Abstract
Simple Summary
Approximately 80% hepatocellular carcinoma (HCC) patients are in intermediate or advanced stages at diagnosis and have lost the chance of curative surgery, resulting in poor prognosis. Lenvatinib was approved in 2018 as a first-line treatment for unresectable HCC. The aim of this study was to evaluate the efficacy and safety of lenvatinib as a first-line treatment for unresectable HCC by reviewing the currently available data and comparing lenvatinib with sorafenib. An overview of 24 studies indicates that lenvatinib can provide better tumor responses and survival benefits as compared with sorafenib for unresectable HCC patients, with a comparable incidence of adverse events.
Abstract
Lenvatinib was approved in 2018 as a first-line treatment for patients with unresectable hepatocellular carcinoma (HCC). This systematic review and meta-analysis aimed to provide the most updated evidence about the efficacy and safety of lenvatinib as a first-line treatment for unresectable HCC. An electronic search of the PubMed database, Web of Science, Embase, and Cochrane Library was undertaken to identify all relevant studies up to May 2022. The pooled effect sizes were calculated based on the random-effects model. One phase III randomized controlled trial and 23 retrospective studies of 2438 patients were eligible for analysis. For patients treated with lenvatinib as first-line treatment, the pooled median overall survival (OS), median progression-free survival (PFS), 1-year OS rate, 1-year PFS rate, objective response rate (ORR), and disease control rate (DCR) were 11.36 months, 6.68 months, 56.0%, 27.0%, 36.0% and 75.0%, respectively. Lenvatinib showed a significantly superior efficacy compared with sorafenib (HR for OS, 0.85 and HR for PFS, 0.72; OR for ORR, 4.25 and OR for DCR, 2.23). The current study demonstrates that lenvatinib can provide better tumor responses and survival benefits than sorafenib as a first-line treatment for unresectable HCC, with a comparable incidence of adverse events.
Keywords: lenvatinib, sorafenib, hepatocellular carcinoma, review, meta-analysis
1. Introduction
Liver cancer is the 6th most common malignant tumor and the 4th leading cause of cancer-related death worldwide, with 841,000 new cases diagnosed per year [1]. Among primary liver cancers, hepatocellular carcinoma (HCC) represents the major histological subtype, accounting for 70–85% of all liver cancer cases [2]. Surgical resection and liver transplantation are effective treatments for patients with early stage HCC. However, due to the insidious onset, approximately 80% HCC patients are already in intermediate or advanced stages at diagnosis and have lost the chance of curative surgery, resulting in poor prognosis [3,4]. Based on the results from the SHARP and Asia-Pacific trials [5,6], sorafenib became the first approved systemic therapy for unresectable HCC in 2007. Lenvatinib, an oral multi-kinase inhibitor of vascular endothelial growth factor receptor (VEGFR) 1-3, platelet-derived growth factor receptor (PDGFR) α, fibroblast growth factor receptor (FGFR) 1-4, and RET and KIT proto-oncogenes, has previously been used in the treatment of papillary differentiated thyroid carcinoma and advanced endometrial cancer [7]. In 2018, supported by a phase III clinical trial, lenvatinib was also approved as a first-line treatment for unresectable HCC [8]. Since then, many researchers have assessed the treatment outcomes of lenvatinib for these patients. However, as most data are based on small single-center series, it is difficult to reach a consensus. In addition, the results of comparative studies of lenvatinib versus sorafenib are conflicting [9].
The aim of this study was to evaluate the efficacy and safety of lenvatinib as a first-line treatment for unresectable HCC by reviewing the currently available data, and comparing lenvatinib with sorafenib.
2. Materials and Methods
Systematic review and meta-analysis were conducted in accordance with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [10]. The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42022356713). Meta-analysis of the present study strictly followed the PRISMA checklist for study search, screening, data extraction and analysis.
2.1. Search Strategy
An electronic search of PubMed database, Web of Science, Embase, and Cochrane Library was undertaken to identify all relevant studies published in English up to May 2022, using the following terms: hepatocellular carcinoma, HCC, liver cancer, lenvatinib and molecular-targeted agents. For additional citations, the reference list of retrieved articles was reviewed.
2.2. Study Selection
The literature search was conducted by two researchers (S.J.W and Y.T.W) independently. The inclusion criteria were as follows: (1) all study types except for case reports; (2) unresectable HCC patients receiving first-line treatment with lenvatinib as the study participants whose information could be extracted from a subgroup; and (3) studies that clearly reported the characteristics of the participants and post-treatment clinical responses.
2.3. Data Extraction
Data were extracted independently by two independent investigators (S.J.W and Y.T.W); any disagreement was resolved through discussion, or through further consultation with a third investigator (Y.M.Z). The first author, year of publication, country, study design, patient details, number of patients, overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs) were extracted. For studies that did not directly indicate OS or PFS, information was derived from the reported survival plots. The hazard ratios (HRs) for OS and PFS with 95% confidence intervals (CIs) were extracted from each controlled study, or from the Kaplan–Meier curves in accordance with the protocol from Tierney et al. [11], if not available. For propensity score matched studies, only data after matching were considered.
2.4. Quality Assessment
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS) for cohort studies [12] or the Jadad Scale for randomized trials [13]. The studies with scores greater than or equal to 6 using NOS or that with scores greater than 2 using Jadad Scale were considered high quality.
2.5. Statistical Analysis
All statistical analyses were performed using Stata statistical software version 14.0. Survival data, including OS and PFS, are presented with HRs and 95% CI. Odds ratios (ORs) and 95% CIs were calculated for the analysis of tumor response and AEs. Meta-analyses of data were estimated with a random effects model. The I2 statistic was used to assess heterogeneity of the studies, with a value more than 50% defined as significant.
3. Results
3.1. Search Results
A total of 2132 articles from the databases and 36 articles from the reference list were identified by the initial search strategy, from which 1449 articles were excluded due to duplication. After screening the titles and abstracts, 464 unrelated studies were excluded, and by reading the full text, an additional 230 articles were excluded. One article was excluded because the study population had been included in a previous study [8]. Eventually, 24 studies involving patients with unresectable HCC were included in this analysis. A flowchart depicting the study selection is shown in Figure 1.
Figure 1.
Flow diagram of the selection process.
