Abstract
Simple Summary
Immune checkpoint inhibitors (ICIs) have made an indelible mark on metastatic colorectal cancer patients with microsatellite instability-high (MSI-H) instead of microsatellite stability (MSS) tumors. Clinical studies have explored the introduction of ICIs into treatment for locally advanced rectal cancer patients with MSS; however, the outcomes are still limited. Different studies have provided valuable information for understanding the expression and clinical significance of PD-1 and PD-L1 during neoadjuvant chemoradiotherapy (nCRT). However, the value of CTLA-4 and CD86 after nCRT has not been fully studied. We found that CD86 expression was significantly lower in the nCRT-treated group rather than CTLA-4. Improved overall survival in patients with lower CD86 expression could only be observed in patients with low rather than high t/sCD8+ cell densities. Our findings reveal the relationship between the immunosuppressive microenvironment and the CTLA-4/CD86 pathway after nCRT.
Abstract
The synergistic effect of combining immune checkpoint inhibitors (ICIs) with neoadjuvant chemo(radio)therapy (nCRT) in colorectal cancer is still limited. We aimed to understand the impact of nCRT on the tumor microenvironment and to explore favorable immune markers of this combination. Herein, we investigated the expression of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), CD86, CD4, and CD8 after nCRT and its association with clinicopathological characteristics. Immunostaining of immune-related molecules was performed in 255 surgically resected specimens from rectal cancer patients treated with nCRT. CD4 and CD8 expression on the tumor (tCD4/CD8), stroma (sCD4/CD8), and invasive front (iCD4/CD8) was evaluated. The expression levels of immune-related molecules were significantly lower in the nCRT-treated group, except for CTLA-4 and sCD8. However, patients with higher sCD8+ cell density and CTLA-4 expression had better progression-free survival (PFS) and distant metastasis-free survival (DMFS). In addition, higher CD86 expression was associated with poorer overall survival (OS). Higher CTLA-4 expression was associated with higher tCD8+ cell density, whereas CD86 expression was correlated with the cell density of t/sCD8. Prognostic analysis confirmed that the relationships between CTLA-4 and DMFS as well as CD86 and OS were significantly correlated in low rather than high CD8+ cell density. Further the combination of CD8+ cell density and CD86 expression was shown to be an independent prognostic factor of OS, whereas the combination of CTLA-4 was not for DMFS. Together, these results demonstrate significant correlations between CD86 expression and t/sCD8+ cell density in rectal cancer after nCRT and could potentially have clinical implications for combining ICIs and nCRT.
Keywords: immune checkpoint molecules, tumor-infiltrating lymphocytes, neoadjuvant chemo(radio)therapy, immunotherapy, rectal cancer
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, and patients with metastatic CRC (mCRC) show a poor prognosis, with a five-year survival lower than 20% [1]. Immune checkpoint inhibitors (ICIs) have been approved to treat mCRC patients with mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) tumors due to their long-term durable remission [2]. However, patients with mismatch repair-proficient (pMMR) or microsatellite stability (MSS) tumors constitute approximately 85% of CRC patients and might not benefit from immunotherapy [3,4]. Neoadjuvant chemo(radio)therapy (nCRT/nCT) has been established as the standard treatment for locally advanced rectal cancer (LARC) and plays a crucial role in pathological downstaging and achieving a higher pathologic response rate [5]. Meanwhile, several preclinical data demonstrate that chemotherapy or radiotherapy might generate an increasing neo-antigen repertoire for T cell priming, which could mediate immunostimulatory effects [6,7]. Considering the rising interest in immunotherapeutic strategies after nCRT/nCT, exploring and evaluating the expression of checkpoint-related molecules after neoadjuvant therapies could provide proof of concept for nCRT/nCT in combination with ICIs for treating LARC patients with pMMR/MSS tumors.
ICIs target programmed cell death-1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) on T cells or their ligands, such as programmed cell death 1 ligand-1 (PD-L1) on tumor cells to enhance antitumor T cell activity [8]. In recent studies, PD-L1 expression in rectal cancer (RC) has been shown to increase or decrease during nCRT, with the prognostic values of PD-L1 expression before or after nCRT being dynamic [9,10,11]. Moreover, CD8+ cell densities show different prognostic patterns according to the expression level of PD-L1 [12]. It has been reported that the expression of CTLA-4 is relatively stable after nCRT and has no relationship with OS and DFS in RC [13]. CD86, one of the ligands of CTLA-4, is associated with a higher risk of relapse in patients with chronic myeloid leukemia after discontinuing a tyrosine kinase inhibitor [14]. However, the expression pattern, correlation with tumor-infiltrating lymphocytes, and prognostic value of CTLA-4 and CD86 in RC after nCRT/nCT are yet to be further elucidated.
CTLA-4, also called CD152, is expressed on the surface of CD4+ and CD8+ T lymphocytes and limits their activation by competing with CD28 for binding to its ligands, CD80/CD86 [15]. While these ligands can both interact with CTLA-4 and CD28, they are only approximately 25% identical in sequence. In recent years, many studies have explored the differences between CD80 and CD86, yet the role of these ligands in T cell regulation is still a paradox. It has been reported that CD80 is the primary ligand mediating CTLA-4 localization, and that CD86 is mainly responsible for CD28 accumulation at the synapse level [16]. On the contrary, in vivo experiments have verified that CTLA-4 can deplete CD80 and CD86 via trogocytosis [17]. However, it is clear that the expression of CD86 is strongly upregulated on dendritic cells by lipopolysaccharide and is generally greater than that of CD80, while CD80 is expressed only after the activation of antigen-presenting cells and temporally after CD86 [18].
Herein, we aimed to detect the expression of CTLA-4, CD86, CD4, and CD8 in surgical specimens from RC patients after nCRT/nCT, and to classify CD4+ and CD8+ cells into stroma, tumor, and invasive categories according to their location. Subsequently, the relationship between these variables and clinicopathological characteristics was evaluated, including tumor regression grade (TRG) and neoadjuvant therapy methods. We analyzed these variables alongside their clinical outcomes. Furthermore, prognosis focused on CTLA-4 and CD86 expression was analyzed according to the status of CD8+ cell density.
