Abstract
Enteroaggregative Escherichia coli (EAEC) strains have been shown to adhere to human intestinal tissue in an in vitro organ culture (IVOC) model, and certain strains manifest mucosal toxicity. We have recently described the EAEC plasmid-encoded toxin (Pet), a member of a specific serine protease subclass of the autotransporter proteins. When injected into rat ileal loops, Pet both elicited fluid accumulation and had cytotoxic effects on the mucosa. Furthermore, the Pet protein caused rises in short circuit current from rat jejunal tissue mounted in a Ussing chamber and rounding of intestinal epithelial cells in culture. We therefore hypothesized that the mucosal pathology induced by EAEC strains in the IVOC model was related to expression of the Pet protein. Here, we have examined the effects of EAEC strain 042 and its isogenic pet mutant in the IVOC model. 042-infected colonic explants exhibited dilation of crypt openings, increased cell rounding, development of prominent intercrypt crevices, and absence of apical mucus plugs. Colonic tissue incubated with the pet mutant exhibited significantly fewer mucosal abnormalities both subjectively and as quantitated morphometrically by measurement of crypt aperture diameter. Mucosal effects were restored upon complementation of the pet mutation in trans. Interestingly, we found that the ability of 042 to damage T84 cells was not dependent upon Pet. The data suggest that the Pet toxin is active on the human intestinal mucosa but that EAEC may have other mechanisms of eliciting mucosal damage.
Enteroaggregative Escherichia coli (EAEC) is an enteric pathogen defined by its distinctive aggregative or “stacked-brick” pattern of adherence to cultured human epithelial cells (27). EAEC strains have been associated with persistent diarrhea in children and with a number of both nosocomial and community outbreaks worldwide (5, 18, 23, 33). The diarrhea elicited by EAEC strains appears to be predominantly secretory in nature; stools from affected patients are watery and contain mucus but are usually not bloody and do not contain polymorphonuclear cells (6, 24). Patients are generally afebrile.
Although the pathogenesis of EAEC infection is not completely understood, a number of features have been defined (26): (i) initial mucosal adherence, mediated by aggregative adherence fimbriae (AAFs) and perhaps other factors; (ii) formation of a mucus-bacteria biofilm on the intestinal surface; and (iii) mucosal toxicity, marked by exfoliation of epithelial cells. Damage to the intestinal epithelium was observed in patients by Eslava et al. (10), who reported the presence of ileal necrosis in infants in Mexican EAEC outbreaks.
The pathogenic sequence described above has been reproduced in human colonic biopsy specimens maintained in an in vitro organ culture (IVOC) model (16, 17, 25). Using this model, Hicks et al. (16) and Nataro et al. (25) demonstrated that EAEC strains (including strain 042, a proven human pathogen) adhere predominantly to the colonic mucosa and that most strains manifest toxic effects, characterized by exfoliation of enterocytes and dilation of crypt openings. Nataro et al. (25) also described characteristic cytopathic changes in T84 cell monolayers infected with EAEC.
The mechanisms of EAEC-induced mucosal toxicity are not known. However, these effects, coupled with the secretory nature of EAEC-associated diarrhea, have led investigators to search for a cytotoxin and/or enterotoxin of EAEC. Recently, Eslava et al. (9, 10) identified two immunogenic proteins (with predicted Mrs of 104,000 and 116,000) which produced both fluid accumulation and cytotoxic effects in rat ligated ileal loops. Navarro-Garcia et al. (28, 29) purified the ca. 104-kDa protein and showed that this species elicits rises in short circuit current (Isc) in rat jejunum specimens mounted in Ussing chambers as well as rounding of HEp-2 and HT29 cells in culture and loss of actin microfilaments. The gene encoding this toxin, designated Pet (for plasmid-encoded toxin), has been cloned and sequenced, and Pet was shown to be a member of the autotransporter class of secreted proteins (9). It is relevant to note that although the autotransporter proteins have been implicated as adhesins and toxins of several important gram-negative pathogens, in no case described thus far has their precise role in virulence been ascertained (14).
