Abstract
Nitisinone (NTBC) was recently approved to treat alkaptonuria (AKU), but there is no information on its impact on oxidative stress and inflammation, which are observed in AKU. Therefore, serum samples collected during the clinical studies SONIA1 (40 AKU patients) and SONIA2 (138 AKU patients) were tested for Serum Amyloid A (SAA), CRP and IL-8 by ELISA; Advanced Oxidation Protein Products (AOPP) by spectrophotometry; and protein carbonyls by Western blot. Our results show that NTBC had no significant effects on the tested markers except for a slight but statistically significant effect for NTBC, but not for the combination of time and NTBC, on SAA levels in SONIA2 patients. Notably, the majority of SONIA2 patients presented with SAA > 10 mg/L, and 30 patients in the control group (43.5%) and 40 patients (58.0%) in the NTBC-treated group showed persistently elevated SAA > 10 mg/L at each visit during SONIA2. Higher serum SAA correlated with lower quality of life and higher morbidity. Despite no quantitative differences in AOPP, the preliminary analysis of protein carbonyls highlighted patterns that deserve further investigation. Overall, our results suggest that NTBC cannot control the sub-clinical inflammation due to increased SAA observed in AKU, which is also a risk factor for developing secondary amyloidosis.
Keywords: amyloidosis, biomarkers, disease severity, inborn errors of metabolism, inflammation, oxidative stress, protein carbonyls, rare disease, serum amyloid A (SAA)
1. Introduction
Alkaptonuria (AKU) is a rare autosomal recessive metabolic disorder (MIM 203500) of phenylalanine and tyrosine catabolism causing considerable morbidity once early onset chronic osteoarthritis-like damage manifests around the third/fourth decade of life. This is due to increased circulating homogentisic acid (HGA, 2,5-dihydroxyphenylacetic acid) leading to the deposition of an ochronotic pigment onto connective tissues in the osteoarticular and cardiac compartments, which is associated with a variety of clinical manifestations [1].
The use of nitisinone (NTBC, Orfadin) was suggested in AKU because it can block the production of HGA by inhibiting the enzyme hydroxyphenylpyruvate dioxygenase (HPPD) (EC 1.13.11.27), which is upstream of the defective 1,2-homogentisate dioxygenase (HGD) found in AKU. Therefore, a 4-week dose-finding study (Suitability of Nitisinone in Alkaptonuria 1, SONIA1) [2], and a four-year, open-label, evaluator-blinded, multicentre, randomized, no-treatment controlled, parallel-group study (SONIA2) were carried out to test the efficacy and safety of NTBC in AKU [3]. Results from these studies led to the approval of the use of NTBC for the treatment of AKU by the European Commission [4].
HGD mutations have no direct effect on the variability of the AKU phenotype, and relevant intra- and interindividual variability is found in urinary HGA levels [5]; therefore, other factors besides HGA should account for the variability observed in disease severity. There are so far no approved established clinical indicators that can describe AKU severity or predict disease prognosis. Hence, we undertook this work with a twofold aim: the evaluation of a potential short- or long-term effect of NTBC in modifying inflammatory and oxidative stress markers and the first longitudinal analysis of such markers in AKU in a 4-year timeframe.
2. Materials and Methods
2.1. Samples
Analyses were carried out in serum samples obtained from two clinical studies, namely SONIA1 (n = 40) and SONIA2 (n = 138). In SONIA1, patients were randomly allocated in five groups (n = 8): untreated (control) or treated with 1, 2, 4 or 8 mg NTBC for 4 weeks [2]. In SONIA2, patients were randomly allocated to two groups (n = 69): untreated (control) or treated with 10 mg NTBC for 4 years. A total of 55 patients in the NTBC group and 53 in the control group completed SONIA2 [3]. Serum samples were collected under fasting conditions and kept at −80 °C before analysis, as agreed within the research consortium. An independent Ethics Committee at each centre approved the study. Additional details on the study design are published elsewhere [2,3].
2.2. Measurements
In SONIA1 samples, serum SAA and interleukin 8 (IL-8) were measured using a magnetic bead-based multiplex assay (kit HCYTOMAG-60K, Milliplex, Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Plates were read on MagPix (Luminex DiaSorin Corporate, Saluggia (VC) Italy) using xPONENT Software (3.1 Rev2, Luminex DiaSorin Corporate, Saluggia (VC) Italy). Test analyte concentrations were obtained using a standard curve generated by Bio-Plex Manager software (version 6.1.1, Bio-Rad Laboratories S.r.l., Segrate (MI), Italy) with a 5-parameter logistic nonlinear curve-fitting method. C-reactive protein (CRP) was measured by means of commercial ELISA (catalog KHA0031, Invitrogen-Life Technologies, Camarillo, CA, USA) according to the manufacturer’s instructions. Plates were read on a VersaMax microplate reader (Molecular Devices, San Jose, CA, USA) using Ascent software (version 2.6, Thermo Scientific, Waltham, MA, USA). Quantification of analytes was obtained against polynomial standard curves generated with appropriate standards.
In SONIA2 samples, serum SAA was measured by means of commercial ELISA (catalog KHA0011, Invitrogen-Life Technologies, Camarillo, CA, USA) according to the manufacturer’s instructions. Plates were read on a VersaMax microplate reader (Molecular Devices, San Jose, CA, USA) using Ascent software (version 2.6, Thermo Scientific, Waltham, MA, USA). Quantification of analytes was obtained against polynomial standard curves generated with the appropriate standards.
AOPP in SONIA1 and SONIA2 samples was measured according to Witko-Sarsat et al. [6] by spectrophotometry on a microplate reader (Envision, Perkin Elmer, Milano, Italy) using Envision Manager software (version1.14, Perkin Elmer, Milano, Italy) as described previously [7].
For all the measurements, blanks, standards, and samples were tested at least in duplicate. Multiple samples from the same patient were always tested in the same plate. Measurements were performed at the end of study for SONIA1, whereas for SONIA2 an interim analysis was planned at year 1 together with a final analysis at year 4 after collection of all the samples. In this work, only the end of study results are reported (i.e., baseline and 1-year SONIA2 samples were re-tested, showing good reproducibility of results).
2.3. Western Blot of Protein Carbonyls
Selected SONIA2 serum samples were submitted to the analysis of protein carbonyls. Samples were first depleted of albumin and IgG with the Aurum Serum Protein Mini Kit (Bio-Rad Laboratories S.r.l., Segrate (MI), Italy), and the protein concentration was then assessed by Bradford’s assay. To derivatize protein carbonyls, ten micrograms of proteins were first incubated in the dark in 6% (w/v) sodium dodecyl sulphate (SDS), 5% (v/v) trifluoroacetic acid (TFA), and 5 mM 2,4-dinitrophenylhydrazine (DNPH); then the buffer reaction was neutralized with 2 M Tris base containing 30% (v/v) glycerol and 2% (v/v) β-mercaptoethanol [8]. Derivatized samples were submitted to SDS-PAGE (12% polyacrylamide), and gels were transferred to nitrocellulose (NC) sheets with the semidry Novablot transblot cell (Bio-Rad Laboratories S.r.l., Segrate (MI), Italy), applying 0.85 mA/cm2 for a total time of 75 min. For the detection of protein carbonyls, NC sheets were incubated overnight at 4 °C with 1:10,000 rabbit anti-dinitrophenyl antibodies (Sigma-Aldrich, Merk Life Science S.r.l., Milan, Italy), followed by 1:7000 HRP-conjugated anti-rabbit antibodies (Sigma-Aldrich) (2 h, room temperature) before enhanced chemiluminescence (Immobilon Crescendo Western HRP substrate, Merk Life Science S.r.l., Milan, Italy). In parallel, non-derivatized serum samples were run by SDS-PAGE (12% polyacrylamide) and gels were stained with Coomassie brilliant blue. A semiquantitative analysis was then undertaken on immunoreactive bands, whose volumes were subtracted of the background and normalized against the volumes of the corresponding bands in the replica Coomassie stained gel by calculating fold-changes values (end-of-study vs. baseline). Thresholds were set at 0.5 and 2.0 for significantly reduced or increased carbonylation, respectively.
