Abstract
Researchers have made crucial advances in understanding the pathogenesis and therapeutics of non-small cell lung cancer (NSCLC), improving our understanding of lung tumor biology and progression. Although the survival of NSCLC patients has improved due to chemoradiotherapy, targeted therapy, and immunotherapy, overall NSCLC recovery and survival rates remain low. Thus, there is an urgent need for the continued development of novel NSCLC drugs or combination therapies with less toxicity. Although the anticancer effectiveness of curcumin (Cur) and some Cur analogs has been reported in many studies, the results of clinical trials have been inconsistent. Therefore, in this review, we collected the latest related reports about the anti-NSCLC mechanisms of Cur, its analogs, and Cur in combination with other chemotherapeutic agents via the Pubmed database (accessed on 18 June 2022). Furthermore, we speculated on the interplay of Cur and various molecular targets relevant to NSCLC with discovery studio and collected clinical trials of Cur against NSCLC to clarify the role of Cur and its analogs in NSCLC treatment. Despite their challenges, Cur/Cur analogs may serve as promising therapeutic agents or adjuvants for lung carcinoma treatment.
Keywords: curcumin, curcumin analogs, non-small cell lung cancer, combined treatment, signaling pathways
1. Introduction
Malignant lung cancer tumors are associated with the highest worldwide morbidity and mortality, with an incidence rate of 14.5 men and 8.4 women per 100,000 individuals [1]. The most common cause is long-term exposure to tobacco carcinogens that result in bronchial mucosal or gland lesions, contributing to the formation of lung tumors. Other related risk factors include environmental pollution, genetic susceptibility, chronic obstructive pneumonia, and infection [2]. The incidence of lung cancer in non-smokers is approximately 10–15 per 100,000 in the US. Notably, results of a meta-analysis indicate that lung cancer in non-smokers seems to be a distinct disease caused by mutations in driver genes, such as those for the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) [3]. Lung cancer has traditionally been categorized into small cell lung cancer and non-small cell lung cancer (NSCLC), the latter of which accounts for approximately 85% of all lung cancers, including lung adenocarcinoma (LUADs), lung squamous cell carcinoma (LUSCs), and large cell carcinoma subtypes [4]. However, some researchers have found that LUADs and LUSCs appear distinct in transcriptomics, pathology, and clinical treatment, suggesting that considering these two types of carcinomas as different—abandoning the existing classifications—may help develop novel specific agents [5]. At present, the clinical treatments for NSCLC include surgery, chemotherapy (such as mitomycin, gemcitabine, ifosfamide, and cisplatin), immunotherapy (including durvalumab), targeted therapy (e.g., EGFR inhibitors, vascular endothelial growth factor [VEGF] receptor inhibitors, and ALK inhibitors), and combined therapy [6]. Although these therapies have greatly improved the survival rate of patients with NSCLC, overall survival and recovery rates are still low, and tumor cells are not completely eliminated. In addition, most lung cancers originate in airway stem cell niches, indicating that lung tumors have cancer stem cells with substantial self-renewing and self-healing abilities [7]. Moreover, multiple drug resistance and cross resistance are not uncommon in NSCLC, and the etiology and pathogenesis are complex, requiring the development of more effective NSCLC treatment strategies.
Curcumin (Cur), a natural polyphenolic compound extracted from the root of turmeric, possesses diverse pharmacologic activities, including anti-diabetes [8], anti-aging [9], anti-Parkinson’s disease and Alzheimer’s disease [10], anti-cardiovascular disease [11], and anti-cancer [12], etc. Specifically, in tumor treatment, modern medicine has demonstrated that Cur exerts therapeutic effects on various cancers, including breast cancer [13], colorectal neoplasm [14,15], liver carcinoma [16,17], glioblastoma [18,19], gastric tumor [20,21], and lung carcinoma [22,23], etc. Many preclinical data have demonstrated that it is an excellent compound for different disease treatments. However, Cur also has a “dark side” exhibited in poor pharmacokinetic/pharmacodynamic (PK/PD) properties, low plasma and tissue levels, and rapid metabolism, which limits its clinical application. Researchers have attempted to change these unfavorable effects by screening Cur analogs, using piperine that interferes with glucuronidation, manufacturing liposomal Cur or nanometer Cur, and a combination of some molecule compounds [24,25].
In preclinical trials, Cur and its analogs have demonstrated control over NSCLC cell proliferation and metastasis, the promotion of cell apoptosis, and the regulation of autophagy via multiple mechanisms. Nevertheless, it is still unclear whether the observed Cur suppression of NSCLC in preclinical experiments occurs in humans. Hence, in this review, the objective is to clarify the role of Cur and its analogs in NSCLC treatment to accelerate the development of associated fields and potential clinical applications.
2. The Impact of Cur on NSCLC
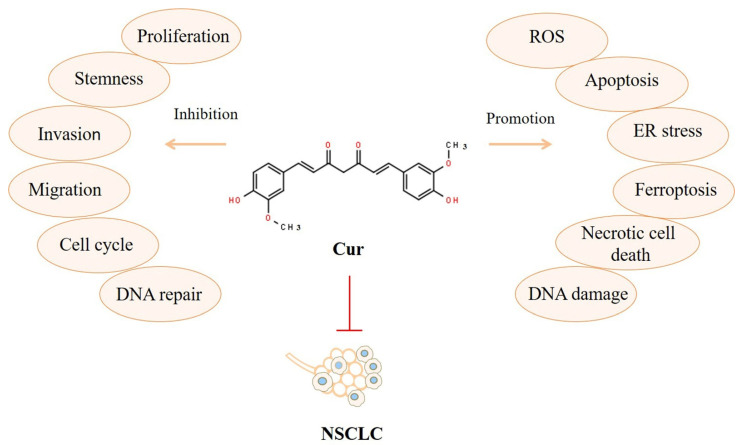
We determined via a PubMed database search that researchers first reported assessing Cur as an anticancer drug in 1985 [26]. Ten years later, investigators found that Cur could suppress lung tumor metastasis and extend the life span of mice [27]. Since then, many NSCLC preclinical studies revealed that Cur could inhibit tumor nodules [27]; restrain cancer stem cells [28]; control the cell cycle [29]; suppress immigration, invasion, and repair [30,31,32]; induce the production of ROS and ER stress [33]; trigger apoptosis [34,35]; elevate DNA damage and ferroptosis [32,36]; and promote necrotic cell death [37], thereby treating and preventing NSCLC. The broad actions through which Cur can affect NSCLC in vitro and in vivo are summarized in Figure 1.
Figure 1.
Broad actions of Cur against NSCLC.
2.1. The Effects of Cur on the Proliferation, Invasion, and Metastasis of Lung Cancer
Malignant proliferation and highly active invasion or migration have long been considered the cause of cancer immortality. In recent years, in-depth studies of cancer progression have revealed that Cur suppresses tumors by interfering with all aspects of tumor progression, which is the action of some of the most promising anticancer drugs. First, at the root of cancer progression, Cur has been shown to elevate the ubiquitination level of TAZ that increases proteasome-degrading TAZ protein, thereby activating the hippo pathway and negatively regulating cancer stem cell function [38]. Additionally, Cur significantly impedes the self-healing of circulating cancer stem cells, limiting stem cell metastasis [28]. Cur also alters the expression of more than 700 genes linked to carcinoma development, such as those involved in DNA recovery or associated with the cell cycle, cell proliferation, or metastasis in NCI-H460 human lung cancer cells [39]. By detecting the entire transcriptome in Cur-controlled A549 cell lines, researchers revealed that Cur not only changes the expression of many genes, but also alters signaling pathways [40]. Through further investigation, it was found that those Cur-altered genes induce cell death and control extracellular matrix receptors, repressing NSCLC cell proliferation and migration [40]. These observations indicate that Cur governs NSCLC tumor growth and exhibits cytotoxic mechanisms at the genetic level.
Cur can directly stop NSCLC cell proliferation through the downregulation of Axl receptor tyrosine kinase and the inhibition of XIAP [41]. Moreover, the blockade of Bcl-2 and stimulation of Bax and cytochrome C by Cur inhibits A549 cell growth, indicating that Cur’s inhibitory action is closely related to the mitochondrial apoptosis pathway [42]. Cur also significantly hindered the angiogenesis of tumors by inhibiting the activation of STAT3 and JAK in an orthotopic xenograft model, reducing tumor size and weight [43]. Notably, heat shock protein 40 (HLJ1), a tumor suppressor, metastasis-associated protein 1 (MTA1), and E-cadherin protein play an important role in the proliferation and metastasis of NSCLC. A study on adenocarcinoma cell lines in mice showed that Cur could activate the JNK/JunD pathway to upregulate tumor suppressor HLJ1 expression and increase E-cadherin expression, blocking lung cancer cell invasion and migration [44]. It was also reported that Cur retards the expression of MTA1 along with the Wnt/β-catenin pathway to restrain proliferation and invasion in NSCLC [45,46]. Furthermore, Cur manipulates pathway crosstalk between the Wnt pathway and the adherens junction by blockading the early growth response protein (EGR-1), thereby exerting anti-proliferation and anti-metastasis effects in NSCLC 95D cells [47]. Cur also directly suppresses the levels of Toll-like receptor 4 (TLR4)/MyD88 and EGFR, thereby controlling cell cycle and epithelial–mesenchymal transition (EMT)-related checkpoints and repressing cell growth and invasion in NSCLC [29].
The hormone adiponectin is linked to insulin resistance and carcinogenesis. Although researchers have reported low levels of adiponectin in gastric and prostate carcinomas [48], the role of this hormone in NSCLC is controversial. Cur was shown to obstruct adiponectin receptor 1, resulting in the downregulation of adiponectin expression and the inhibition of metastasis and tumor growth of A549 cells [31]. A preclinical study of A549 cells showed that Cur blocks the migration and invasion of NSCLC by decreasing matrix metalloproteinase (MMP)-2 and 9 and VEGF; p-ERK and MEKK3 signaling pathways are also involved [49]. Notably, an investigation of MMP-9 expression and E-cadherin expression in radiation-treated A549 cells revealed markedly richer EMT than in untreated A549 cells. However, Cur inhibits the expression of MMP-9 and E-cadherin, reversing radiation-induced EMT and mitigating the invasion and metastasis of NSCLC [50].
In smokers, cigarette smoke is a potential tumor promoter because of various established carcinogens, such as polycyclic aromatic hydrocarbons, benzo(a)pyrenes, and nicotine-derived nitrosamines. These substances can alter the normal structure of lung tissue via stimulating the secretion of growth factors, neurotransmitters, and cytokines, and promote cell proliferation and cancer metastases by interfering with cell cycle progression, migration, invasion, and angiogenesis [51]. Most of these influences are due to tobacco activating cell surface neuronal nicotinic acetylcholine receptors (nAChRs) or a certain amount of β-adrenergic receptors (β-AR) to trigger downstream intracellular signaling cascades [51,52]. Considerable evidence has showed that α7-nAChR—a subtype of nAChRs—is the most powerful regulator in the oncogenic process [53,54,55]. Furthermore, in 2009, Hildegard M. Schuller explained in detail that α7-nAChR agonists can induce NSCLC cells proliferation and angiogenesis and inhibit NSCLC apoptosis through multiple pathways [56]. Therefore, nAChRs subtype-selective antagonists are regarded as a good target for the treatment of NSCLC. However, the latest research findings have revealed that Cur has been confirmed to serve as a α7-nAChR-positive allosteric modulator (PAM) [57]. Cur not only drives more Ca2+ entry into the cell by modulating α7-nAChR allosterically, thereby exerting neuroprotective effects [58], but also attenuates disturbed oxidative stress and improves autistic spectrum disorder by potentiating α7-nAChR [59]. Consequently, Cur may activate α7-nAChR to promote the effects of nicotine to which the lungs of smokers are exposed. If so, Cur may have detrimental effects on the treatment of NSCLC. However, there are no reports on the influence of Cur on α7-nAChR in NSCLC. In addition, due to the extensive pharmacological activities of Cur, it is possible that different doses of Cur have different effects on diverse diseases. Therefore, more research is needed to clarify the relationship between Cur and α7-nAChR to provide more evidence for the use of Cur in the treatment of NSCLC.
Nevertheless, it has also been reported that tobacco activates NF-κB, subsequently inhibiting apoptosis, promoting proliferation, and provoking tumorigenesis [60]. Cur was found to retard levels of cyclin D1, COX-2, and matrix MMP-9 to inactivate NF-κB, attenuating lung carcinogenesis in smokers [60].
2.2. The Effects of Cur on the Autophagy and Apoptosis of Lung Cancer
The impact of autophagy on cancers has been controversial, as autophagy can inhibit or promote tumors depending on the specific context and carcinoma progression [61]. In this way, autophagy acts dynamically in early and late-stage tumorigenesis. Cur, as an autophagy activator, causes ferroptosis, increasing the protein levels of ACSL4 and decreasing the expression of SLC7A11 and GPX4 to suppress growth and facilitate NSCLC cell death [36]. The number of NSCLC cases dramatically declines in Cur-exposed A549 cells by triggering apoptosis and autophagy through PI3K/AKT/mTOR pathway inhibition [62,63]. In addition to autophagy, Cur can induce NSCLC apoptosis by elevating the [Ca2+] level, resulting in Ca2+ overload [64]. Cur also induces oxidative stress-mediated Bcl-2 ubiquitination and Bax upregulation, causing NSCLC apoptosis [35]. The apoptosis of NCI-H460 cells is linked to an increase of ROS, intracellular Ca2+ and ER stress, and the FAS-caspase-8 (extrinsic) pathway in the Cur treatment group [33]. Moreover, increased ROS continually stimulates mitochondria to induce cell death and promote DNA damage, resulting in G2/M arrest and NSCLC cytotoxicity [65]. The suppression of the PI3K/AKT/mTOR pathway by Cur also provokes NSCLC apoptosis, indicating that the pathway exerts dual effects on autophagy and apoptosis [62]. Moreover, Cur also induces orthotopic NSCLC xenograft apoptosis, the mechanisms of which are associated with the suppression of the expressions of IkB, nuclear p65, COX-2, and p-ERK1/2 [66].
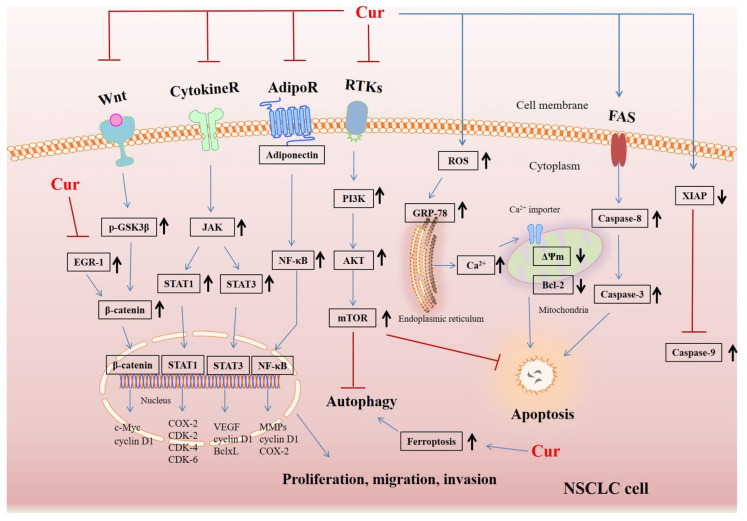
The many kinds of related signaling pathways obstructed or activated by Cur that inhibit NSCLC in vitro and in vivo are presented in Figure 2.
Figure 2.
The associated signaling pathways through which Cur suppresses NSCLC.