Patient Characteristics
Of the 24 studies included, one was a phase III randomized controlled trial (RCT) [8], and the other 23 were retrospective studies [8,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35], involving 2438 patients with unresectable HCC who received first-line treatment with lenvatinib. The initial dose of oral lenvatinib was 12 mg QD for patients with a body weight of ≥60 kg, or 8 mg QD for those with a body weight of <60 kg. Of the 23 studies that assessed the tumor response, nineteen studies [9,14,15,16,17,18,19,20,22,23,24,25,27,28,31,32,33,34,35] applied the mRECIST, one [32] applied the RECIST 1.1, one [21] applied the RECIST 1.1 and mRECIST, and two [26,30] were unknown. Details of all studies and the characteristics of the patients are presented in Table 1.
Table 1.
Baseline characteristics of patients recruited in the included studies.
| Study (Years) | Country | Regimen | MFUT | Patients | Age | Female | ECOG PS: 0/1/2 |
Child–Pugh Class: A/B/C |
HBV/HCV | MVI | EHS | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-arm study | ||||||||||||
| Takahashi, 2019 [14] | Japan | Lenvatinib | 6.6 | 50 | Median = 78 | 12 | 37/12/1 | 47/3/0 | 9/18 | 8 | 13 | 6 a |
| Hayashi, 2020 [15] | Japan | Lenvatinib | 6.9 | 53 | Median = 73 | 11 | NA | 53/0/0 | 7/15 | 10 | 18 | 6 a |
| Maruta, 2020 [16] | Japan | Lenvatinib | 6.7 | 95 | 47 (≤50 years) | 20 | 90 (0 or 1) | 84/11/0 | 13/41 | 25 | 33 | 7 a |
| Sho, 2019 [17] | Japan | Lenvatinib | NA | 18 | Median = 75 | 0 | 11/7/0 | 18/0/0 | 3/2 | NA | 4 | 7 a |
| Goh, 2021 [18] | Korea | Lenvatinib | 7.2 | 48 | Median = 57 | 7 | 38/NA/NA | 48/0/0 | NA | 20 | 39 | 7 a |
| Koroki, 2021 [19] | Japan | Lenvatinib | NA | 178 | 94 (≤73 years) | 33 | 169 (0 or 1) | 150/NA/NA | 25/70 | 45 | 64 | 7 a |
| Shimozato, 2022 [20] | Japan | Lenvatinib | NA | 98 | Median = 76 | 20 | 91/7/0 | 98/0/0 | 19/34 | 14 | 20 | 6 a |
| Singal, 2021 [21] | USA | Lenvatinib | 9.1 | 233 | Median = 62.9 | 75 | 75/147/NA | 104/92/17 | 36/84 | NA | NA | 7 a |
| Kobayashi,2022 [22] | Japan | Lenvatinib | NA | 31 | Median = 77 | 2 | 31/0/0 | 31/0/0 | 3/6 | 0 | NA | 6 a |
| Double-arm study | ||||||||||||
| Kudo, 2018 [8] | Multinational | Lenvatinib vs. Sorafenib | 27.7 | 478 476 |
Mean = 61.3 Mean = 61.2 |
73 75 |
304/174/0 301/175/0 |
475/3/0 471/5/0 |
251/91 228/126 |
329 336 |
291 295 |
2 b |
| Kudo, 2019 [23] | Japan | Lenvatinib vs. TACE | 23 | 30 60 |
Mean = 68.2 Mean = 72.4 |
6 18 |
30/0/0 60/0/0 |
30/0/0 60/0/0 |
7/12 29/10 |
0 0 |
0 0 |
7 a |
| Kuzuya, 2020 [24] | Japan | Lenvatinib vs. Sorafenib | NA | 13 13 |
Median = 70 Median = 67 |
2 2 |
12/1/0 8/5/0 |
13/0/0 13/0/0 |
2/2 2/5 |
NA | 3 7 |
6 a |
| Nakano, 2020 [25] | Japan | Lenvatinib vs. Sorafenib | 7.3 10.5 |
146 146 |
Mean = 72.8 Mean = 72.8 |
21 25 |
NA | 134/12/0 137/9/0 |
25/77 24/81 |
21 21 |
56 55 |
6 a |
| Gardini, 2021 [26] | Multinational | Lenvatinib vs. Sorafenib | 15.8 30.7 |
360 562 |
Mean = 66.7 Mean = 66.2 |
67 105 |
110 (1 or 2) 197 (1 or 2) |
324/NA 498 |
93/138 164/206 |
196 156 |
146 255 |
7 a |
| Kim, 2021 [27] | Korean | Lenvatinib vs. Sorafenib | NA | 44 61 |
Median = 56 Median = 64 |
17 10 |
41 (0 or 1) 59 (0 or 1) |
36 (A and B) 56 (A and B) |
27/NA 45/NA |
26 23 |
25 32 |
7 a |
| Hatanaka, 2021 [28] | Japan | Lenvatinib vs. Sorafenib | 9.8 10 |
56 375 |
Median = 73.5 Median = 71.0 |
14 75 |
NA | 50/6/0 304/71/0 |
3/32 40/224 |
NA | NA | 6 a |
| Kuo, 2021 [29] | Taiwan (China) | Lenvatinib vs. Sorafenib | NA | 70 140 |
Mean = 65 Mean = 65.7 |
20 40 |
NA | 68/2/0 138/2/0 |
36/22 75/34 |
33 62 |
28 64 |
7 a |
| Rimini, 2021 [30] | Multinational | Lenvatinib vs. Sorafenib | NA | 92 92 |
23 (<65 years) 33 (<65 years) |
17 11 |
70/22/0 65/27/0 |
87/5/0 85/7/0 |
18/38 15/41 |
8 10 |
44 44 |
7 a |
| Tomonari, 2021 [31] | Japan | Lenvatinib vs. Sorafenib | 9.6 | 52 52 |
Median = 70.0 Median = 71.0 |
16 17 |
38/14/0 37/15/0 |
52/0/0 52/0/0 |
15/18 10/19 |
11 9 |
10 9 |
7 a |
| Xu, 2021 [32] | China | Lenvatinib vs. Toripalimab plus HAIC | NA | 47 47 |
19 (≤50 years) 21 (≤50 years) |
6 5 |
4/32/11 5/35/7 |
47/0/0 47/0/0 |
42/NA 43/0 |
NA | 18 15 |
6 a |
| Choi, 2022 [33] | Korea | Lenvatinib vs. Sorafenib | 4.7 6.7 |
44 88 |
Median = 58.0 Median = 58.0 |
4 8 |
23/9/NA 48/29/NA |
29/13/2 63/19/6 |
44/NA 88/NA |
NA | 31 59 |
6 a |
| Kim, 2022 [34] | Korea | Lenvatinib vs. Atezolizumab/Bevacizumab | 7.2 7.7 |
146 86 |
Median = 62 Median = 62 |
22 16 |
105/41(1 or 2) 36/50 (1 or 2) |
127/19/0 82/4/0 |
90/19 62/3 |
76 43 |
91 37 |
7 a |
| Lee, 2022 [35] | Taiwan (China) | Lenvatinib vs. Sorafenib | NA | 22 44 |
Mean = 63.95 Mean = 63.77 |
4 8 |
NA | 22/0/0 44/0/0 |
12/6 24/13 |
13 25 |
11 23 |
7 a |
| Park, 2022 [9] | Korea | Lenvatinib vs. Sorafenib | 3.7 | 34 60 |
Mean = 62 Mean = 65 |
5 13 |
NA | 0/30/4 0/56/4 |
26/NA 43/NA |
21 40 |
23 37 |
6 a |
Abbreviations: MFUT, median follow-up time; ECOG, Eastern Cooperative Oncology Group; PS, performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; MVI, macroscopic vascular invasion; EHS, extrahepatic spread; a, quality assessed using Newcastle–Ottawa Scale (NOS); b, study quality assessed using JADAD score.
