor size | 1.44 (0.0709) | Stromal CD8+ T | 2.19 (0.0165) |
| Parenchymal PD1+CD8+ T | 0.52 (0.0003) | Stromal PD1+CD8+ T | 0.30 (0.0000) |
In the models where the IHC markers were evaluated without tissue segmentation but HPV status was included, HPV RNA positivity, higher number of all PD-1+CD8+ T cells, and lower tumor stage or nodal status were connected with better OS and DSS, and low number of all CTLA4+CD8+ T cells was linked to better OS (Table 4 and Supplement Table S3).
In models not including the HPV status, the main predictors of worse OS were positive smoking history, a lower number of parenchymal PD-1+CD8+ T cells, a higher number of all CTLA4+CD8+ T, and tumor size or tumor stage. Similarly, for DSS, a lower number of stromal PD-1+CD8+ T cells, together with the number of stromal CD8+ T cells, and higher age related to worse survival (Table 4 and Table S3).
We have shown the level of stromal or parenchymal PD-1+CD8+ T cells to be a positive prognostic predictor in all models. Additionally, rising tumor stage or nodal status appeared to be a negative predictor for both OS and DSS (Table S3). Our data suggest that the level of PD-1+CD8+ TIL can serve as an independent prognostic marker for HNSCC patients and may improve the current prognosis assessment.
4. Discussion
The major aim of this retrospective study was to analyze the tumor microenvironment in HNSCC of different etiology in order to explain the better prognosis of patients with HPV-positive tumors and to evaluate non-viral markers in the populations of TILs as a possible independent prognostic tool for HNSCC patients.
The prognostic significance of the TIL count was evaluated in several malignancies and recently this parameter has been approved as an additional characteristic to the TNM classification for colorectal carcinomas [5,6,7,8,9]. It has also been proposed that the spatial distribution of TILs in the tumor parenchyma or stroma relates to their different biological function in the process of tumorigenesis [20].
In our study, HPV-induced tumors were more infiltrated by CD3+CD4+ and CD3+CD8+ T cells. Higher infiltration by T cells of HPV+ tumors than HPV− tumors may be explained by the presence of viral antigens, which stimulate the immune response more strongly. Despite the differences in their designs, several studies have come to similar results. Badoual et al. found higher CD4+ T cell counts in the stroma of HNSCC tumors [21]. Other studies regardless of the method used detected a statistically significantly higher infiltration of CD3+, CD4+, and CD8+ T lymphocytes in both the parenchyma and the stroma of HPV+ tumors [22,23,24,25,26,27,28]. Although we observed higher Treg cell infiltration in HPV+ tumors, the difference from HPV− tumors was not statistically significant. The same was shown by IHC [25], gene expression [28], and flow cytometry analyses [27,29]. Significantly higher Treg counts in the HPV+ cohort, in comparison to HPV− tumors, were only reported by a single study for tonsillar SCC [23]. To analyze Treg cells more thoroughly, their functional status was evaluated according to the expression of the ICOS molecule. ICOS serves as a co-stimulatory receptor of immunogenic T cells, but it is also involved in the activation of Treg cells resulting in the immunosuppression [15]. ICOS+ Treg cells were more prevalent in the stroma and parenchyma of HPV− tumors, but the difference was statistically significant only for the stroma. Inversely, ICOS− Treg counts were higher in both compartments of HPV+ tumors with a statistically significant difference for parenchyma location. To our knowledge, this is the first study analyzing the spatial prevalence of ICOS on Treg cells in HNSCC of different etiology, and the results suggest that these cells can have different function in HPV+ and HPV− tumors.
Furthermore, we have detected significantly higher counts of PD-1+CD4+ and PD-1+CD8+ T cells in both the parenchyma and the stroma of HPV+ tumors, compared to HPV− tumors. Higher levels of PD-1+CD4+ and/or PD-1+CD8+ T cells and PD-1 mRNA were also observed in HPV+ than in HPV− HNSCC by others [27,30,31]. It was also shown that PD-1 expression was more frequent on CD4+ than CD8+ T cells in HNSCC [21,32], which agrees with our study. Oguejiofor et al. observed a higher level of PD-1+CD8+ cells in the stroma, compared to the parenchyma but with no difference in the PD-1+CD8+ counts between HPV+ and HPV− OPSCC [22].
