Abstract
The ability of innate immune cells to differentially respond to various bacterial components provides a mechanism by which the acquired immune response may be tailored to specific pathogens. The response of innate immune cells to bacterial components provides regulatory signals to cognate immune cells. These signals include secreted cytokines and costimulatory molecules, and to a large extent they determine the quantitative and qualitative nature of the immune response. In order to determine if innate immune cells can differentially respond to bacterial components, we compared the responses of macrophages to two bacterially derived molecules, cholera toxin (CT) and lipopolysaccharide (LPS). We found that CT and LPS differentially regulated the expression of interleukin-12 (IL-12) and CD80-CD86 but not that of IL-1β. LPS and CT each induced IL-1β expression in macrophages, while only LPS induced IL-12 and only CT induced CD80-CD86. These differences were markedly potentiated in gamma interferon (IFN-γ)-treated macrophages, in which LPS potently induced IL-12 and CD80-CD86 expression. In contrast, IFN-γ treatment had no effect on the expression of IL-1β. These results define a molecular basis for the differential pathogenicities of bacterial toxins and are relevant to the design of vaccine adjuvants able to selectively induce desired types of immunity.
Bacteria and bacterial components have potent effects on the immune system (15). Bacterial endotoxins and exotoxins profoundly affect the immune response both to the bacteria and to coincidental antigens. Thus, coadministration of lipopolysaccharide (LPS) from a variety of gram-negative bacteria results in an immune response to proteins that are otherwise nonimmunogenic (23), and cholera toxin (CT) potently increases the immune response to orally administered antigens (28). While this “adjuvanticity” (9) of bacterial components has long been observed, more recent studies have sought to elucidate their specific cellular and molecular mechanisms, including effects on the expression of cytokines and costimulatory molecules by antigen-presenting cells (APC).
A number of mechanisms for the adjuvanticity of LPS, including effects on macrophages and lymphocytes, have been suggested (41). Recent evidence supports the importance of proinflammatory cytokines, including interleukin-1 (IL-1) and IL-12, as mediators of LPS adjuvanticity. Vella et al. (39) demonstrated the ability of LPS-induced IL-1 to “provide long term survival signals to activated T cells,” thereby preventing deletion of superantigen-stimulated T cells. In addition, IL-1 plays a key role in immunity via the generation of functional T helper cells. Pape et al. (33) demonstrated that the ability of LPS to “enhance the clonal expansion, follicular migration and T helper function of Ag-specific T cells” could be mimicked by IL-1 and IL-12. There is also evidence for IL-1 playing a specific role in Th2 cell proliferation. Huber et al. (22) demonstrated that IL-1β provides a costimulatory signal that, along with a T-cell receptor signal, induces IL-4-independent proliferation of Th2 cells mediated by cell-associated IL-1α.
Cholera toxin (CT), a multimeric protein produced by Vibrio cholerae, consists of a pentameric ring of B subunits associated with a single A subunit (CT-A). The B subunits (CT-B) bind to intestinal epithelial cells and mediate uptake of the toxic A subunit (37). The adjuvanticity of CT, especially when administered orally, is associated with its A subunit (35) but may vary with the species and route of administration. For example, CT-A activity is required for adjuvanticity of orally administered CT in swine (14) but not for intranasal administration in mice (6). The ability of CT to profoundly affect the physiology of many cell types suggests many possible mechanisms for its adjuvanticity (35), including induction of IL-1 production (25) and expression of the costimulatory molecules CD80 and CD86 (4).
While LPS and CT may have some common mechanisms of adjuvanticity (e.g., IL-1 induction), there is also evidence of divergent effects. The adjuvanticity of CT has generally been associated with a Th2-type cytokine response and immunoglobulin E (IgE) production (32) and may inhibit Th1 cytokine secretion (10). In contrast, LPS induces IL-12 (7), which promotes Th1-type responses, both directly (21) and indirectly via the induction of gamma interferon (IFN-γ) (34). In sum, the immunomodulatory activity of bacterial toxins may ultimately be determined by the cytokine and costimulatory environment they induce via their interactions with innate immune cells, including macrophages. Therefore, in order to better understand the ability of bacterial toxins to differentially regulate immunity, we have directly compared the abilities of CT and LPS to induce IL-1β, IL-12, major histocompatibility complex (MHC) II, and CD80-CD86 expression by macrophages.
MATERIALS AND METHODS
Materials.
Recombinant CT-B was obtained from a human cholera vaccine (SBL Vaccine, Stockholm, Sweden) which contains whole, heat-inactivated V. cholerae and recombinant CT-B. The recombinant CT-B was separated from the whole killed cells by centrifugation and filtration through a 0.2-μm-pore-size filter and was examined by polyacrylamide gel electrophoresis (14). The endotoxin contents of CT (Sigma Chemical Co., St. Louis, Mo.) and CT-B, as determined by a Limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, Md.), were 1.5 μg/mg of CT and 460 μg/mg of CT-B. Porcine IFN-γ (Endogen, Inc., Woburn, Mass.) and LPS (Sigma Chemical Co.) were obtained from commercial sources.
Antibodies and reagents for flow cytometry included soluble cytotoxic T lymphocyte-associated molecule 4 fused to an immunoglobulin Fc region (CTLA-4–Ig; Peter Linsley, Bristol-Myers Squibb, Seattle, Wash.), fluorescein isothiocyanate-conjugated anti-human IgG (γ-chain specific) (Sigma Chemical Co.), anti-porcine MHC II (MSA3; Joan Lunney, U.S. Department of Agriculture Agricultural Research Service, Beltsville, Md.), and R-phycoerythrin conjugated anti-mouse IgG (Sigma Chemical Co.).