3.2. Therapeutic Efficacy Assessment
3.2.1. Overall Survival and Progression-Free Survival
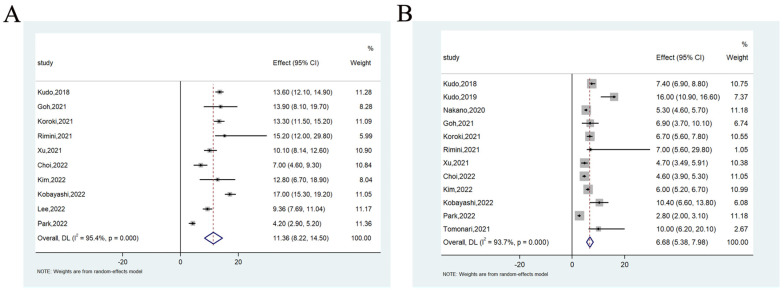
The median OS, median PFS, 1-year OS rate and 1-year PFS rate were analyzed, respectively. Data were available on median OS in 10 studies involving 1120 patients, ranging from 4.2 to 15.2 months. The meta-analysis showed that the pooled median OS was 11.36 months (95%CI = 8.22–14.50) (heterogeneity analysis: I2 = 95.4%) (Figure 2A). Data were available on median PFS in 12 studies involving 1326 patients, ranging from 2.8 to 16.0 months. The meta-analysis also showed that the pooled median PFS was 6.68 months (95%CI = 5.38–7.98) (heterogeneity analysis: I2 = 93.7%) (Figure 2B).
Figure 2.
The median OS (A) and median PFS (B) of lenvatinib in the treatment of unresectable hepatocellular carcinoma.
The 1-year OS rate was available in 17 studies involving 1751 patients, ranging from 0% to 80.3%. The meta-analysis showed that the pooled 1-year OS rate was 56.0% (95%CI = 35.0–76.0%) (heterogeneity analysis: I2 = 99.4%) (Figure 3A). The 1-year PFS rate was available in 15 studies involving 1692 patients, ranging from 0% to 64.9%. The meta-analysis showed that the pooled 1-year PFS rate was 27.0% (17.0–36.0%) (heterogeneity analysis: I2 = 98.5%) (Figure 3B).
Figure 3.
The 1-year OS rate (A) and 1-year PFS rate (B) of lenvatinib for unresectable hepatocellular carcinoma.
The I2 statistic indicated that data were heterogeneous in our analyses and therefore these summary measures should be interpreted with appropriate caution. Due to the limited studies in Western countries, subgroup analysis was not possible, and our results showed no significant decrease in heterogeneity after deleting the limited Western and multicenter studies (Supplementary Figures S1 and S2).
3.2.2. Objective Response Rate and Disease Control Rate
The ORR was available in 23 studies involving 2394 patients, ranging from 7.1% to 73.3%. The meta-analysis showed that the pooled ORR was 36.0% (95%CI = 29.0–44.0%) (heterogeneity analysis: I2 = 94.1%) (Figure 4A). The DCR was available in 21 studies involving 1856 patients, ranging from 38.2% to 100.0%. The meta-analysis showed that the pooled DCR was 75.0% (68.0–83.0%) (heterogeneity analysis: I2 = 97.0%) (Figure 4B). Heterogeneity was not substantially reduced after removing the limited Western and multicenter studies (Supplementary Figure S3).
Figure 4.
The ORR (A) and DCR (B) of lenvatinib for unresectable hepatocellular carcinoma.
3.2.3. Adverse Events
The AEs of unresectable HCC patients who received first-line treatment with lenvatinib are shown in Table 2. The most common AEs were hypertension (36.8%), followed by decreased appetite (36.4%), fatigue (34.5%), palmar–plantar erythrodysaesthesia syndrome (PPES) (29.4%), decreased weight (29.1%), proteinuria (25.6%), diarrhea (24.6%), and hypothyroidism (22.5%). Other less common AEs included elevated aspartate aminotransferase (AST) (18.0%), dysphonia (17.7%), vomiting (12.1%), nausea (16.4%), constipation (15.0%), abdominal pain (16.9%), decreased platelet count (18.2%), and bilirubin elevation (16.6%) (Table 2).
Table 2.