We hypothesized that the mucosal damage induced by EAEC strains is associated with production of the Pet protein (9, 28). To test this hypothesis, we have constructed an isogenic Pet-negative strain and have characterized this mutant in the IVOC model, on T84 cells in culture, and on rat jejunum mounted in a Ussing chamber. Based upon these studies we conclude that the 104-kDa Pet is an EAEC toxin that is active on whole human tissue but that EAEC has additional pathogenic mechanisms.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Strain 042 was isolated from a child with diarrhea in the course of an epidemiologic study conducted in Lima, Peru, in 1983; this strain has been shown to cause diarrhea in adult volunteers (24). E. coli DH5α λpir and S17-1 λpir were used as recipient strains for genetic manipulations. Strains were generally stored at −70°C in Trypticase soy broth with 15% glycerol and were passed routinely on Luria-Bertani broth (LB broth) or agar with the following antibiotics where appropriate: ampicillin (100 μg/ml), kanamycin (50 μg/ml), and nalidixic acid (50 μg/ml). Strains used in IVOC were stored in a Microbank system at −70°C.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| E. coli strains | ||
| 042 | Wild-type EAEC strain from Peru | 24 |
| DH5α λpir | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoR Δ(lacZYA-argF)U169 λpir+ | 8 |
| S17-1 λpir | pro res mod+ RP4-2 Tet::Mu-Kan::Tn7 | 32 |
| HB101 | K-12/B hybrid | 2 |
| JIF1 | 042 pet::pJIF | This study |
| JIF2 | JIF1 harboring pCEFN1 | This study |
| Plasmids | ||
| pJP5603 | 3.1-kb R6K-based suicide vector (Kanr) | 30 |
| pJIF | 1,120-bp internal fragment of pet cloned into pJP5603 (Kanr) | This study |
| pCEFN1 | 3.9-kb PCR-derived fragment encoding Pet protein, cloned into pSPORT1 (Ampr) | 9 |
Preparation and analysis of cellular fractions.
Bacteria were harvested in the late logarithmic phase of growth by centrifugation at 16,000 × g for 10 min at 4°C. Envelopes were prepared by a modification of the method outlined by Caffrey and Owen (4). Briefly, envelopes isolated following French pressure lysis of bacterial cells were sedimented by centrifugation (48,000 × g for 60 min at 4°C) and washed twice in 30-ml volumes of 10 mM Tris-HCl (pH 7.2) and once in 3 ml of the same buffer. The standard conditions for sedimentation of envelope fractions were 48,000 × g, 60 min, and 4°C. The envelopes were finally resuspended in 1 ml of the same buffer and aliquoted for storage at −70°C for further manipulations.
To prepare culture supernatant fractions, strains were grown overnight at 37°C in 100 ml of LB broth. After centrifugation at 12,000 × g for 10 min, supernatants were concentrated and size fractionated (50 kDa) by using Ultrafilters (Biomax-50 Ultrafree; Millipore, Bedford, Mass.).
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (21) was performed by using 12.5% (wt/vol) acrylamide separating gels and 4.5% (wt/vol) acrylamide stacking gels. Samples were routinely heated for 5 min at 100°C in Laemmli sample buffer (21) prior to loading. Proteins were detected by staining with Coomassie brilliant blue R250. Western immunoblotting was performed essentially as described by Caffrey et al. (3). Dried skimmed milk (5% [wt/vol]) was used as a blocking reagent. Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G was used as the localizing reagent, and reacting bands were visualized by reaction with BCIP (5-bromo-4-chloro-3-indolylphosphate) and nitroblue tetrazolium as described previously (1).
Mutagenesis and complementation.
To construct the pet mutant (JIF1), an internal portion of the pet gene (nucleotides 312 to 1460; see GenBank nucleotide sequence accession no. AF056581) was generated by PCR and cloned into the KpnI and SacI sites of the suicide vector pJP5603, whose replication requires a copy of the R6K pir-encoded π protein supplied in trans (30). The resulting plasmid, pJIF, was propagated in E. coli DH5α λpir prior to transformation into the donor E. coli strain S17-1 λpir. The mutant strain JIF1 was then obtained by conjugal mating between the wild-type parent strain 042 (which is nalidixic acid resistant) and E. coli S17-1 λpir (pJIF). Transconjugants were selected on LB agar supplemented with kanamycin and nalidixic acid. This process resulted in integration of pJIF into the homologous site in the pet gene (and hence a merodiploid state), generating strain JIF1. Notably, the requirement of autotransporter proteins for both N- and C-terminal secretion domains implies that no Pet-derived product is secreted from strain JIF1.
trans complementation of the JIF1 mutation was accomplished by the introduction of pCEFN1, which contains the complete pet gene cloned into the vector pSPORT1 (9). For these constructions, agglutination with antiserum to the 042 O antigen (serogroup O44) and the ability to utilize lactose on MacConkey plates were confirmed. The correct location of the merodiploid moiety was confirmed by Southern analysis.
