Abstract
Haemophilus ducreyi causes chancroid, a sexually transmitted cutaneous genital ulcer disease associated with increased heterosexual transmission of human immunodeficiency virus. H. ducreyi expresses a periplasmic copper-zinc superoxide dismutase (Cu,Zn SOD) that protects the bacterium from killing by exogenous superoxide in vitro. We hypothesized that the Cu,Zn SOD would protect H. ducreyi from immune cell killing, enhance survival, and affect ulcer development in vivo. In order to test this hypothesis and study the role of the Cu,Zn SOD in H. ducreyi pathogenesis, we compared a Cu,Zn SOD-deficient H. ducreyi strain to its isogenic wild-type parent with respect to survival and ulcer development in immunocompetent and immunosuppressed pigs. The Cu,Zn SOD-deficient strain was recovered from significantly fewer inoculated sites and in significantly lower numbers than the wild-type parent strain or a merodiploid (sodC+ sodC) strain after infection of immunocompetent pigs. In contrast, survival of the wild-type and Cu,Zn SOD-deficient strains was not significantly different in pigs that were rendered neutropenic by treatment with cyclophosphamide. Ulcer severity in pigs was not significantly different between sites inoculated with wild type and sites inoculated with Cu,Zn SOD-deficient H. ducreyi. Our data suggest that the periplasmic Cu,Zn SOD is an important virulence determinant in H. ducreyi, protecting the bacterium from host immune cell killing and contributing to survival and persistence in the host.
Haemophilus ducreyi is the causative agent of chancroid, a sexually transmitted cutaneous genital ulcer disease (13, 18). The observation that chancroid facilitates human immunodeficiency virus transmission (16, 19, 22–24) has provided additional impetus for the study of the pathogenesis of H. ducreyi. Several potential H. ducreyi virulence factors have been identified, including lipooligosaccharide (3), fine tangled pili (2), a cytolethal distending toxin (5), and a hemolysin (21). A recent study has established a requirement for the hemoglobin binding protein for survival of H. ducreyi in a temperature-dependent rabbit model of infection (29).
Histological features characteristic of chancroid include micropustules on the skin surface consisting of neutrophils in necrotic debris and dermal infiltrates of activated T cells and macrophages (8, 14, 26, 27). Despite the density of immune cells in chancroid lesions, viable H. ducreyi organisms are commonly recovered from ulcers (18, 26, 27), suggesting that H. ducreyi possesses defense mechanisms against immune cell killing.
There is evidence from in vitro studies that H. ducreyi is resistant to the oxidative burst products of neutrophils (15, 20). Neutrophilic bactericidal activity is primarily attributed to an oxidative burst reaction that leads to the evolution of toxic oxygen radicals, including superoxide (4, 10). In other pathogenic bacteria such as Nocardia asteroides, Salmonella typhimurium, and Shigella flexneri, the expression of superoxide dismutase (SOD) confers protection from oxidative damage caused by exposure to activated neutrophils (1, 6, 7).
SODs are metalloenzymes that catalyze the conversion of superoxide radical to oxygen and hydrogen peroxide (reviewed in references 9 and 30). Inhibition of superoxide accumulation by SOD prevents the formation of the highly mutagenic and cytotoxic hydroxyl radical (10). Thus, bacterial SODs may constitute the first line of defense against oxidative killing by host immune cells.
H. ducreyi expresses a periplasmic copper-zinc SOD (Cu,Zn SOD) (28) that protects against superoxide killing in vitro (25). In this study, we examined the contribution of the periplasmic Cu,Zn SOD to H. ducreyi survival and the early development of chancroid skin lesions in vivo. We have previously developed a swine model of H. ducreyi infection (12) that exploits the high degree of structural and physiological similarity between juvenile pig skin and human skin. In this model, ulcers histologically resembling human chancroid lesions are produced by inoculating H. ducreyi into the skin of young pigs by using an allergen delivery device. As in human infection, viable H. ducreyi can be recovered from pig skin weeks after inoculation. In addition, major features of the pig immune response are similar to the human immune response to H. ducreyi; neutrophils, T cells, and macrophages are predominant at the site of infection, and protective immunity does not develop as a consequence of infection (12).
Herein, we report the first use of the swine model for comparing wild-type H. ducreyi to an isogenic mutant strain with an insertion at a single chromosomal locus, sodC. Since we hypothesized that the Cu,Zn SOD enzyme protects H. ducreyi from killing by host neutrophils, we also compared survival and lesion formation of wild-type and sodC mutant strains in neutrophil-depleted (neutropenic) pigs.
MATERIALS AND METHODS
Animals.