Adverse events of lenvatinib in patients with unresectable hepatocellular carcinoma.
| Adverse Events | All Grades | Grades 3 and 4 | ||||
|---|---|---|---|---|---|---|
| No. of Studies Included | Total Events | Total Patients | No. of Studies Included | Total Events | Total Patients | |
| Diarrhea | 19 | 493 | 2015 | 14 | 53 | 1726 |
| Hypertension | 18 | 604 | 1664 | 14 | 174 | 1397 |
| PPES | 18 | 552 | 1869 | 12 | 35 | 1220 |
| Fatigue | 17 | 586 | 1642 | 12 | 80 | 1353 |
| Proteinuria | 17 | 394 | 1633 | 13 | 83 | 1366 |
| Decreased appetite | 16 | 573 | 1568 | 12 | 76 | 1301 |
| Hypothyroidism | 16 | 343 | 1602 | 12 | 9 | 1335 |
| Decreased PC | 10 | 187 | 1030 | 7 | 36 | 909 |
| Rash | 10 | 82 | 1048 | 9 | 2 | 1030 |
| Elevated AST | 10 | 118 | 1045 | 9 | 47 | 995 |
| Hepatic encephalopathy | 7 | 33 | 431 | 5 | 12 | 363 |
| Bilirubin elevation | 7 | 79 | 476 | 7 | 14 | 476 |
| Dysphonia | 6 | 138 | 780 | 6 | 2 | 780 |
| Nausea | 6 | 133 | 812 | 5 | 5 | 762 |
| Vomiting | 6 | 99 | 817 | 6 | 7 | 817 |
| Abdominal pain | 5 | 113 | 668 | 5 | 9 | 668 |
| Decreased weight | 5 | 203 | 698 | 4 | 38 | 680 |
| Fever | 3 | 4 | 83 | 2 | 0 | 65 |
| Stomatitis | 3 | 8 | 101 | 2 | 0 | 83 |
| Alopecia | 3 | 14 | 572 | 3 | 0 | 572 |
| Constipation | 2 | 78 | 520 | 2 | 3 | 520 |
| Increased SCR | 2 | 4 | 62 | 2 | 0 | 62 |
| Decreased albumin | 2 | 4 | 83 | 1 | 0 | 65 |
Abbreviations: PPES, palmar–plantar erythrodysaesthesia; PC, platelet count; AST, aspartate aminotransferase; SCR, serum creatinine.
Not all studies reported severe forms of (grade 3–4) AEs. According to the valid data, the most common severe AEs were hypertension (12.5%), followed by proteinuria (6.02%), fatigue (5.9%), decreased appetite (5.8%), and decreased weight (5.6%) (Table 2).
3.3. Lenvatinib Versus Sorafenib
3.3.1. Overall Survival
A total of 11 studies compared the OS of lenvatinib and sorafenib as first-line treatment for unresectable HCC patients. Of them, two studies showed that lenvatinib prolonged OS compared with sorafenib, and nine studies showed no significant difference. The meta-analysis showed that the pooled HR was 0.85 (95%CI = 0.73–0.99) (heterogeneity analysis: I2 = 41.1%), and lenvatinib mediated significantly better OS than Sorafenib (Figure 5A).
Figure 5.
Forest plots of the OS (A) and PFS (B) associated with lenvatinib vs. sorafenib.
3.3.2. Progression-Free Survival
A total of eight studies compared PFS between lenvatinib and sorafenib as first-line treatment for unresectable HCC patients. Of them, five studies showed that lenvatinib prolonged PFS compared with sorafenib, and three studies showed no significant difference. Meanwhile, the meta-analysis showed that the pooled HR was 0.72 (95%CI = 0.61–0.84) (heterogeneity analysis: I2 = 47.2%), and lenvatinib mediated significantly better OS than Sorafenib did (Figure 5B).
3.3.3. Objective Response Rate
A total of 10 studies reported ORR of lenvatinib and sorafenib as first-line treatment for unresectable HCC patients. Of them, eight studies showed that lenvatinib was associated with a higher ORR as compared with sorafenib, and two studies showed no significant difference. The meta-analysis showed that the pooled OR was 4.25 (95%CI = 2.78–6.48) (heterogeneity analysis: I2 = 47.8%), and lenvatinib reported a higher ORR than sorafenib (Figure 6A).
Figure 6.
Forest plots of the ORR (A) and DCR (B) associated with lenvatinib vs. sorafenib.
3.3.4. Disease Control Rate
A total of ten studies reported DCR of lenvatinib and sorafenib as first-line treatment for unresectable HCC patients. Of them, six studies showed a higher DCR compared with sorafenib, and four studies showed no significant difference. The meta-analysis showed that the pooled OR was 2.23 (95%CI = 1.70–2.93) (heterogeneity analysis: I2 = 39.0%), and lenvatinib reported a higher DCR than sorafenib (Figure 6B).
3.3.5. Adverse Events
AEs between lenvatinib and sorafenib were compared based on the total number of AEs and severe (grade 3–4) AEs. The meta-analysis showed that there was no significant difference between the lenvatinib and sorafenib for all-grade (OR = 0.74, 95%CI: 0.20–2.79) (Figure 7A) and severe AEs (OR = 1.15, 95%CI: 0.88–1.50) (Figure 7B).
Figure 7.
Forest plots of the all-grade (A) and severe adverse events (B) associated with lenvatinib vs. sorafenib.
Subgroup analysis was performed on the 10 most reported AEs of lenvatinib and sorafenib in the original articles (Supplementary Figure S4). The meta-analysis showed that in comparison with sorafenib, lenvatinib did not significantly increase the incidence of AEs including fatigue (OR = 1.54, 95%CI: 0.98–2.40) and elevated AST (OR = 0.78, 95%CI: 0.57–1.07). In contrast, lenvatinib significantly reduced the incidence of PPES (OR = 0.49, 95%CI: 0.26–0.95), diarrhea (OR = 0.69, 95%CI: 0.50–0.96) and rash (OR = 0.51, 95%CI: 0.28–0.93). However, for decreased appetite (OR = 1.97, 95%CI: 1.18–3.29), decreased platelet count (OR = 1.66, 95%CI: 1.19–2.32), hypertension (OR = 2.91, 95%CI: 1.86–4.54), hypothyroidism (OR = 12.05, 95%CI: 6.32–22.98) and proteinuria (OR = 2.82, 95%CI: 2.06–3.84), the incidence was significantly higher in the lenvatinib group.
3.4. Subgroup Analysis
Subgroup analyses were performed to explore the treatment effect of lenvatinib according to the eligibility criteria of the study population and the study background.
3.4.1. Therapeutic Efficacy Assessment According to the Reflect Criteria
Overall and Progression-Free Survival
Eight of the ten studies with median OS did not meet the REFLECT criteria, and the meta-analysis showed that the pooled median OS was 10.80 months (95%CI = 7.17–14.43) (heterogeneity analysis: I2 = 95.6%) (Supplementary Figure S5A). Nine of the twelve studies with median PFS did not meet the REFLECT criteria, and the meta-analysis showed that the pooled median PFS was 6.44 months (95%CI = 5.01–7.86) (heterogeneity analysis: I2 = 94.5%) (Supplementary Figure S5A).