2.4. Patients’ Data
SONIA2 patient’s age, clinical and quality of life data are reported in Table 1 according to information collected at investigative sites at yearly visits by using the following validated health questionnaires (additional information in Supplementary Table S1):
Knee injury and Osteoarthritis Outcome Score (KOOS)
Health Assessment Questionnaire (HAQ)
Short Form-36 (SF-36)
Table 1.
SONIA2 patients’ demographic, clinical and quality of life data.
| Baseline | Year 4 | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Control | NTBC | P § | Control | NTBC | P § | |
| n | 69 | 69 | 58 | 55 | |||
| age | 47.59 ± 10.10 | 49.10 ± 11.39 | 0.4121 | 50.91 ± 9.778 | 52.07 ± 10.99 | 0.5554 | |
| BMI | 26.00 (24.00, 28.35) |
26.80 (24.25, 29.75) |
0.3093 |
26.10
(23.70, 28.43) |
28.50
(24.25, 31.60) |
0.0275 | |
| HAQ | visual analog scale | 48.00 (24.25, 70.75) |
48.00 (25.00, 70.75) |
0.6777 | 52.00 (27.00, 72.00) |
49.00 (26.00, 70.00) |
0.7214 |
|
disability
index |
0.9348 ± 0.6480 | 0.8739 ± 0.6434 | 0.8739 | 0.880 (0.500, 1.380) |
1.130 (0.630, 1.500) |
0.2770 | |
| SF-36 | physical | 35.60 ± 10.87 | 35.46 ± 9.237 | 0.9349 | 35.71 ± 10.38 | 35.33 ± 9.715 | 0.8442 |
| mental | 49.72 (38.12, 55.89) |
49.72 (38.12, 55.89) |
0.2064 | 42.00 (34.73, 52.49) |
44.52 (37.02, 57.27) |
0.5722 | |
| KOOS | pain | 66.67 (47.22, 84.03) |
65.28 (47.22, 90.98) |
0.8965 | 66.67 (47.92, 90.98) |
59.38 (50.00, 83.33) |
0.6242 |
| symptoms | 69.65 (49.11, 89.29) |
69.65 (50.00, 89.29) |
0.9336 | 75.00 (4732, 88.40) |
67.86 (53.57, 85.71) |
0.9715 | |
| activities of daily living | 66.18 (51.47, 91.55) |
60.29 (42.28, 93.02) |
0.5717 | 59.79 (45.96, 90.81) |
57.35 (42.65, 80.88) |
0.4988 | |
| sport | 45.00 (16.25, 80.00) |
45.00 (10.00, 78.75) |
0.7845 | 40.00 (16.25, 80.00) |
40.00 (15.00, 71.25) |
0.6486 | |
| quality of life | 53.13 (25.00, 81.25) | 50.00 (18.75, 75.00) | 0.7258 | 50.00 (25.00, 81.25) |
43.75 (25.00, 75.00) |
0.4206 | |
| cAKUSSI | 82.00 (57.50, 105.0) |
91.00 (56.00, 113.0) |
0.2285 | 95.00 (69.25, 122.5) |
97.00 (63.50, 123.8) |
0.9354 | |
| sHGA (µmol/L) | 27.30 (22.45, 32.90) |
28.00 (22.45, 35.85) |
0.4452 |
33.10
(24.28, 44.30) |
0.5100
(0.3400, 1.385) |
<0.0001 | |
| uHGA (µmol/L) | 33,742 (26,102, 44,220) |
33,291 (26,148, 42,767) |
0.8023 |
31,689
(28,244, 38,260) |
111.0
(66.00, 312.0) |
<0.0001 | |
| sTyr (µmol/L) | 64.52 ± 15.46 | 65.35 ± 15.46 | 0.7490 |
61.50
(54.75, 69.00) |
813
(692, 1013) |
<0.0001 | |
Data expressed as mean ± SD (normally distributed variables) or median (25, 75 percentiles) (non-normally distributed variables). § P calculated with unpaired t-test (normally distributed variables) or Mann-Whitney test (non-normally distributed variables). Significant differences highlighted in bold. Abbreviations. HAQ: Health Assessment Questionnaire; SF-36: Short Form-36; KOOS: Knee injury and Osteoarthritis Outcome Score; cAKUSSI: clinical AKU Severity Score Index; sHGA: serum HGA; uHGA: urinary HGA; sTyr: serum tyrosine.
Furthermore, specific items were extrapolated from a structured questionnaire named AKUSSI [9] that is specific to describe AKU: ear and eye ochronosis, patient joint and spinal pain, scintigraphy-based evaluation of osteoarticular disease of joints and spine, and overall clinical AKUSSI (cAKUSSI) score.
Serum HGA, serum tyrosine and 24-h urinary HGA were measured at Liverpool University Hospital [3].
2.5. Statistical Analysis
Raw data were stored in Excel (Microsoft 365, Microsoft corporation) and processed with GraphPad Prism 8 (version8.0.2, GraphPad Software Inc., San Diego, CA, USA); graphs were generated with GraphPad Prism 8 or Plotly [10] (Plotly Technologies Inc., Montréal, QC, Canada, available at https://plot.ly, accessed on 1 November 2022). Normal distribution was analyzed with a D’Agostino-Pearson test. Normally distributed data were expressed as mean ± standard deviation, and differences between groups were assessed using t-tests. Non- normally distributed data were expressed as median with 25 and 75 percentiles, and differences between groups were assessed using Mann–Whitney U-tests. A mixed-effects model analysis (REML) was carried out on log-transformed normalized data from repeated measurements to check for a statistically significant effect of treatment or the combination of time and treatment, followed by Dunnett’s or Sidak’s test for multiple comparisons. A Kruskal-Wallis’ test followed by Dunn’s multiple comparisons test was used to assess differences between groups, and Spearman’s test was used for correlation analyses. The statistical significance threshold was set at p ≤ 0.05.
3. Results
In this work, we investigated the effects of the drug NTBC on oxidative and inflammatory markers in alkaptonuric subjects after 4 weeks (SONIA1) and 4 years (SONIA2) of treatment. Untreated alkaptonuric subjects were in the control groups of both the studies. The measured markers were agreed upon within the research consortium and they were also based on previous results from our group [7].
3.1. SONIA1
The results obtained measuring IL-8, CRP, and SAA in SONIA1 are available in Supplementary Table S2. Since data were non normally distributed, they were log-transformed and analyzed by two-way repeated measures ANOVA corrected for multiple comparisons. We found that time, treatment with NTBC, and the combination of time and treatment had no significant effect on CRP (p = 0.0722, p = 0.729, and p = 0.5310, respectively) and SAA (p = 0.1039, p = 0.3065, and p = 0.6519, respectively). For IL-8, time (p = 0.569) and treatment (p = 0.3043) had no significant effect, whereas their interaction produced a statistically significant effect (p = 0.0194). A multiple comparison analysis (Table 2) showed an isolated a statistically significant decrease in IL-8 for patients treated with 4 mg NTBC/day compared to the untreated control (log mean control = 0.8711, log mean NTBC 4 mg = 0.3608, 95% CI [0.05383; 0.9669]) at the end of the study, which, however, is more likely due to the small size of groups or inter-individual variability, since a clear dose-response trend was missing (i.e., a higher NTBC dosage produced no statistically significant effect).
Table 2.