Isolation of cells.
Alveolar macrophages, collected from euthanized (Beuthanasia-D Special; Schering-Plough Animal Health, Kenilworth, N.J.) 6-week-old pigs by lung lavage, were washed three times with phosphate-buffered saline (PBS) and plated in complete medium (RPMI medium 1640 with l-glutamine [Irvine Scientific, Santa Ana, Calif.] with 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% fetal bovine serum). Cell viability was confirmed by trypan blue exclusion. Reagents were prepared with endotoxin-free water in glassware heated at 200°C for 4 h.
RNA isolation and Northern hybridization.
Total RNA was isolated by a modification of previously described methods (3). Briefly, cells were lysed with 5 M guanidine thiocyanate and extracted at least twice with phenol and chloroform at pH 4. RNA was precipitated with isopropyl alcohol, washed with 75% ethanol, dried under a vacuum, and resuspended in RNase-free water. The total RNA concentration was calculated from the A260 values. In some experiments, total RNA was also isolated with TRIzol Reagent (Life Technologies, Gaithersburg, Md.) or with an RNeasy Mini Kit (Qiagen Inc., Santa Clara, Calif.). Total RNA was denatured with formaldehyde and separated by electrophoresis on a 0.8% agarose gel. Relative loading of lanes was monitored by including ethidium bromide in the loading buffer and visualizing RNA with UV light. RNA was transferred to a nylon membrane (Micron Separations Inc., Westborough, Mass.) by capillary transfer, fixed to the membrane with UV light, and hybridized to a random-primed, 32P-labeled cDNA probe (Prime-it II; Stratagene, La Jolla, Calif.). Membranes were quantitated by phosphorimaging (Molecular Dynamics, Sunnyvale, Calif.).
GM1-ELISA.
Ninety-six-well enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with 100 ng of monosialoganglioside (GM1) (Sigma Chemical Co.) per well in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3), blocked with 2% bovine serum albumin (BSA) or 5% nonfat dry milk, and washed with PBS with 0.05% Tween 20. Samples were diluted in PBS with 1% BSA, detected with serum from CT-immunized pigs (14) and a peroxidase-labeled goat anti-swine IgA (Bethyl Laboratories, Inc.), and visualized with 3,3′,5,5′-tetramethylbenzidine (TMB) microwell peroxidase substrate system (KPL Inc., Gaithersburg, Md.).
IL-12 bioassay.
Porcine IL-12 bioactivity was measured by modifications of a method (16) used to measure human IL-12, based on the ability of IL-12 to induce proliferation of phytohemagglutinin (PHA)-activated lymphoblasts. Peripheral blood mononuclear cells were isolated by using LSM lymphocyte separation medium (Organon Teknika-Cappel, Durham, N.C.), and their viability was confirmed by trypan blue exclusion. Cells were then resuspended in complete medium (50% RPMI 1640, 50% Dulbecco modified Eagle medium with 1% nonessential amino acids, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 10 mM HEPES, 0.006% [wt/vol] l-arginine, 0.1% [wt/vol] dextrose, and 5% human AB serum) containing 10 μg of PHA/ml for 5 days, with IL-2 (25 ng of recombinant human IL-2/ml) added on the last day. After a wash with complete medium to remove the PHA and IL-2, the lymphoblasts were plated at 20,000 cells per well in a 96-well tissue culture plate, dilutions of test samples or standard cytokine were added, and the mixture was incubated for 48 h. Proliferation was measured by [3H]thymidine (1.0 μCi/well) uptake for the final 16 h of incubation. Cells were harvested onto glass filters, and the amount of incorporated [3H]thymidine was determined (Matrix 9600 Direct Beta Counter; Packard, Downers Grove, Ill.). Porcine and human IL-12 are equally potent in this bioassay and are both neutralized by the anti-human IL-12 monoclonal antibody C8.6 (Pharmingen, San Diego, Calif.) (14a). Therefore, the specificity of the IL-12 activity in culture supernatants was confirmed by its inhibition with this antibody. Furthermore, there were no direct effects of the macrophage treatments on the bioassay (data not shown).
Competitive PCR.
Since IL-12 mRNA is not readily detected by Northern hybridization (13), it was determined by a competitive PCR assay. This method uses plasmid DNA competitors containing the respective genes with a portion of the sequence deleted (12, 31). Construction of IL-12 p40 and hypoxanthine phosphoribosyltransferase (HPRT) competitors has been described previously (31). A similar competitor for IL-12 p35 was prepared by digesting a p35-containing plasmid with the restriction enzymes EcoRV and StuI. These enzymes each cut a unique site in the p35 sequence, 33 nucleotides apart and between the primer sequences used for PCR. The resulting linearized plasmid was religated and used to transform competent Escherichia coli (DH5α), and bacterial colonies containing plasmids with the predicted deletion were identified by PCR. For some experiments, a competitor with an additional deletion was generated by digestion with HindIII and partial digestion with Exo III nuclease followed by religation, transformation, and screening as above. This resulted in a competitor with an additional 87-bp deletion.