2. Materials and Methods
2.1. Patients and Treatment
Patients with LARC who underwent nCRT following surgical resection at the Sixth Affiliated Hospital of Sun Yat-sen University (SYSU) from 2010 through 2015 were enrolled in the study, and 255 post-nCRT/nCT surgical tissues were collected. All patients signed informed consent, and this study was approved by the Clinical Ethics Review Committee at the Sixth Affiliated Hospital of SYSU. Clinicopathological parameters and follow-up information were retrieved from the Follow-up Database of the Sixth Affiliated Hospital of SYSU. Primary endpoints used in this study included the following: overall survival (OS) was defined as the duration from first diagnosis to death from any cause or last follow-up; progression free survival (PFS) was defined as the duration from total mesorectal excision (TME) surgery to first recurrence or metastasis; distant metastasis free survival (DMFS) was defined as the duration from TME surgery to the first distant relapse (recurrence outside the pelvis).
One hundred and forty-nine patients have been treated with nCRT. For neoadjuvant radiotherapy, the gross tumor volume was treated with 50 Gy in 25 fractions. These patients also received neoadjuvant induction (pre-radiotherapy), as well as concurrent (during treatment) and/or consolidation (post-radiotherapy) chemotherapy according to the LARC guidelines. One hundred and six patients have been treated with nCT. nCT consisted of a fluoropyrimidine-based regimen, involving one of the following: Folinic acid, fluorouracil, and oxaliplatin (FOLFOX); capecitabine and oxaliplatin (CAPOX); capecitabine (Xeloda); folinic acid and fluorouracil (DeGramont). The majority of patients in this cohort also received a fluoropyrimidine-based adjuvant chemotherapy regimen. Total neoadjuvant therapy (TNT) in this study was defined as receiving neoadjuvant radiotherapy, eight or more cycles of nCT, and no adjuvant chemotherapy [19]. The clinical response was assessed four to eight weeks after the completion of chemoradiation according to TME principles.
2.2. Grading Standard and Evaluation Method for TRG
The TRG of a primary tumor after preoperative nCRT was evaluated on hematoxylin and eosin-stained slides according to the seventh edition of the American Joint Committee on Cancer-Tumor Regression Grade (AJCC-TRG) [20]: TRG 0—no residual tumor cells, TRG 1—single cell or small group of cells, TRG 2—residual cancer with a desmoplastic response, and TRG 3—minimal evidence of tumor response. Two experienced pathology specialists independently reviewed the tissue sections and evaluated the TRG. When the evaluation results were inconsistent, both discussed and reviewed the sections together and provided the final evaluation. In this study, TRG 0—1 was categorized as the good response group and TRG 2—3 as the poor response group [10,11,13].
2.3. Immunohistochemistry (IHC)
The post-treatment surgically resected specimens of all the patients were subjected to an IHC assay. One thousand and twenty sections were deparaffinized in xylene and rehydrated through a series of ethanol solutions, and then the endogenous peroxidase was blocked with a 1% hydrogen peroxide solution. Antigen retrieval was induced with a microwave oven. The sections were incubated with anti-CTLA-4 (1:200, ab19792, Abcam, Cambridge, MA, USA), anti-CD86 (1:200, ab269587, Abcam), anti-CD8 (without dilution, ZA-0508, Zhongshan Golden Bridge Biotechnology, Beijing, China) and anti-CD4 (without dilution, ZA-0519, Zhongshan Golden Bridge Biotechnology) at 4 °C overnight. After washing with PBS, the sections were incubated with secondary antibodies (without dilution, MP-7401/4702, Vectorlabs, Burlingame, CA, USA) at room temperature for 30 min. Immunostaining was performed using a 3,3′-diaminobenzidine (DAB) kit (ZLI-9018, Zhongshan Golden Bridge Biotechnology), which was visualized as a brown-colored precipitate at the antigen site. The sections were then counterstained with hematoxylin, followed by dehydration and mounting. The sections were visualized under a microscope at a 40× magnification (Olympus, DP 27, Tokyo, Japan).
2.4. Quantification of the Expression of Immune-Related Molecules
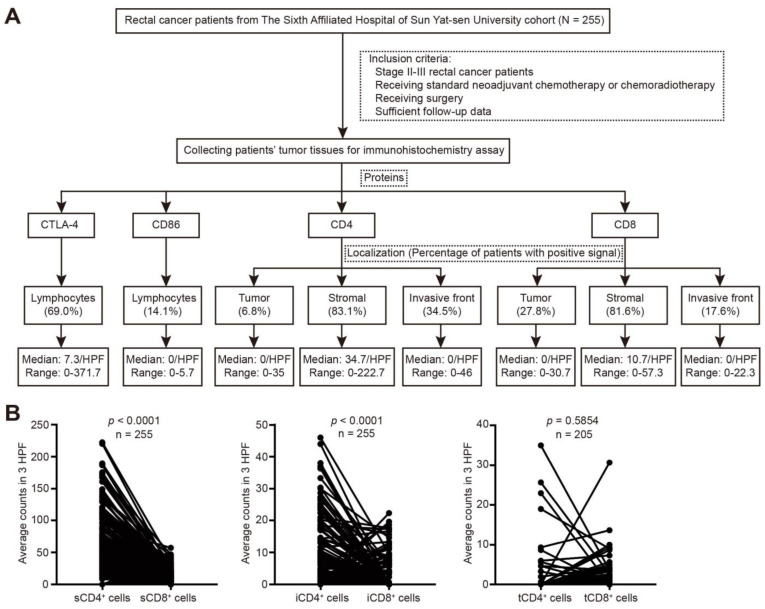
The results were assessed by two pathologists who were blinded to the clinical outcomes. CTLA-4 (Figure 1A) and CD86 (Figure 1B) expressed on the immune cells were counted. The expression levels of CD4 and CD8 were evaluated according to the location of the tumors, and then divided into tCD4+ and tCD8+ cells (lymphocytes on the tumor; Figure 1 C,D), sCD4+ and sCD8+ cells (lymphocytes on the stroma; Figure 1E,F) and iCD4+ and iCD8+ cells (lymphocytes on the invasion front; Figure 1G,H). Only s/iCD4+ and s/iCD8+ cells were counted according to the fibrotic adjacent area in case pathological complete response was evaluated. All the sections were evaluated according to the number of positively stained cells in three randomly selected high-power fields (HPFs) and then averaged. The median counts were set as the thresholds to subdivide the patients.