Analysis of the effect of NTBC on inflammatory markers measured in SONIA1. Two-way repeated measures ANOVA corrected for multiple comparisons (Dunnett’s test, adjusted p values) was carried out on log transformed, normalized data. Statistically significant values are highlighted in bold.
| Baseline | Week 4 | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Mean Diff. | 95% CI | Mean | Mean Diff. | 95% CI | ||
| log IL-8 (pg/mL) | control | 0.7384 | 0.8711 | ||||
| NTBC 1 mg | 0.8264 | −0.08796 | [−0.5445; 0.3686] | 0.7995 | 0.07169 | [−0.3849; 0.5282] | |
| NTBC 2 mg | 0.7413 | −0.002917 | [−0.4595; 0.4536] | 0.6696 | 0.2015 | [−0.2550; 0.6581] | |
| NTBC 4 mg | 0.6581 | 0.08028 | [−0.3763; 0.5368] | 0.3608 | 0.5104 | [0.05383; 0.9669] | |
| NTBC 8 mg | 0.9006 | −0.1622 | [−0.6188; 0.2943] | 0.7926 | 0.07851 | [−0.3780; 0.5351] | |
| log CRP (mg/L) | control | 0.07317 | 0.2461 | ||||
| NTBC 1 mg | 0.1300 | −0.05686 | [−0.6992; 0.5855] | 0.3718 | −0.1257 | [−0.7680; 0.5166] | |
| NTBC 2 mg | 0.1834 | −0.1102 | [−0.7525; 0.5322] | 0.2459 | 0.0001408 | [−0.6422; 0.6425] | |
| NTBC 4 mg | −0.1318 | 0.2049 | [−0.4374; 0.8473] | 0.09233 | 0.1538 | [−0.4886; 0.7961] | |
| NTBC 8 mg | 0.3177 | −0.2445 | [−0.8869; 0.3978] | 0.2368 | 0.009251 | [−0.6331; 0.6516] | |
| log SAA (mg/L) | control | 0.7569 | 0.7030 | ||||
| NTBC 1 mg | 0.8183 | −0.06140 | [−0.5853; 0.4625] | 1.007 | −0.3045 | [−0.8284; 0.2194] | |
| NTBC 2 mg | 1.014 | −0.2569 | [−0.7808; 0.2669] | 1.083 | −0.3801 | [−0.9040; 0.1437] | |
| NTBC 4 mg | 0.7530 | 0.003929 | [−0.5199; 0.5278] | 0.8957 | −0.1927 | [−0.7166; 0.3312] | |
| NTBC 8 mg | 1.053 | −0.2958 | [−0.8197; 0.2281] | 1.141 | −0.4383 | [−0.9622; 0.08554] | |
3.2. SONIA2
The results obtained measuring AOPP and SAA in SONIA2 are shown in Supplementary Table S3. Most of the patients either in the control or the NTBC-treated group presented with SAA levels above the reference threshold of 10 mg/L [11], with percentages spanning from 75.4% (control group, year 3) to 89.6% (NTBC-treated group, year 1). Notably, 30 patients (43.5%) in the control group and 40 patients (58.0%) in the NTBC-treated group showed persistently elevated SAA levels > 10 mg/L at each visit during the whole 4-year study (Supplementary Table S3).
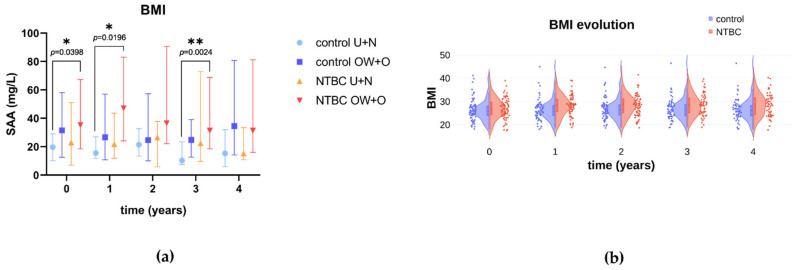
Since data were non normally distributed, they were log-transformed and analyzed by a mixed effect repeated measures analysis. We found that neither the treatment with NTBC (p = 0.8173), nor the combination of time and treatment (p = 0.4860) had statistically significant effects on AOPP levels. Further analyses corrected for multiple comparisons showed no differences in AOPP values between the control and NTBC-group at each visit (Table 3). On the contrary, we found a slight but statistically significant effect for the treatment with NTBC (p = 0.022), but not for the combination of time and treatment (p = 0.2077), on SAA levels. Further analyses corrected for multiple comparisons showed that SAA levels were significantly higher in the NTBC-treated group compared to the untreated control at year 3 (log mean control = 1.265; log mean NTBC = 1.503; 95% CI [−0.4398; −0.03570]) (Table 3). However, a clear trend was lacking since SAA levels at year 4 did not show a statistically significant difference. Inter-individual variability along with the wide dynamic range of SAA might explain this isolated finding. Additionally, a multiple comparison analysis on SAA stratified by subjects’ BMI revealed that SAA was higher in NTBC-treated overweight/obese subjects compared to underweight/normal weight untreated subjects (Figure 1a). This phenomenon reached statistical significance at baseline, year 1 and year 3 (but the trend was observed along the entire study), and might contribute to the increase in SAA in the NTBC group. In fact, NTBC had a statistically significant effect on BMI (Table 1), whose evolution shows a clear increasing trend in the NTBC group compared to the control (Figure 1b).
Table 3.
Analysis of the effect of NTBC on markers measured in SONIA2. A mixed-effects model and Sidak’s multiple comparisons test (adjusted p value) were carried out on log transformed, normalized data. Statistically significant value(s) are highlighted in bold.
| Analyte | Time (Years) | Mean (Control) | Mean (NTBC) | Mean Diff. | 95% CI |
|---|---|---|---|---|---|
| AOPP | 0 | 1.026 | 1.014 | 0.01177 | [−0.1136; 0.1372] |
| 1 | 1.024 | 0.9941 | 0.03017 | [−0.09484; 0.1552] | |
| 2 | 0.9165 | 0.945 | −0.02851 | [−0.1424; 0.08537] | |
| 3 | 0.9097 | 0.9251 | −0.0154 | [−0.1229; 0.09211] | |
| 4 | 0.9653 | 0.9704 | −0.005068 | [−0.1251; 0.1149] | |
| SAA | 0 | 1.367 | 1.448 | −0.08166 | [−0.2986; 0.1263] |
| 1 | 1.346 | 1.533 | −0.1871 | [−0.4007; 0.02657] | |
| 2 | 1.369 | 1.533 | −0.1644 | [−0.3764; 0.04761] | |
| 3 | 1.265 | 1.503 | −0.2378 | [−0.4398; −0.03570] | |
| 4 | 1.389 | 1.451 | −0.06242 | [−0.3035; 0.1786] |
Figure 1.
SAA and BMI in SONIA2. (a) SAA serum levels according to BMI in AKU subjects classified as: U + N, underweight and normal weight (BMI < 25); OW + O, overweight and obese (BMI ≥ 25). Median values are indicated with symbols, 25–75 percentiles with vertical lines. (b) Violin plot showing the evolution of BMI in control and NTBC. Dots indicate single values; internal boxes show median and 25–75 percentiles. A Kruskal-Wallis test followed by Dunn’s test for multiple comparisons was carried out. * p ≤ 0.05; ** p ≤ 0.01 (adjusted values).