Serial dilutions of each competitor were added to a series of PCRs containing an aliquot of cDNA. Each 10-μl PCR mixture contained cDNA synthesized from approximately 5 ng (HPRT) or 50 ng (IL-12, p35, or p40) of total RNA and 2- or 2.5-fold dilutions of competitor. Depending on the target and treatment condition, 101 to 105 copies of competitor were used per PCR. Amplification conditions were the same for all three genes (93°C for 5 min; cycles of 93°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by 7 min at 72°C). HPRT was amplified for 30 cycles, and p35 and p40 were amplified for 35 cycles. Primer sequences were as follows: for HPRT, TGATG AAAGA GATGG GAGGC (upstream) and ATAGC CAACA TTCGA GGGG (downstream); for IL-12 p35, CCTGC ACTTC AGAAG AGATC G (upstream) and CTCAC TGTTG AAATT CAGAG CC (downstream); for IL-12 p40, GATGC TGGCC AGTAC ACCTG TCG (upstream) and TCCCT GATGA AGAAG CTGCT GG (downstream). Following amplification, products were separated by electrophoresis on a 1.5% agarose gel and visualized with ethidium bromide and UV light. Images were recorded digitally (Eagle Eye; Stratagene) and computer analyzed (National Institutes of Health Image software). The amplified target/amplified competitor ratio and the known starting concentration of the competitor were used to calculate the starting concentration of the target in the PCR. The log10 of the ratio of the amplified target to the amplified competitor was plotted against the log10 of the number of competitor molecules present in each reaction. This resulted in a graph with a line whose slope was approximately equal to −1. At the point where the log10 of the amplified target/amplified competitor ratio equaled 0, the concentration of competitor was equal to the concentration of target. The starting concentration of target cDNA was calculated from the amount of competitor that resulted in equal amplification.
Flow cytometry.
Following culture with the indicated treatments, plates were placed on ice and adherent cells were removed by gentle scraping. Cells were washed and blocked in PBS with 1% BSA, followed by incubation of 106 cells with (or without, for controls) 100 μl of the primary antibody (a 1:10 dilution of MSA3-producing hybridoma supernatant) or 35 μg of CTLA-4–Ig (35 μg/ml), washing, and incubation with 100 μl of the secondary antibody. After a final wash, cells were analyzed by flow cytometry (FACScan; Becton Dickinson, San Jose, Calif.).
RESULTS
Induction of IL-1 by LPS.
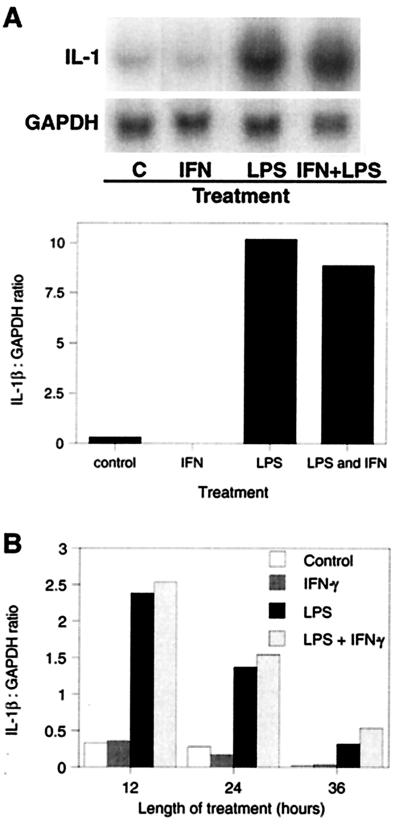
LPS markedly increased IL-1β mRNA levels in alveolar macrophages (Fig. 1A). This increase in mRNA levels was correlated with an increase in bioactive IL-1β levels (data not shown) (1). The levels of IL-1β mRNA declined to control levels by 36 h even with continued LPS stimulation (Fig. 1B). The induction of IL-1β by LPS was completely inhibited by preincubation with a 50-fold excess (wt/wt) of polymyxin B but was not diminished by boiling (data not shown). Since IFN-γ may be indirectly induced by LPS and since it is a potent activator of macrophages, we also determined its effect on macrophage IL-1β secretion. In contrast to treatment with LPS, treatment of alveolar macrophages with 5 ng of IFN-γ/ml had no effect on IL-1β mRNA levels, either alone or combined with LPS (Fig. 1). Durations of IFN-γ treatment up to 36 h failed to induce any IL-1β (Fig. 1B). This concentration of IFN-γ had a marked effect on the expression of other genes (e.g., IL-12; see below), indicating that it was biologically active.
FIG. 1.
(A) Induction of IL-1β by LPS. Alveolar macrophages, isolated by lung lavage, were cultured for 12 h in complete medium alone and then were incubated with 1 μg of LPS/ml and/or 5 ng of IFN-γ/ml for 12 h. Total RNA was isolated, and IL-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were determined by Northern hybridization and phosphorimaging (top panel). The graph shows IL-1β mRNA normalized to GAPDH and expressed as the ratio of arbitrary phosphorimaging units. Results are representative of four experiments with alveolar macrophages and three with peripheral blood monocytes. (B) Time course of IL-1β induction by LPS. Alveolar macrophages were isolated, cultured, and treated as described above, and IL-1β mRNA levels were determined at 12, 24, or 36 h.
Induction of IL-1β by CT.