PCR and molecular cloning procedures.
Amplifications were performed by using 500 ng of purified pAA2 plasmid DNA as a template and 0.2 μM each primer in a 100-μl volume of reaction mixture containing 2 U of Taq DNA polymerase (Stratagene, Inc., La Jolla, Calif.), 50 μM each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 10 μl of the buffer provided by Stratagene. Forty cycles of 1-min denaturation at 94°C, 1-min primer annealing at 60°C, and 1-min extension by Taq polymerase at 72°C were carried out. PCR was performed by using the following primers: a forward primer (5′-CCGCAAATGGAGCTGCAAC-3′), corresponding to a region 312 to 334 bp from the first nucleotide of the pet gene, and a reverse primer (5′-CGAGTTTTCCGCCGTTTTC-3′), which is complementary to a region 1,432 to 1,460 bp from the first nucleotide. The forward and reverse primers contained engineered KpnI and SacI sites, respectively. The 1,148-bp PCR product recovered from an 0.8% agarose gel was subsequently cleaved with SacI and KpnI and ligated into the corresponding sites in pJP5603.
DNA analysis and manipulations were performed according to standard methods (1). Plasmid DNA was extracted by using the Plasmid Midi kit (Qiagen Inc., Chatsworth, Calif.). Purification of DNA fragments and extraction from agarose gel slices were performed by using the PCR Wizard kit (Promega, Madison, Wis.). Plasmid DNA was introduced into E. coli DH5α λpir and S17-1 λpir by transformation of competent cells according to the method of Hanahan (12).
Ussing chamber experiments.
Six pieces of rat jejunum removed from adult male Sprague-Dawley rats under sodium pentobarbital anesthesia were placed in ice-cold Ringer’s solution for mammals and gassed with an O2-CO2 (95%:5%) mixture. The excised segments were cut open along their mesenteric borders, washed with cold Ringer’s solution, and mounted between the circular openings of six adjacent Ussing hemichambers; a known positive control and an appropriate negative control were always assayed in parallel with the test samples. Each hemichamber was filled with 10 ml of gassed Ringer’s solution and kept at 37°C under constant O2-CO2 bubbling. The transepithelial electrical potential difference (PD) was measured at 10-min intervals, and the total tissue conductance and Isc were calculated (11). Tested concentrated culture supernatants contained 25 μg of total protein/ml. Statistical differences were tested by using Student’s t test, with a P value of <0.05 taken as the level of significance.
IVOC.
Histologically normal mucosal samples from the transverse colon were obtained from pediatric patients undergoing endoscopic investigation. Institutional ethical approval and the consent of the parents after they had been fully informed were obtained. Tissue from seven patients (five male and two female patients) ranging in age from 62 to 182 months (median age, 129 months) was available. By the IVOC method (17), done essentially as described by Knutton et al. (19, 20), each intestinal biopsy specimen was mounted (mucosa side up) on a sponge in a petri dish at 37°C in an atmosphere composed of 95% O2 and 5% CO2. Tissue was partially submerged in a medium consisting of a 1:1 mixture of NCTC-135 medium and Eagle’s minimum essential medium containing 0.5% (wt/vol) d-mannose, with 10% (vol/vol) newborn calf serum. The medium was changed every 2 h to maintain the pH and nutrient supply. Bacteria were grown in brain heart infusion broth for 18 h at 37°C without agitation. Fifty microliters of the overnight culture was applied to the tissue and incubated for 8 h; an uninoculated specimen was included in each experiment as a negative control. At the conclusion of the experiment, tissue specimens were washed three times in fresh IVOC medium to remove any nonadherent bacteria and were processed for scanning electron microscopy (SEM) with a JEOL JSM 5300 microscope as described previously (19).
Enumeration of adherent bacteria was performed by counting bacteria in 10 random fields per specimen at a fixed magnification of ×3,500. The median number of adhering bacteria per field was calculated, and the data among tissue sections were compared by using the Mann-Whitney test, in which a P value of <0.05 was taken as significant. Crypt openings were measured from SEM photomicrographs at a magnification of ×500. An average of 19 openings (range, 11 to 31 openings) were measured per sample, in a blinded fashion, and the median diameter, range, and standard deviation were calculated. Statistical comparisons were performed by using the Mann-Whitney test as described above.
