Abstract
We tested the virulence in mice of Toxoplasma gondii RH strain tachyzoites containing various copies of the chloramphenicol acetyl transferase-herpes simplex virus thymidine kinase fusion sequence (CAT-HSTK). Tachyzoite isolates containing ≥five copies of the fusion sequence were not lethal to female CD-1 outbred or BALB/c inbred mice, at doses up to 106 parasites, while the parental RH strain caused 100% mortality within 2 weeks at doses as low as 10 parasites. Mice infected with CTK11, an isolate containing five copies of the fusion sequence, showed no overt symptoms of disease and were protected from lethal challenge with the parental RH strain. The CTK11 isolate showed no difference in growth rate, the rate of host cell invasion, or extracellular viability in cell culture compared with parental RH parasites, demonstrating that the CAT-HSTK fusion protein does not affect the normal viability of this isolate. B11, B11C, and D1 isolates contained one or two copies of the CAT-HSTK coding sequence, were not sensitive to thymidine in cell culture, and caused 100% mortality in CD-1 outbred mice in <12 days. A fourth isolate, D1C, contained seven copies of the CAT-HSTK fusion sequence and was sensitive to exogenous thymidine (50% inhibitory concentration = 5.5 μM). Mice infected with D1C showed no symptoms of disease and survived beyond 90 days, thus correlating increased CAT-HSTK gene copies with thymidine sensitivity in cell culture and attenuated virulence in mice. BALB/c mice containing a targeted disruption of the gamma interferon gene (gko) were also susceptible to infection with CTK11 parasites but could be rescued by administration of subcutaneous thymidine once each day for 5 or 10 days following infection. These results suggest that the attenuation of CAT-HSTK+ isolates in mice is directly due to active thymidine kinase that likely alters the pyrimidine biosynthetic pathway in these parasites.
Toxoplasma gondii is a protozoan parasite that proliferates in a wide range of vertebrate hosts. Infections in humans are generally asymptomatic, although this microorganism remains an important cause of morbidity and mortality among individuals immunosuppressed due to AIDS (27–29). Similarly, a primary infection acquired during pregnancy can also lead to serious neurological disorders of the infant and neonatal death (32). T. gondii tachyzoite isolates can be divided between strains exhibiting a dose-dependent virulence in mice (50% lethal dose [LD50] = 102 to 105 parasites) and acutely virulent strains that cause mortality at doses of ≤102 parasites (20). The rate of tachyzoite replication is one determinant of virulence (23), and fast-growing strains are often acutely virulent in mice, where rapid parasite multiplication overwhelms host immune defenses. Tachyzoite isolates that demonstrate dose-dependent virulence in mice undergo a comparatively slower rate of replication. This slow-growth phenotype has been correlated with the capacity to differentiate (5, 21) and form tissue cysts that can recrudesce and become fully proliferative if the host immune system is compromised (29).
Transmission of toxoplasmosis to humans can occur via direct contact with oocysts or the consumption of meat products contaminated with viable tissue cysts (12). Thus, the effective vaccination of domestic livestock remains pertinent to the prevention of T. gondii infections in humans. Mouse models demonstrate the induction of protective immunity to T. gondii following vaccination with purified p30 (SAG1), a tachyzoite surface protein (6, 22). However, live tachyzoite strains that confer some level of immunity against subsequent infections are more common but not without problems (9, 38, 40). Live strains have been used to vaccinate both cats (T-263) (17, 18) and sheep (S48) (7, 8, 39). Additionally, the temperature-sensitive mutant TS-4 confers protection in mice and has been proposed for vaccination in swine (13–15). A live T. gondii vaccine for livestock requires strains that are unable to form tissue cysts and whose growth is strictly limited. Current vaccine strains are blocked from cyst formation (S48 and TS-4) yet remain fully proliferative and, thus, pathogenic to some mammalian hosts (14, 26). Little research effort has been focused on strains whose growth is attenuated in animals.
We have developed CTK11, a tachyzoite strain that expresses the herpes simplex virus thymidine kinase (HSTK) (31). The alteration of pyrimidine biosynthesis renders the parasite susceptible to increased levels of thymidine in cell culture and stops parasite growth at the G1-S boundary of the cell cycle (31). In this study, we demonstrate that strains expressing thymidine kinase are avirulent in mice, and the degree of attenuation in gko mice can be further modulated by the addition of exogenous thymidine. Vaccination of mice with HSTK+ strains protects against a lethal challenge from virulent RH strain parasites.
MATERIALS AND METHODS
Parasite strains and cell culture.
CTK11 is an RH strain tachyzoite that expresses a fusion of the chloramphenicol acetyltransferase and herpes simplex virus thymidine kinase (CAT-HSTK+) coding sequences (31). pCAT-GFP is a parental RH strain tachyzoite that expresses a fusion of the chloramphenicol acetyltransferase and green fluorescent protein coding sequences (CAT-GFP) (35). The parental RH, RH/ΔHXGPRT knockout (11), and pCAT-GFP tachyzoite strains were provided by David Roos (University of Pennsylvania, Philadelphia). All tachyzoite strains were maintained by serial passage in human foreskin fibroblasts (HFF) according to standard methods (33). HFF cells were grown in Dulbecco’s modified Eagle medium (Gibco BRL, Grand Island, N.Y.) supplemented with 10% (vol/vol) newborn calf serum (Hyclone Laboratories Inc., Logan, Utah).
