Abstract
Haemophilus ducreyi is the etiologic agent of chancroid, a sexually transmitted genital ulcer disease. Keratinocytes are likely the first cell type encountered by H. ducreyi upon infection of human skin; thus, the interaction between H. ducreyi and keratinocytes is probably important for the ability of H. ducreyi to establish infection. We have used the HaCaT keratinocyte cell line grown in monolayers and in cocultures with HS27 fibroblasts to investigate H. ducreyi interactions with keratinocytes and the host-cell response to H. ducreyi infection. Using quantitative adherence and gentamicin protection assays, we determined that approximately 13% of H. ducreyi adhered to HaCaT cell monolayers, while only a small proportion (0.0052%) was intracellular. By transmission electron microscopy, we observed numerous H. ducreyi organisms adherent to but rarely within HaCaT cells cocultured with fibroblasts. Both live H. ducreyi and purified H. ducreyi lipooligosaccharide (LOS) induced significant interleukin 8 (IL-8) expression from HaCaT cell-HS27 cell cocultures. However, the level of IL-8 expression in response to LOS alone was not as pronounced. H. ducreyi LOS was a more potent inducer of IL-8 from cocultures than Escherichia coli lipopolysaccharide (LPS) at the same concentration, suggesting a unique effect of H. ducreyi LOS on cocultures. Neither live H. ducreyi nor purified H. ducreyi LOS or E. coli LPS induced tumor necrosis factor alpha expression from cocultures. H. ducreyi induced drastically different cytokine profiles from cocultures than from HS27 or HaCaT cells cultured separately. IL-8 expression by skin cells in response to H. ducreyi infection in vivo may be responsible for the massive influx of polymorphonuclear leukocytes and other inflammatory cells to the site of infection. This influx of inflammatory cells may be partly responsible for the tissue destruction characteristic of chancroid.
Haemophilus ducreyi is the gram-negative bacterium responsible for causing the sexually transmitted genital ulcer disease chancroid (29). Chancroid is prevalent in Southeast Asia and Africa and is uncommon in the United States, with the incidence of reported cases decreasing from 3,476 in 1991 to only 243 in 1997 (9a). The finding that chancroid is a risk factor for increased transmission of human immunodeficiency virus (25, 30, 36, 37, 39, 62) has prompted further study into the mechanisms by which H. ducreyi causes chancroid in the hope of discovering ways to prevent chancroid.
The genital ulcer caused by H. ducreyi begins with the entry of the organism through tiny breaks in keratinized skin (12, 17, 53). A papular lesion that is characterized by a disorganization of the epidermal layer and an infiltration of polymorphonuclear leukocytes (PMN) develops (15, 53). Within several days, a pustule forms and ruptures, leaving an ulcer that contains necrotic tissue, PMN, macrophages, and CD4+ T cells but few B cells (52, 53). Although a humoral immune response to H. ducreyi infection occurs as the disease progresses to the ulcerative stage (10), there is no documented immunity to reinfection with H. ducreyi in natural infection.
Despite recent research efforts, little is known regarding the H. ducreyi-specific factors responsible for ulcer formation in humans. Several putative virulence determinants have been identified; these include lipooligosaccharide (LOS) (3, 8, 16), pili (1, 6, 9, 51), a hemolysin (32, 33, 35, 59), a secreted toxin (11, 22, 23, 41), an outer membrane hemoglobin binding protein (13, 56), and a copper-zinc superoxide dismutase (46, 55). However, the molecules involved in the ability of H. ducreyi to directly interact with host cells and the host cells with which H. ducreyi associates remain largely unknown.
Several in vitro models that utilize dermal foreskin fibroblasts (2, 4, 24) or primary keratinocytes (7, 16, 58) grown as monolayers have been developed to study H. ducreyi adherence to and entry into host cells. Although it is clear that H. ducreyi is capable of adhering to both cell types in vitro (2, 4, 7, 16, 24, 58), whether the organism is intracellular (16, 24, 58) or remains extracellular (2, 4) is controversial. Analyzing H. ducreyi interactions with individual cell types grown in monolayers is convenient but may not accurately reflect the in vivo situation, as skin contains both fibroblasts and keratinocytes in a highly ordered structure. Recently, using an in vitro skin model (Advanced Tissue Sciences, La Jolla, Calif.) consisting of a lower layer containing dermal fibroblasts grown in a collagen matrix and an upper, epidermis-like layer containing stratified, differentiated keratinocytes, we observed H. ducreyi adherent to keratinocytes and occasionally within suprabasal keratinocytes (19). In this in vitro skin model (19), H. ducreyi induced the secretion of interleukin 8 (IL-8) and IL-6 but not tumor necrosis factor alpha (TNF-α). Since IL-8 is a potent chemoattractant for neutrophils, its induction by H. ducreyi in the in vitro skin model may mimic an important early event in natural infection. IL-8 is also expressed in lesions of experimentally infected human volunteers (34).