Cell culture methods.
T84 cells were grown as polarized monolayers on 12-mm-diameter Snapwell polyester membranes (Costar). Bacterial samples consisting of 20 μl of an overnight LB broth culture of the test strain were added to each well along with 1 ml of fresh medium; assay cultures were incubated for 3 h. At the end of the 3-h incubation the cells were washed, and they were incubated for a further 3 h in the presence of fresh medium. To assess the effects of purified Pet protein, each monolayer was incubated with tissue culture medium containing 20 μg of Pet protein/ml, prepared from the supernatant of HB101(pCEFN1) as described previously (29). Incubation was performed for 3 h and then the cells were washed and fresh medium containing a similar concentration of Pet protein was reapplied; incubation was continued for a further 3 h. At the end of the 6-h incubation, tissue culture monolayers were glutaraldehyde fixed and embedded as described previously (22) and were examined on a JEOL JEM 1200EX11 transmission electron microscope.
RESULTS
Construction and complementation of a pet mutant.
To test the effect of Pet in the IVOC system, we constructed an isogenic pet mutant of strain 042. Sequence analysis of the region downstream of the pet gene (see GenBank nucleotide sequence accession no. AF053947) suggested that pet (carried on plasmid pAA2 in the prototype EAEC strain 042) is followed by a hairpin termination motif (9) and subsequently by an insertion-like open reading frame in the opposite orientation. Since polar effects were therefore unlikely to occur, the pet gene was inactivated by integration of a suicide plasmid, pJP5603, into which had been cloned an internal fragment of pet (see Materials and Methods). This single crossover strategy yielded a mutant (JIF1) which expressed only the N-terminal 100 amino acids of Pet.
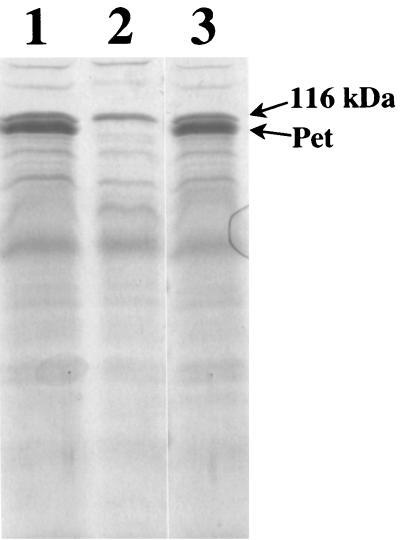
Culture supernatants of the wild-type 042 and mutant JIF1 strains were analyzed by SDS-PAGE (Fig. 1) and Western immunoblotting. These experiments documented the absence of either the mature Pet protein or any Pet-derived truncation product in JIF1 supernatants. To complement the pet mutation, the pet minimal clone pCEFN1 was electroporated into JIF1 (to create strain JIF2). Analysis of culture supernatants from JIF2 indicated that expression of Pet was restored (Fig. 1). In vitro growth rates of both the pet mutant and JIF2 were reduced compared to that of the wild-type strain. When grown in IVOC media, 042, JIF1, and JIF2 displayed generation times of 48, 62, and 81 min, respectively.
FIG. 1.
SDS-PAGE analysis of pet mutants. Concentrated culture supernatants from strains 042 (lane 1), JIF1 (lane 2), and JIF2 (lane 3), which have the presence, absence, and complemented expression of Pet, respectively, are shown. The positions of Pet and the 116-kDa secreted protein are indicated.
Effect of 042, JIF1, and JIF2 on intestinal explants.
