Abstract
Simple Summary
Near-infrared photoimmunotherapy (NIR-PIT) represents a potential novel treatment modality for a range of cancer types, including head and neck cancers. NIR-PIT is based on the conjugation of photoactivating chemicals to cancer cell-specific antibodies. Antibody-photoabsorber-conjugate causes killing of cancer cells when activated by near-infrared light. NIR-PIT is considered to have particularly promising applications in head and neck cancers and these tumors are typically more easily accessed for illumination. Two patients with oropharyngeal lesions treated with NIR-PIT at our institution had good response with no serious adverse events and no functional disorders.
Abstract
Human papillomavirus (HPV)-associated oropharyngeal cancer has a better prognosis than other head and neck cancers. However, rates of recurrence and metastasis are similar and the prognosis of recurrent or metastatic HPV-associated oropharyngeal cancer is poor. Near-infrared photoimmunotherapy (NIR-PIT) is a treatment involving administration of a photosensitizer (IRDye®700DX) conjugated to a monoclonal antibody followed by activation with near-infrared light illumination. It is a highly tumor-specific therapy with minimal toxicity in normal tissues. Moreover, NIR-PIT is expected to have not only direct effects on a treated lesion but also immune responses on untreated distant lesions. NIR-PIT with cetuximab-IR700 (AlluminoxTM) has been in routine clinical use since January 2021 for unresectable locally advanced or locally recurrent head and neck cancer in patients that have previously undergone radiotherapy in Japan. NIR-PIT for head and neck cancer (HN-PIT) is expected to provide a curative treatment option for the locoregional recurrent or metastatic disease after radiotherapy and surgery. This article reviews the mechanism underlying the effect of NIR-PIT and recent clinical trials of NIR-PIT for head and neck cancers, treatment-specific adverse events, combination treatment with immune checkpoint inhibitors, illumination approach and posttreatment quality of life, and provides a case of series of two patients who receive NIR-PIT for oropharyngeal cancer at our institution.
Keywords: oropharyngeal cancer, head and neck cancer, photoimmunotherapy, EGFR, recurrence and metastasis
1. Introduction
Head and neck cancers are the seventh most common type of cancer worldwide [1,2]. Among head and neck cancers, cancers of the oropharynx are the most common followed by cancers of the oral cavity, hypopharynx, and larynx. While most head and neck cancers are associated with smoking and alcohol, the incidence of HPV-associated oropharyngeal cancer is increasing [3,4]. HPV-associated oropharyngeal cancer differs biochemically and histopathologically from non-HPV-associated oropharyngeal cancer and is reportedly more responsive to chemotherapy and radiation therapy [5].
Although HPV-associated oropharyngeal cancer has a better prognosis than other head and neck cancers, rates of recurrence and metastasis are similar and metastatic recurrence of HPV-associated oropharyngeal cancer has a poor prognosis [6].
Surgery for recurrent or metastatic head and neck cancer in patients that have previously undergone radiation therapy or surgery has a high risk of serious complications including dehiscence, delayed wound healing, and carotid bleeding [7,8,9,10]. Accordingly, chemotherapy and treatment with molecularly targeted drugs or immune checkpoint inhibitors (ICI) are the current mainstays of treatment for recurrent head and neck cancer [11,12,13]. However, these therapies currently provide a very low chance of cure [14,15], even in case of locoregional disease. There is therefore an urgent clinical need for novel therapies for recurrent or metastatic head and neck cancer that can control locoregional disease without causing severe functional impairment.
Near-infrared photoimmunotherapy (NIR-PIT) was first reported in 2011 as a novel method of tumor-specific cancer treatment [16]. NIR-PIT involves administration of a light-activatable dye (IRDye®700DX, abbreviated to IR700) conjugated to a monoclonal antibody followed by activation with near-infrared non-thermal light. NIR-PIT is a highly tumor-specific therapy with minimal toxicity in normal tissues [17,18]. The first human phase I/IIa multicenter study of NIR-PIT with a cetuximab-IR700 conjugate (cetuximab sarotalocan sodium; RM1929) targeting epidermal growth factor receptor (EGFR) in patients with unresectable head and neck squamous cell carcinoma (HNSCC) was completed in 2017 [19]; with a “fast-tracked” global phase 3 clinical trial opening in 2019 and scheduled to be completed in 2024 (https://clinicaltrials.gov/ct2/show/NCT03769506, accessed on 12 October 2022). Although a global phase III trial is ongoing, NIR-PIT with cetuximab-IR700 (AlluminoxTM) was conditionally approved by the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan in 2020 and has been in routine clinical use since January 2021 for unresectable locally advanced or locally recurrent head and neck cancer in patients that have previously undergone radiotherapy. NIR-PIT for head and neck cancer (HN-PIT) is expected to provide a curative treatment option for the locoregional recurrent or metastatic disease after radiotherapy and surgery.
In the present review, we describe the mechanisms underlying the cytotoxicity of NIR-PIT and summarize relevant clinical studies including adverse events. In addition, we present two cases of oropharyngeal cancer treated with HN-PIT at our institution and discuss future perspectives.
2. Mechanism of NIR-PIT Cytotoxicity
NIR-PIT comprises the administration of IR700, a photoactivating chemical, conjugated to monoclonal antibodies specific to a cell surface marker on cancer cells. Excitation of antibody-bound IR700 with near-infrared light at 690 nm causes IR700 to undergo a photochemical ligand reaction that releases the hydrophilic side chain of IR700 and hydrophobizes the remaining molecules. This reaction leads to damage to transmembrane target proteins thereby reducing cell membrane integrity. Damage to the cell membrane causes cancer cells to expand approximately 3-fold due to influx of extracellular fluid [20]. This rapid expansion destabilizes the cell membrane leading to the release of cytoplasmic contents. Injury to the cell membrane also leads to the activation of stress markers such as heat shock proteins 70 and 90 and pro-apoptotic signaling molecules such as calreticulin, ATP, and HMGB1 [21]. Host immune responses are then initiated against antigens released from dying cancer cells. The rapid release of cancer-specific antigens and signals induced by cell membrane damage activate local dendritic cells that then stimulate and educate cancer-specific naive T cells. Activated T cells then contribute to cell-mediated cancer cell death in a process known as immunogenic cell death (ICD) [22,23].
Near-infrared light is nonionizing, does not damage DNA, is harmless to normal cells, and penetrates a few centimeters into tissues [24,25]. Monoclonal antibody-photosensitive dye conjugates predominantly bind to cancer cells overexpressing cancer-associated antigens. Furthermore, IR700 is a water-soluble fluorescent molecule with negligible phototoxicity and biotoxicity [26,27] and IR700 dissociated from antibody-photoabsorber conjugate is easily excreted in urine [28]. Therefore, NIR-PIT has efficacy in selectively killing cancer cells without harming adjacent normal cells [20,29,30,31].