Chemicals.
Ganciclovir [CYMEVENE (or CYTOVENE); 9-(1,3-dihydroxy-2-propoxymethyl) guanine] was obtained from the Roche Research Centre (Hertfordshire, United Kingdom). Thymidine [1-(2-deoxy-β-d-ribofuranosyl)-5-methyluracil], mycophenolic acid, and chloramphenicol were purchased from Sigma (St. Louis, Mo.). Thymidine stock solutions to treat mice were 100 mg/ml in phosphate-buffered saline (PBS) and were sterile filtered.
Experimental infections in mice.
Female CD-1 outbred mice, BALB/c inbred mice, and BALB/c mice containing a targeted disruption of the gamma interferon (IFN-γ) gene (gko) (10) were used for experimental infections. Parasite inoculum (101 to 106 parasites) was purified from tachyzoite-infected HFF cell monolayers by filtration through a 3-μm-pore-size polycarbonate membrane (Nucleopore, Pleasanton, Calif.). At the appropriate parasite doses, 5 to 10 mice were infected by subcutaneous (s.q.) inoculation and monitored until death or for 90 days. Some IFN-γ knockout mice were also treated with exogenous thymidine following infection with parasites. Single, 20-mg (in 200 μl of PBS) doses of thymidine were administered s.q. once each 24 h for 5 or 10 days, and mice were monitored until death or at least for 90 days. To ensure that surviving mice were infected, serum was analyzed by using immunofluorescence, and titers of >1:100 were considered confirmation of a Toxoplasma infection.
Comparing parasite growth rate, invasion, and extracellular viability.
To measure replication rates, HFF monolayers infected with tachyzoites at mid-log growth (4 to 32 parasites per vacuole) were filter purified and passed into fresh HFF cultures (25-cm2 T flask) as previously described (21). Replication rate was evaluated by using the average vacuole size at 12-, 24-, 36-, and 48-h intervals (average number of tachyzoites per vacuole based on a minimum of 50 randomly chosen vacuoles, 5 to 10 fields at 40× magnification).
The ability of parasite strains to invade a new host cell was compared by using immunofluorescence. HFF cells grown in 8-well chamber slides were inoculated with ≈105 tachyzoites and allowed to invade 1 h. Slides were washed three times in PBS, fixed by using 3% paraformaldehyde in PBS, and treated 10 min in acetone at 4°C. The slides were incubated with antitachyzoite mouse antiserum for 1 h in a humid chamber, washed three times in PBS, and treated for 1 h with secondary fluorescein-conjugated anti-mouse IgG (Sigma) antibody diluted 1:64 in PBS. Slides were washed four times in PBS, mounted in a solution containing 2.5% (wt/vol) diazabicyclo(2.2.2.7)octane, and evaluated by using an Olympus BX60 epifluorescence microscope. Parasites that had invaded host cells were enumerated by using the average number per 20 randomly selected fields.
To determine relative parasite survivability outside of the host cell, tachyzoites were filter purified as described above, diluted, and allowed to incubate in normal 1% Dulbecco modified Eagle medium (DMEM) at 37°C. Aliquots containing 150 and 300 parasites were removed at various time intervals (1, 2, 4, 8, 12, and 24 h) and plated in a fresh HFF monolayer. Survivability was evaluated by percent plaque formation after 7 days compared to control parasites plated directly and without extended incubation outside the host cell.
Preparation of TK+ parasites with variable copies of the ptubCAT-HSTK+ plasmid construct.
The pdhfrCAT-HSTK+ plasmid used to transform RH strain tachyzoites and select for CTK11 has been previously described (31). A new plasmid containing the CAT-HSTK+ coding sequence under the control of the tubulin promoter sequence was constructed by first removing the tubulin promoter sequence from the plasmid ptubROPI-GFP (35) with a single HindIII-BglII restriction endonuclease digest. This sequence was used to replace the HindIII/BglII-liberated dihydrofolate reductase (DHFR) promoter from the original pdhfrCAT-HSTK+ plasmid. The new ptubCAT-HSTK plasmid contains both the HXGPRT (11)- and CAT (24)-positive selectable markers. RH/ΔHXGPRT tachyzoites were electroporated by using 50 μg of the ptubCAT-HSTK+ plasmid (31, 33), and stable transformants were selected in medium containing 25 μg of mycophenolic acid/ml as previously described (11). Mycophenolic acid is 100% lethal to RH/ΔHXGPRT parasites; thus, tachyzoites resistant to selection must contain at least one stable copy of the ptubCAT-HSTK plasmid. Drug-resistant parasites were subsequently cloned by limiting dilution in 96-well plates (33), and selected clonal isolates were then screened for increased sensitivity to ganciclovir. The growth of RH/ΔHXGPRT tachyzoites is not inhibited by ganciclovir (50% inhibitory concentration [IC50], >5 mM), but more copies of the CAT-HSTK fusion sequence will render the parasite increasingly sensitive to lower concentrations of this drug (31). Isolates with variable sensitivities to ganciclovir were subsequently cultured in 20 μM chloramphenicol to select tachyzoites in which the CAT-HSTK fusion sequence was duplicated at least once (31, 35), and the population was cloned again as described above.