2.3. The Effects of Cur on the miRNAs and LncRNAs of Lung Cancer
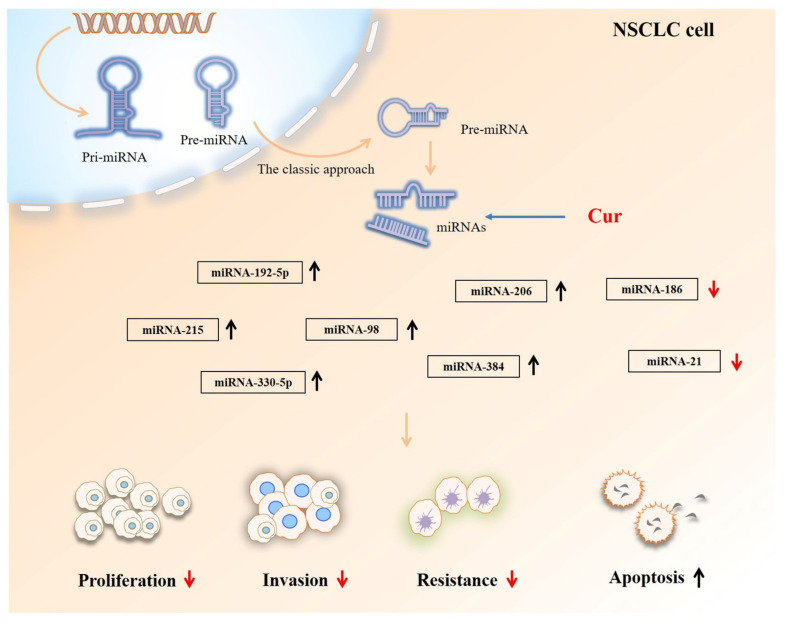
Abnormal miRNA can influence cancer pathogenesis, as epigenetic modulations are considered important for carcinoma development. Natural compounds such as Cur manipulate multiple miRNAs to restore the epigenetic balance. For example, Cur upregulates miR-192-5p expression by targeting cMyc and suppressing the Wnt/β catenin pathway to limit the growth, migration, and invasion of NSCLC [67]. Furthermore, silencing miR-192-5p was found to reverse the inhibitory activity of Cur [67]. In addition to inhibiting proliferation, Cur promotes the apoptosis of H460 and A427 cells by upregulating the p53-miR-192-5p and miR-215-XIAP pathways, thereby inducing NSCLC death [34]. Similarly, Cur increases miR-206 expression by blocking the PI3K/AKT/mTOR pathway, obstructing NSCLC migration and invasion [30]. Cur has also been reported to elevate the levels of miR-330-5p (of the miR-330 family), thereby retarding A549 cell proliferation. These effects are reduced when the miR-330-5p inhibitor is added to Cur-treated A549 cells [68]. Cur was found to induce miR-98 overexpression, targeting the gene LIN28A. This activity subsequently inhibits the production of MMP-2 and MMP-9, which suppresses A549 cell migration and invasion [69]. Furthermore, the antitumor effects of Cur on NSCLC are reduced when miR-21 expression is inhibited [70]. Cur decreases circ-PRKCA levels, which is accompanied by the downregulation of ITGB1 expression and increased levels of miR-384 to repress NSCLC progression [71]. Notably, in A549/DDP multidrug-resistant human lung adenocarcinoma cells, Cur remarkably reduced the levels of miR-186 to accelerate A549/DDP cell apoptosis [72]. These findings suggest that Cur is involved in multiple crucial components of NSCLC treatment by manipulating the levels of key miRNAs (shown in Figure 3). In gemcitabine-resistant NSCLC, Cur promotes the expression of lncRNA-MEG3 and PTEN, which is linked to the inhibition of cancer growth [73]. Unfortunately, studies on Cur’s effect on NSCLC cells via interference with lncRNA expression are sparse.
Figure 3.
Cur controls the levels of specific key miRNAs against NSCLC.
3. The Combination of Cur and Some Small Molecular Compounds in NSCLC
Drug resistance, which is key to chemotherapy failure in tumors, occurs due to tumor heterogeneity, immune system dysfunction, microenvironment alterations, genetic influences, and other increasingly novel mechanisms [74,75]. Researchers attempt to overcome or minimize drug resistance in lung tumors to boost the efficacy of chemotherapy drugs by identifying novel mechanisms, changing therapeutic strategies, creating small-molecule therapeutic compounds or peptides, and using intensive drug combination treatments. Many studies have reported that Cur is a perfect adjunctive agent as it increases the sensitivity of NSCLC to some chemotherapeutic drugs or other anticancer agents, synergistically retarding the growth of NSCLC by regulating different mechanisms (Table 1).
Table 1.
Combination of Cur and some small molecular compounds against NSCLC in preclinical studies.
| Items | Dosage | Assay Type | Mechanisms | Outcomes | References |
|---|---|---|---|---|---|
| Cur plus inorganic chemotherapy drug | |||||
| Cur plus cisplatin | 41 μM Cur and 30 μM cisplatin for A549 cells; 33 μM Cur and 7 μM cisplatin for H2170 cells | A549 and H2170 cells | Suppression of the self-renewal capability of cancer stem cells | Synergistic inhibition of NSCLC | [76] |
| Cur plus cisplatin | In vitro: 2–32 µM Cur and 0.5–8 µg/mL cisplatin In vivo: 2.5 mg/kg cisplatin and 50 mg/kg Cur |
A549, H1299, and NCI-H460 cells and BALB/c mice | Upregulating the levels of CTR1 and Sp1 to increase more Pt2+ uptake | Enhancing sensitivity and antitumor effects of cisplatin in NSCLC | [77] |
| Cur plus cisplatin | 40 μM Cur and 20 μM cisplatin | A549 and H1703 cells | Decreasing XRCC1 expression to restrain the repair of platinum-DNA in NSCLC cells. | Enhancing cytotoxic effect in NSCLC cells | [78] |
| Cur plus cisplatin | 10–40 µM Cur and ≤3 µM cisplatin | A549 and H2170 cells | Triggering intrinsic apoptotic pathway to limit CSC growth | Elevating cisplatin sensitivity of the double-positive (CD166+/EpCAM+) CSC subpopulation in NSCLC cells | [79] |
| Cur plus cisplatin | 0–20 µg/mL Cur and 0–20 µg/mL cisplatin | A549 and A549/DDP cells | Downregulation of FA/BRCA pathway DNA damage repair processes to induce apoptosis in NSCLC | Sensitizing cisplatin-resistant NSCLC cells to cisplatin | [32] |
| Cur plus cisplatin | 0–40 µM Cur and 0–20 µg/mL cisplatin | A549 and H1975 cells | Inactivating ERK pathway to decrease expression of TP and ERCC1 | Synergistic suppression of NSCLC | [80] |
| Cur plus cisplatin | 0–100 μmol/L Cur and 0–100 μmol/L cisplatin | NCI-H460 cells | Raising superoxide anion generation to diminish Bcl-2 protein | Enhancing cytotoxic effect of cisplatin in NSCLC cells to induce apoptosis | [81] |
| Cur plus honokiol (HNK) plus cisplatin | 10 µg/mL Cur and 5 µg/mL cisplatin and 5 µg/mL HNK | A549 and A549/DDP cells | Downregulation of P-gp expression and inactivating AKT/ERK pathway to promote apoptosis and suppress migration and invasion | Synergistically elevating sensitivity of multidrug-resistant NSCLC to cisplatin | [82] |
| Cur plus cisplatin plus X-ray | 10 µmol/L Cur and 1 mg/L cisplatin | A549 cells | Associated with blocking the EGFR-related signaling pathway. | Augmenting radiosensitization effects against NSCLC | [83] |
| Cur plus carboplatin | 10 µM Cur and 50 or 100 µM carboplatin | A549 cells | Activation of ERK1/2 and suppression of Akt/IKKα pathway to inhibit NF-Κb | Synergistically promoting apoptosis and suppressing tumor cell growth, migration, and invasion in NSCLC | [84] |
| Cur plus palladium complex | 0.78–100 μM Cur and 0.39–50 μM Pd (II) complex | H1299 and A549 cells | Activation of d caspase 3/7 to induce apoptosis | Exhibiting a superior cytotoxic activity to suppress tumor growth | [85] |
| Cur plus organic chemotherapy drug | |||||
| Cur plus mitomycin C (MMC) | 5–50 µM Cur and 0.5–5 µM MMC | H1975 and H1650 cells | Downregulating TP expression and inactivating ERK1/2 pathway | Synergistic increasement of MMC-induced cytotoxicity | [86] |
| Cur plus MMC | 0–40 µM Cur and 0–10 µM MMC | A549 and H1975 cells | Decreasing the expression of Rad51 and blocking the MKK1/2–ERK1/2 pathway | Synergistic increasement of MMC-induced cytotoxicity | [87] |
| Cur plus vinorelbine | 25 μM Cur and 0.1 μg/mL vinorelbine | NCI-H520 cells | Releasing cytochrome C and activating caspase-9 and downstream caspase-3 in mitochondria to promote apoptosis |
Synergistic inhibition of NSCLC | [88] |
| Cur plus gemcitabine (GEM) | 3 μmol/L Cur and 58.2 μmol/L GEM for A549 cells; 3 μmol/L Cur and 98.72 μmol/L GEM for A549/GEM cells | A549 and A549/GEM drug-resistant cells | Downregulating expression of MMP-9, vimentin, and N-cadherin and upregulating E-cadherin to slow EMT | Elevating sensitivity of GEM-resistant NSCLC and decreasing migration and invasion | [89] |
| Cur plus paclitaxel | 30 µM Cur and 30 µM paclitaxel | A549 and H460 cells and paclitaxel-resistant lines A549 and H460 | Upregulation of miR-30c-5p to decrease levels of MTA1 | Increasing paclitaxel sensitivity to paclitaxel resistant NSCLC cells | [90] |
| Cur plus docetaxel (Doc) | In vitro: 2 µM Cur and 2 nM Doc or 0.5 µM Cur and 0.5 nM Doc In vivo: 15 mg/kg Cur and 10 mg/kg Doc |
A549 cells and nude mice | Not clarified | Synergistic suppression of NSCLC | [91] |
| Cur plus targeted agents | |||||
| Cur plus crizotinib | 30 μM Cur and 20 μM crizotinib | A549, H460, H1299, and H1066 cells | Increasing the levels of miR-142-5p through epigenetic and suppressing autophagy | Enhancing NSCLC sensitivity to crizotinib treatment | [92] |
| Cur plus erlotinib | 12.5 µM Cur and 1 µM erlotinib | H1650 and H1975 cells | The blockade of NF-κB activation and reducing the expressions of EGFR, p-EGFR, and survivin | Lowering erlotinib-resistant NSCLC cells with mutated EGFR | [93] |
| Cur plus gefitinib (Gef) | In vitro: 5–10 μM Cur and 0–20 μM Gef In vivo: 1 g/kg Cur and 100 mg/kg Gef |
H157, H1299, and PC-9 cells and BALBL/c mice | Inhibition of Sp1/EGFR activity to induce autophagy-mediated apoptosis | Elevating the sensitivity to Gef in NSCLC patients with mutated EGFR | [94] |
| Cur plus Gef | In vitro: 1–20 µM Cur and 1–20 µM Gef In vivo: 1 g/kg Cur and 120 mg/kg Gef |
CL1-5, A549, H1299, H1650, and H1975 cells and SCID mice | Reducing the levels of EGFR and altering p38MAPK or inhibiting AKT to promote apoptosis | Improving the treatment in NSCLC patients with mutated EGFR | [95] |
| Cur plus bioactive molecules | |||||
| Cur plus (-)-epigallocatechin gallate (EGCG) | In vitro: 10 μM/L Cur, 10 μM/L EGCGIn vivo: (20 mg/kg) EGCG and Cur | A549 and NCI-H460 cells and BALB/c nude mice | Suppression of proteins cyclin D1 and cyclin B1 to enhance cell cycle arrest | Synergistic inhibition of NSCLC | [96] |
| Cur plus the purine analog sulfinosine (SF) | 7.5 or 35 µM Cur and 1–10 µM SF for NCI-H460; 15 or 55 µM Cur and 5–25 µM SF for NCI-H460/R | NCI-H460 and NCI-H460/R cells | Linked with cell cycle distribution | Synergistic inhibition of multidrug-resistant NSCLC | [97] |
| Cur plus interferon-α (IFN-α) | 0–50 µM Cur and 1000U/mL IFN-α | A549 cells | Inhibition of COX-2 and NF-κB activation |
Overcoming the resistance of NSCLC to IFN-α | [98] |
| Cur plus galbanic acid (GBA) | 10–20 μM Cur and 40 μM GBA | A549 cells | Suppression of Akt/mTOR pathway | Potentiating the antitumor effect of GBA and inhibiting cancer migration | [99] |
| Cur plus (-)-epicatechin (EC) | 15–25 μmol/L Cur and 100 or 200 μmol/L EC | PC-9 and A549 cells | Upregulating the levels of GADD153 and GADD45 | Remarkable improvement in growth inhibition and apoptosis of NSCLC | [100] |
| Cur plus fenretinide (Fen) | In vitro: 10–20 μM Cur and 4–6 μM Fen In vivo: 40 mg/kg Cur and 1 mg/kg Fen |
A549 and LLC cells and C57BL/6 mice | Related to regulating ER chaperone protein GRP78 | Synergistic suppression of NSCLC | [101] |
| Cur plus multiple small molecular agents | 5–10 µM Cur and 0.1–2.5 µM AG1478, AG1024, PD173074, LY294002, or caffeic acid phenethyl ester (CAPE) | H1299 and A549 cells | Associated with EGFR, insulin-like growth factor 1, fibroblast growth factors receptor, PI3K, or NF-κB signaling pathway | Cooperating with these small molecules to inhibit tumor growth | [102] |
3.1. Cur Plus an Inorganic Chemotherapy Drug
Cisplatin and carboplatin-based chemotherapy remain standard regimens in most patients with late-stage NSCLC [103]. However, recent evidence has emphasized the toxicity of and tumors’ inherent resistance to platinum-based chemotherapy, requiring researchers to consider combining platinum with other agents to minimize these adverse effects [104].
One study demonstrated that Cur plus cisplatin effectively inhibits the self-renewal capability of cancer stem cells (CSCs) and prevents the emergence of chemo-resistance, as these cells possess a pronounced ability to amplify, differentiate, and metastasize, resulting in cancer escape and recurrence [76]. In addition to controlling the cells’ self-renewal capacity, Cur combined with cisplatin directly induces death in the highly migratory CSC subpopulation by changing cyclin D1 and p21 expression while enhancing NSCLC sensitivity to cisplatin [79]. In addition, co-treatment with Cur and cisplatin promotes the uptake of platinum ions to suppress A549 cell survival and mediate A549 cell apoptosis by targeting the Cu-Sp1-CTR1 regulatory loop [77]. This Cur-elevated cisplatin-induced cytotoxicity in NSCLC has been linked to the downregulation of p38 MAPK-dependent X-ray repair cross-complementing group 1 (XRCC1) [78], an important mediator of DNA repair and the reparative process of cisplatin-mediated DNA injury in HepG2 cells [105]. Previous studies have indicated that XRCC1 is involved in cisplatin resistance, and the inhibition of XRCC1 contributes to facilitating DNA single-strand breaks in breast cancer cells [106,107]. In addition to XRCC1, excision repair cross-complementary 1 (ERCC1) exerts a key role in removing DNA adducts to permit the repair of damaged DNA [108]. An investigation into the potential mechanisms responsible for the anticancer effects of the co-administration of Cur and cisplatin demonstrated that combined utilization sensitizes cisplatin resistance cells to cisplatin and synergistically inhibits the proliferation of NSCLC by inactivating the ERK pathway with the subsequent suppression of ERCC1 and thymidine phosphorylase (TP) expression [80]. Based on these outcomes, the suppression of XRCC1 or ERCC1 expression by Cur combined with cisplatin provides a promising strategy for NSCLC treatment.
