Abstract
Background:
The value of contrast-enhanced harmonic EUS (CH-EUS)for diagnosis of portal vein invasion in patients with pancreatic cancer was evaluated.
Patients and Methods:
This single-center, retrospective study included consecutive patients with pancreatic cancer who underwent both surgical resection after preoperative EUS, CH-EUS, and contrast-enhanced computed tomography (CE-CT) examinations between April 2015 and August 2017. CH-EUS evaluation was performed during the late phase. Portal vein invasion on EUS and CH-EUS was defined as no continuity in the line of the vessel wall. Definition of portal vein invasion on CE-CT was based on the Loyer's criteria. The accuracy of three modalities for diagnosis of invasion into the portal vein was compared using the McNemar's test.
Results:
Eighty-eight patients (mean age: 71.0 years, ratio of male to female: 48:40) were eligible. Postoperative pathological results were as follows: seven cases of portal vein invasion; 81 cases without. Diagnostic accuracy of EUS, CH-EUS, and CE-CT for diagnosing invasion into the portal vein was 72.7%, 93.2%, and 81.8%, respectively. The differences between CH-EUS and CE-CT (P = 0.0094) and CH-EUS and EUS (P = 0.0022) were significant. EUS and CE-CT were comparable.
Conclusion:
CH-EUS is useful for diagnosis of portal vein invasion by pancreatic cancer.
Keywords: contrast-enhanced harmonic EUS, portal vein invasion, pancreatic cancer, contrast-enhanced computed tomography, Loyer's Criteria
INTRODUCTION
In clinical practice, clinical symptoms, blood tests, and transabdominal ultrasound are the first steps in the diagnosis of pancreatic cancer. Contrast-enhanced computed tomography CT (CE-CT), the second step, is widely used for diagnosis of pancreatic cancer and for the assessment of resectability after a definitive diagnosis.[1,2,3,4,5] Resectability depends on the presence or absence of vascular and lymph node invasion and on the presence of distant metastasis. Although EUS is unsuitable for assessing lymph nodes or metastases distant from the gastrointestinal tract, it is useful for assessing vascular invasion.[4,6,7] In particular, the portal vein is visualized by EUS at a position close to the ultrasonic probe, which leads to easier evaluation. To support this, one comparable study shows that the sensitivity and the accuracy of EUS for diagnosis of portal vein invasion by pancreatobiliary cancer are 95% and 93%, respectively, whereas those of CE-CT are 65% and 74%, respectively.[7] Few studies have examined contrast-enhanced harmonic EUS (CH-EUS) for pancreatic cancer T-staging.[8,9] One study reported that the portal vein was visualized clearly by the contrast method and that CH-EUS was superior to EUS alone with respect to accuracy for T-staging of pancreatobiliary cancer (92.4% vs. 69.2%, respectively; P < 0.05).[8] By contrast, another study did not show superiority of CH-EUS over EUS alone for T-staging of pancreatic cancer.[9] Thus, little is known about the utility of CH-EUS for this application. Currently, the presence or absence of portal vein invasion has been important for resectability classification. Here, we report a retrospective study of the value of CH-EUS for diagnosis of portal vein invasion by pancreatic cancer and compared the results with those of EUS and CE-CT.
PATIENTS AND METHODS
Study design
This was a retrospective cohort study. The study protocol was approved by the Ethics Committee of Kindai University Faculty of Medicine (approval number: R02-321). The primary endpoint was the accuracy of EUS, CH-EUS, and CE-CT for diagnosing portal vein invasion by pancreatic cancer. The gold standard was obtained from intraoperative findings or postoperative pathological diagnoses. The surgical indication was determined by comprehensively evaluating the results of CE-CT, EUS, and CH-EUS. Pancreatic cancer in contact with portal and arterial systems of ≥180° suspected by any of three modalities was considered inoperable.