Thanks to the long-term follow-up, we were able to explore the impact of TILs infiltration on prognosis, which is the main benefit of this study. We fitted several models, including clinicopathological characteristics, HPV RNA status, and tissue compartment. We were also interested if any of the studied population of TILs would be an independent prognostic marker for HNSCC regardless of the HPV association. The influence of TILs on prognosis was also analyzed by others who, unfortunately, did not take account of HPV status and/or spatial distribution was not considered, which makes the comparison of results difficult. We found that a higher number of parenchymal or stromal PD-1+CD8+ T cells is associated with both better OS and DSS of OC and OPSCC patients in all of the models. When HPV status was included, the multivariate analysis confirmed HPV positivity as the strongest independent positive prognostic factor for OS and DSS [3,4].
In OC and OPSCC, a higher number of CD8+ T cells was associated with better OS or DFS [23,24,33,34,35]. However, other studies did not confirm this observation [30,36,37,38]. The interplay between subfamilies of TILs in different tumor compartments is as complex as the outcome of these interactions. Therefore, it is important to analyze TILs more deeply with respect to their functional status. The PD-1 molecule is expressed by activated T lymphocytes, pointing to the persistent antigen stimulation, and subsequent T cell exhaustion. However, in the TME context, the PD-1 expression may not be followed by the expression of co-inhibitory molecules and, therefore, is not a marker of T cell exhaustion [30,39]. In the HPV− cohort, it has been suggested that PD1+CD8+ TIL cells act as a tumor-specific population, compared to the PD1−CD8+ TILs and play a key anti-tumor role [39]. It was also shown that high infiltration by PD-1+CD4+ T cells in HPV+ tumors and by PD-1+CD8+ T cells in HPV+ and HPV− tumors related to better OS than low infiltration by these cell populations [30,39]. The increased numbers of PD-1+CD8+ T cells in tumors suggest that patients contain tumor-specific T cells that can be activated and—before silencing by inhibitory molecules, such as PD-1—exert an antitumor effect, supplementing both radiotherapy and surgery and enhancing survival. Due to the limited number of markers, which can be detected using multispectral fIHC, we are not able to characterize the PD-1+CD8+ T cells in the more detailed manner, which is the limitation of this study. To describe the status of PD-1+CD8+ T cells in the TME, expression of other activation and/or exhaustion markers can be examined on a fresh tumor tissue, using other methods, such as flow cytometry. However, the results of our study and of others indicate that this marker should be included in the prognostic immunoscore for HNSCC [30,39]. The multivariate analyses did not reveal the influence of the counts of CD4+ T lymphocytes on prognosis, which was also observed by others [24,30]. In our study, the levels of PD-1, CTLA4, and ICOS-expressing CD4+ T cells were analyzed in the context of prognosis, and none of these subpopulations influenced OS or DSS.
The level of Treg cells itself did not affect OS or DSS but was positively correlated with a better locoregional control in HNSCC [21]. It was proposed that ICOS+ Treg cells in the TME have higher immunosuppressive capacity than the ICOS− Treg population [15]. The higher ICOS+ Treg counts found in the HPV− tumors may relate to worse outcomes of HPV− patients. In our study, despite the findings of higher ICOS+Treg cell counts in HPV− tumors, no influence of this subpopulation on OS or DSS was detected. Similar to Lukesova et al. who observed the phenomenon in the blood, in our models, we found that a low CD8+/FOXP3+ ratio related to improved OS and DSS [40]. In the study of de Ruiter et al., the CD8+/FOXP3+ ratio measured in HPV− tumor tissue microarray cores did not corelate with improved survival [41]. However, in agreement with our study, Nasman identified a high CD8+/FOXP3+ ratio, connected with better DFS in both HPV+ and HPV− groups [23].
5. Conclusions
In this study, we evaluated the level of TILs in different compartments of the HNSCC microenvironment using multispectral IHC. We compared the TILs levels with respect to the presence of an active HPV infection and tumor compartment in a retrospective cohort of patients under long-term follow-up. The impact of different TIL infiltration levels on prognosis was studied. We revealed that higher levels of PD-1+CD8+ T lymphocytes improved the prognosis of the patients in all our models, which points out the importance of this marker and entitles it to be included in the prognostic immunoscore for patients with OC and OPSCC of different etiology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10112794/s1, Table S1:Reaction conditions for each panel of antibodies; Table S2: Cell counts/Mpx in the parenchyma and stroma of HNSCC tumors; Table S3: Models of DSS and OS; Figure S1: Optimization of algorithms for automatic picture analysis.