Combining Cur and cisplatin has also shown that Cur intensifies apoptosis in NSCLS cells attacked by cisplatin by increasing the ROS-mediated degradation of anti-apoptotic Bcl-2 [81]. Notably, the combined use of Cur and cisplatin is also responsible for enhancing the sensitization of A549 cells to X-rays, diminishing cancer cell growth, possibly by blocking EGFR-related signaling pathways [83]. Furthermore, in cisplatin-resistant lung tumor cells, Cur plus cisplatin eliminates resistance and facilitates cytotoxic effects by blocking the FA/BRCA pathway-mediated process of DNA repair [32]. A recent study found that combining Cur, honokiol, and cisplatin increases the susceptibility of multidrug-resistant lung tumor cells to chemotherapeutics. The proposed mechanism was the inactivation of the AKT/ERK signaling pathway and the reduction of P-gp expression to limit NSCLC occurrence and progression [82]. The co-treatment of A549 cells with Cur and carboplatin revealed the suppression of the AKT-IKKα axis and downstream NF-κB and the inhibition of ERK1/2 activation, ultimately contributing to reduced cancer cell growth and elevated apoptosis [84]. Cur and palladium (II) complex combination treatment could potentially activate caspase 3/7, inducing apoptosis, thereby limiting NSCC growth and providing a new, effective method for treating lung cancer [85]. While co-treatment with Cur plus platinum or palladium could offer a promising clinical lung cancer treatment with or without platinum resistance, many challenges remain to be considered before the co-treatment can be applied.
3.2. Cur Plus an Organic Chemotherapy Drug
Mitomycin C, vinorelbine, gemcitabine, paclitaxel, and docetaxel are common chemotherapeutic drugs for lung cancer treatment. However, with the emergence of multiple drug-resistant tumors, clinicians are more aware of drug resistance in patients, a phenomenon that leads to the gradual weakening of a single drug’s efficacy.
Mitomycin C and Cur in combination inhibit the activation of the ERK pathway, leading to the downregulation of TP expression, which promotes DNA damage in NSCLC cells [86]. This treatment combination was also found to augment mitomycin C cytotoxicity in NSCLC by blocking the ERK pathway and suppressing the expression of Rad51, which controls tolerance in chemo- or radio-resistant neoplasms [87]. Cur combined with vinorelbine also promotes the apoptosis of NSCLC, which by mechanism was closely associated with releasing cytochrome C and activating caspase-9 along with downstream caspase-3 through the mitochondrial pathway [88].
Notably, for gemcitabine-resistant cells, the co-administration of Cur and gemcitabine greatly enhances the sensitivity of resistant cells to gemcitabine and does not appear to increase toxicity in mice [89]. Similarly, treatment with Cur and paclitaxel also sensitizes paclitaxel-resistant NSCLC cells to paclitaxel via epigenetic modification involving the upregulation of the miRNA-30c-mediated decrease of MTA1 expression [90]. Unfortunately, even though Cur plus docetaxel also synergistically retards the proliferation of NSCLC, the detailed mechanisms have not been elucidated [91].
3.3. Cur Plus Targeted Agents
The chemotherapy drugs crizotinib, erlotinib, and gefitinib are usually used to treat NSCLC with gene mutations. However, several exposures to crizotinib or erlotinib may result in drug resistance and therapy failure. It is reported that co-treatment with Cur and crizotinib upregulates the expression of miR-142-5p to target Ulk1 and inhibit autophagy in NSCLC cells, lowering the resistance of lung carcinoma to crizotinib [92]. Researchers also found that the co-treatment of erlotinib and Cur greatly increases the mortality of erlotinib-resistant cells due to the decreased expression of EGFR and repression of NF-κB activation in EGFR-mutant NSCLC cells [93]. Moreover, co-treatment with Cur and gefitinib significantly inactivates EGFR by retarding Sp1, influencing the interaction between Sp1 and HDAC1 and markedly promoting autophagy and autophagy-mediated apoptosis in resistant NSCLC cells [94]. Another study demonstrated that Cur and gefitinib co-treatment modulates the AKT or the p38MAPK pathway, thereby inducing apoptosis in vitro and in vivo [95]. These findings signify that Cur and targeted agents might act in concert to provide an effective therapy for advanced NSCLC.
3.4. Cur Plus Bioactive Molecules
When combined with Cur, other natural agents or small synthetic compounds, such as (-)-epigallocatechin gallate (EGCG), the purine analog sulfinosine, interferon-α, and galbanic acid, have been effective in NSCLC treatment. Co-treatment with Cur and EGCG, two natural agents, noticeably impedes the expression of cyclin D1 and cyclin B1, inducing NSCLC arrest at the G1 and S/G2 phases and preventing weight loss caused by tumor burden in nude mice with lung tumor xenografts [96]. Co-treatment with Cur and the natural agent galbanic in A549 cells markedly increases cancer cell apoptosis, autophagy, and other antitumor effects by suppressing the AKT/mTOR axis relative to treatment with a single chemotherapeutic agent [99]. Cur also promotes the anticancer activity of (-)-epicatechin against NSCLC by potentiating the expression of GADD153 and GADD45 genes, causing apoptosis and inhibiting cell proliferation [100]. In addition, Cur plus interferon-α could reverse interferon-α-induced NF-κB and COX-2 activation, suggesting that Cur counterbalances the negative effects of interferon-α and improves its antitumor activity [98]. Moreover, Cur improves the cytotoxicity of fenretinide in NSCLC by governing the expression of the ER chaperone protein GRP78 [101]. Finally, co-treatment with Cur plus multiple small molecular antagonists, such as AG1024, PD173074, LY294002, or caffeic acid phenethyl ester, also improves proliferation suppression in NSCLC by blocking the corresponding signaling pathway [102]. Although these data indicate that Cur, combined with other small agents, operates on multiple signaling pathways or integrates pathways to eliminate NSCLC, research on new compounds and combination therapies is needed to supply more clinical trial candidates.
4. Predicting the Interaction of Cur and Various NSCLC Molecular Targets
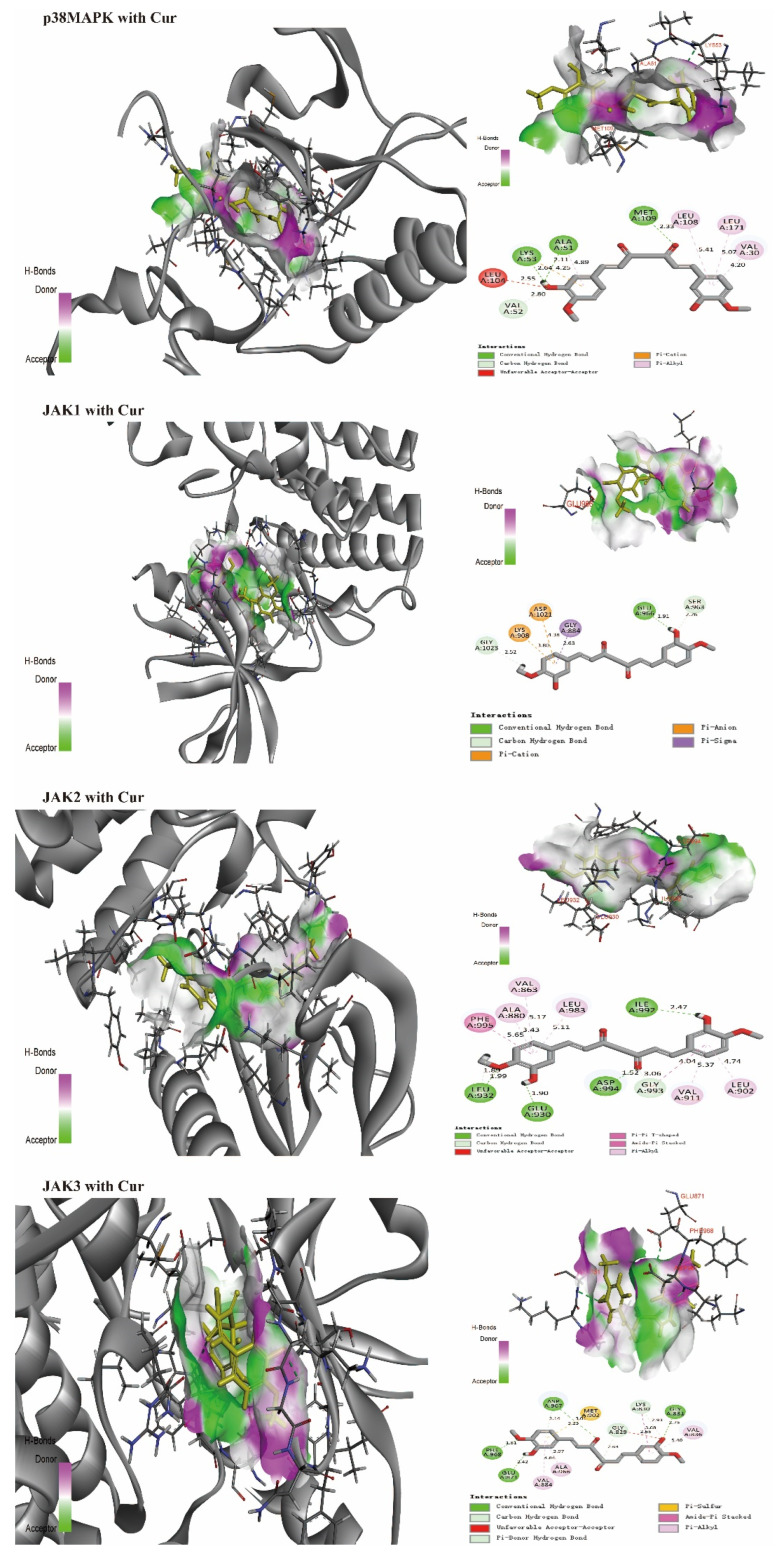
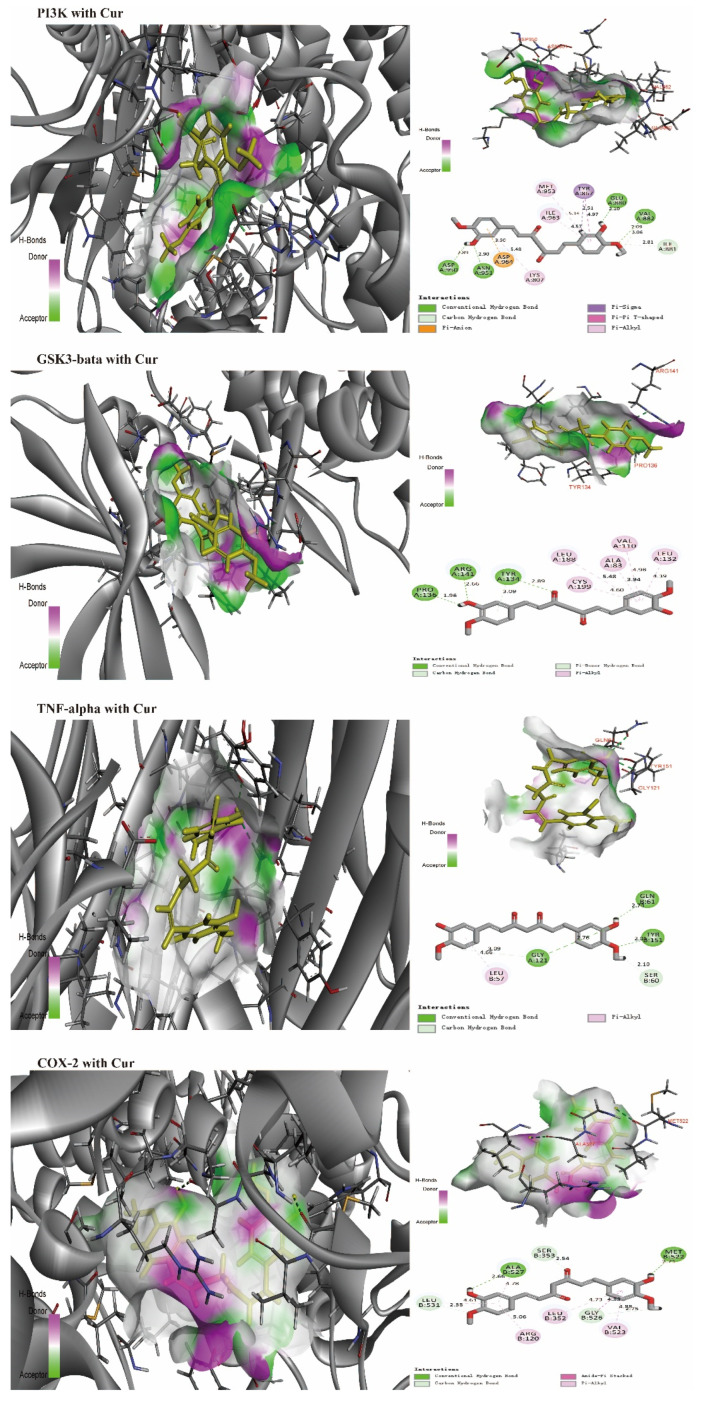
Discovery studio software (2019 version) was used to evaluate the interaction of Cur with amino acid residues in the binding positions of various target proteins (in 3D and 2D conformational structures, Figure 4). Various target protein (PDB) formats (www.rcsb.org, accessed on 15 August 2022) include p38MAPK [109], JAK1, JAK2, JAK3 [110,111], PI3K [112,113], GSK3-bata [114,115], TNF-alpha [116,117], and COX-2 [118]. Even though the affinity of Cur for different amino acid residues results from hydrogen, carbon–hydrogen, pi-anion, or pi-cation bonds, all target proteins can form hydrogen bonds with Cur, which disturb the activities of specific enzymes to enhance sensitivity to chemotherapy drugs or evoke apoptosis, preventing NSCLC proliferation. Preclinical and clinical studies are needed for confirmation.
Figure 4.
Interactions (3D and 2D) of Cur with various amino acid residues in NSCLC-related proteins. p38MAPK (hydrogen bonds: ALA51, LYS53, and MET109), PDB: 5WJJ; JAK1 (hydrogen bond: GLU966), PDB: 6SMB; JAK2 (hydrogen bonds: GLU930, LEU932, ILE992, and ASP994), PDB: 3UGC; JAK3 (hydrogen bonds: GLY831, GLU871, ASP967, and PHE968), PDB: 3ZC6; PI3K (hydrogen bonds: GLU880, VAL882, ASP950, and ASN951), PDB: 3TL5; GSK3-bata (hydrogen bonds: TYR134, PRO136, and ARG141), PDB: 7OY5; TNF-alpha (hydrogen bonds: GLN61, GLY121, and TYR151), PDB: 2AZ5; COX-2 (hydrogen bonds: MET522 and ALA527), PDB: 4M10.
5. The Impact of Cur Analogs on NSCLC
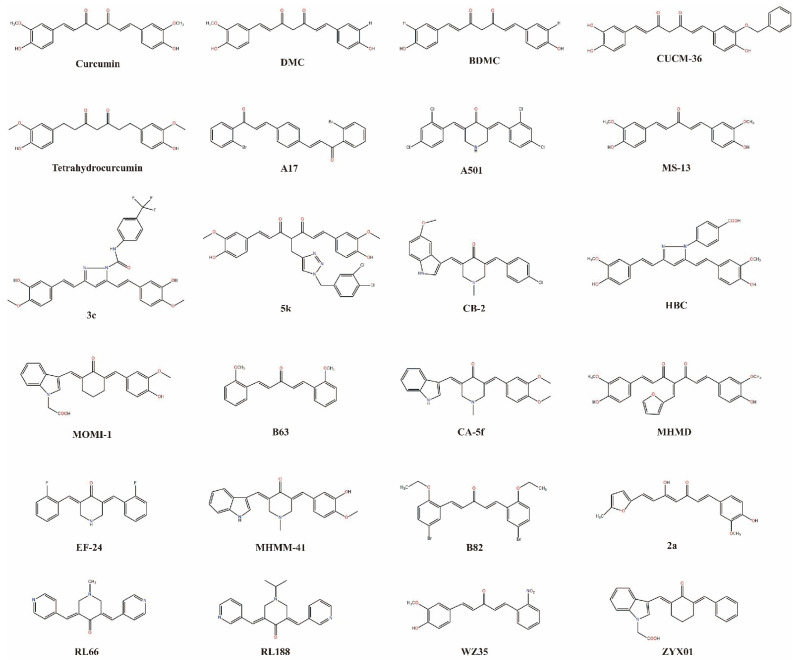
Cur analogs, including those that are semisynthetic and synthetic, have developed rapidly in the last 20 years. One study demonstrated that Cur exhibits anticancer effects against different types of cancer, including NSCLC in vivo and in vitro, via substituent modifications, such as dimethoxy substitution, piperidine-4-one core, or fluorination [119]. Many Cur analogs have been revealed to decrease the survival of NSCLC (Figure 5).