Patients
Consecutive patients with pathologically proven pancreatic cancer who underwent surgical resection at Kindai University Hospital between April 2015 and August 2017 were enrolled. All patients underwent EUS, CH-EUS, and CE-CT before surgery. Patients who received neoadjuvant chemotherapy or patients for whom there was more than 2 months between diagnostic imaging and surgery were excluded.
EUS and contrast-enhanced harmonic EUS
EUS including CH-EUS was performed by endosonographers, each with the experience of more than 1000 EUS procedures. The procedure was conducted using a linear-type echoendoscope (GF-UCT260; Olympus Medical Systems Co. Ltd., Tokyo, Japan) and imaging equipment (ALOKA Prosound SSD α-10 or F75 System; ALOKA Co. Ltd., Tokyo, Japan). Extended pure harmonic detection-CHE and/or -THE mode was used to perform CH-EUS. Sonazoid (0.015 mL/kg body weight; Daiichi-Sankyo, Tokyo, Japan) was used as the ultrasound contrast agent. EUS and CH-EUS videos were stored in a recording system. Invasion of the portal vein was evaluated during the late phase after 50–60 s after injection of Sonazoid. In accordance with a previous study, portal vein invasion was defined as disruption of the continuity of the vascular wall [Figure 1].[8] Absence of portal invasion was defined as noninterruption of the line of the portal vein wall [Figure 2]. Sweeping scanned EUS and CH-EUS videos were evaluated separately. Two readers (S. Omoto and T. Yamazaki for EUS, A. Nakai and K. Kamata for CH-EUS), both of whom were blinded to the clinical findings, reviewed the stored data. When the independent conclusions of the two reviewers differed, the reviewers re-evaluated the stored videos until agreement was reached.
Figure 1.
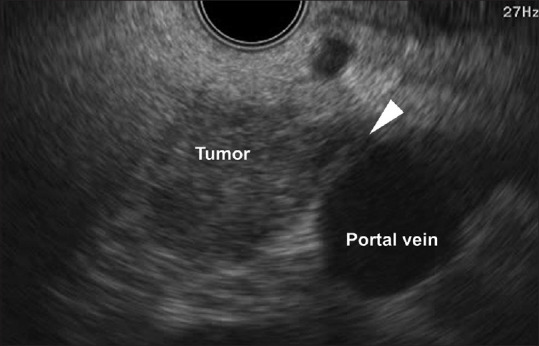
EUS image showing the contact between the tumor and the portal vein and that portal vein wall is unclear (arrowhead)
Figure 2.
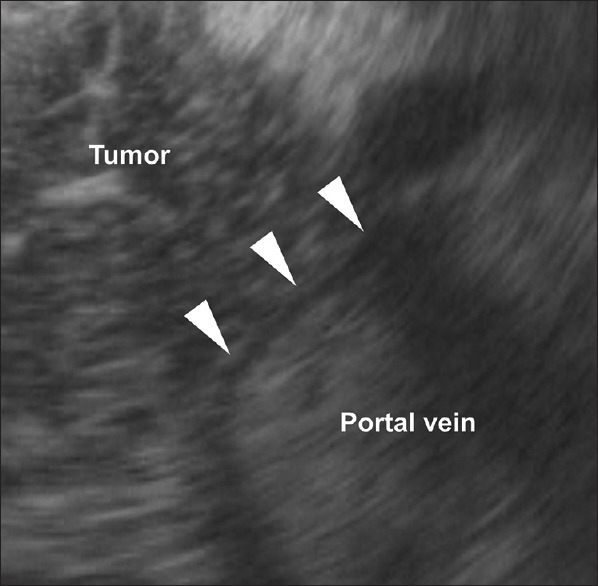
Contrast-enhanced harmonic EUS image using THE mode showing no portal vein invasion. The portal vein wall is depicted as a single-layer avascular line, with no interruption (arrowheads)
Contrast-enhanced computed tomography
Two 64-channel multidetector CT scanners (Discovery CT750 HD and LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA) were used. After the noncontrast-enhanced scan, patients were given nonionic contrast material containing 300–370 mg/mL iodine over a fixed duration of 30 s (equating to 510 mg iodine per kilogram body weight).[10] Manual early arterial phase scanning began 10 s after the attenuation value in the region of interest of the abdominal aorta reached >200 HU (approximately 30 s after injection). The portal venous phases were scanned at 55 s. CT images were reviewed in the axial and coronal planes (5 and 3 mm thick, respectively). Based on the Loyer et al.'s criteria, portal vein invasion by pancreatic cancer was defined as contact between the tumor and the portal vein or invasion of the tumor into the portal vein [Figure 3].[11] The absence of portal invasion was defined as the presence of intervening fatty tissue or normal pancreatic parenchyma between the tumor and the portal vein wall. Two readers (K. Minaga and T. Hyodo), both of whom were blinded to the results of EUS, CH-EUS, and pathological findings, reviewed the CE-CT images. When the independent conclusions of the two reviewers differed, the images were reviewed together and re-evaluated until agreement was reached.
