Abstract
Cryptosporidiosis, caused by the apicomplexan parasite Cryptosporidium parvum, has become a well-recognized diarrheal disease of humans and other mammals throughout the world. No approved parasite-specific drugs, vaccines, or immunotherapies for control of the disease are currently available, although passive immunization with C. parvum-specific antibodies has some efficacy in immunocompromised and neonatal hosts. We previously reported that CSL, an ∼1,300-kDa conserved apical glycoprotein of C. parvum sporozoites and merozoites, is the antigenic species mechanistically bound by neutralizing monoclonal antibody 3E2 which elicits the circumsporozoite precipitate (CSP)-like reaction and passively protects against C. parvum infection in vivo. These findings indicated that CSL has a functional role in sporozoite infectivity. Here we report that CSL has properties consistent with being a sporozoite ligand for intestinal epithelial cells. For these studies, native CSL was isolated from whole sporozoites by isoelectric focusing (IEF) following observations that the ∼1,300-kDa region containing CSL as seen by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was comprised of approximately 15 molecular species (pI 3 to 10) when examined by two-dimensional (2-D) electrophoresis and silver staining. A subset of six ∼1,300-kDa species (pI 4.0 to 6.5) was specifically recognized by 3E2 in 2-D Western immunoblots of IEF-isolated CSL. Isolated native CSL bound specifically and with high affinity to permissive human intestinal epithelial Caco-2 cells in a dose-dependent, saturable, and self-displaceable manner. Further, CSL specifically bound to the surface of live Caco-2 cells inhibited sporozoite attachment and invasion. In addition, sporozoites having released CSL after incubation with 3E2 and occurrence of the CSP-like reaction did not attach to and invade Caco-2 cells. These findings indicate that CSL contains a sporozoite ligand which facilitates attachment to and invasion of Caco-2 cells and, further, that ligand function may be disrupted by CSL-reactive monoclonal antibody. We conclude that CSL is a rational target for passive or active immunization against cryptosporidiosis.
Cryptosporidium parvum is an apicomplexan parasite that commonly causes diarrhea in humans, calves, and other economically important food animals throughout the world (14). The infection is transmitted by fecal, food, and waterborne routes and is initiated when sporozoites released from oocysts in the intestinal tract attach to and invade mucosal epithelial cells (14). Although progress has been made, control of cryptosporidiosis remains problematic due to the absence of approved vaccines or immunotherapies and lack of consistently effective parasite-specific pharmaceuticals (3, 35). Because apical complex and surface molecules of the apicomplexa are involved in attachment, invasion, and intracellular development, these molecules may provide targets for immunological or pharmacological therapy against cryptosporidiosis (4, 7, 12, 25, 43, 50). To this end, we recently reported the production and characterization of a panel of mouse monoclonal antibodies (MAbs) against multiple epitopes of immunoaffinity-purified apical complex and surface antigens of C. parvum sporozoites (40). The ability of these MAbs to neutralize C. parvum infectivity and thereby identify parasite molecules involved in the pathogenesis of infection was determined (22, 40). One of the MAbs, designated 3E2, was central in these studies because of its ability to elicit distinctive morphologic changes in both sporozoites and merozoites, neutralize their infectivity in vitro, and control infection in vivo (40). The structural and functional consequences of zoite exposure to MAb 3E2 (40) closely paralleled the neutralization-associated circumsporozoite precipitate (CSP) reaction, originally described for Plasmodium spp. sporozoites after incubation with antibody against the circumsporozoite protein, an apical-complex-derived sporozoite exoantigen (10, 30). The CSP reaction is hypothesized to mimic a process normally initiated by sporozoite attachment to host cell receptors during infection and result in premature shedding of attachment and invasion molecules on, or translocated to, the sporozoite surface (30). MAb 3E2 recognizes multiple sporozoite glycoproteins of 46 to 230 kDa, ∼770 kDa, and ∼1,300 kDa in Western blots (40). The ∼1,300-kDa glycoprotein, an apical exoantigen designated CSL, is the antigen species mechanistically targeted by MAb 3E2 and hyperimmune bovine colostral antibody in the CSP-like reaction of C. parvum (36, 40). CSL is conserved on geographically diverse C. parvum isolates and expresses a repetitive carbohydrate-dependent epitope recognized by MAb 3E2 (40). The sporozoite neutralizing activity of MAb 3E2 in vitro and its ability to control infection in vivo are profoundly greater than that of 116 additional MAbs produced in our laboratory against distinct epitopes of other neutralization-sensitive C. parvum antigens, including CPS-500 (22, 37, 38), GP25-200 (22, 35, 36, 40), and P23 (22, 32, 35). Collectively, these observations provided the rationale for the present study to further characterize the function of CSL in the pathogenesis of infection and the mechanism by which MAb 3E2 neutralizes infectivity. Because region II-plus of the circumsporozoite protein target of the CSP reaction in Plasmodium spp. has been shown to contain a sporozoite ligand for host cell receptors (8, 9, 33), we hypothesized that CSL functions as a sporozoite ligand for epithelial cells in the initiation of C. parvum infection. Indeed, the high efficiency of C. parvum infection indicated by low infective doses (14) and the rapidity with which C. parvum sporozoites locate, attach to, and invade epithelial cells after excystation suggest a ligand-receptor relationship (14, 25, 35). We further hypothesized that MAbs against CSL which elicit the CSP-like reaction inhibit infection of epithelial cells by preventing sporozoite attachment and/or invasion.
Here we report that CSL binds specifically and with high affinity to the surface of viable Caco-2 intestinal epithelial cells and, once bound, significantly diminishes their permissiveness to infection by C. parvum sporozoites. Further, after binding of MAb 3E2 to sporozoites and occurrence of the CSP-like reaction with accompanying release of CSL, the attachment of sporozoites to Caco-2 cells is inhibited. Finally, we demonstrate that CSL, which previous studies suggested was a single glycoprotein (40), is comprised of multiple ∼1,300-kDa molecular species with differing isoelectric points (pI). We conclude that CSL functions as a sporozoite ligand, facilitating the attachment to and invasion of intestinal epithelial cells.
(Some of the research findings contained here were presented in part as a preliminary report at the Fourth International Workshops on Opportunistic Protists on 13 June 1996 in Tucson, Ariz. [23]).
MATERIALS AND METHODS
Oocyst and sporozoite isolation.
The Iowa C. parvum isolate (19) used in all experiments was propagated in newborn Cryptosporidium-free Holstein bull calves to obtain parasite material for study (39). Oocysts were isolated by sucrose density gradient centrifugation and stored in 2.5% (wt/vol) KCr2O7 (4°C) prior to use (1). Immediately prior to excystation, oocysts were treated with hypochlorite (39). Sporozoites were isolated from excysted oocyst preparations by passage through a polycarbonate filter (2.0 μm, pore size; Poretics, Livermore, Calif.).
Isolation of CSL and control glycoproteins CPC205 and Tf190.
CSL was isolated from whole C. parvum by isoelectric focusing (IEF) as follows. Excysted oocysts were solubilized in lysis buffer [50 mM Tris (pH 8.0), 5 mM 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), 0.3 μM aprotinin, 10 μM E-64, 0.01 mM leupeptin, 5 mM EDTA, 130 μM bestatin, and 1% (wt/vol) octyl-glucoside]. Parasite molecules in the soluble fraction were then separated by preparative IEF (12 W, 4 h, 4°C) according to the manufacturer’s protocol (Rotofor; Bio-Rad, Hercules, Calif.), enriching for those of pI 3.5 to 5.0. CSL-containing fractions were identified by dot immunoblot by using MAb 3E2 (immunoglobulin M [IgM] isotype) as previously described (40) and then combined and concentrated by centrifugation (10,000 × g, 4°C; Centriprep 30; Amicon, Beverly, Mass.). The purity of isolated CSL was determined by silver staining the preparation resolved by 10 to 20% and 2 to 12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) reducing gels (40). Immunoreactivity and identity of isolated CSL were determined by Western blotting the preparation resolved in 10 to 20% and 2 to 12% gradient SDS-PAGE reducing gels (40). Blots were probed with MAb 3E2 or isotype-matched control MAb of irrelevant specificity (each at 8 μg/ml), and bound MAb was detected with affinity-purified phosphatase-conjugated goat anti-mouse IgM (Zymed, San Francisco, Calif.) and phosphatase substrate. MAbs used in these and all experiments described below were derived from hybridoma culture supernatant.