Crossbred (Yorkshire, Landrace, and Hampshire cross) 6- to 12-week-old female pigs were used in experiments as described previously (12). Briefly, pigs were housed at ambient temperatures (20 to 25°C) in an American Association for Accreditation of Laboratory Animal Care-accredited P2 containment facility at North Carolina State University. Water and antibiotic-free growth ration were provided ad libitum. Animals were sedated for all procedures with 2 mg each of ketamine HCl (Fort Dodge Laboratories, Fort Dodge, Iowa) and xylazine (Miles Laboratories, Shawnee Mission, Kans.) per kg of body weight. Pigs were kept in individual enclosures after inoculation and biopsied on either the second or the seventh day postinoculation. Pigs were rendered immune cell deficient by administration of cyclophosphamide (Mead Johnson, Princeton, N.J.) at 50 mg per kg 4 days preinoculation and 20 mg per kg every other day thereafter until the day of biopsy (17). Peripheral blood was drawn every 48 h to monitor numbers of circulating immune cells.
Bacterial inoculum preparation.
H. ducreyi 35000 (ATCC 33922), the isogenic mutant strain 35000-sodC-cat, and the merodiploid strain 35000-sodC+-sodC (25) were grown from frozen stocks and passaged once on chocolate agar plates consisting of 2.5% brain heart infusion, 1.5% Bacto agar (Difco, Detroit, Mich.), 1% hemoglobin, 1% IsoVitaleX (Becton Dickinson, Cockeysville, Md.), and 10% fetal calf serum (Life Technologies, Gaithersburg, Md.) at 35°C in a humidified atmosphere with 5% CO2. Chloramphenicol and vancomycin were used at 1 and 3 μg/ml, respectively.
Inocula were prepared essentially as described elsewhere (12) except that H. ducreyi cells harvested from plates with swabs and resuspended in phosphate-buffered saline (PBS) were forced through 30-gauge needles to create single-cell suspensions. Bacterial cell suspensions were quantitated by duplicate culture of serial 10-fold dilutions. Heat-killed H. ducreyi organisms used as controls were prepared by heating inocula for 10 min in a boiling water bath.
Inoculation.
Each pig was inoculated with the wild-type, mutant, and merodiploid strains or the wild-type and mutant strains and heat-killed controls. The dorsal surface of each pig ear was cleansed with 95% ethanol and inoculated at multiple sites with a single strain of H. ducreyi at different doses. Bacterial inocula were vortexed vigorously prior to each inoculation. At each site, 10 μl of bacterial suspension containing approximately 105, 106, or 107 organisms was applied to the skin by using a multitest applicator (MTA; Lincoln Diagnostics, Decatur, Ill.) as described elsewhere (12, 27).
Biopsy and sample preparation.
Lesion biopsy specimens were taken with disposable 6-mm-diameter skin punches (Acuderm, Ft. Lauderdale, Fla.). For histological study and observations of H. ducreyi recovery, biopsy specimens were cut in half. Sample halves for histological observation were fixed in 4% paraformaldehyde in PBS at 4°C, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (Histopathology Reference Laboratory, Richmond, Calif.). All slides were coded and evaluated blindly by one of the authors. Sample halves for assessment of H. ducreyi recovery were minced and cultured on chocolate agar.
Quantitation of H. ducreyi organisms delivered into pig skin by MTA.
Inocula were prepared as described above and serially diluted. H. ducreyi organisms at different doses from 103 to 107 CFU were loaded in 10-μl volumes onto MTA pads and applied with pressure to ethanol-cleansed pig skin for 10 s. Five minutes after application, inoculated sites were washed with streams of 5 ml of PBS and removed with 6-mm-diameter biopsy punches. The 6-mm-diameter punch biopsy specimens were minced finely with scalpels, suspended in 5 ml of PBS in 50-ml polypropylene tubes, and homogenized with a rotor-stator homogenizer (Tissue-Tearor; Biospec Products, Bartlesville, Okla.) at 30,000 rpm for 30 s. Dilutions of the homogenate were cultured on chocolate agar. The undelivered H. ducreyi organisms were quantitated by vigorously washing the MTA pads five times with 100 μl of PBS, pooling all washes, and culturing dilutions.
Statistical procedures.
Statistical analyses were performed with SPSS version 7.5 (SPSS, Inc., Chicago, Ill.) and Sigma Stat version 2.0 (Jandel Scientific, San Rafael, Calif.). Data from pig lesions were analyzed within a hierarchical linear model to accommodate the nested nature of observing several lesions on each pig. Calculations were performed with (i) pig and strain and (ii) strain only as factors or covariates. Analysis of variance (ANOVA) was used for comparing histology scores. Logistic regression was used to analyze recovery data as a dichotomous dependent variable. The Kruskal-Wallis ANOVA on ranks and multiple comparisons by Dunn’s method were used to analyze numerical recovery data. The Mann-Whitney rank sum test was used to determine correlation between lesion histology and presence of live bacteria. A P value of <0.05 was considered significant.
RESULTS
Survival of the wild-type and Cu,Zn SOD-deficient H. ducreyi in the pig.