Figure 3.
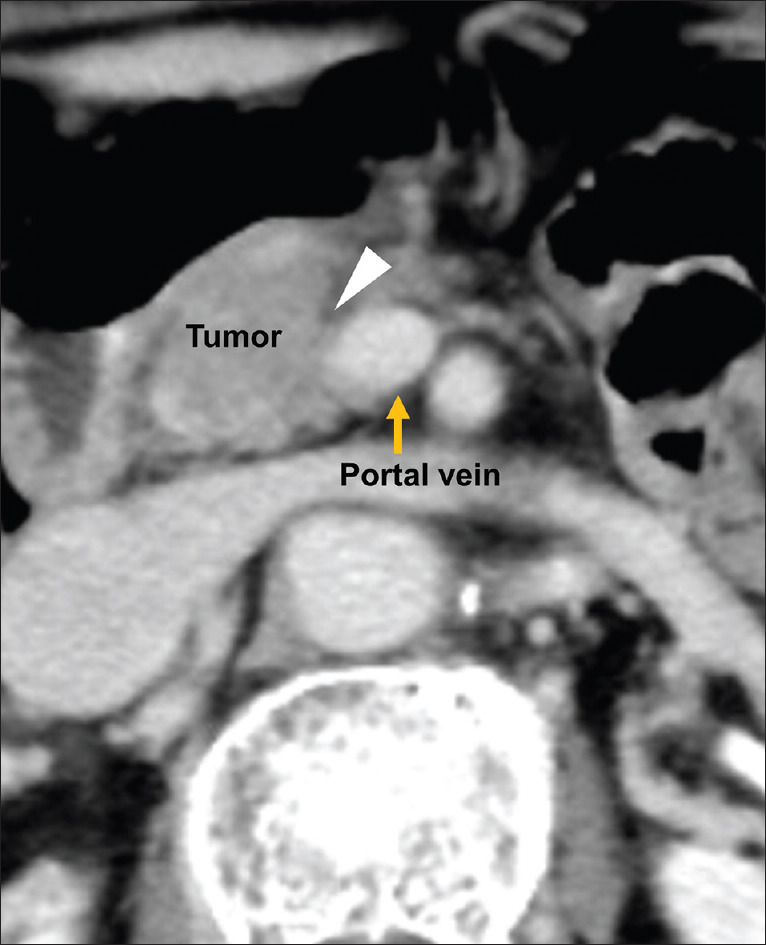
Contrast-enhanced computed tomography image showing portal vein invasion. The tumor is in contact with the portal vein (arrowhead)
Statistical analysis
All analyses were performed using Bell Curve for Excel (Social Survey Research Information Co., Ltd.). The accuracy of EUS, CH-EUS, and CE-CT for diagnosis of portal vein invasion was compared using the McNemar's test. Interobserver variations in EUS, CH-EUS, and CE-CT results were assessed by calculating the κ-coefficient. Values &0.8, &0.6, and &0.4 were considered to indicate excellent, good, and moderate agreement, respectively. P < 0.05 was considered statistically significant.