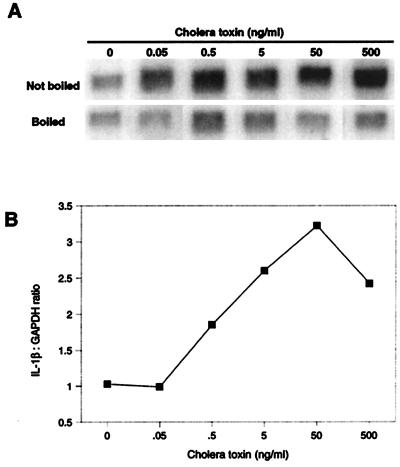
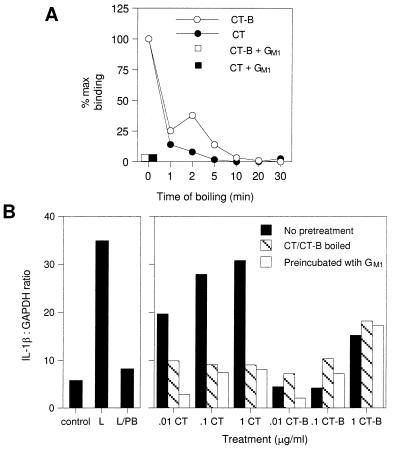
CT induced IL-1β in porcine macrophages in a dose-dependent manner at doses from 0.5 to 50 ng/ml (Fig. 2). CT was similar to LPS in its ability to induce IL-1β (Fig. 3 and Table 1). Several experiments were conducted to demonstrate that this effect was specifically due to the activity of CT-A and not to CT-B binding or endotoxin contamination. First, all cells were cultured in the presence of 10 μg of polymyxin B/ml. This level of polymyxin B completely blocked the activity of 0.2 μg of LPS/ml (data not shown), well above the highest concentration (1.5 ng/ml) of endotoxin present in any CT-treated cells. Second, the effect of CT was inhibited by preincubation with GM1. These treatments blocked the binding of CT and CT-B to GM1 as measured by an ELISA (Fig. 3A). Boiling or preincubation with GM1 blocked the IL-1-inducing activity of CT (Fig. 3B) but had no effect on the activity of endotoxin (data not shown).
FIG. 2.
Effect of dose of CT on the induction of IL-1β mRNA expression. Alveolar macrophages isolated by lung lavage were cultured for 12 h in complete medium alone and then with 10 μg of polymyxin B/ml and the indicated concentration of CT. Total RNA was isolated, and IL-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were determined by Northern hybridization and phosphorimaging (A). The graph (B) shows IL-1β mRNA expression as arbitrary units normalized to GAPDH and expressed as the ratio of the IL-1β mRNA induced by the nonboiled CT to that induced by the boiled CT.
FIG. 3.
(A) Inhibition of the GM1 binding or immunoreactivity of CT and CT-B by boiling or preincubation with GM1. CT or CT-B was boiled or preincubated with 10 μg of GM1/ml and then tested for GM1 binding and immunoreactivity by a GM1-ELISA as described in Materials and Methods. (B) Induction of IL-1β mRNA by whole CT but not CT-B. Alveolar macrophages isolated by lung lavage were cultured for 12 h in complete medium alone and then with 10 μg of polymyxin B/ml and the indicated concentration of CT or CT-B which had been boiled for 15 min or preincubated with 10 μg of GM1/ml. Additional cells were cultured with medium alone (control), with LPS (L), or with LPS and polymyxin B (10 μg/ml) (L/PB). Total RNA was isolated, and IL-1β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were determined by Northern hybridization and phosphorimaging. The graph shows IL-1β mRNA expression as arbitrary units normalized to GAPDH and is representative of two additional experiments.
TABLE 1.
Approximate relative effects of various treatments on cytokine or costimulatory molecule expression in porcine alveolar macrophages
| Treatment | Fold induction of:
|
|||
|---|---|---|---|---|
| IL-1βa | IL-12b | CD80-CD86c | MHC IId | |
| None (control) | 1 | 0e | 1 | 1 |
| CT (0.5 μg/ml) | 10 | 0e | 1.5 | 1 |
| 0.2f | ||||
| IFN-γ (5 ng/ml) | 1 | 0e | <1 | 1 |
| LPS (1 μg/ml) | 10 | 1 | 1 | 1 |
| LPS plus IFN-γ | 10 | 20 | 3.7 | 1 |
IL-1β mRNA levels were measured by Northern hybridization.
IL-12 activity was measured by a bioassay.
Cell surface CD80-CD86 levels were measured by CTLA-4 binding by flow cytometry.
Cell surface MHC II levels were measured by flow cytometry.
Below the sensitivity of the assay.
Effect of CT plus IFN-γ.
In contrast to CT, CT-B did not increase IL-1β expression. While cells treated with the highest doses of CT-B expressed more IL-1β than controls, the activity was not blocked by boiling or preincubation with GM1 (Fig. 3B). The failure of CT-B to induce IL-1β suggests that the cyclic AMP (cAMP)-elevating activity of CT-A is required. Consistent with this conclusion, the cAMP analog dibutyryl cAMP was a potent inducer of IL-1β in these cells (data not shown).
Induction of IL-12 by LPS.