Author Contributions
Conceptualization, B.P. and R.T.; methodology, B.P.; software, B.P.; validation, B.P., R.T., J.N. and O.V.; formal analysis, B.P.; investigation, B.P.; resources, J.K. and M.G.; data curation, J.N., O.V. and B.P.; writing—original draft preparation, B.P.; writing—review and editing, R.T., M.G. and J.K.; visualization, B.P.; supervision, R.T.; project administration, R.T.; funding acquisition, R.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Motol University Hospital (IRB 00000947, 4/25/2001) and of the Institute of Hematology and Blood Transfusion (IRB 00001238, 4/26/2001).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are publicly available on Zenodo. Barbora Pokryvkova, Marek Grega, Jan Klozar, Ondrej Vencalek, Jaroslav Nunvar, Ruth Tachezy (2022), “PD1+CD8+ cells are an independent prognostic marker in patients with head and neck cancer”, doi: 10.5281/zenodo.6874821.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Czech Health Research Council, grant number 17-28055A and the European Regional Development Fund and the Ministry of Education, Youth and Sports of the Czech Republic, grant number CZ.02.1.01/0.0/0.0/16_019/0000785, by the project National Institute of Virology and Bacteriology (Programme EXCELES, ID Project No. LX22NPO5103)—Funded by the European Union—Next Generation EU, and by the Charles University, grant number SVV 260568.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tumban E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses. 2019;11:922. doi: 10.3390/v11100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison M.L., Chaturvedi A.K., Anderson W.F., Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koslabova E., Hamsikova E., Salakova M., Klozar J., Foltynova E., Salkova E., Rotnaglova E., Ludvikova V., Tachezy R. Markers of HPV infection and survival in patients with head and neck tumors. Int. J. Cancer. 2013;133:1832–1839. doi: 10.1002/ijc.28194. [DOI] [PubMed] [Google Scholar]
- 4.Rotnaglova E., Tachezy R., Salakova M., Prochazka B., Kosl’abova E., Vesela E., Ludvikova V., Hamsikova E., Klozar J. HPV involvement in tonsillar cancer: Prognostic significance and clinically relevant markers. Int. J. Cancer. 2011;129:101–110. doi: 10.1002/ijc.25889. [DOI] [PubMed] [Google Scholar]
- 5.Ward M.J., Thirdborough S.M., Mellows T., Riley C., Harris S., Suchak K., Webb A., Hampton C., Patel N.N., Randall C.J., et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer. 2014;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagès F., Mlecnik B., Marliot F., Bindea G., Ou F.S., Bifulco C., Lugli A., Zlobec I., Rau T.T., Berger M.D., et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 7.Wong P.F., Wei W., Smithy J.W., Acs B., Toki M.I., Blenman K.R.M., Zelterman D., Kluger H.M., Rimm D.L. Multiplex Quantitative Analysis of Tumor-Infiltrating Lymphocytes and Immunotherapy Outcome in Metastatic Melanoma. Clin. Cancer Res. 2019;25:2442–2449. doi: 10.1158/1078-0432.CCR-18-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnem T., Hald S.M., Paulsen E.E., Richardsen E., Al-Saad S., Kilvaer T.K., Brustugun O.T., Helland A., Lund-Iversen M., Poehl M., et al. Stromal CD8+ T-cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 9.Borsetto D., Tomasoni M., Payne K., Polesel J., Deganello A., Bossi P., Tysome J.R., Masterson L., Tirelli G., Tofanelli M., et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers. 2021;13:781. doi: 10.3390/cancers13040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonson W.T.N., Allison K.H. Tumour-infiltrating lymphocytes in cancer: Implications for the diagnostic pathologist. Diagn. Histopathol. 2011;17:80–90. doi: 10.1016/j.mpdhp.2010.10.006. [DOI] [Google Scholar]
- 11.Nakano O., Sato M., Naito Y., Suzuki K., Orikasa S., Aizawa M., Suzuki Y., Shintaku I., Nagura H., Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 12.Oudejans J.J., Jiwa N.M., Kummer J.A., Ossenkoppele G.J., van Heerde P., Baars J.W., Kluin P.M., Kluin-Nelemans J.C., van Diest P.J., Middeldorp J.M., et al. Activated cytotoxic T cells as prognostic marker in Hodgkin’s disease. Blood. 1997;89:1376–1382. doi: 10.1182/blood.V89.4.1376. [DOI] [PubMed] [Google Scholar]