RESULTS
Eighty-eight patients with pancreatic cancer were diagnosed by surgical resection during the study period and were eligible for analysis. The interval between EUS, CH-EUS, and CE-CT was less than 2 weeks in all patients. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics
| Total (n=88) | |
|---|---|
| Age (years), mean (range) | 71.0 (46-84) |
| Sex, n (%) | |
| Male | 48 (54.5) |
| Female | 40 (45.5) |
| Tumor size (mm), mean (range) | 28 (15–42) |
| Location of tumor, n (%) | |
| Pancreatic head | 55 (62.5) |
| Pancreatic body/tail | 33 (37.5) |
| Portal vein invasion, n (%) | |
| Presence | 7 (8.0) |
| Absence | 81 (92.0) |
Pathological diagnoses showed that portal vein invasion was observed in five cases of pancreatic head cancer and two cases of pancreatic body and tail cancer. Concomitant portal vein resection was performed in four cases. The concordance rates (percentages of agreement between the two modalities on the presence or absence of portal vein invasion) were 81.8% (72/88) for CH-EUS and CE-CT, 80.7% (71/88) for CH-EUS and EUS, and 76.1% (67/88) for EUS and CE-CT, respectively. The sensitivities, specificities, positive predictive values, negative predictive values, and accuracies with 95% confidence intervals of three modalities are presented in Table 2. All these parameters of CH-EUS were higher than those of EUS and CE-CT. There was a significant difference between the diagnostic accuracy of CH-EUS and that of CE-CT (P = 0.0094). The difference between CH-EUS and EUS was also significant (P = 0.0022). By contrast, EUS and CE-CT were comparable (P = 0.2864). Regarding interobserver variation, the κ-values for EUS, CH-EUS, and CE-CT regarding diagnosis of portal vein invasion were 0.767, 0.821, and 0.590, respectively.
Table 2.
Utility of EUS, CH-EUS and CH-CT for diagnosis of portal vein invasion by pancreatic cancer
| 95% CI | |||||
|---|---|---|---|---|---|
|
| |||||
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| EUS | 71.4 (37.2-91.6) | 72.8 (69.9-74.6) | 18.5 (9.7-23.8) | 96.7 (92.8-99.0) | 72.7 (67.3-75.9) |
| CH-EUS | 85.7 (52.8-97.4) | 93.8 (91.0-94.8) | 54.5 (33.6-62.0) | 98.7 (95.7-99.8) | 93.2 (87.9-95.0) |
| CE-CT | 57.1 (26.2-83.4) | 84.0 (81.3-86.2) | 23.5 (10.8-34.3) | 95.8 (92.7-98.4) | 81.8 (76.9-86.0) |
CE-CT: Contrast-enhanced computed tomography; CH-EUS: Contrast-enhanced harmonic EUS; CI: Confidence interval; NPV: Negative predictive value; PPV: Positive predictive value
The number of overdiagnosed cases (i.e., misdiagnosis of a negative case as positive) of EUS, CH-EUS, and CE-CT was 22, 5, and 13, respectively. By contrast, the number of underdiagnosed cases (i.e., misdiagnosis of a positive case as negative) of EUS, CH-EUS, and CE-CT was 2, 1, and 3, respectively. Figures 1-3 are the images of the same patient. Portal vein invasion was suspected by EUS and CE-CT [Figures 1 and 3]. In this case, CH-EUS showed no portal vein invasion, which is consistent with the results of pathological diagnosis after surgical resection [Figure 2]. In one underdiagnosed case of CH-EUS, the tumor invaded the tunica adventitia of the portal vein, but not the tunica intima [Figure 4]. In this case, CE-CT detected portal vein invasion although EUS did not.
Figure 4.