Figure 5.
Various Cur analogs used against NSCLC.
5.1. Demethoxycurcumin (DMC)
DMC, one of the most important curcuminoids, possesses antitumor activities against various human cancer cells, including leukemia, glioma cells, baller cancer, cervical cancer carcinomas, and kidney tumors [120,121,122,123,124], and is an effective adjuvant. Although few investigations have been conducted to assess the effects of DMC on NSCLC, one study on A549 cells showed that DMC downregulates TP expression and represses the expression of ERCC1. This DMC downregulation occurs by DMC acting on PI3K-AKT-snail pathways to enhance the sensitivity of NSCLC to cisplatin, thereby increasing cisplatin-induced cytotoxicity and inducing cancer cell death [125]. A recent study in A549/DDP cells revealed that DMC also markedly increases DDP sensitivity relative to DDP-resistant A549 cells. Moreover, DMC also demonstrates low toxicity to normal lung fibroblast MRC-5 cells, which indicates that the co-administration of DMC and cisplatin directly induces the apoptosis of NSCLC and enhances the anticancer effects of cisplatin for A549/DDP cells [126]. Additionally, DMC increases ROS and Ca2+ production and elevates the production of ER stress-related proteins, such as GRP78, GADD153, IRE1α, IRE1β, ATF-6α, ATF-6β, and caspase-4, demonstrating the beneficial effects of promoting NSCLC apoptosis [127].
However, similarly to Cur, DMC has poor water solubility, gastrointestinal absorption, and low bioavailability, which is not conducive to clinical applications [128]. Researchers have consistently focused on improving its bioavailability and changing its delivery mode into cancer cells. When cisplatin induces TP and ERCC1 overexpression, DMC-loaded amphiphilic chitosan nanomatrix (DMC–CHC) significantly enhances cisplatin-mediated cytotoxicity via the high-performance intracellular transmission of the encapsulated DMC. Moreover, DMC–CHC combined with cisplatin greatly suppresses cisplatin resistance protein ERCC1 and TP through the PI3K-AKT pathway, permitting increased cisplatin-induced NSCLC apoptosis [129]. Notably, DMC–CHC covered with an anti-EGFR antibody layer to assist DMC entrance into the cytoplasmic region exhibits high cytotoxicity against multidrug-resistant lung cancer, particularly NSCLC [130]. This antibody shell layer is accurately positioned on the cell against tumor cells and slows down drug release. This technique is valuable for developing drug delivery systems for NSCLC treatment.
5.2. Bisdemethoxycurcumin (BDMC)
BDMC comprises approximately 3% of curcuminoids and is more stable than others in vivo [131]. Recent studies have suggested that BDMC plays an antitumor role via multiple biological mechanisms or synergistic modes of action, from the suppression of cell growth, proliferation, invasion, and migration, to the induction of programmed cell death in various cancers [132,133,134,135,136,137].
First, BDMC significantly induces DNA lesions and restrains DNA repair by inhibiting the expression of the proteins 14-3-3σ, MGMT, BRCA1, and MDC1 and increasing p-p53 and p-H2A.X in NSCLC [138]. BDMC also alters the expression of specific genes, including CCNE2, linked to the cell cycle, and ERCC6L, involved in DNA damage and recovery, which mediates the cytotoxicity, disturbing cell migration, invasion, and tumor progression for NSCLC treatment [139]. In addition to affecting the levels of genes, BDMC can block the activity of DNA methyltransferase-1. Blocking this DNA activity decreases Wnt inhibitory factor-1 (WIF-1) promoter demethylation associated with the activation of the Wnt pathway, resulting in epigenetic alterations that inhibit the Wnt pathway and induce apoptosis in NSCLC [140]. Moreover, the Wnt pathway also participates in the progression of EMT and cancer metastasis [141]. When the Wnt pathway is activated, secreted Wnt proteins operate on E-cadherin to promote the EMT [142]. BDMC negatively regulates the Wnt/β-catenin pathway by elevating WIF-1 expression, which subsequently blocks TGF-β1-induced EMT to inhibit migration and invasion in highly metastatic NSCLC [143]. In addition, BDMC causes autophagy, upregulating the levels of E-cadherin and downregulating vimentin to limit the migration and invasion of highly metastatic NSCLC [144].
Accordingly, the BDMC induction of autophagy accelerates cell apoptosis via the inactivation of the hedgehog signaling pathway in NSCLC [145]. Moreover, BDMC strongly induces S-phase arrest by suppressing the protein levels of Cdc25A and cyclins A and E, promoting DNA damage by increasing ROS and Ca2+ production and raising ER stress by altering the protein expression of GRP78, GADD153, IRE1α, IRE1β, ATF-6α, ATF-6β, and caspase-4 expression to induce lung tumor cells apoptosis [146].
Two studies have demonstrated that BDMC enhances the sensitivity of NSCLC to chemotherapeutic drugs. BDMC combined with icotinib exhibits potent, synergistic growth suppression in EGFR-TKI-resistant NSCLC via multiple pathways, such as ROS accumulation, autophagy induction, or DNA damage [147]. Furthermore, there may be crosstalk between several signaling pathways because single-target agents, such as ROS antagonists (n-acetylcysteine) or autophagy antagonists (chloroquine or 3-MA), only partially abate growth inhibition induced by BDMC plus icotinib in NSCLC cells [147]. Furthermore, BDMC and cisplatin co-treatment regulates the chemo-sensitivity response for cisplatin-resistant NSCLC by repressing the expression of CA916798 and PI3K/AKT activities [148]. CA916798 is a recently identified protein that interacts with AKT in BDMC-exposed A549 and A549/CDDP cells. Moreover, the phosphorylation of CA916798 at the S20 residue exerts a key role in mediating BDMC anti-carcinoma activity [148]. All these data demonstrate that BDMC inhibits NSCLC cell growth, proliferation, invasion, and migration by extrinsic, intrinsic, and ER stress pathways to drive cell apoptosis in NSCLC.
5.3. Cur Analogs with Targeted Therapy
Oncogenic receptor tyrosine kinases are definitive drug therapy targets for the treatment of NSCLC patients containing gene mutations, especially EGFR mutations and ALK rearrangements that are targets of the precision medicine management of chest neoplasms [149,150]. A meta-data analysis showed that individuals who never smoked were more susceptible to the EGFR and ALK-EML4 mutations than ever-smokers [3]. In ALK+ cells, multiple effective ALK tyrosine kinase inhibitors (TKIs), such as crizotinib, ceritinib, alectinib, brigatinib, or lorlatinib, contribute to substantial improvement [151]. However, with increased adverse effects and the appearance of molecular resistance, the identification of new TKIs for NSCLC targets or combinations is urgent.
Two Cur analogs, RL66 and RL118, showed powerful anticancer activities against various tumor cells [152,153]. An investigation into ALK+ (H3122) and ALK− (A549) cells revealed that RL66 and RL118 demonstrate greater potency in ALK+ cells than in ALK− A549 cells. Moreover, co-treatment with crizotinib and RL66 or RL118 promotes ALK+ H3122 cell death more than either drug alone [136]. Moreover, in crizotinib-resistant H3122 cells, RL66 and RL118 demonstrate negligible cross resistance with crizotinib, indicating that RL66 or RL118 act on independent targets, requiring further exploration in vivo [154].
In NSCLC patients with EGFR mutations, EGFR-TKIs such as gefitinib and erlotinib are the first-line clinical drugs, dramatically improving quality of life. However, most of these patients obtain resistance to EGFR-TKIs via a secondary mutation within EGFR [155,156]. However, Cur analog WZ35 suppresses the proliferation of gefitinib- and erlotinib-resistant H1975 cancers via increased ROS generation, accompanied by ER stress and mitochondrial disorder, which trigger apoptosis [157]. Cur analog CUCM-36 targets EGFR to prevent NSCLC via diverse mechanisms, whereas the actual synthesis of the CUCM-36 compound is required to verify its anti-EFGR action in vivo and in vitro [158]. These data offer insight to accelerate studies on Cur analog-targeted therapy for NSCLC patients with gene mutations.
5.4. Other Cur Analogs
CA-5f, a Cur analog that is well tolerated in vivo, is toxic to NSCLC via elevated ROS production in mitochondria and diminishes the growth of NSCLC by blocking autophagosome–lysosome fusion [159]. Similarly, Cur analog EF24 also demonstrates excellent cytotoxicity against NSCLC cells by increasing ROS deposition and subsequent mitochondrial disorder-mediated apoptosis to repress tumors [160]. However, in contrast to CA-5f, EF24 induces autophagy to prevent the survival of NSCLC rather than inhibit autophagy. Another Cur analog, MS13, demonstrates potential anticancer activities that inhibit most carcinomas, such as colon cancer, prostate tumor, glioblastoma, cervical carcinoma, and neuroblastoma [161]. Studies exploring the influence of MS13 on NCI-H520 and NCI-H23 lung cancer cells showed that MS13 promotes anti-proliferation and apoptosis activity in NSCLC, involving the PI3K-AKT axis, cell cycle-apoptosis, and MAPK signaling pathways [161]. Unfortunately, researchers have not fully elucidated the mechanisms of MS13 against NSCLC, so further analysis is needed. With a triazole ring, the analog compound 5k regulates multiple signaling pathways, including MAPK activation and STAT3 and NF-κB inhibition, to reduce lung tumor development [162]. Although PI3K-AKT pathways play a significant role in tumor growth, invasion, migration, and apoptosis, 5k does not influence PI3K-AKT pathways [162].
ER stress has recently been regarded as a significant driver of cell apoptosis [163], so disrupting ER functions to elevate ER stress may be a treatment for various cancers. One investigation indicated that Cur analog B86 diminishes cancer growth by inducing ER stress-mediated apoptosis, whereas silencing the CHOP gene reverses the inhibitory effects of B86 in NSCLC [164]. Similarly, Cur analog B63-treated NSCLC has been shown to cause lung tumor cell apoptosis by regulating ER Ca2+ stores and stimulating the production of ER stress, ultimately triggering the caspase cascades [165].
Cur derivative MHMM-41 appears to be a more promising agent against NSCLC in vitro. MHMM-41 increases ROS production, alters mitochondrial permeability or mitochondrial membrane potential, and activates caspase-3, 8, 9, 12, Bax, and PARP proteins to cause A549 cell apoptosis. These results suggest that MHMM-41 exerts inhibitory effects on NSCLC through extrinsic and intrinsic mitochondrial pathways [166]. Similarly, Cur analog A501 also promotes the apoptosis of NSCLC cells by inhibiting cyclinB1, cdc-2, and Bcl-2 and activating p53 and caspase-3 [149]. Analog A501 induces G2/M arrest by interfering with cyclin expression to limit cancer development in NSCLC, as cyclins play a vital role in cell proliferation, irreplaceable for cell cycle transitions [167]. In another study, investigators synthesized Cur analog A17 (with a double carbonyl group) and explored its antitumor effects against NSCLC. The data revealed that A17 exerts potential anticancer activity by increasing CHOP expression and some crucial constituents to induce ER stress. Moreover, this research reported that A17 induces H460 cell apoptosis by successively increasing the production of downstream proteins, such as p53, p-JNK, and Bax, ultimately activating caspase-3 to induce NSCLC apoptosis [168].
Two Cur derivatives, MOMI-1 and HBC, repress the proliferation of A549 cells by causing autophagy rather than cell apoptosis. Autophagy has been regarded as a crucial intracellular mechanism for the degradation of damaged proteins and organelles, while the activation of autophagy can suppress NSCLC progression [169]. Microtubule-associated protein LC3 is an important marker for estimating whether autophagy is activated. When autophagy occurs, the LC3-I is converted to LC3 II rapidly, increasing the LC3-II/I ratio. However, LC3-I/II conversion is not the only measure used to judge autophagy. MOMI-1 not only promotes the conversion of LC3-I to LC II to induce autophagy, but also inhibits the migration of A549 cells and disturbs the cell cycle [170]. By contrast, HBC promotes the fusion of autophagosomes with lysosomes and increases the conversion of LC3-I to LC3 II to induce autophagy, ultimately repressing A549 proliferation [171]. Another Cur derivative, ZYX01, also upregulates the ratio of LC3-II/LC3-I and beclin-1 and reduces p62 expression to provoke autophagy [172]. Mechanistic studies show that ZYX01 activates the AMPK pathway, which further facilitates ULK1 phosphorylation to inhibit A549 cells, while using autophagy inhibitors eliminates the effect of ZYX01 on the AMPK/ULK1/beclin-1 pathway and autophagy [172]. Notably, tetrahydrocurcumin increases the formation of autophagosomes by elevating beclin 1 expression and advancing autophagic cell death by blocking the PI3K/AKT/mTOR pathway, inducing autophagy [173]. In the early stages after tetrahydrocurcumin administration into A549 cell cultures, the LC3-II/LC3-I ratio is low. The ratio increases over time [173]. These findings demonstrate that the activation of the AMPK/ULK1/beclin-1 pathway or the inhibition of the PI3K/AKT/mTOR pathway promotes the autophagy of NSCLC cells. However, studies have revealed that autophagy activation prompts several types of cancer cells to obtain the energy and metabolites required to maintain their growth and decreases the sensitivity of NSCLC to chemo/radio-therapeutic drugs [174,175,176,177,178]. Preclinical and clinical research has shown that the inhibition of autophagy could attenuate tumor development or improve chemo/radio-therapy resistance in NSCLC [115,179]. Notably, Cur analog CB-2 increased the accumulation of LC3B-II, yet this did not change other key proteins related to autophagy. CB-2 also reduces tumor growth by inhibiting autophagosome–lysosome fusion [180]. The results of additional studies suggest that lower-dose CB-2 represses the migration of A549 cells while slightly affecting apoptosis, whereas higher doses of CB-2 directly promote apoptosis and necrosis in A549 cells [180]. Therefore, autophagy inhibitors have also emerged as efficient NSCLC antitumor agents. It is unclear why both autophagy inducers and inhibitors provoke the apoptosis of NSCLC. The interactions between autophagy and apoptosis are intricate, and autophagy acts as a double-edged sword in NSCLC treatment [181].
For cisplatin-resistant A549 cells, Cur analog 2a effectively enhances the sensitivity of A549/CDDP cells to cisplatin by increasing oxidative stress. This is because 2a, as a thioredoxin reductase (TrxR) inhibitor, can deplete glutathione and disrupt intracellular redox homeostasis, thereby restraining cell growth [182]. TrxR with selenocysteine exhibits strong anti-oxidant stress, possibly regulating redox balance to influence the development of cancers by mediating signal transduction [183,184]. Moreover, the suppression of TrxR augments the radio-sensitization of cancer to accelerate cell apoptosis [185]. Hence, using TrxR inhibitors or the co-administration of TrxR suppressants and chemoradiotherapy drugs provides a new NSCLC treatment methodology [186,187,188]. Another study revealed that Cur analog MHMD regulates cell death by apoptosis and necrosis pathways, although the detailed regulation mechanisms are still unclear [189]. Notably, however, MHMD activates caspases to induce apoptosis rather than autophagy, inhibiting NSCLC cell growth [189]. Unfortunately, these studies suffer from a lack of research in animal samples, and data on the inhibition of NSCLC require corroboration via larger preclinical studies. The associated analogs are listed in Table 2.
Table 2.