Figure 1.
Immunohistochemical staining of immune-related molecules. (A) CTLA-4+ cells were detected on the stroma and tumor. (B) The expression of CD86 was detected on the lymphocytes. (C–H) CD8 and CD4 positive cells were divided into tCD8+/tCD4+ cells (lymphocytes on the tumors, (C,D)), sCD8+/sCD4+ cells (lymphocytes on the stroma where the curve labeled, (E,F)), and iCD8+/iCD4+ cells (lymphocytes on the invasive front, (G,H)).
2.5. Statistical Analysis
The association between the CTLA-4+, CD86+, CD8+, and CD4+ cells and the patients’ clinicopathological characteristics, therapy method, and treatment response were evaluated using the chi-square test. The Kaplan-Meier curve with the log-rank test were used for survival analysis. Univariate and multivariate analyses were performed using Cox proportional hazard regression via SPSS 25.0 (IBM, Chicago, IL, USA). Forest plots and assumptions for the Cox PH models were performed using the R Survival (v3.4–0) and Survminer (v0.4.9) packages. Differences in CD4+ and CD8+ cell densities were compared with the paired t test using GraphPad Prism 7.0. All p values less than 0.05 were considered statistically significant.
3. Results
3.1. Patient Characteristics
After applying the inclusion criteria, 255 patients from the Sixth Affiliated Hospital of SYSU cohort were included. The clinicopathological characteristics of the patients are described in Table 1. In brief, there were 178 males (69.8%) and 77 females (30.2%) with a median age of 55 (22–77) years. As for the therapy method, nCRT was given in 149 patients (58.4%), whereas 106 patients (41.6%) received nCT. After treatment, 201 patients were in the N0 stage (78.8% vs. 22.4%) and 124 patients were of T0–T2 stage (48.6% vs. 0%), and 131 patients (51.4%) were categorized as having a good response.
Table 1.
Patient characteristics.
| Characteristics | Number of Patients |
|---|---|
| Gender | |
| Male | 178 (69.8) |
| Female | 77 (30.2) |
| Age | |
| ≤55 | 128 (50.2) |
| >55 | 127 (49.8) |
| cT stage | |
| cT3 | 203 (79.6) |
| cT4 | 52 (20.4) |
| cN stage | |
| cN0 | 57 (22.4) |
| cN1-2 | 198 (77.6) |
| Therapy method | |
| nCRT | 149 (58.4) |
| nCT | 106 (41.6) |
| ypT | |
| ypT0-2 | 124 (48.6) |
| ypT3-4 | 131 (51.4) |
| ypN | |
| ypN0 | 201 (78.8) |
| ypN1- | 54 (21.2) |
| Tumor Regression Grade | |
| TRG 0 | 50 (19.6) |
| TRG 1 | 81 (31.8) |
| TRG 2 | 82 (32.2) |
| TRG 3 | 42 (16.5) |
TRG, tumor regression grade; nCRT, neoadjuvant chemoradiotherapy; nCT, neoadjuvant chemotherapy.
3.2. Expression of CTLA-4, CD86, CD4, and CD8 in Rectal Cancer after nCRT
The expression of immune-related molecules in rectal cancer patients was quantified (Figure 2A). CTLA-4 expression was detected in 176 of the 255 patients (69.0%), and the median counts were 7.3/HPF (range = 0–371.7), while CD86 was only present in 36 of the 255 patients (14.1%) with a median count of 0. CD4 and CD8 were mainly detected in the stroma (34.7/HPF and 10.7/HPF, respectively). The percentage of patients with tCD4+ cells was lower than that with tCD8+ cells (6.8% vs. 27.8%), and for the invasive front, the ratio of patients with iCD4+ cells was higher than that of iCD8+ cells (34.5% vs. 17.6%). In addition, CD4 expression was higher than CD8 expression at the stroma and invasive front (p < 0.001), whereas the number of tCD4+ cells was similar to that of tCD8+ cells (p = 0.5854; Figure 2B).
Figure 2.
Expression of CTLA-4, CD86, CD4, and CD8 in rectal cancer after nCRT. (A) Schematics showing the workflow for detecting and quantifying the expression of immune-related molecules. (B) Paired t test was performed between s/t/iCD4+ and s/t/iCD8+ cells.
3.3. Association between Expression of Immune-Related Molecules and Clinicopathologic Characteristics
The relationship between the expression level of immune-related molecules and clinicopathological characteristics was explored. There were no significant differences between the expression of immune-related molecules and ypT or ypN (all p > 0.05), expect that high tCD8+ cell density was correlated with low ypN grade (p = 0.032; Supplementary Table S1). CD86 expression, t/s/iCD4+, and t/iCD8+ cell densities were significantly lower in the nCRT-treated group (all p < 0.05), while CTLA-4 expression and sCD8+ cell density were not (both p > 0.05; Table 2). Those patients treated with nCRT were more likely to achieve a better response compared to the nCT-treated group (p < 0.001; Table 2). In addition, similar associations were observed between the expression of the molecules and TRG categories (Table 2). However, only s/iCD8+ cell densities were correlated with TRG categories in patients treated with nCRT (p = 0.033 and 0.005, respectively; Supplementary Table S2).
Table 2.