To determine gene copy, genomic DNA was isolated from tachyzoites by phenol extraction as described previously (34). Genomic DNA (3 μg) was digested overnight with BglII restriction endonuclease, and the fragments were separated by agarose gel electrophoresis (0.8%) and then transferred to nitrocellulose. A PstI/NotI fragment from ptubCAT-HSTK+ plasmid corresponding to the DHFR-thymidylate synthase (DHFR-TS) 3′ untranslated sequence was 32P-labeled via nick translation and used as a probe (35). Following overnight hybridization (42°C) in a 50% formamide solution (1), nitrocellulose blots were washed three times in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% sodium dodecyl sulfate (SDS) at room temperature (10 min each) and then twice in 0.1× SSC containing 0.1% SDS at 42°C (30 min each). The CAT-HSTK gene copy number was estimated based on comparison of the hybridization signal between clones before and after selection in 20 μM chloramphenicol, and the single endogenous copy of DHFR-TS (31, 35). Hybridization signal strength was quantified by using a molecular imager FX (Bio-Rad, Hercules, Calif.) that integrates the area under the density curve for each band and expresses the result in units of optical density (OD) × millimeters.
Thymidine kinase assay.
Thymidine kinase activity was measured in tachyzoite protein extracts made by using the M-PER extraction buffer (Pierce, Rockford, Ill.), with added protease inhibitors (10 μg [each] of antipain, pepstatin A, chymostatin, and leupeptin/ml, 1 mM 1,10-phenanthroline, and 1 mM phenylmethylsulfonyl fluoride). Protein content was determined by using the Pierce bicinchoninic acid protein assay kit. Kinase activity was evaluated by the ability of extracts to phosphorylate [3H]thymidine over a 30-min period and measured via scintillation based on the binding of radioactive thymidine-monophosphate to DE81 ion-exchange paper (Whatman, Maidstone, United Kingdom).
RESULTS
Expression of thymidine kinase in tachyzoites attenuates virulence in mice.
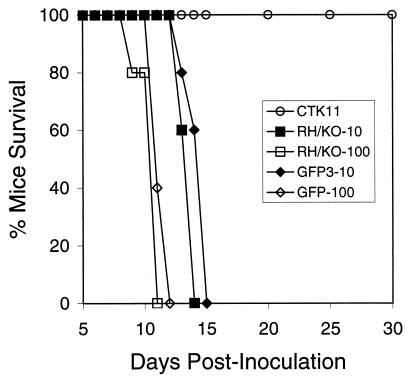
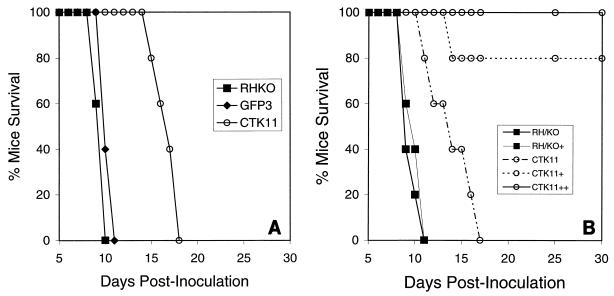
CTK11 tachyzoites express thymidine kinase and are very sensitive to low levels of exogenous thymidine (IC50 = 2.3 μM) (31). To explore the effects of thymidine kinase on the virulence of CTK11 parasites in mice, we infected female CD-1 outbred mice and compared the time-to-death with those for the parental strain, RH/ΔHXGPRT, and pCAT-GFP, an independent isolate containing multiple copies of an unrelated CAT-fusion sequence (35). CTK11 parasites were not lethal at doses up to 106 (Fig. 1), while the parental RH/ΔHXGPRT and pCAT-GFP strains (Fig. 1) caused 100% mortality within 11 days at doses as low as 10 parasites. Serum titers for surviving mice were >1:4,000 at 30 days postinfection, and mice monitored beyond 90 days showed no symptoms of disease. Forty-five days postinfection, 10 mice initially immunized with 1,000 CTK11 parasites were able to successfully survive a subsequent challenge with 200 tachyzoites of the virulent parental RH strain—a dose that causes 100% mortality in ≤12 days (21). Identical results were obtained by using female BALB/c inbred mice (data not shown).
FIG. 1.
The relative times-to-death of mice infected with CTK11, RH/ΔHXGPRT (RH/KO), and pCAT-GFP (GFP3) were compared. Female CD-1 mice (groups of five) were inoculated s.q. with various parasite doses, and mortality was monitored over 90 days. Tachyzoites of CTK11 are avirulent, and mice infected with doses as high as 106 parasites survived indefinitely, showing no symptoms of disease. RH/ΔHXGPRT and pCAT-GFP tachyzoites were virulent and caused 100% mortality within 2 weeks at parasite doses as low as 10. All surviving mice showed serum titers to T. gondii antigens of >1:4,000, indicating that they were infected.
Tachyzoites of CTK11 show no measurable defect in growth rate, invasion, or extracellular viability.