Various Cur analogs and their mechanisms of action against NSCLC.
| Analogs | Dosage | Assay Type | Mechanisms | Outcomes | References |
|---|---|---|---|---|---|
| CA-5f | 0–40 µM | A549 cells and BALB/c nude mice | Increasing ROS production in mitochondria and inhibiting autophagy | Suppressing the growth of NSCLC | [159] |
| EF24 | 2 μM, 4 μM/ 5, 10 or 20 mg/kg |
A549 and H520 cells and BALB/c nude mice | Increasing ROS production in mitochondria, autophagy, and apoptosis | Inhibiting the growth of NSCLC | [160] |
| MS13 | 6.3 µM, 12.5 µM | NCI-H520 and NCI-H23 cells | Greatly concerned with PI3K-AKT signaling pathways, cell cycle-apoptosis, and MAPK pathways | Inducing anti-proliferation and apoptosis activities of NSCLC | [161] |
| MHMM-41 | 8 μM and 16 μM | A549 cells | Activating caspase-3, 8, 9, 12, Bax, and PARP proteins and increasing the levels of ROS | Inducing apoptosis of cancer cells and inhibiting tumor migration | [166] |
| A17 | 1, 5, 10 µM | NCI-H460 and A549 cells | Activating PERK, ATF-6, and IRE1 to increase ER stress | ER stress-induced cell apoptosis | [168] |
| A501 | 0.5, 1, 2, 4 µM | A549, H460, H1975, and HCC827 cells | Inhibiting the levels of cyclinB1, cdc-2, and Bcl-2 and increasing p53, cleaved caspase-3, and Bax | Inhibition of cancer cell proliferation and causing apoptosis of NSCLC cells. | [167] |
| B82 | 2.5, 5, 10 µM and 5 mg/kg/day |
H460 cells and BALB/c nude mice | Inhibiting the levels of cyclinB1 and Bcl-2 and increased cleaved caspase-3 and Bax | Inhibition of cancer cell proliferation and causing apoptosis of NSCLC | [164] |
| B63 | 10 and 20 μM | H460 and H358 cells and BALB/c nude mice | Regulating intracellular Ca2+ in ER to increase cleaved caspase-3 and cleaved caspase-9 | ER stress-induced cell apoptosis | [165] |
| 5 k | 0.625 μM–2.5 μM | A549 cells and zebrafish larvae | Activation of the mitogen-activated protein kinases and suppression of NF-κB/STAT3 signaling pathways | Inhibition of cancer cell proliferation | [162] |
| MOMI-1 | 5, 20, 80 μM | A549 cells | Through the LC3-I/II conversion and reducing the levels of cyclin D1 and cyclin E1 protein | Inhibition of cancer cell proliferation and migration | [170] |
| HBC | 0–80 μM | A549 cells | Increasing amassment of autophagosomes and inducing the conversion of LC3-I to LC3-II | Inducing cell autophagy to suppress tumor proliferation | [171] |
| MHMD | 0–20 µM | A549 cells | Unclearly specific mechanisms | Inducing cell necrosis and death | [189] |
| ZYX01 | 15 μM | A549 cells | Activation of AMPK- ULK1-Beclin-1 signaling pathway | Inducing autophagy and inhibiting migration in NSCLC cells | [172] |
| CB-2 | 0–40 μM | A549 cells | Elevating ROS production in mitochondria and inhibiting autophagy | Suppressing the growth and migration of NSCLC cells | [180] |
| 2a | 0–100 μM | A549 and A549/CDDP cells | Depleting GSH to promote oxidative stress damage and apoptosis | Enhancing drug sensibility to cisplatin against A549/CDDP cells | [182] |
6. Clinical Trials of Cur and Its Analogs in NSCLC
In a phase I clinical trial (NCT02321293), the aim of co-administration of Cur and TKIs for EGFR-mutant-advanced NSCLC was to assess the safety and feasibility of using Cur in conjunction with an EGFR-TKI in patients with advanced NSCLC. The 20 study subjects (≥18 years) were given CURCUVivaTM (an enhanced bioavailable formulation of Cur) at 80 mg per day (in capsule form) for eight weeks, after which the patients continued taking EGFR-TKI (gefitinib, 250 mg/once daily or erlotinib, 150 mg/once daily) without Cur until progression.
Researchers also investigated (JPRN-UMIN000006892) the safety and feasibility of erlotinib and theracurmin (nanoparticle Cur) co-treatment in patients with advanced or recurrent NSCLC. Patients were treated with theracurmin and erlotinib for eight weeks (erlotinib, 150 mg per day; theracurmin, three cohorts: 180 mg per day, 240 mg per day, and 360 mg per day). In another phase I/Π clinical trial (NCT01048983), researchers compared armodafinil, bupropion, Cur, and minocycline alone or in combination to determine the most appropriate treatment for controlling symptoms of lung cancer. Unfortunately, this project was withdrawn. In the latest phase Π clinical trial (NCT03598309), researchers attempted to determine whether co-treatment with Lovaza (made with fish oils) and Cur C3 complex was nontoxic and tolerable and could help to reduce the size of lung nodules. After searching multiple clinical research websites, including https://www.isrctn.com/ (accessed on 3 July 2022), https://www.anzctr.org.au/ (accessed on 3 July 2022), https://www.clinicaltrialsregister.eu/ (accessed on 3 July 2022), https://irct.ir/page/about_irct (accessed on 3 July 2022), http://ctri.nic.in/Clinicaltrials/login.php (accessed on 3 July 2022), and http://apps.who.int/trialsearch/ (accessed on 3 July 2022), we identified no current studies on Cur and its analogs for NSCLC treatment.
7. Conclusions
Considerable preclinical evidence has revealed that Cur and its analogs affect NSCLC via various mechanisms, such as inducing ROS production, increasing ferroptosis, changing mitochondrial potential, and disturbing cellular signaling pathways. Additionally, co-treatment with Cur and other agents synergistically enhances cytotoxicity in NSCLC cells to suppress tumor cell growth, migration, and invasion. This evidence suggests that Cur and its analogs offer promise to prevent NSCLC in humans. However, substantial clinical research is required before Cur, Cur analog, and Cur co-treatment with other chemotherapeutic agents can be implemented as anti-lung cancer drugs in clinical settings. First, some results have been less than encouraging. Second, critical issues remain regarding dosage, formulations and bioavailability, optimal combinations, potential adverse reactions, and other factors; thus, more studies are required.
Abbreviations
EGFR: epidermal growth factor receptor; ALK: anaplastic lymphoma kinase; VEGF: vascular endothelial growth factor; ROS: reactive oxygen species; ER: endoplasmic reticulum; TAZ: tafazzin; XIAP: X-linked inhibitor of apoptosis protein; Bax: apoptosis regulator Bax; Bcl-2: apoptosis regulator Bcl-2; STAT3: signal transducer and activator of transcription 3; JAK: Janus kinase; MTA1: metastasis-associated protein 1; JNK: C-Jun kinase enzyme; EGR-1: early growth response protein 1; TLR4: Toll-like receptor 4; MyD88: myeloid differentiation factor 88; MMP-2: matrix metalloproteinase-2; ERK: extracellular regulated protein kinases; MMP-9: matrix metalloproteinase-9; nAChRs: neuronal nicotinic acetylcholine receptors; NF-κB: nuclear factor kappa-B; COX-2: cyclooxygenase-2; ACSL4: acyl—CoA synthetase long-chain family member-4; SLC7A11: solute carrier family 7 member 11; GPX4: glutathione peroxidase 4; PI3K/AKT: phosphatidylinositol 3 kinase/protein kinase B; GRP78: glucose regulated protein 78; Cytokine R: cytokine receptor; Adipo R: adiponectin receptor; ITGB1: integrin beta 1; Sp1: specificity protein 1; CTR1: copper transporter 1; XRCC1: X-ray repair cross-complementing group 1; ERCC1: excision repair cross-complementary 1; p38 MAPK: p38 mitogen activated protein kinases; FA/BRCA: Fanconi anemia/BRCA; TP: thymidine phosphorylase; P-gp: P-glycoprotein; PTEN: phosphatase and tensin homologue deleted on chromosome 10; HDAC1: histone deacetylase 1; GADD153: growth arrest and DNA damage 153; GADD45: growth arrest and DNA damage 45; GSK3-bata: glucogen synthase kinase-3 beta; TNF-alpha: tumor necrosis factor-alpha; ATF-6α/6β: activating transcription factor 6α/6β; A549/DDP cells: human lung adenocarcinoma drug-resistant cell line; IRE1α/1β: inositol-requiring enzyme-1α/1β; MGMT: O6-methyl-guanine DNA methyltransferase; p53: cellular tumor antigen protein 53; 14-3-3σ: an important checkpoint keeper of DDR; p-H2A.X: phospho Ser140; MDC1: mediator of DNA damage checkpoint 1; EMT: epithelial mesenchymal transformation; TGF-β1: transforming growth factor-β1; Cdc25A: cell division cycle 25A; CHOP: C/EBP-homologous protein; PARP: poly ADP-ribose polymerase; TrxR: thioredoxin reductase.
Author Contributions
Conceptualization, X.Y. and Y.L.; methodology, J.M.; software, X.C.; validation, J.L., C.Y. and D.L.; formal analysis, C.T.; investigation, Y.Z.; resources, W.Z.; data curation, C.Y.; writing—original draft preparation, C.T.; writing—review and editing, X.Y. and J.L.; visualization, Y.L.; supervision, J.M.; project administration, D.L.; funding acquisition, X.Y. and Y.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest regarding this manuscript.
Funding Statement
This research was funded by the Department of Science and Technology of Sichuan Province (Grant NO. 2019YFS0343 to Yunzhu Lin); Fundamental Research Funds for the Central Universities (SCU2021E4251 to Yunzhu Lin) and The Doctoral Research Foundation of Mudanjiang Medical University (2021-MYBSKY-060 to Xiaohuan Yuan).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bade B.C., Dela Cruz C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz A.G., Cote M.L. Epidemiology of Lung Cancer. Adv. Exp. Med. Biol. 2016;893:21–41. doi: 10.1007/978-3-319-24223-1_2. [DOI] [PubMed] [Google Scholar]
- 3.Chapman A.M., Sun K.Y., Ruestow P., Cowan D.M., Madl A.K. Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–134. doi: 10.1016/j.lungcan.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Travis W.D. Lung Cancer Pathology: Current Concepts. Clin. Chest Med. 2020;41:67–85. doi: 10.1016/j.ccm.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Relli V., Trerotola M., Guerra E., Alberti S. Abandoning the Notion of Non-Small Cell Lung Cancer. Trends Mol. Med. 2019;25:585–594. doi: 10.1016/j.molmed.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Duma N., Santana-Davila R., Molina J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Suresh R., Ali S., Ahmad A., Philip P.A., Sarkar F.H. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv. Exp. Med. Biol. 2016;890:57–74. doi: 10.1007/978-3-319-24932-2_4. [DOI] [PubMed] [Google Scholar]
- 8.Tang C., Liu Y., Liu S., Yang C., Chen L., Tang F., Wang F., Zhan L., Deng H., Zhou W., et al. Curcumin and Its Analogs as Potential Epigenetic Modulators: Prevention of Diabetes and Its Complications. Pharmacology. 2022;107:1–13. doi: 10.1159/000520311. [DOI] [PubMed] [Google Scholar]
- 9.Zia A., Farkhondeh T., Pourbagher-Shahri A.M., Samarghandian S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021;134:111119. doi: 10.1016/j.biopha.2020.111119. [DOI] [PubMed] [Google Scholar]
- 10.Stacchiotti A., Corsetti G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front. Cell Dev. Biol. 2020;8:555409. doi: 10.3389/fcell.2020.555409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Sureda A., Devkota H.P., Pittalà V., Barreca D., Silva A.S., Tewari D., Xu S., Nabavi S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020;38:107343. doi: 10.1016/j.biotechadv.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Giordano A., Tommonaro G. Curcumin and Cancer. Nutrients. 2019;11:2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcelos K.A., Mendonça C.R., Noll M., Botelho A.F., Francischini C.R.D., Silva M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers. 2022;14:2165. doi: 10.3390/cancers14092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrero de la Parte B., Rodeño-Casado M., Iturrizaga Correcher S., Mar Medina C., García-Alonso I. Curcumin Reduces Colorectal Cancer Cell Proliferation and Migration and Slows In Vivo Growth of Liver Metastases in Rats. Biomedicines. 2021;9:1183. doi: 10.3390/biomedicines9091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pricci M., Girardi B., Giorgio F., Losurdo G., Ierardi E., Di Leo A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020;21:2364. doi: 10.3390/ijms21072364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren B., Luo S., Tian X., Jiang Z., Zou G., Xu F., Yin T., Huang Y., Liu J. Curcumin inhibits liver cancer by inhibiting DAMP molecule HSP70 and TLR4 signaling. Oncol. Rep. 2018;40:895–901. doi: 10.3892/or.2018.6485. [DOI] [PubMed] [Google Scholar]
- 17.Darvesh A.S., Aggarwal B.B., Bishayee A. Curcumin and liver cancer: A review. Curr. Pharm. Biotechnol. 2012;13:218–228. doi: 10.2174/138920112798868791. [DOI] [PubMed] [Google Scholar]
- 18.Walker B.C., Mittal S. Antitumor Activity of Curcumin in Glioblastoma. Int. J. Mol. Sci. 2020;21:9435. doi: 10.3390/ijms21249435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., Liu F., Liao W., Yu L., Hu Z., Li M., Xia H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch. Biochem. Biophys. 2020;689:108412. doi: 10.1016/j.abb.2020.108412. [DOI] [PubMed] [Google Scholar]
- 20.Shetty N.P., Prabhakaran M., Srivastava A.K. Pleiotropic nature of curcumin in targeting multiple apoptotic-mediated factors and related strategies to treat gastric cancer: A review. Phytother. Res. PTR. 2021;35:5397–5416. doi: 10.1002/ptr.7158. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X., Zhang C., Ren Z., Zhang F., Xu J., Zhang X., Zheng H. Curcumin Affects Gastric Cancer Cell Migration, Invasion and Cytoskeletal Remodeling Through Gli1-β-Catenin. Cancer Manag. Res. 2020;12:3795–3806. doi: 10.2147/CMAR.S244384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan Mohd Tajuddin W.N.B., Lajis N.H., Abas F., Othman I., Naidu R. Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients. 2019;11:2989. doi: 10.3390/nu11122989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye M.X., Li Y., Yin H., Zhang J. Curcumin: Updated molecular mechanisms and intervention targets in human lung cancer. Int. J. Mol. Sci. 2012;13:3959–3978. doi: 10.3390/ijms13033959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 26.Kuttan R., Bhanumathy P., Nirmala K., George M.C. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 27.Menon L.G., Kuttan R., Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995;95:221–225. doi: 10.1016/0304-3835(95)03887-3. [DOI] [PubMed] [Google Scholar]