3. Clinical Studies
The first clinical trials of NIR-PIT were conducted in patients with head and neck cancers for the following reasons: (1) approximately 90% of head and neck cancers have cell surface expression of EGFR; (2) light illumination of head and neck cancer sites is technically feasible; and (3) there is an urgent clinical need for novel treatments for recurrent metastatic head and neck cancer due to poor prognosis.
A phase I/IIa, first-in-human study (RM-1929-101) of RM-1929 for the treatment of unresectable locally recurrent HNSCC was conducted in the United States in 2015 [19]. RM-1929 is a conjugate of IR700 and cetuximab, an antibody targeting EGFR which is highly expressed in HNSCC. A Japanese phase I trial (RM-1929-102) was conducted in Japanese patients with unresectable locally recurrent HNSCC in 2017 [32]. These two studies demonstrated the safety profile and efficacy RM-1929 in treating unresectable locally recurrent HNSCC. A global, multicenter, international phase III study of RM-1929 was initiated in 2018 and remains ongoing in 2022. Following the results of the RM-1929-101 and RM-1929-102 studies, RM-1929 was approved by the Japanese Ministry of Health, Labor and Welfare for the indication of “unresectable locally advanced or unresectable locally recurrent head and neck cancer” in January 2021.
3.1. RM-1929-101 Study
The RM-1929-101 study was a multicenter, open-label, phase I/IIa study consisting of two parts. Part 1 was designed to determine the maximum feasible dose (MFD) of RM-1929 in patients with unresectable, locally recurrent HNSCC and to evaluate the safety profile of RM-1929. The eligibility criteria of the RM-1929-101 study were patients who could not be adequately treated with surgery, radiation, or platinum-based chemotherapy with an ECOG performance status (PS) of 0 to 2. Patients must have previously received platinum-based chemotherapy. Nine patients received 160, 320, or 640 mg/m2 of RM-1929 intravenously over 2 h on day 1 and one cycle of near-infrared light illumination on day 2 at approximately 24 h after the completion of RM-1929 administration.
Oropharyngeal cancer was present in five out of nine patients (55.6%). All nine patients had treatment-related adverse events. The most common adverse event was pain in the treated area in three cases (33.3%). Serious adverse events (SAEs) were observed in six patients (one case each of dehydration, decreased level of consciousness, and aspiration pneumonia at 160 mg/m2; tumor pain and oral pain at 320 mg/m2; and tumor bleeding at 640 mg/m2). No adverse events leading to death or treatment discontinuation were observed. No dose limiting toxicity (DLT) occurred at any dose. Complete response (CR) or partial response (PR) were not observed at 160 mg/m2 and 320 mg/m2. CR was observed in one of three patients treated at a dose of 640 mg/m2. The mean AUC0-∞ at 640 mg/m2 of RM-1929 was within the range to achieve optimal EGFR saturation in tumors. Accordingly, the MFD of RM-1929 was determined as 640 mg/m2.
In Part 2, 30 patients received up to a maximum of 4 cycles of 640 mg/m2 RM-1929 intravenously and light illumination at intervals of 4 to 8 weeks. Oropharyngeal carcinoma was present in seven out of 30 patients (23.3%). The median number of cycles was one cycle in 11 patients (36.7%), two cycles in seven patients (23.3%), three cycles in eight patients (26.7%), and four cycles in four patients (13.3%). SAEs occurred in 13 patients (43.3%) including three cases of pneumonia and two cases of tumor hemorrhage. Adverse events leading to death occurred in a total of 3 patients comprising tumor hemorrhage in cycle 2, arterial hemorrhage in cycle 3, and pneumonia in cycle 4. Adverse events leading to death were determined not to be related to the study treatment. Adverse events leading to discontinuation of treatment occurred in a total of five patients and included one case each of tumor hemorrhage and peripheral swelling in cycle 1, increased creatinine in cycle 2, and arterial bleeding and rash in cycle 3. Adverse events related to the study treatment were observed in 25 patients (83.3%). Major events included facial edema, fatigue, erythema, and dysphagia in five patients (16.7%), respectively, and peripheral edema, rash, tongue edema, oropharyngeal pain, and tumor pain in four patients (13.3%), respectively.
Out of the 30 patients, four patients (13.3%) achieved CR, nine patients (30.0%) achieved PR, and 11 (36.7%) had stable disease (SD) according to the modified response evaluation criteria in solid tumors (mRECIST) ver. 1.1. The median overall survival was 9.30 months (95% confidence interval [CI] 5.16–16.92). Six-, 12- and 18-month survival rates were 63.3% (19/30), 46.7% (14/30), and 20.0% (6/30), respectively. The median progression-free survival was 5.16 months (95% CI 2.10–5.52).
3.2. RM-1929-102 Study
The RM-1929-102 study was a single-center, open-label, phase I study conducted in Japan. Eligibility criteria were: patients with ECOG PS of 0 to 2 who could not be satisfactorily treated with surgery, radiotherapy, or platinum chemotherapy; and prior systemic platinum-based chemotherapy for HNSCC. This study comprised three patients administered 640 mg/m2 of RM-1929 intravenously over 2 h on day 1 and one cycle of near-infrared light illumination on day 2 approximately 24 h after the completion of RM-1929 administration. Oropharyngeal carcinoma was present in one of three patients (33.3%). Adverse events were observed in all three cases (100%), with 13 adverse events (pain in the treated area, edema, glossitis, liver enzyme elevation, hypertension, and increased gamma-glutamyl transferase) determined to be related to the study treatment. No SAEs, deaths, or adverse events leading to treatment discontinuation were observed. No DLTs were observed. Two of the three patients achieved PR and one patient had disease progression (PD) according to mRECIST ver. 1.1.
4. Treatment Flow of HN-PIT in Clinical Practice
4.1. Day 1: Administration of RM-1929
On day 1 or the day of RM-1929 administration, the environment around the patient was prepared for RM-1929 administration. Patients were asked to avoid direct sunlight and room illuminance was kept below 120 lux. RM-1929 was administered at a dose of 640 mg/m2 by intravenous infusion over 2 h.
4.2. Day 2: Laser Illumination
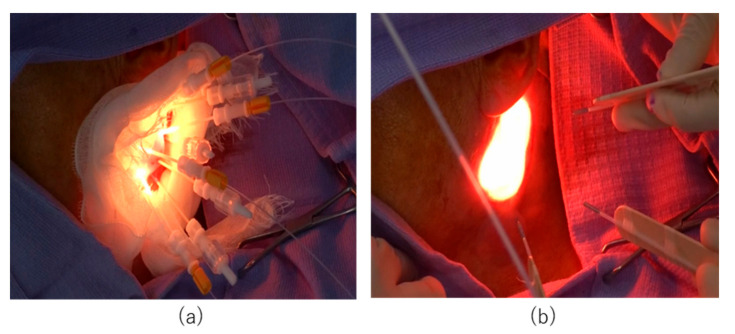
At 20–28 h after completion of the RM-1929 infusion, near-infrared laser illumination was performed using the BioBlade® laser system (Rakuten Medical, Inc., San Diego, CA, USA). Laser diffusers were selected according to tumor location. For deep lesions (tumors deeper than 10 mm below the skin or mucosal surface) or thick lesions (tumors thicker than 10 mm), a cylindrical diffuser was used for laser illumination (Figure 1a). Superficial lesions (tumors located within 10 mm from the skin or mucosal surface) were treated using a frontal diffuser (Figure 1b).