We previously created a Cu,Zn SOD-deficient strain of H. ducreyi isogenic to H. ducreyi 35000 by inserting a chloramphenicol acetyltransferase cassette into the open reading frame of the chromosomal sodC gene. We showed that the Cu,Zn SOD-deficient strain had a significantly increased susceptibility to killing by exogenous superoxide compared to the wild-type parent or merodiploid sodC+ sodC H. ducreyi strains (25). We hypothesized that Cu,Zn SOD activity confers resistance to superoxide generated by neutrophils and phagocytes during oxidative burst reactions in the course of the host immune response to H. ducreyi infection. Consequently, the Cu,Zn SOD-deficient strain would be predicted to have a decreased rate of survival in vivo.
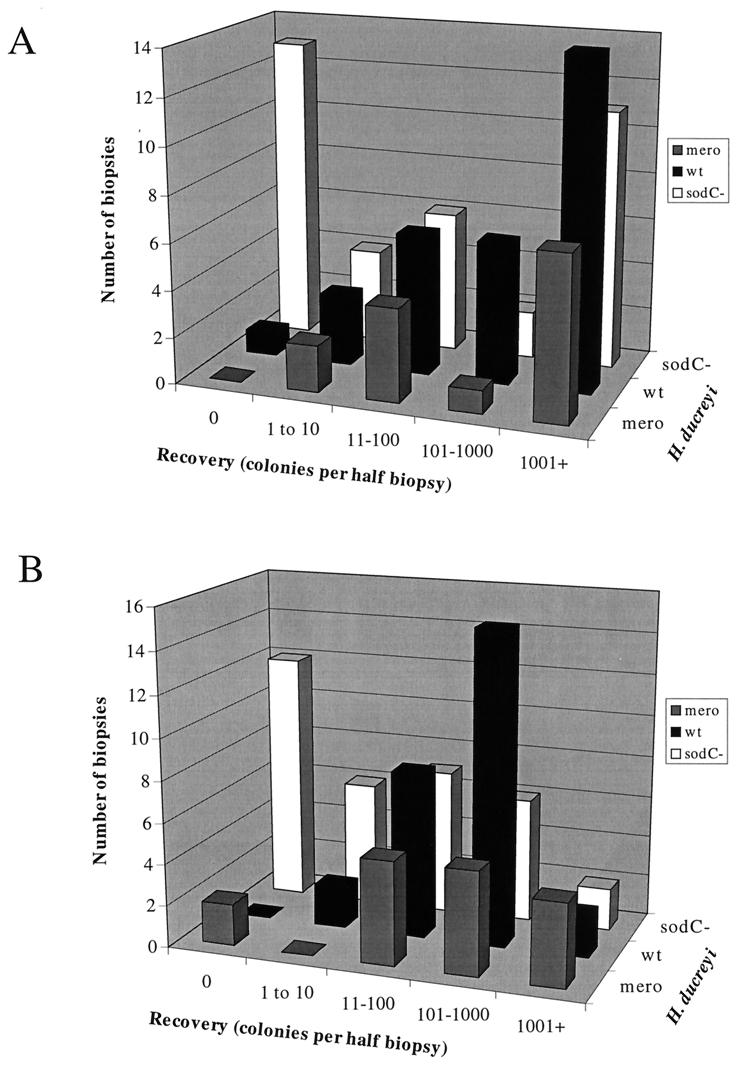
We inoculated pigs at multiple sites with 2.5 × 106 to 2.7 × 107 CFU (delivering an estimated dose of 104 to 105 CFU; see following section) of each strain individually to directly compare the ability of the wild-type, Cu,Zn SOD-deficient, and merodiploid sodC+ sodC H. ducreyi strains to survive and persist in vivo. We harvested biopsy specimens 2 and 7 days postinfection, halved the biopsy specimens, and cultured minced halves on chocolate agar. We compared the numbers of bacteria recovered from biopsy specimens of sites inoculated with the three strains. We found significant differences among the three strains with respect to the numbers of bacteria recovered from biopsy specimens taken 2 and 7 days postinoculation (P = 0.049 and <0.001, respectively [Kruskal-Wallis ANOVA]) (Fig. 1). Viable Cu,Zn SOD-deficient H. ducreyi organisms were recovered from day 7 pig biopsy specimens less often and in significantly fewer numbers than wild-type (P < 0.01 [Dunn’s method]) and merodiploid sodC+ sodC (P < 0.01 [Dunn’s method]) H. ducreyi. The recovery of merodiploid sodC+ sodC H. ducreyi was not significantly different from that of the wild type, demonstrating that the cat insertion in the chromosome at the sodC locus was not in itself responsible for the decreased recovery of the Cu,Zn SOD-deficient strain. Recovery of H. ducreyi strains 2 days postinoculation followed the same trend, with Cu,Zn SOD-deficient H. ducreyi recovered from fewer biopsy specimens and in lower numbers than wild-type and merodiploid bacteria.
FIG. 1.