- 13.de Ruiter E.J., Ooft M.L., Devriese L.A., Willems S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1356148. doi: 10.1080/2162402X.2017.1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb E.S., Liu P., Baleeiro R., Lemoine N.R., Yuan M., Wang Y.H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018;32:317–326. doi: 10.7555/jbr.31.20160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amatore F., Gorvel L., Olive D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin. Ther. Targets. 2018;22:343–351. doi: 10.1080/14728222.2018.1444753. [DOI] [PubMed] [Google Scholar]
- 16.Vojtechova Z., Sabol I., Salakova M., Turek L., Grega M., Smahelova J., Vencalek O., Lukesova E., Klozar J., Tachezy R. Analysis of the integration of human papillomaviruses in head and neck tumours in relation to patients’ prognosis. Int. J. Cancer. 2016;138:386–395. doi: 10.1002/ijc.29712. [DOI] [PubMed] [Google Scholar]
- 17.Tachezy R., Klozar J., Rubenstein L., Smith E., Saláková M., Smahelová J., Ludvíková V., Rotnáglová E., Kodet R., Hamsíková E. Demographic and risk factors in patients with head and neck tumors. J. Med. Virol. 2009;81:878–887. doi: 10.1002/jmv.21470. [DOI] [PubMed] [Google Scholar]
- 18.Edge S.B. AJCC Cancer Staging Manual. Volume 7. Springer; Berlin/Heidelberg, Germany: 2010. pp. 97–100. [Google Scholar]
- 19.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 20.Khoury T., Nagrale V., Opyrchal M., Peng X., Wang D., Yao S. Prognostic Significance of Stromal Versus Intratumoral Infiltrating Lymphocytes in Different Subtypes of Breast Cancer Treated With Cytotoxic Neoadjuvant Chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2018;26:523–532. doi: 10.1097/PAI.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badoual C., Hans S., Rodriguez J., Peyrard S., Klein C., Agueznay Nel H., Mosseri V., Laccourreye O., Bruneval P., Fridman W.H., et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 22.Oguejiofor K., Galletta-Williams H., Dovedi S.J., Roberts D.L., Stern P.L., West C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 2017;8:14416–14427. doi: 10.18632/oncotarget.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasman A., Romanitan M., Nordfors C., Grun N., Johansson H., Hammarstedt L., Marklund L., Munck-Wikland E., Dalianis T., Ramqvist T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS ONE. 2012;7:e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordfors C., Grun N., Tertipis N., Ahrlund-Richter A., Haeggblom L., Sivars L., Du J., Nyberg T., Marklund L., Munck-Wikland E., et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer. 2013;49:2522–2530. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Oguejiofor K., Hall J., Slater C., Betts G., Hall G., Slevin N., Dovedi S., Stern P.L., West C.M. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Cancer. 2015;113:886–893. doi: 10.1038/bjc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Succaria F., Kvistborg P., Stein J.E., Engle E.L., McMiller T.L., Rooper L.M., Thompson E., Berger A.E., van den Brekel M., Zuur C.L., et al. Characterization of the tumor immune microenvironment in human papillomavirus-positive and -negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother. 2021;70:1227–1237. doi: 10.1007/s00262-020-02747-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partlova S., Boucek J., Kloudova K., Lukesova E., Zabrodsky M., Grega M., Fucikova J., Truxova I., Tachezy R., Spisek R., et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4:e965570. doi: 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mito I., Takahashi H., Kawabata-Iwakawa R., Ida S., Tada H., Chikamatsu K. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci. Rep. 2021;11:16134. doi: 10.1038/s41598-021-95718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poropatich K., Fontanarosa J., Swaminathan S., Dittmann D., Chen S., Samant S., Zhang B. Comprehensive T-cell immunophenotyping and next-generation sequencing of human papillomavirus (HPV)-positive and HPV-negative head and neck squamous cell carcinomas. J. Pathol. 2017;243:354–365. doi: 10.1002/path.4953. [DOI] [PubMed] [Google Scholar]