- 28.Mirza S., Vasaiya A., Vora H., Jain N., Rawal R. Curcumin Targets Circulating Cancer Stem Cells by Inhibiting Self-Renewal Efficacy in Non-Small Cell Lung Carcinoma. Anti-Cancer Agents Med. Chem. 2017;17:859–864. doi: 10.2174/1871520616666160923102549. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L., Tao X., Fu Q., Ge C., Li R., Li Z., Zhu Y., Tian H., Li Q., Liu M., et al. Curcumin inhibits cell proliferation and migration in NSCLC through a synergistic effect on the TLR4/MyD88 and EGFR pathways. Oncol. Rep. 2019;42:1843–1855. doi: 10.3892/or.2019.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N., Feng T., Liu X., Liu Q. Curcumin inhibits migration and invasion of non-small cell lung cancer cells through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR signaling pathway. Acta Pharm. 2020;70:399–409. doi: 10.2478/acph-2020-0029. [DOI] [PubMed] [Google Scholar]
- 31.Tsai J.R., Liu P.L., Chen Y.H., Chou S.H., Cheng Y.J., Hwang J.J., Chong I.W. Curcumin Inhibits Non-Small Cell Lung Cancer Cells Metastasis through the Adiponectin/NF-κb/MMPs Signaling Pathway. PLoS ONE. 2015;10:e0144462. doi: 10.1371/journal.pone.0144462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen P., Li J., Jiang H.G., Lan T., Chen Y.C. Curcumin reverses cisplatin resistance in cisplatin-resistant lung caner cells by inhibiting FA/BRCA pathway. Tumour Biol. 2015;36:3591–3599. doi: 10.1007/s13277-014-2996-4. [DOI] [PubMed] [Google Scholar]
- 33.Wu S.H., Hang L.W., Yang J.S., Chen H.Y., Lin H.Y., Chiang J.H., Lu C.C., Yang J.L., Lai T.Y., Ko Y.C., et al. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer. Res. 2010;30:2125–2133. [PubMed] [Google Scholar]
- 34.Ye M., Zhang J., Zhang J., Miao Q., Yao L., Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357:196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Pongrakhananon V., Nimmannit U., Luanpitpong S., Rojanasakul Y., Chanvorachote P. Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis. 2010;15:574–585. doi: 10.1007/s10495-010-0461-4. [DOI] [PubMed] [Google Scholar]
- 36.Tang X., Ding H., Liang M., Chen X., Yan Y., Wan N., Chen Q., Zhang J., Cao J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac. Cancer. 2021;12:1219–1230. doi: 10.1111/1759-7714.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F., Chen X., Xu B., Zhou H. Curcumin induces p53-independent necrosis in H1299 cells via a mitochondria-associated pathway. Mol. Med. Rep. 2015;12:7806–7814. doi: 10.3892/mmr.2015.4395. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y., Yang X., Tan J., Tian R., Shen P., Cai W., Liao H. Curcumin suppresses the stemness of non-small cell lung cancer cells via promoting the nuclear-cytoplasm translocation of TAZ. Environ. Toxicol. 2021;36:1135–1142. doi: 10.1002/tox.23112. [DOI] [PubMed] [Google Scholar]
- 39.Chiang I.T., Wang W.S., Liu H.C., Yang S.T., Tang N.Y., Chung J.G. Curcumin alters gene expression-associated DNA damage, cell cycle, cell survival and cell migration and invasion in NCI-H460 human lung cancer cells in vitro. Oncol. Rep. 2015;34:1853–1874. doi: 10.3892/or.2015.4159. [DOI] [PubMed] [Google Scholar]
- 40.Li H., Wu H., Zhang H., Li Y., Li S., Hou Q., Wu S., Yang S.Y. Identification of curcumin-inhibited extracellular matrix receptors in non-small cell lung cancer A549 cells by RNA sequencing. Tumour Biol. 2017;39:1010428317705334. doi: 10.1177/1010428317705334. [DOI] [PubMed] [Google Scholar]
- 41.Kim K.C., Baek S.H., Lee C. Curcumin-induced downregulation of Axl receptor tyrosine kinase inhibits cell proliferation and circumvents chemoresistance in non-small lung cancer cells. Int. J. Oncol. 2015;47:2296–2303. doi: 10.3892/ijo.2015.3216. [DOI] [PubMed] [Google Scholar]
- 42.Li Y., Zhang S., Geng J.X., Hu X.Y. Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac. J. Cancer Prev. 2013;14:4599–4602. doi: 10.7314/APJCP.2013.14.8.4599. [DOI] [PubMed] [Google Scholar]
- 43.Xu X., Zhu Y. Curcumin inhibits human non-small cell lung cancer xenografts by targeting STAT3 pathway. Am. J. Transl. Res. 2017;9:3633–3641. [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H.W., Lee J.Y., Huang J.Y., Wang C.C., Chen W.J., Su S.F., Huang C.W., Ho C.C., Chen J.J., Tsai M.F., et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–7438. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y., Wei C., Xi Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitr. Cell. Dev. Biology. Anim. 2014;50:840–850. doi: 10.1007/s11626-014-9779-5. [DOI] [PubMed] [Google Scholar]
- 46.Wang J.Y., Wang X., Wang X.J., Zheng B.Z., Wang Y., Wang X., Liang B. Curcumin inhibits the growth via Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7492–7499. doi: 10.26355/eurrev_201811_16290. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q.Y., Jiao D.M., Wang L.F., Wang L., Hu H.Z., Song J., Yan J., Wu L.J., Shi J.G. Curcumin inhibits proliferation-migration of NSCLC by steering crosstalk between a Wnt signaling pathway and an adherens junction via EGR-1. Mol. Biosyst. 2015;11:859–868. doi: 10.1039/C4MB00336E. [DOI] [PubMed] [Google Scholar]
- 48.Dalamaga M., Diakopoulos K.N., Mantzoros C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin S.S., Lai K.C., Hsu S.C., Yang J.S., Kuo C.L., Lin J.P., Ma Y.S., Wu C.C., Chung J.G. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett. 2009;285:127–133. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 50.Deng X., Chen C., Wu F., Qiu L., Ke Q., Sun R., Duan Q., Luo M., Luo Z. Curcumin Inhibits the Migration and Invasion of Non-Small-Cell Lung Cancer Cells Through Radiation-Induced Suppression of Epithelial-Mesenchymal Transition and Soluble E-Cadherin Expression. Technol. Cancer Res. Treat. 2020;19:1533033820947485. doi: 10.1177/1533033820947485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Mucchietto V., Crespi A., Fasoli F., Clementi F., Gotti C. Neuronal Acetylcholine Nicotinic Receptors as New Targets for Lung Cancer Treatment. Curr. Pharm. Des. 2016;22:2160–2169. doi: 10.2174/1381612822666160203144114. [DOI] [PubMed] [Google Scholar]
- 52.Schaal C., Chellappan S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egleton R.D., Brown K.C., Dasgupta P. Nicotinic acetylcholine receptors in cancer: Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol. Sci. 2008;29:151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y., Ritzenthaler J.D., Roman J., Han S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am. J. Respir. Cell Mol. Biol. 2007;37:681–690. doi: 10.1165/rcmb.2007-0051OC. [DOI] [PubMed] [Google Scholar]
- 55.Schuller H.M. Regulatory role of the α7nAChR in cancer. Curr. Drug Targets. 2012;13:680–687. doi: 10.2174/138945012800398883. [DOI] [PubMed] [Google Scholar]
- 56.Schuller H.M. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 57.Ximenis M., Mulet J., Sala S., Sala F., Criado M., González-Muñiz R., Pérez de Vega M.J. Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as α7 nAChRs Positive Allosteric Modulators. Int. J. Mol. Sci. 2021;22:973. doi: 10.3390/ijms22020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nebrisi E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021;22:11248. doi: 10.3390/ijms222011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jayaprakash P., Isaev D., Shabbir W., Lorke D.E., Sadek B., Oz M. Curcumin Potentiates α7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice. Int. J. Mol. Sci. 2021;22:7251. doi: 10.3390/ijms22147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shishodia S., Potdar P., Gairola C.G., Aggarwal B.B. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: Correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–1279. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 61.Li X., He S., Ma B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu F., Gao S., Yang Y., Zhao X., Fan Y., Ma W., Yang D., Yang A., Yu Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol. Rep. 2018;39:1523–1531. doi: 10.3892/or.2018.6188. [DOI] [PubMed] [Google Scholar]
- 63.Wang A., Wang J., Zhang S., Zhang H., Xu Z., Li X. Curcumin inhibits the development of non-small cell lung cancer by inhibiting autophagy and apoptosis. Exp. Ther. Med. 2017;14:5075–5080. doi: 10.3892/etm.2017.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X., Chen D., Ye B., Zhong F., Chen G. Curcumin induces the apoptosis of non-small cell lung cancer cells through a calcium signaling pathway. Int. J. Mol. Med. 2015;35:1610–1616. doi: 10.3892/ijmm.2015.2167. [DOI] [PubMed] [Google Scholar]
- 65.Wang C., Song X., Shang M., Zou W., Zhang M., Wei H., Shao H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019;15:1243–1253. doi: 10.2217/fon-2018-0708. [DOI] [PubMed] [Google Scholar]
- 66.Lev-Ari S., Starr A., Katzburg S., Berkovich L., Rimmon A., Ben-Yosef R., Vexler A., Ron I., Earon G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J. Nutr. Biochem. 2014;25:843–850. doi: 10.1016/j.jnutbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Pan Y., Sun Y., Liu Z., Zhang C. miR-192-5p upregulation mediates the suppression of curcumin in human NSCLC cell proliferation, migration and invasion by targeting c-Myc and inactivating the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020;22:1594–1604. doi: 10.3892/mmr.2020.11213. [DOI] [PubMed] [Google Scholar]
- 68.Zhan J.W., Jiao D.M., Wang Y., Song J., Wu J.H., Wu L.J., Chen Q.Y., Ma S.L. Integrated microRNA and gene expression profiling reveals the crucial miRNAs in curcumin anti-lung cancer cell invasion. Thorac. Cancer. 2017;8:461–470. doi: 10.1111/1759-7714.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W.L., Chang J.M., Chong I.W., Hung Y.L., Chen Y.H., Huang W.T., Kuo H.F., Hsieh C.C., Liu P.L. Curcumin Inhibits LIN-28A through the Activation of miRNA-98 in the Lung Cancer Cell Line A549. Molecules. 2017;22:929. doi: 10.3390/molecules22060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W., Bai W., Zhang W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin. Transl. Oncol. 2014;16:708–713. doi: 10.1007/s12094-013-1135-9. [DOI] [PubMed] [Google Scholar]
- 71.Xu X., Zhang X., Zhang Y., Wang Z. Curcumin suppresses the malignancy of non-small cell lung cancer by modulating the circ-PRKCA/miR-384/ITGB1 pathway. Biomed. Pharmacother. 2021;138:111439. doi: 10.1016/j.biopha.2021.111439. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J., Zhang T., Ti X., Shi J., Wu C., Ren X., Yin H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem. Biophys. Res. Commun. 2010;399:1–6. doi: 10.1016/j.bbrc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Gao L., Shao T., Zheng W., Ding J. Curcumin suppresses tumor growth of gemcitabine-resistant non-small cell lung cancer by regulating lncRNA-MEG3 and PTEN signaling. Clin. Transl. Oncol. 2021;23:1386–1393. doi: 10.1007/s12094-020-02531-3. [DOI] [PubMed] [Google Scholar]
- 74.Haider T., Pandey V., Banjare N., Gupta P.N., Soni V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020;72:1125–1151. doi: 10.1007/s43440-020-00138-7. [DOI] [PubMed] [Google Scholar]
- 75.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdul Satar N., Ismail M.N., Yahaya B.H. Synergistic Roles of Curcumin in Sensitising the Cisplatin Effect on a Cancer Stem Cell-Like Population Derived from Non-Small Cell Lung Cancer Cell Lines. Molecules. 2021;26:1056. doi: 10.3390/molecules26041056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang W., Shi H., Chen C., Ren K., Xu Y., Liu X., He L. Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine. 2018;48:51–61. doi: 10.1016/j.phymed.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 78.Tung C.L., Jian Y.J., Chen J.C., Wang T.J., Chen W.C., Zheng H.Y., Chang P.Y., Liao K.S., Lin Y.W. Curcumin downregulates p38 MAPK-dependent X-ray repair cross-complement group 1 (XRCC1) expression to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2016;389:657–666. doi: 10.1007/s00210-016-1235-5. [DOI] [PubMed] [Google Scholar]
- 79.Baharuddin P., Satar N., Fakiruddin K.S., Zakaria N., Lim M.N., Yusoff N.M., Zakaria Z., Yahaya B.H. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol. Rep. 2016;35:13–25. doi: 10.3892/or.2015.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai M.S., Weng S.H., Kuo Y.H., Chiu Y.F., Lin Y.W. Synergistic effect of curcumin and cisplatin via down-regulation of thymidine phosphorylase and excision repair cross-complementary 1 (ERCC1) Mol. Pharmacol. 2011;80:136–146. doi: 10.1124/mol.111.071316. [DOI] [PubMed] [Google Scholar]
- 81.Chanvorachote P., Pongrakhananon V., Wannachaiyasit S., Luanpitpong S., Rojanasakul Y., Nimmannit U. Curcumin sensitizes lung cancer cells to cisplatin-induced apoptosis through superoxide anion-mediated Bcl-2 degradation. Cancer Investig. 2009;27:624–635. doi: 10.1080/07357900802653472. [DOI] [PubMed] [Google Scholar]
- 82.Qi M., Chen X., Bian L., Zhang H., Ma J. Honokiol combined with curcumin sensitizes multidrug-resistant human lung adenocarcinoma A549/DDP cells to cisplatin. Exp. Ther. Med. 2021;22:1301. doi: 10.3892/etm.2021.10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai Y., Sheng Z., Liang S. Radiosensitization effects of curcumin plus cisplatin on non-small cell lung cancer A549 cells. Oncol. Lett. 2019;18:529–534. doi: 10.3892/ol.2019.10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang J.H., Kang H.S., Kim I.K., Lee H.Y., Ha J.H., Yeo C.D., Kang H.H., Moon H.S., Lee S.H. Curcumin sensitizes human lung cancer cells to apoptosis and metastasis synergistically combined with carboplatin. Exp. Biol. Med. (Maywood) 2015;240:1416–1425. doi: 10.1177/1535370215571881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tunc D., Dere E., Karakas D., Cevatemre B., Yilmaz V.T., Ulukaya E. Cytotoxic and apoptotic effects of the combination of palladium (II) 5,5-diethylbarbiturate complex with bis(2-pyridylmethyl)amine and curcumin on non small lung cancer cell lines. Bioorganic Med. Chem. 2017;25:1717–1723. doi: 10.1016/j.bmc.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 86.Weng S.H., Tsai M.S., Chiu Y.F., Kuo Y.H., Chen H.J., Lin Y.W. Enhancement of mitomycin C-induced cytotoxicity by curcumin results from down-regulation of MKK1/2-ERK1/2-mediated thymidine phosphorylase expression. Basic Clin. Pharmacol. Toxicol. 2012;110:298–306. doi: 10.1111/j.1742-7843.2011.00806.x. [DOI] [PubMed] [Google Scholar]
- 87.Ko J.C., Tsai M.S., Weng S.H., Kuo Y.H., Chiu Y.F., Lin Y.W. Curcumin enhances the mitomycin C-induced cytotoxicity via downregulation of MKK1/2-ERK1/2-mediated Rad51 expression in non-small cell lung cancer cells. Toxicol. Appl. Pharmacol. 2011;255:327–338. doi: 10.1016/j.taap.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Sen S., Sharma H., Singh N. Curcumin enhances Vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem. Biophys. Res. Commun. 2005;331:1245–1252. doi: 10.1016/j.bbrc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 89.Dong Z., Feng Q., Zhang H., Liu Q., Gong J. Curcumin enhances drug sensitivity of gemcitabine-resistant lung cancer cells and inhibits metastasis. Die Pharm. 2021;76:538–543. doi: 10.1691/ph.2021.0927. [DOI] [PubMed] [Google Scholar]