Two additional glycoproteins, CPC205 and Tf190, were isolated for use as negative controls in experiments to assess the binding specificity of CSL to Caco-2 cells (described below). CPC205, a 205-kDa C. parvum oocyst wall glycoprotein, is defined by mouse MAb 4D3 (IgM isotype). MAb 4D3 was prepared against immunoaffinity chromatography-isolated GP25-200 as previously described (40), binds to oocyst shells but not sporozoites or merozoites in immunofluorescence assays (IFA), and recognizes a carbohydrate-dependent epitope on multiple oocyst shell glycoproteins of >40 kDa in Western blots. CPC205 was isolated from oocyst shells by continuous elution gel electrophoresis according to the manufacturer’s protocol (Bio-Rad) as follows. Oocysts (5 × 108) were excysted, layered onto a Percoll (Pharmacia, Alameda, Calif.) gradient (Percoll [nine parts], 10× Alsever’s solution [one part], 1× Alsever’s solution [nine parts]), and centrifuged (40,000 × g, 30 min, 4°C). Gradient fractions (1 ml) were examined by phase-contrast microscopy, and those containing excysted oocyst shells, but not intact oocysts or sporozoites, were pooled and washed four times (16,000 × g, 10 min, 4°C) with phosphate-buffered saline (PBS; pH 7.4) containing protease inhibitors (5 mM AEBSF, 0.3 μM aprotinin, 10 μM E-64, 0.01 mM leupeptin, 5 mM EDTA, and 130 μM bestatin). Oocyst shells were then resuspended in reducing SDS-PAGE sample buffer (0.06 M Tris HCl [pH 6.8], 2% [wt/vol] SDS, 5% [vol/vol] β-mercaptoethanol, 10% [vol/vol] glycerol, 0.025% [wt/vol] bromophenol blue), boiled (4 min), centrifuged (20,000 × g, 10 min, 4°C) to remove insoluble material, and resolved in an SDS–4% PAGE gel by using a Prep Cell (Bio-Rad) for fractionation. Samples from eluted fractions were resolved in 2 to 12% gradient SDS-PAGE reducing gels and silver stained to identify those containing a 205-kDa band corresponding to CPC205 and to determine purity. Immunoreactivity and identity of isolated CPC205 were determined by Western blotting of silver stain-positive fractions resolved in 2 to 12% gradient SDS-PAGE reducing gels. Blots were probed with MAb 4D3 or isotype-matched control MAb (each at 8 μg/ml), and bound MAb was detected with affinity-purified phosphatase-conjugated goat anti-mouse IgM (Zymed) and phosphatase substrate. Tf190, the second negative control glycoprotein, is an adhesion molecule of Tritrichomonas foetus defined by mouse MAb 32.3B3.5 (IgG1 isotype) (5, 46). MAb 32.3B3.5 recognizes a carbohydrate-dependent epitope on an ∼190-kDa surface-exposed glycoprotein complex in Western blots (5). Tf190 was isolated from whole T. foetus by preparative electrophoresis as follows. The KV-1 isolate of T. foetus was cultured in Diamond’s medium (48 h, 37°C) (11), centrifuged (15,000 × g, 10 min, 4°C), resuspended in lysis buffer, disrupted by sonication (4°C), and ultracentrifuged (50,000 × g, 30 min, 4°C) to remove insoluble material. The soluble fraction was diluted with non-reducing sample buffer, boiled (4 min), and resolved in an SDS–7.5%-PAGE gel under nonreducing conditions. A gel strip spanning the 180- to 200-kDa region containing Tf190 was excised and electroeluted according to the manufacturer’s protocol (Six-Pack gel eluter; Hoeffer Scientific, San Francisco, Calif.). Purity of isolated Tf190 was determined by silver staining the electroeluted preparation resolved in an SDS–7.5%-PAGE nonreducing gel. Immunoreactivity and identity of isolated Tf190 were determined by Western blotting of the preparation resolved in an SDS–7.5%-PAGE nonreducing gel. Blots were probed with MAb 32.3B3.5 or isotype-matched control mAb (each at 1 μg/ml), and bound MAb was detected with affinity-purified alkaline phosphatase-conjugated goat anti-mouse IgG1 (Zymed) and phosphatase substrate.
To determine if the biologically relevant CSL species bound by MAb 3E2 during the CSP-like reaction had been isolated by the IEF method described above, the ability of isolated CSL to competitively inhibit the reaction was evaluated as follows. Viable, purified sporozoites (1.2 × 105 in 10 μl PBS) were coincubated (30 min, 37°C) with MAb 3E2 (0.5 μg) and IEF-isolated CSL (2.5 μg) and then observed by phase-contrast microscopy to determine the percentage of sporozoites undergoing the CSP-like reaction ([number of sporozoites undergoing CSP-like reaction ÷ total number of sporozoites counted] × 100). A total of 200 sporozoites was counted in each of two replicate experiments. In parallel, the percentage of sporozoites undergoing the CSP-like reaction was determined in control preparations incubated under identical conditions with (i) MAb 3E2 (0.5 μg), (ii) isotype-matched control MAb of irrelevant specificity (0.5 μg), (iii) MAb 3E2 (0.5 μg) and isolated CPC205 (2.5 μg), (iv) MAb 3E2 (0.5 μg) and isolated Tf190 (2.5 μg); or (v) MAb 3E2 (0.5 μg) and the soluble fraction from C. parvum sporozoites (2.5 μg). For the latter control, sporozoites were disrupted by freeze-thawing and sonication (4°C) in lysis buffer and then ultracentrifuged to remove insoluble material and dialyzed (12- to 14-kDa exclusion limit) against PBS (4°C) prior to use. The glycoprotein concentration in CSL, CPC205, Tf190, and solubilized sporozoite samples was determined by the microbicinchoninic acid method (Pierce, Rockford, Ill.) with bovine glycoprotein purified from Cohn fraction VI (G9014; Sigma, St. Louis, Mo.) as a standard. Percent CSP-like reaction values for test and control preparations were examined for significant differences by using the Student’s one-tailed t test.
2-D electrophoretic analysis of whole C. parvum and isolated CSL.
The glycoprotein species composition of IEF-isolated CSL was analyzed by two-dimensional (2-D) electrophoresis (2-D Electrophoresis Cell; Bio-Rad) and compared to that of whole C. parvum by using a previously described method with some modifications (29). For whole C. parvum analysis, excysted oocysts were boiled (3 min) in 2-D sample buffer (0.06 M Tris [pH 6.8], 5% [wt/vol] SDS, 5% [vol/vol] β-mercaptoethanol, 10% [vol/vol] glycerol), sonicated (4°C), and subjected to freeze-thawing, after which the insoluble fraction was removed by ultracentrifugation. CSL was isolated by preparative IEF as described above, solubilized in 2-D sample buffer, and ultracentrifuged to remove insoluble material prior to analysis. First-dimension tube gels (9.2 M urea, 4% [wt/vol] acrylamide, 20% [vol/vol] Triton X-100, 1.6% [vol/vol] Biolyte 5/7, 0.4% [vol/vol] Biolyte 3/10, 0.01% [wt/vol] ammonium persulfate, 0.1% [vol/vol] TEMED [N,N,N′,N′-tetramethylethylenediamine]) were cast in 1 mm (inner diameter) glass capillary tubes, loaded with the soluble fraction from excysted oocysts or isolated CSL, and subjected to IEF (6 h, 750 V). After IEF, tube gels were laid onto 2 to 12% gradient SDS-PAGE reducing gels and resolved in the second dimension (200 V, 1 h). Second-dimension gels were then silver stained to identify the ∼1,300-kDa (i.e., 1,200 to 1,400 kDa) (glyco)protein species in whole C. parvum (3 × 107 excysted oocysts) or isolated CSL (10 μg). To characterize the ∼1,300-kDa species specifically recognized by MAb 3E2, second-dimension gels containing isolated CSL (6 μg) were electrotransferred (100 V, 1 h; Trans-Blot Cell; BioRad) to polyvinylidene difluoride membranes for Western blotting. Briefly, the membranes were incubated (1 h, 21°C) with MAb 3E2 or isotype-matched control MAb (each at 1 μg/ml), washed 12 times with buffer B (36) containing 5% (wt/vol) nonfat dry milk, incubated (1 h, 21°C) with affinity-purified alkaline phosphatase-conjugated goat anti-mouse IgM (Zymed), washed four times with buffer B containing 5% (wt/vol) nonfat dry milk, washed four times with buffer B, washed two times with 0.1 M Tris (pH 9.0), and then developed with chemiluminescence substrate (Bio-Rad) and photographed. The same membranes were then stripped of antibodies (0.1M Tris [pH 6.7], 20% [wt/vol] SDS, 0.7% [vol/vol] β-mercaptoethanol; 12 h, 21°C) as previously described (17) and reprobed identically with mouse MAb 4D10 (IgM isotype). MAb 4D10 binds to an ∼1,300-kDa glycoprotein in Western blots of whole C. parvum or IEF-isolated CSL but, unlike MAb 3E2, recognizes a peptide epitope and does not elicit the CSP-like reaction (40). Therefore, it was of interest to compare the reactivity patterns of MAbs 3E2 and 4D10 with IEF-isolated CSL in 2-D Western blots.