Association of immune-related molecules with therapy method and response.
| Variables | Therapy Method | p | Tumor Regression Grade | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| nCT | nCRT | TRG 0 | TRG 1 | TRG 2 | TRG 3 | ||||
| CTLA-4+ cells | Low | 54 (50.9) | 75 (50.3) | 0.924 | 26 (52.0) | 45 (55.6) | 42 (51.2) | 16 (38.1) | 0.324 |
| High | 52 (49.1) | 74 (49.7) | 24 (48.0) | 36 (44.4) | 40 (48.8) | 26 (61.9) | |||
| CD86+ cells | Low | 85 (80.2) | 134 (89.9) | 0.028 * | 47 (94.0) | 72 (88.9) | 67 (81.7) | 33 (78.6) | 0.096 |
| High | 21 (19.8) | 15 (10.1) | 3 (6.0) | 9 (11.1) | 15 (18.3) | 15 (21.4) | |||
| tCD4+ cells | Low | 85 (88.5) | 106 (97.2) | 0.014 * | 0 | 79 (97.5) | 77 (93.9) | 35 (83.3) | 0.012 * |
| High | 11 (11.5) | 3 (2.8) | 0 | 2 (2.5) | 5 (6.1) | 7 (16.7) | |||
| NA | 10 | 40 | 50 | 0 | 0 | 0 | |||
| sCD4+ cells | Low | 41 (38.7) | 85 (57.0) | 0.004 * | 31 (62.0) | 45 (55.6) | 37 (45.1) | 13 (31.0) | 0.013 * |
| High | 65 (61.3) | 64 (43.0) | 19 (38.0) | 36 (44.4) | 45 (54.9) | 29 (69.0) | |||
| iCD4+ cells | Low | 52 (49.1) | 115 (77.2) | 0.000 * | 44 (88.0) | 61 (75.3) | 44 (53.7) | 18 (42.9) | 0.000 * |
| High | 54 (50.9) | 34 (22.8) | 6 (12.0) | 20 (24.7) | 38 (46.3) | 24 (57.1) | |||
| tCD8+ cells | Low | 58 (60.4) | 90 (82.6) | 0.000 * | 0 | 64 (79.0) | 58 (70.7) | 26 (61.9) | 0.124 |
| High | 38 (39.6) | 19 (17.4) | 0 | 17 (21.0) | 24 (29.3) | 16 (38.1) | |||
| NA | 10 | 40 | 50 | 0 | 0 | 0 | |||
| sCD8+ cells | Low | 51 (48.1) | 80 (53.7) | 0.380 | 33 (66.0) | 40 (49.4) | 35 (42.7) | 23 (54.8) | 0.069 |
| High | 55 (51.9) | 69 (46.3) | 17 (34.0) | 41 (50.6) | 47 (57.3) | 19 (45.2) | |||
| iCD8+ cells | Low | 79 (74.5) | 131 (87.9) | 0.006 * | 50 (100.0) | 67 (82.7) | 61 (74.4) | 32 (76.2) | 0.002 * |
| High | 27 (25.5) | 18 (12.1) | 0 (0.0) | 14 (17.3) | 21 (25.6) | 10 (23.8) | |||
| TRG | TRG 0 | 10 (9.4) | 40 (26.8) | 0.000 * | - | - | - | - | - |
| TRG 1 | 24 (22.6) | 57 (38.3) | - | - | - | - | |||
| TRG 2 | 35 (33.0) | 47 (31.5) | - | - | - | - | |||
| TRG 3 | 37 (34.9) | 5 (3.4) | - | - | - | - | |||
*, p < 0.05, statistically significant; the p value of 0.000 indicated p < 0.001.
3.4. Survival Analysis
The median follow-up duration for PFS, DMFS, and OS was 58.0 months (9–101), 57.0 months (9–101), and 60.0 months (9–101), respectively. Kaplan-Meier curve analysis revealed that compared to the lower expression group, those patients in the higher sCD8+ and CTLA-4+ cell groups had better PFS and DMFS, respectively (p = 0.046 and 0.028; Figure 3A,B), while no correlation was found with OS (p = 0.845 and 0.876; Supplementary Figure S1A,B; Supplementary Table S3). However, OS was significantly improved in those patients with lower CD86 expression compared to those with higher CD86 expression (p = 0.024; Figure 3C, Supplementary Figure S1C,D; Supplementary Table S3). Furthermore, multivariate analysis showed that sCD8+ cell density and CTLA-4 expression were not the independent prognostic factor of PFS and DMFS, respectively (both p > 0.05; Figure 3D,E). CD86 was an independent prognostic factor of OS (HR = 2.12, 95% CI = 1.03–4.37, p = 0.042, Figure 3F). Meanwhile, the ypT or ypN stage was an independent prognostic factor of PFS (HR = 4.35, 95% CI = 1.91–9.88, p < 0.001; HR = 2.25, 95% CI = 1.38–3.69, p = 0.001; respectively), DMFS (HR = 6.93, 95% CI = 2.43–19.82, p < 0.001; HR = 2.38, 95% CI = 1.40–4.02, p = 0.001; respectively), and OS (HR = 2.72, 95% CI = 1.42–5.22, p = 0.003; Figure 3D–F; Supplementary Figure S1E–G). However, the TRG categories were not the independent prognostic factor for OS, PFS, or DMFS (all p > 0.05; Figure 3D–F; Supplementary Figure S1E–G).
Figure 3.