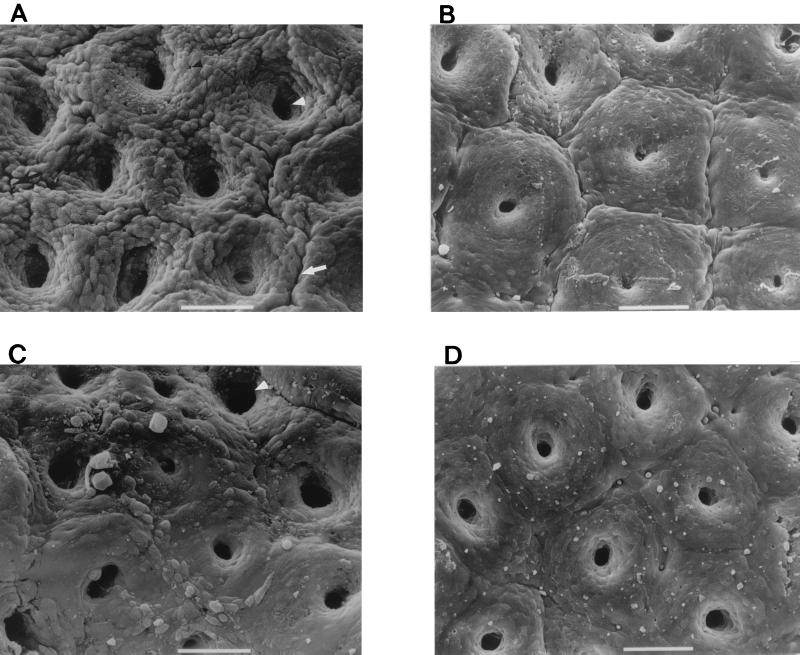
Fresh colonic mucosal biopsy specimens obtained from seven pediatric patients were divided into quarters, and sections were infected with 042, JIF1, JIF2, or no bacteria for 8 h (Fig. 2). Uninoculated control IVOC specimens appeared morphologically normal; i.e., few bacteria were noted on the mucosal surface. There was no evidence of extrusion of enterocytes or of mucus release (Fig. 2D). SEM of 042-infected tissue revealed the presence of many bacteria adhering to the mucosal surface of each specimen; the number of adherent bacteria was significantly greater than that in uninfected (control) tissue samples (P < 0.0001). Notably, although the bacteria had access to all sides of the tissue sections, adherence was observed only on the mucosal surface. As previously reported (25), dramatic abnormalities of the mucosal surface were apparent in 042-infected tissue (Fig. 2A). These effects included dilation of the crypt openings, development of prominent intercrypt crevices, and rounding and extrusion of colonic enterocytes. The stomata of goblet cells were pitted (i.e., devoid of their apical mucus plugs).
FIG. 2.
Scanning electron photomicrographs of in vitro-cultured human colonic tissues infected with the EAEC strain 042 (A), the pet mutant strain JIF1 (B), and the complemented strain JIF2 (C) for 8 h, and of uninoculated colonic tissue (D). All of these tissue sections derived from the same intestinal biopsy specimen. For the tissues shown in panels A and C the surface of the colon is markedly abnormal, as manifested by increased crypt apertures (white arrowheads), prominent mucosal crevices (white arrow), goblet cell pitting (black arrowhead), and rounding of epithelial cells (black arrow). Bars, 50 μm.
JIF1-infected tissue contained significantly more bacteria than did control specimens, and numbers of bacteria were similar to those observed for 042. However, JIF1 was found to induce substantially less damage to the colonic mucosa than its wild-type parent. The diameters of crypt openings in JIF1-infected tissue were appreciably smaller than those induced by 042, and intercrypt crevices were not apparent (Fig. 2B). However, some rounding of epithelial cells and minimal mucus release were apparent in JIF1-exposed specimens; qualitatively, these effects appeared slightly greater in degree compared to the appearance of the uninoculated controls. For the specimens infected with the pet-complemented strain JIF2, significantly fewer adhering bacteria were found than for 042-infected specimens (P < 0.0001) or JIF1-infected specimens (P < 0.006), but the number of bacteria on JIF2-infected tissue was significantly greater than that on negative controls (P < 0.0001). JIF2-infected tissue induced morphologic changes similar to those induced by 042 (Fig. 2C).
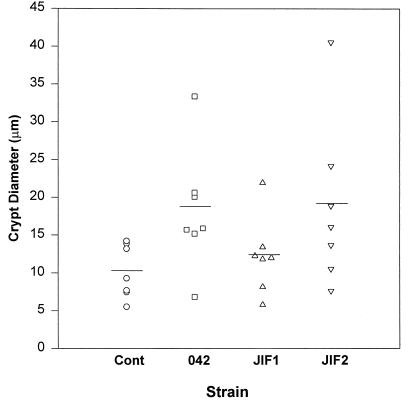
Noting that dilation of crypt apertures appeared to be a consistent manifestation of EAEC-induced mucosal changes, we utilized the measurement of crypt apertures as a quantitative morphometric method to assess the effects induced by 042 and its derivatives. As shown in Fig. 3, the mean crypt aperture diameter was greater in 042-infected tissue than in control tissue (P < 0.02 by Mann-Whitney test) or tissue infected with JIF1 (P < 0.05). JIF2 induced a crypt aperture diameter that was significantly greater than those in control tissue (P < 0.02) and JIF1-infected tissue (P < 0.04), but the diameter was not different from that in 042-infected tissue (P > 0.05).