Effect of MAb 3E2 on sporozoite attachment and invasion.
To quantitate specific neutralizing activity of MAb 3E2 against the infective sporozoite stage, an in vitro neutralization assay was performed. Purified sporozoites (1.2 × 105 in 200 μl of minimum essential medium [MEM]) were incubated (15 min, 37°C, 10% CO2) with MAb 3E2 or isotype-matched control MAb (10 μg/ml each) and then inoculated onto individual Caco-2 cell monolayer cultures (three replicates per treatment). Monolayers had been grown to ∼90% confluency on glass coverslips (12 mm in diameter) in complete MEM (MEM containing 10% fetal bovine serum, 1% nonessential amino acids, 100 U of penicillin per ml, and 100 μg of streptomycin per ml) prior to inoculation. At 24 h postinoculation (p.i.), coverslip cultures were washed with PBS, methanol fixed (30 s), blocked (PBS containing 3.2% [wt/vol] fish gelatin and 2% [wt/vol] bovine serum albumin [BSA]), and processed for IFA by using MAb 4B10 and affinity-purified fluoresceinated goat anti-mouse IgG-IgM-IgA (Kirkegaard & Perry, Gaithersburg, Md.) to detect intracellular stages. MAb 4B10, prepared against immunoaffinity chromatography-isolated GP25-200 as previously described (40), recognizes C. parvum stages in Caco-2 cells through 72 h p.i. (22, 23). Each coverslip culture was systematically examined in its entirety by the same investigator by epifluorescence microscopy to directly quantitate the number of intracellular stages per monolayer. The mean numbers of intracellular stages in test and control cultures were examined for significant differences by using Student’s one-tailed t test. After quantitation of MAb 3E2-mediated sporozoite neutralization, specific effects of the MAb on attachment and invasion events of the infection process were examined. Purified sporozoites (6 × 104 in 30 μl of MEM) were incubated (15 min, 37°C, 10% CO2) with MAb 3E2 or isotype-matched control MAb (final concentration, 15 μg/ml each) and then inoculated onto individual Caco-2 cell monolayers immediately after aspiration of the culture medium. Monolayers had been grown in complete MEM to ∼90% confluency in eight-well-chamber coverglass slides (Nalge Nunc International, Naperville, Ill.) prior to inoculation. Interaction of MAb-treated sporozoites with Caco-2 cells was observed with a Nomarski differential interference contrast inverted microscope (Ziess) and recorded by video photomicroscopy (Cohu, San Diego, Calif.). In each of eight replicate experiments, 15 MAb 3E2- or control mAb-treated sporozoites were observed over a 30-min period by the same investigator.
Effect of CSL incubation with Caco-2 cells on sporozoite attachment and invasion.
To determine if CSL could bind specifically to Caco-2 cells, an in vitro binding assay was performed as follows. After dialysis (exclusion limit of 12 to 14 kDa) against PBS (4°C) and ultracentrifugation (50,000 × g, 30 min, 4°C) to remove insoluble material, 2 μg each of isolated CSL, CPC205, or Tf190 was incubated (15 min, 21°C) with viable Caco-2 cell monolayers grown to ∼90% confluency on coverslips as described above (three replicates per treatment). Coverslip cultures were then washed six times with PBS and processed for IFA as described above. The binding specificity was assessed by epifluorescence microscopy with MAb 3E2 for cultures incubated with CSL, MAb 4D3 for cultures incubated with CPC205, or MAb 32.3B3.5 for cultures incubated with Tf190. In parallel, replicates of each culture preparation were processed identically by using isotype-matched control MAbs of irrelevant specificity. Observations were validated in three separate replicate experiments.
To determine whether incubation of Caco-2 cells with CSL would affect their permissiveness to sporozoite attachment and/or invasion, coverslip cultures were incubated as described above with increasing quantities (0.25 to 2.0 μg/monolayer) of isolated CSL, CPC205, or Tf190 (three replicates per treatment). Cultures were then inoculated with purified sporozoites (1.2 × 105/monolayer), incubated (24 h, 37°C, 10% CO2), washed with PBS, and processed for epifluorescence microscopy as described above with MAb 7B6 to quantitate intracellular stages. MAb 7B6 recognizes C. parvum stages in Caco-2 cells through 72 h p.i., binds to multiple 58- to 200-kDa sporozoite glycoproteins in Western blots, and is unreactive with CSL (22, 23). MAb 7B6 was used in these experiments to facilitate accurate identification and quantitation of intracellular stages because it was observed in the in vitro binding assay described above that MAbs 3E2 and 4B10 detected CSL specifically bound to Caco-2 cells. Cell viability was determined in parallel monolayer cultures after incubation with CSL, CPC205, or Tf190. For each preparation, a minimum of 200 cells was observed by epifluorescence microscopy by using an acridine orange and ethidium bromide viability assay (13).
Characterization of binding kinetics of CSL to Caco-2 cells.
For use in binding kinetics studies, CSL was radioiodinated by the Iodobead method (Pierce). Briefly, Iodobeads, IEF-isolated CSL (100 μg in 1 ml of PBS), and 125I-labeled Na (0.5 mCi) (Dupont-NEN, Wilmington, Del.) were incubated (30 min, 4°C) with rocking, after which KI (0.5 mM) and protease inhibitors were added, and the supernatant was collected. The solution containing 125I-CSL was then dialyzed (exclusion limit of 12 to 14 kDa) against PBS (4°C). Trichloroacetic acid (TCA)-precipitable incorporation of 125I was approximately 4,240 cpm/μg of CSL (18). To determine if radioiodination affected CSL binding to Caco-2 cells, 125I-CSL or unlabelled CSL was incubated (30 min, 4°C) with viable Caco-2 cells (4 μg of CSL/monolayer), which were then processed for IFA as described above with MAb 3E2 or isotype-matched control MAb to detect bound CSL. The specific immunofluorescence pattern and intensity with MAb 3E2 were indistinguishable in Caco-2 cell monolayers which had been incubated with either unlabeled CSL or 125I-CSL.