Fourteen of the seventeen studies with a 1-year OS rate did not meet the REFLECT criteria, and the meta-analysis showed that the pooled 1-year OS rate was 54.0% (95%CI = 32.0–77.0%) (heterogeneity analysis: I2 = 99.3%) (Supplementary Figure S6A); no significant difference was observed when compared with the overall population (p = 0.776). Twelve of the fifteen studies with a 1-year PFS rate did not meet the REFLECT criteria, and the meta-analysis showed that the pooled 1-year PFS rate was 28.0% (15.0–42.0%) (heterogeneity analysis: I2 = 97.6%) (Supplementary Figure S6B); no significant difference was observed when compared with the overall population (p = 0.874).
Objective Response Rate and Disease Control Rate
Eighteen of the twenty-three studies with ORR did not meet the REFLECT criteria, and the meta-analysis showed that the pooled ORR was 36.0% (26.0–45.0%) (heterogeneity analysis: I2 = 95.0%) (Supplementary Figure S7A); no significant difference was observed when compared with the overall population (p = 1.000). Eighteen of the twenty-three studies with DCR did not meet the REFLECT criteria, and the meta-analysis showed that the pooled DCR was 74.0% (65.0–83.0%) (heterogeneity analysis: I2 = 96.8%) (Supplementary Figure S7B); no significant difference was observed when compared with the overall analysis population (p = 0.871).
3.4.2. Therapeutic Efficacy Assessment According to the Study Background
To make the included studies mimic the clinical settings as much as possible, nine studies representing only a specific population with different clinical settings were removed.
Overall Survival and Progression-Free Survival
Five of the ten studies with median OS were included, and the meta-analysis showed that the pooled median OS was 12.31 months (95%CI = 9.50–15.13) (heterogeneity analysis: I2 = 74.3%) (Supplementary Figure S8A). Six of the twelve studies with median PFS were included, and the meta-analysis showed that the pooled median PFS was 6.31 months (95%CI = 5.30–7.32) (heterogeneity analysis: I2 = 68.7%) (Supplementary Figure S8B).
Nine of the seventeen studies with a 1-year OS rate were included, and the meta-analysis showed that the pooled 1-year OS rate was 61.0% (95%CI = 57.0–65.0%) (heterogeneity analysis: I2 = 48.4%) (Supplementary Figure S9A). Eight of the fifteen studies with a 1-year PFS rate were included, and the meta-analysis showed that the pooled 1-year PFS rate was 29.0% (13.0–45.0%) (heterogeneity analysis: I2 = 98.3%) (Supplementary Figure S9B).
Objective Response Rate and Disease Control Rate
Fourteen of the twenty-three studies with ORR were included, and the meta-analysis showed that the pooled ORR was 35.0% (26.0–44.0%) (heterogeneity analysis: I2 = 95.1%) (Supplementary Figure S10A). Thirteen of the twenty-three studies with DCR were included, and the meta-analysis showed that the pooled DCR was 76.0% (70.0–82.0%) (heterogeneity analysis: I2 = 87.3%) (Supplementary Figure S10B).
4. Discussion
As many HCC patients have lost the chance of surgery at the time of diagnosis, it is primarily important to find an optimal systemic treatment for them. Currently, sorafenib and lenvatinib are the only approved first-line systemic agents for unresectable HCC. As lenvatinib was approved as the first-line treatment for unresectable HCC only a few years ago, reports about its therapeutic efficacy are inconsistent. It is therefore necessary to conduct a meta-analysis to assess its efficacy and safety. To the best of our knowledge, this is the first and largest single-arm meta-analysis to evaluate the efficacy and safety of lenvatinib versus sorafenib as a first-line treatment for unresectable HCC patients.
Antitumor efficacy is the most predominant cornerstone in evaluating lenvatinib. Our study showed that the pooled median OS was 11.36 months, which is worse than the result of the previous phase III clinical trial [8]. The possible reason is due to the presence of patients with Child–Pugh class C in some of our included studies [9,21,33]. When we excluded these studies for subgroup analysis, the result showed that the pooled median OS was 12.90 months (Supplementary Figure S11). This obvious change also indirectly suggests that liver function is an important factor affecting the anti-tumor efficacy of lenvatinib. Regarding the survival benefit of lenvatinib in patients with hepatic decompensation (Child–Pugh class B and C), Park et al. reported a worse median OS (1.5 months) in the untreated group versus the lenvatinib group (4.2 months), which still could not prove the survival benefit of lenvatinib due to patient selection bias [9]. As with sorafenib, the first approved systemic agent for advanced HCC, lenvatinib is also recommended with caution in patients with hepatic decompensation due to the unsatisfactory survival benefits [36]. There is still some way to go before lenvatinib can be used as a first-line treatment for unresectable HCC patients with hepatic decompensation.
In addition to OS, PFS, ORR and DCR were also analyzed in this study. The results showed that the pooled median PFS, ORR and DCR were 6.68 months, 35.0% and 75.0%, respectively. Interestingly, the best median OS, median PFS, ORR, and DCR were obtained from the same study reported by Kudo et al. in 2019 [23]. The most likely reason for this favorable antitumor effect is that no patient included in their study had macroscopic vascular invasion and extrahepatic spread, and all had a Child–Pugh A liver function and Eastern Cooperative Oncology Group performance status of 0. These factors have proved to be favorable predictors of survival in patients with HCC, which are also important components of the REFLECT criteria [8,37]. However, not all patients could meet the REFLECT criteria in real-world settings, so we performed a subgroup analysis to evaluate the efficacy of lenvatinib in these patients. The results showed that the mOS and mPFS of patients who did not meet the REFLECT criteria were only slightly shorter than those in the overall population cohort, and there was no significant difference in tumor response. This proves that lenvatinib can still provide a favorable efficacy, even for patients who do not meet the REFLECT criteria. A similar result was also demonstrated in patients with advanced HCC in the study of Welland et al. [38], despite the fact that some surgically treated patients were included. When lenvatinib was compared with sorafenib, unlike the previous phase III clinical trial that showed no significant difference in OS, our meta-analysis showed that lenvatinib could significantly improve OS and PFS and offer better ORR and DCR. In addition, lenvatinib is significantly superior to sorafenib for patients with HBV infection in terms of OS (14.9 vs. 9.9 months), which further validates the antitumor efficacy of lenvatinib [39].