Frequency distribution histogram showing number of colonies of H. ducreyi strains recovered from biopsy specimens 2 (A) and 7 (B) days postinoculation. Recovery numbers were not determined for five and four sites infected with wild-type and sodC mutant H. ducreyi, respectively. mero, merodiploid; wt, wild type.
Using logistic regression analysis, we determined that wild-type H. ducreyi was significantly more likely than Cu,Zn SOD-deficient H. ducreyi to be recovered from inoculated sites on day 2 (97 versus 59% recovery [Table 1]; odds ratio, 15.7; 95% confidence interval, 1.9 to 128.9; P = 0.010) and day 7 (97 versus 64% recovery [Table 2]; odds ratio, 17.5; 95% confidence interval, 2.1 to 143.7; P = 0.008) postinoculation.
TABLE 1.
Recovery of viable H. ducreyi on day 2 postinfectiona
| Pig no. | Value for inoculation with strain:
|
|||||
|---|---|---|---|---|---|---|
| Wild type
|
sodC mutant
|
sodC+ sodC mutant
|
||||
| n | Recovery (% live/total) | n | Recovery (% live/total) | n | Recovery (% live/total) | |
| 1 | 3 | 100 | 3 | 100 | 3 | 100 |
| 2 | 1 | 100 | 3 | 0 | 3 | 100 |
| 3 | 3 | 100 | 3 | 100 | 4 | 100 |
| 4 | 3 | 100 | 3 | 100 | 4 | 100 |
| 5 | 2 | 100 | 2 | 0 | 0 | |
| 6 | 2 | 100 | 2 | 0 | 0 | |
| 7 | 4 | 100 | 10 | 100 | 0 | |
| 8 | 4 | 100 | 4 | 0 | 0 | |
| 9 | 4 | 100 | 3 | 100 | 0 | |
| 10 | 4 | 75 | 4 | 50 | 0 | |
| Total | 30 | 37 | 14 | |||
n indicates number of sites inoculated. Mean percent recovery was 97, 59, and 100 for the wild-type, sodC mutant, and sodC+ sodC mutant strains, respectively.
TABLE 2.
Recovery of viable H. ducreyi on day 7 postinfectiona
| Pig no. | Value for inoculation with strain:
|
|||||
|---|---|---|---|---|---|---|
| Wild type
|
sodC mutant
|
sodC+ sodC mutant
|
||||
| n | Recovery (% live/total) | n | Recovery (% live/total) | n | Recovery (% live/total) | |
| 1 | 3 | 100 | 3 | 100 | 4 | 100 |
| 2 | 3 | 100 | 3 | 0 | 4 | 50 |
| 3 | 3 | 100 | 3 | 100 | 4 | 100 |
| 4 | 3 | 100 | 3 | 100 | 4 | 100 |
| 11 | 2 | 100 | 2 | 0 | 0 | |
| 12 | 2 | 100 | 2 | 50 | 0 | |
| 13 | 4 | 100 | 8 | 75 | 0 | |
| 14 | 4 | 100 | 4 | 100 | 0 | |
| 15 | 4 | 75 | 4 | 25 | 0 | |
| 16 | 4 | 100 | 4 | 50 | 0 | |
| Total | 32 | 36 | 16 | |||
n indicates number of sites inoculated. Mean percent recovery was 97, 64, and 88 for the wild-type, sodC mutant, and sodC+ sodC mutant strains, respectively.
In contrast with the near-100% recovery of the wild-type strain, live recovery of the sodC mutant strain varied from pig to pig (Tables 1 and 2). To illustrate, 2 days postinoculation, the sodC mutant strain was recovered from all the inoculated sites on five pigs but from none of the inoculated sites on the other four pigs.
The recovery rate of the sodC mutant strain 2 days postinoculation was not significantly different from its day 7 recovery rate. In order to test whether the Cu,Zn SOD-null H. ducreyi that persisted in pig skin to days 2 and 7 still contained the cat insertion, colonies from recovery plates were picked and replated on chocolate agar containing chloramphenicol. One hundred percent of colonies tested were chloramphenicol resistant and deficient in Cu,Zn SOD activity.
Our observations are consistent with the notion that the ability of H. ducreyi to survive in normal immunocompetent pigs was significantly impaired by the loss of periplasmic SOD activity.
Survival of the wild-type and Cu,Zn SOD-null H. ducreyi strains in neutropenic pigs.
We hypothesized that the Cu,Zn SOD-deficient H. ducreyi strain survived less efficiently in the pig because it lacks a protective mechanism against neutrophil killing. If so, the null strain should not differ from the wild type with respect to survival in an in vivo system without these immune cells. To test this hypothesis, we pretreated pigs with cyclophosphamide, a widely used immunosuppressive agent. Cyclophosphamide treatment of pigs leads to depletion of circulating neutrophils (data not shown) and reduction of B- and T-cell numbers (17).