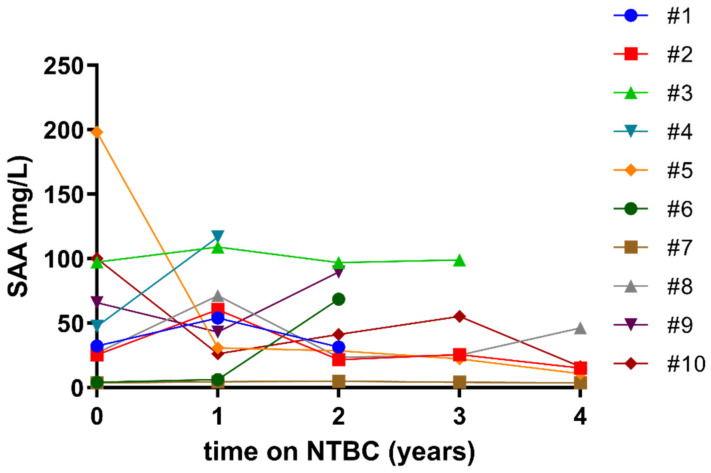
Variability in SAA levels was observed in the small subset of NTBC-treated patients (n = 10) that developed keratopathy due to the NTBC-induced hypertyrosinemia (Figure 2): their serum SAA ranged from 4.2 mg/L (case #6, baseline) to 198.2 mg/L (case #5, baseline). In six patients out of ten, serum SAA remained stable (patients #1, #2, #3, #7, #8 and #9). For cases #5 and #10, who presented with high serum SAA at baseline (198 and 100 mg/L, respectively), sustained reduction in SAA was observed at the following visits, and both of the patients were able to complete the study. Conversely, for case #4, a >2-fold-increase was observed from baseline to year 1 before the patient’s dropout; similarly, for case #6 a 100-fold increase was observed from year 1 to year 2 before patient’s dropout.
Figure 2.
SAA serum levels in SONIA2 NTBC-treated subjects who developed keratopathy due to the NTBC-induced hypertyrosinemia. The graph shows the trend of serum SAA in these ten subjects for the period they could remain in the study.
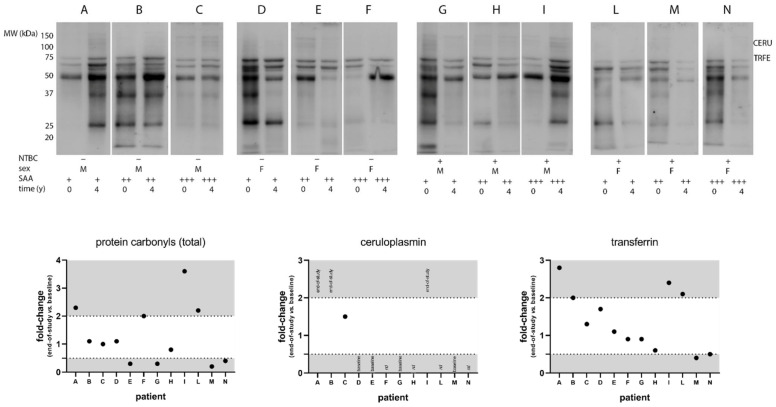
A preliminary analysis of protein carbonylation was carried out on a selected group of SONIA2 samples chosen to represent males and females (n = 6) equally, control and NTBC-treated subjects (n = 6), and SAA levels found to be low (<10 mg/L, n = 4), medium (10–50 mg/L, n = 4) or high (>50 mg/L, n = 4). Additional details on these samples can be found in Supplementary Table S4. To better visualize low abundant proteins, serum samples were previously depleted of albumin and immunoglobulins before being submitted to SDS-PAGE and Western blot. The semi-quantitative analysis suggested that despite no quantitative differences in AOPP levels in SONIA2 (Table 3), serum proteins could undergo quite variable carbonylation patterns (Figure 3). By looking at total carbonyls, increased levels were found in four cases (A, F, I, and L), reduced levels in four cases (E, G, M, and N) and no significant differences in the remaining four cases (B, C, D, and H) comparing end of study to baseline regardless of treatment or SAA levels (Supplementary Table S4). It was also interesting to note that specific protein bands whose apparent molecular weight was consistent with that of ceruloplasmin (≈115–135 kDa) and transferrin (≈75 kDa), as well as various protein bands that still need identification (data not shown), followed a similar trend. An increase in protein carbonylation was previously found when treating in vitro human serum with hypochlorous acid, which corroborates the finding that inflammation and protein oxidation are linked in several diseases [12]. Therefore, future investigations will be undertaken to shed light on this phenomenon and to evaluate if the oxidation of specific serum proteins can be used as an (early) biomarker of inflammatory conditions.
Figure 3.
Analysis of protein carbonyls in a small group of SONIA2 patients’ sera. Baseline and end-of-study samples were selected according to sex, treatment, and SAA levels [low (+) if < 10 mg/L, medium (++) if between 10 and 50 mg/L, and high (+++) if > 50 mg/L]. Representative images from Western blot (upper panel) underwent a semi-quantitative analysis (lower panel) by calculating end-of-study vs. baseline fold-change values on normalized band volumes. Thresholds were set at 0.5 and 2 (dotted lines) for significantly reduced or increased carbonylation, respectively. Total carbonyls and specific proteins whose molecular weight is compatible with that of ceruloplasmin (CERU) and transferrin (TRFE) are reported. For CERU, the labels “end-of-study” and “baseline” indicate carbonylation only at these specific time points; nd: not detected. Additional details on these samples are available in Supplementary Table S4.
3.3. Correlation analysis in SONIA2
A correlation analysis carried out on end of study measurements showed that SAA was significantly correlated to patients’ BMI but not age (Table 4). Additionally, statistically significant correlations were found between SAA and patients’ reported outcomes on QoL (Table 4), as follows:
-
i.
A positive correlation with the disability index of HAQ (haqdi), which describes the difficulty in performing some daily life activities; the higher the haqdi, the higher the difficulty.
-
ii.
Negative correlations with the physical component of SF-36 (a lower score indicates higher disability), as well as activities of daily living, sport, and quality of life scores from the KOOS questionnaire (the lower the scores, the higher the knee-related problems).
-
iii.
A positive correlation with the eye pigment score from cAKUSSI (the higher the score, the more abundant the ochronotic pigment).
Table 4.
Correlation matrix for serum SAA in SONIA 2 patients at the end of study (year 4). Spearman’s r and p values are reported, and statistically significant correlations highlighted in bold. * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
| Source | Indicator | r | P |
|---|---|---|---|
| age | −0.0278 | ns | |
| BMI | 0.369 | *** <0.0001 | |
| HAQ | hapvas | 0.136 | ns |
| haqdi | 0.360 | *** 0.0001 | |
| SF-36 | physical | −0.283 | ** 0.0030 |
| mental | 0.0320 | ns | |
| KOSS | pain | −0.170 | ns |
| symptoms | −0.180 | ns | |
| ADL | −0.247 | * 0.0102 | |
| sport | −0.231 | * 0.0175 | |
| QoL | −0.227 | * 0.0188 | |
| AKUSSI | joint pain | 0.0445 | ns |
| spinal pain | −0.089 | ns | |
| eye pigment | 0.309 | ** 0.0012 | |
| ear pigment | 0.072 | ns | |
| ostearticular disease joints | 0.194 | ns | |
| ostearticular disease spine | 0.101 | ns | |
| cAKUSSI | 0.181 | ns | |
| metabolites | sHGA | −0.061 | ns |
| u24HGA | −0.078 | ns | |
| sTyr | 0.141 | ns |
Abbreviations: ns: not significant; hapvas: HA visual analog scale; haqdi: HA disability index; ADL: activities of daily living; QoL: quality of life. sHGA: serum HGA; u24HGA: 24 h urinary HGA; sTyr: serum Tyrosine.
No significant correlations were found for SAA and serum/urinary HGA or serum tyrosine levels (Table 4).