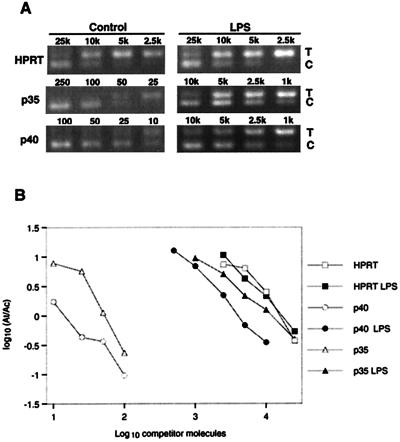
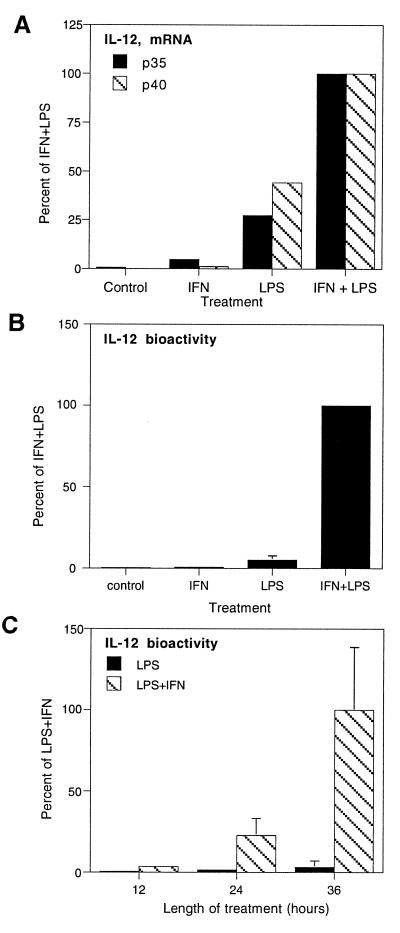
Studies of the regulation of IL-12 are complicated by its heterodimeric structure and the independent regulation of its two subunits (17). Expression of bioactive protein requires expression of both subunits, so we examined the regulation of mRNA for both subunits of IL-12 by competitive PCR (12). Figure 4A shows the ethidium bromide-stained agarose gel of a series of PCRs spiked with dilutions of competitor for each subunit of IL-12 and HPRT. The four dilutions closest to the point of equivalent amplification are shown. The amount of competitor that must be added to give an amplified product equal to the amplified target is relative to the amount of starting target in the sample. Therefore, the right shifts of the graphs for IL-12 p35 and p40 (Fig. 4B) indicate that LPS increased the levels of mRNA for both subunits of IL-12.
FIG. 4.
Quantitation of IL-12 p35 and p40 subunit mRNA by competitive PCR. Alveolar macrophages were cultured for 48 h in complete medium, with 1 μg of LPS/ml added to selected cultures for the final 36 h. Total RNA was isolated and converted to cDNA, which was added to eight replicate PCR mixtures containing 2- or 2.5-fold dilutions of a competitor construct. Following amplification, the PCR products were electrophoresed and visualized with ethidium bromide and UV transillumination. The digital image was used for quantitation. (A) The four dilutions closest to the point of equivalent amplification for each subunit of IL-12 and for HPRT are shown. (B) The resulting ratio of amplified target to amplified competitor was plotted versus the starting concentration of competitor in order to calculate the starting concentration of target as described in Materials and Methods.
The relevance of increased levels of IL-12 p35 and p40 mRNAs was demonstrated by analyzing the effects of LPS and IFN-γ on the secretion of bioactive IL-12 (Fig. 5). LPS (1 μg/ml for 12 h) increased mRNA levels of both subunits of IL-12, with no effect on HPRT (Fig. 4). The IL-12-inducing activity of LPS was completely blocked by preincubation with 10 μg of polymyxin B/ml (data not shown). Treatment of macrophages with IFN-γ (5 ng/ml for 12 h) had little or no effect on the mRNA of either subunit of IL-12 but markedly potentiated the ability of LPS to induce both p35 and p40 mRNAs (Fig. 5A).
FIG. 5.
Induction of IL-12 by LPS and IFN-γ. (A) IL-12 mRNA. Alveolar macrophages were cultured for 24 h with 1 μg of LPS/ml and/or 5 ng of IFN-γ/ml. IL-12 p35, IL-12 p40, and HPRT mRNAs in total RNA were measured as described in the legend to Fig. 4. The resulting values for the IL-12 subunits were normalized to HPRT and plotted as fractions of the LPS- and IFN-γ-induced expression of each subunit. Each data point represents the average of two separate experiments and ≥8 PCRs. The same results were obtained from cells cultured for 36 h. (B) IL-12 activity. IL-12 bioactivity was measured by a lymphoblast proliferation assay, and the results are expressed as the relative concentrations of IL-12 as determined by comparison of the proliferation results to a standard curve generated with porcine IL-12. Each bar represents the mean ± 1 standard deviation from four experiments. (C) Time course of IL-12 expression. Alveolar macrophages were cultured with 1 μg of LPS/ml and/or 5 ng of IFN-γ/ml for 36 h. Samples of media were assayed for IL-12 bioactivity by lymphoblast proliferation assay at the times indicated, and the results are expressed as the relative concentrations of IL-12. Each bar represents the mean ± 1 standard deviation of triplicate determinations.
The potentiating effect of IFN-γ on IL-12 secretion was most dramatic at the level of bioactive protein. Addition of IFN-γ to medium containing LPS increased the expression of active IL-12 20-fold compared to treatment with LPS alone (Fig. 5B). The effect required the presence of LPS, as IFN-γ alone was inactive. Moreover, IL-12 expression was induced for extended periods of culture, in contrast to IL-1β expression, which is transient (compare Fig. 5C and 1B).
Induction of IL-12 by CT.
