Abstract
Interleukin-5 (IL-5) transgenic mice are highly resistant to primary infections with the intestinal nematode Nippostrongylus brasiliensis; few parasites are found in the intestines of infected animals, and egg production is minimal. While adult worms may be damaged in the intestine, larval migration, development, and viability may also be impaired in other tissues. This study addresses the migration of N. brasiliensis larvae through the skin and lungs and associated cellular responses in primary infections of IL-5 transgenic mice. Although some larvae may have been trapped and killed in the lungs of IL-5 transgenic mice, most apparently failed to reach this site. Two or more hours after infection of IL-5 transgenic mice, eosinophils were a major component of the cellular infiltrate at the subcutaneous site of injection, and localized eosinophil degranulation was extensive. Seventy-five to ninety-five percent of the larvae injected into subcutaneous air pouches in IL-5 transgenic mice were retained there for at least 24 h. In contrast, in nontransgenic mice, less than 20% of larvae could be recovered from the skin 2 or more h postinjection, and eosinophil activity was modest at all times. The data strongly suggest that eosinophils can restrict the movement of N. brasiliensis larvae in the first few hours of a primary infection and that this has profound effects on later stages of parasite development. Preexisting eosinophilia, due either to allergy or to infection with tissue-invasive helminth species, may therefore confer some degree of immediate and nonspecific resistance in primary infections with parasitic worms.
Eosinophils are polymorphonuclear granular leukocytes produced within the bone marrow and normally represent only a small proportion of leukocytes found in blood and most tissues. Accumulation of eosinophils (eosinophilia) in peripheral blood and tissues is considered a hallmark of many tissue-invasive helminthic infections. In some geographical localities, eosinophilia is present in a very large proportion of the human population (35). Eosinophilia is associated with absence of reinfection of children with Schistosoma species (15, 34), and atopy, which often includes eosinophilia, has been associated with increased resistance to helminth infections (14). Eosinophils can kill a range of helminth species in vitro (2), and major basic protein, which represents a large proportion of protein in eosinophil granules, is highly toxic in vitro for schistosomula (3), Trichinella spiralis (16), and Trypanosoma cruzi (19). Other eosinophil granule proteins such as eosinophil peroxidase (EPO), eosinophil cationic protein, and eosinophil-derived neurotoxin are also toxic for a number of parasites (12, 17, 22). Such findings therefore suggest a major role for eosinophils in protection against helminths and possibly in other parasitic infections.
The cytokine interleukin-5 (IL-5) is a growth and differentiation factor crucial to the regulation of eosinophilia. Constitutive expression of IL-5 transgenes induces lifelong eosinophilia in mice (9). IL-5 transgenic mice are no more resistant than nontransgenic littermates to several helminth species (6, 8, 32), but they do show greater resistance to the intestinal nematode Nippostrongylus brasiliensis (6, 7). Arrival of adult N. brasiliensis worms into the small intestine is delayed in IL-5 transgenic mice relative to nontransgenic mice. In addition, fewer parasites survive passage through the usual migratory pathway from the subcutaneous inoculation site to the small intestine (6, 7). Surviving worms recovered from the small intestines of IL-5 transgenic mice exhibit reduced fecundity, fail to grow, and are less likely to occupy the preferred site of attachment in the anterior section of the small intestine (7). Such findings suggest that while parasite growth and development may be adversely affected in the small intestines of IL-5 transgenic mice, it is also likely that migration and maturation of N. brasiliensis larvae is inhibited prior to the intestinal stage of the life cycle. To address this possibility, we examined the migration of N. brasiliensis larvae from a subcutaneous inoculation site to the lungs and analyzed the inflammatory response induced in both sites. Our data suggest that the entrapment and killing of larvae begin within a few hours of initiation of a primary infection in IL-5 transgenic mice and that this process is likely to be mediated by eosinophils. It is possible that eosinophils have a previously unrecognized role to play in primary resistance to any of a number of helminth infections in humans, a matter worthy of consideration when new therapies designed to regulate the putative actions of eosinophils are applied in cases of asthma and allergy.
MATERIALS AND METHODS
Animals.
Female heterozygous IL-5 transgenic (Tg5C2) CBA mice, carrying approximately 49 copies of the transgene (9), and their normal littermates were bred in the University of Adelaide Medical School Animal House. The immunology and parasite biology characteristics of these mice have been described previously (6–8, 31, 32). All mice were closely aged matched within experiments. Hooded Wistar (randomly bred) and dark agouti rats (inbred) produced by Laboratory Animal Services at the University of Adelaide were used for passage of N. brasiliensis. Animals were handled according to Animal Ethics Committee guidelines of the University of Adelaide.
Parasitological techniques.
Infective N. brasiliensis third-stage larvae (L3) were obtained by the fecal culture method, washed twice in saline, and injected subcutaneously (s.c.) or into air pouches at an approximate dose of 500 larvae per mouse. At designated time points following infection, mice were sacrificed by CO2 asphyxiation. Whole lungs were removed, finely minced, and incubated in saline at 37°C for at least 2 h before emergent larvae were enumerated under a dissecting microscope.
Histology.
Lungs were inflated and fixed in situ by tracheal infusion of 10% buffered formalin before removal and further fixation in the same solution. Five-micrometer-thick sections of paraffin-embedded tissues were stained with hematoxylin and eosin (H&E.) and evaluated by light microscopy. The shaved skin together with the underlying fat pad at the site of injection of L3 were removed, pinned to a cork board fur side down, fixed in 10% buffered formalin, and processed as described above.
Generation of air pouches.
Air pouches were formed as described previously (11), with modifications. Briefly, 2.5 ml of sterile air was injected s.c. into a shaved skin site on the back of each mouse. This was allowed to settle for 3 days to permit healing of the wound. The pouch was then reinflated with 2 ml of sterile air and left for a further 3 days before injection of either 500 N. brasiliensis L3 in 1 ml of endotoxin-free phosphate-buffered saline (PBS) or PBS alone. All air pouch procedures were conducted under light ether anesthesia. Mice were sacrificed by CO2 asphyxiation, and the pouches were lavaged with 1 ml of mouse osmolality PBS (MPBS), followed by two separate washes with 2 ml of MPBS for each. Pouch washings were centrifuged at 100 × g for 10 min before enumeration of total and viable cells by using trypan blue exclusion and a hemocytometer. Supernatants were snap frozen for analysis of EPO. The number of larvae recovered was estimated from a sample of the resuspended pellet. Pouch exudate cells were cytocentrifuged onto glass slides, air dried, fixed with methanol, and stained with Giemsa (BDH Chemicals, Gurr, Australia). Differential cell counts were estimated by enumeration of a minimum of 200 cells per sample.