For all binding kinetics experiments, Caco-2 cells were cultured in complete MEM in 96-well plates to ∼90% confluency prior to use. To construct a binding curve, monolayers were incubated (30 min, 4°C) in quadruplicate with increasing quantities of 125I-CSL (0.06, 0.13, 0.25, 0.50, 1.0, 2.0, or 4.0 μg/monolayer). Unlabeled CSL (0.25 μg/monolayer) was included with 125I-CSL (1.0, 2.0, and 4.0 μg) to level the binding curve in the upper half of the titration (6, 44). After incubation, cells were transferred to microfuge tubes, centrifuged (4,000 × g, 10 min, 4°C) to remove incubation medium, and washed three times with PBS (4°C). Cells were then resuspended in PBS (100 μl/monolayer), placed in scintillation vials, and bound 125I-CSL (in counts per minute) was determined for each treatment group with a gamma counter. Nonspecific 125I-CSL binding was determined by incubating (30 min, 4°C) monolayers in quadruplicate with an amount of unlabelled CSL (7.0 μg/monolayer) exceeding that required for saturation of binding sites, as determined from the preceding experiment and saturability experiments described below, and repeating the titration with 125I-CSL (0.06 to 4.0 μg/monolayer). These data were used to correct the binding curve for nonspecific binding (6, 24, 44). To determine if CSL binding was self-displaceable, monolayers were incubated (30 min, 4°C) in quadruplicate with a fixed quantity of 125I-CSL (0.25 μg/monolayer) and increasing quantities of unlabeled CSL (0.06, 0.13, 0.25, 0.50, 1.0, 2.0, or 4.0 μg/monolayer) (6, 24). After incubation, cells were washed with PBS, and bound counts per minute were determined. To determine if CSL binding was saturable, monolayers were incubated (30 min, 4°C) in quadruplicate with increasing quantities of 125I-CSL (0.06, 0.13, 0.25, 0.50, 1.0, 2.0, or 4.0 μg/monolayer). After incubation, the medium was collected, and the cells were washed with PBS, after which cell-bound and free counts per minute were determined. Bound-to-free ratios of 125I-CSL (in counts per minute) were then plotted against the corresponding quantity of 125I-CSL (in micrograms) incubated with each culture. A Scatchard plot was constructed by using B/F ratios and the corresponding 125I-CSL counts per minute bound by each culture (24). To calculate specific activity of the 125I-CSL used in binding saturability experiments and Scatchard plot construction, the maximal binding capacity of 125I-CSL was determined (6, 44). In brief, increasing numbers of Caco-2 cells (105, 2 × 105, 3 × 105, 4 × 105, or 5 × 105) were incubated (30 min, 4°C) in triplicate with a fixed quantity of 125I-CSL (0.25 μg/cell preparation). Cells were then PBS washed, and the amounts of bound counts per minute were determined. All data from binding kinetics experiments were analyzed by nonlinear least-squares fit by using the program LIGAND to calculate the association (KA) and dissociation (KD) constants (28).
RESULTS
Isolation of CSL, CPC205, and Tf190.
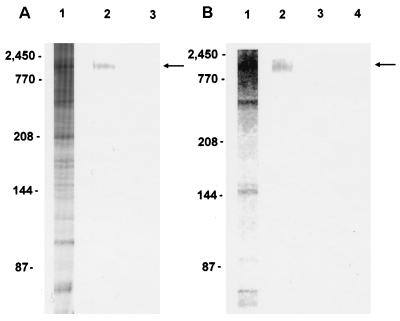
IEF-isolated CSL comigrated with an ∼1,300-kDa band in solubilized whole C. parvum and was relatively free of contaminating lower Mr (glyco)proteins as determined by SDS-PAGE and silver staining (Fig. 1A). In Western blots, isolated CSL was specifically recognized by MAb 3E2, comigrated with an ∼1,300-kDa antigen recognized in whole C. parvum, and was free of lower Mr antigens (Fig. 1B). These data confirmed the utility of IEF for isolation of native CSL from whole C. parvum.
FIG. 1.
(A) Silver-stained 2 to 12% gradient SDS-PAGE gel of solubilized oocysts before (lane 1, 105) and after (lane 2, 1.4 μg) IEF isolation of CSL (arrow). Lane 3 was loaded with sample buffer to identify silver-stain artifacts. (B) Western blot recognition of IEF-isolated CSL (lane 2, 0.5 μg) (arrow) and an ∼1,300-kDa comigrating antigen in solubilized oocysts (lane 1, 7 × 106) by MAb 3E2. Lane 3 (7 × 106 solubilized oocysts) and lane 4 (0.5 μg of CSL) were probed with isotype control MAb. Molecular mass standards are indicated on the left (titin, 2,450 kDa, and nebulin, 770 kDa [obtained from Kuan Wang and Gustavo Gutierrez, University of Texas, Austin]; myosin, 208 kDa; β-galactosidase, 144 kDa; and BSA, 87 kDa [Bio-Rad]).
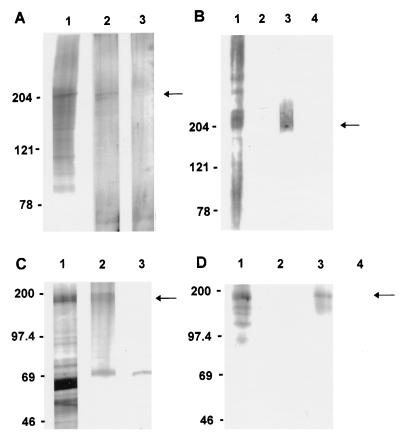
Because CPC205 is oocyst wall-specific and is not expressed by the infective sporozoite or merozoite stages, it was considered an appropriate C. parvum-derived control glycoprotein having no known biologic role in the infection process. Because Tf190 functions in adhesion of T. foetus to epithelial cells, it was considered an appropriate C. parvum-related protozoan control glycoprotein having a known biologic role in the pathogenesis of infection. In silver-stained SDS-PAGE gels, electrophoretically isolated CPC205 (Fig. 2A) and Tf190 (Fig. 2C) comigrated with bands of corresponding Mr in the whole-organism preparations from which they were derived and were relatively free of contaminating (glyco)proteins. Similarly, in Western blots, isolated CPC205 (Fig. 2B) and Tf190 (Fig. 2D) were specifically recognized by MAbs 4D3 and 32.3B3.5, respectively, and comigrated with antigens of corresponding Mr recognized in whole organism preparations. Isolated CPC205 was free of the higher and lower Mr antigens recognized by MAb 4D3 in whole C. parvum (Fig. 2B) and was unreactive with MAb 3E2 in Western blots. A 160-kDa antigen in the Tf190 preparation which was not visualized by silver staining (Fig. 2C) was recognized by MAb 32.3B3.5 in Western blots and comigrated with an antigen recognized in whole T. foetus (Fig. 2D). The Tf190 preparation was free of other lower Mr antigens recognized by MAb 32.3B3.5 (Fig. 2D).
FIG. 2.
(A) Silver-stained 2 to 12% gradient SDS-PAGE gel of solubilized oocysts before (lane 1, 1.5 × 106) and after (lane 2, 10 μg) electrophoretic isolation of CPC205 (arrow). Lane 3 was loaded with sample buffer to identify silver-stain artifacts. (B) Western blot recognition of isolated CPC205 (lane 3, 10 μg) (arrow) and a 205-kDa comigrating antigen in solubilized oocysts (1.5 × 106, lane 1) by MAb 4D3. Lane 2 (1.5 × 106 solubilized oocysts) and lane 4 (10 μg of CPC205) were probed with isotype control MAb. Molecular mass standards (Bio-Rad) are indicated on the left (myosin, 204 kDa; β-galactosidase, 121 kDa; BSA, 78 kDa). (C) Silver-stained SDS–7.5% PAGE gel of solubilized T. foetus before (lane 1, 300 μg) and after (lane 2, 10 μg) electrophoretic isolation of Tf190 (arrow). Lane 3 was loaded with sample buffer, identifying a 70-kDa silver-stain artifact. (D) Western blot recognition of isolated Tf190 (lane 3, 10 μg) (arrow) and comigrating antigen in solubilized organisms (300 μg, lane 1) by MAb 32.3B3.5. Lane 2 (300 μg of solubilized T. foetus) and lane 4 (10 μg of Tf190) were probed with isotype control MAb. Molecular mass standards (Amersham) are indicated on the left (myosin, 200 kDa; β-galactosidase, 97.4 kDa; BSA, 69 kDa; carbonic anhydrase, 46 kDa).
IEF-isolated CSL, but not CPC205 or Tf190, inhibited the CSP-like reaction by 100% when coincubated with viable sporozoites and MAb 3E2 (Table 1). This observation indicated that the relevant glycoprotein species mechanistically involved in the CSP-like reaction had been successfully isolated and retained biologic activity. Further, observations that CPC205 or Tf190 did not affect the CSP-like reaction validated these glycoproteins as appropriate negative controls for binding assays.
TABLE 1.
Inhibition of the MAb 3E2-elicited CSP-like reaction by CSL
| Sporozoite treatment | % CSP-like reaction
|
Pa | |
|---|---|---|---|
| Expt 1 | Expt 2 | ||
| MAb 3E2 | 78 | 76 | |
| Isotype control MAb | 0 | 0 | <0.004 |
| MAb 3E2 + CSL | 0 | 0 | <0.004 |
| MAb 3E2 + solubilized sporozoites | 0.06 | 0.01 | <0.007 |
| MAb 3E2 + CPC205 | 78 | 82 | <0.25 |
| MAb 3E2 + Tf190 | 79 | 76 | <0.25 |
Compared to MAb 3E2 treatment.