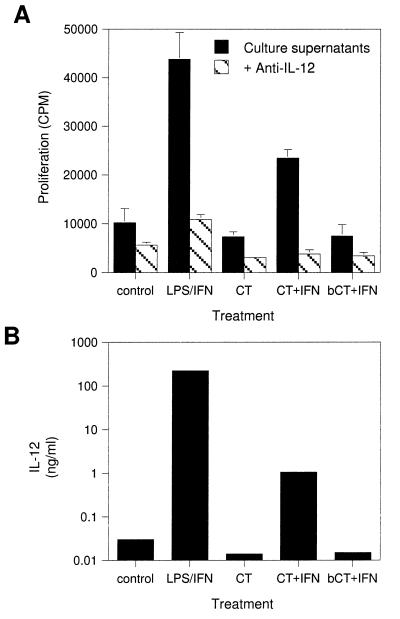
CT alone did not induce IL-12 secretion in macrophages, but the combination of CT and IFN-γ was effective (Fig. 6). This induction was not observed with CT-B or with boiled CT (Fig. 6 and data not shown). Furthermore, preincubation of CT with 10 μg of polymyxin B/ml, a dose capable of blocking the induction of IL-12 by 10 μg of LPS/ml, had no effect on IL-12 levels, suggesting that the activity was not due to endotoxin contamination. While both LPS and CT induced IL-12 secretion in combination with IFN-γ, the amount of IL-12 induced by CT plus IFN-γ was much lower (<1%) than that induced by LPS plus IFN-γ (Fig. 6B).
FIG. 6.
Induction of IL-12 by CT and IFN-γ. Alveolar macrophages were cultured for 12 h in complete medium alone and then were cultured with the indicated treatments for 36 h. Samples were assayed for IL-12 bioactivity by lymphoblast proliferation assay. bCT, boiled CT. (A) Proliferation of lymphoblasts treated with culture supernatants that were preincubated with (hatched bars) or without (solid bars) an IL-12-neutralizing antibody. (B) IL-12 bioactivity determined from a standard curve generated with porcine IL-12.
Induction of CD80-CD86 costimulatory molecule expression by LPS.
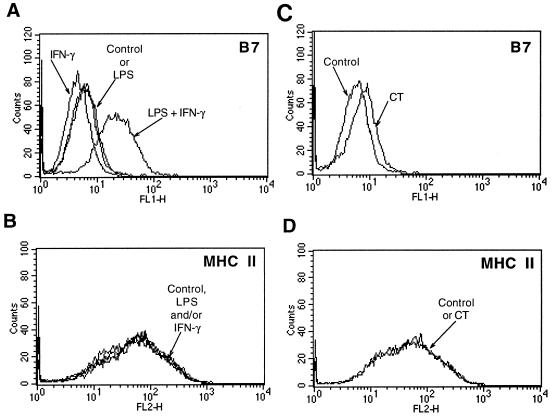
The key costimulatory pathway for T-cell activation is through CD28 by CD80-CD86. Signaling through CD28 by CD80-CD86 is essential both for the initiation of T-cell expansion and differentiation (26) and for the adjuvant activity of LPS (24). Therefore, we compared the ability of LPS alone to induce CD80-CD86 expression with that of LPS plus IFN-γ. We found that, while LPS had no effect and IFN-γ alone decreased the expression of CD80-CD86, the combination of IFN-γ and LPS increased the expression of macrophage CD80-CD86 about fourfold, as determined by relative fluorescence intensity (Fig. 7A).
FIG. 7.
Induction of CD80 and CD86 costimulatory molecules. Alveolar macrophages were cultured for 12 h in complete medium alone and then were incubated with LPS and/or IFN-γ (A and B) or CT (C and D) for 24 h. Cells were analyzed by flow cytometry for expression of CD80 and CD86 as CTLA-4–Ig ligands (A and C) and for expression of MHC II as described in the text. Results are representative of three (LPS plus IFN-γ) or four (CT) experiments.
Induction of CD80-CD86 costimulatory molecule expression by CT.
CT (0.1 to 0.5 μg/ml for 24 to 48 h) increased the expression of macrophage CD80-CD86, although somewhat less than the combination of LPS and IFN-γ (Fig. 7A and C). In four experiments, the mean fluorescent intensity increased an average of 1.5-fold over that of control cells or those treated with boiled CT (Fig. 7C and data not shown).
Expression of MHC II.
LPS and IFN-γ had no effect, either alone or together, on MHC II expression (Fig. 7B). Likewise, CT did not alter the levels of MHC II in these cells (Fig. 7D).
DISCUSSION
The immune-modulating activities of bacteria and bacterial components may be related to their abilities to induce cytokines, costimulatory molecules, and MHC in APC. In order to better understand the mechanisms of bacterial virulence factors such as LPS and CT, we compared their relative abilities to induce IL-1β, IL-12, CD80-CD86, and MHC II. An overview of their relative effects is summarized in Table 1.
CT and LPS induced IL-β similarly in porcine macrophages. This is consistent with the findings of previous studies in which CT and LPS induced similar levels of IL-1 in a murine macrophage cell line (27). The induction of IL-1β by CT was not due to endotoxin contamination, since the CT-mediated induction of IL-1β was specifically blocked by preincubation with GM1 and by boiling, treatments that do not block LPS induction of IL-1β. In addition, the effect of CT on IL-1β secretion was mimicked by dibutyryl cAMP but not by CT-B, suggesting that CT-A was necessary for CT activity. The ability of LPS to induce IL-1β was not affected by or dependent on the presence of IFN-γ. Since CT and LPS induce similar levels of IL-1β, any differential effects of CT and LPS on immunity are not mediated by IL-1β.