Drug toxicity is an indispensable factor affecting the quality of life of patients and should be taken into account when choosing optimal systemic treatment. The results in a study [40] showed that hypertension was the most common all-grade and severe AE in unresectable HCC patients who received first-line treatment with lenvatinib, which was similar to lenvatinib for other solid tumors. Interestingly, it was reported that lenvatinib-induced hypertension may be associated with better prognosis [41]. Although the specific mechanism of lenvatinib-induced hypertension is not fully understood, the possible mechanism is supposed to be associated with the interaction of neurostimulators factors, endothelin signaling pathway, renin–angiotensin aldosterone system and nitric oxide signaling pathway [42,43,44]. When compared with sorafenib, a higher incidence of decreased appetite, decreased platelet count, hypertension, hypothyroidism and proteinuria was observed in patients receiving lenvatinib, but the opposite was true for HFSR, diarrhea and rash. Overall, lenvatinib does not differ significantly from sorafenib in both the incidence of all-grade AEs and the incidence of severe AEs (grade 3–4), proving that lenvatinib is a relatively safe drug. Although lenvatinib treatment usually only lasts for months, timely recognition and management of AEs are still crucial, and avoidance of unnecessary dose reductions or interruption of the treatment is the key to ensure the antitumor efficacy.
With the increasing understanding of tumor pathogenesis, it has been found that tumor cells can evade immune system attacks through the stimulation of immune checkpoint targets; therefore, immune checkpoint inhibitors have received increasing attention in recent years [45]. In 2021, atezolizumab plus bevacizumab treatment was recommended as the first-line treatment for unresectable HCC, due to the superior survival to sorafenib [46]. However, the recently published results of a multicenter study showed that the treatment with lenvatinib is associated with a significant survival benefit compared to atezolizumab plus bevacizumab [47]. Meanwhile, the efficacy of anti-PD1 therapy in a phase III randomized trial of patients with advanced HCC has not shown statistically significant improvement due to drug resistance in some patients [48]. The mechanism of resistance was theorized in a recent study [49] arguing that the PKCa/ZFP64/CSF1 axis played a critical role in triggering immune evasion. Interestingly, lenvatinib was found to decrease PKC expression and inhibited the PKCa/ZFP64/CSF1 axis, thereby overcoming anti-PD1 resistance in HCC, while sorafenib did not [49]. Meanwhile, a real-world study reported that lenvatinib combined with PD-1-targeted immunotherapy sintilimab may lead to better long-term outcomes than lenvatinib alone [50]. Therefore, lenvatinib seems to show a survival advantage compared with sorafenib not only as monotherapy but also plays an important role in the combination of immune checkpoint inhibitors. However, the combination therapy may lead to immune checkpoint inhibitor-associated AEs, and dose personalization can reduce the related AEs and maximize patient outcomes [50,51]. Given the therapeutic advantages and synergies of lenvatinib, the potential of combination of lenvatinib and immune checkpoint inhibitors to become the new treatment trend in clinical practice is promising, which may bring hope to unresectable HCC patients.
There are several limitations in our study. First, most included studies were of a retrospective nature, which may weaken the quality of their outcomes. Second, most studies were conducted in Eastern countries, and there was a lack of studies from Western counties to perform subgroup analyses. Third, selection bias may have existed because the included articles were limited to the literature published in English. Finally, due to the limited number of studies, subgroup analysis of patients with hepatic decompensation could not be performed.
5. Conclusions
Our study demonstrated that lenvatinib provided better tumor response and survival benefits than sorafenib as first-line therapy for unresectable HCC patients, with a comparable incidence of AEs, presenting competitive therapeutic efficacy. More high-impact studies with larger samples are needed to further validate our conclusions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14225525/s1, Figure S1: The median OS (A) and median PFS (B) after deleting the limited Western and multicenter studies. Figure S2: The 1-year OS rate (A) and 1-year PFS rate (B) after deleting the limited Western and multicenter studies. Figure S3: The ORR (A) and DCR (B) after deleting the limited Western and multicenter studies. Supplementary Figure S4: Forest plots of the 10 most reported adverse events associated with lenvatinib vs. sorafenib (A, hypertension; B, diarrhea; C, decreased appetite; D, palmar–plantar erythrodysaesthesia; E, fatigue; F, proteinuria; G, rash; H, hypothyroidism; I, elevated aspartate aminotransferase; J, decreased platelet count). Figure S5: The median OS (A) and median PFS (B) for patients not meeting the REFLECT criteria. Figure S6: The 1-year OS rate (A) and 1-year PFS rate (B) for patients not meeting the REFLECT criteria. Figure S7: The ORR (A) and DCR (B) for patients not meeting the REFLECT criteria. Figure S8: The median OS (A) and median PFS (B) for patients with similar study background. Figure S9: The 1-year OS rate (A) and 1-year PFS rate (B) for patients with similar study background. Figure S10: The ORR (A) and DCR (B) for patients with similar study background. Figure S11: Median OS in patients with Child–Pugh class A or B treated with Lenvatinib.
Author Contributions
Conceptualization, S.W. and Y.Z.; methodology, S.W. and Y.W.; software, S.W. and Y.W.; validation, S.W., Y.W. and Y.Z.; formal analysis, S.W.; investigation, Y.Z.; resources, Y.Z.; data curation, J.Y. and H.W.; writing—original draft preparation, S.W.; writing—review and editing, Y.Z.; visualization, Y.Z.; supervision, Y.Z.; project administration, Y.Z. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data are available in the manuscript and its supplements.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Perz J.F., Armstrong G.L., Farrington L.A., Hutin Y.J., Bell B.P. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Jiang M., Zhu J., Qu J., Qin K., Zhao D., Wang L., Dong L., Zhang X. The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed. Pharmacother. 2020;132:110797. doi: 10.1016/j.biopha.2020.110797. [DOI] [PubMed] [Google Scholar]
- 4.Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., de Oliveira A.C., Santoro A., Raoul J.L., Forner A., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., Luo R., Feng J., Ye S., Yang T.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Rehman O., Jaferi U., Padda I., Khehra N., Atwal H., Mossabeh D., Bhangu R. Overview of lenvatinib as a targeted therapy for advanced hepatocellular carcinoma. Clin. Exp. Hepatol. 2021;7:249–257. doi: 10.5114/ceh.2021.109312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.Park M.K., Lee Y.B., Moon H., Choi N.R., Kim M.A., Jang H., Nam J.Y., Cho E.J., Lee J.H., Yu S.J., et al. Effectiveness of Lenvatinib Versus Sorafenib for Unresectable Hepatocellular Carcinoma in Patients with Hepatic Decompensation. Dig. Dis. Sci. 2022;67:4939–4949. doi: 10.1007/s10620-021-07365-9. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 18 May 2022)]. Available online: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp.