Figure 1.
Laser illuminating devices. (a) Near-infrared laser illumination using cylindrical diffusers for a deep or thick lesion. (b) Near-infrared laser illumination using frontal diffusers for a superficial lesion.
When using a cylindrical diffuser, a needle catheter was first inserted into the tumor. If tumors were large in diameter, multiple needle catheters were inserted at a maximum distance of 18 mm to avoid overlap with the illuminated area, thus allowing the laser beam to illuminate the entire tumor. A cylindrical diffuser was inserted into the lumen of the needle catheter to illuminate the laser beam. When treating with a frontal diffuser, the area to be illuminated was first determined including a margin of at least 5 mm. The minimum diameter of the laser beam that can be emitted by a single frontal diffuser is 17 mm and the maximum diameter is 38 mm. If the planned illumination area was larger than 38 mm, multiple frontal diffusers were used or illumination was divided into multiple sessions. The two types of diffusers were used in combination according to lesion location and size. Subsequent treatment cycles were performed up to a maximum of four cycles spaced at least four weeks apart.
5. Treatment-Specific Adverse Events
Patients become photosensitive after RM-1929 administration because as EGFR is also expressed by normal skin and mucosal cells [33,34,35,36]. Therefore, appropriate measures to protect against light exposure should be taken. The environment around patients should be maintained below 120 lux for one week after the administration of RM-1929. Patients should avoid skin exposure when in brighter areas. Sun exposure should be avoided for four weeks after the administration of RM-1929. The incidence of photosensitivity in the RM-1929-101 study was 3/39 patients (7.7%), with grade 1 photosensitivity in two patients and grade 2 photosensitivity in one patient. This incidence of photosensitivity is considerably lower than the severity and frequency of photosensitivity associated with photodynamic therapy [37]. Other adverse events requiring caution include carotid and tumor hemorrhage, tongue edema and laryngeal edema, infusion reaction, severe skin reactions, and hypomagnesemia. HN-PIT causes rapid tumor collapse which can lead to fatal bleeding after treatment in patients with tumor invasion of large blood vessels. Therefore, the treatment of tumors with carotid invasion is contraindicated due to the risk of carotid rupture. Severe edema can develop after HN-PIT due to increased levels of reactive oxygen species generated by the treatment [17,38]. As laryngeal edema leads to airway obstruction, tracheostomy should be performed prior to light illumination when treating areas close to the airway.
6. Treatment Indications
HN-PIT, NIR-PIT for head and neck cancer, is indicated in Japan for unresectable locally recurrent or locally advanced head and neck cancer with prior radiotherapy. In other words, HN-PIT is considered appropriate for the treatment of unresectable tumors only. Common reasons for unresectable head and neck cancer include carotid artery invasion, extensive skull base invasion, and mediastinal or vertebral invasion. However, the use of HN-PIT is not indicated for the treatment of these lesions. Therefore, the lesion must be determined to be unresectable based on other factors. In our previous study of HN-PIT, tumors were considered unresectable for the following reasons: (1) the high risk of further surgery or reconstructive surgery; (2) the decreased likelihood of complete cure after further surgery due to multiple previous resections; (3) poor general condition limiting reconstructive surgery; and (4) patient refusal of further surgery despite clinical recommendations.
Decisions regarding treatment options are often difficult in cases of locoregional recurrence of oropharyngeal lesions that have previously undergone reconstructive surgery or radiation. Additional open surgery is high risk and transoral resection is often technically challenging due to changes in anatomical structure. HN-PIT is a tumor-specific treatment that has demonstrated efficacy and less damage to normal tissue even in patients that have previously undergone multiple treatments.
7. Case Presentation
Ten patients underwent a total of 19 cycles of HN-PIT at Aichi Cancer Center Hospital (Table 1). Herein, we have described two patients of oropharyngeal lesions treated with HN-PIT. Tumor response evaluation was conducted using modified RECIST 1.1 [19].
Table 1.
Characteristics of patients treated with HN-PIT.
| Case | Gender | Age | ECOG PS | Histology | Primary site | Location of Target Lesion |
Diffuser | Cycle | Complication | BOR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 84 | 1 | SCC | Floor of mouth | Cervical skin | Cylindrical, frontal |
3 | Pain G2 Bleeding G2 Edema G1 |
PR |
| 2 | M | 84 | 1 | SCC | Upper gingiva | Oropharynx | Cylindrical | 2 | Pain G1 | PR |
| 3 | M | 54 | 0 | SCC | Upper gingiva | Subcutaneous tissue of face | Cylindrical | 2 | Pain G2 Edema G1 Fistula G1 |
CR |
| 4 | M | 77 | 0 | SCC | Oropharynx | Oropharynx | Cylindrical | 1 | Pain G2 Edema G1 |
PR |
| 5 | M | 68 | 0 | SCC | Larynx | Glottis | Cylindrical, frontal |
3 | Edema G1 | PR |
| 6 | M | 79 | 1 | SCC | Oropharynx | Cervical skin | Cylindrical, frontal |
2 | Pain G2 | PR |
| 7 | M | 42 | 0 | SCC | Buccal mucosa | Tongue | Cylindrical | 1 | Pain G2 Edema G2 |
CR |
| 8 | M | 88 | 1 | SCC | Lower gingiva | Lower gingiva | Cylindrical, frontal |
1 | Edema G4 | CR |
| 9 | F | 74 | 1 | SCC | Maxilla | Nasal cavity | Cylindrical | 3 | Pain G1 | PR |
| 10 | M | 80 | 1 | SCC | Oral cavity | Subcutaneous tissue of face | Cylindrical | 1 | Fistula G2 | PR |
ECOG-PS, Eastern Cooperative Oncology Group Performance Status; BOR, best overall response; SCC, squamous cell carcinoma; PR, partial response; CR, complete response.
7.1. Case 1
An 80-year-old man had undergone partial tongue resection and radiotherapy for tongue cancer 40 years previously. Maxillary, oropharyngeal, and mandibular resection, left neck dissection, and free rectus abdominis musculocutaneous flap reconstruction for mandibular gingival carcinoma T4aN0M0 (UICC 8th edition) had been performed one year previously. Local recurrence in the lateral wall of the oropharynx developed two months prior to attending our institution. Endoscopy demonstrated a lesion on the dorsal side of the musculocutaneous flap of the lateral wall of the left oropharynx (Figure 2a). No carotid infiltration was observed (Figure 2b). The tumor was 14 mm in anterior-posterior diameter and 34 mm in superior-inferior diameter. Pharyngocutaneous fistulation was considered unlikely due to the distance between the tumor and the skin surface. The tumor was considered unresectable for the following reasons. Transoral resection was not considered possible as it was difficult to resect the appropriate layer due to previous surgery. Open surgery for resection and reconstruction was considered high risk. Other treatment options including chemotherapy and ICI were excluded as they were not curative treatments. Accordingly, HN-PIT was selected in this case.