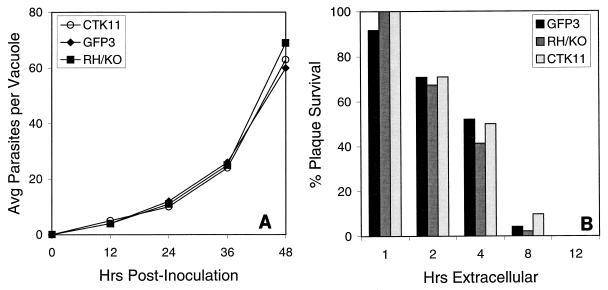
Growth rate is one determinant of tachyzoite virulence (21). Thus, a slow-growth phenotype, resulting from the insertion of one or more copies of the CAT-HSTK fusion sequence, could account for the decreased virulence of CTK11 parasites in mice. We compared the growth rates of CTK11 with the parental RH/ΔHXGPRT and pCAT-GFP strains. Tachyzoite cultures were purified and plated onto a fresh HFF cell monolayer, and parasite growth was measured by using the average number of parasites per vacuole at 12-h intervals. The parental RH/ΔHXGPRT strain (Fig. 2A), pCAT-GFP (Fig. 2A), and CTK11 parasites (Fig. 2A) all had similar growth characteristics, replicating on average once every 7 h. For each strain, growth ultimately lysed the host cell, beginning at ≈48 h, when vacuoles contained 64 to 128 parasites.
FIG. 2.
(A) The growth rate of CTK11 was similar to that of RH/ΔHXGPRT (RH/KO) and pCAT-GFP (GFP3) tachyzoites in cell culture. Each strain doubles approximately once every 7 h and begins lysis of the host cell by 48 h. Growth was determined each 12 h postinoculation by using the average number of parasites per vacuole, determined from a minimum of 50 randomly selected vacuoles. (B) The parental RH/ΔHXGPRT (gray bar), pCAT-GFP (black bar) and CTK11 (white bar) strains all remained equally viable outside the host cell. Tachyzoites were filter purified and allowed to stand in normal 1% DMEM at 37°C. At 1-, 2-, 4-, 8-, and 12-h intervals, duplicate 150 and 300 parasite aliquots were plated in separate culture flasks at 37°C, and viability was measured by plaque formation after 1 week. Following 1 h outside the host cell, ≥94% of the parasites were able to form plaques, and by 2 h, ≈75% of parasites still formed plaques. However, the viability of each strain declined steadily, until by 8 h, <10% of the parasites from each strain were able to form plaques.
Tachyzoites with a reduced capacity to invade a new host cell will not readily colonize tissue and thus may be more susceptible to the pressure of sustaining extracellular viability for an extended period. We compared the ability of each strain to invade a host cell, using immunofluorescence to evaluate the number of parasites that can invade a new cell within 1 h. CTK11 and pCAT-GFP invaded an average of 10 ± 2.21 and 10.45 ± 3.10 new cells, while the parental RH/ΔHXGPRT strain invaded 12 cells per field (±4.43). The average number of new cells invaded was not significantly different (P = 0.20), suggesting that the decreased virulence observed for CTK11 tachyzoites in mice is not the result of an invasion mechanism compromised by the presence of the CAT-HSTK fusion sequence.
To investigate the comparative viability of CTK11 for extended periods outside a host cell, tachyzoites were purified, diluted, and allowed to stand in normal 1% DMEM at 37°C. At subsequent time intervals, 150 and 300 parasite aliquots were plated in separate culture flasks at 37°C, and viability was measured by plaque formation after 1 week. The parental RH/ΔHXGPRT, pCAT-GFP, and CTK11 strains all remained equally viable after 1 h, when ≥94% of the parasites from each strain plated were able to form plaques (Fig. 2B). After 2 h, ≈75% of parasites from each strain formed plaques, but viability continued to decline, until by 8 h, <10% of all parasite strains were still able to form plaques. Thus, the attenuation of CTK11 in mice, in contrast to the sustained virulence of the parental RH/ΔHXGPRT and pCAT-GFP strains, is not likely the result of decreased tachyzoite viability outside the host cell.
Tachyzoites expressing active thymidine kinase are sensitive to exogenous thymidine.