Measurement of EPO.
EPO was measured in lungs and washings from air pouches by using a modification of techniques described previously (10, 33). Whole lungs were removed immediately following death, homogenized in MPBS in a mechanical homogenizer (Ultra-Turrex), and subjected to three cycles of freezing and thawing. EPO activity was determined in fluid phase by oxidation of o-phenylene diamine (OPD Sigma Fast tablets; Sigma, St. Louis, Mo.) in the presence of hydrogen peroxide. Samples were run in duplicate with and without resorcinol (1.5 mg/ml; Sigma), an eosinophil peroxidase-specific inhibitor, to differentiate EPO from neutrophil and macrophage myeloperoxidases (27, 29). Absorbance of the reactions was measured with a Biolumin-960 plate reader and Xperiment software (Molecular Dynamics Inc, Sunnyvale, Calif.). Absorbance values with resorcinol were subtracted from those without the inhibitor to estimate the level of EPO activity. Results are expressed as mean arbitrary absorbance units at 490 nm ± standard error of the mean (SEM).
Statistics.
Where appropriate, data were analyzed by two-tailed Student’s t test using Excel for Macintosh 4.0 (Microsoft), with P < 0.05 considered significant.
RESULTS
Recovery of larvae from lungs.
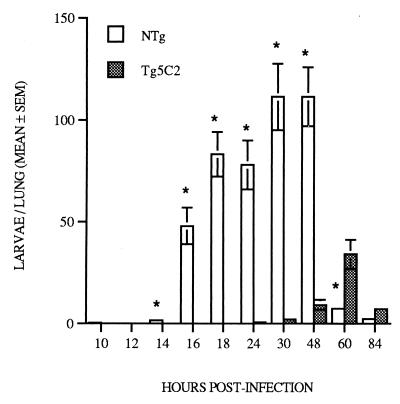
N. brasiliensis larvae migrate from the skin to the lungs, where they undergo a molt and maturation phase en route to the small intestine (18). In secondary infections in nontransgenic mice, larvae appear to be surrounded by eosinophil-rich leukocyte infiltrates in the lungs (36). To determine whether the lungs might also be a site of entrapment and killing of larvae in primary infections in IL-5 transgenic mice, we assessed lung larval burdens from 10 to 84 h postinfection (p.i.).
Significant numbers of larvae were first detected in the lungs of nontransgenic mice 16 hours p.i., with peak larval recovery 30 to 48 h p.i. (Fig. 1). Larvae were relatively rare in the lungs of nontransgenic mice later than 60 h p.i. (Fig. 1), in keeping with the kinetics of parasite migration to the small intestine (7). In contrast, few larvae were recovered from the lungs of IL-5 transgenic mice prior to 48 h p.i., and maximum numbers detected at 60 h p.i. were approximately 30% of the peak levels observed in nontransgenic control mice (Fig. 1). These data suggest that migration of larvae was delayed and that large numbers of N. brasiliensis larvae were prevented from reaching the lungs of IL-5 transgenic mice.
FIG. 1.
Recovery of N. brasiliensis larvae from the lungs of nontransgenic and Tg5C2 IL-5 transgenic mice following primary infection with 500 infective larvae. Nontransgenic mice exhibited a significantly higher parasite burden than similarly infected IL-5 transgenic mice. Each bar represents the mean number of larvae recovered ± SEM from three to seven mice per group. ∗, significantly different from nontransgenic littermates at the same stage of infection; P ≤ 0.01.
Eosinophil responses in the lungs.
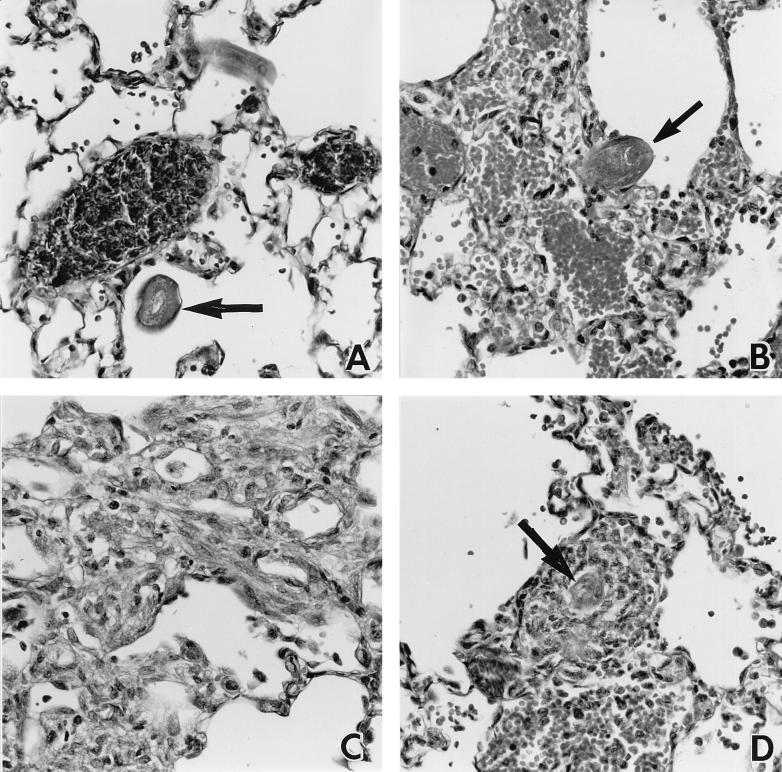
Twenty-four hours p.i. larvae could be detected in histological sections of lungs recovered from both nontransgenic and IL-5 transgenic mice (Fig. 2A and B), though as indicated above, they were relatively rare in the latter. Many erythrocytes could be found in the immediate vicinity of larvae, in keeping with the hemorrhagic foci seen macroscopically on the surfaces of the lungs. At this stage of the infection, eosinophils were not prominent in the lungs of either nontransgenic or transgenic mice. Those leukocytes which were present were mostly mononuclear cells, many of which appeared to be macrophages. However, by 60 h p.i., substantial eosinophil-rich infiltrates were observed in the lungs of both host types (Fig. 2C and D). In nontransgenic mice, eosinophils became obvious only after most larvae had left the lung (Fig. 2C), whereas in IL-5 transgenic mice, the presence of large numbers of eosinophils in lung sections was approximately coincident with the later appearance of lung-stage larvae. Sixty hours p.i., some parasites detected in sections of lungs from transgenic mice were surrounded by large inflammatory infiltrates (Fig. 2D); 6 days p.i., very large granulomatous structures, which were apparently free of larvae, were found (data not shown).