Comparing the abilities of the H. ducreyi strains to replicate in neutropenic pigs required quantitating the numbers of bacteria inoculated and recovered. The tendency of H. ducreyi to clump is a significant source of inaccuracy in determining numbers of bacteria used in in vitro and in vivo experiments. In a series of experiments, we loaded MTAs with single-cell suspensions of H. ducreyi that had been forced through a 30-gauge needle and applied the inocula into pig skin. Bacteria in suspensions thus treated were visible microscopically as mostly single cells with some small groups of cells (data not shown). Five minutes after application, we washed the inoculated pig skin surface with streams of sterile PBS, harvested the sites with a skin punch, and homogenized the biopsy specimen. Of bacteria loaded onto the MTA pads, (0.42 ± 0.22)% was delivered into the skin. This result was corroborated by quantitative culture of the bacteria remaining on the pads after application and the bacteria that were recovered from the PBS rinses. Thus, 2.5 × 106 to 2.7 × 107 CFU loaded onto MTA pads corresponds to 1.0 × 104 to 1.1 × 105 CFU of H. ducreyi delivered into live pig skin.
Using these quantitative methods to assess inocula and recovery of H. ducreyi from pigs, we found that total numbers of both wild-type and sodC mutant H. ducreyi strains increased in neutropenic pigs. In a representative experiment (of two experiments performed), wild-type recovery on day 2 was (1.2 ± 0.5) × 104 CFU (average of four sites) from an inoculum of (1.1 ± 0.6) × 104 CFU. Recovery of sodC mutant H. ducreyi was (2.7 ± 0.3) × 105 CFU (average of four sites) from a delivered inoculum of (1.7 ± 0.9) × 104 CFU. In contrast, drastic reductions in viability of both strains, but particularly the Cu,Zn SOD-deficient strain, were observed with immunocompetent pigs. In a representative quantitative experiment (of two), wild-type H. ducreyi numbers decreased from (1.7 ± 0.8) × 104 CFU on day of inoculation to (1.7 ± 1.0) × 103 CFU on day 2 (average of four sites). Numbers of sodC mutant H. ducreyi organisms decreased from (7.3 ± 3.0) × 104 to (1.2 ± 0.9) × 101 CFU (average of four sites, of which two were negative for recovery).
In addition to the quantitative experiments above, we inoculated seven neutropenic pigs with both H. ducreyi strains and harvested biopsy specimens on days 2 and 7. Biopsy specimen halves were studied with respect to histology and recovery of viable bacteria. There was no significant difference between the probability of recovering viable wild type and that of recovering Cu,Zn SOD-deficient H. ducreyi on either day 2 or day 7 postinfection (logistic regression [Tables 3 and 4]).
TABLE 3.
Recovery of viable H. ducreyi from neutropenic pigs day 2 postinfectiona
| Pig no. | Value for inoculation with strain:
|
|||
|---|---|---|---|---|
| Wild type
|
sodC mutant
|
|||
| n | Recovery (% live/total) | n | Recovery (% live/total) | |
| 13 | 4 | 100 | 4 | 100 |
| 14 | 2 | 100 | 2 | 100 |
| 15 | 8 | 100 | 8 | 100 |
| 16 | 4 | 100 | 4 | 100 |
| Total | 18 | 18 | ||
Half of sites on pigs 14 and 15 were inoculated with 2.7 × 105 to 3.7 × 105 CFU of H. ducreyi. n indicates number of sites inoculated. Mean percent recovery was 100 for both the wild-type and sodC mutant strains.
TABLE 4.
Recovery of viable H. ducreyi from neutropenic pigs day 7 postinfectiona
| Pig no. | Value for inoculation with strain:
|
|||
|---|---|---|---|---|
| Wild type
|
sodC mutant
|
|||
| n | Recovery (% live/total) | n | Recovery (% live/total) | |
| 17 | 4 | 100 | 4 | 100 |
| 18 | 3 | 100 | 3 | 67 |
| 19 | 4 | 75 | 4 | 100 |
| Total | 11 | 11 | ||
n indicates number of sites inoculated. Mean percent recovery was 91 for both the wild-type and sodC mutant strains.
Development of lesions caused by wild-type and Cu,Zn SOD-deficient H. ducreyi.
Because heat-killed H. ducreyi produces only mild inflammation and no ulceration in the pig model of infection (reference 12 and data not shown), we hypothesized that an attenuated strain with a decreased ability to survive in vivo might show a different histological picture or time course of ulcer development. Thus, we hypothesized that there might be a significantly different histological manifestation in skin infected with an H. ducreyi strain without periplasmic SOD activity.