We continue to be interested in examining the interaction between H. ducreyi and keratinocytes on the premise that keratinocytes may be one of the first cell types encountered by H. ducreyi during natural infection. Literature regarding the interaction between H. ducreyi and keratinocytes is limited. High cost and infrequent availability of the in vitro skin model preclude its use in large-scale quantitative analyses of H. ducreyi adherence and entry. Although primary keratinocytes may be an ideal cell type for the analysis of H. ducreyi-host cell interactions, human foreskin can be difficult to obtain, tissue characteristics may differ from donor to donor, keratinocyte isolation is time-consuming, and primary keratinocytes are slow growing. For these reasons, we used the HaCaT keratinocyte cell line for our studies.
HaCaT cells are spontaneously transformed human adult skin keratinocytes that were selected for immortality by propagation under low-Ca2+ conditions and an elevated temperature (38°C) (5). In contrast to other transformed keratinocyte cell lines, HaCaT cells retain the ability to express keratinocyte differentiation-specific markers and are not tumorigenic when transplanted onto nude mice (5). Furthermore, the growth of HaCaT cells to confluence on tissue culture plastic, on collagen I matrices, and in cocultures with fibroblasts induces differentiation-specific markers, such as the adult skin suprabasal keratins 1 and 10 and the foreskin, neonatal skin, and mucosal epithelium suprabasal keratins 4 and 13 (45, 48). HaCaT cells have been used to study aspects of H. ducreyi (26), Streptococcus pyogenes (44, 61), Staphylococcus aureus (14), and herpes simplex virus (57) infections in vitro. Furthermore, HaCaT cells, like normal keratinocytes, are capable of expressing a number of cytokines in response to bacterial pathogens (14, 44, 61) and other perturbations (28).
In this study, we examined the interaction between H. ducreyi and HaCaT cells in monolayer cultures. Furthermore, we constructed an in vitro skin-like model consisting of dermal fibroblasts and HaCaT cells and used it to examine H. ducreyi adherence to and entry into host cells quantitatively and microscopically and to examine the host cell cytokine response to H. ducreyi colonization. Since H. ducreyi LOS has been implicated as a mediator of ulcer formation in some animal models (8, 16), we also used the coculture system to investigate the role of LOS in the induction of cytokine expression by host cells.
MATERIALS AND METHODS
Cell cultures.
Cell cultures were maintained at 35°C in a humidified atmosphere with 5% CO2, and medium was replaced every third day or as needed. The HaCaT keratinocyte cell line (5) was a gift from Bernard Weissman (Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill) and was cultured in RPMI 1640 medium (Gibco, Grand Island, N.Y.) supplemented with 20 mM HEPES and 10% fetal bovine serum (FBS; Gibco) (RPMI-10). With the addition of FBS, the final calcium concentration in the medium would be greater than 0.9 mM, a concentration expected to induce full differentiation of HaCaT cells in cultures (45). Human foreskin fibroblasts (HS27; American Type Culture Collection, Manassas, Va.; 1634-CRL) were cultured in Dulbecco’s modified Eagle medium with high glucose (Gibco) and supplemented with 20 mM HEPES and 10% FBS (DMEM-10).
HS27 cell-HaCaT cell cocultures were constructed as follows. HS27 cells (105) were seeded on 3-μm collagen I-coated tissue culture inserts in 24-well tissue culture dishes (Becton Dickinson, Bedford, Mass). HS27 cells were grown for 9 to 11 days in DMEM-10 supplied above and below the insert. HaCaT cells were seeded on top of the HS27 cells, and the cocultures were grown in RPMI-10 supplied above and below the insert for an additional 9 to 11 days before use in experiments. For some experiments (see below), HS27 or HaCaT cells were cultured separately on tissue culture inserts for 9 to 11 days in DMEM-10 or RPMI-10, respectively.