Kaplan-Meier curves and multivariate analysis of survival. (A) PFS according to the status of sCD8+ cell (low or high). (B) DMFS according to CTLA-4+ cell density (low or high). (C) OS according to CD86 expression (low or high) level. PFS, progression-free survival; DMFS, distant metastasis-free survival; OS, overall survival. (D–F) Forest plots of PFS (D), DMFS (E), and OS (F) for variables by multivariate Cox analysis. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
3.5. Combined Status of CD8+ Cell Density and CTLA-4/CD86 Expression and Survival
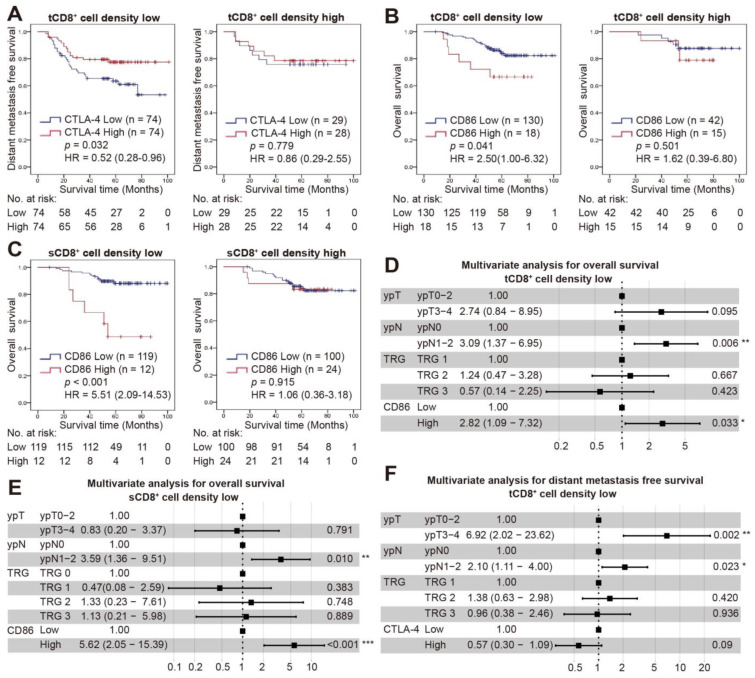
The correlation between CTLA-4/CD86 expression and CD4+/CD8+ cell density was examined (Table 3). CTLA-4 expression was correlated with the density of tCD8+ cells (p = 0.003), and the improved DMFS with high CTLA-4 expression was observed in a low, not high tCD8+ cell density group (p = 0.032 and 0.779, respectively; Figure 4A). In addition, the tCD8+/sCD8+ cell density was significantly higher in those patients with higher CD86 expression (p = 0.013 and 0.019, respectively). CD86 expression was inversely correlated with OS in those patients with a low t/sCD8+ cell density (p = 0.041 and <0.001, respectively), while it was not observed in those patients with a high t/sCD8+ cell density (p = 0.501 and 0.915, respectively; Figure 4B,C). Furthermore, besides the ypN stage (HR = 3.09, 95% CI = 1.37–6.95, p = 0.006; HR = 3.59, 95% CI = 1.36–9.51, p = 0.010), the combination of t/sCD8+ cell density and CD86 expression was also an independent prognostic factor of OS (HR = 2.82, 95% CI = 1.09–7.32, p = 0.033; HR = 5.62, 95% CI = 2.05–15.39, p < 0.001; respectively; Figure 4D,E; Supplementary Table S4; Supplementary Figure S2A,B), while the combination of tCD8+ cell density and CTLA-4 expression was not the independent prognostic factor of DMFS (HR = 0.57, 95% CI = 0.30–1.09, p = 0.09; Figure 4F; Supplementary Figure S2C).
Table 3.
Correlation between CTLA-4/CD86 expression and CD4+/CD8+ cells.
| Variables | CTLA-4 Expression | p | CD86 Expression | p | |||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| tCD4+ cells | Low | 98 (95.1) | 93 (91.2) | 0.260 | 162 (94.2) | 29 (87.9) | 0.188 |
| High | 5 (4.9) | 9 (8.8) | 10 (5.8) | 4 (12.1) | |||
| sCD4+ cells | Low | 66 (51.2) | 60 (47.6) | 0.571 | 109 (49.8) | 17 (47.2) | 0.777 |
| High | 63 (48.8) | 66 (52.4) | 110 (50.2) | 19 (52.8) | |||
| iCD4+ cells | Low | 90 (69.8) | 77 (61.1) | 0.146 | 146 (66.7) | 21 (58.3) | 0.330 |
| High | 39 (30.2) | 49 (38.9) | 73 (33.3) | 15 (41.7) | |||
| tCD8+ cells | Low | 84 (81.6) | 64 (62.7) | 0.003 * | 130 (75.6) | 18 (54.5) | 0.013 * |
| High | 19 (18.4) | 38 (37.3) | 42 (24.4) | 15 (45.5) | |||
| sCD8+ cells | Low | 68 (52.7) | 63 (50.0) | 0.665 | 119 (54.3) | 12 (33.3) | 0.019 * |
| High | 61 (47.3) | 63 (50.0) | 100 (45.7) | 24 (66.7) | |||
| iCD8+ cells | Low | 108 (83.7) | 102 (81.0) | 0.562 | 184 (84.0) | 26 (72.2) | 0.085 |
| High | 21 (16.3) | 24 (19.0) | 35 (16.0) | 10 (27.8) | |||
*, p < 0.05, statistically significant.
Figure 4.
Combined status of CD8+ cell density and CTLA-4/CD86 expression with survival. (A) Kaplan-Meier curve for DMFS in low or high tCD8+ cell density groups according to CTLA-4 expression. (B,C) Kaplan-Meier curves for OS in low or high tCD8+ (B)/sCD8+ (C) cell density groups according to the CD86 expression. (D–F) Forest plots of PFS and DMFS for variables by multivariate Cox analysis in low tCD8+ (D,F)/sCD8+ (E) cell density groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
4. Discussion
Radiotherapy- or chemotherapy-induced tumor cell death can disrupt tumor escape strategies and recruit immune cells to enhance antitumor immunity. Clinical studies have explored the synergy between immunotherapy and conventional therapies [21], as monotherapy no longer meets the actual clinical needs. The effect of combining ICIs with chemo(radio)therapy in RC is still limited. Further understanding of the impact of chemo(radio)therapy on the tumor microenvironment, particularly the CTLA-4 and CD86 signatures, is required to optimize the design of clinical trials. To the best of our knowledge, this is the first study to demonstrate the relationship between CTLA-4, CD86, CD4, and CD8 expression and prognosis after nCRT in RC. Our results showed that the expression of CD86 but not CTLA-4 was significantly lower in the nCRT groups, and that CD86 was an independent prognostic factor of OS. Specifically, those patients with lower CD86 expression could achieve better survival outcomes when their t/sCD8+ cell densities were low, and the combined status of t/sCD8+ cell density with CD86 expression was an independent prognostic factor of OS.