- 13.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi A., Moriguchi M., Seko Y., Ishikawa H., Yo T., Kimura H., Fujii H., Shima T., Mitsumoto Y., Ishiba H., et al. Impact of Relative Dose Intensity of Early-phase Lenvatinib Treatment on Therapeutic Response in Hepatocellular Carcinoma. Anticancer Res. 2019;39:5149–5156. doi: 10.21873/anticanres.13710. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T., Shibata M., Oe S., Miyagawa K., Honma Y., Harada M. C-reactive protein can predict dose intensity, time to treatment failure and overall survival in HCC treated with lenvatinib. PLoS ONE. 2020;15:e0244370. doi: 10.1371/journal.pone.0244370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruta S., Ogasawara S., Ooka Y., Obu M., Inoue M., Itokawa N., Haga Y., Seki A., Okabe S., Azemoto R., et al. Potential of Lenvatinib for an Expanded Indication from the REFLECT Trial in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2020;9:382–396. doi: 10.1159/000507022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sho T., Suda G., Ogawa K., Kimura M., Shimazaki T., Maehara O., Shigesawa T., Suzuki K., Nakamura A., Ohara M., et al. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open. 2020;4:54–60. doi: 10.1002/jgh3.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh M.J., Oh J.H., Park Y., Kim J., Kang W., Sinn D.H., Gwak G.Y., Paik Y.H., Choi M.S., Lee J.H., et al. Efficacy and Safety of Lenvatinib Therapy for Unresectable Hepatocellular Carcinoma in a Real-World Practice in Korea. Liver Cancer. 2021;10:52–62. doi: 10.1159/000512239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koroki K., Kanogawa N., Maruta S., Ogasawara S., Iino Y., Obu M., Okubo T., Itokawa N., Maeda T., Inoue M., et al. Posttreatment after Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2021;10:473–484. doi: 10.1159/000515552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimozato N., Namisaki T., Okano A., Ohana M., Kinoshita D., Kawasaki T., Aihara Y., Nakatani T., Kinoshita H., Ann T., et al. Efficacy and Safety of Lenvatinib for Patients With Advanced Hepatocellular Carcinoma: A Retrospective, Real-world Study Conducted in Japan. Anticancer Res. 2022;42:173–183. doi: 10.21873/anticanres.15471. [DOI] [PubMed] [Google Scholar]
- 21.Singal A.G., Nagar S.P., Hitchens A., Davis K.L., Iyer S. Real-world effectiveness of lenvatinib monotherapy among unresectable hepatocellular carcinoma patients in the USA. Future Oncol. 2021;17:2759–2768. doi: 10.2217/fon-2021-0242. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi S., Fukushima T., Ueno M., Moriya S., Chuma M., Numata K., Tsuruya K., Hirose S., Kagawa T., Hattori N., et al. A prospective observational cohort study of lenvatinib as initial treatment in patients with BCLC-defined stage B hepatocellular carcinoma. BMC Cancer. 2022;22:517. doi: 10.1186/s12885-022-09625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M., Ueshima K., Chan S., Minami T., Chishina H., Aoki T., Takita M., Hagiwara S., Minami Y., Ida H., et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers. 2019;11:1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzuya T., Ishigami M., Ito T., Ishizu Y., Honda T., Ishikawa T., Fujishiro M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis. Anticancer Res. 2020;40:2283–2290. doi: 10.21873/anticanres.14193. [DOI] [PubMed] [Google Scholar]
- 25.Nakano M., Kuromatsu R., Niizeki T., Okamura S., Iwamoto H., Shimose S., Shirono T., Noda Y., Kamachi N., Koga H., et al. Primary Treatment with Molecular-Targeted Agents for Hepatocellular Carcinoma: A Propensity Score-matching Analysis. Hepatol. Commun. 2020;4:1218–1228. doi: 10.1002/hep4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casadei-Gardini A., Scartozzi M., Tada T., Yoo C., Shimose S., Masi G., Lonardi S., Frassineti L.G., Nicola S., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: An inverse probability of treatment weighting analysis. Liver Int. 2021;41:1389–1397. doi: 10.1111/liv.14817. [DOI] [PubMed] [Google Scholar]
- 27.Kim S., Kim K.H., Kim B.K., Park J.Y., Ahn S.H., Kim D.Y., Kim S.U. Lenvatinib is independently associated with the reduced risk of progressive disease when compared with sorafenib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2021;36:1317–1325. doi: 10.1111/jgh.15355. [DOI] [PubMed] [Google Scholar]
- 28.Hatanaka T., Kakizaki S., Nagashima T., Ueno T., Namikawa M., Tojima H., Takizawa D., Naganuma A., Arai H., Sato K., et al. A change in the timing for starting systemic therapies for hepatocellular carcinoma: The comparison of sorafenib and lenvatinib as the first-line treatment. Acta Gastroenterol. Belg. 2021;84:65–72. doi: 10.51821/84.1.109. [DOI] [PubMed] [Google Scholar]
- 29.Kuo Y.H., Lu S.N., Chen Y.Y., Kee K.M., Yen Y.H., Hung C.H., Hu T.H., Chen C.H., Wang J.H. Real-World Lenvatinib Versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Front. Oncol. 2021;11:737767. doi: 10.3389/fonc.2021.737767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimini M., Shimose S., Lonardi S., Tada T., Masi G., Iwamoto H., Lai E., Burgio V., Hiraoka A., Ishikawa T., et al. Lenvatinib versus Sorafenib as first-line treatment in hepatocellular carcinoma: A multi-institutional matched case-control study. Hepatol. Res. 2021;51:1229–1241. doi: 10.1111/hepr.13718. [DOI] [PubMed] [Google Scholar]