FIG. 2.
Representative photomicrographs of leukocyte infiltrates and larvae in the lungs of nontransgenic (A and C) and IL-5 transgenic (B and D) mice. Lungs were recovered 24 (A and B) and 60 (C and D) h p.i. Larvae (arrows) but few leukocytes were present in the lungs of nontransgenic and transgenic mice 24 h p.i. At 60 h p.i., eosinophils and other leukocytes were present in the lungs of nontransgenic and transgenic hosts, but larvae were detected only in the latter. H&E-stained 5-μm sections; ×320 magnification.
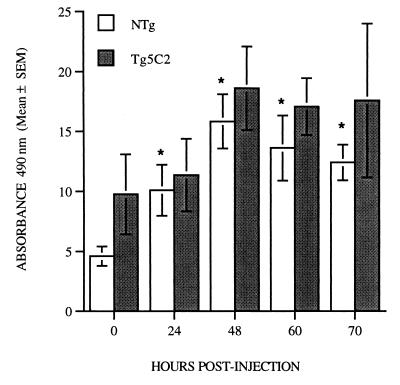
Quantitation of leukocytes in lung sections was difficult, but it was possible to estimate the extent of eosinophilia in this tissue by measuring EPO activity in whole-lung homogenates. EPO activity in the lungs of uninfected IL-5 transgenic mice was approximately twice as great as the levels measured in uninfected nontransgenic animals. Relative to uninfected mice, the levels of EPO activity in the lungs of nontransgenic mice had increased significantly by 24 h p.i., reached maximum at 48 h, and was maintained until at least 70 h p.i. (Fig. 3, P < 0.01). In contrast, lungs recovered from IL-5 transgenic mice showed no changes in EPO activity within the first 24 h of infection (Fig. 3). A modest and sustained increase in EPO activity over uninfected levels was observed in lung homogenates from transgenic mice from 48 to 70 h p.i., but this failed to reach statistical significance.
FIG. 3.
Mean EPO activity ± SEM expressed as arbitrary absorbance units at 490 nm in whole-lung homogenates of uninfected (day 0) mice and mice at various times after infection with 500 N. brasiliensis L3. EPO activity increases in both nontransgenic and Tg5C2 IL-5 transgenic mice over the course of the infection. Each bar represents the mean of four to six mice per group. ∗, significantly different from matched uninfected mice.
Detection of larvae and eosinophilic infiltrates in subcutaneous tissue.
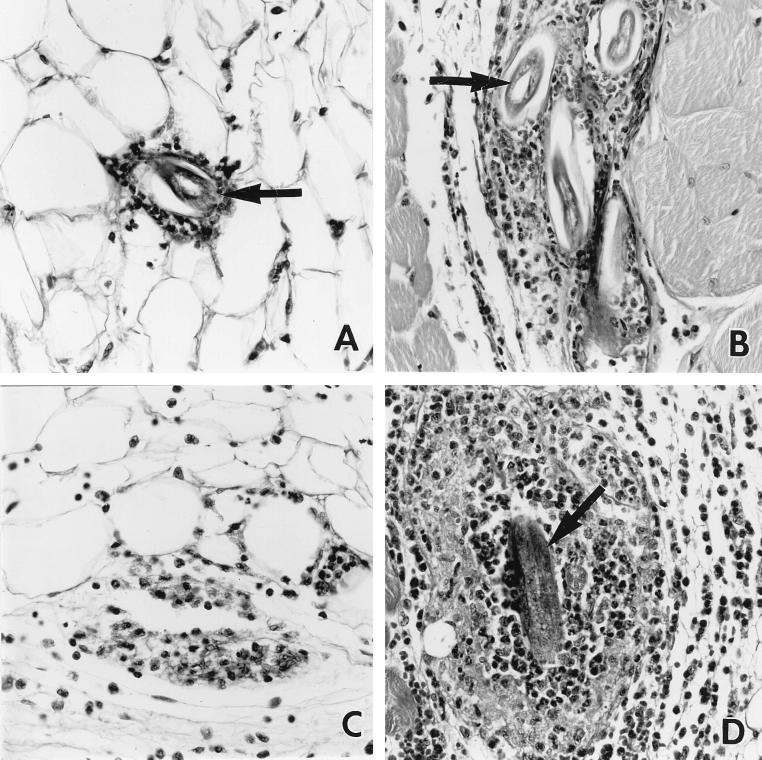
Larval migration to the lungs of IL-5 transgenic mice is clearly inhibited compared to that in nontransgenic controls, suggesting entrapment and/or killing of the parasite prior to this stage of the life cycle. The subcutaneous inoculation site was analyzed, since it is the first site at which larvae are localized in the host. Cellular infiltrates composed principally of eosinophils were observed to surround larvae in skin from both nontransgenic and IL-5 transgenic mice from as early as 2 h p.i. However, many more leukocytes, and especially eosinophils, were evident in the latter (Fig. 4A and B). Larvae were identified infrequently at the inoculation site at times later than 6 h p.i. in nontransgenic mice (Fig. 4C, 24 h p.i.). Both eosinophil-rich leukocyte infiltrates and larvae were detected for at least 48 h after infection at the site of inoculation in IL-5 transgenic mice (Fig. 4D, 24 h p.i.).
FIG. 4.
Representative photomicrographs of leukocyte infiltrates and larvae at the site of inoculation in nontransgenic (A and C) and Tg5C2 IL-5 transgenic (B and D) mice. Tissues were recovered 2 (A and B) and 12 (C and D) h p.i. At 2 h p.i., inflammatory infiltrates had formed around larvae (arrows) in both nontransgenic and transgenic mice, with both larvae and leukocytes more numerous in the latter. At 12 h p.i., leukocyte infiltrates had often largely resolved in nontransgenic mice and larvae were absent (C), whereas eosinophil-rich infiltrates had developed further around larvae still retained at the site of inoculation in transgenic mice (D). H&E-stained 5-μm sections; ×320 magnification.
Quantitation of leukocytes and larvae in air pouches.
To quantitate and further characterize the early leukocyte response to N. brasiliensis, L3 were injected into air pouches raised in the dorsal skin. No more than a mean of 2 × 105 cells were recovered from air pouches in nontransgenic mice injected only with endotoxin-free PBS (maximum detected 4 h p.i., compared to 6 × 104 cells for uninjected air pouches) and of these, 40% were neutrophils and 10% were eosinophils. Injection of endotoxin-free PBS alone into air pouches raised in IL-5 transgenic mice induced a significant increase in the cellular infiltrate by 2 h p.i., and this reached maximum levels at 4 h p.i. (mean of 2 × 106 leukocytes/pouch; 25% neutrophils, 53% eosinophils). This number of cells was significantly higher than the mean of 3 × 105 cells/pouch (22% neutrophils, 55% eosinophils) recovered from uninjected transgenic mice.