Bacterial cultures and inoculation protocols.
H. ducreyi 35000 (ATCC 33922) was cultured (i) on chocolate agar plates consisting of 2.5% brain heart infusion (BHI), 1.5% agar, and 1% hemoglobin (Difco Laboratories, Detroit, Mich.) plus 1% IsoVitaleX (Becton Dickinson, Cockeysville, Md.) and 10% FBS or (ii) in BHI (3.79%) broth supplemented with 1% IsoVitaleX and 50 μg of hemin (Sigma, St. Louis, Mo.) per ml at 35°C in 5% CO2. As a negative control for adherence and entry experiments, we used Escherichia coli ORN200, which was a gift from Paul Orndorff (College of Veterinary Medicine, North Carolina State University, Raleigh). ORN200 is a nonpiliated E. coli strain that adheres minimally to but does not enter epithelial cells. ORN200 was grown on Luria-Bertani (LB) agar plates or in LB broth at 37°C.
H. ducreyi was recovered from freezer stocks on one chocolate agar plate 24 h before each experiment. On the day of the experiment, the overnight H. ducreyi growth was suspended in 1 ml of BHI broth and vortexed vigorously for 10 s, and clumps were allowed to settle for 5 min. Bacteria in the supernatant were resuspended in 10 ml of BHI broth to approximately 2.5 × 108 CFU/ml and were grown to approximately 109 CFU/ml (plate counts were determined for each experiment) at 35°C on a rotary shaker set at 100 rotations per min. One milliliter of broth culture was removed and vortexed for 10 s, and clumps were allowed to settle for 5 min before H. ducreyi in the supernatant was resuspended to 106 CFU/ml in RPMI-10 or DMEM-10 for infection of HS27 cells. A 500-μl portion of this suspension (∼5 × 105 CFU) was inoculated onto HaCaT cells grown in monolayers, HS27 or HaCaT cells grown separately on tissue culture inserts, or HS27 cell-HaCaT cell cocultures, and the cultures were incubated at 35°C with 5% CO2 for various times. With this inoculation protocol, a multiplicity of infection (MOI) of between 1 and 5 bacteria per HaCaT cell in monolayer cultures was achieved. Since we could not remove intact cells from the collagen substratum, an accurate MOI could not be calculated when cells were cultured separately or in cocultures on the tissue culture inserts. E. coli ORN200 was grown in LB broth overnight, diluted 1:100 on the morning of the experiment, grown to the mid-log phase, resuspended in RPMI-10 to 106 CFU/ml, and inoculated onto HaCaT cell monolayers or HS27 cell-HaCaT cell cocultures as described above.
Quantitative adherence and gentamicin protection assays.
HaCaT cells (5 × 104 cells/well) were seeded in four-well dishes (Nunc, Rochester, N.Y.) and grown to confluence (∼5 × 105 cells/well). H. ducreyi or E. coli ORN200 was adsorbed to HaCaT cells for 2 h, and the total CFU present in two wells after 2 h was determined by collecting the contents of the wells (inoculum plus HaCaT cells) and plating serial dilutions on chocolate agar or LB agar plates in duplicate. HaCaT cells were removed from the tissue culture plastic by scraping with sterile wooden sticks. To determine the number of H. ducreyi or E. coli bacteria adherent to HaCaT cells after 2 h, we removed the nonadherent bacteria in two wells by washing infected HaCaT cells five times with phosphate-buffered saline (PBS). We collected the washed HaCaT cells plus associated bacteria and plated dilutions in duplicate. The percentage of bacterial adherence to HaCaT cells is expressed as the number of cell-associated bacteria per well at 2 h divided by the total number of bacteria per well at 2 h, multiplied by 100. Adherence kinetics were determined similarly, except that H. ducreyi was adsorbed to HaCaT cells for 0.5, 1, 2, 3, or 4 h before harvest and dilutions were plated to determine the percentage of H. ducreyi adherent to HaCaT cells at each time point. As observed by light and electron microscopy, H. ducreyi adhered to HaCaT cells predominantly as individual cells during the adsorption times of the adherence assays.