The combination of LPS and IFN-γ was a potent inducer of IL-12. Bioactive IL-12 p35 and p40 mRNAs were regulated in parallel. Previous reports showed that the p35 subunit of IL-12 is constitutively expressed, while the p40 subunit is induced by various stimuli (13, 20). In contrast, others indicated that regulation of IL-12 secretion is a function of p35 expression (36) and that p35 mRNA is also regulated by various factors (19). Our results demonstrate that the steady-state level of mRNA for both subunits is increased by the combination of LPS and IFN-γ and that this elevation results in the secretion of bioactive IL-12. These results also suggest that the IL-12 gene promoter elements of the pig are similar to those of the human and the mouse, which contain multiple sequences responsive to both LPS and IFN-γ (29, 42).
This synergistic regulation of IL-12 by LPS and IFN-γ is important during bacterial infections. During a bacterial infection, the release of LPS would initially induce some IL-12 secretion, which results in IFN-γ secretion from NK cells and T cells. IFN-γ and the continued presence of LPS potently stimulate the secretion of IL-12. Therefore, the presence of bacteria would initiate a positive-feedback loop of IL-12 and IFN-γ expression. After bacteria are cleared and LPS is eliminated, IFN-γ alone would no longer induce IL-12, thus preventing an excessive response. In addition, APC may later secrete IL-10, perhaps by an IL-1-dependent mechanism (11), which would suppress both IL-12 (5) and CD80-CD86 (40), further dampening the response.
CT and LPS–IFN-γ increased macrophage CD80-CD86 1.5- and 3.7-fold, respectively. The detection of CD80-CD86 expression by CTLA-4 binding does not distinguish between CD80 (B7-1) and CD86 (B7-2) (18), so it is not directly evident from these results if the increase represents CD80, CD86, or both. Since the functions of these two ligands may be different (38), additional knowledge of their regulation may be significant for predicting the immunological consequences of the increase in expression. While human CTLA-4 has been shown to bind to porcine CD86 (30), there is no reason to expect that it would not also bind to porcine CD80. CT selectively induces CD86 (B7-2) in macrophages activated by granulocyte-macrophage colony-stimulating factor (4), suggesting that the increase in CTLA-4 ligands measured in our experiment was an increase in CD86. The observation that IFN-γ decreased CD80-CD86 expression is consistent with the presence of either CD80 or CD86, both of which may be downregulated by IFN-γ (2, 18). In either event, CT increased the expression of “CTLA-4 ligands,” consistent with previous evidence that the upregulation of B7.2 plays a role in the adjuvanticity of CT (4).
Our observation that IFN-γ did not enhance LPS-induced IL-1 secretion is in contrast to previous results for other species. For example, the secretion of IL-1 by human monocytes treated with 1 to 10 ng of LPS/ml is enhanced by IFN-γ (8). This discrepancy may be due to differences in the activity of IFN-γ between species or cell types or in the doses of LPS used in each experiment.
These results suggest that IFN-γ may play a key role in differentiating the immune response to CT and LPS. CT, with or without endotoxin or IFN-γ, increases IL-1β and CD80-CD86 expression, but not IL-12 expression, in APC. Likewise, without IFN-γ, LPS would induce IL-1β but little IL-12. In contrast, in a high-IFN-γ environment, LPS would induce more CD80-CD86 and high levels of IL-12, while IL-1β levels would be similar regardless of IFN-γ levels. Therefore, the immune response to bacteria expressing both LPS and CT may be determined by the relative invasiveness of the organism and the resulting exposure of immune cells to each toxin. Noninvasive bacteria, such as V. cholerae, may mediate immunity via toxin effects, while invasive bacteria, such as Salmonella spp., may have more LPS-mediated effects.
In conclusion, CT and LPS differentially regulate IL-12 and CD80-CD86 expression, and the presence of IFN-γ markedly enhances the induction of IL-12 and CD80-CD86 by LPS. Therefore, the immune response to bacterial pathogens may be, in part, determined by specific immunoregulatory effects of various bacterial toxins and the context of the ongoing host immune response in which they are encountered.
ACKNOWLEDGMENTS
We thank Mark Moody (Endogen, Inc.) for providing porcine IL-12, Edward Janoff and David Brown (University of Minnesota) for many helpful discussions and technical advice, and Marc Jenkins (University of Minnesota) for reading the manuscript.
This work was supported by National Institutes of Health grant K08AI01396 (to D.L.F.).