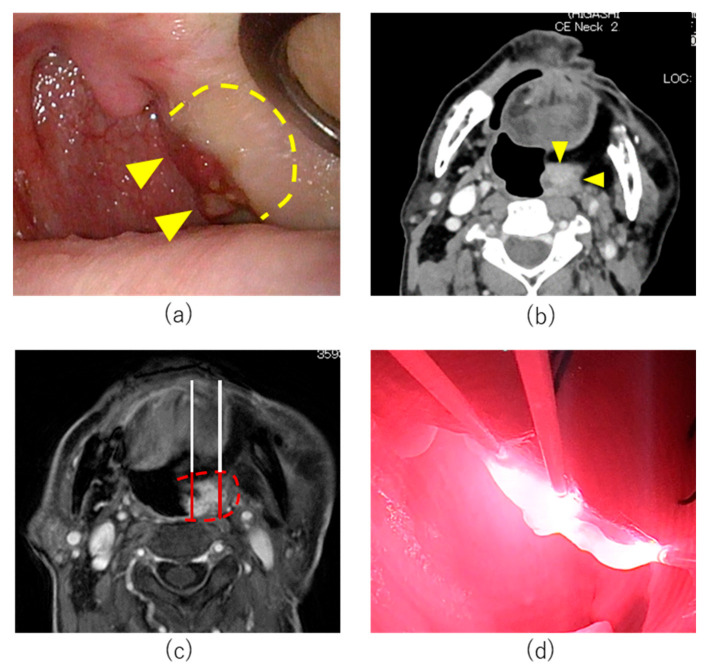
Figure 2.
Treatment course of Case 1. (a) Pretreatment endoscopic findings: Left oropharyngeal tumor (arrowheads) and tumor induration (dotted line). (b) Axial section of Gd-enhanced MRI T1 weighted image. (c) Treatment plan of cycle 1 HN-PIT treatment. Planned illumination area (dotted line). (d) Intraoperative image of HN-PIT in Cycle 1. Near-infrared laser was illuminated using 20 mm cylindrical diffusers.
On day 1, RM-1929 was administered without complications. On day 2, tracheostomy was performed under general anesthesia prior to laser illumination. The pharynx was exposed using a Feyh-Kastenbauer Weinstein-O’Malley (FK-WO) retractor (Olympus, Tokyo, Japan). The tumor location and border were confirmed using ultrasonography. A treatment margin of 5 mm was set around the entire circumference of the tumor and six needle catheters were inserted under ultrasonography (SonoSite SII, Fujifilm, Tokyo, Japan) and endoscopy (ENDOEYE FLEX, Olympus, Tokyo, Japan) (Figure 2c). Laser illumination was performed with cylindrical diffusers of 20 mm length (Figure 2d).
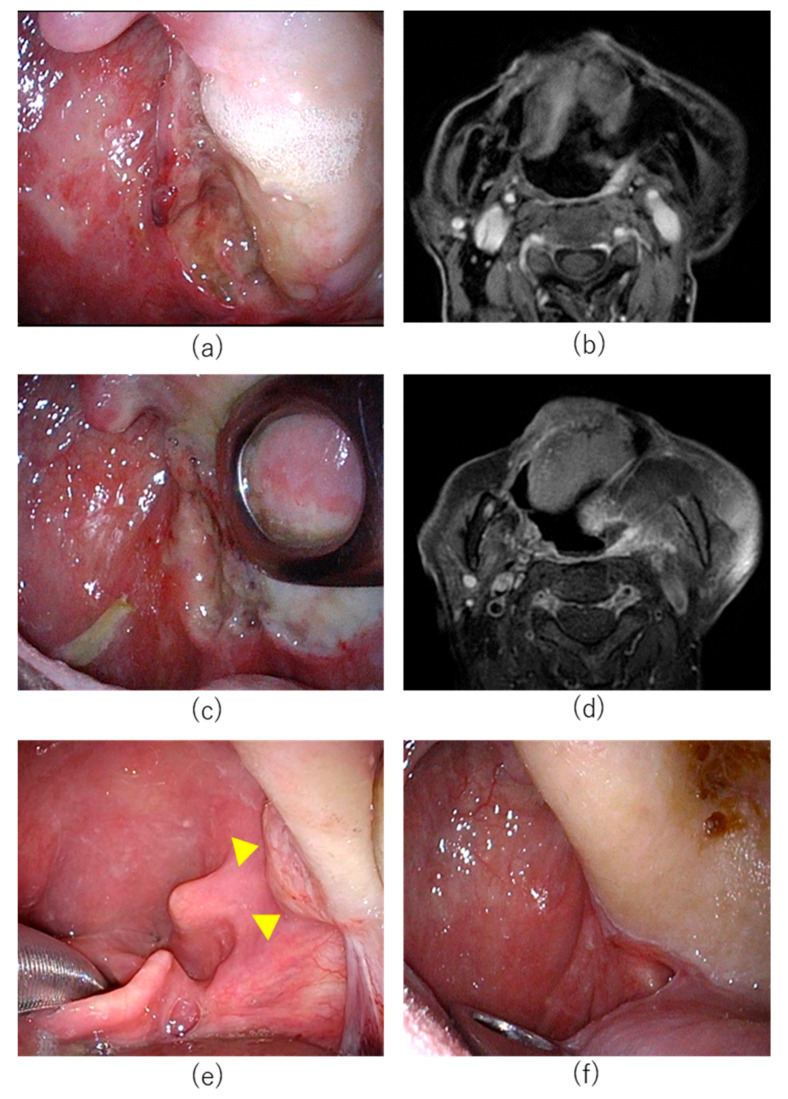
Continuous intravenous fentanyl was administered intraoperatively for prophylaxis of postoperative pain. No postoperative pain, edema, or bleeding was observed. On postoperative day (POD) 1, fentanyl was stopped and tube feeding was initiated through a previously created gastrostomy. The patient was discharged on POD 14. On POD 9, the tumor had almost disappeared with necrotic ulceration observed (Figure 3a). On POD 23, the ulcer had shrunk and tissue granulation had increased. However, biopsy of the granulated area revealed residual tumor cells. Magnetic resonance imaging (MRI) on POD 21 demonstrated the tumor size had decreased compared to pretreatment images (Figure 3b). As biopsy confirmed residual tumor cells, a second round HN-PIT was administered. In an attempt to increase treatment effect, an increased number of diffusers were used and seven needle catheters were inserted. The postoperative course of the second cycle was similar to cycle 1. Although the tracheostomy was closed at the patient’s request after cycle 1, pharyngolaryngeal edema did not occur after illumination in cycle 2. Postoperative pain was mild and fentanyl was not used. Rapid tumor shrinkage was again observed (Figure 3c). The patient was discharged on POD 7 after cycle 2. MRI imaging on POD 27 demonstrated further decreases in tumor size; however, residual tumor was again suspected (Figure 3d). We therefore recommended a third round of HN-PIT; however, the patient declined further treatment with HN-PIT and instead elected to be carefully followed up without further treatment. Four months after the second round of PIT, a biopsy of granulation tissue revealed residual tumor cells (Figure 3e). As the size of the tumor had decreased significantly, we considered transoral resection to be feasible at this time. Accordingly, transoral resection was performed five months after the second cycle of HN-PIT. The tumor was resected from the middle of the pharyngeal constrictor muscle. No scarring or wound healing complications were observed. Pathological analysis of the surgical specimen demonstrated that the oropharyngeal lesion had differing histological features from the original maxillary gingival carcinoma. Additionally, immunohistochemical staining revealed that p16 was negative. Therefore, the oropharyngeal lesion was considered a new p16-negative oropharyngeal carcinoma rather than recurrence of the original maxillary gingival carcinoma. No recurrence was observed at 16 months postoperatively and the patient remains under observation (Figure 3f).