Gentamicin protection assays (54) and electron microscopy were used to determine if H. ducreyi invaded HaCaT cells. After H. ducreyi or E. coli was adsorbed to HaCaT cells for 2 h, we incubated two wells of HaCaT cells plus associated bacteria with 100 μg of gentamicin (Gibco) per ml in RPMI-10 for 2 h to kill extracellular bacteria. This concentration of gentamicin kills 100% of 108 H. ducreyi and E. coli organisms within 2 h (data not shown). Dead bacteria and antibiotic were removed by washing the HaCaT cells five times with PBS. The HaCaT cells containing bacteria protected from gentamicin were removed from the wells as described above for adherence assays, pelleted in a microcentrifuge, and plated on the appropriate media. The percentage of bacteria protected from gentamicin was determined by dividing the number of bacteria recovered per well after gentamicin exposure by the number of bacteria present before gentamicin exposure, multiplied by 100.
Two modifications of the gentamicin protection assay were used to determine whether H. ducreyi protected from gentamicin replicated inside HaCaT cells following 2 h of antibiotic exposure. In one experiment, infected HaCaT cells were exposed for 2 h to gentamicin; then, the antibiotic was removed and replaced with fresh RPMI-10. At 24 and 48 h post-gentamicin removal, the entire contents of the wells were harvested, dilutions were plated, and the number of bacteria per well was calculated. To determine if the bacteria present at 24 and 48 h were gentamicin accessible, we added gentamicin back to the cultures for 2 h prior to harvesting the well contents and plating. In a second experiment, gentamicin was incubated with infected HaCaT cells for 2, 4, 8, 24, and 48 h prior to the harvesting of well contents and plating.
TEM.
For transmission electron microscopy (TEM), H. ducreyi was inoculated onto the apical surface of HS27 cell-HaCaT cell cocultures. After 2, 4, 8, 24, 48, or 72 h, the nonadherent bacteria were removed with one wash in PBS, and the cells were fixed in 2.5% glutaraldehyde in 0.15 M sodium phosphate buffer (pH 7.6) for processing for TEM as previously described (38). Mock-infected cocultures served as controls for comparison. Sections were cut, and specimens were viewed with an EM910 electron microscope (LEO Electron Microscopy, Thornwood, N.Y.).
LOS preparation.
H. ducreyi LOS was purified by the method of Westphal and Jann (63) and was resuspended in endotoxin-free water (Sigma). E. coli lipopolysaccharide (LPS) from strain J5 (rough LPS) was purchased from Sigma (L7520) and was resuspended in endotoxin-free water. Rough E. coli LPS was chosen for comparison with H. ducreyi LOS (see cytokine experiments below) because it more closely resembles H. ducreyi LOS than smooth LPS in structure. The molar concentrations of LOS and LPS in LOS and LPS preparations and whole H. ducreyi organisms were determined by the 2-keto-3-deoxyoctonate assay (20). All LOS and LPS preparations contained <5% protein, as determined with the Bradford protein assay reagent (Bio-Rad, Hercules, Calif.) (data not shown).
Cytokine assays.
HS27 cell-HaCaT cell cocultures were inoculated with either live H. ducreyi, as described earlier, purified H. ducreyi LOS, or E. coli LPS. To analyze H. ducreyi-induced cytokine expression by HS27 or HaCaT cells, the cells were cultured separately on tissue culture inserts prior to being inoculated with H. ducreyi. As a control for TNF-α assays, cocultures were also inoculated with live E. coli ORN200. The medium under the filters (subnatant medium) was removed from the filters and replaced with 1 ml of fresh medium at different times. Therefore, the cytokine accumulation in the medium at each time point is indicative of the cytokine expression that occurred since the previous time point. Samples were frozen at −70°C until assayed for IL-8 or TNF-α by an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn.) according to the manufacturer’s instructions. Samples for IL-8 assays were diluted as high as 1:1,000 in some circumstances. The limits of detection for IL-8 and TNF-α were 31.3 and 15.6 pg/ml, respectively.
Statistical analysis.