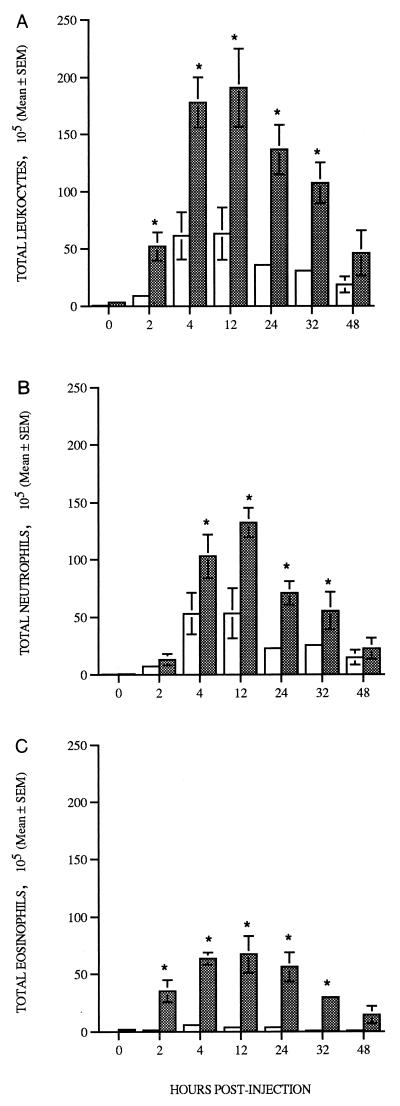
Two hours after injection of larvae, total leukocyte numbers in air pouches rose dramatically in both nontransgenic and IL-5 transgenic mice (Fig. 5A). Total air pouch leukocyte counts of both nontransgenic and transgenic mice infected for 2 or more h were always at least threefold higher than values from mice injected with PBS alone (full data not shown). From 2 to 32 h p.i., at least three- to fivefold more cells were recovered from air pouches in IL-5 transgenic mice injected with larvae than from pouches in similarly treated nontransgenic hosts (Fig. 5A). The presence of large numbers of neutrophils was noted in the first 2 h of infection in both lines of mice. In nontransgenic animals, neutrophil numbers peaked 4 to 12 h p.i. (Fig. 5B) and remained at approximately 80% of total leukocytes until at least 48 h p.i. Eosinophils also became more numerous in air pouches in nontransgenic mice from 2 h p.i., and numbers peaked 4 to 24 h p.i. However, eosinophils never represented more than 10% of the total leukocytes recovered from air pouches in nontransgenic mice (Fig. 5C). The numbers of eosinophils recovered from air pouches in transgenic mice 2 h after injection of larvae were approximately 5-fold higher than those from PBS-injected IL-5 transgenic mice (full data not shown) and 20-fold higher than those from pouches in nontransgenic mice infected with larvae for the same period of time (Fig. 5C). Peak eosinophil numbers were observed 4 to 12 h p.i. in IL-5 transgenic mice, declining gradually thereafter but nevertheless remaining significantly greater than those in nontransgenic animals for at least 32 h p.i. (Fig. 5C). Relatively few lymphocytes were detected in air pouches at any time point in either transgenic or nontransgenic mice infected with the parasite (range of 1 to 3% of total leukocytes [data not shown]). Monocyte numbers in the air pouches of parasite-infected IL-5 transgenic mice were higher than those in the air pouches of infected nontransgenic mice between 12 and 32 h p.i. (e.g., 1.9 × 106 and 9 × 104 respectively at 32 h PI; P < 0.02 [data not shown]).
FIG. 5.
Total leukocytes (A), neutrophils (B), and eosinophils (C) recovered by lavage of subcutaneous air pouches of nontransgenic (open bars) and Tg5C2 IL-5 transgenic (shaded bars) mice previously injected with 500 N. brasiliensis L3. Data are expressed as the mean number of leukocytes (105) ± SEM for two to six mice per group. ∗, significantly different from nontransgenic animals at the same time point; P < 0.05.
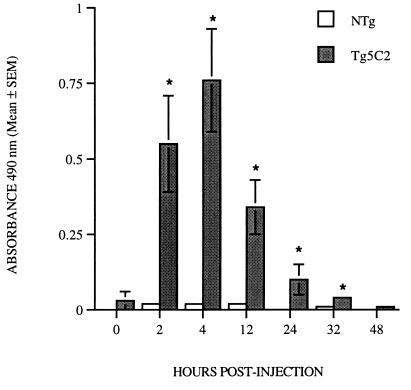
Neutrophils were apparently more numerous than eosinophils in larva-infected air pouches of transgenic mice at most time points and were also usually more common in transgenic than in nontransgenic hosts (Fig. 5B and C). We considered the possibility that degranulated eosinophils had been incorrectly scored as neutrophils or at least that they may not be readily detected by standard differential staining techniques. In addition, eosinophils attached either to the wall of the air pouch or to larvae were not quantitated in the analysis represented in Fig. 5. It is therefore possible that eosinophil levels may be underestimated in the data presented above. To obtain an estimate of the extent of eosinophil degranulation, EPO was measured in cell-free air pouch lavage fluids (Fig. 6). No cell-free EPO was detected in lavage fluids from uninjected air pouches in either nontransgenic or transgenic mice (data not shown). In both transgenic and nontransgenic mice, EPO activity in samples from air pouches injected with PBS (data not shown) did not exceed 15% of the corresponding mean value recorded for pouches injected with larvae. In pouch fluids recovered from IL-5 transgenic mice from 2 to 12 h p.i., EPO activity was elevated 10- to 25-fold above uninfected levels (P < 0.02) and gradually subsided to background levels by 48 h p.i. No substantial changes in EPO activity were detected at any stage of infection in nontransgenic mice (Fig. 6). These results clearly indicate that eosinophil degranulation was significant in the air pouches of parasite-infected transgenic mice, and so it is likely that the eosinophil counts depicted in Fig. 5 for these animals are significantly underestimated. It is also possible that as a consequence, the neutrophil counts were overestimated.
FIG. 6.
EPO activity in cell-free lavage fluid from air pouches in nontransgenic and Tg5C2 IL-5 transgenic mice after injection of 500 N. brasiliensis L3. EPO activity is abundant from 2 to 32 h p.i. in transgenic but not nontransgenic mice. Samples are from the same experiment represented in Fig. 5. ∗, significantly different from nontransgenic mice at the same stage of infection; P < 0.01.
