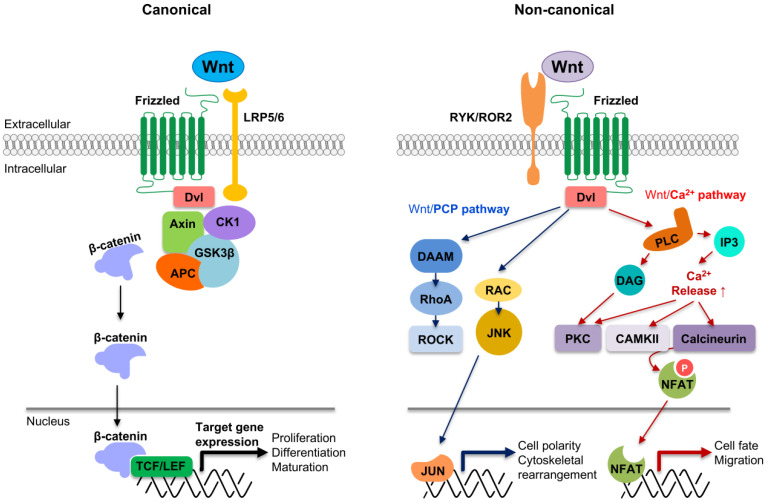
Figure 1.
The canonical and non-canonical Wnt signaling pathways. In the canonical Wnt pathway, the Wnt signaling is activated upon binding Wnt ligands such as Wnt3a to Frizzled (FZD) and co-receptor LRP5/6. Then, the Disheveled (Dvl) recruits axis inhibition protein (Axin), the casein kinase 1 (CK1), and glycogen synthase kinase 3 β (GSK3β) to the plasma membrane, inactivating the β-catenin destruction complex and weakening phosphorylation and degradation of β-catenin. This results in the accumulation of the stabilized β-catenin in the cytoplasm and the translocation of it into the nucleus. β-catenin in the nucleus forms an active transcriptional complex with T-cell factor (TCF) and lymphoid enhancer factor (LEF), leading to canonical Wnt target gene expression. Upon the non-canonical Wnt ligands such as Wnt5a binding to the RYK/ROR2-FZD complex, Dvl is recruited, and Wnt/PCP or Wnt/Ca2+ signaling pathway is activated. In the Wnt/PCP pathway, the scaffold protein Dvl stimulates the activation of the small GTPase Rho and RAC to induce Rho-associated kinase (ROCK) and c-Jun N-terminal kinase (JNK), respectively. ROCK and JNK trigger gene expression associated with cell polarization and cytoskeletal rearrangement. In the Wnt/Ca2+ pathway, Dvl activates phospholipase C (PLC), stimulating 1,2-diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). The activated IP3 promotes the release of Ca2+ within the cytoplasm, and protein kinase C (PKC), CAMKII, and calcineurin are subsequently induced. The nuclear factor of activated T-cells (NFAT), a transcriptional factor, is then activated through dephosphorylation to induce calcium-dependent cytoskeletal and transcriptional responses.