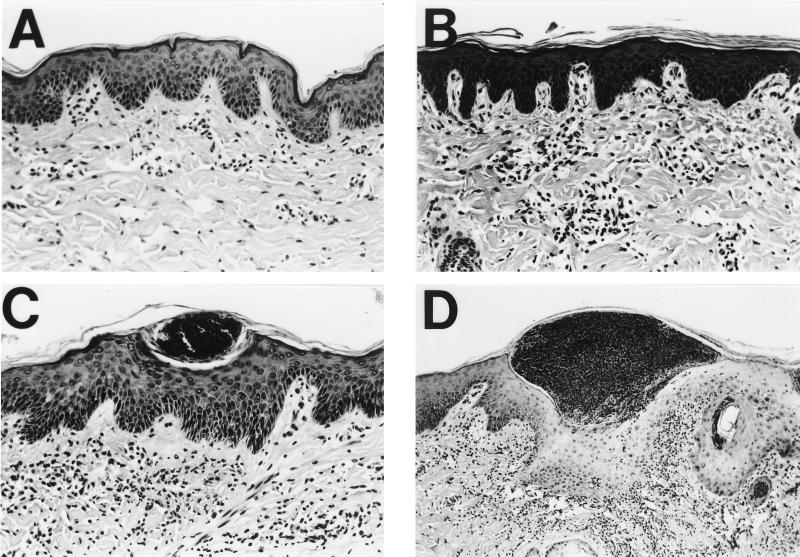
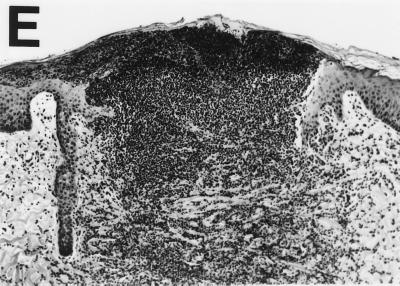
To test this hypothesis, we compared lesions caused by wild-type and mutant H. ducreyi with respect to histology. Microscopic examination of biopsy specimens revealed no gross histological differences between lesions caused by wild type and those caused by sodC mutant H. ducreyi. To allow statistical comparisons of relative lesion severity, we devised a numerical scoring system for the stages of ulcer development (Fig. 2). Skin of normal appearance was scored as 1; the presence of dermal perivascular and interstitial mononuclear and plasma cell infiltrate was assigned a score of 2; the presence of an intraepidermal pustule consisting of neutrophils, fibrin, and necrotic debris was assigned a score of 3; an epidermal pustule accompanied by keratinocyte cytopathology and diffuse mononuclear and polymorphonuclear dermal infiltrate was scored as 4; and ulceration or epidermal necrosis and dermal erosion accompanied by confluence of immune cells were scored as 5. Biopsy specimens were coded and scored blindly. Inoculation of pig skin with either strain most often led to the formation of an epidermal pustule, erosion of epidermal layers, cytopathology of keratinocytes around the pustule, and a diffuse mononuclear cell infiltrate in the dermis (Table 5 and Fig. 2D). No significant difference in severity was found between the lesions induced by the wild type and those induced by Cu,Zn SOD-deficient strains on either day 2 or day 7 (Tables 5 and 6, ANOVA). However, there was a significant correlation between decreased lesion severity and the absence of live bacteria in the same biopsy specimen (P = 0.035 [Mann-Whitney test]).
FIG. 2.
Hematoxylin- and eosin-stained cross sections of pig skin inoculated with H. ducreyi, demonstrating method of scoring lesion severity. (A) Skin of normal appearance, score = 1; (B) Presence of dermal perivascular and interstitial mononuclear cell infiltrate, score = 2; (C) Presence of intraepidermal pustule consisting of neutrophils, fibrin, and necrotic debris, score = 3; (D) Epidermal pustule accompanied by keratinocyte cytopathology and diffuse mononuclear and polymorphonuclear dermal infiltrate, score = 4; (E) Ulceration or epidermal necrosis and dermal erosion accompanied by confluence of immune cells, score = 5. Magnifications, ×103 for panels A to C and ×52 for panels D and E.
TABLE 5.
Histology scores for lesions induced by H. ducreyi strains 2 days postinfection
| Pig no. | Value for inoculation with strain:
|
|||||
|---|---|---|---|---|---|---|
| Wild type
|
sodC mutant
|
|||||
| n | Mean score | SD | n | Mean score | SD | |
| 1 | 2 | 4.0 | 1.4 | 2 | 4.0 | 0.0 |
| 2 | 2 | 4.0 | 0.0 | 2 | 4.0 | 1.4 |
| 3 | 4 | 4.5 | 0.6 | 8 | 4.5 | 0.5 |
| 5 | 0 | NAa | NA | 2 | 4.0 | 0.0 |
| 6 | 2 | 4.5 | 0.7 | 2 | 5.0 | 0.0 |
| Total | 10 | 4.3 | 0.0 | 16 | 4.4 | 0.0 |
NA, data not available.
TABLE 6.