- 31.Tomonari T., Sato Y., Tani J., Hirose A., Ogawa C., Morishita A., Tanaka H., Tanaka T., Taniguchi T., Okamoto K., et al. Comparison of therapeutic outcomes of sorafenib and lenvatinib as primary treatments for hepatocellular carcinoma with a focus on molecular-targeted agent sequential therapy: A propensity score-matched analysis. Hepatol. Res. 2021;51:472–481. doi: 10.1111/hepr.13597. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y.J., Lai Z.C., He M.K., Bu X.Y., Chen H.W., Zhou Y.M., Xu L., Wei W., Zhang Y.J., Chen M.S., et al. Toripalimab Combined with Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib for Advanced Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2021;20:15330338211063848. doi: 10.1177/15330338211063848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi N.R., Kim J.Y., Hong J.H., Hur M.H., Cho H., Park M.K., Kim J., Lee Y.B., Cho E.J., Lee J.H., et al. Comparison of the outcomes between sorafenib and lenvatinib as the first-line systemic treatment for HBV-associated hepatocellular carcinoma: A propensity score matching analysis. BMC Gastroenterol. 2022;22:135. doi: 10.1186/s12876-022-02210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim B.K., Cheon J., Kim H., Kang B., Ha Y., Kim D.Y., Hwang S.G., Chon Y.E., Chon H.J. Atezolizumab/Bevacizumab vs. Lenvatinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Real-World, Multi-Center Study. Cancers. 2022;14:1747. doi: 10.3390/cancers14071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S.W., Yang S.S., Lien H.C., Peng Y.C., Ko C.W., Lee T.Y. Efficacy of Lenvatinib and Sorafenib in the Real-World First-Line Treatment of Advanced-Stage Hepatocellular Carcinoma in a Taiwanese Population. J. Clin. Med. 2022;11:1444. doi: 10.3390/jcm11051444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki E., Kaneko S., Okusaka T., Ikeda M., Yamaguchi K., Sugimoto R., Aramaki T., Asagi A., Yasui K., Sano K., et al. A multicenter Phase II study of sorafenib in Japanese patients with advanced hepatocellular carcinoma and Child Pugh A and B class. Jpn J. Clin. Oncol. 2018;48:317–321. doi: 10.1093/jjco/hyy010. [DOI] [PubMed] [Google Scholar]
- 37.Inghilesi A.L., Gallori D., Antonuzzo L., Forte P., Tomcikova D., Arena U., Colagrande S., Pradella S., Fani B., Gianni E., et al. Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib. World J. Gastroenterol. 2014;20:786–794. doi: 10.3748/wjg.v20.i3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welland S., Leyh C., Finkelmeier F., Jefremow A., Shmanko K., Gonzalez-Carmona M.A., Kandulski A., Jeliazkova P., Best J., Fründt T.W., et al. Real-World Data for Lenvatinib in Hepatocellular Carcinoma (ELEVATOR): A Retrospective Multicenter Study. Liver Cancer. 2022;11:219–232. doi: 10.1159/000521746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai H., Zhang L., Li N., Zheng B., Liu M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: A cost-effectiveness analysis. J. Comp. Eff. Res. 2020;9:553–562. doi: 10.2217/cer-2020-0041. [DOI] [PubMed] [Google Scholar]
- 40.Sato J., Satouchi M., Itoh S., Okuma Y., Niho S., Mizugaki H., Murakami H., Fujisaka Y., Kozuki T., Nakamura K., et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020;21:843–850. doi: 10.1016/S1470-2045(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 41.Wu H., Ding X., Zhang Y., Li W., Chen J. Incidence and risk of hypertension with lenvatinib in treatment of solid tumors: An updated systematic review and meta-analysis. J. Clin. Hypertens. 2022;24:667–676. doi: 10.1111/jch.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tlemsani C., Mir O., Boudou-Rouquette P., Huillard O., Maley K., Ropert S., Coriat R., Goldwasser F. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target. Oncol. 2011;6:253–258. doi: 10.1007/s11523-011-0201-x. [DOI] [PubMed] [Google Scholar]
- 43.Rizzoni D., De Ciuceis C., Porteri E., Agabiti-Rosei C., Agabiti-Rosei E. Use of Antihypertensive Drugs in Neoplastic Patients. High Blood Press Cardiovasc. Prev. 2017;24:127–132. doi: 10.1007/s40292-017-0198-z. [DOI] [PubMed] [Google Scholar]
- 44.Bair S.M., Choueiri T.K., Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: Emerging evidence and evolving perspectives. Trends Cardiovasc. Med. 2013;23:104–113. doi: 10.1016/j.tcm.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzo A., Ricci A.D., Gadaleta-Caldarola G., Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: Current management and future challenges. Expert Rev. Gastroenterol. Hepatol. 2021;15:1245–1251. doi: 10.1080/17474124.2021.1973431. [DOI] [PubMed] [Google Scholar]
- 46.Benson A.B., D’Angelica M.I., Abbott D.E., Anaya D.A., Anders R., Are C., Bachini M., Borad M., Brown D., Burgoyne A., et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 47.Rimini M., Rimassa L., Ueshima K., Burgio V., Shigeo S., Tada T., Suda G., Yoo C., Cheon J., Pinato D.J., et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: An international propensity score matching analysis. ESMO Open. 2022;7:100591. doi: 10.1016/j.esmoop.2022.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finn R.S., Ryoo B.Y., Merle P., Kudo M., Bouattour M., Lim H.Y., Breder V., Edeline J., Chao Y., Ogasawara S., et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 49.Wei C.Y., Zhu M.X., Zhang P.F., Huang X.Y., Wan J.K., Yao X.Z., Hu Z.T., Chai X.Q., Peng R., Yang X., et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 2022;77:163–176. doi: 10.1016/j.jhep.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Zhao L., Chang N., Shi L., Li F., Meng F., Xie X., Xu Z., Wang F. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: A retrospective, real-world study. Heliyon. 2022;8:e09538. doi: 10.1016/j.heliyon.2022.e09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzo A., Nannini M., Novelli M., Dalia Ricci A., Scioscio V.D., Pantaleo M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020;12:1758835920936932. doi: 10.1177/1758835920936932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript and its supplements.