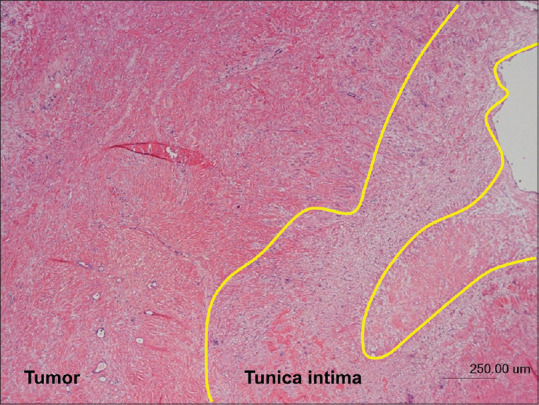
Histopathological image of the tumor (hematoxylin and eosin stain) in a case with underdiagnosis of contrast-enhanced harmonic EUS (i.e., misdiagnosis of a positive case as negative). The tumor has invaded the tunica adventitia of the portal vein, but not the tunica intima (area surrounded by yellow line)
DISCUSSION
CH-EUS is used for the evaluation of patients with pancreatobiliary diseases.[12,13,14,15,16] Regarding use of CH-EUS for pancreatic cancer staging, only two prospective studies have examined T-staging and only one retrospective study has examined N-staging.[8,9,17] Both of these studies were limited with respect to small sample size and lack of normal controls. Two of the three studies included both pancreatic and biliary cancers. The present study included only patients with pancreatic cancer and used both EUS and CE-CT as a control. Moreover, only resected cases were included so as to obtain a pathological diagnosis after surgery (the gold standard). Thus, the present study is the first report to compare the utility of EUS, CH-EUS, and CE-CT with respect to assessing portal vein invasion by pancreatic cancer. Based on the results of a previous study, we hypothesized that CH-EUS is superior to EUS and CE-CT for diagnosis of portal vein invasion.[7,8] The results showed that CH-EUS was significantly better than CE-CT for diagnosis of portal vein invasion (accuracy: 93.2% vs. 81.8%, respectively; P = 0.020). During CH-EUS, continuous observation is possible after administration of the contrast agent, and the method provides more temporal information than CE-CT. Generally, CT is performed using 2–4 time-discontinuous scans, which might not capture the optimal contrast that distinguishes the portal vein from cancer tissue, showing increased enhancement. In addition, the axial and coronal CT images do not always match the short-axis image of the portal vein, leading to under- or over-diagnosis due to possible partial volume effects. Increased fibrous growth in tumors or inflammatory changes caused by concomitant pancreatitis might also lead to overdiagnosis by both modalities. By contrast, tumors that invade only the tunica adventitia of the portal vein might be associated with underdiagnosis [Supplementary Figures 1 (254.5KB, tif) and 2 (233.5KB, tif) ].
The sensitivity and specificity of CE-CT for diagnosis of portal vein invasion by pancreatic cancer are 52%−80% and 76%−96%, respectively.[18,19,20] The sensitivity and specificity of CE-CT in the present study were comparable (57.1% and 84.0%, respectively), although the sensitivity was low. One reason for the low sensitivity is that we examined only seven cases with portal vein invasion, which led to wide variations in sensitivity. As described previously, Sugiyama et al. found that the diagnostic ability of conventional EUS was better than that of CE-CT for portal vein invasion in patients with pancreatobiliary carcinoma.[7] In the present study including only patients with pancreatic cancer, EUS and CE-CT were comparable. One factor is the recent improvement in CE-CT accuracy. Imazu et al. compared EUS with CH-EUS in 11 patients with pancreatic cancer and found that CH-EUS provided accurate diagnosis of portal vein invasion in all patients, including two who were not correctly diagnosed by conventional EUS.[8] Similarly, CH-EUS had significant better accuracy than EUS in the present study. CH-EUS depicts the portal vein wall clearly as a single-layer avascular line [Figure 2]. This makes it easier to determine whether pancreatic cancer has invaded the portal vein. In fact, interobserver agreement for CH-EUS was excellent.