In both transgenic and nontransgenic mice injected with larvae, over the course of the infection, there was an increase in EPO activity associated with the pellet recovered after centrifugation of air pouch lavage fluids (i.e., containing cells and parasites). EPO was maximal between 4 and 12 h p.i. (data not shown). At all time points, EPO values calculated for lavage pellets from infected transgenic mice were much higher than those for similarly treated nontransgenic animals.
Retention of larvae in air pouches.
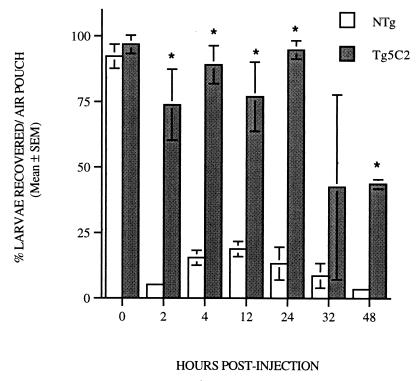
Retention of larvae within air pouches was monitored over a 48-h period (Fig. 7). In both transgenic and nontransgenic hosts, almost 100% of the estimated dose was recoverable when air pouches were washed out immediately following injection. At all other time points assessed, larval recovery was greater from air pouches in transgenic mice than from those in nontransgenic littermates. From 2 to 24 h p.i., only 6 to 20% of the estimated dose of larvae injected could be recovered from nontransgenic hosts, whereas 75 to 95% of the estimated original inoculum was retained in air pouches in IL-5 transgenic mice (Fig. 7). Larval numbers in air pouch washings from transgenic hosts were not significantly reduced until 48 h after injection (P < 0.01), and even at this time, more than 45% of the estimated original inoculum was still recoverable.
FIG. 7.
Recovery of larvae from parasite-infected air pouches in nontransgenic and Tg5C2 IL-5 transgenic mice represented in Fig. 5. Most larvae migrate out of air pouches in nontransgenic mice within 2 h of infection, whereas the majority of larvae injected into air pouches in transgenic mice are retained for at least 32 h. Each bar represents the mean percentage of larvae recovered relative to the estimated original inoculum ± SEM for two to six mice per group. ∗, significantly different from nontransgenic mice at the same stage of infection; P ≤ 0.01.
DISCUSSION
We have previously demonstrated that IL-5 transgenic mice are very resistant to primary infections with the intestinal nematode N. brasiliensis. Relative to infections in their nontransgenic counterparts, few parasites complete migration to the small intestines of IL-5 transgenic mice (6, 7). This suggests that N. brasiliensis larvae are trapped and killed along the normal migratory route. In the present study we have analyzed the earlier lung and skin phases of infections with this parasite. The larval burden in the lungs of IL-5 transgenic mice was less than that in nontransgenic mice, and the time of arrival of larvae was delayed. It appeared, therefore, that larval entrapment in transgenic mice occurred within the first 24 h of infection and prior to arrival in the lungs. By 2 h p.i., leukocyte infiltrates were evident at the subcutaneous inoculation site in both host types. Larvae and high numbers of eosinophils were detected at the site of inoculation for at least 48 h in IL-5 transgenic mice, whereas in nontransgenic hosts larvae had mostly disseminated by 6 h p.i. When quantified in the air pouch model, most larvae had left the site of inoculation within 2 h of injection into nontransgenic hosts, and the involvement of eosinophils, although detectable, was always modest. In contrast, eosinophil recruitment and degranulation in IL-5 transgenic hosts occurred rapidly in air pouches injected with larvae, and few larvae migrated from this site within the first 32 h. Collectively these results suggest that in IL-5 transgenic mice, the majority of larvae are trapped and destroyed at the site of initiation of the primary infection. Such resistance has previously been clearly demonstrated only in secondary infections of nontransgenic mice. While eosinophils are prominent in secondary immune responses to this parasite (26), our very novel data suggest that most of the damage to N. brasiliensis larvae can occur before the development of parasite-specific humoral and cell-mediated immunity.
Following subcutaneous (or percutaneous) infection, N. brasiliensis L3 migrate to the lungs, where they are first evident approximately 15 h p.i. The larvae then molt before migrating by day 3 p.i. to the small intestine, via the trachea and esophagus (36, 37). Our data for nontransgenic mice are in keeping with these observations. In contrast, the arrival of larvae into the lungs of IL-5 transgenic mice was delayed, and at no stage did parasite numbers reach the levels detected in nontransgenic hosts. Both histological analysis and whole-tissue measurements of EPO suggest that eosinophil numbers increase in the lungs of nontransgenic mice over the course of infection. However, eosinophils were not prominent in histological sections of the lungs of these animals when sampled before 60 h p.i., even though peak larval numbers were recorded between 30 and 48 h p.i. It is significant, therefore, that EPO levels increased from as early as 24 h p.i. and remained elevated throughout this period. These data suggest that those few eosinophils present in the lungs at the same time as the parasite may have been more difficult to detect in histological sections because they had already degranulated. In contrast, over the first 24 to 48 h of infection, lungs recovered from transgenic mice showed little evidence of eosinophilic infiltrates or increased EPO activity, which is in keeping with our observation that the arrival of larvae is delayed. Eosinophil-rich infiltrates were detected from 60 h p.i., with some clearly focused on larvae. This suggests that some of the larvae which reach the lungs in IL-5 transgenic mice are trapped and killed there. Nevertheless, since few larvae ever reach the lungs in these mice, an appreciable level of entrapment and killing must occur elsewhere.
Associations between immunity to parasites and eosinophils within the skin of immunized animals have been demonstrated in studies with a variety of parasite species (23, 30, 37, 40). Immobilization of N. brasiliensis larvae occurs 2 to 3 h after penetration of the skin in mice immunized by prior infection (36). At the site of inoculation in both IL-5 transgenic and nontransgenic mice, we detected N. brasiliensis larvae which were surrounded by eosinophil-rich leukocyte infiltrates within 2 h of initiation of a primary infection. However, in nontransgenic mice, most larvae had disseminated from the skin by 6 h p.i. and the leukocyte accumulations were less evident from this time. Larval migration was even more rapid from air pouches in nontransgenic mice. A small but statistically significant increase in larval recovery from air pouches of nontransgenic mice was noted 4 to 12 h p.i., with moderate increases in eosinophil numbers and activity recorded for the same period. The developing inflammatory response within the air pouch or the surrounding environment may cause the detachment of some larvae from the air pouch wall, resulting in a transient rise in the number of larvae recovered by lavage of the air pouch. However, it is striking that in the IL-5 transgenic mice, the larvae do not attach firmly to the pouch wall at any time and few leave the pouch in the first 24 h p.i.