FIG. 3.
Scattergram illustrating crypt aperture diameters induced by 042 and mutant strains in the IVOC model. An average of 19 openings (range, 11 to 31 openings) were measured per sample, in blinded fashion. Means per sample are illustrated by separate data points, and overall means are indicated by the horizontal bars. P values are noted in the text. Cont, control.
Effect of Pet on T84 cells.
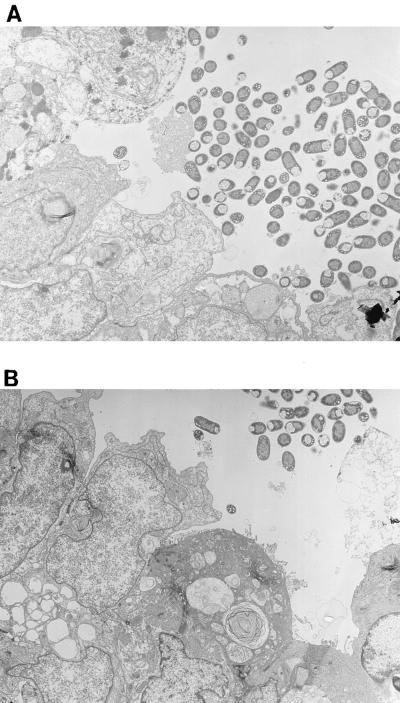
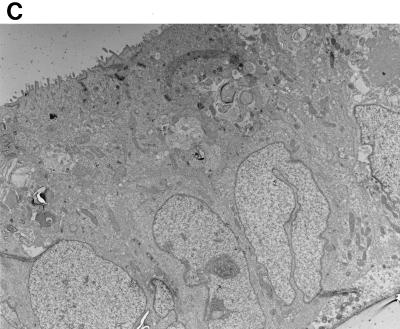
Previous work by Nataro et al. (25) demonstrated that strain 042 adhered in loose aggregates to the surfaces of T84 cells and induced marked cytopathic changes, characterized by denudation of microvilli, increased cytoplasmic vacuolization, apical plasma membrane vesiculation, and ballooning of the apical cytoplasm. In view of the Pet-associated effects observed in IVOC, we sought to determine whether the toxicity observed in T84 cells was also dependent on the Pet toxin. Incubation of T84 cells with 042 produced the changes previously reported (25). However, the pet mutant strain JIF1 did not display noticeably attenuated cytotoxic effects, and the cells appeared similar to those of the parent strain, 042 (Fig. 4A and B), and to those of the complemented mutant strain, JIF2 (data not shown). To determine whether effects of Pet were being masked by other 042 products, T84 cells were incubated with purified Pet protein. The purified Pet protein did not induce appreciable cytopathic changes of the T84 cells when added to either the apical or basolateral compartments (Fig. 4C).
FIG. 4.
Transmission electron photomicrographs of T84 cell monolayers infected for 6 h. (A) EAEC strain 042. The appearance of the cells is comparable to that in images previously published by Nataro et al. (25); i.e., the monolayer demonstrates adherent bacteria, loss of microvilli, ballooning of the apical cytoplasm, and subnuclear vacuolization. (B) The pet mutant strain JIF1. The effects induced by the pet mutant strain are similar to those observed for the wild-type strain (042). (C) Pet-treated T84 cells. The appearance of the cells is similar to that of uninfected T84 cells.
Ussing chamber experiments.
Previously published results of Ussing chamber experiments indicated that an enterotoxic moiety of EAEC strain 042 was contained in the culture supernatant fraction with molecular mass of >100 kDa (28). Additional experimentation indicated that the most likely candidate for the enterotoxin was Pet, and not the 116-kDa secreted protein that is also present in these fractions. In agreement with these prior observations, the mean ΔIsc and ΔPD after addition of the 042 supernatant fraction were significantly greater (P < 0.001) than those induced by the JIF1 supernatant fraction (Fig. 5). However, JIF1-induced rises were significantly greater than the changes produced by LB medium alone (P < 0.01). In all of the above-described experiments, the changes in Isc observed were accompanied by parallel increases in PD after addition of the test samples but not by changes in tissue conductance.