All statistical analyses were performed with SigmaStat 2.0 (Jandel Scientific). The Mann-Whitney rank sum test was used to compare the difference between the percentages of H. ducreyi and E. coli ORN200 adherence to and entry into HaCaT cell monolayers. The Kruskal-Wallis test and Dunn’s multiple-comparison procedure were used to analyze the differences in H. ducreyi adherence to HaCaT cell monolayers at different time points in kinetics assays. Student’s t test was used to compare the differences in cytokine levels between treated (H. ducreyi, LOS, or LPS inoculated) cells and mock-treated cells, and an analysis of variance followed by the Tukey test was used for multiple-comparison analyses of cytokine levels between treated groups.
RESULTS
H. ducreyi adheres to HaCaT keratinocyte monolayers.
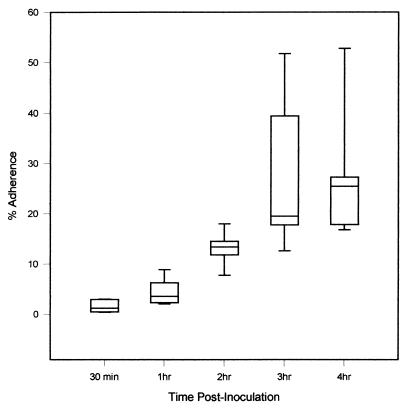
We used a quantitative adherence assay to determine if H. ducreyi adhered to HaCaT cells and to examine the kinetics of H. ducreyi adherence. We inoculated HaCaT cell monolayers with H. ducreyi at an MOI of between 1 and 5 and calculated the percentage of adherent H. ducreyi by dividing the number of HaCaT cell-associated organisms at 0.5, 1, 2, 3, and 4 h postinoculation by the total number of H. ducreyi organisms at each time point. H. ducreyi adherence to HaCaT cells reached saturation at between 1 and 3 h postinoculation (Fig. 1; P, <0.05) and did not increase significantly by 4 h. Thus, a 2-h incubation was chosen for all subsequent adherence assays. Using the quantitative adherence assay, we found that of the total bacteria present after 2 h, a higher percentage of H. ducreyi (13.2% ± 1.16%) than of E. coli (0.39% ± 0.10%) adhered to HaCaT cells (P < 0.001). The results of the quantitative adherence assay and the gentamicin protection assay (see below) are expressed as means ± SEM and are from at least five independent experiments performed in duplicate.
FIG. 1.
Maximum adherence of H. ducreyi 35000 to HaCaT cell monolayers occurs between 1 and 3 h postinoculation. The results are from five independent experiments performed in duplicate. Boxes represent data in the 25th and 75th percentiles; the horizontal line in the boxes represents the median. Error bars encompass data points in the 5th and 95th percentiles.
H. ducreyi is protected from gentamicin killing following adsorption to HaCaT cells.
We used a gentamicin protection assay to quantitate H. ducreyi entry into HaCaT cells. Viable H. ducreyi organisms possibly representing intracellular bacteria were routinely recovered following a 2-h exposure to gentamicin, whereas viable E. coli was rarely recovered. When calculated based on the total number of bacteria present per well, the proportion of H. ducreyi protected from gentamicin (0.0052% ± 0.00087%) at 2 h was low but was significantly higher than that of E. coli (0.000063% ± 0.000032%) (P < 0.001). However, when the percentage of organisms protected from gentamicin was calculated based on the number of adherent bacteria, there was no difference in the percentages of H. ducreyi and E. coli protected from gentamicin.
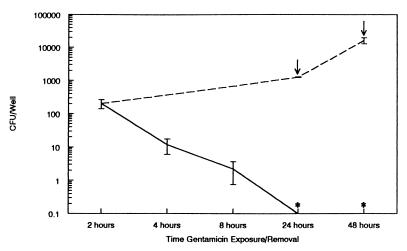
To determine if H. ducreyi protected from gentamicin replicated inside HaCaT cells, we extended the experiment by following the 2-h gentamicin exposure with incubation in antibiotic-free medium and plating dilutions of the well contents 24 or 48 h later. H. ducreyi replicated from an average of 200 bacteria per well immediately following gentamicin exposure to between 104 and 105 bacteria per well by 48 h (Fig. 2). H. ducreyi was recovered from both the supernatant and HaCaT cell-associated fractions (data not shown). If HaCaT cells plus associated bacteria were exposed to gentamicin for 2 h at 24 or 48 h following gentamicin removal, no viable H. ducreyi was recovered (Fig. 2). Furthermore, when infected HaCaT cells were exposed to gentamicin for 2, 4, 8, 24, and 48 h, the number of viable organisms recovered decreased with increasing time of gentamicin exposure, reaching zero by 24 h (Fig. 2). Combined, the results presented in Fig. 2 suggest that organisms protected from gentamicin at 2 h after antibiotic exposure become accessible to gentamicin within 24 h. It is likely that H. ducreyi protected from gentamicin was initially intracellular but was toxic for HaCaT cells, allowing gentamicin eventual access to the previously protected H. ducreyi.