2-D electrophoretic analysis of whole C. parvum and isolated CSL.
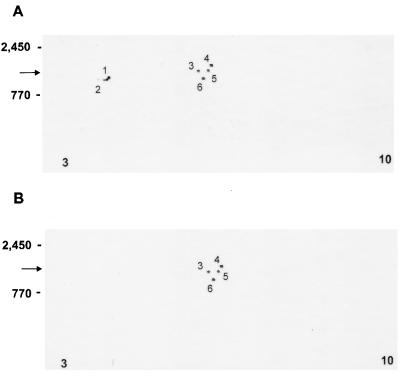
Because more than one CSL species or isoforms differing in glycosylation state could exist and not have been identified by silver-staining SDS-PAGE gels or Western blotting, further characterization was of interest. In silver-stained 2-D gels, approximately 15 (glyco)protein species (pI 3 to 10) in whole C. parvum were identified in the 1,200- to 1,400-kDa region known to contain CSL, a subset (pI 4 to 6.5) of which was identified in IEF-isolated CSL (data not shown). In 2-D Western blots of IEF-isolated CSL, four species having similar masses (1,200 to 1,400 kDa) and pI values (∼6) were corecognized by MAbs 3E2 and 4D10 (Fig. 3). However, MAb 3E2 recognized two additional species (∼1,300 kDa, pI 4) (Fig. 3A) which were not recognized by MAb 4D10 (Fig. 3B). No immunoreactivity was observed in 2-D Western blots probed with isotype-matched control MAb.
FIG. 3.
2-D Western blots of isolated CSL (6 μg) demonstrating antigen species migrating in the 1,200- to 1,400-kDa region (arrows) which are recognized by MAb 3E2 (A) or MAb 4D10 (B). Note the recognition of species 1 and 2 by 3E2 but not by 4D10. Molecular mass standards (titin, 2,450 kDa; nebulin, 770 kDa) and pI values are indicated on the left and bottom, respectively, of each panel.
MAb 3E2 inhibits sporozoite attachment and invasion.
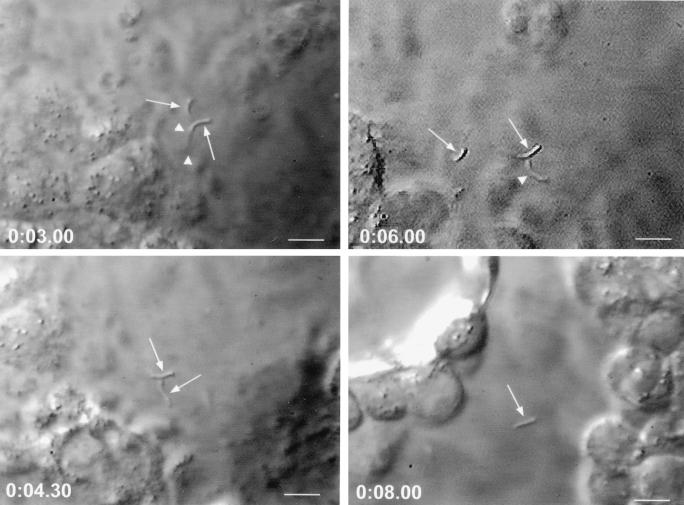
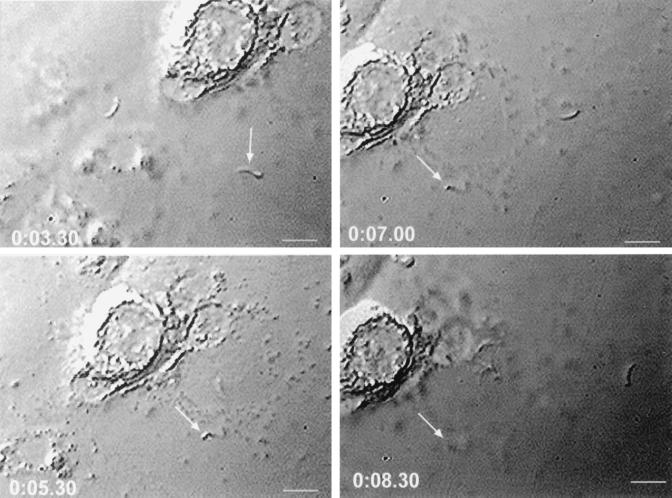
Caco-2 cells were selected for use in all in vitro assays because of their human intestinal epithelial origin, permissiveness to C. parvum infection and ability to support complete development of all life cycle stages, and widespread use in quantitating the activity of anticryptosporidial agents (21, 52). Caco-2 cell cultures inoculated with MAb 3E2-treated sporozoites contained significantly (P < 0.0001) fewer intracellular stages (217 ± 23) than cultures inoculated with isotype control MAb-treated sporozoites (2,735 ± 210), representing an ∼92% reduction in sporozoite infectivity after exposure to MAb 3E2. These findings and significance conclusions were verified in a replicate experiment. The magnitude of sporozoite infectivity reduction observed in vitro, the ability of MAb 3E2 to passively protect against C. parvum infection in vivo (40), and the inability of certain other IgM or IgG MAbs reactive with surface-exposed CSL epitopes distinct from that recognized by 3E2 to neutralize infectivity (22, 40) suggested that ligand-mediated attachment and/or invasion processes had been inhibited by 3E2. To determine whether these potential mechanisms of neutralization were operative, MAb 3E2-treated sporozoites were inoculated onto Caco-2 monolayers and observed microscopically. MAb 3E2-treated sporozoites (Fig. 4), but not isotype control mAb-treated sporozoites (Fig. 5), underwent the CSP-like reaction and did not attach to Caco-2 cells during a 30-min observation period. Consistent with previous observations (35, 36, 40), the majority of sporozoites undergoing the CSP-like reaction remained individualized; significant agglutination was not observed. These findings were consistent in eight replicate experiments, indicating that the initial attachment step of the infection process is inhibited by MAb 3E2.
FIG. 4.
Time-lapse video photomicroscopic depiction of sporozoite interactions with Caco-2 cells after incubation with MAb 3E2. Note that sporozoites (arrows) undergoing the CSP-like reaction (arrowheads) after treatment with MAb 3E2 fail to attach and invade. Bars, 7 μm. The time p.i. (in minutes) is indicated in each frame.
FIG. 5.
Time-lapse video photomicroscopic depiction of sporozoite interactions with Caco-2 cells after incubation with isotype control MAb. Note a sporozoite (arrow) probing the cell surface (0:03.30), attaching (0:05.30), invading (0:07.00), and becoming intracellular (0:08.30). Bars, 7 μm. The time p.i. (in minutes) is indicated in each frame.
CSL specifically bound to Caco-2 cells inhibits sporozoite attachment and invasion.
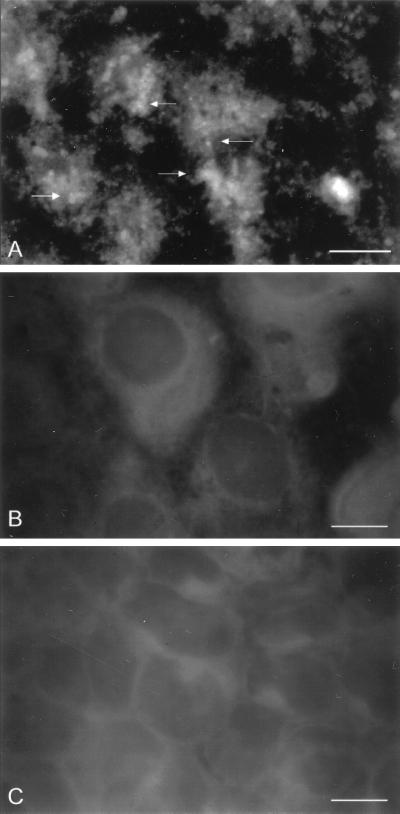
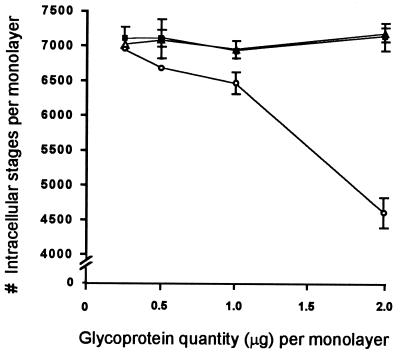
IEF-isolated CSL, but not CPC205 or Tf190, bound to multifocal areas on the surface of viable Caco-2 cells as determined by IFA with MAbs specific for each glycoprotein (Fig. 6). These observations demonstrated that CSL binding was specific. Further, after incubation of Caco-2 cells with CSL, a dose-dependent decrease in permissiveness to infection by sporozoites was observed. Specifically, cultures incubated with CSL (0.5 to 2.0 μg/monolayer) prior to sporozoite inoculation contained significantly fewer intracellular stages than cultures incubated with CPC205 or Tf190 (0.5 to 2.0 μg each/monolayer), or MEM, prior to inoculation (Fig. 7). Because cell viability exceeded 90% after incubation with MEM (92% viable) or 2.0 μg each of CSL (93% viable), CPC205 (91% viable), or Tf190 (91% viable), the observed reduction in permissiveness in cultures incubated with CSL was specific and not due to reduced cell viability caused by toxicity or other nonspecific factors.