Histology scores for lesions induced by H. ducreyi strains 7 days postinfection
| Pig no. | Value for inoculation with strain:
|
|||||
|---|---|---|---|---|---|---|
| Wild type
|
sodC mutant
|
|||||
| n | Mean score | SD | n | Mean score | SD | |
| 7 | 2 | 5.0 | 0.0 | 2 | 2.5 | 0.7 |
| 8 | 2 | 4.5 | 0.7 | 2 | 3.5 | 0.7 |
| 9 | 3 | 3.3 | 1.1 | 8 | 4.1 | 0.3 |
| 11 | 0 | NAa | NA | 2 | 2.5 | 0.7 |
| 12 | 2 | 3.0 | 2.8 | 2 | 4.0 | 0.0 |
| Total | 9 | 3.9 | 0.2 | 16 | 3.6 | 0.0 |
NA, data not available.
DISCUSSION
H. ducreyi expresses a periplasmic copper- and zinc-cofactored SOD (28) that protects this bacterium from killing by chemically generated superoxide in vitro (25). We hypothesized that periplasmic SOD activity protects H. ducreyi from bactericidal oxygen radicals evolved by host immune cells during infection. In this study, we tested that hypothesis by comparing a Cu,Zn SOD-deficient H. ducreyi strain to its wild-type parent with respect to its survival and ability to induce chancroid-like ulcer formation in swine.
Our results suggest that the periplasmic Cu,Zn SOD is an important virulence determinant of H. ducreyi. Periplasmic SOD-deficient H. ducreyi was recovered from pigs less often and in significantly fewer numbers than wild-type or merodiploid sodC+ sodC H. ducreyi. Whereas the recovery of wild-type and merodiploid sodC+ sodC H. ducreyi was consistent, the recovery of the Cu,Zn SOD-deficient H. ducreyi differed markedly between pigs. Viable Cu,Zn SOD-deficient H. ducreyi organisms were recovered from only 6 of the 10 pigs inoculated, while wild-type H. ducreyi was recovered from all 10 pigs 2 days after inoculation. It could be that the degree of attenuation of the Cu,Zn SOD-deficient strain is such that genetic differences among the outbred pigs, such as major histocompatibility complex alleles, or individual differences in the efficiency of nonspecific immune defenses, may have had an impact on the survival rate of the bacterium.
The differences between the recovery rates of Cu,Zn SOD-null and wild type and those of merodiploid H. ducreyi were statistically significant, although there was variability in the actual number of colonies recovered from different sites, even for the same strain infecting adjacent sites on the same pig. There is evidence from studies of naturally occurring chancroid (18), as well as from human experimental H. ducreyi challenge studies (26), that the outcome of infection can vary widely among lesions on the same subject. We do not know whether the H. ducreyi organisms that persisted within skin lesions were selected in any way from the initial infecting population.
The recovery data from cyclophosphamide-treated pigs infected with mutant and wild-type strains contrasted with the data gathered from immunocompetent pigs. Both wild-type and Cu,Zn SOD-deficient H. ducreyi strains survived equally well in skin of neutropenic pigs. The difference in rates of recovery between the two strains that was evident in immunocompetent pigs was not observed with neutropenic pigs. These contrasting results suggest that the absence of host neutrophils (and perhaps monocytes and lymphocytes) allowed the survival of H. ducreyi in the absence of bacterial periplasmic SOD activity. Conversely, the periplasmic Cu,Zn SOD protected H. ducreyi from host neutrophils in immunocompetent pigs.
The severity and gross appearance of 2- and 7-day lesions caused by the Cu,Zn SOD-deficient strain were not significantly different from those of lesions caused by the wild type. Pig skin inoculated with SOD-deficient H. ducreyi showed histological changes similar to those of skin inoculated with the wild-type strain and consistent with chancroid ulcer development, even though roughly half of the sodC strain-inoculated sites had no detectable live bacteria on the day of biopsy. These observations would seem to suggest that live bacteria were not required for chancroid ulcer formation. However, sites inoculated with heat-killed H. ducreyi never progressed to pustule formation and never received scores greater than 2, and statistical analysis of our data demonstrated a correlation between decreased lesion severity and the absence of viable bacteria on the day of biopsy. It is possible that the initial input of live bacteria may be sufficient to induce ulcer formation (11) and that further development of the ulcer requires the persistence of a very small number of live H. ducreyi organisms. We predict that the sodC mutant strain would not induce chronic ulcers because of its decreased ability to survive in vivo.
In conclusion, we observed that lack of periplasmic Cu,Zn SOD activity was detrimental to survival of H. ducreyi in immunocompetent pigs but not in neutropenic pigs. Our observations suggest that the Cu,Zn SOD protects H. ducreyi from neutrophil killing and contributes to the persistence of the bacterium in chancroid lesions.
ACKNOWLEDGMENTS
This work was supported by grants NIAID AI42824 (T.H.K.) and NRSA 1 F31 AI09565-01 (L.R.S.M.).