- 30.Badoual C., Hans S., Merillon N., Van Ryswick C., Ravel P., Benhamouda N., Levionnois E., Nizard M., Si-Mohamed A., Besnier N., et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 31.Kansy B.A., Concha-Benavente F., Srivastava R.M., Jie H.B., Shayan G., Lei Y., Moskovitz J., Moy J., Li J., Brandau S., et al. PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017;77:6353–6364. doi: 10.1158/0008-5472.CAN-16-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lechner A., Schlößer H., Rothschild S.I., Thelen M., Reuter S., Zentis P., Shimabukuro-Vornhagen A., Theurich S., Wennhold K., Garcia-Marquez M., et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget. 2017;8:44418–44433. doi: 10.18632/oncotarget.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spector M.E., Bellile E., Amlani L., Zarins K., Smith J., Brenner J.C., Rozek L., Nguyen A., Thomas D., McHugh J.B., et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2019;145:1012–1019. doi: 10.1001/jamaoto.2019.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee G., Bag S., Chakraborty P., Dey D., Roy S., Jain P., Roy P., Soong R., Majumder P.P., Dutt S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLoS ONE. 2020;15:e0242058. doi: 10.1371/journal.pone.0242058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon B., Young R.J., Bressel M., Urban D., Hendry S., Thai A., Angel C., Haddad A., Kowanetz M., Fua T., et al. Prognostic Significance of PD-L1(+) and CD8(+) Immune Cells in HPV(+) Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018;6:295–304. doi: 10.1158/2326-6066.CIR-17-0299. [DOI] [PubMed] [Google Scholar]
- 36.Quan H., Shan Z., Liu Z., Liu S., Yang L., Fang X., Li K., Wang B., Deng Z., Hu Y., et al. The repertoire of tumor-infiltrating lymphocytes within the microenvironment of oral squamous cell carcinoma reveals immune dysfunction. Cancer Immunol. Immunother. 2020;69:465–476. doi: 10.1007/s00262-020-02479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poropatich K., Hernandez D., Fontanarosa J., Brown K., Woloschak G., Paintal A., Raparia K., Samant S. Peritumoral cuffing by T-cell tumor-infiltrating lymphocytes distinguishes HPV-related oropharyngeal squamous cell carcinoma from oral cavity squamous cell carcinoma. J. Oral. Pathol. Med. 2017;46:972–978. doi: 10.1111/jop.12605. [DOI] [PubMed] [Google Scholar]
- 38.Wolf G.T., Chepeha D.B., Bellile E., Nguyen A., Thomas D., McHugh J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral. Oncol. 2015;51:90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu K., Fu Y., Han Y., Xia R., Xu S., Duan S., Zhang Z., Li J. Fewer tumour-specific PD-1(+)CD8(+) TILs in high-risk "Infiltrating" HPV(-) HNSCC. Br. J. Cancer. 2020;123:932–941. doi: 10.1038/s41416-020-0966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukesova E., Boucek J., Rotnaglova E., Salakova M., Koslabova E., Grega M., Eckschlager T., Rihova B., Prochazka B., Klozar J., et al. High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. Biomed. Res. Int. 2014;2014:303929. doi: 10.1155/2014/303929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Ruiter E.J., de Roest R.H., Brakenhoff R.H., Leemans C.R., de Bree R., Terhaard C.H.J., Willems S.M. Digital pathology-aided assessment of tumor-infiltrating T lymphocytes in advanced stage, HPV-negative head and neck tumors. Cancer Immunol. Immunother. 2020;69:581–591. doi: 10.1007/s00262-020-02481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are publicly available on Zenodo. Barbora Pokryvkova, Marek Grega, Jan Klozar, Ondrej Vencalek, Jaroslav Nunvar, Ruth Tachezy (2022), “PD1+CD8+ cells are an independent prognostic marker in patients with head and neck cancer”, doi: 10.5281/zenodo.6874821.