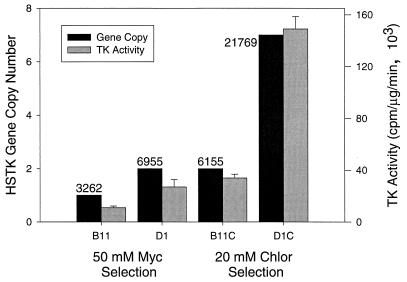
To confirm the correlation of tachyzoite thymidine kinase activity and the observed attenuation of virulence in mice, we developed new CAT-HSTK+ isolates and examined the relationship between gene copy number, thymidine kinase activity, and attenuated virulence. RH/ΔHXGPRT tachyzoites were transfected with the ptubCAT-HSTK construct, selected in 50 μM mycophenolic acid, and cloned as previously described (33). Following initial selection in mycophenolic acid, the B11 and D1 isolates contain one and two copies of the CAT-HSTK+ coding sequence, respectively (Fig. 3), and cell extracts demonstrated minimal levels of thymidine kinase activity (Fig. 3, 11,000 and 24,000 cpm/μg/min). These isolates were subsequently cultured in 20 μM chloramphenicol to select parasites containing duplicated copies of the CAT-HSTK fusion sequence (31, 35) and cloned as described above. B11C gained one additional copy of the insert (Fig. 3) and an approximately-threefold-increase in thymidine kinase activity (Fig. 3, 34,000 cpm/μg/min). We estimate that D1C has seven copies of the CAT-HSTK insert (Fig. 3) and observed a >sixfold increase in thymidine kinase activity (Fig. 3, 149,000 cpm/μg/min). B11, B11C, and D1 isolates were all sensitive to ganciclovir, but not to thymidine, in cell culture (data not shown). D1C was very sensitive to low levels of thymidine (IC50 = 5.5 μM), indicating that a higher CAT-HSTK copy number can be correlated with increased thymidine kinase activity and suggesting that a threshold level of thymidine kinase is necessary for parasite sensitivity to thymidine.
FIG. 3.
Isolates with multiple copies of the CAT-HSTK coding sequence have higher levels of thymidine kinase (TK) activity. Southern analysis and densitometry comparing a single-copy DHFR-TS gene control was used to quantify gene copy number. The hybridization signal strength was evaluated automatically using a model Quantity One (Bio-Rad) molecular imager FX to plot an intensity curve for each band in the analysis, integrate the area under the curve, and express the result in units of OD (OD × millimeters). Thymidine kinase activity was determined from lysates as described previously. B11 and D1 isolates contain one copy (black bar, 3,262 OD × mm) or two copies (black bar, 6,955 OD × mm) of the CAT-HSTK+ coding sequence following selection in mycophenolic acid (Myc). Lysates demonstrate minimal levels of thymidine kinase activity (gray bars, 11,000 and 24,000 cpm/μg/min). After selection in chloramphenicol (Chlor), B11C contains two copies of the CAT-HSTK insert (black bar, 6,155 OD × mm) and an approximately-threefold-increase in thymidine kinase activity (gray bar, 34,000 cpm/μg/min). D1C has ≈7 copies of the insert (black bar, 21,769 OD × mm) and an >sixfold-increase in thymidine kinase activity (gray bar, 149,000 cpm/μg/min).
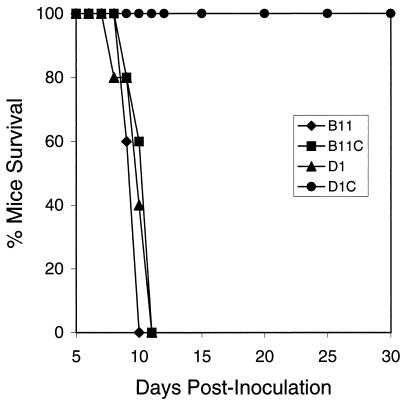
We compared the virulence of B11 and D1 isolates with the virulence of B11C and D1C in female CD-1 outbred mice (dose = 103 parasites). B11 (Fig. 4), D1 (Fig. 4), and B11C (Fig. 4) tachyzoites, which are not sensitive to thymidine in cell culture, caused 100% mortality in 12 days or less. However, mice infected with the thymidine-sensitive D1C isolate (Fig. 4) survived this infection beyond 90 days (serum titers >1:4,000). Together, these results confirm the positive relationship between thymidine sensitivity and the attenuation of virulence in mice.
FIG. 4.
Tachyzoite isolates containing multiple copies of the CAT-HSTK+ coding sequence and comparatively high levels of thymidine kinase activity are avirulent in mice. B11, B11C, and D1 isolates contain one or two gene copies and a commensurate level of thymidine kinase activity but remain virulent in female CD-1 outbred mice, causing 100% mortality within 11 days. The D1C isolate contains seven copies of the CAT-HSTK insert and a >6-fold-higher thymidine kinase activity, and mice survived infection beyond 90 days, showing no symptoms of disease. All doses were 103 parasites, and serum titers from surviving mice were >1:4,000, confirming a tachyzoite infection.
The virulence of CTK11 parasites in gko mice can be modulated by using exogenous thymidine.
IFN-γ knockout mice (gko) mice are unable to control tachyzoite infections even with avirulent strains (21). Tachyzoites of CTK11, RH/ΔHXGPRT, and pCAT-GFP strains were lethal to gko mice, and the time-to-death was correlated with the relative virulence observed in CD-1 and BALB/c mice reported above. RH/ΔHXGPRT (Fig. 5A) and pCAT-GFP (Fig. 5A) tachyzoites killed gko mice within 11 days, whereas mice inoculated with CTK11 survived an additional week. scid mice infected with CTK11 survived 23 days on average (data not shown). Although the growth rate of CTK11 in cell culture is similar to that for the parental RH and pCAT-GFP strains, the extended time-to-death suggests that these parasites grow more slowly in gko mice.
FIG. 5.