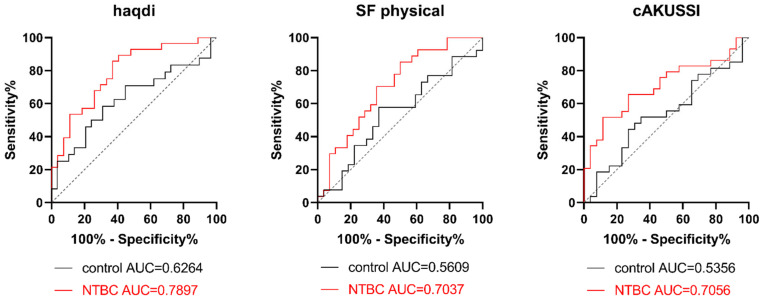
Based on these findings, SAA levels at the end of SONIA2 were generally correlated with lower QoL and higher morbidity in AKU. Furthermore, a ROC analysis showed that SAA could help differentiate SONIA2 patients according to the reported quality of life scores (haqdi and SF physical component) or disease severity (cAKUSSI), especially in the NTBC cohort (Figure 4).
Figure 4.
ROC curve of SAA for differentiating SONIA2 alkaptonuric subjects according to quality of life and disease severity in control and NTBC-treated groups. Abbreviations AUC: area under the curve; haqdi: Health Assessment Questionnaire disability index. SF physical: physical component of Short Form-36; cAKUSSI: clinical AKU Severity Score Index.
4. Discussion
AKU represents an iconic inborn error of metabolism well characterized for its manifestations but whose molecular mechanisms are still incomplete [13,14,15,16,17,18]. Notably, AKU still lacks clinically relevant biomarkers to monitor severity and progression, and it was only recently that NTBC was approved for its treatment [4]. In such a framework, we undertook this work to monitor inflammation and oxidative stress biomarkers in AKU patients during treatment with NTBC.
Confirming previous reports [7,19,20,21,22,23,24] serum SAA levels but not AOPP were significantly increased in the vast majority of SONIA2 AKU patients regardless of treatment. In nearly half of them, SAA was persistently high throughout the study, which may be a risk factor for the development of AA amyloidosis [11], as observed in other rheumatological conditions [25,26,27,28]. Even younger AKU subjects showed significantly high SAA, which could help explain why cartilage deterioration, synovial inflammation, alterations in collagen composition, and proteoglycan depletion may be found in early asymptomatic stages [29]. In this work we also investigated for the first time the short- and long-term impact of NTBC on inflammatory and oxidative markers. If no effects were generally observed in the short term (SONIA1), in the long term (SONIA2) NTBC was associated with higher SAA. NTBC-treated patients were slightly older and with a more severe disease (i.e., higher cAKUSSI) at baseline (Table 1), which might represent potential confounding factors. More importantly, NTBC-treated patients gained weight due to a protein restricted diet to reduce the risk of keratopathy [30], which supports the positive correlation between SAA and BMI that we detected in SONIA2 patients at baseline [7] and confirmed here with end-of-study results. Obesity is linked to low-grade inflammation, the adipose tissue being a major source of SAA [31,32,33,34,35], and we have previously reported low HDL levels and altered lipoprotein profiles in AKU [7,20], which suggests the existence of a persistent inflammatory and amyloidogenic stimulus sustained by SAA similar to other rheumatological conditions [36,37,38,39]. We previously raised the possibility that HGA could sustain SAA production [7], but here we showed that SAA levels were persistently increased and pathologically relevant in vivo even when the HGA stimulus was removed by NTBC. Regardless, the cause of the SAA increase and the presence of potential confounding factors such as obesity or other concomitant inflammatory conditions, assessment and pharmacological control of SAA may be suggested in AKU [11,40,41,42,43]. This is also because HGA was found to promote the aggregation of SAA even at nearly physiological concentrations in vitro [44]; therefore, amyloid formation could represent an issue even for NTBC-treated subjects.
Despite the finding of normal AOPP ranges, our analysis showed that serum proteins in AKU can undergo variable carbonylation patterns. HGA and AKU were already linked to oxidation and carbonylation of serum proteins both in vitro and in vivo [20,45]. Here we showed that carbonylation might significantly affect important anti-oxidant serum proteins such as ceruloplasmin and transferrin [46,47,48], whose consequences deserve investigation.
Eye keratopathy is a major side effect of NTBC that seems to involve other factors than increased ocular tyrosine [49,50,51,52,53]. Inflammation and SAA-related pathways are associated to retinal microvascular changes [54] and pathological corneal neo-vascularization [55]. Furthermore, a role for SAA was suggested in the association between ankylosing spondylitis and acute anterior uveitis [56]. Since we highlighted a positive and significant correlation between SAA and eye ochronotic pigmentation here, we suggest that future research investigate whether SAA could concur with increased tyrosine to produce the ocular toxicity observed in AKU in NTBC-treated patients.
Though a systemic increase in HGA is observed in AKU, only tissues undergoing loading and stresses seem to be severely affected by ochronosis. Unfortunately, the measurement of SAA in the synovial fluid of SONIA2 patients was out of the scope of the trials, hence we can only speculate about the possibility of passive diffusion of SAA from blood to synovial joints, as already suggested by others [28,57,58]. Since both systemic and local SAA production can play a role in joint inflammation and destruction by matrix metalloproteinases [58], our findings suggest that persistently elevated SAA may represent a continuous stimulus promoting joint damage. This stress would be paralleled by HGA-induced mechanical and rheological alterations of cartilage and the presence of ochronotic pigment [16] co-localizing with amyloid deposits, further triggering local oxidative stress and inflammation [22,24,29,59,60].
An important limitation of SONIA2 was the fact that patients could not be blinded since NTBC, by blocking HGA production, prevents the typical urine discoloration which is pathognomonic of the disease [3]. Hence, bias could have affected patients’ reported outcomes about joint and spinal pain or QoL [61]. Other confounding factors are the presence of concomitant diseases contributing to morbidity, the use of medications other than NTBC (which were allowed during the study) to ease the pain, and prosthesis replacement that may have occurred during the study, all of them deserving further investigations. Nevertheless, as previously shown with SONIA2 baseline data [7], SAA was significantly correlated to various QoL and disease severity scores at the end of the study as well, with patients presenting with higher SAA reporting a lower QoL and higher morbidity. The ROC curve analysis presented here also suggests that SAA levels might be able to discriminate AKU patients based on disease severity. In line with previous reports in rheumatological conditions [28,62,63], our findings might thus support the use of serum SAA as a disease activity and severity marker in AKU. Similarly, it has been recently proposed that SAA could be used as a significant marker to track inflammation in COVID-19 infected patients, helping in the evaluation of disease severity and prognosis and allowing for personalization of treatment [64,65]. Although other biomarkers of inflammation have overwhelmed the use of SAA in the clinical practice, SAA has been demonstrated to provide more information and higher sensitivity, especially when subclinical inflammation is involved [64], emphasizing its clinical utility to monitor disease activity and treatment response.
5. Conclusions
To the best of our knowledge, this work analyzes for the first time the effects of the drug NTBC on biomarkers related to inflammation, oxidative stress, and amyloidosis in a relatively large cohort of subjects with the rare disease AKU. It also provides a longitudinal analysis of such markers in AKU, so far missing, as AKU patients were monitored at regular intervals for 4 years. The findings presented here suggest that: (i) NTBC cannot control the low grade, sub-clinical, chronic inflammation found in AKU due to increased SAA; and (ii) serum SAA should be monitored in AKU, as it represents a risk factor for the development of secondary amyloidosis and may promote local damage. Furthermore, our results might support the use of SAA as a clinically relevant disease activity and severity marker in AKU. Overall, the datasets generated here along with those already stored in our AKU database [66,67,68] could be amenable to further analyses through artificial intelligence tools [66,67,68,69,70,71], helping us to better understand AKU and the analysis of genotype-phenotype correlations, possibly leading to a precision-medicine approach to AKU.
Acknowledgments
The authors thank aimAKU, Associazione Italiana Malati di Alcaptonuria (ORPHA263402) and all the patients who participated in the studies. The authors also thank Aasia Bibi for technical assistance. This work is in memory of Duccio Calamandrei, a man of science and a friend.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11223668/s1. Table S1. Health questionnaires for SONIA2 patients. Table S2: Summary of measurements in SONIA1 patients. Table S3. Summary of measurements in SONIA2 patients. Table S4. Summary of measurements in SONIA2 samples submitted to the analysis of protein carbonyls.