Usually, flow cytometry is used to identify CD86 expression on B or plasma cells in hematologic malignancies, with a detection rate of 58.0% or 47.5% [22,23]. In solid tumors, CD86 expression can be detected by multiplexed immunofluorescence and is used as the phenotypic biomarker of M1 macrophages, together with Interferon Regulatory Factor 5 (IRF5) [24]. In addition, qRT-PCR, Western blot, and IHC assays can also be used to detect CD86 expression. Herein, CD86 was immunohistochemically detected in 36 of the 255 patients (14.1%), which is similar to nasopharyngeal carcinoma patients (20.0%) [25] and lower in non-small cell lung cancer (NSCLC) patients (53%) [26]. The positive rate of CD86 expression detected by IHC in the present study was slightly lower than in other reports. The levels of soluble immune checkpoint molecules have been reported to decrease during nCRT [27], and CD86 expression showed a different pattern between the nCRT and nCT groups in this study. This indicates that nCRT/nCT might cause a low rate of CD86 expression, and further experiments should be conducted to identify whether the expression of CD86 changes before or after nCRT/nCT.
Studies have also demonstrated that combining ICIs with chemotherapy improves survival not only in patients with metastatic squamous NSCLC [28], but also in patients with stage IB–IIIA NSCLC [29]. The survival advantage is observed only in stage III NSCLC when ICIs are administered after chemoradiotherapy [30,31,32]. For CRC patients with pMMR/MSS tumors, the most favorable time point or therapy methods for an immunotherapy combination remains a great challenge. A subset of tumors might display significant adaptive resistance after post-combined modality therapy, manifested by an active immune microenvironment characterized by increased CD8 infiltration and IFNγ, leading to the compensatory induction of multiple checkpoints, including PD-1, PD-L1, CTLA-4, LAG-3, and IDO, to prevent tumor cell death [33,34]. Furthermore, the expression pattern of the tumor microenvironment shows different changes in different cancer patients after receiving chemo(radio)therapy [35]. Our results showed that the expression of CD86 but not CTLA-4 was significantly higher in the nCT-treated group compared to patients treated with nCRT. Consistent with our study, Teng et al. also verified that CTLA-4 is not associated with the response in rectal cancer [13]. Prognostic analysis showed that CD86 expression was associated with OS, but not with PFS or DMFS. Since a tumor could become more malignant after recurrence or metastasis, the role of CD86 in this stage and the relationship with OS could be further explored. In addition, a previous study shows that the correlation between homologous recombination mutation and prognosis is inconsistent when patients are receiving different types of treatment [36]. Thus, the prognostic value of CD86 could also be explored in patients receiving ICIs, as CD86 plays a vital role in immune response.
In RC, higher densities of pretreatment CD8+ and CD4+ cells are associated with a better response and OS [13], while the associations after neoadjuvant treatment have not been thoroughly investigated. We showed that patients with a good response had lower s/iCD8+ cell densities only in nCRT-treated group. Moreover, only the expression of sCD8 was associated with PFS after neoadjuvant treatment. Matsutani et al. demonstrated that CD8 expression was significantly higher in the responder group in patients treated with nCT rather than nCRT [37]. However, their 64 patients, whose specimens were available in their study, was much lower than the 255 patients in the present study. In addition, their study did not analyze the relationship between CD8 expression and prognosis according to the tumor’s location, as previous studies have demonstrated the importance of TILs in different regions of the tumor microenvironment for predicting prognosis [38]. Previous studies have demonstrated that improved disease-free survival was only seen in patients with high immune-expressed-PD-L1 [12]. Interestingly, we found that improved DMFS and OS were only observed in patients with low instead of high s/tCD8+ cell densities. This suggests that the immunosuppressive microenvironment could affect the function of the CTLA-4/CD86 pathway. In particular, CTLA-4 played a converse role to CD86 in terms of prognosis, with patients with higher CTLA-4 expression having better DMFS. A reduced death rate was observed in NSCLC when considering tumors with CTLA-4 > 20 and Ki67 ≤ 15 [39]. The high CTLA-4 H-score level was correlated with prolonged OS and DFS in extrahepatic bile duct cancer patients plus adjuvant chemoradiotherapy [40]. Although CTLA-4 was not associated with OS and DFS in RC after nCRT [13], these data indicated that the positive prognostic role of CTLA-4 was similar to that of other tumor types. Preclinical models indicate that high-order combined therapy could promote a durable benefit and tumor regression in pancreatic ductal adenocarcinoma [41]. Therefore, in future clinical studies, considering neoadjuvant therapy modalities and CTLA-4/CD86 expression during ICIs, combination therapy might effectively block more immune-evasive tumor mechanisms.
The limitations of this study include its retrospective design at a single institution and its lack of functional experiments. Further in vivo and in vitro studies are needed to verify the role of the CD86 pathway during nCRT. Antibodies and assessment methods for CD86 immunostaining have not been well established, and we used only one antibody to evaluate CD86 staining. In addition, the expression of CD86 was evaluated only in surgical specimens after neoadjuvant therapy for more than four weeks. It would be fascinating to explore whether CD86 expression changes during nCRT. Finally, we only described four immune-related molecules in the present study, and it was hard to build predictive models. Therefore, more immune markers are recommended to establish a comprehensive immune score for ICIs in combination with nCRT.