(A) Comparative mortality of gko mice infected with CTK11, RH/ΔHXGPRT (RH/KO), and pCAT-GFP (GFP3) tachyzoites. RH/ΔHXGPRT and pCAT-GFP caused mortality within 11 days, as expected; however, gko mice did not succumb to CTK11 until day 18. (B) Administration of exogenous thymidine in gko mice can further attenuate the virulence of CTK11. One hundred percent of gko mice infected with CTK11 succumbed within 18 days (A), but 80% of mice infected with CTK11 and then administered 20 mg of thymidine in PBS (s.q.) once daily for 5 days survived this infection indefinitely (CTK11+), and 100% of mice survived when treated for 10 days (CTK11++). RH/ΔHXGPRT strain tachyzoites caused 100% mortality within 11 days regardless of thymidine treatment (RH/KO+). All mice were inoculated s.q. (groups of 5 or 10) with doses of 250 parasites.
Since CTK11 replication can be progressively inhibited in culture by increasing levels of thymidine (31), we predicted that serum thymidine levels could also be modulated to affect the growth of CTK11 in mice. We infected gko mice with 250 CTK11 tachyzoites and then treated each animal with a course of thymidine administered s.q. once daily for 5 or 10 days postinfection (20 mg/200 μl of sterile PBS). Eight of 10 mice treated with thymidine for 5 days following infection survived ≥90 days (Fig. 5B), while 100% of infected mice treated for 10 days (Fig. 5B) survived. Thus, the virulence of CTK11 parasites in gko mice could be abrogated by the administration of daily thymidine injections.
DISCUSSION
We have demonstrated that tachyzoite isolates expressing sufficient thymidine kinase are avirulent in mice. Female CD-1 outbred mice were able to survive infections of 106 CTK11 tachyzoites with no overt symptoms of disease. Identical results with BALB/c inbred mice suggest that the observed attenuation is independent of host genetic factors (3, 36, 41). The pCAT-GFP isolate (34) is derived from the parental RH/ΔHXGPRT strain and contains multiple copies of the CAT coding sequence. The acute virulence of pCAT-GFP in mice (Fig. 1) suggests that the attenuation of CTK11 cannot be attributed to the CAT sequence or to CAT activity in these parasites. Additionally, there is no significant difference (P = 0.20) in a comparison of the ability of CTK11 to invade new host cells, and decreased extracellular viability does not sufficiently explain the avirulent phenotype in mice, since each strain remains equally viable outside host cells. The replication rate of CTK11 is similar to that observed for the parental RH/ΔHXGPRT and pCAT-GFP strains. Thus, it is not likely that insertion of one or more copies of the CAT-HSTK fusion sequence alone gave rise to a phenotype that attenuates the virulence of CTK11 independently of the demonstrated activity of thymidine kinase.
The specific effect of thymidine kinase activity on the virulence of CAT-HSTK+ tachyzoites in mice was demonstrated directly by using clonal isolates that differ in thymidine sensitivity. Following selection in mycophenolic acid, B11 and D1 isolates, as well as B11C, after further selection in chloramphenicol, had one or two copies of the CAT-HSTK fusion sequence and minimal thymidine kinase activity but were not sensitive to thymidine. In CD-1 outbred mice, these strains cause 100% mortality in 11 days or less (Fig. 4). D1C were extremely sensitive to thymidine (IC50 = 5.5 μM), and infected mice survive indefinitely. Thus, it appears that a threshold level of thymidine kinase activity is required to attenuate the virulence of tachyzoites in mice.
The observed time-to-death for IFN-γ knockout (gko) mice infected with CTK11 tachyzoites was 1 week longer than that for mice infected with the parental RH/ΔHXGPRT (Fig. 5A), and scid mice survived an additional 2 weeks (data not shown). The extended time-to-death is likely due to a slower rate of CTK11 replication in animals. The levels of endogenous thymidine in mouse serum are 1 to 2 μM (19), but concentrations can reach 10 μM in some tissues (25), a level sufficient to slow or inhibit CTK11 growth (IC50 = 2.3 μM) (31). In cell culture, the growth of CTK11 is unaffected by existing thymidine levels because HFF host cells are growth arrested and cultured in thymidine-free media, suggesting that cell culture contributes little to intracellular thymidine nucleotide pools. Tachyzoites lack the kinases to salvage thymidine nucleosides, but active HSTK in CAT-HSTK+ parasites serves to phosphorylate exogenous thymidine as well as other nucleoside analogues. While the mechanism of thymidine action in Toxoplasma has not been determined, the effect of high levels of thymidine on animal cells has been published previously (2, 4). Similarly, we speculate that increased dTTP levels that result from the presence of an active thymidine kinase lower the affinity of ribonucleotide reductase for the pyrimidine diphosphates CDP and TDP. This quickly depletes dCTP pools during DNA synthesis and slows or stops cell growth (16, 30). In immunocompetent mice, the slower CTK11 replication that results from endogenous thymidine is sufficient to allow immunity to control proliferation without the administration of exogenous thymidine. However, gko and scid mice are not immunocompetent, and as a result, even slow-growing parasites cause eventual mortality. Since CTK11 tachyzoites are sensitive to thymidine, raising serum thymidine levels in gko mice infected with CTK11 should ultimately stop parasite growth and prevent mortality. Indeed, gko mice were fully protected by treatment with exogenous thymidine (Fig. 5B), further demonstrating the relationship between parasite sensitivity to thymidine and avirulence in mice.