We believe that CH-EUS has the potential to exceed both EUS and CE-CT for evaluating portal vein invasion by pancreatobiliary cancer. However, the use of CE-CT for diagnosis of pancreatic cancer is widespread and enables both definitive diagnosis and TNM staging. CE-CT has some drawbacks; it is difficult to perform in patients with asthma or iodine allergy and in those at risk of allergic reactions and contrast-induced nephropathy. Further, the radiation dose associated with multiphase scanning must be considered. By contrast, multicenter clinical trials report no serious adverse events after the use of Sonazoid, which was used as a contrast agent during CH-EUS in the present study.[21] A drawback of CH-EUS is that it can be performed only in a limited number of facilities. Moreover, the diagnostic ability of EUS is different according to the target vessels on T-staging of pancreatic cancer. The sensitivity and specificity of EUS for diagnosis of invasion of the superior mesenteric artery were 17% and 67%, respectively, which are lower than those for CE-CT (50% and 100%, respectively).[22] Collectively, we propose additional performance of CH-EUS, especially for diagnosis of portal vein invasion when pancreatic cancer staging cannot be confirmed by EUS or CE-CT.
The present study has several limitations. First, it was a single-center retrospective design. Only resected cases were enrolled and some cases with portal vein invasion on imaging were excluded as inoperable case, which might lead to selection bias. Moreover, only seven cases with portal vein invasion were included. Vascular invasion other than portal vein invasion was not evaluated because there were no cases with invasion of the superior mesenteric or common hepatic arteries: this was due to the limited patients with surgical resection. Convex-type EUS might not be suitable difficult for cross-section evaluation, and it might be difficult to accurately evaluate the positional relationship between pancreatic cancer and blood vessels. Therefore, further studies assessing the role of CH-EUS for diagnosis of vascular invasion by pancreatic cancer are required.
CONCLUSION
CH-EUS achieves a clear distinction between the portal vein wall and its surroundings, making it more useful than EUS and CE-CT for diagnosis of portal vein invasion by pancreatic cancer.
Supplementary materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Financial support and sponsorship
This work was supported by Grants-in-Aid from Japan Research Foundation for Clinical Pharmacology.
Conflicts of interest
There are no conflicts of interest.
Schematic showing a cross-section of the portal vein and tumor. Disruption of the continuity of the vascular wall is seen when the tumor invades the lumen of the portal vein
Schematic showing a cross-section of the portal vein and tumor. No disruption of the continuity of the vascular wall is seen when the tumor invades only the tunica adventitia of the portal vein
REFERENCES
- 1.Freeny PC, Traverso LW, Ryan JA. Diagnosis and staging of pancreatic adenocarcinoma with dynamic computed tomography. Am J Surg. 1993;165:600–6. doi: 10.1016/s0002-9610(05)80443-x. [DOI] [PubMed] [Google Scholar]
- 2.Laghi A, Iannaccone R, Catalano C, et al. Multislice spiral computed tomography in diagnosis and staging of pancreatic carcinoma: Preliminary experience. Dig Liver Dis. 2002;34:732–8. doi: 10.1016/s1590-8658(02)80025-1. [DOI] [PubMed] [Google Scholar]
- 3.Catalano C, Laghi A, Fraioli F, et al. Pancreatic carcinoma: The role of high-resolution multislice spiral CT in the diagnosis and assessment of resectability. Eur Radiol. 2003;13:149–56. doi: 10.1007/s00330-002-1473-4. [DOI] [PubMed] [Google Scholar]