FIG. 6.
Immunofluorescence photomicrographs of Caco-2 cell monolayers incubated with IEF-isolated CSL (A), CPC205 (B), or Tf190 (C) and probed with MAbs 3E2, 4D3, or 32.3B3.5, respectively. Note the specific binding of CSL (A), indicated by immunofluorescence reactivity (arrows), and the absence of binding of control glycoproteins CPC205 (B) and Tf190 (C). Bars, 7 μm.
FIG. 7.
Dose-dependent reduction in Caco-2 cell permissiveness to sporozoite infection by specifically bound CSL. Caco-2 cells were incubated with MEM, or increasing amounts (0.25, 0.50, 1.0, or 2.0 μg) of IEF-isolated CSL (○), CPC205 (■), or Tf190 (▵) prior to inoculation with sporozoites. The mean numbers of intracellular stages in cells incubated with CSL were significantly lower than in those incubated with CPC205 (P < 0.002) or Tf190 (P < 0.03) at 0.50 μg, CPC205 (P < 0.004) or Tf190 (P < 0.002) at 1.0 μg, and CPC205 (P < 0.00002) or Tf190 (P < 0.00005) at 2.0 μg. The mean numbers of intracellular stages in cultures incubated with MEM (6,866 ± 126), CPC205, or Tf190 were not significantly different. Bars represent the standard deviation.
The kinetics of CSL binding to Caco-2 cells are consistent with a sporozoite ligand function.
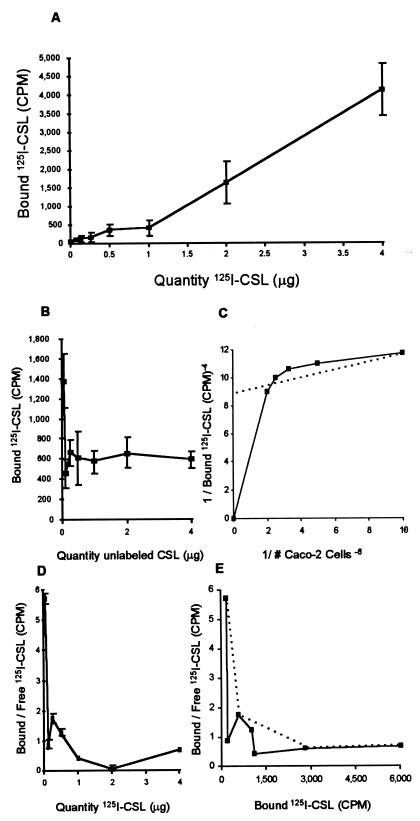
When Caco-2 cells were incubated with increasing quantities of 125I-CSL, dose-dependent binding was observed (Fig. 8A). Incubation of cells with a fixed quantity of 125I-CSL and increasing quantities of unlabeled CSL demonstrated self-displaceability. As the amount of unlabelled CSL approached and then exceeded that of 125I-CSL (0.25 μg), bound counts per minute were significantly reduced (Fig. 8B). These findings further supported the specificity of CSL binding initially observed in binding experiments evaluated by IFA. Specific radioactivity of 125I-CSL, calculated from TCA precipitation values (0.48 μCi/μg of CSL), represented an average specific radioactivity of all molecules comprising the preparation, assuming a 100% maximal binding capacity (44). The actual maximal binding capacity, determined to be 88% (Fig. 8C), was used to calculate a more accurate specific radioactivity (0.42 μCi/μg of CSL) for CSL binding saturability determination and Scatchard plot construction. When 125I-CSL (in micrograms) incubated with Caco-2 cells was plotted against the corresponding B/F 125I-CSL values (in counts per minute), decreasing B/F values and leveling of the resulting curve with increasing 125I-CSL indicated that binding was saturable (Fig. 8D). Concave upward curvature of the Scatchard plot suggested that CSL bound to Caco-2 cells with negative cooperativity (Fig. 8E) (24). KA and KD were calculated at 3.65 × 108 M−1 and 2.74 × 10−9 M, respectively (Fig. 8E) (28).
FIG. 8.
Kinetics of CSL binding to Caco-2 cells. (A) Dose-dependent binding of CSL to Caco-2 cells, corrected for nonspecific binding. (B) Self-displaceable binding of CSL to Caco-2 cells. The counts per minute of 125I-CSL bound in the presence of 0.13 to 4 μg of unlabeled CSL compared to that of 0.06 μg of unlabelled CSL are significantly (P < 0.02) lower. Bars represent the standard deviation (A and B). (C) Maximal binding capacity of biologically active 125I-CSL as determined from the ordinate of the y intercept for the best fit line (dashed line). (D) Saturability of CSL binding to Caco-2 cells. Bars represent the standard deviation. (E) Scatchard plot depicting the best-fit concave upward curvature line (dashed line).
DISCUSSION
We previously reported that CSL, an ∼1,300-kDa apical complex glycoprotein of sporozoites and merozoites, is the molecular species mechanistically bound by neutralizing antibodies in the CSP-like reaction of C. parvum (36, 40). Because the infectivity of Plasmodium spp. sporozoites is neutralized after the malarial CSP reaction (10, 30) and region II-plus of the circumsporozoite protein bound in the reaction contains a ligand for host cell receptors (8, 9, 16, 33), it was of interest to further characterize the CSP-like reaction of C. parvum and the function of CSL in the pathogenesis of infection. In the present study we examined the role of CSL in sporozoite attachment and invasion events. To perform the functional studies reported herein, isolation of native CSL with preservation of biological activity was required. An IEF method was used and allowed recovery of adequate quantities of native CSL. IEF-isolated CSL was capable of inhibiting the CSP-like reaction when incubated with MAb 3E2 and viable sporozoites, indicating retention of biological activity after purification.
2-D electrophoretic analysis of whole C. parvum identified approximately 15 1,200- to 1,400-kDa (glyco)protein species. Accurate quantitation was made difficult by the large number and the indiscreet silver staining pattern of the species identified, a feature which may be observed in 2-D analysis of glycoproteins (31). Nonetheless, this finding indicated that the ∼1,300-kDa band recognized by 3E2 in single-dimension Western blots (40) could have been comprised of more than one antigenic species which differed in pI and Mr. Indeed, preparative IEF isolated a subset of six 1,200- to 1,400-kDa species specifically recognized by 3E2 in 2-D Western blots, at least one of which was capable of inhibiting the CSP-like reaction. Previous studies determined that the CSL epitope recognized by 3E2 is periodate sensitive (40), indicating carbohydrate dependency and possible sialic acid dependency (54). Therefore, the six species recognized by 3E2 in 2-D Western blots of CSL may reflect differences in glycosylation and/or sialysation states of a single glycoprotein, either of which could alter pI and Mr (29, 31). Alternatively, the six species recognized may be distinct glycoproteins. Of the six species in IEF-isolated CSL recognized by 3E2, four were corecognized by MAb 4D10. Importantly, the two species (∼1,300 kDa, pI 4) recognized by 3E2, but not by 4D10, may be mechanistically important in the CSP-like reaction because 4D10, unlike 3E2, does not elicit this reaction.