FIG. 5.
Enterotoxic activity of concentrated culture supernatants derived from 042 and the pet mutant strain JIF1. Supernatants from overnight cultures were size fractionated (saving the fraction with mass >50 kDa), and equivalent loadings were added to each Ussing chamber, into which had been mounted full-thickness rat jejunal tissue. Data points are the means of at least three experiments; error bars represent standard errors of the means. The supernatants from 042 and JIF1 generated significant rises in PD and Isc compared with those of negative controls (P < 0.05, Student’s t test).
DISCUSSION
A plausible explanation for EAEC-induced diarrhea, and its persistent nature, involves mucosal damage, possibly mediated by a secreted cytotoxin. Indeed, previous studies have reported that the proven human pathogen, EAEC strain 042, induces mucosal damage in the IVOC model (25). Although the factor(s) inducing the observed toxic manifestations was not identified, it was shown that the presence of the large AA plasmid (pAA2) of 042 was required for induction of these effects. In other studies, we have shown that the plasmid pAA2 encodes a high-molecular-weight secreted autotransporter protein (Pet) that induces the loss of actin microfilaments and cell rounding of HEp-2 and HT29 cells in culture (9, 29). Here, using the IVOC model, we found that the mucosal abnormalities induced by the wild-type EAEC strain (dilation of the crypt openings, extrusion of colonic enterocytes, development of intercrypt crevices, and loss of apical mucus from goblet cells) are not induced by a pet mutant. To fulfill the molecular requirements of Koch’s postulates, the pet mutation was complemented with a pet minimal clone, which restored both Pet expression and the capacity to induce toxic effects on the intestinal explants. These data indicate that the pet gene is required for the full spectrum of 042-induced mucosal toxicity.
Of note, our pet mutant was complemented with a high-copy-number plasmid clone, which apparently resulted in prolonged generation times yet a higher level of toxin expression per bacterial cell (13). As might be expected, therefore, the complemented construction yielded a significantly diminished level of bacterial adherence compared with the wild-type parent but, notably, a similar degree of mucosal toxicity.
We cannot yet conclude that Pet is itself sufficient to cause the mucosal effects we have described. Indeed, the dose of Pet required to elicit HT29 cell rounding is relatively high (>10 nM), and preliminary experiments applying Pet to IVOC specimens have not resulted in substantial crypt dilation (15). Thus, it is likely that other EAEC factors contribute to mucosal toxicity; our data for T84 cells also support this conclusion. T84 cells are considered to be most similar to intestinal crypt cells (25), which are not visualized by scanning electron microscopy in the IVOC model and which may indeed be resistant to the effects of Pet.
The nature of EAEC-related diarrhea suggests the presence of one or more enterotoxins. This and previous studies have shown that a substance secreted by EAEC strain 042 induces ion transport alterations in rat ileal mucosa, consistent with a secretory response. That Pet contributes to the enterotoxic activity of 042 is supported by the significant differences for the ΔIsc and ΔPD values observed between 042 culture supernatants and JIF1 culture supernatants. Despite the evidence for enterotoxicity demonstrated by Pet, the significant rises in Isc and PD induced by the pet mutant suggest the presence of another EAEC enterotoxin. Further work will address the nature of this enterotoxic moiety; candidates include EAST1 (31) and Shigella enterotoxin 1 (7, 13).
Our data support an important role for Pet in 042-induced mucosal toxicity, yet the results for the T84 cell model, in conjunction with the Ussing chamber data, suggest that other factors also contribute to EAEC pathogenesis. It is important to note that Pet appears to be expressed by only a minority of EAEC strains; whether these strains are of increased virulence is currently being addressed in epidemiologic studies. The search for other virulence factors of EAEC constitutes a high research priority.
ACKNOWLEDGMENTS
This work was supported by U.S. Public Health Service grants AI33096 and AI43615 to J.P.N.