- 90.Lu Y., Wang J., Liu L., Yu L., Zhao N., Zhou X., Lu X. Curcumin increases the sensitivity of Paclitaxel-resistant NSCLC cells to Paclitaxel through microRNA-30c-mediated MTA1 reduction. Tumour Biol. 2017;39:1010428317698353. doi: 10.1177/1010428317698353. [DOI] [PubMed] [Google Scholar]
- 91.Yin H., Guo R., Xu Y., Zheng Y., Hou Z., Dai X., Zhang Z., Zheng D., Xu H. Synergistic antitumor efficiency of docetaxel and curcumin against lung cancer. Acta Biochim. Et Biophys. Sin. 2012;44:147–153. doi: 10.1093/abbs/gmr106. [DOI] [PubMed] [Google Scholar]
- 92.He Y.Z., Yu S.L., Li X.N., Bai X.H., Li H.T., Liu Y.C., Lv B.L., Zhao X.M., Wei D., Zhang H.L., et al. Curcumin increases crizotinib sensitivity through the inactivation of autophagy via epigenetic modulation of the miR-142-5p/Ulk1 axis in non-small cell lung cancer. Cancer Biomark. 2022;34:297–307. doi: 10.3233/CBM-210282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S., Liu Z., Zhu F., Fan X., Wu X., Zhao H., Jiang L. Curcumin lowers erlotinib resistance in non-small cell lung carcinoma cells with mutated EGF receptor. Oncol. Res. 2013;21:137–144. doi: 10.3727/096504013X13832473330032. [DOI] [PubMed] [Google Scholar]
- 94.Chen P., Huang H.P., Wang Y., Jin J., Long W.G., Chen K., Zhao X.H., Chen C.G., Li J. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J. Exp. Clin. Cancer Res. 2019;38:254. doi: 10.1186/s13046-019-1234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J.Y., Lee Y.M., Chang G.C., Yu S.L., Hsieh W.Y., Chen J.J., Chen H.W., Yang P.C. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE. 2011;6:e23756. doi: 10.1371/journal.pone.0023756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou D.H., Wang X., Yang M., Shi X., Huang W., Feng Q. Combination of low concentration of (-)-epigallocatechin gallate (EGCG) and curcumin strongly suppresses the growth of non-small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int. J. Mol. Sci. 2013;14:12023–12036. doi: 10.3390/ijms140612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andjelkovic T., Pesic M., Bankovic J., Tanic N., Markovic I.D., Ruzdijic S. Synergistic effects of the purine analog sulfinosine and curcumin on the multidrug resistant human non-small cell lung carcinoma cell line (NCI-H460/R) Cancer Biol. Ther. 2008;7:1024–1032. doi: 10.4161/cbt.7.7.6036. [DOI] [PubMed] [Google Scholar]
- 98.Lee J., Im Y.H., Jung H.H., Kim J.H., Park J.O., Kim K., Kim W.S., Ahn J.S., Jung C.W., Park Y.S., et al. Curcumin inhibits interferon-alpha induced NF-kappaB and COX-2 in human A549 non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2005;334:313–318. doi: 10.1016/j.bbrc.2005.06.093. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Q., Qiao H., Wu D., Lu H., Liu L., Sang X., Li D., Zhou Y. Curcumin potentiates the galbanic acid-induced anti-tumor effect in non-small cell lung cancer cells through inhibiting Akt/mTOR signaling pathway. Life Sci. 2019;239:117044. doi: 10.1016/j.lfs.2019.117044. [DOI] [PubMed] [Google Scholar]
- 100.Saha A., Kuzuhara T., Echigo N., Suganuma M., Fujiki H. New role of (-)-epicatechin in enhancing the induction of growth inhibition and apoptosis in human lung cancer cells by curcumin. Cancer Prev. Res. 2010;3:953–962. doi: 10.1158/1940-6207.CAPR-09-0247. [DOI] [PubMed] [Google Scholar]
- 101.Chen H., Chen L., Wang L., Zhou X., Chan J.Y., Li J., Cui G., Lee S.M. Synergistic effect of fenretinide and curcumin for treatment of non-small cell lung cancer. Cancer Biol. Ther. 2016;17:1022–1029. doi: 10.1080/15384047.2016.1219810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin H.P., Kuo L.K., Chuu C.P. Combined treatment of curcumin and small molecule inhibitors suppresses proliferation of A549 and H1299 human non-small-cell lung cancer cells. Phytother. Res. 2012;26:122–126. doi: 10.1002/ptr.3523. [DOI] [PubMed] [Google Scholar]
- 103.Rossi A., Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: Optimal number of treatment cycles. Expert Rev. Anticancer. Ther. 2016;16:653–660. doi: 10.1586/14737140.2016.1170596. [DOI] [PubMed] [Google Scholar]
- 104.Cosaert J., Quoix E. Platinum drugs in the treatment of non-small-cell lung cancer. Br. J. Cancer. 2002;87:825–833. doi: 10.1038/sj.bjc.6600540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang R., Niu Y., Zhou Y. Increase the cisplatin cytotoxicity and cisplatin-induced DNA damage in HepG2 cells by XRCC1 abrogation related mechanisms. Toxicol. Lett. 2010;192:108–114. doi: 10.1016/j.toxlet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 106.Brem R., Hall J. XRCC1 is required for DNA single-strand break repair in human cells. Nucleic acids research. 2005;33:2512–2520. doi: 10.1093/nar/gki543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kudo K., Gavin E., Das S., Amable L., Shevde L.A., Reed E. Inhibition of Gli1 results in altered c-Jun activation, inhibition of cisplatin-induced upregulation of ERCC1, XPD and XRCC1, and inhibition of platinum-DNA adduct repair. Oncogene. 2012;31:4718–4724. doi: 10.1038/onc.2011.610. [DOI] [PubMed] [Google Scholar]
- 108.Niedernhofer L.J., Odijk H., Budzowska M., van Drunen E., Maas A., Theil A.F., de Wit J., Jaspers N.G., Beverloo H.B., Hoeijmakers J.H., et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sahu V., Mohan A., Dey S. p38 MAP kinases: Plausible diagnostic and prognostic serum protein marker of non small cell lung cancer. Exp. Mol. Pathol. 2019;107:118–123. doi: 10.1016/j.yexmp.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Prabhu K.S., Bhat A.A., Siveen K.S., Kuttikrishnan S., Raza S.S., Raheed T., Jochebeth A., Khan A.Q., Chawdhery M.Z., Haris M., et al. Sanguinarine mediated apoptosis in Non-Small Cell Lung Cancer via generation of reactive oxygen species and suppression of JAK/STAT pathway. Biomed. Pharmacother. 2021;144:112358. doi: 10.1016/j.biopha.2021.112358. [DOI] [PubMed] [Google Scholar]
- 111.Yao Y., Yang G., Lu G., Ye J., Cui L., Zeng Z., Chen J., Zhou J. Th22 Cells/IL-22 Serves as a Protumor Regulator to Drive Poor Prognosis through the JAK-STAT3/MAPK/AKT Signaling Pathway in Non-Small-Cell Lung Cancer. J. Immunol. Res. 2022;2022:8071234. doi: 10.1155/2022/8071234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng M., Zheng Z., Chen S., Fang L., Feng R., Zhang L., Tang Q., Liu X. Sensitization of Non-Small Cell Lung Cancer Cells to Gefitinib and Reversal of Epithelial-Mesenchymal Transition by Aloe-Emodin Via PI3K/Akt/TWIS1 Signal Blockage. Front. Oncol. 2022;12:908031. doi: 10.3389/fonc.2022.908031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vora M., Mondal A., Jia D., Gaddipati P., Akel M., Gilleran J., Roberge J., Rongo C., Langenfeld J. Bone morphogenetic protein signaling regulation of AMPK and PI3K in lung cancer cells and C. elegans. Cell Biosci. 2022;12:76. doi: 10.1186/s13578-022-00817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao F., Yu X., Li M., Zhou L., Liu W., Li W., Liu H. Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization. Cell Death Dis. 2020;11:143. doi: 10.1038/s41419-020-2344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren J., Liu T., Han Y., Wang Q., Chen Y., Li G., Jiang L. GSK-3β inhibits autophagy and enhances radiosensitivity in non-small cell lung cancer. Diagn. Pathol. 2018;13:33. doi: 10.1186/s13000-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Gao Y., Lin T. Expression of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) in non-small cell lung cancer and its relationship with the occurrence and prognosis of cancer pain. Ann. Palliat. Med. 2021;10:12759–12766. doi: 10.21037/apm-21-3471. [DOI] [PubMed] [Google Scholar]
- 117.Lin A., Zhang H., Meng H., Deng Z., Gu T., Luo P., Zhang J. TNF-Alpha Pathway Alternation Predicts Survival of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Front. Immunol. 2021;12:667875. doi: 10.3389/fimmu.2021.667875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang H., Wang J., Zhao W. Cox-2 in non-small cell lung cancer: A meta-analysis. Clin. Chim. Acta Int. J. Clin. Chem. 2013;419:26–32. doi: 10.1016/j.cca.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 119.Adeluola A., Zulfiker A.H.M., Brazeau D., Amin A. Perspectives for synthetic curcumins in chemoprevention and treatment of cancer: An update with promising analogues. Eur. J. Pharmacol. 2021;906:174266. doi: 10.1016/j.ejphar.2021.174266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin Y.J., Chen C.J., Hsueh S.C., Lee M.H., Peng S.F., Lu H.F., Lu K.W., Huang W.W., Liu K.C., Chen Y.L., et al. Demethoxycurcumin Promotes Macrophage Cell Population and Phagocytosis in WEHI-3 Cell-generated Leukemia BALB/c Mice In Vivo. In Vivo. 2021;35:3253–3260. doi: 10.21873/invivo.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luo S.M., Wu Y.P., Huang L.C., Huang S.M., Hueng D.Y. The Anti-Cancer Effect of Four Curcumin Analogues on Human Glioma Cells. Onco Targets Ther. 2021;14:4345–4359. doi: 10.2147/OTT.S313961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kao C.C., Cheng Y.C., Yang M.H., Cha T.L., Sun G.H., Ho C.T., Lin Y.C., Wang H.K., Wu S.T., Way T.D. Demethoxycurcumin induces apoptosis in HER2 overexpressing bladder cancer cells through degradation of HER2 and inhibiting the PI3K/Akt pathway. Environ. Toxicol. 2021;36:2186–2195. doi: 10.1002/tox.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chueh F.S., Lien J.C., Chou Y.C., Huang W.W., Huang Y.P., Huang J.Y., Kuo J.Y., Huang W.N., Sheng S.Y., Tung H.Y., et al. Demethoxycurcumin Inhibits In Vivo Growth of Xenograft Tumors of Human Cervical Cancer Cells. In Vivo. 2020;34:2469–2474. doi: 10.21873/invivo.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ibáñez Gaspar V., McCaul J., Cassidy H., Slattery C., McMorrow T. Effects of Curcumin Analogues DMC and EF24 in Combination with the Cytokine TRAIL against Kidney Cancer. Molecules. 2021;26:6302. doi: 10.3390/molecules26206302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin C.Y., Hung C.C., Wang C.C.N., Lin H.Y., Huang S.H., Sheu M.J. Demethoxycurcumin sensitizes the response of non-small cell lung cancer to cisplatin through downregulation of TP and ERCC1-related pathways. Phytomedicine. 2019;53:28–36. doi: 10.1016/j.phymed.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 126.Chen Y., Hong C., Chen X., Qin Z. Demethoxycurcumin increases the sensitivity of cisplatin-resistant non-small lung cancer cells to cisplatin and induces apoptosis by activating the caspase signaling pathway. Oncol. Lett. 2020;20:209. doi: 10.3892/ol.2020.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ko Y.C., Lien J.C., Liu H.C., Hsu S.C., Ji B.C., Yang M.D., Hsu W.H., Chung J.G. Demethoxycurcumin induces the apoptosis of human lung cancer NCI-H460 cells through the mitochondrial-dependent pathway. Oncol. Rep. 2015;33:2429–2437. doi: 10.3892/or.2015.3865. [DOI] [PubMed] [Google Scholar]
- 128.Datta S., Misra S.K., Saha M.L., Lahiri N., Louie J., Pan D., Stang P.J. Orthogonal self-assembly of an organoplatinum(II) metallacycle and cucurbit[8]uril that delivers curcumin to cancer cells. Proc. Natl. Acad. Sci. USA. 2018;115:8087–8092. doi: 10.1073/pnas.1803800115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y.Y., Lin Y.J., Huang W.T., Hung C.C., Lin H.Y., Tu Y.C., Liu D.M., Lan S.J., Sheu M.J. Demethoxycurcumin-Loaded Chitosan Nanoparticle Downregulates DNA Repair Pathway to Improve Cisplatin-Induced Apoptosis in Non-Small Cell Lung Cancer. Molecules. 2018;23:3217. doi: 10.3390/molecules23123217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang W.T., Larsson M., Wang Y.J., Chiou S.H., Lin H.Y., Liu D.M. Demethoxycurcumin-carrying chitosan-antibody core-shell nanoparticles with multitherapeutic efficacy toward malignant A549 lung tumor: From in vitro characterization to in vivo evaluation. Mol. Pharm. 2015;12:1242–1249. doi: 10.1021/mp500747w. [DOI] [PubMed] [Google Scholar]
- 131.Ramezani M., Hatamipour M., Sahebkar A. Promising anti-tumor properties of bisdemethoxycurcumin: A naturally occurring curcumin analogue. J. Cell. Physiol. 2018;233:880–887. doi: 10.1002/jcp.25795. [DOI] [PubMed] [Google Scholar]
- 132.Qiu C., Liu K., Zhang S., Gao S., Chen W., Li D., Huang Y. Bisdemethoxycurcumin Inhibits Hepatocellular Carcinoma Proliferation Through Akt Inactivation via CYLD-Mediated Deubiquitination. Drug Des. Dev. Ther. 2020;14:993–1001. doi: 10.2147/DDDT.S231814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsia T.C., Peng S.F., Chueh F.S., Lu K.W., Yang J.L., Huang A.C., Hsu F.T., Wu R.S. Bisdemethoxycurcumin Induces Cell Apoptosis and Inhibits Human Brain Glioblastoma GBM 8401/Luc2 Cell Xenograft Tumor in Subcutaneous Nude Mice In Vivo. Int. J. Mol. Sci. 2022;23:538. doi: 10.3390/ijms23010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chou C.H., Wang H.K., Lin Y.C., Tsai D.H., Lu M.T., Ho C.T., Hseu Y.C., Yang H.L., Way T.D. Bisdemethoxycurcumin Promotes Apoptosis and Inhibits the Epithelial-Mesenchymal Transition through the Inhibition of the G-Protein-Coupled Receptor 161/Mammalian Target of Rapamycin Signaling Pathway in Triple Negative Breast Cancer Cells. J. Agric. Food Chem. 2021;69:14557–14567. doi: 10.1021/acs.jafc.1c05585. [DOI] [PubMed] [Google Scholar]
- 135.Luo C., Du Z., Wei X., Chen G., Fu Z. Bisdemethoxycurcumin attenuates gastric adenocarcinoma growth by inducing mitochondrial dysfunction. Oncol. Lett. 2015;9:270–274. doi: 10.3892/ol.2014.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shao Y., Zhu W., Da J., Xu M., Wang Y., Zhou J., Wang Z. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther. 2017;10:2675–2683. doi: 10.2147/OTT.S130653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liao C.L., Chu Y.L., Lin H.Y., Chen C.Y., Hsu M.J., Liu K.C., Lai K.C., Huang A.C., Chung J.G. Bisdemethoxycurcumin Suppresses Migration and Invasion of Human Cervical Cancer HeLa Cells via Inhibition of NF-ĸB, MMP-2 and -9 Pathways. Anticancer. Res. 2018;38:3989–3997. doi: 10.21873/anticanres.12686. [DOI] [PubMed] [Google Scholar]