The transmission of toxoplasmosis occurs primarily via consumption of contaminated meat products, and thus vaccination of commercial livestock would likely reduce the incidence of human infection. Vaccination in animals with live tachyzoite strains has achieved some success; however, the global efficacy of this strategy is, in part, limited, since candidate strains remain fully proliferative and pathogenic to some hosts (15, 26). We have developed a strategy to attenuate tachyzoite proliferation by transfecting parasites with the HSTK gene. The observed attenuation is dramatic, and mice that routinely succumb to fewer than 10 parental RH parasites (21) survive indefinitely when they are infected with HSTK+ parasites at doses up to 106. Moreover, the degree of attenuation can be directly controlled in animals, as observed for infected gko mice following the administration of thymidine nucleosides. Even parasite isolates expressing low levels of thymidine kinase remain sensitive to ganciclovir, a commercially available drug that targets HSTK, making HSTK+ strains labile in response to effective drug treatment. Additionally, the parental RH is unable to produce competent tissue cysts (37), and there is no evidence of cyst formation among mice infected with high doses of HSTK+ parasites, suggesting that this modification does not promote cyst formation (data not shown). Infected mice show no symptoms of disease and are fully protected from subsequent challenge with a lethal dose of the parental RH strain. Thus, HSTK+ tachyzoite strains represent a potential model vaccine candidate unable to persist or continuously proliferate but capable of affording mice complete protection from challenge with a virulent strain.
ACKNOWLEDGMENTS
We thank David Roos and Boris Striepen (University of Pennsylvania) for kindly providing the ptubROPI-GFP and pCAT-GFP plasmids.
This work was supported in part by USDA CSREES NRI competitive grants 98-442 (J.R.R.) and 97-02461 (M.W.W.) and by NIH AI44600 (M.W.W.).
Footnotes
This study is a contribution from the Montana State University Agriculture Experiment Station, Bozeman.
REFERENCES
- 1.Abrahamsen M S, Clark T G, Mascolo P, Speer C A, White M W. Developmental gene expression in Eimeria bovis. Mol Biochem Parasitol. 1993;57:1–14. doi: 10.1016/0166-6851(93)90239-t. [DOI] [PubMed] [Google Scholar]
- 2.Adams R L P. The effect of endogenous pools of thymidilate on the apparent rate of DNA synthesis. Exp Cell Res. 1969;56:55–58. doi: 10.1016/0014-4827(69)90393-0. [DOI] [PubMed] [Google Scholar]
- 3.Araujo F G, Williams S M, Grumet F C, Remington J S. Strain-dependent differences in murine susceptibility to Toxoplasma. Infect Immun. 1976;13:1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjursell G, Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973;24:3904–3909. [PubMed] [Google Scholar]
- 5.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulow R, Boothroyd J. Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- 7.Buxton D, Thomson K, Maley S, Wright S, Bos H J. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet Rec. 1991;129:89–93. doi: 10.1136/vr.129.5.89. [DOI] [PubMed] [Google Scholar]
- 8.Buxton D, Thomson K M, Maley S, Wright S, Bos H J. Experimental challenge of sheep 18 months after vaccination with a live (S48) Toxoplasma gondii vaccine. Vet Rec. 1993;133:310–312. doi: 10.1136/vr.133.13.310. [DOI] [PubMed] [Google Scholar]
- 9.Choromanski L, Freyre A, Popiel R, Brown K, Grieve R, Shibley G. Safety and efficacy of modified live feline Toxoplasma gondii vaccine. Non-target effects of live vaccines. Dev Biol Stand. 1995;84:269–281. [PubMed] [Google Scholar]
- 10.Dalton D K, Pitts-Meek S, Kesha S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 11.Donald R G K, Roos D S. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol Biochem Parasitol. 1998;91:295–305. doi: 10.1016/s0166-6851(97)00210-7. [DOI] [PubMed] [Google Scholar]
- 12.Dubey J P. Long-term persistence of Toxoplasma gondii in tissues of pigs inoculated with T. gondii oocysts and effect of freezing on viability of tissue cysts in pork. Am J Vet Res. 1988;49:910–913. [PubMed] [Google Scholar]
- 13.Dubey J P, Urban J F, Davis S W. Protective immunity to toxoplasmosis in pigs vaccinated with a nonpersistent strain of Toxoplasma gondii. Am J Vet Res. 1991;52:1316–1319. [PubMed] [Google Scholar]
- 14.Dubey J P. Evaluation of the safety and efficacy of vaccination of nursing pigs with living tachyzoites of two strains of Toxoplasma gondii. J Parasitol. 1994;80:438–448. [PubMed] [Google Scholar]
- 15.Dubey J P, Baker D G, Davis S W, Urban J F, Shen S K. Persistence of immunity to toxoplasmosis in pigs vaccinated with a nonpersistent strain of Toxoplasma gondii. Am J Vet Res. 1994;55:982–987. [PubMed] [Google Scholar]
- 16.Eriksson S, Skog S, Tribukait B, Jaderberg K. Deoxyribonucleoside triphosphate metabolism and the mammalian cell cycle. Exp Cell Res. 1984;155:129–140. doi: 10.1016/0014-4827(84)90774-2. [DOI] [PubMed] [Google Scholar]