Detection of EPO within air pouch supernatants is significant not only because it suggests that eosinophils are activated and degranulating but also because EPO is toxic for a number of parasite species (1, 17, 25, 42). Other eosinophil granule proteins not analyzed in our study also have larvicidal properties (22, 41) and may be released at or around the same time as EPO. The extent of eosinophil degranulation, as indicated by the high levels of cell-free EPO detected in parasite-infected air pouches, also suggests that eosinophil numbers may have been underestimated. After the introduction of larvae, eosinophils are also very prominent in the wall of the air pouch in IL-5 transgenic mice, and these cells are not removed or quantitated with our lavage technique (unpublished data). Nevertheless, they may make a major contribution to containing the larvae within the pouch.
Air pouches have been used to great effect in analyzing inflammatory responses induced by a wide range of materials, including bacteria and protozoan parasites. A similar technique has also been used to assess inflammatory responses induced by eggs from the helminth Schistosoma mansoni (28). The work described above would appear to be the first published report of the use of the air pouch technique to study the inflammatory response induced by a skin-invasive stage of a helminth infection. It is particularly relevant for this application because it permits assessment of the inflammatory response in the skin itself, i.e., at or near the first point of contact of parasite and host. With this method, the extent of the cellular response can be estimated much more reliably and accurately than by quantitation of cells in histological sections of tissues recovered from the injection site. Where required, the extracellular fluid can also be analyzed. However, perhaps the greatest advantage of this approach is that the retention of larvae at the site of inoculation can be quantitated over an extended period and without the difficulties often inherent with alternative techniques requiring radioactive labelling of the parasite.
It is perhaps noteworthy that although a potent local inflammatory response was detected in IL-5 transgenic mice after injection of N. brasiliensis larvae into the skin (directly s.c. or into air pouches), there is no evidence of inordinate skin hypersensitivity or localized toxicity. The concentration of the EPO measured in this study, and presumably of other components released from eosinophils, increased rapidly during the initial stages of the infection, but this did not lead to overt tissue damage. Other experiments suggest that the IL-5 transgenic mice (9) used in the present study are no more sensitive to skin pathology than their wild-type counterparts. Skin thickening after two exposures to the contact-sensitizing agent trinitrochlorobenzene was similar in IL-5 transgenic mice and in nontransgenic controls (unpublished results). Our IL-5 transgenic mice were also similar to wild-type mice in terms of immediate hypersensitivity reactions measured in two different assays (unpublished results). In contrast, skin abnormalies or enhanced hypersensitivity may be a feature of other IL-5 transgenic lines. Five exposures to dinitrofluorobenzene causes dermatitis in another line of IL-5 transgenic mice (38), and it is more severe than that seen in similarly treated wild-type mice (24). In a third line of IL-5 transgenic mice established by using a transgene driven by the CD3δ gene promoter/enhancer, ulcerating skin lesions and hair loss occur spontaneously (20). Thus, under the conditions that we have tested to date, marked skin pathology is not a feature of our experimental model and extreme hypersensitivity does not appear to be necessary for the primary resistance to N. brasiliensis which we have described here. However, more frequent exposure to an antigen, or the nature of the IL-5 transgene construct itself, may lead to more severe skin lesions or toxicity which could prevent dissemination of larvae via less physiological mechanisms. This should be considered in any comparisons between different IL-5 transgenic mouse models.
Eosinophils are probably not the only cell type active in the entrapment of N. brasiliensis larvae in this model, though we propose that their role is elemental to the process. For many hours after injection of larvae, neutrophils appear to be more numerous than eosinophils in air pouches in IL-5 transgenic mice. While this may be an artifact caused by the misidentification of eosinophils which have degranulated, unfortunately it is a difficult issue to resolve. There are no antibodies available to clearly differentiate mouse neutrophils and eosinophils on the basis of cell surface phenotype, and eosinophils lacking their characteristic granules could well be misclassified. However, in our IL-5 transgenic model, events vital to larval entrapment clearly occur within the first 2 h of infection. By this time, most larvae have migrated out of air pouches in nontransgenic mice, but this is not so in transgenic hosts. Neutrophil numbers in air pouches at this early stage of infection were approximately equal in the two hosts. In contrast, eosinophils were clearly far more numerous in transgenic than in nontransgenic hosts. Therefore, we propose that eosinophils rather than neutrophils are likely to be the critical factor in larval retention.
Although larvae may be trapped within the first few hours of infection, parasite killing probably proceeds at a relatively low rate since motile larvae can be recovered from air pouches for at least 48 h after inoculation (unpublished data). The decline in larval recovery after this time may reflect a combination of larval destruction and escape into other tissues. In support of the former, preliminary experiments in our laboratory have demonstrated immobilization of infective-stage larvae following 6 to 20 h of in vitro coculture with eosinophil-rich leukocytes from IL-5 transgenic mice (reference 5 and unpublished data). Larvae again become more motile after 24 h, coinciding with a precipitous decline in eosinophil viability. While eosinophils are recruited into the skin of nontransgenic mice early in infection, there may be insufficient numbers of these cells available in the tissue pool to significantly affect parasite migration. This view is generally supported by the size of infiltrates seen in histological sections and the numbers of leukocytes recovered from air pouches injected with larvae. On the other hand, IL-5 transgenic mice have an expanded pool of eosinophils which can be readily recruited to sites of antigenic challenge (32), and larvae in these hosts may eventually be overwhelmed by the early and qualitatively more appropriate inflammatory response at the site of inoculation.
Eosinophils in IL-5 transgenic mice may be activated by prolonged exposure to this cytokine, perhaps enhancing antilarval activity in mice infected with N. brasiliensis. Our earlier results suggest that if activation does occur, it does not appear to be excessive for a number of parameters assessed (6, 8). It is also worth noting that eosinophils from parasite-infected nontransgenic mice can be chronically exposed to levels of IL-5 comparable to those seen in uninfected IL-5 transgenic mice (9, 32). So, at least for the early stages of N. brasiliensis infection, the exposure of eosinophils to IL-5 in IL-5 transgenic animals is likely to be within normal physiological limits.