- 138.Yu C.C., Yang S.T., Huang W.W., Peng S.F., Huang A.C., Tang N.Y., Liu H.C., Yang M.D., Lai K.C., Chung J.G. Bisdemethoxycurcumin induces DNA damage and inhibits DNA repair associated protein expressions in NCI-H460 human lung cancer cells. Environ. Toxicol. 2016;31:1859–1868. doi: 10.1002/tox.22187. [DOI] [PubMed] [Google Scholar]
- 139.Yu C.C., Yang M.D., Lin H.Y., Huang A.C., Lin J.P., Kuo C.L., Liu K.C., Liu H.C., Yang S.T., Chung J.G. Bisdemethoxycurcumin (BDMC) Alters Gene Expression-associated Cell Cycle, Cell Migration and Invasion and Tumor Progression in Human Lung Cancer NCI-H460 Cells. In Vivo. 2015;29:711–728. [PubMed] [Google Scholar]
- 140.Liu Y.L., Yang H.P., Zhou X.D., Gong L., Tang C.L., Wang H.J. The hypomethylation agent bisdemethoxycurcumin acts on the WIF-1 promoter, inhibits the canonical Wnt pathway and induces apoptosis in human non-small-cell lung cancer. Curr. Cancer Drug Targets. 2011;11:1098–1110. doi: 10.2174/156800911798073041. [DOI] [PubMed] [Google Scholar]
- 141.Valenta T., Hausmann G., Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee J.S., Hur M.W., Lee S.K., Choi W.I., Kwon Y.G., Yun C.O. A novel sLRP6E1E2 inhibits canonical Wnt signaling, epithelial-to-mesenchymal transition, and induces mitochondria-dependent apoptosis in lung cancer. PLoS ONE. 2012;7:e36520. doi: 10.1371/journal.pone.0036520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu J.H., Yang H.P., Zhou X.D., Wang H.J., Gong L., Tang C.L. Role of Wnt Inhibitory Factor-1 in Inhibition of Bisdemethoxycurcumin Mediated Epithelial-to-Mesenchymal Transition in Highly Metastatic Lung Cancer 95D Cells. Chin. Med. J. 2015;128:1376–1383. doi: 10.4103/0366-6999.156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu J., Yang H., Zhou X., Wang H., Gong L., Tang C. Bisdemethoxycurcumin suppresses migration and invasion of highly metastatic 95D lung cancer cells by regulating E-cadherin and vimentin expression, and inducing autophagy. Mol. Med. Rep. 2015;12:7603–7608. doi: 10.3892/mmr.2015.4356. [DOI] [PubMed] [Google Scholar]
- 145.Xu J.H., Yang H.P., Zhou X.D., Wang H.J., Gong L., Tang C.L. Autophagy Accompanied with Bisdemethoxycurcumin-induced Apoptosis in Non-small Cell Lung Cancer Cells. Biomed. Environ. Sci. 2015;28:105–115. doi: 10.3967/bes2015.013. [DOI] [PubMed] [Google Scholar]
- 146.Yang S.T., Huang A.C., Tang N.Y., Liu H.C., Liao C.L., Ji B.C., Chou Y.C., Yang M.D., Lu H.F., Chung J.G. Bisdemethoxycurcumin-induced S phase arrest through the inhibition of cyclin A and E and induction of apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathways in human lung cancer NCI H460 cells. Environ. Toxicol. 2016;31:1899–1908. doi: 10.1002/tox.22191. [DOI] [PubMed] [Google Scholar]
- 147.Xiang M., Jiang H.G., Shu Y., Chen Y.J., Jin J., Zhu Y.M., Li M.Y., Wu J.N., Li J. Bisdemethoxycurcumin Enhances the Sensitivity of Non-small Cell Lung Cancer Cells to Icotinib via Dual Induction of Autophagy and Apoptosis. Int. J. Biol. Sci. 2020;16:1536–1550. doi: 10.7150/ijbs.40042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang H.J., Yang Z.X., Dai X.T., Chen Y.F., Yang H.P., Zhou X.D. Bisdemethoxycurcumin sensitizes cisplatin-resistant lung cancer cells to chemotherapy by inhibition of CA916798 and PI3K/AKT signaling. Apoptosis. 2017;22:1157–1168. doi: 10.1007/s10495-017-1395-x. [DOI] [PubMed] [Google Scholar]
- 149.Takeuchi K., Ito F. Receptor tyrosine kinases and targeted cancer therapeutics. Biol. Pharm. Bull. 2011;34:1774–1780. doi: 10.1248/bpb.34.1774. [DOI] [PubMed] [Google Scholar]
- 150.Pisano C., De Filippis M., Jacobs F., Novello S., Reale M.L. Management of Oligoprogression in Patients with Metastatic NSCLC Harboring ALK Rearrangements. Cancers. 2022;14:718. doi: 10.3390/cancers14030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin J.J., Riely G.J., Shaw A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yadav B., Taurin S., Rosengren R.J., Schumacher M., Diederich M., Somers-Edgar T.J., Larsen L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorganic Med. Chem. 2010;18:6701–6707. doi: 10.1016/j.bmc.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 153.Chen S., Nimick M., Cridge A.G., Hawkins B.C., Rosengren R.J. Anticancer potential of novel curcumin analogs towards castrate-resistant prostate cancer. Int. J. Oncol. 2018;52:579–588. doi: 10.3892/ijo.2017.4207. [DOI] [PubMed] [Google Scholar]
- 154.Bland A.R., Bower R.L., Nimick M., Hawkins B.C., Rosengren R.J., Ashton J.C. Cytotoxicity of curcumin derivatives in ALK positive non-small cell lung cancer. Eur. J. Pharmacol. 2019;865:172749. doi: 10.1016/j.ejphar.2019.172749. [DOI] [PubMed] [Google Scholar]
- 155.Gazdar A.F. Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28((Suppl. 1)):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balak M.N., Gong Y., Riely G.J., Somwar R., Li A.R., Zakowski M.F., Chiang A., Yang G., Ouerfelli O., Kris M.G., et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin. Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 157.Dai X., Zhang J., Guo G., Cai Y., Cui R., Yin C., Liu W., Vinothkumar R., Zhang T., Liang G., et al. A mono-carbonyl analog of curcumin induces apoptosis in drug-resistant EGFR-mutant lung cancer through the generation of oxidative stress and mitochondrial dysfunction. Cancer Manag. Res. 2018;10:3069–3082. doi: 10.2147/CMAR.S159660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shaik N.A., Al-Kreathy H.M., Ajabnoor G.M., Verma P.K., Banaganapalli B. Molecular designing, virtual screening and docking study of novel curcumin analogue as mutation (S769L and K846R) selective inhibitor for EGFR. Saudi J. Biol. Sci. 2019;26:439–448. doi: 10.1016/j.sjbs.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L., Qiang P., Yu J., Miao Y., Chen Z., Qu J., Zhao Q., Chen Z., Liu Y., Yao X., et al. Identification of compound CA-5f as a novel late-stage autophagy inhibitor with potent anti-tumor effect against non-small cell lung cancer. Autophagy. 2019;15:391–406. doi: 10.1080/15548627.2018.1511503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang M., Shang M., Yuan F., Guo W., Wang C. EF24 exerts cytotoxicity against NSCLC via inducing ROS accumulation. Cancer Cell Int. 2021;21:531. doi: 10.1186/s12935-021-02240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wan Mohd Tajuddin W.N.B., Abas F., Othman I., Naidu R. Molecular Mechanisms of Antiproliferative and Apoptosis Activity by 1,5-Bis(4-hydroxy-3-methoxyphenyl)1,4-pentadiene-3-one (MS13) on Human Non-Small Cell Lung Cancer Cells. Int. J. Mol. Sci. 2021;22:7424. doi: 10.3390/ijms22147424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhi T.X., Liu K.Q., Cai K.Y., Zhao Y.C., Li Z.W., Wang X., He X.H., Sun X.Y. Anti-Lung Cancer Activities of 1,2,3-Triazole Curcumin Derivatives via Regulation of the MAPK/NF-κB/STAT3 Signaling Pathways. ChemMedChem. 2022;17:e202100676. doi: 10.1002/cmdc.202100676. [DOI] [PubMed] [Google Scholar]
- 163.Faitova J., Krekac D., Hrstka R., Vojtesek B. Endoplasmic reticulum stress and apoptosis. Cell. Mol. Biol. Lett. 2006;11:488–505. doi: 10.2478/s11658-006-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu Z., Sun Y., Ren L., Huang Y., Cai Y., Weng Q., Shen X., Li X., Liang G., Wang Y. Evaluation of a curcumin analog as an anti-cancer agent inducing ER stress-mediated apoptosis in non-small cell lung cancer cells. BMC Cancer. 2013;13:494. doi: 10.1186/1471-2407-13-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiao J., Wang Y., Peng J., Guo L., Hu J., Cao M., Zhang X., Zhang H., Wang Z., Li X., et al. A synthetic compound, 1,5-bis(2-methoxyphenyl)penta-1,4-dien-3-one (B63), induces apoptosis and activates endoplasmic reticulum stress in non-small cell lung cancer cells. Int. J. Cancer. 2012;131:1455–1465. doi: 10.1002/ijc.27406. [DOI] [PubMed] [Google Scholar]
- 166.Zhou G.Z., Li A.F., Sun Y.H., Sun G.C. A novel synthetic curcumin derivative MHMM-41 induces ROS-mediated apoptosis and migration blocking of human lung cancer cells A549. Biomed. Pharmacother. 2018;103:391–398. doi: 10.1016/j.biopha.2018.04.086. [DOI] [PubMed] [Google Scholar]
- 167.Xia Y.Q., Wei X.Y., Li W.L., Kanchana K., Xu C.C., Chen D.H., Chou P.H., Jin R., Wu J.Z., Liang G. Curcumin analogue A501 induces G2/M arrest and apoptosis in non-small cell lung cancer cells. Asian Pac. J. Cancer Prev. 2014;15:6893–6898. doi: 10.7314/APJCP.2014.15.16.6893. [DOI] [PubMed] [Google Scholar]
- 168.Ye H., Wei X., Wang Z., Zhang S., Ren J., Yao S., Shi L., Yang L., Qiu P., Wu J., et al. A novel double carbonyl analog of curcumin induces the apoptosis of human lung cancer H460 cells via the activation of the endoplasmic reticulum stress signaling pathway. Oncol. Rep. 2016;36:1640–1648. doi: 10.3892/or.2016.4911. [DOI] [PubMed] [Google Scholar]
- 169.Wang J., Gong M., Fan X., Huang D., Zhang J., Huang C. Autophagy-related signaling pathways in non-small cell lung cancer. Mol. Cell. Biochem. 2022;477:385–393. doi: 10.1007/s11010-021-04280-5. [DOI] [PubMed] [Google Scholar]
- 170.Zhou G.Z., Shi Y.Y., Wei L.L., Sun G.C. Autophagy induction and antiproliferative effect of a novel curcumin derivative MOMI-1 on the human lung cancer cells A549. J. Biochem. Mol. Toxicol. 2019;33:e22280. doi: 10.1002/jbt.22280. [DOI] [PubMed] [Google Scholar]
- 171.Zhou G.Z., Zhang S.N., Zhang L., Sun G.C., Chen X.B. A synthetic curcumin derivative hydrazinobenzoylcurcumin induces autophagy in A549 lung cancer cells. Pharm. Biol. 2014;52:111–116. doi: 10.3109/13880209.2013.816971. [DOI] [PubMed] [Google Scholar]
- 172.Zhou G.Z., Wang Q.Q., Wang P.B., Wang Z.C., Sun G.C. One novel curcumin derivative ZYX01 induces autophagy of human non-small lung cancer cells A549 through AMPK/ULK1/Beclin-1 signaling pathway. Cell. Mol. Biol. 2019;65:1–6. doi: 10.14715/cmb/2019.65.2.1. [DOI] [PubMed] [Google Scholar]
- 173.Song G., Lu H., Chen F., Wang Y., Fan W., Shao W., Lu H., Lin B. Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells. Mol. Med. Rep. 2018;17:5964–5969. doi: 10.3892/mmr.2018.8600. [DOI] [PubMed] [Google Scholar]
- 174.White E., Mehnert J.M., Chan C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015;21:5037–5046. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Karsli-Uzunbas G., Guo J.Y., Price S., Teng X., Laddha S.V., Khor S., Kalaany N.Y., Jacks T., Chan C.S., Rabinowitz J.D., et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014;4:914–927. doi: 10.1158/2159-8290.CD-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee J.G., Shin J.H., Shim H.S., Lee C.Y., Kim D.J., Kim Y.S., Chung K.Y. Autophagy contributes to the chemo-resistance of non-small cell lung cancer in hypoxic conditions. Respir. Res. 2015;16:138. doi: 10.1186/s12931-015-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen X., Wang P., Guo F., Wang X., Wang J., Xu J., Yuan D., Zhang J., Shao C. Autophagy enhanced the radioresistance of non-small cell lung cancer by regulating ROS level under hypoxia condition. Int. J. Radiat. Biol. 2017;93:764–770. doi: 10.1080/09553002.2017.1325025. [DOI] [PubMed] [Google Scholar]
- 178.Wang J., Liu Z., Hu T., Han L., Yu S., Yao Y., Ruan Z., Tian T., Huang T., Wang M., et al. Nrf2 promotes progression of non-small cell lung cancer through activating autophagy. Cell Cycle. 2017;16:1053–1062. doi: 10.1080/15384101.2017.1312224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Malhotra J., Jabbour S., Orlick M., Riedlinger G., Guo Y., White E., Aisner J. Phase Ib/II study of hydroxychloroquine in combination with chemotherapy in patients with metastatic non-small cell lung cancer (NSCLC) Cancer Treat. Res. Commun. 2019;21:100158. doi: 10.1016/j.ctarc.2019.100158. [DOI] [PubMed] [Google Scholar]
- 180.Liu Z., Zhang L., Liu Y., Zhang H., Chen J., Feng G., Yang P., Sha F., Cui L., Sun G. Identification of Compound CB-2 as a Novel Late-Stage Autophagy Inhibitor Exhibits Inhibitory Potency against A549 Cells. Life. 2021;11:865. doi: 10.3390/life11080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu G., Pei F., Yang F., Li L., Amin A.D., Liu S., Buchan J.R., Cho W.C. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2017;18:367. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou B., Huang J., Zuo Y., Li B., Guo Q., Cui B., Shao W., Du J., Bu X. 2a, a novel curcumin analog, sensitizes cisplatin-resistant A549 cells to cisplatin by inhibiting thioredoxin reductase concomitant oxidative stress damage. Eur. J. Pharmacol. 2013;707:130–139. doi: 10.1016/j.ejphar.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 183.Chupakhin E., Krasavin M. Thioredoxin reductase inhibitors: Updated patent review (2017-present) Expert Opin. Ther. Pat. 2021;31:745–758. doi: 10.1080/13543776.2021.1899160. [DOI] [PubMed] [Google Scholar]
- 184.Duan D., Feng X., Pan D., Wang L., Wang Y., Wang X. Oridonin induces oxidative stress-mediated cancer cells apoptosis via targeting thioredoxin reductase. Curr. Pharm. Biotechnol. 2021;23:1647–1657. doi: 10.2174/1389201023666211217151955. [DOI] [PubMed] [Google Scholar]
- 185.Patwardhan R.S., Sharma D., Sandur S.K. Thioredoxin reductase: An emerging pharmacologic target for radiosensitization of cancer. Transl. Oncol. 2022;17:101341. doi: 10.1016/j.tranon.2022.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Y., Sun S., Xu W., Yang R., Yang Y., Guo J., Ma K., Xu J. Thioredoxin reductase 1 inhibitor shikonin promotes cell necroptosis via SecTRAPs generation and oxygen-coupled redox cycling. Free. Radic. Biol. Med. 2022;180:52–62. doi: 10.1016/j.freeradbiomed.2021.12.314. [DOI] [PubMed] [Google Scholar]
- 187.Li X., Fan X.X., Jiang Z.B., Loo W.T., Yao X.J., Leung E.L., Chow L.W., Liu L. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol. Res. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 188.Wang L., Fu J.N., Wang J.Y., Jin C.J., Ren X.Y., Tan Q., Li J., Yin H.W., Xiong K., Wang T.Y., et al. Selenium-containing thioredoxin reductase inhibitor ethaselen sensitizes non-small cell lung cancer to radiotherapy. Anti-Cancer Drugs. 2011;22:732–740. doi: 10.1097/CAD.0b013e32834618bc. [DOI] [PubMed] [Google Scholar]
- 189.Zhou G.Z., Cao F.K., Chang J.M., Sun G.C., Chen X.B. Mechanism of curcumin analog MHMD-induced cell death in A549 lung cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2014;18:3134–3138. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.