Figure 3.
Posttreatment change after HN-PIT for Case 1. (a) Endoscopic image on postoperative day 9 after cycle 1. Ulceration was observed in the illuminated area. (b) MRI image after cycle 1. (c) Endoscopic image on postoperative day 6 after cycle 2. (d) MRI image after cycle 2. (e) Intraoperative image of the tumor (arrowheads) before transoral resection. (f) endoscopic image on postoperative 4 months.
7.2. Case 2
A 77-year-old male patient had undergone concurrent chemoradiotherapy after neck dissection for hypopharyngeal cancer 6 years previously. Transoral resection for cancer of the base of tongue had been attempted one year previously; however, the tumor had been resected using a mandibular swing approach as exposure of the tumor had been technically challenging using a retractor. In the same year, he had undergone transoral resection of soft palate cancer. Subsequently, cancer of the base of tongue (SCC, p16 negative, cT1N0M0 UICC 8th edition) was detected and the patient was referred to Aichi Cancer Center Hospital from another hospital (Figure 4). The tumor was located from the base of the tongue to the vallecula. Although the tumor diameter was unmeasurable on CT and MRI, based on the endoscopic findings, we determined that the tumor was a superficial lesion of <20 mm in diameter. It did not involve the carotid or lingual arteries. The tumor was considered unresectable for the following reasons. Regarding transoral resection, pharyngeal exposure was considered technically challenging. The risk of open surgery for resection was considered high due to previous surgery. As chemotherapy and ICI were not curative treatments, HN-PIT was selected in this case.
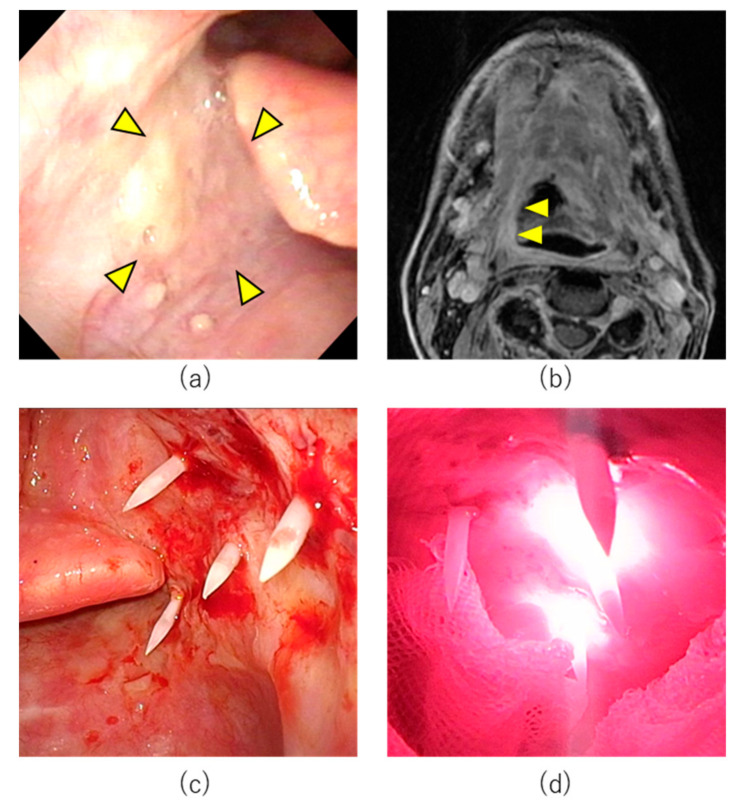
Figure 4.
Treatment course of Case 2. (a) Pretreatment endoscopic findings with white light. Right base of tongue tumor (arrowheads). (b) Axial section of Gd-enhanced MRI T1 weighted image. The tumor was enhanced (arrowheads). (c) Needle catheters were punctured percutaneously into the pharynx in HN-PIT. (d) Near-infrared laser illuminated the tumor.
On day 1, the patient received intravenous RM-1929 with no complications. On day 2, tracheostomy was first performed under general anesthesia followed by pharyngeal exposure with an FK-WO retractor. The tumor was a superficial tumor located on the right base of tongue. Near-infrared light must be directed perpendicularly to the lesion when using a frontal diffuser. As vertical illumination for this area was technically challenging, we selected laser illumination with cylindrical diffusers. Percutaneous insertion of four needle catheters and laser illumination with cylindrical diffusers of 20 mm length was performed under endoscopy and ultrasonography. Near-infrared laser illumination was then performed.