Eosinophils were usually closely associated with larvae seen in histological sections of tissue from the inoculation site. Mechanisms through which these cells may interact with and damage migrating larvae are not completely understood, but innate pattern recognition systems are likely to be involved in the initial steps. Complement, natural antibodies, and lectin receptors may all contribute, but further study of this issue is necessary. It is likely that eosinophil adherence to the parasite surface is an important trigger for the release of toxic granule proteins. Since EPO levels in the fluid phase of air pouch washings were maximal 4 h p.i., it is evident that eosinophils begin to degranulate soon after coming into contact with larvae. It is likely that eosinophil-derived neurotoxin, another eosinophil granule protein and potent neurotoxin, contributes to the initial immobilization of the larvae. This could be crucial in keeping the larvae in a state and location in which they are most susceptible to further immune attack.
To date, studies analyzing eosinophil associations with this and other parasites have shown dependence on the presence of parasite-specific antibody, either in vitro or in immunized animals (3, 4, 39). In direct contrast, the most important features of our study were that resistance was evident in IL-5 transgenic animals exposed to primary infections and that this occurred within the first 24 h of exposure to the parasite. From these very critical observations, we conclude that parasite-specific antibodies are not likely to be essential for the processes of eosinophil attachment, activation, and degranulation.
In summary, entrapment and/or killing of N. brasiliensis larvae may occur within the lungs of IL-5 transgenic mice, but our data suggest that the majority of larvae are eliminated at the site of inoculation. This process begins within a few hours of injection of larvae into naive animals and may be largely complete within the first 48 h of infection. Responses observed in IL-5 transgenic mice are similar in quality and initial time of onset to those detected in nontransgenic mice, but the magnitude and duration of the reactions are significantly greater in transgenic hosts. Though we cannot at this stage exclude other mechanisms induced by IL-5, eosinophils would appear to be responsible for the enhanced resistance observed in IL-5 transgenic mice. In more general terms, our data also suggest that animals with preexisting eosinophilia, induced by either previous or current exposure to parasites, may have an enhanced degree of innate resistance to primary infections with other helminth species. This adds a new dimension to the role of eosinophils in innate immunity and should encourage a reassessment of concurrent infections in experimental animals, with the focus now shifting to the earliest stages of exposure with the challenge parasite species.
Our results may have considerable significance to management in humans of diseases in which eosinophils are a prominent feature. The high levels of IL-5 and eosinophilia detected in uninfected IL-5 transgenic mice are seen not only in helminth-infected nontransgenic mice (9, 32) but also in humans infected with similar parasites (21). Eosinophilia occurs at very high frequency in human populations in some regions, most probably due to recent or ongoing parasite infections (35), and this may provide some protection against reinfection with helminths (15, 34), or even against infection with helminth species encountered for the first time. Field studies in several countries also suggest that humans with atopic diseases may be more resistant to helminth infections (13, 14), possibly in part due to priming of eosinophilopoiesis and enhanced activation and survival of eosinophils. Therapeutic measures designed to control asthma and allergic diseases by down-regulating eosinophils may therefore have the adverse effect of rendering patients more susceptible to acquisition or exacerbation of helminth infections. In particular, such treatments may impair at least partial protection afforded by a previously undervalued component of the innate immune response.
ACKNOWLEDGMENTS
Thanks go to Ann Hallett and Hans Scoppe for technical assistance, Leon Bignold for helpful discussions and assistance with histology, and Dale Caville for assistance with photography.
This work was supported in part by the Australian Research Council and the University of Adelaide Faculty of Medicine.
REFERENCES
- 1.Bass D A, Szejda P. Mechanism of killing newborn larvae of T. spiralis by neutrophils and eosinophils. Killing by generators of hydrogen peroxide in vitro. J Clin Investig. 1979;64:1558–1564. doi: 10.1172/JCI109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterworth A E. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth A E, Wassom D L, Gleich G J, Loegering D A, David J R. Damage to schistosomula of S. mansoni induced directly by eosinophil major basic protein. J Immunol. 1979;122:222–229. [PubMed] [Google Scholar]
- 4.Capron A, Dessain J P, Haque A, Capron M. Antibody-dependent cell-mediated cytotoxicity against parasites. Prog Allergy. 1982;31:234–267. [PubMed] [Google Scholar]
- 5.Daly C M, Mayrhofer G, Dent L A. Trapping and killing of N. brasiliensis larvae in IL-5 transgenic mice. Immunologist Suppl. 1998;1:511. . (Abstract). [Google Scholar]
- 6.Dent L A, Daly C, Geddes A, Cormie J, Finlay D A, Bignold L, Hagan P, Parkhouse R M E, Garate T, Parsons J, Mayrhofer G. Immune responses of IL-5 transgenic mice to parasites and aeroallergens. Mem Inst Oswaldo Cruz. 1997;92(Suppl. II):45–54. doi: 10.1590/s0074-02761997000800008. [DOI] [PubMed] [Google Scholar]
- 7.Dent L A, Daly C M, Mayrhofer G, Zimmermann T, Hallett A, Bignold L P, Creaney J, Parsons J C. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dent L A, Munro G H, Piper K P, Sanderson C J, Finlay D A, Dempster R K, Bignold L P, Harkin D G, Hagan P. Eosinophilic interleukin 5 (IL-5) transgenic mice: eosinophil activity and impaired clearance of Schistosoma mansoni. Parasite Immunol. 1997;19:291–300. doi: 10.1046/j.1365-3024.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- 9.Dent L A, Strath M, Mellor A L, Sanderson C J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimayuga E, Stober M, Kayes S G. Eosinophil peroxidase levels in hearts and lungs of mice infected with Toxocara canis. J Parasitol. 1991;77:461–466. [PubMed] [Google Scholar]
- 11.Edwards J C W, Sedgwick A D, Willoughby D A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 12.Gleich G J, Loegering D A. Immunobiology of eosinophils. Annu Rev Immunol. 1984;2:249–259. doi: 10.1146/annurev.iy.02.040184.002241. [DOI] [PubMed] [Google Scholar]