5. Conclusions
In conclusion, tumor immunity was different according to the neoadjuvant therapy method and response, with CD86, CD4, and t/iCD8 expression, but not CTLA-4 and sCD8 expression, being significantly higher in the nCT-treated and poor response groups. However, CTLA-4 and sCD8 expression was correlated with DMFS and PFS, respectively. Furthermore, CTLA-4 expression was related to tCD8+ cell density, whereas CD86 expression was associated with t/sCD8+ cell density. The relationship between CTLA-4 and DMFS was independent of tCD8+ cell density. At the same time, improved OS in patients with lower CD86 expression could only be observed in patients with low t/sCD8+ cell density. These findings might have important implications for a possible combined therapy using ICIs after nCRT/nCT in rectal cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14225573/s1, Supplementary Table S1, Correlation between the expression of immune-related molecules and clinicopathologic characteristics; Supplementary Table S2, Association of immune-related molecules with TRG categories in nCT- and nCRT-treated groups; Supplementary Table S3, Univariate analysis for survival; Supplementary Table S4, Multivariate analysis for overall survival for patients with TRG 1-3 and low sCD8+ cell density; Supplementary Figure S1, Kaplan-Meier curves and multivariate analysis of survival; and Supplementary Figure S2, The assumption of Cox PH model test.
Author Contributions
Conceptualization, Q.-Y.Q.; Data curation, X.-K.Y. and L.-L.F.; Funding acquisition, X.-K.Y., X.-J.F. and Q.-Y.Q.; Investigation, W.-X.F.; Methodology, C.W., S.-M.B. and X.-J.F.; Resources, Z.-H.C.; Supervision, Q.-Y.Q.; Writing—original draft, X.-K.Y., C.W. and L.-L.F.; Writing—review & editing, N.-T.O., Z.-H.C. and X.-J.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Clinical Ethics Review Committee at The Sixth Affiliated Hospital of SYSU (No. 2022ZSLYEC-361).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the Natural Science Foundation for Excellent Young Scholars (No. 82122057), Natural Science Foundation of China (No. 82203965), Guangdong Science and Technology Project (No. 2019B030316003), and China Postdoctoral Science Foundation (No. 2022M713620), Science and Technology Plan of Guangzhou (No. 202102020009) and National Key Clinical Discipline.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biller L.H., Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 2.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland C.R., Thibodeau S.N., Hamilton S.R., Sidransky D., Eshleman J.R., Burt R.W., Meltzer S.J., Rodriguez-Bigas M.A., Fodde R., Ranzani G.N., et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 4.Huyghe N., Baldin P., Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: What is the future beyond deficient mismatch-repair tumours? Gastroenterol. Rep. 2020;8:11–24. doi: 10.1093/gastro/goz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrelli F., Trevisan F., Cabiddu M., Sgroi G., Bruschieri L., Rausa E., Ghidini M., Turati L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann. Surg. 2020;271:440–448. doi: 10.1097/SLA.0000000000003471. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L., Humeau J., Buqué A., Zitvogel L., Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020;17:725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 7.Pfirschke C., Engblom C., Rickelt S., Cortez-Retamozo V., Garris C., Pucci F., Yamazaki T., Poirier-Colame V., Newton A., Redouane Y., et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44:343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagchi S., Yuan R., Engleman E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 9.Hecht M., Büttner-Herold M., Erlenbach-Wünsch K., Haderlein M., Croner R., Grützmann R., Hartmann A., Fietkau R., Distel L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer. 2016;65:52–60. doi: 10.1016/j.ejca.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Chiang S.F., Huang C.Y., Ke T.W., Chen T.W., Lan Y.C., You Y.S., Chen W.T., Chao K.S.C. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol. Immunother. CII. 2019;68:283–296. doi: 10.1007/s00262-018-2275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huemer F., Klieser E., Neureiter D., Schlintl V., Rinnerthaler G., Pagès F., Kirilovsky A., El Sissy C., Iglseder W., Singhartinger F., et al. Impact of PD-L1 Scores and Changes on Clinical Outcome in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. J. Clin. Med. 2020;9:2775. doi: 10.3390/jcm9092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura A., Akiyoshi T., Yamamoto N., Kawachi H., Ishikawa Y., Mori S., Oba K., Nagino M., Fukunaga Y., Ueno M. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur. J. Cancer. 2018;91:11–20. doi: 10.1016/j.ejca.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Teng F., Meng X., Kong L., Mu D., Zhu H., Liu S., Zhang J., Yu J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl. Res. J. Lab. Clin. Med. 2015;166:721–732.e721. doi: 10.1016/j.trsl.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Schütz C., Inselmann S., Saussele S., Dietz C.T., Mu Ller M.C., Eigendorff E., Brendel C.A., Metzelder S.K., Bru Mmendorf T.H., Waller C., et al. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia. 2017;31:829–836. doi: 10.1038/leu.2017.9. [DOI] [PubMed] [Google Scholar]
- 15.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pentcheva-Hoang T., Egen J.G., Wojnoonski K., Allison J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Tekguc M., Wing J.B., Osaki M., Long J., Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA. 2021;118:e2023739118. doi: 10.1073/pnas.2023739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sansom D.M., Manzotti C.N., Zheng Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003;24:314–319. doi: 10.1016/S1471-4906(03)00111-X. [DOI] [PubMed] [Google Scholar]