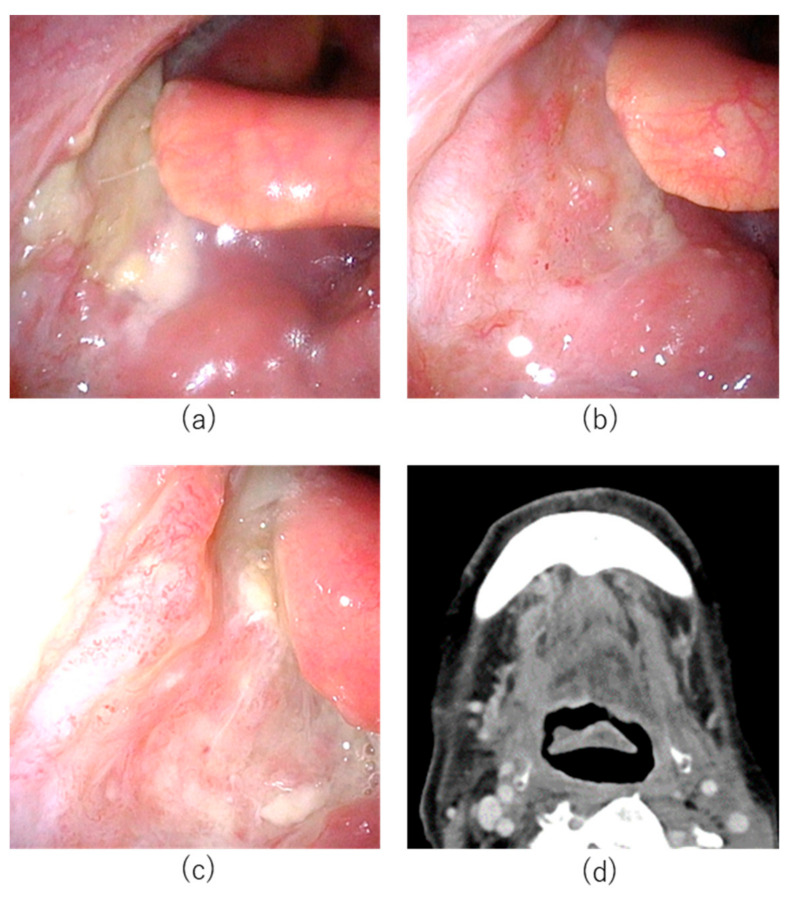
The patient had postoperative pain which was treated with intravenous administration of fentanyl, non-steroidal anti-inflammatory drugs (NSAIDs), and acetaminophen. Severe pain had resolved by POD 1 and fentanyl administration was discontinued. Edema of the neck, face, and larynx was observed on POD 1 but had almost completely resolved by POD 6. No other complications were observed and the patient was discharged on POD 7. Although CR could not be confirmed, the tracheal cannula was removed one month postoperatively as the patient declined a second cycle of HN-PIT. Eleven months after cycle 2, the mucosal surface appeared slightly irregular. However, biopsy did not reveal obvious residual cancer cells. The patient remains under observation with no obvious recurrence at 13 months after the first cycle of HN-PIT (Figure 5).
Figure 5.
Posttreatment change after HN-ALX for Case 2. (a) Endoscopic image on postoperative day 21 after cycle 1. White coating was observed on the illuminated area. (b) Endoscopic image on postoperative day 98 after cycle 1. The mucosa was almost normalized with no obvious tumor. (c) Endoscopic image 11 months after cycle 2. The mucosal surface was slightly irregular. Biopsy did not detect evidence of cancer. (d) CT image 11 months after cycle 2. No tumor mass was observed.
8. Immune Activation
As mentioned above, NIR-PIT has been posited to cause ICD that promotes maturation of immature dendritic cells in the cancer microenvironment [21]. Newly primed CD8+ T cells after NIR-PIT have been shown to response to a greater range of cancer antigens [39]. Although NIR-PIT may not kill all cancer cells immediately, activation of host immunity may lead to the clearance of a high proportion of remaining tumor cells. Conventional systemic cancer immunotherapies include T cell activating type 1 cytokines (e.g., IL-2 and IL-15) and ICI (e.g., anti-CTLA4 and anti-PD1/PDL1 antibodies). These therapies work on the principle of activating existing CD8+ T cells and, therefore, may induce autoimmune disease [40]. Unlike these therapies, NIR-PIT enhances host immunity locally without systemic adverse events.
NIR-PIT selectively eliminates immunosuppressive cells in the local tumor environment when used with antibodies against CD25 or CCR4 on the surface of Treg cells and CXCR2 on the surface of MDSCs [41]. Depletion of local Treg cells using Treg-targeted NIR-PIT against CD25 was highly effective in a syngeneic mouse model [42]. CD8+ T cells and NK cells in the treated tumor bed were fully activated within hours of Treg depletion by NIR-PIT. Treg-targeted NIR-PIT also affected non-treated tumors despite the treatment being directed at a single target lesion. When targeting CD25 systemically, activated effector cells expressing CD25 are also depleted. Furthermore, systemic blockade of immunosuppressive regulatory mechanisms may induce adverse autoimmune events. On the other hand, Treg-targeted NIR-PIT, which selectively depletes intra-tumoral Treg cells, avoids systemic adverse effects.
9. Combined Treatment with ICI
The use of NIR-PIT alone has been largely unable to induce sustained antitumor responses in syngeneic tumor mouse models. Adaptive immune resistance may limit sustained responses after treatment with NIR-PIT. The combination of NIR-PIT with an anti-PD-1 inhibitor has been shown to reverse adaptive immune resistance and enhance preexisting tumor antigen-specific T cell responses and de novo T cell responses induced by NIR-PIT in multiple allogeneic tumor models [39]. Combination therapy led to complete rejection of tumors treated with NIR-PIT and untreated distant tumors. Tumor antigen-specific T cell responses were observed in both treated and untreated tumors, demonstrating the development of systemic antitumor immunity. Although this strategy has demonstrated efficacy in animal models, this approach may have undesirable side effects associated with the use of ICI. The combination of NIR-PIT and ICI resulted in rapid tumor shrinkage within a few days of treatment. Even after tumor removal, the immune system of treated animals was able to reject tumors after re-inoculation, indicating the persistence of systemic immune memory of the initial tumor. A clinical study of NIR-PIT in combination with ICI for HNSCC and cutaneous squamous cell carcinoma is currently ongoing (ClinicalTrials.gov identifier: NCT04305795 accessed on 12 October 2022).
10. Laser Illumination Approach
NIR-PIT requires precise illumination of target lesions with near-infrared light, which is a major factor affecting efficacy and safety. Treatment is performed using a combination of a cylindrical diffusers to illuminate deep or thick lesions or a frontal diffuser to illuminate superficial lesions. Although the head and neck region is an easier area to illuminate than other parts of a body, accurate illumination of deep lesions under the skin and mucosa and lesions deep in the upper aerodigestive tract, such as hypopharynx, is technically challenging. In the present cases, needle catheter insertion was performed under endoscopic and ultrasound guidance after pharyngeal exposure with a retractor and illumination was performed with cylindrical diffusers. There have been several reports of HN-PIT using imaging modalities. Omura et al. treated a nasopharyngeal lesion with a frontal diffuser under rigid endoscope assistance [43]. Okamoto et al. illuminated a lesion within the lateral pterygoid muscle with cylindrical diffusers and performed needle catheter insertion using the Surgical Navigation System [44]. Koyama et al. reported needle catheter insetion and cylindrical diffuser illumination of a lesion in the maxillary sinus, which was not visible from the outside, using the Surgical Navigation System and CT guidance [45]. Thus, laser illumination must be performed precisely using a combination of imaging modalities.
However, there are areas where illumination is technically challenging even with use of the above imaging modalities, such as the hypopharynx. As the frontal diffuser is only able to illuminate lesions in front of the light socket, there is a need for the development of diffusers that are able to illuminate lesions in the lateral wall of the upper aerodigestive system.