Author Contributions
Conceptualization, D.B., M.G. (Michela Geminiani), G.B., L.R.R. and A.S.; Methodology, D.B., E.E.P.; Formal Analysis, D.B.; Data curation, J.-B.A., J.A.G., K.-H.L.Q.S., R.I., M.S.A.-S., E.E.P., M.G. (Matthew Gornall), R.J. and L.R.R.; Resources, A.S.; Writing—Original Draft Preparation, D.B., M.G. (Michela Geminiani), D.G., B.M., R.R., G.B. and O.S.; Writing—Review & Editing, D.B., L.R.R. and A.S.; Supervision, A.S.; Project Administration, L.R.R.; Funding Acquisition, D.B. and L.R.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Independent Ethics Committee at each center as detailed in [2,3]. EC Liverpool (NRES Committee North-West – Liverpool Central). Reference number: 13/NW/0567. EC Piešťany (NURCH Ethica Committee, National Institute of Rheumatic Diseases, Ivana Krasku 4,92101 Piešťany, Slovak Republic). Reference number: 04196/0029/001/001. EC Paris (EC Ile De France II, hospital Necker 149 Rue de Sevres 75 743 Paris Cedex 15, Porte N2, 1er etage, France). Reference number: 2013-08-08.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable and qualified research request from the corresponding author. Proposals should be directed to daniela.braconi@unisi.it. Data requestors will need to sign a data access agreement.
Conflicts of Interest
The authors declare that they have no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Funding Statement
This work was supported by European Commission Seventh Framework Programme funding granted in 2012 (DevelopAKUre, project number: 304985) and University of Siena PSR 2021 F-CUR grant to D.B.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phornphutkul C., Introne W.J., Perry M.B., Bernardini I., Murphey M.D., Fitzpatrick D.L., Anderson P.D., Huizing M., Anikster Y., Gerber L.H., et al. Natural history of alkaptonuria. N. Engl. J. Med. 2002;347:2111–2121. doi: 10.1056/NEJMoa021736. [DOI] [PubMed] [Google Scholar]
- 2.Ranganath L.R., Milan A.M., Hughes A.T., Dutton J.J., Fitzgerald R., Briggs M.C., Bygott H., Psarelli E.E., Cox T.F., Gallagher J.A., et al. Suitability of nitisinone In alkaptonuria 1 (SONIA 1): An international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid. Ann. Rheum. Dis. 2016;75:362–367. doi: 10.1136/annrheumdis-2014-206033. [DOI] [PubMed] [Google Scholar]
- 3.Ranganath L.R., Psarelli E.E., Arnoux J.B., Braconi D., Briggs M., Bröijersén A., Loftus N., Bygott H., Cox T.F., Davison A.S., et al. Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): An international, multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:762–772. doi: 10.1016/S2213-8587(20)30228-X. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt L., Sehic O., Wild C. EU FP7 research funding for an orphan drug (Orfadin®) and vaccine (Hep C) development: A success and a failure? J. Pharm. Policy Pract. 2021;14:37. doi: 10.1186/s40545-021-00317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranganath L. Alkaptonuria: Current Perspectives. Appl. Clin. Genet. 2020;13:37–47. doi: 10.2147/TACG.S186773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witko-Sarsat V., Friedlander M., Capeillère-Blandin C., Nguyen-Khoa T., Nguyen A.T., Zingraff J., Jungers P., Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 7.Braconi D., Giustarini D., Marzocchi B., Peruzzi L., Margollicci M., Rossi R., Bernardini G., Millucci L., Gallagher J.A., Le Quan Sang K.H., et al. Inflammatory and oxidative stress biomarkers in alkaptonuria: Data from the DevelopAKUre project. Osteoarthr. Cartil. 2018;26:1078–1086. doi: 10.1016/j.joca.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 9.Cox T.F., Ranganath L. A quantitative assessment of alkaptonuria: Testing the reliability of two disease severity scoring systems. J. Inherit. Metab. Dis. 2011;34:1153–1162. doi: 10.1007/s10545-011-9367-8. [DOI] [PubMed] [Google Scholar]
- 10.Plotly Technologies Inc. Collaborative Data Science; Plotly Technologies Inc: Montréal, QC, Canada, 2015. [(accessed on 1 November 2022)]. Available online: https://plot.ly.
- 11.Lachmann H.J., Goodman H.J.B., Gilbertson J.A., Gallimore J.R., Sabin C.A., Gillmore J.D., Hawkins P.N. Natural History and Outcome in Systemic AA Amyloidosis. N. Engl. J. Med. 2007;356:2361–2371. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 12.Colombo G., Clerici M., Altomare A., Rusconi F., Giustarini D., Portinaro N., Garavaglia M.L., Rossi R., Dalle-Donne I., Milzani A. Thiol oxidation and di-tyrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid. J. Proteom. 2017;152:22–32. doi: 10.1016/j.jprot.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Mitri E., Millucci L., Merolle L., Bernardini G., Vaccari L., Gianoncelli A., Santucci A. A new light on Alkaptonuria: A Fourier-transform infrared microscopy (FTIRM) and low energy X-ray fluorescence (LEXRF) microscopy correlative study on a rare disease. Biochim. Biophys. Acta Gen. Subj. 2017;1861:1000–1008. doi: 10.1016/j.bbagen.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Gambassi S., Geminiani M., Thorpe S.D., Bernardini G., Millucci L., Braconi D., Orlandini M., Thompson C.L., Petricci E., Manetti F., et al. Smoothened-antagonists reverse homogentisic acid-induced alterations of Hedgehog signaling and primary cilium length in alkaptonuria. J. Cell. Physiol. 2017;232:3103–3111. doi: 10.1002/jcp.25761. [DOI] [PubMed] [Google Scholar]
- 15.Thorpe S.D., Gambassi S., Thompson C.L., Chandrakumar C., Santucci A., Knight M.M. Reduced primary cilia length and altered Arl13b expression are associated with deregulated chondrocyte Hedgehog signaling in alkaptonuria. J. Cell. Physiol. 2017;232:2407–2417. doi: 10.1002/jcp.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernardini G., Leone G., Millucci L., Consumi M., Braconi D., Spiga O., Galderisi S., Marzocchi B., Viti C., Giorgetti G., et al. Homogentisic acid induces morphological and mechanical aberration of ochronotic cartilage in alkaptonuria. J. Cell. Physiol. 2019;234:6696–6708. doi: 10.1002/jcp.27416. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher J.A., Ranganath L.R., Boyde A. Lessons from rare diseases of cartilage and bone. Curr. Opin. Pharmacol. 2015;22:107–114. doi: 10.1016/j.coph.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher J.A., Dillon J.P., Sireau N., Timmis O., Ranganath L.R. Alkaptonuria: An example of a “fundamental disease”-A rare disease with important lessons for more common disorders. Semin. Cell Dev. Biol. 2016;52:53–57. doi: 10.1016/j.semcdb.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Millucci L., Braconi D., Bernardini G., Lupetti P., Rovensky J., Ranganath L., Santucci A. Amyloidosis in alkaptonuria. J. Inherit. Metab. Dis. 2015;38:797–805. doi: 10.1007/s10545-015-9842-8. [DOI] [PubMed] [Google Scholar]