- 19.Wan X.B., Zhang Q., Chen M., Liu Y., Zheng J., Lan P., He F. Prognostic Value of Interval Between the Initiation of Neoadjuvant Treatment to Surgery for Patients With Locally Advanced Rectal Cancer Following Neoadjuvant Chemotherapy, Radiotherapy and Definitive Surgery. Front. Oncol. 2020;10:1280. doi: 10.3389/fonc.2020.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trakarnsanga A., Gönen M., Shia J., Nash G.M., Temple L.K., Guillem J.G., Paty P.B., Goodman K.A., Wu A., Gollub M., et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J. Natl. Cancer Inst. 2014;106:dju248. doi: 10.1093/jnci/dju248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap T.A., Parkes E.E., Peng W., Moyers J.T., Curran M.A., Tawbi H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021;11:1368–1397. doi: 10.1158/2159-8290.CD-20-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sędek Ł., Theunissen P., Sobral da Costa E., van der Sluijs-Gelling A., Mejstrikova E., Gaipa G., Sonsala A., Twardoch M., Oliveira E., Novakova M., et al. Differential expression of CD73, CD86 and CD304 in normal vs. leukemic B-cell precursors and their utility as stable minimal residual disease markers in childhood B-cell precursor acute lymphoblastic leukemia. J. Immunol. Methods. 2019;475:112429. doi: 10.1016/j.jim.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Brown R., Kabani K., Favaloro J., Yang S., Ho P.J., Gibson J., Fromm P., Suen H., Woodland N., Nassif N., et al. CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood. 2012;120:2055–2063. doi: 10.1182/blood-2012-03-416792. [DOI] [PubMed] [Google Scholar]
- 24.Väyrynen J.P., Haruki K., Lau M.C., Väyrynen S.A., Zhong R., Dias Costa A., Borowsky J., Zhao M., Fujiyoshi K., Arima K., et al. The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol. Res. 2021;9:8–19. doi: 10.1158/2326-6066.CIR-20-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C.S., Chang J.H., Hsu N.C., Lin H.Y., Chung C.Y. Expression of CD80 and CD86 costimulatory molecules are potential markers for better survival in nasopharyngeal carcinoma. BMC Cancer. 2007;7:88. doi: 10.1186/1471-2407-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato T., Takagi K., Higuchi M., Abe H., Kojimahara M., Sagawa M., Tanaki M., Miki Y., Suzuki T., Hojo H. Immunolocalization of CD80 and CD86 in Non-Small Cell Lung Carcinoma: CD80 as a Potent Prognostic Factor. Acta Histochem. Cytochem. 2022;55:25–35. doi: 10.1267/ahc.21-00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Wang P., Sun Y., Dou X., Hu X., Zou W., Sun Y., Hu Q., Yue J. Neoadjuvant Chemoradiotherapy Changes the Landscape of Soluble Immune Checkpoint Molecules in Patients With Locally Advanced Rectal Cancer. Front. Oncol. 2022;12:756811. doi: 10.3389/fonc.2022.756811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paz-Ares L., Vicente D., Tafreshi A., Robinson A., Soto Parra H., Mazières J., Hermes B., Cicin I., Medgyasszay B., Rodríguez-Cid J., et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Felip E., Altorki N., Zhou C., Csőszi T., Vynnychenko I., Goloborodko O., Luft A., Akopov A., Martinez-Marti A., Kenmotsu H., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 30.Spigel D.R., Faivre-Finn C., Gray J.E., Vicente D., Planchard D., Paz-Ares L., Vansteenkiste J.F., Garassino M.C., Hui R., Quantin X., et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faivre-Finn C., Vicente D., Kurata T., Planchard D., Paz-Ares L., Vansteenkiste J.F., Spigel D.R., Garassino M.C., Reck M., Senan S., et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2021;16:860–867. doi: 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Kurata T., Chiappori A., Lee K.H., de Wit M., et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 33.Kelly R.J., Zaidi A.H., Smith M.A., Omstead A.N., Kosovec J.E., Matsui D., Martin S.A., DiCarlo C., Werts E.D., Silverman J.F., et al. The Dynamic and Transient Immune Microenvironment in Locally Advanced Esophageal Adenocarcinoma Post Chemoradiation. Ann. Surg. 2018;268:992–999. doi: 10.1097/SLA.0000000000002410. [DOI] [PubMed] [Google Scholar]
- 34.Llosa N.J., Cruise M., Tam A., Wicks E.C., Hechenbleikner E.M., Taube J.M., Blosser R.L., Fan H., Wang H., Luber B.S., et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Ende T., van den Boorn H.G., Hoonhout N.M., van Etten-Jamaludin F.S., Meijer S.L., Derks S., de Gruijl T.D., Bijlsma M.F., van Oijen M.G.H., van Laarhoven H.W.M. Priming the tumor immune microenvironment with chemo(radio)therapy: A systematic review across tumor types. Biochim. Biophys. Acta Rev. Cancer. 2020;1874:188386. doi: 10.1016/j.bbcan.2020.188386. [DOI] [PubMed] [Google Scholar]
- 36.Zhou P., Wu X., Chen H., Hu Y., Zhang H., Wu L., Yang Y., Mao B., Wang H. The mutational pattern of homologous recombination-related (HRR) genes in Chinese colon cancer and its relevance to immunotherapy responses. Aging. 2020;13:2365–2378. doi: 10.18632/aging.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsutani S., Shibutani M., Maeda K., Nagahara H., Fukuoka T., Nakao S., Hirakawa K., Ohira M. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018;109:966–979. doi: 10.1111/cas.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards C.H., Roxburgh C.S., Powell A.G., Foulis A.K., Horgan P.G., McMillan D.C. The clinical utility of the local inflammatory response in colorectal cancer. Eur. J. Cancer. 2014;50:309–319. doi: 10.1016/j.ejca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Salvi S., Fontana V., Boccardo S., Merlo D.F., Margallo E., Laurent S., Morabito A., Rijavec E., Dal Bello M.G., Mora M., et al. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol. Immunother. CII. 2012;61:1463–1472. doi: 10.1007/s00262-012-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim Y.J., Koh J., Kim K., Chie E.K., Kim S., Lee K.B., Jang J.Y., Kim S.W., Oh D.Y., Bang Y.J. Clinical Implications of Cytotoxic T Lymphocyte Antigen-4 Expression on Tumor Cells and Tumor-Infiltrating Lymphocytes in Extrahepatic Bile Duct Cancer Patients Undergoing Surgery Plus Adjuvant Chemoradiotherapy. Target. Oncol. 2017;12:211–218. doi: 10.1007/s11523-016-0474-1. [DOI] [PubMed] [Google Scholar]
- 41.Winograd R., Byrne K.T., Evans R.A., Odorizzi P.M., Meyer A.R., Bajor D.L., Clendenin C., Stanger B.Z., Furth E.E., Wherry E.J., et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol. Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.