11. Impact on Quality of Life
In patients with unresectable head and neck cancer, quality of life (QOL) can be compromised before and after treatment [46]. The progression of tumors in the head and neck region can result in marked functional decline in swallowing, speech, and breathing as well as symptoms of severe pain and bleeding [47]. Previous health-related QOL studies in patients with head and neck cancers have shown that QOL is particularly affected by oropharyngeal cancer, including increasing the risk of malnutrition [48]. In addition, conventional treatments of unresectable head and neck cancer after radiation include chemotherapy and ICI, which may reduce QOL due to treatment-related adverse events [11,12,13]. Anticipated functional declines due to treatment have been shown to influence treatment choice [49]. In HN-PIT, adverse events are predominantly localized while systemic adverse events are rare. Therefore, HN-PIT may represent a promising treatment option for unresectable head and neck cancer. Okamoto et al. assessed functional scales (physical, role, emotional, cognitive, and social functioning) and global health status as well as domain scales (pain, swallowing, sense problems, speech problems, trouble with social eating, trouble with social contact, and reduced sexuality) using the EORTC QLQ-C30 and QLQ-H&N35 questionnaires, with no significant changes in any of the QOL endpoints reported after HN-PIT [50]. Head and neck cancer survivors may suffer from treatment sequelae over a long period of time even in cases of CR to treatment [51]. HN-PIT may contribute to the maintenance of QOL after treatment.
12. Conclusions
We report the treatment of two patients with oropharyngeal lesions at our institution. Treatment responses were good in both cases with no severe adverse events and no functional disorders observed after HN-PIT. At present, HN-PIT is a predominantly local treatment. However, future approaches combining HN-PIT with ICI or Treg-targeting NIR-PIT may also have efficacy in the treatment of distant metastatic disease.
Acknowledgments
We would like to thank Enago (https://www.enago.jp) for their writing support.
Author Contributions
Conceptualization, D.N.; methodology, D.N.; validation, D.N.; investigation, D.N., H.T. and M.S.; writing—original draft preparation, D.N.; writing—review and editing, H.S., S.B., H.T., M.S. and N.H.; visualization, D.N. supervision, N.H.; project administration, N.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval is not required by the Institutional Review Board of Aichi Cancer Center Hospital for this type of study. We hereby declare that we fully understand our local ethical guidelines: the ethical guidelines for medical research concerning humans of the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chow L.Q.M. Head and Neck Cancer. Reply. N. Engl. J. Med. 2020;382:e57. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chenevert J., Chiosea S. Incidence of human papillomavirus in oropharyngeal squamous cell carcinomas: Now and 50 years ago. Hum. Pathol. 2012;43:17–22. doi: 10.1016/j.humpath.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Ang K.K., Harris J., Wheeler R., Weber R., Rosenthal D.I., Nguyen-Tan P.F., Westra W.H., Chung C.H., Jordan R.C., Lu C., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellin H., Friesland S., Lewensohn R., Dalianis T., Munck-Wikland E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int. J. Cancer. 2000;89:300–304. doi: 10.1002/1097-0215(20000520)89:3<300::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Temam S., Pape E., Janot F., Wibault P., Julieron M., Lusinchi A., Mamelle G., Marandas P., Luboinski B., Bourhis J. Salvage surgery after failure of very accelerated radiotherapy in advanced head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:1078–1083. doi: 10.1016/j.ijrobp.2004.12.062. [DOI] [PubMed] [Google Scholar]
- 8.Khan N., Clemens M., Liu J., Garden A.S., Lawyer A., Weber R., Gunn G.B., Morrison W.H., Kupferman M.E. The role of salvage surgery with interstitial brachytherapy for the Management of Regionally Recurrent Head and Neck Cancers. Cancers Head Neck. 2019;4:4. doi: 10.1186/s41199-019-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee N., Chan K., Bekelman J.E., Zhung J., Mechalakos J., Narayana A., Wolden S., Venkatraman E.S., Pfister D., Kraus D., et al. Salvage re-irradiation for recurrent head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:731–740. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H., Hanai N., Nishikawa D., Fukuda Y., Hasegawa Y. Complication and surgical site infection for salvage surgery in head and neck cancer after chemoradiotherapy and bioradiotherapy. Auris Nasus Larynx. 2017;44:596–601. doi: 10.1016/j.anl.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., Erfan J., Zabolotnyy D., Kienzer H.R., Cupissol D., et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 12.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burtness B., Harrington K.J., Greil R., Soulieres D., Tahara M., de Castro G., Jr., Psyrri A., Baste N., Neupane P., Bratland A., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 14.Hanai N., Shimizu Y., Kariya S., Yasumatsu R., Yokota T., Fujii T., Tsukahara K., Yoshida M., Hanyu K., Ueda T., et al. Effectiveness and safety of nivolumab in patients with head and neck cancer in Japanese real-world clinical practice: A multicenter retrospective clinical study. Int. J. Clin. Oncol. 2021;26:494–506. doi: 10.1007/s10147-020-01829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa D., Suzuki H., Beppu S., Terada H., Sawabe M., Kadowaki S., Sone M., Hanai N. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 2021;112:339–346. doi: 10.1111/cas.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsunaga M., Ogawa M., Kosaka N., Rosenblum L.T., Choyke P.L., Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto S., Bernardo M., Saito K., Koyasu S., Mitchell J.B., Choyke P.L., Krishna M.C. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR-700-antibody conjugates. Free Radic. Biol. Med. 2015;85:24–32. doi: 10.1016/j.freeradbiomed.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K., Ando K., Okuyama S., Moriguchi S., Ogura T., Totoki S., Hanaoka H., Nagaya T., Kokawa R., Takakura H., et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018;4:1559–1569. doi: 10.1021/acscentsci.8b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cognetti D.M., Johnson J.M., Curry J.M., Kochuparambil S.T., McDonald D., Mott F., Fidler M.J., Stenson K., Vasan N.R., Razaq M.A., et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck. 2021;43:3875–3887. doi: 10.1002/hed.26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsunaga M., Nakajima T., Sano K., Kramer-Marek G., Choyke P.L., Kobayashi H. Immediate in vivo target-specific cancer cell death after near infrared photoimmunotherapy. BMC Cancer. 2012;12:345. doi: 10.1186/1471-2407-12-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa M., Tomita Y., Nakamura Y., Lee M.J., Lee S., Tomita S., Nagaya T., Sato K., Yamauchi T., Iwai H., et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425–10436. doi: 10.18632/oncotarget.