REFERENCES
- 1.Baarsch M J, Scamurra R W, Burger K, Foss D L, Maheswaran S K, Murtaugh M P. Inflammatory cytokine expression in swine experimentally infected with Actinobacillus pleuropneumoniae. Infect Immun. 1995;63:3587–3594. doi: 10.1128/iai.63.9.3587-3594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chelen C J, Fang Y, Freeman G J, Secrist H, Marshall J D, Hwang P T, Frankel L R, Dekruyff R H, Umetsu D T. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J Clin Investig. 1995;95:1415–1421. doi: 10.1172/JCI117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Cong Y Z, Weaver C T, Elson C O. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;59:5301–5308. [PubMed] [Google Scholar]
- 5.D’Andrea A, Aste Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Haan L, Verweij W R, Feil I K, Lijnema T H, Hol W G J, Agsteribbe E, Wilschut J. Mutants of the Escherichia coli heat-labile enterotoxin with reduced ADP-ribosylation activity or no activity retain the immunogenic properties of the native holotoxin. Infect Immun. 1996;64:5413–5416. doi: 10.1128/iai.64.12.5413-5416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekruyff R H, Gieni R S, Umetsu D T. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;58:359–366. [PubMed] [Google Scholar]
- 8.Donnelly R P, Fenton M J, Finbloom D S, Gerrard T L. Differential regulation of IL-1 production in human monocytes by IFN-γ and IL-4. J Immunol. 1990;145:569–575. [PubMed] [Google Scholar]
- 9.Dresser D W. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- 10.Elson C O, Beagley K W. Cytokines and immune mediators. In: Johnson L R, editor. Physiology of the gastrointestinal tract. New York, N.Y: Raven Press; 1994. pp. 243–256. [Google Scholar]
- 11.Foey A D, Parry S L, Williams L M, Feldmann M, Foxwell B M J, Brennan F M. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha—role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- 12.Foss D L, Baarsch M J, Murtaugh M P. Regulation of hypoxanthine phosphoribosyltransferase, glyceraldehyde-3-phosphate dehydrogenase and β-actin mRNA expression in porcine immune cells and tissues. Anim Biotechnol. 1998;9:67–78. doi: 10.1080/10495399809525893. [DOI] [PubMed] [Google Scholar]
- 13.Foss D L, Murtaugh M P. Molecular cloning and mRNA expression of porcine interleukin-12. Vet Immunol Immunopathol. 1997;57:121–134. doi: 10.1016/s0165-2427(96)05773-x. [DOI] [PubMed] [Google Scholar]
- 14.Foss D L, Murtaugh M P. Mucosal immunogenicity and adjuvanticity of cholera toxin in swine. Vaccine. 1999;17:788–801. doi: 10.1016/s0264-410x(98)00263-1. [DOI] [PubMed] [Google Scholar]
- 14a.Foss, D. L., et al. Unpublished data.
- 15.Freund J, Casals J, Hosmer E P. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc Soc Exp Biol Med. 1937;37:509–513. [Google Scholar]
- 16.Gately M K, Chizzonite R. Measurement of human and mouse interleukin 12. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1992. pp. 6.16.1–6.16.8. [DOI] [PubMed] [Google Scholar]
- 17.Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. The interleukin-12/interleukin-12-receptor system—role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 18.Hathcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes M P, Wang J H, Norcross M A. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 20.Heinzel F P, Rerko R M, Ling P, Hakimi J, Schoenhaut D S. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 22.Huber M, Beuscher H U, Rohwer P, Kurrle P, Rollinghoff M, Lohoff M. Costimulation via TCR and IL-1 receptor reveals a novel IL-1-alpha-mediated autocrine pathway of Th2 cell proliferation. J Immunol. 1998;60:4242–4247. [PubMed] [Google Scholar]
- 23.Johnson A G, Gaines S, Landy M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956;103:225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoruts A, Mondino A, Pape K A, Reiner S L, Jenkins M K. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krakauer T. Evidence for protein kinase C pathway in the response of human peripheral blood mononuclear cells to cholera toxin. Cell Immunol. 1996;172:224–228. doi: 10.1006/cimm.1996.0236. [DOI] [PubMed] [Google Scholar]
- 26.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 27.Lycke N, Bromander A, Ekman L, Karlsson U, Holmgren J. Cellular basis of immunomodulation by cholera toxin in vitro with possible association to the adjuvant function in vivo. J Immunol. 1989;142:20–27. [PubMed] [Google Scholar]
- 28.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 29.Ma X J, Chow J M, Gri G, Carra G, Gerosa F, Wolf S E, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher S E, Karmann K, Min W, Hughes C C W, Pober J S, Bothwell A L M. Porcine endothelial CD86 is a major costimulator of xenogeneic human T cells—cloning, sequencing, and functional expression in human endothelial cells. J Immunol. 1996;157:3838–3844. [PubMed] [Google Scholar]
- 31.Mansfield L S, Urban J F, Holley-Shanks R R, Murtaugh M P, Zarlenga D S, Foss D L, Canals A, Gause W, Lunney J K. Construction of internal cDNA competitors for measuring IL-10 and IL-12 cytokine gene expression in swine. Vet Immunol Immunopathol. 1998;65:63–74. doi: 10.1016/s0165-2427(98)00106-8. [DOI] [PubMed] [Google Scholar]
- 32.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 33.Pape K A, Khoruts A, Mondino A, Jenkins M K. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;59:591–598. [PubMed] [Google Scholar]
- 34.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol. 1994;24:793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 35.Snider D P. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15:317–348. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 36.Snijders A, Hilkens C M U, van der Pouw Kraan T C T M, Engel M, Aarden L A, Kapsenberg M L. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–1212. [PubMed] [Google Scholar]
- 37.Spangler B D. Structure and function of cholera toxin and the Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson C B. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 39.Vella A T, Mitchell T, Groth B, Linsley P S, Green J M, Thompson C B, Kappler J W, Marrack P. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J Immunol. 1997;158:4714–4720. [PubMed] [Google Scholar]
- 40.Villanueva P O F, Reiser H, Stadecker M J. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10—effect on expression of B7 and B7-2 costimulatory molecules by macrophages. J Immunol. 1994;153:5190–5199. [PubMed] [Google Scholar]
- 41.Warren H S, Vogel F R, Chedid L A. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–388. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimoto T, Kojima K, Funakoshi T, Endo Y, Fujita T, Nariuchi H. Molecular cloning and characterization of murine IL-12 genes. J Immunol. 1996;156:1082–1088. [PubMed] [Google Scholar]