The magnitude of reduction in intracellular stages in Caco-2 cells inoculated with 3E2-treated sporozoites suggested that the attachment and/or invasion processes had been inhibited. To better characterize the mechanism of neutralization, sporozoite interactions with Caco-2 cells following 3E2 treatment were monitored by video microscopy, allowing observation of events not readily examined in vivo. 3E2-treated sporozoites having undergone the CSP-like reaction did not probe Caco-2 cells or attach, indicating that the initial step in infection had been inhibited. However, IFA detection of small numbers of intracellular stages in such cultures at 24 h p.i. indicates that inhibition was not 100%. It is possible that subpopulations of sporozoites which differ in the expression of CSL and ability to undergo the CSP-like reaction exist and that those which do not undergo the reaction remain infectious. Consistent with this possibility, 76 to 78% of the sporozoites in the present study, and a similar percentage in previous studies (34), underwent the reaction after incubation with 3E2. Alternatively, it is possible that CSL or other functional sporozoite molecules contain additional ligands or that C. parvum has redundant systems for mediating infection.
Failure of sporozoites to attach and invade after CSL binding by 3E2 first suggested that CSL might function as a sporozoite ligand. Several subsequent lines of evidence from studies designed to investigate this possibility indicate that CSL does indeed contain a sporozoite ligand for Caco-2 cells. IEF-isolated CSL bound specifically to the surface of viable Caco-2 cells and, once bound, decreased their permissiveness to infection by sporozoites. These findings suggested CSL occupancy of one or more host cell surface receptors specifically recognized during infection. Additionally, in kinetics studies CSL bound to Caco-2 cells with apparent high affinity in a dose-dependent, saturable, and self-displaceable manner. Scatchard plot curvature suggested that binding occurs with true or apparent negative cooperativity, resulting in an increasing KD as Caco-2 receptor occupancy by CSL increases (24). It is also possible that the concave upward curvature of the Scatchard plot observed could be explained by binding of more than one of the six CSL species identified in 2-D Western blots. Taken together, these findings indicate that CSL meets functional and biochemical definitions required for unequivocal designation as a ligand-containing parasite glycoprotein. Studies to determine which region(s) of CSL is involved in receptor recognition are in progress.
While (glyco)protein ligands involved in attachment have been described for other protozoans including Trypanosoma cruzi (20, 42), Entamoeba histolytica (45), and Tritrichomonas foetus (5, 46), and apicomplexan parasites closely related to C. parvum, including Toxoplasma gondii (15, 27), Plasmodium spp. (8, 16, 33), and Eimeria tenella (51), there have been relatively few reports of such molecules in C. parvum. TRAP-C1, a C. parvum sporozoite apical protein having structural and sequence similarities to the thrombospondin family of adhesion molecules and a predicted molecular mass of 76 kDa has recently been reported (47). Other investigators have described a C. parvum sporozoite surface protein having Gal-GalNAc lectin activity which may function in adhesion (21, 53). In addition to CSL, it has been proposed that at least one other high-Mr C. parvum sporozoite apical glycoprotein, designated GP900, functions as a sporozoite ligand (2). The presence of multiple, distinct high-Mr sporozoite glycoproteins in C. parvum (2, 26, 40, 41, 48, 49) is consistent with the 2-D electrophoresis findings in the present study. The relationship, if any, between CSL (40), GP900 (2), and other high-Mr C. parvum (glyco)proteins (26, 41, 48) is presently unclear, although differences are evident (35). For example, while both CSL and GP900 are contained in sporozoite and merozoite micronemes, only CSL is also contained in zoite dense granules (35, 40). In addition, while both CSL and GP900 are also exposed on the surface of zoites, only CSL has been reported to be translocated posteriorly along the sporozoite pellicle by a cytoskeletal dependent, cytochalasin-d inhibitable process (35, 40); GP900 is thought not to be bound to the sporozoite cytoskeleton (2). Collectively, these studies (2, 21, 40, 47, 53) and the present report suggest that C. parvum zoites may use more than one ligand during infection, each having functional domains which differ biochemically and in binding specificity. For example, it is possible that proteins such as TRAP-C1, which contain peptide motifs known to bind sulfated glycoconjugates, or sporozoite surface lectins which have Gal-GalNAc binding specificity, may allow localization and initial attachment to the host glycocalyx overlying intestinal mucosa. After glycocalyx binding, zoite glycoproteins such as CSL or GP900 may mediate specific attachment to enterocyte surface membrane receptors and subsequent invasion. A functional role for both carbohydrate-dependent and lectin ligands in C. parvum infection is consistent with the apparent negative cooperativity binding of CSL observed in the present study. Binding with negative cooperativity may result from steric effects related to multivalent receptors or ligands or to multiple receptor or ligand subpopulations (24).
In this report we have demonstrated that CSL has properties which are consistent with the expression of a sporozoite ligand for intestinal epithelial cells. The defined functional role of CSL makes it a rational target for passive immunization against cryptosporidiosis in immunocompromised or neonatal hosts and for active immunization in immunocompetent hosts. Ongoing molecular studies on CSL, as well as on the host cell receptor to which it binds, may provide insight into additional modalities to structurally or functionally disrupt ligand-receptor interactions involved in the pathogenesis of infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 30223 from the National Institutes of Health, Bethesda, Md., and U.S. Department of Agriculture NRICGP grant 37204-0496. Rebecca C. Langer was supported in part by funds from the Pathobiology Graduate Program at the University of Arizona.
We thank Beth A. Auerbach-Dixon for excellent technical assistance, Deborah A. Schaefer and Kathryn Huey Tubman for assistance with figure preparation, Lynn A. Joens and J. Glenn Songer (University of Arizona) for valuable discussions, John D. Dame (University of Florida, Gainesville) for T. foetus, Donald E. Burgess (Montana State University, Bozeman) for MAb 32.3B3.5, and Kuan Wang and Gustavo Gutierrez (University of Texas, Austin) for the titin and nebulin molecular weight standards.
REFERENCES
- 1.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 2.Barnes D A, Bonnin A, Huang J X, Gousset L, Wu J, Gut J, Doyle P, Dubremetz J F, Ward H, Petersen C. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol Biochem Parasitol. 1998;96:93–110. doi: 10.1016/s0166-6851(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 3.Blagburn B L, Soave R. Prophylaxis and chemotherapy. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 111–123. [Google Scholar]
- 4.Brown W C, McElwain T F, Hotzel I, Suarez C E, Palmer G H. Helper T-cell epitopes encoded by the Babesia bigemina rap-1 gene family in the constant and variant domains are conserved among parasite strains. Infect Immun. 1998;66:1561–1569. doi: 10.1128/iai.66.4.1561-1569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess D E, McDonald C M. Analysis of adhesion and cytotoxicity of Tritrichomonas foetus to mammalian cells by use of monoclonal antibodies. Infect Immun. 1992;60:4253–4259. doi: 10.1128/iai.60.10.4253-4259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo J C, Radicella J P, Charreau E H. Measurement of specific radioactivities in labeled hormones by self-displacement analysis. Biochem J. 1983;212:259–264. doi: 10.1042/bj2120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 8.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos M J, Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 9.Cerami C, Frevert U, Sinnis P, Takacs B, Nussenzweig V. Rapid clearance of malaria circumsporozoite protein (CS) by hepatocytes. J Exp Med. 1994;179:695–701. doi: 10.1084/jem.179.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane A H, Aikawa M, Jeng M, Nussenzweig R S. Antibody-induced ultrastructural changes of malarial sporozoites. J Immunol. 1976;116:859–867. [PubMed] [Google Scholar]
- 11.Diamond L S. Lumen dwelling protozoa: Entamoeba, trichomonads, and Giardia. In: Jensen J B, editor. In vitro cultivation of protozoan parasites. Boca Raton, Fla: CRC Press; 1987. pp. 65–109. [Google Scholar]