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green D.R., Ferguson T., Zitvogel L., Kroemer G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obeid M., Panaretakis T., Tesniere A., Joza N., Tufi R., Apetoh L., Ghiringhelli F., Zitvogel L., Kroemer G. Leveraging the immune system during chemotherapy: Moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007;67:7941–7944. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 24.Henderson T.A., Morries L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakiyama H., Kato T., Furusawa A., Choyke P.L., Kobayashi H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics. 2021;10:3135–3151. doi: 10.1515/nanoph-2021-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Railkar R., Krane L.S., Li Q.Q., Sanford T., Siddiqui M.R., Haines D., Vourganti S., Brancato S.J., Choyke P.L., Kobayashi H., et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol. Cancer Ther. 2017;16:2201–2214. doi: 10.1158/1535-7163.MCT-16-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maczynska J., Da Pieve C., Burley T.A., Raes F., Shah A., Saczko J., Harrington K.J., Kramer-Marek G. Immunomodulatory activity of IR700-labelled affibody targeting HER2. Cell Death Dis. 2020;11:886. doi: 10.1038/s41419-020-03077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi H., Choyke P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019;52:2332–2339. doi: 10.1021/acs.accounts.9b00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato K., Hanaoka H., Watanabe R., Nakajima T., Choyke P.L., Kobayashi H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol. Cancer Ther. 2015;14:141–150. doi: 10.1158/1535-7163.MCT-14-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato K., Choyke P.L., Kobayashi H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS ONE. 2014;9:e113276. doi: 10.1371/journal.pone.0113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirasu N., Yamada H., Shibaguchi H., Kuroki M., Kuroki M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int. J. Cancer. 2014;135:2697–2710. doi: 10.1002/ijc.28907. [DOI] [PubMed] [Google Scholar]
- 32.Tahara M., Okano S., Enokida T., Ueda Y., Fujisawa T., Shinozaki T., Tomioka T., Okano W., Biel M.A., Ishida K., et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021;26:1812–1821. doi: 10.1007/s10147-021-01960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan M., Jewell S.D. Evaluation of TGF-alpha and EGFR expression in oral leukoplakia and oral submucous fibrosis by quantitative immunohistochemistry. Oncology. 2001;61:284–292. doi: 10.1159/000055335. [DOI] [PubMed] [Google Scholar]
- 34.Mukaida H., Toi M., Hirai T., Yamashita Y., Toge T. Clinical significance of the expression of epidermal growth factor and its receptor in esophageal cancer. Cancer. 1991;68:142–148. doi: 10.1002/1097-0142(19910701)68:1<142::AID-CNCR2820680126>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 35.Jones N.R., Rossi M.L., Gregoriou M., Hughes J.T. Epidermal growth factor receptor expression in 72 meningiomas. Cancer. 1990;66:152–155. doi: 10.1002/1097-0142(19900701)66:1<152::AID-CNCR2820660127>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Mascia F., Mariani V., Girolomoni G., Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zumsteg Z.S., Luu M., Yoshida E.J., Kim S., Tighiouart M., David J.M., Shiao S.L., Mita A.C., Scher K.S., Sherman E.J., et al. Combined High-Intensity Local Treatment and Systemic Therapy in Metastatic Head and Neck Squamous Cell Carcinoma: An Analysis of the National Cancer Data Base. Cancer. 2017;123:4583–4593. doi: 10.1002/cncr.30933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato T., Okada R., Goto Y., Furusawa A., Inagaki F., Wakiyama H., Furumoto H., Daar D., Turkbey B., Choyke P.L., et al. Electron Donors Rather Than Reactive Oxygen Species Needed for Therapeutic Photochemical Reaction of Near-Infrared Photoimmunotherapy. ACS Pharmacol. Transl. Sci. 2021;4:1689–1701. doi: 10.1021/acsptsci.1c00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagaya T., Friedman J., Maruoka Y., Ogata F., Okuyama S., Clavijo P.E., Choyke P.L., Allen C., Kobayashi H. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol. Res. 2019;7:401–413. doi: 10.1158/2326-6066.CIR-18-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haanen J., Ernstoff M.S., Wang Y., Menzies A.M., Puzanov I., Grivas P., Larkin J., Peters S., Thompson J.A., Obeid M. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 2020;31:724–744. doi: 10.1016/j.annonc.2020.03.285. [DOI] [PubMed] [Google Scholar]
- 41.Barnett J.D., Jin J., Penet M.F., Kobayashi H., Bhujwalla Z.M. Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice. Cancers. 2022;14:3578. doi: 10.3390/cancers14153578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato K., Sato N., Xu B., Nakamura Y., Nagaya T., Choyke P.L., Hasegawa Y., Kobayashi H. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 2016;8:352ra110. doi: 10.1126/scitranslmed.aaf6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omura G., Honma Y., Matsumoto Y., Shinozaki T., Itoyama M., Eguchi K., Sakai T., Yokoyama K., Watanabe T., Ohara A., et al. Transnasal photoimmunotherapy with cetuximab sarotalocan sodium: Outcomes on the local recurrence of nasopharyngeal squamous cell carcinoma. Auris Nasus Larynx. 2022 doi: 10.1016/j.anl.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Okamoto I., Okada T., Tokashiki K., Tsukahara K. A Case Treated With Photoimmunotherapy Under a Navigation System for Recurrent Lesions of the Lateral Pterygoid Muscle. In Vivo. 2022;36:1035–1040. doi: 10.21873/invivo.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyama S., Ehara H., Donishi R., Morisaki T., Ogura T., Taira K., Fukuhara T., Fujiwara K. Photoimmunotherapy with surgical navigation and computed tomography guidance for recurrent maxillary sinus carcinoma. Auris Nasus Larynx. 2022 doi: 10.1016/j.anl.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Kraaijenga S.A., Oskam I.M., van der Molen L., Hamming-Vrieze O., Hilgers F.J., van den Brekel M.W. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51:787–794. doi: 10.1016/j.oraloncology.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Ojo B., Genden E.M., Teng M.S., Milbury K., Misiukiewicz K.J., Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48:923–937. doi: 10.1016/j.oraloncology.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao L.J., Hsu W.L., Lo W.C., Cheng P.W., Shueng P.W., Hsieh C.H. Health-related quality of life and utility in head and neck cancer survivors. BMC Cancer. 2019;19:425. doi: 10.1186/s12885-019-5614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metcalfe C.W., Lowe D., Rogers S.N. What patients consider important: Temporal variations by early and late stage oral, oropharyngeal and laryngeal subsites. J. Craniomaxillofac. Surg. 2014;42:641–647. doi: 10.1016/j.jcms.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto I., Okada T., Tokashiki K., Tsukahara K. Quality-of-Life Evaluation of Patients with Unresectable Locally Advanced or Locally Recurrent Head and Neck Carcinoma Treated with Head and Neck Photoimmunotherapy. Cancers. 2022;14:4413. doi: 10.3390/cancers14184413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hammerlid E., Adnan A., Silander E. Population-based reference values for the European Organization for Research and Treatment of Cancer Head and Neck module. Head Neck. 2017;39:2036–2047. doi: 10.1002/hed.24870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.