- 20.Braconi D., Bernardini G., Paffetti A., Millucci L., Geminiani M., Laschi M., Frediani B., Marzocchi B., Santucci A. Comparative proteomics in alkaptonuria provides insights into inflammation and oxidative stress. Int. J. Biochem. Cell Biol. 2016;81:271–280. doi: 10.1016/j.biocel.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Millucci L., Ghezzi L., Paccagnini E., Giorgetti G., Viti C., Braconi D., Laschi M., Geminiani M., Soldani P., Lupetti P., et al. Amyloidosis, inflammation, and oxidative stress in the heart of an alkaptonuric patient. Mediat. Inflamm. 2014;2014:258471. doi: 10.1155/2014/258471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millucci L., Spreafico A., Tinti L., Braconi D., Ghezzi L., Paccagnini E., Bernardini G., Amato L., Laschi M., Selvi E., et al. Alkaptonuria is a novel human secondary amyloidogenic disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012;1822:1682–1691. doi: 10.1016/j.bbadis.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spreafico A., Millucci L., Ghezzi L., Geminiani M., Braconi D., Amato L., Chellini F., Frediani B., Moretti E., Collodel G., et al. Antioxidants inhibit SAA formation and pro-inflammatory cytokine release in a human cell model of alkaptonuria. Rheumatology. 2013;52:1667–1673. doi: 10.1093/rheumatology/ket185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braconi D., Millucci L., Bernardini G., Santucci A. Oxidative stress and mechanisms of ochronosis in alkaptonuria. Free Radic. Biol. Med. 2015;88:70–80. doi: 10.1016/j.freeradbiomed.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Shen C., Sun X.G., Liu N., Mu Y., Hong C.C., Wei W., Zheng F. Increased serum amyloid A and its association with autoantibodies, acute phase reactants and disease activity in patients with rheumatoid arthritis. Mol. Med. Rep. 2015;11:1528–1534. doi: 10.3892/mmr.2014.2804. [DOI] [PubMed] [Google Scholar]
- 26.Connolly M., Veale D.J., Fearon U. Acute serum amyloid A regulates cytoskeletal rearrangement, cell matrix interactions and promotes cell migration in rheumatoid arthritis. Ann. Rheum. Dis. 2011;70:1296–1303. doi: 10.1136/ard.2010.142240. [DOI] [PubMed] [Google Scholar]
- 27.Vallon R., Freuler F., Desta-Tsedu N., Robeva A., Dawson J., Wenner P., Engelhardt P., Boes L., Schnyder J., Tschopp C., et al. Serum Amyloid A (apoSAA) Expression Is Up-Regulated in Rheumatoid Arthritis and Induces Transcription of Matrix Metalloproteinases. J. Immunol. 2001;166:2801–2807. doi: 10.4049/jimmunol.166.4.2801. [DOI] [PubMed] [Google Scholar]
- 28.De Seny D., Cobraiville G., Charlier E., Neuville S., Esser N., Malaise D., Malaise O., Calvo F.Q., Relic B., Malaise M.G. Acute-Phase Serum Amyloid A in Osteoarthritis: Regulatory Mechanism and Proinflammatory Properties. PLoS ONE. 2013;8:e66769. doi: 10.1371/journal.pone.0066769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millucci L., Bernardini G., Spreafico A., Orlandini M., Braconi D., Laschi M., Geminiani M., Lupetti P., Giorgetti G., Viti C., et al. Histological and Ultrastructural Characterization of Alkaptonuric Tissues. Calcif. Tissue Int. 2017;101:50–64. doi: 10.1007/s00223-017-0260-9. [DOI] [PubMed] [Google Scholar]
- 30.Olsson B., Ranganath L., Arnoux J., Imrich R., Milan A., Rudebeck M. Effects of a protein-restricted diet on body weight and serum tyrosine concentrations in patients with alkaptonuria. JIMD Rep. 2021;63:41–49. doi: 10.1002/jmd2.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R.Z., Lee M.J., Hu H., Pollin T.I., Ryan A.S., Nicklas B.J., Snitker S., Horenstein R.B., Hull K., Goldberg N.H., et al. Acute-phase serum amyloid A: An inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:0884–0894. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y., He X., Shi X., Huang C., Liu J., Zhou S., Heng C.K. Association between serum amyloid A and obesity: A meta-analysis and systematic review. Inflamm. Res. 2010;59:323–334. doi: 10.1007/s00011-010-0163-y. [DOI] [PubMed] [Google Scholar]
- 33.Gómez-Ambrosi J., Azcona C., Patiño-García A., Frühbeck G. Serum Amyloid A Concentration is Increased in Obese Children and Adolescents. J. Pediatr. 2008;153:71–75. doi: 10.1016/j.jpeds.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Poitou C., Coussieu C., Rouault C., Coupaye M., Cancello R., Bedel J.F., Gouillon M., Bouillot J.L., Oppert J.M., Basdevant A., et al. Serum amyloid A: A marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity. 2006;14:309–318. doi: 10.1038/oby.2006.40. [DOI] [PubMed] [Google Scholar]
- 35.Reddy P., Lent-Schochet D., Ramakrishnan N., McLaughlin M., Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta. 2019;496:35–44. doi: 10.1016/j.cca.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Han C.Y., Tang C., Guevara M.E., Wei H., Wietecha T., Shao B., Subramanian S., Omer M., Wang S., O’Brien K.D., et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016;126:266–281. doi: 10.1172/JCI83475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosso L.G., Lhomme M., Meroño T., Sorroche P., Catoggio L., Soriano E., Saucedo C., Malah V., Dauteuille C., Boero L., et al. Altered lipidome and antioxidative activity of small, dense HDL in normolipidemic rheumatoid arthritis: Relevance of inflammation. Atherosclerosis. 2014;237:652–660. doi: 10.1016/j.atherosclerosis.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 38.Da Fonseca L.J.S., Nunes-Souza V., Goulart M.O.F., Rabelo L.A. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxid. Med. Cell. Longev. 2019;2019:7536805. doi: 10.1155/2019/7536805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uslu A.U., Aydin B., Icagasıoğlu I.S., Balta S., Deveci K., Alkan F., Yıldız G., Sahin A. The Relationship Among the Level of Serum Amyloid A, High-Density Lipoprotein and Microalbuminuria in Patients With Familial Mediterranean Fever. J. Clin. Lab. Anal. 2016;30:1003–1008. doi: 10.1002/jcla.21971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malaviya A.N., Sharma A., Agarwal D., Kapoor S., Garg S., Sawhney S. Low-dose and high-dose methotrexate are two different drugs in practical terms. Int. J. Rheum. Dis. 2010;13:288–293. doi: 10.1111/j.1756-185X.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda T., Wada Y., Nakano M. Diagnosis and Treatment of AA Amyloidosis with Rheumatoid Arthritis: State of the Art. In: Feng D., editor. Amyloidosis. IntechOpen; London, UK: 2013. [DOI] [Google Scholar]
- 42.Nakamura T. Amyloid A amyloidosis secondary to rheumatoid arthritis: Pathophysiology and treatment. Clin. Exp. Rheumatol. 2011;29:850–857. doi: 10.5822/978-1-59726-228-6_3_WATER. [DOI] [PubMed] [Google Scholar]
- 43.Picken M.M. Modern approaches to the treatment of amyloidosis: The critical importance of early detection in surgical pathology. Adv. Anat. Pathol. 2013;20:424–439. doi: 10.1097/PAP.0b013e3182a92dc3. [DOI] [PubMed] [Google Scholar]
- 44.Braconi D., Millucci L., Bernini A., Spiga O., Lupetti P., Marzocchi B., Niccolai N., Bernardini G., Santucci A. Homogentisic acid induces aggregation and fibrillation of amyloidogenic proteins. Biochim. Biophys. Acta Gen. Subj. 2017;1861:135–146. doi: 10.1016/j.bbagen.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 45.Braconi D., Bianchini C., Bernardini G., Laschi M., Millucci L., Spreafico A., Santucci A. Redox-proteomics of the effects of homogentisic acid in an in vitro human serum model of alkaptonuric ochronosis. J. Inherit. Metab. Dis. 2011;34:1163–1176. doi: 10.1007/s10545-011-9377-6. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 47.Van Rensburg S.J., Van Zyl J., Hon D., Daniels W., Hendricks J., Potocnik F., Erasmus R. Biochemical model for inflammation of the brain: The effect of iron and transferrin on monocytes and lipid peroxidation. Metab. Brain Dis. 2004;19:97–112. doi: 10.1023/B:MEBR.0000027421.33085.8b. [DOI] [PubMed] [Google Scholar]