- 4.Nawaz H, Fan CY, Kloke J, et al. Performance characteristics of endoscopic ultrasound in the staging of pancreatic cancer: A meta-analysis. JOP. 2013;14:484–97. doi: 10.6092/1590-8577/1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treadwell JR, Zafar HM, Mitchell MD, et al. Imaging tests for the diagnosis and staging of pancreatic adenocarcinoma: A meta-analysis. Pancreas. 2016;45:789–95. doi: 10.1097/MPA.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 6.Kulig J, Popiela T, Zajac A, et al. The value of imaging techniques in the staging of pancreatic cancer. Surg Endosc. 2005;19:361–5. doi: 10.1007/s00464-004-9056-x. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama M, Hagi H, Atomi Y, et al. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: Value of endoscopic ultrasonography. Abdom Imaging. 1997;22:434–8. doi: 10.1007/s002619900227. [DOI] [PubMed] [Google Scholar]
- 8.Imazu H, Uchiyama Y, Matsunaga K, et al. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010;45:732–8. doi: 10.3109/00365521003690269. [DOI] [PubMed] [Google Scholar]
- 9.Seicean A, Badea R, Stan-Iuga R, et al. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571–6. doi: 10.1055/s-0029-1245833. [DOI] [PubMed] [Google Scholar]
- 10.Awai K, Hiraishi K, Hori S. Effect of contrast material injection duration and rate on aortic peak time and peak enhancement at dynamic CT involving injection protocol with dose tailored to patient weight. Radiology. 2004;230:142–50. doi: 10.1148/radiol.2301021008. [DOI] [PubMed] [Google Scholar]
- 11.Loyer EM, David CL, Dubrow RA, et al. Vascular involvement in pancreatic adenocarcinoma: Reassessment by thin-section CT. Abdom Imaging. 1996;21:202–6. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 12.Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–10. doi: 10.1038/ajg.2011.354. [DOI] [PubMed] [Google Scholar]
- 13.Omoto S, Takenaka M, Kitano M, et al. Characterization of pancreatic tumors with quantitative perfusion analysis in contrast-enhanced harmonic endoscopic ultrasonography. Oncology. 2017;93(Suppl 1):55–60. doi: 10.1159/000481231. [DOI] [PubMed] [Google Scholar]
- 14.Kamata K, Takenaka M, Omoto S, et al. Impact of avascular areas, as measured by contrast-enhanced harmonic EUS, on the accuracy of FNA for pancreatic adenocarcinoma. Gastrointest Endosc. 2018;87:158–63. doi: 10.1016/j.gie.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Kamata K, Kitano M, Omoto S, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35–41. doi: 10.1055/s-0034-1393564. [DOI] [PubMed] [Google Scholar]
- 16.Kamata K, Takenaka M, Kitano M, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig Endosc. 2018;30:98–106. doi: 10.1111/den.12900. [DOI] [PubMed] [Google Scholar]
- 17.Miyata T, Kitano M, Omoto S, et al. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J Gastroenterol. 2016;22:3381–91. doi: 10.3748/wjg.v22.i12.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa H, Uesaka K, Boku N. Treatment decision making in pancreatic adenocarcinoma: Multidisciplinary team discussion with multidetector-row computed tomography. Arch Surg. 2008;143:275–80. doi: 10.1001/archsurg.2007.78. [DOI] [PubMed] [Google Scholar]
- 19.Teramura K, Noji T, Nakamura T, et al. Preoperative diagnosis of portal vein invasion in pancreatic head cancer: Appropriate indications for concomitant portal vein resection. J Hepatobiliary Pancreat Sci. 2016;23:643–9. doi: 10.1002/jhbp.387. [DOI] [PubMed] [Google Scholar]
- 20.Marinelli T, Filippone A, Tavano F, et al. A tumour score with multidetector spiral CT for venous infiltration in pancreatic cancer: Influence on borderline resectable. Radiol Med. 2014;119:334–42. doi: 10.1007/s11547-013-0349-9. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto Y, Ito T, Takada E, et al. Efficacy of sonazoid (perflubutane) for contrast-enhanced ultrasound in the differentiation of focal breast lesions: Phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2014;202:W400–7. doi: 10.2214/AJR.12.10518. [DOI] [PubMed] [Google Scholar]
- 22.Midwinter MJ, Beveridge CJ, Wilsdon JB, et al. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg. 1999;86:189–93. doi: 10.1046/j.1365-2168.1999.01042.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic showing a cross-section of the portal vein and tumor. Disruption of the continuity of the vascular wall is seen when the tumor invades the lumen of the portal vein
Schematic showing a cross-section of the portal vein and tumor. No disruption of the continuity of the vascular wall is seen when the tumor invades only the tunica adventitia of the portal vein