- 12.Dubremetz J F. Apical organelles (rhoptries, micronemes, dense granules) and host cell invasion by coccidia: what do we know now? Proc VIth Int Coccidiosis Conf. 1993;6:3–9. [Google Scholar]
- 13.Duke R C, Cohen J J. Morphological and biochemical assays of apoptosis. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1992. pp. 3.17.1–3.17.16. [Google Scholar]
- 14.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 1–41. [Google Scholar]
- 15.Fourmaux M N, Achbarou A, Mercereau-Puijalon O, Biderre C, Briche I, Loyens A, Odberg-Ferragut C, Camus D, Dubremetz J F. The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol Biochem Parasitol. 1996;83:201–210. doi: 10.1016/s0166-6851(96)02773-9. [DOI] [PubMed] [Google Scholar]
- 16.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparin sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillespie P G, Hudspeth A J. Chemiluminescence detection of proteins from single cells. Proc Natl Acad Sci USA. 1991;88:2563–2567. doi: 10.1073/pnas.88.6.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goding J W. Monoclonal antibodies: principles and practice. San Diego, Calif: Academic Press; 1996. Analysis of antigens recognized by monoclonal antibodies; pp. 234–326. [Google Scholar]
- 19.Heine J, Pohlenz J F L, Moon H W, Woode G N. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J Infect Dis. 1984;150:768–775. doi: 10.1093/infdis/150.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivan-Ramirez M, De Cassia-Ruiz R, Enrique-Araya J, Franco-Da Silveria J, Yoshida N. Involvement of the stage specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect Immun. 1993;61:3636–3641. doi: 10.1128/iai.61.9.3636-3641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joe A, Verdon R, Tzipori S, Keusch G T, Ward H D. Attachment of Cryptosporidium parvum sporozoites to human intestinal epithelial cells. Infect Immun. 1998;66:3429–3432. doi: 10.1128/iai.66.7.3429-3432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer R C. Ph.D. dissertation. Tucson: University of Arizona; 1998. [Google Scholar]
- 23.Langer R C, Riggs M W. Proceedings of the Fourth International Workshops on Opportunistic Protists, 13 June 1996, Tucson, Ariz. J. Eukaryot. Microbiol. 43:76S. 1996. Neutralizing monoclonal antibody protects against Cryptosporidium parvum infection by inhibiting sporozoite attachment and invasion. [DOI] [PubMed] [Google Scholar]
- 24.Lauffenburger D A, Linderman J J. Cell surface receptor/ligand binding fundamentals. In: Lauffenburger D A, Linderman J J, editors. Receptors: models for binding, trafficking and signaling. New York, N.Y: Oxford Press; 1993. pp. 9–72. [Google Scholar]
- 25.Lumb R, Smith K, O’Donoghue P J, Lanser J A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol Res. 1988;74:531–536. doi: 10.1007/BF00531630. [DOI] [PubMed] [Google Scholar]
- 26.McDonald V, McCrossan M V, Petry F. Localization of parasite antigens in Cryptosporidium parvum-infected epithelial cells using monoclonal antibodies. Parasitology. 1995;110:259–268. doi: 10.1017/s0031182000080847. [DOI] [PubMed] [Google Scholar]
- 27.Mineo J R, Kasper L H. Attachment of Toxoplasma gondii to host cells involves major surface protein, SAG-1 (P30) Exp Parasitol. 1994;79:11–20. doi: 10.1006/expr.1994.1054. [DOI] [PubMed] [Google Scholar]
- 28.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 29.Nag B, Arimilli S, Koukis B, Rhodes E, Baichwal V, Sharma S D. Intramolecular charge heterogeneity in purified major histocompatibility class II α polypeptide and β polypeptide chains. J Biol Chem. 1994;269:10061–10070. [PubMed] [Google Scholar]
- 30.Nussenzweig V, Nussenzweig R S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- 31.Otto V I, Fried R, Wiederkehr F, Hanseler E. Separation of the two most closely related isoenzymes of alkaline phosphatase by two-dimensional electrophoresis. Electrophoresis. 1995;16:1284–1288. doi: 10.1002/elps.11501601210. [DOI] [PubMed] [Google Scholar]
- 32.Perryman L E, Jasmer D P, Riggs M W, Bohnet S G, McGuire T C, Arrowood M J. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 33.Rich K A, George F W, Law J L, Martin W J. Cell adhesive motif in region II of malarial circumsporozoite protein. Science. 1990;249:1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- 34.Riggs, M. W. Unpublished data.
- 35.Riggs M W. Immunology: host response and development of passive immunotherapy and vaccines. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 129–162. [Google Scholar]
- 36.Riggs M W, Cama V A, Leary H L, Jr, Sterling C R. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect Immun. 1994;62:1927–1939. doi: 10.1128/iai.62.5.1927-1939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riggs M W, McGuire T C, Mason P H, Perryman L E. Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J Immunol. 1989;143:1340–1345. [PubMed] [Google Scholar]
- 38.Riggs M W, McNeil M R, Perryman L E, Stone A L, Scherman M S, O’Connor R M. Cryptosporidium parvum sporozoite pellicle antigen recognized by a neutralizing monoclonal antibody is a β-mannosylated glycolipid. Infect Immun. 1999;67:1317–1322. doi: 10.1128/iai.67.3.1317-1322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riggs M W, Perryman L E. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun. 1987;55:2081–2087. doi: 10.1128/iai.55.9.2081-2087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riggs M W, Stone A L, Yount P A, Langer R C, Arrowood M J, Bentley D L. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol. 1997;158:1787–1795. [PubMed] [Google Scholar]
- 41.Robert B, Antoine H, Dreze F, Coppe P, Collard A. Characterization of a high molecular weight antigen of Cryptosporidium parvum micronemes possessing epitopes that are cross-reactive with all parasitic life cycle stages. Vet Res. 1994;25:384–398. [PubMed] [Google Scholar]
- 42.Ruiz R C, Rigoni V L, Gonzalez J, Yoshida N. The 35/50 kDa surface antigen of Trypanosoma cruzi metacyclic trypomastigotes, an adhesion molecule involved in host cell invasion. Parasite Immunol. 1993;15:121–125. doi: 10.1111/j.1365-3024.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 43.Sam-Yellowe T Y. Rhoptry organelles of the apicomplexa: their role in host invasion and intracellular survival. Parasitol Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 44.Schumacher C, von Tscharner V. Practical instructions for radioactively labeled ligand-receptor binding studies. Anal Biochem. 1994;222:262–269. doi: 10.1006/abio.1994.1483. [DOI] [PubMed] [Google Scholar]
- 45.Seguin R, Mann B J, Keller K, Chadee K. Identification of the galactose-adherence lectin epitopes of Entamoeba histolytica that stimulate tumor necrosis factor-alpha production by macrophages. Proc Natl Acad Sci. 1995;92:12175–12179. doi: 10.1073/pnas.92.26.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaia C I, Voyich J, Gillis S J, Singh B N, Burgess D E. Purification and expression of the Tf190 adhesin in Tritrichomonas foetus. Infect Immun. 1998;66:1100–1105. doi: 10.1128/iai.66.3.1100-1105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spano F, Putignani L, Naitza S, Puri C, Wright S, Crisanti A. Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol Biochem Parasitol. 1998;92:147–162. doi: 10.1016/s0166-6851(97)00243-0. [DOI] [PubMed] [Google Scholar]
- 48.Tilley M, Eggleston M T, Upton S J. Multiple oral inoculations with Cryptosporidium parvum as a means of immunization for production of monoclonal antibodies. FEMS Microbiol Lett. 1993;113:235–240. doi: 10.1111/j.1574-6968.1993.tb06520.x. [DOI] [PubMed] [Google Scholar]
- 49.Tilley M, Upton S J. Biochemistry of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 163–180. [Google Scholar]
- 50.Tomley F M, Bumstead J M, Billington K J, Dunn P P J. Molecular cloning and characterization of a novel acidic microneme protein (Et mic-2) from the apicomplexan protozoan parasite, Eimeria tenella. Mol Biochem Parasitol. 1996;79:195–206. doi: 10.1016/0166-6851(96)02662-x. [DOI] [PubMed] [Google Scholar]
- 51.Tomley F M, Clark L E, Kawazoe U, Dijkema R, Kok J J. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. Mol Biochem Parasitol. 1991;49:277–288. doi: 10.1016/0166-6851(91)90071-d. [DOI] [PubMed] [Google Scholar]
- 52.Upton S J, Tilley M, Brillhart D B. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol Lett. 1994;118:233–236. doi: 10.1111/j.1574-6968.1994.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 53.Ward H D. Glycobiology of parasites: role of carbohydrate-binding proteins and their ligands in the host-parasite interaction. In: Gabius H-J, Gabius S, editors. Glycosciences. Weinheim, Germany: Chapman & Hall; 1997. pp. 399–413. [Google Scholar]
- 54.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]