- 48.Schrag M., Mueller C., Zabel M., Crofton A., Kirsch W.M., Ghribi O., Squitti R., Perry G. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol. Dis. 2013;59:100–110. doi: 10.1016/j.nbd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Lock E.A., Gaskin P., Ellis M., Provan W.M., Smith L.L. Tyrosinemia produced by 2-(2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione (NTBC) in experimental animals and its relationship to corneal injury. Toxicol. Appl. Pharmacol. 2006;215:9–16. doi: 10.1016/j.taap.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 50.White A., Tchan M.C. Nitisinone-induced keratopathy in alkaptonuria: A challenging diagnosis despite clinical suspicion. JIMD Rep. 2018;40:7–9. doi: 10.1007/8904_2017_56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al Zubi K.M., Alsbou M.S., Alkhasawneh M.H., Al-Shagahin H.M. Nitisinone-induced keratopathy in alkaptonuria disease: A case report and literature review. J. Clin. Diagnostic Res. 2018;12:ND01–ND03. doi: 10.7860/JCDR/2018/35741.11629. [DOI] [Google Scholar]
- 52.Khedr M., Judd S., Briggs M.C., Hughes A.T., Milan A.M., Stewart R.M.K., Lock E.A., Gallagher J.A., Ranganath L.R. Asymptomatic Corneal Keratopathy Secondary to Hypertyrosinaemia Following Low Dose Nitisinone and a Literature Review of Tyrosine Keratopathy in Alkaptonuria. JIMD Rep. 2018;40:31. doi: 10.1007/8904_2017_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolffenbuttel B.H.R., Heiner-Fokkema M.R., van Spronsen F.J. Preventive use of nitisinone in alkaptonuria. Orphanet J. Rare Dis. 2021;16:4–9. doi: 10.1186/s13023-021-01977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stettler C., Witt N., Tapp R.J., Thom S., Allemann S., Tillin T., Stanton A., O’Brien E., Poulter N., Gallimore J.R., et al. Serum amyloid A, C-reactive protein, and retinal microvascular changes in hypertensive diabetic and nondiabetic individuals: An Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) substudy. Diabetes Care. 2009;32:1098–1100. doi: 10.2337/dc08-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren S.W., Qi X., Wang Y.Q. Serum amyloid A and pairing formyl peptide receptor 2 are expressed in corneas and involved in inflammation-mediated neovascularization. Int. J. Ophthalmol. 2014;7:187–193. doi: 10.3980/j.issn.2222-3959.2014.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai M.L., Fan S., Li Z., Yu X., Lin D., Huang X.F., Wang Y. Correlation of serum amyloid A levels, clinical manifestations, treatment, and disease activity in patients with acute anterior uveitis. Eye. 2020;34:1672. doi: 10.1038/s41433-019-0740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson C.S., Singer E.R., Piviani M., Rubio-Martinez L.M. Are serum amyloid A or D-lactate useful to diagnose synovial contamination or sepsis in horses? Vet. Rec. 2017;181:425. doi: 10.1136/vr.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connolly M., Mullan R.H., McCormick J., Matthews C., Sullivan O., Kennedy A., FitzGerald O., Poole A.R., Bresnihan B., Veale D.J., et al. Acute-phase serum amyloid A regulates tumor necrosis factor α and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012;64:1035–1045. doi: 10.1002/art.33455. [DOI] [PubMed] [Google Scholar]
- 59.Millucci L., Ghezzi L., Braconi D., Laschi M., Geminiani M., Amato L., Orlandini M., Benvenuti C., Bernardini G., Santucci A. Secondary amyloidosis in an alkaptonuric aortic valve. Int. J. Cardiol. 2014;172:e121–e123. doi: 10.1016/j.ijcard.2013.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geminiani M., Gambassi S., Millucci L., Lupetti P., Collodel G., Mazzi L., Frediani B., Braconi D., Marzocchi B., Laschi M., et al. Cytoskeleton Aberrations in Alkaptonuric Chondrocytes. J. Cell. Physiol. 2017;232:1728–1738. doi: 10.1002/jcp.25500. [DOI] [PubMed] [Google Scholar]
- 61.Orbai A.M., Bingham C.O. Patient Reported Outcomes in Rheumatoid Arthritis Clinical Trials. Curr. Rheumatol. Rep. 2015;17:501. doi: 10.1007/s11926-015-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cantarini L., Giani T., Fioravanti A., Iacoponi F., Simonini G., Pagnini I., Spreafico A., Chellini F., Galeazzi M., Cimaz R. Serum amyloid A circulating levels and disease activity in patients with juvenile idiopathic arthritis. Yonsei Med. J. 2012;53:1045–1048. doi: 10.3349/ymj.2012.53.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ally M.M., Hodkinson B., Meyer P.W.A., Musenge E., Tikly M., Anderson R. Serum matrix metalloproteinase-3 in comparison with acute phase proteins as a marker of disease activity and radiographic damage in early rheumatoid arthritis. Mediators Inflamm. 2013;2013:183653. doi: 10.1155/2013/183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorić Hosman I., Kos I., Lamot L. Serum Amyloid A in Inflammatory Rheumatic Diseases: A Compendious Review of a Renowned Biomarker. Front. Immunol. 2021;11:631299. doi: 10.3389/fimmu.2020.631299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J. Infect. 2020;80:646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cicaloni V., Spiga O., Dimitri G.M., Maiocchi R., Millucci L., Giustarini D., Bernardini G., Bernini A., Marzocchi B., Braconi D., et al. Interactive alkaptonuria database: Investigating clinical data to improve patient care in a rare disease. FASEB J. 2019;33:12696–12703. doi: 10.1096/fj.201901529R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiga O., Cicaloni V., Bernini A., Zatkova A., Santucci A. ApreciseKUre: An approach of Precision Medicine in a Rare Disease. BMC Med. Inform. Decis. Mak. 2017;17:42. doi: 10.1186/s12911-017-0438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spiga O., Cicaloni V., Fiorini C., Trezza A., Visibelli A., Millucci L., Bernardini G., Bernini A., Marzocchi B., Braconi D., et al. Machine learning application for development of a data-driven predictive model able to investigate quality of life scores in a rare disease. Orphanet J. Rare Dis. 2020;15:46. doi: 10.1186/s13023-020-1305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossi A., Giacomini G., Cicaloni V., Galderisi S., Milella M.S., Bernini A., Millucci L., Spiga O., Bianchini M., Santucci A. AKUImg: A database of cartilage images of Alkaptonuria patients. Comput. Biol. Med. 2020;122:103863. doi: 10.1016/j.compbiomed.2020.103863. [DOI] [PubMed] [Google Scholar]
- 70.Spiga O., Cicaloni V., Dimitri G.M., Pettini F., Braconi D., Bernini A., Santucci A. Machine learning application for patient stratification and phenotype/genotype investigation in a rare disease. Brief. Bioinform. 2021;22:bbaa434. doi: 10.1093/bib/bbaa434. [DOI] [PubMed] [Google Scholar]
- 71.Spiga O., Cicaloni V., Visibelli A., Davoli A., Paparo M.A., Orlandini M., Vecchi B., Santucci A. Towards a Precision Medicine Approach Based on Machine Learning for Tailoring Medical Treatment in Alkaptonuria. Int. J. Mol. Sci. 2021;22:1187. doi: 10.3390/ijms22031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on reasonable and qualified research request from the corresponding author. Proposals should be directed to daniela.braconi@unisi.it. Data requestors will need to sign a data access agreement.