- 13.Grove D I. What is the relationship between asthma and worms? Allergy. 1982;37:139. doi: 10.1111/j.1398-9995.1982.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 14.Grove D I, Forbes I J. Increased resistance to helminth infestation in an atopic population. Med J Aust. 1975;1:336–338. doi: 10.5694/j.1326-5377.1975.tb111423.x. [DOI] [PubMed] [Google Scholar]
- 15.Hagan P, Wilkins H A, Blumenthal U J, Hayes R J, Greenwood B M. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 1985;7:625–631. doi: 10.1111/j.1365-3024.1985.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamann K H, Barker R L, Loegering D A, Gleich G J. Comparative toxicity of purified human eosinophil granule proteins for newborn larvae of Trichinella spiralis. J Parasitol. 1987;73146:523–529. [PubMed] [Google Scholar]
- 17.Jong E C, Henderson W R, Klebanoff S J. Peroxidase mediated toxicity to schistosomula of Schistosoma mansoni. J Immunol. 1980;126:468–471. [PubMed] [Google Scholar]
- 18.Kassai T. Handbook of Nippostrongylus brasiliensis (Nematode). Slough, United Kingdom: Commonwealth Agricultural Bureaux; 1982. [Google Scholar]
- 19.Kierszenbaum F, Ackerman S J, Gleich G J. Destruction of bloodstream forms of Trypanosoma cruzi by eosinophil granule major basic protein. Am J Trop Med Hyg. 1981;30:775–779. doi: 10.4269/ajtmh.1981.30.775. [DOI] [PubMed] [Google Scholar]
- 20.Lee N A, McGarry M P, Larson K A, Horton M A, Kristensen A B, Lee J J. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 21.Limaye A P, Abrams J S, Silver J E, Ottesen E A, Nutman T B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990;172:399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren D J, Paterson C E, Venge P. Schistosoma mansoni: further studies of the interaction between schistosomula and granulocyte derived cationic proteins in vitro. Parasitology. 1984;88:491–503. doi: 10.1017/s0031182000054755. [DOI] [PubMed] [Google Scholar]
- 23.Moqbel R. Histopathological changes following primary, secondary and repeated infection of rats with Strongyloides ratti, with special reference to eosinophils. Parasite Immunol. 1980;B2:11–27. [Google Scholar]
- 24.Nagai H, Ueda Y, Tanaka H, Hirano Y, Nakamura N, Inagaki N, Takatsu K, Kawada K. Effect of overproduction of interleukin 5 on dinitrofluorobenzene-induced allergic cutaneous response in mice. J Pharmacol Exp Ther. 1999;288:43–50. [PubMed] [Google Scholar]
- 25.Nogueira N M, Klebanoff S J, Cohn Z A. Trypanosoma cruzi: sensitisation to macrophage killing by EPO. J Immunol. 1987;128:1705–1708. [PubMed] [Google Scholar]
- 26.Ogilvie B M, Jones V E. Nippostrongylus brasiliensis: a review of immunity and the host/parasite relationship in the rat. Exp Parasitol. 1971;29:138–177. doi: 10.1016/0014-4894(71)90021-x. [DOI] [PubMed] [Google Scholar]
- 27.Ornstein L, Anstey H, Saunders A. Improving manual differential white blood cell counts with cytochemistry. Blood Cells. 1976;2:557–561. [Google Scholar]
- 28.Pacheco R G, Lenzi H L. Systemic modulation of peripheral eosinophilia (air pouch model) in Schistosoma mansoni infection. Mem Inst Oswaldo Cruz. 1997;92(Suppl. II):165–172. doi: 10.1590/s0074-02761997000800022. [DOI] [PubMed] [Google Scholar]
- 29.Patterson K G, Linch D C, Rosendaal M. In situ cytoenzymatic identification of human bone marrow colonies grown in agar: a simple method with automated cytochemistry reagents. J Clin Pathol. 1981;34:935–938. doi: 10.1136/jcp.34.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotman H L, Yutanawiboonchai W, Brigandi R A, Leon O, Gleich G J, Nolan T J, Schad G A, Abraham D. Strongyloides stercoralis-eosinophil-dependent immune-mediated killing of third stage larvae in Balb/CBYJ mice. Exp Parasitol. 1996;82:267–278. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson C J, Strath M, Mudway I, Dent L A. Transgenic experiments with interleukin-5. In: Gleich G J, Kay A B, editors. Eosinophils in allergy and inflammation. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 335–351. [Google Scholar]
- 32.Strath M, Dent L A, Sanderson C J. Infection of IL-5 transgenic mice with Mesocestoides corti induces high levels of IL-5 but depressed production of eosinophils. Exp Hematol. 1992;120:229–234. [PubMed] [Google Scholar]
- 33.Strath M, Warren D J, Sanderson C J. Detection of eosinophils using an eosinophil peroxidase assay: its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985;83:209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- 34.Sturrock R F, Kimani R, Cottrell B J, Butterworth A E, Seitz H M, Siongok T K, Houba V. Observations on possible immunity to reinfection among Kenyan schoolchildren after treatment for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1983;77:363–371. doi: 10.1016/0035-9203(83)90166-9. [DOI] [PubMed] [Google Scholar]
- 35.Supanaranond W, Migasena S, Pitisuttitham P, Suntharasamai P. Health status of Thai volunteers in a cholera vaccine trial. J Med Assoc Thailand. 1990;73:548–551. [PubMed] [Google Scholar]
- 36.Taliaferro W H, Sarles M P. The cellular reactions in the skin, lungs and intestine of normal and immune rats after infection with Nippostrongylus muris. J Infect Dis. 1939;64:157–192. [Google Scholar]
- 37.Tindall N R, Wilson P A G. An extended proof of migration routes of immature parasites inside hosts: pathways of Nippostrongylus brasiliensis and Strongyloides ratti in the rat are mutually exclusive. Parasitology. 1990;100:281–288. doi: 10.1017/s003118200006128x. [DOI] [PubMed] [Google Scholar]
- 38.Tominga A, Yamauchi S, Kanai Y, Miyazaki J I, Usuku G, Yamamura K I, Takatsu K. Transgenic mice expressing a B cell growth and differentiation factor gene (IL-5) develop eosinophilia and auto-antibody production. J Exp Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vadas M A, Butterworth A E, Sherry B, Dessein A, Hogan M, Bout D, David J R. Interaction between human eosinophils and schistosomula of S. mansoni. I. Stable and irreversible antibody-dependent adherence. J Immunol. 1980;124:1441–1448. [PubMed] [Google Scholar]
- 40.Ward R E, McLaren R J. Schistosoma mansoni: evidence that eosinophils and/or macrophages contribute to skin-phase challenge attrition in vaccinated CBA/Ca mice. Parasitology. 1980;96:63–84. doi: 10.1017/s003118200008166x. [DOI] [PubMed] [Google Scholar]
- 41.Wassom D L, Gleich G J. Damage to Trichinella spiralis new born larvae by eosinophil major basic protein. Am J Trop Med Hyg. 1979;28:860–863. [PubMed] [Google Scholar]
- 42.Yoshimura K, Sugaya H, Ishida K. The role of eosinophils in Angiostrongylus cantonesis infection. Parasitol Today. 1994;10:231–233. doi: 10.1016/0169-4758(94)90124-4. [DOI] [PubMed] [Google Scholar]