We gratefully acknowledge our collaborator, Glen Almond, for valuable advice on pigs; Patty Routh, John Horton, and Re Bai for technical assistance with the pigs; Stephen Knight for help with light micrography; Gary Gaddy and Walter Davis for advice on statistics with hierarchical linear models; John Woosley for help with dermatohistopathology; Janne Cannon for helpful discussions and critical review of the manuscript; and past and present members of the Kawula lab, particularly Marcia Hobbs, Franca Zaretzky, and Gina Donato, for advice and practical and moral support. We are especially grateful to Myron Cohen for his idea to use induced neutropenia to analyze the sodC mutant.
REFERENCES
- 1.Beaman B L, Black C M, Doughty F, Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985;47:135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark R A, Leidel K G, Pearson D W, Nauseef W M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987;262:4065–4074. [PubMed] [Google Scholar]
- 5.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan G, Kroll J S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to pathogenesis of systemic salmonellosis. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 7.Franzon V L, Arondel J, Sansonetti P J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990;58:529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freinkel A L. Histological aspects of sexually transmitted genital lesions. Histopathology. 1987;11:819–831. doi: 10.1111/j.1365-2559.1987.tb01885.x. [DOI] [PubMed] [Google Scholar]
- 9.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 10.Hassett D J, Cohen M S. Bacterial adaptation to oxidative stress: implications for the pathogenesis and interaction with phagocytic cells. FASEB J. 1989;3:2574–2582. doi: 10.1096/fasebj.3.14.2556311. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs M M, Paul T R, Wyrick P B, Kawula T H. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect Immun. 1998;66:2914–2921. doi: 10.1128/iai.66.6.2914-2921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs M M, San Mateo L R, Orndorff P E, Almond G, Kawula T H. Swine model of Haemophilus ducreyi infection. Infect Immun. 1995;63:3094–3100. doi: 10.1128/iai.63.8.3094-3100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan W C. Chancroid: a review for the family practitioner. J Natl Med Assoc. 1991;83:724–726. [PMC free article] [PubMed] [Google Scholar]
- 14.King R, Gough J, Ronald A, Nasio J, Ndinya-Achola J O, Plummer F, Wilkins J A. An immunohistochemical analysis of naturally occurring chancroid. J Infect Dis. 1996;174:427–430. doi: 10.1093/infdis/174.2.427. [DOI] [PubMed] [Google Scholar]
- 15.Lagergard T, Frisk A, Purven M, Nilsson L A. Serum bactericidal activity and phagocytosis in host defence against Haemophilus ducreyi. Microb Pathog. 1995;18:37–51. [PubMed] [Google Scholar]
- 16.Le-Bacq F, Mason P R, Gwanzura L, Robertson V J, Latif A S. HIV and other sexually transmitted diseases at a rural hospital in Zimbabwe. Genitourin Med. 1993;69:352–356. doi: 10.1136/sti.69.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie E J. Immunosuppressive effects of cyclophosphamide in pigs. Am J Vet Res. 1981;42:189–194. [PubMed] [Google Scholar]
- 18.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nzila N, Laga M, Thiam M A, Mayimona K, Edidi B, Van-Dyck E, Behets F, Hassig S, Nelson A, Mokwa K, Ashley R L, Piot P, Ryder R W. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991;5:715–721. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Odumeru J A, Wiseman G M, Ronald A R. Virulence factors of Haemophilus ducreyi. Infect Immun. 1984;43:607–611. doi: 10.1128/iai.43.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer K L, Goldman W E, Munson R S., Jr An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytotoxic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 22.Pepin J, Dunn D, Gaye I, Alonso P, Egboga A, Tedder R, Piot P, Berry N, Schellenberg D, Whittle H. HIV-2 infection among prostitutes working in the Gambia: association with serological evidence of genital ulcer diseases and with generalized lymphadenopathy. AIDS. 1991;5:69–75. [PubMed] [Google Scholar]
- 23.Pepin J, Quigley M, Todd J, Gaye I, Janneh M, Van Dyck E, Piot P, Whittle H. Association between HIV-2 infection and genital ulcer diseases among male sexually transmitted disease patients in the Gambia. AIDS. 1992;6:489–493. doi: 10.1097/00002030-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Plummer F A, Simonsen J N, Cameron D W, Ndinya-Achola J O, Kreiss J K, Gakinya M N, Waiyaki P, Cheang M, Piot P, Ronald A R, Ngugi E N. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 25.San Mateo L R, Hobbs M M, Kawula T H. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol Microbiol. 1998;27:391–404. doi: 10.1046/j.1365-2958.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 26.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4+ cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 27.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 28.Stevens M K, Hassett D J, Radolf J D, Hansen E J. Cloning and sequencing of the gene encoding the Cu, Zn-superoxide dismutase of Haemophilus ducreyi. Gene. 1996;183:35–40. doi: 10.1016/s0378-1119(96)00417-9. [DOI] [PubMed] [Google Scholar]
- 29.Stevens M K, Porcella S, Klesney-Tait J, Lumbley S R, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touati D. Superoxide dismutase in bacteria and pathogen protists. In: Scandalios J G, editor. Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 447–493. [Google Scholar]