We thank Klara Margaretten and Alessio Fasano for assistance with Ussing chamber experiments.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1989. [Google Scholar]
- 2.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 3.Caffrey P, McVeigh T, Owen P. Western immunoblotting. In: Owen P, Foster T J, editors. Immunochemical and molecular genetic analysis of bacterial pathogens. Amsterdam, The Netherlands: Elsevier Science Publishing; 1988. pp. 255–266. [Google Scholar]
- 4.Caffrey P, Owen P. Purification and N-terminal sequence of the alpha subunit of antigen 43, a unique protein complex associated with the outer membrane of Escherichia coli. J Bacteriol. 1989;171:3634–3640. doi: 10.1128/jb.171.7.3634-3640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobeljic M, Miljkovic-Selimovic B, Paunovic-Todosijevic D, Velickovic Z, Lepsanovic Z, Zec N, Savic D, Ilic R, Konstantinovic S, Jovanovic B, Kostic V. Enteroaggregative Escherichia coli associated with an outbreak of diarrhoea in a neonatal nursery ward. Epidemiol Infect. 1996;117:11–16. doi: 10.1017/s0950268800001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 7.Czeczulin J R, Whittam T S, Henderson I R, Nataro J P. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect Immun. 1999;67:2692–2699. doi: 10.1128/iai.67.6.2692-2699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott S J, Kaper J B. Role of type 1 fimbriae in EPEC infections. Microb Pathog. 1997;23:113–118. doi: 10.1006/mpat.1997.0135. [DOI] [PubMed] [Google Scholar]
- 9.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eslava C, Villaseca J, Morales R, Navarro A, Cravioto A. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Identification of a protein with toxigenic activity produced by enteroaggregative Escherichia coli, abstr. B105; p. 44. [Google Scholar]
- 11.Guandalini S, Fasano A, Migliavacca M, Marchesano G, Ferola A, Rubino A. Effects of berberine on basal and secretagogue-modified ion transport in the rabbit ileum in vitro. J Pediatr Gastroenterol Nutr. 1987;6:953–960. doi: 10.1097/00005176-198711000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, I. R., and J. P. Nataro. 1999. Unpublished observations.
- 14.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:337–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 15.Hicks, S., I. Henderson, A. D. Phillips, and J. P. Nataro. Unpublished observations.
- 16.Hicks S, Candy D C, Phillips A D. Adhesion of enteroaggregative Escherichia coli to formalin-fixed intestinal and ureteric epithelia from children. J Med Microbiol. 1996;44:362–371. doi: 10.1099/00222615-44-5-362. [DOI] [PubMed] [Google Scholar]
- 17.Hicks S, Candy D C, Phillips A D. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun. 1996;64:4751–4760. doi: 10.1128/iai.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y, Nagano I, Kunishima M, Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable:H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol. 1997;35:2546–2550. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton S, Shaw R K, Bhan M K, Smith H R, McConnell M M, Cheasty T, Williams P H, Baldwin T J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Maunsbach A B. Fixation of cells and tissues for transmission electron microscopy. In: Celis J E, editor. Cell biology: a laboratory handbook. San Diego, Calif: Academic Press; 1994. pp. 105–116. [Google Scholar]
- 23.Morabito S, Karch H, Mariani-Kurkdjian P, Schmidt H, Minelli F, Bingen E, Caprioli A. Enteroaggregative, Shiga toxin-producing Escherichia coli O111:H2 associated with an outbreak of hemolytic-uremic syndrome. J Clin Microbiol. 1998;36:840–842. doi: 10.1128/jcm.36.3.840-842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nataro J P, Deng Y, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 25.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Garcia F, Eslava C, Villaseca J M, Lopez-Revilla R, Czeczulin J R, Srinivas S, Nataro J P, Cravioto A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3149–3154. doi: 10.1128/iai.66.7.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro-Garcia F, Sears C, Eslava C, Cravioto A, Nataro J. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–2192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penfold R J, Pemberton J M. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene. 1992;118:145–146. doi: 10.1016/0378-1119(92)90263-o. [DOI] [PubMed] [Google Scholar]
- 31.Savarino S J, Fasano A, Watson J, Martin B M, Levine M M, Guandalini S, Guerry P. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 represents another subfamily of E. coli heat-stable toxin. Proc Natl Acad Sci USA. 1993;90:3093–3097. doi: 10.1073/pnas.90.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon R, Priefer U, Pohler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 33.Smith H R, Cheasty T, Rowe B. Enteroaggregative Escherichia coli and outbreaks of gastroenteritis in the UK. Lancet. 1997;350:814–815. doi: 10.1016/s0140-6736(05)62611-6. [DOI] [PubMed] [Google Scholar]