- 17.Frenkel J K, Pfefferkorn E R, Smith M S, Fishback J L. Prospective vaccine prepared from a new mutant of Toxoplasma gondii for use in cats. Am J Vet Res. 1991;52:759. [PubMed] [Google Scholar]
- 18.Freyre A, Choromanski L, Fishback J L, Popiel I. Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J Parasitol. 1993;79:716–719. [PubMed] [Google Scholar]
- 19.Hill D L, Noker P E, Duncan G F, El Dareer S M. Disposition of thymidine administered as large doses to rats and mice. Can Treat Rep. 1980;65:495–499. [PubMed] [Google Scholar]
- 20.Howe D K, Summers B C, Sibley L D. Acute virulence in mice is associated with markers on chromosome VIII in Toxoplasma gondii. Infect Immun. 1996;64:5193–5198. doi: 10.1128/iai.64.12.5193-5198.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerome M E, Radke J R, Bohn W, Roos D S, White M W. Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infect Immun. 1998;66:4838–4844. doi: 10.1128/iai.66.10.4838-4844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn I A, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 23.Kaufman H E, Remington J S, Jacob L. Toxoplasmosis: the nature of virulence. Am J Opthalmol. 1958;46:255–261. doi: 10.1016/0002-9394(58)90805-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim K, Soldati D, Boothroyd J C. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993;262:911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- 25.Lee D J, Prensky W, Krause G, Hughes W L. Blood thymidine level and iododeoxyuridine incorporation and reutilization in DNA in mice given long-acting thymidine pellets. Can Res. 1976;36:4577–4583. [PubMed] [Google Scholar]
- 26.Lindsay D S, Blagburn B L, Dubey J P. Safety and results of challenge of weaned pigs given a temperature-sensitive mutant of Toxoplasma gondii. J Parasitol. 1993;79:71–76. [PubMed] [Google Scholar]
- 27.Luft B J, Brooks R G, Conley F K, McCabe R E, Remington J S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- 28.Luft B J, Remington J S. AIDS commentary: toxoplasmic encephalitis. J Infect Dis. 1988;157:1. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Luft B J, Castro K G. An overview of the problem of toxoplasmosis and pneumocystosis in AIDS in the USA: implications for future therapeutic trials. Eur J Clin Microbiol Infect Dis. 1992;10:178. doi: 10.1007/BF01964455. [DOI] [PubMed] [Google Scholar]
- 30.Mortensen B T, Hartmann N R, Christensen I J, Larsen J K, Kristensen T, Wieslander S B, Nissen N I. Synchronization of the human promyelocytic cell line HL 60 by thymidine. Cell Tissue Kinet. 1986;19:351–364. doi: 10.1111/j.1365-2184.1986.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 31.Radke J R, White M W. A cell cycle model for the tachyzoite of Toxoplasma gondii using the herpes simplex virus thymidine kinase. Mol Biochem Parasitol. 1998;94:237–247. doi: 10.1016/s0166-6851(98)00074-7. [DOI] [PubMed] [Google Scholar]
- 32.Remington J S, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious disease of the fetus and newborn infant. 3rd ed. Philadelphia, Pa: W. B. Saunders Co.; 1990. pp. 89–195. [Google Scholar]
- 33.Roos D S, Donald R G K, Morrissette N S, Moulton A L C. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1995;45:25–61. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 35.Striepen B, He C Y, Natrajt M, Soldati D, Roos D S. Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92:325–338. doi: 10.1016/s0166-6851(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Orellana M A, Wong S Y, Copnley F K, Remington J S. Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect Immun. 1993;61:2284–2288. doi: 10.1128/iai.61.6.2284-2288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villard O, Candolfi E, Ferguson D J P, Marcellin L, Kien T. Loss of oral infectivity of tissue cysts of Toxoplasma gondii RH strain to outbred swiss webster mice. Int J Parasitol. 1997;27:1555–1559. doi: 10.1016/s0020-7519(97)00144-6. [DOI] [PubMed] [Google Scholar]
- 38.Waldeland H, Frenkel J K. Live and killed vaccines against toxoplasmosis in mice. J Parasitol. 1983;69:60. [PubMed] [Google Scholar]
- 39.Wastling J M, Harkins D, Maley S, Innes E, Panton W, Thomson K, Buxton D. Kinetics of the local and systemic antibody response to primary and secondary infection with S48 Toxoplasma gondii in sheep. J Comp Pathol. 1995;112:53–62. doi: 10.1016/s0021-9975(05)80089-1. [DOI] [PubMed] [Google Scholar]
- 40.Wilkins M F, O’Connel E, TePunga W A. Toxoplasmosis in sheep. III. Further evaluation of the ability of a live Toxoplasma gondii vaccine to prevent lamb losses and reduce congenital infection following experimental oral challenge. N Z Vet J. 1988;36:86–89. doi: 10.1080/00480169.1988.35489. [DOI] [PubMed] [Google Scholar]
- 41.Williams D M, Grumet F C, Remington J S. Genetic control of murine resistance to Toxoplasma gondii. Infect Immun. 1978;19:416–420. doi: 10.1128/iai.19.2.416-420.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]