FIG. 2.
Recovery of H. ducreyi over time following gentamicin removal (broken line) or upon prolonged gentamicin exposure (solid line). Values are means ± SEM. Arrows represent times after gentamicin removal when gentamicin was added back to wells run in parallel for 2 h prior to plating for viable bacteria; zero bacteria were recovered following this treatment (asterisks). The data are from two independent experiments performed in duplicate.
H. ducreyi can be found inside HaCaT cells cocultured with HS27 fibroblasts by electron microscopy.
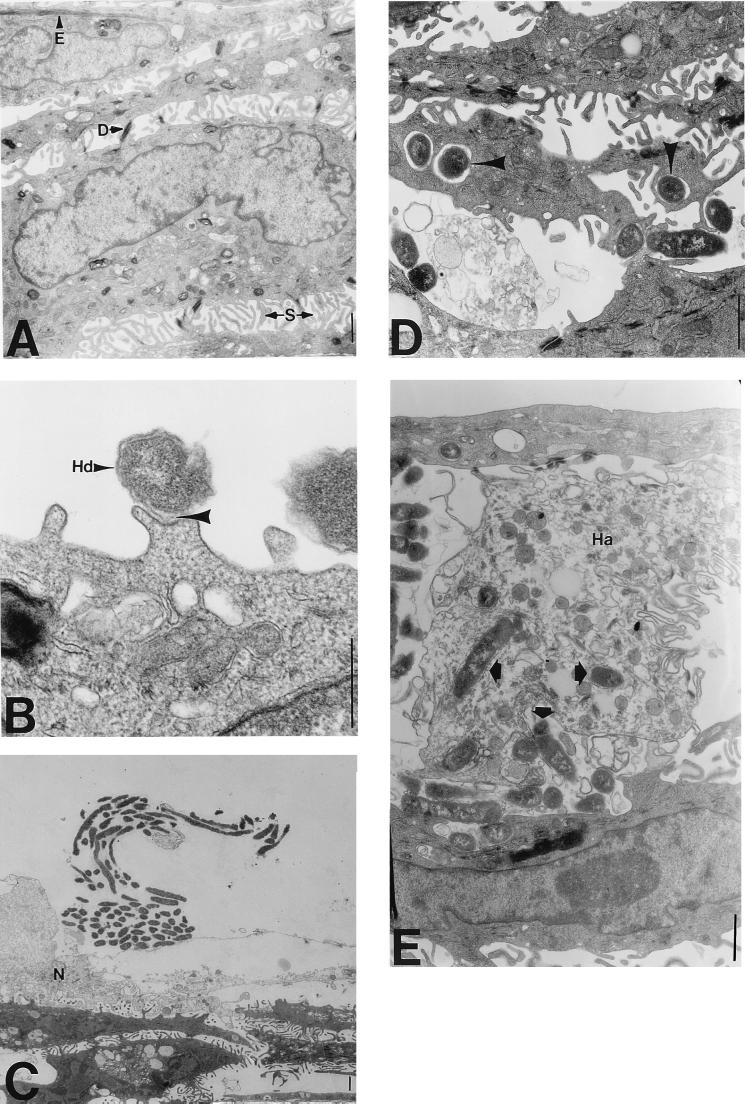
For TEM examination of H. ducreyi-host cell interactions, we developed an in vitro skin-like model (HS27 cell-HaCaT cell cocultures) that more closely represents the in vivo skin structure than do keratinocyte monolayers. Primary foreskin fibroblasts (HS27 cells) were cultured on collagen I-coated tissue culture inserts for 9 to 11 days, HaCaT cells were seeded on top of the fibroblasts, and the cocultures were grown for another 9 to 11 days. Interestingly, the fibroblasts migrated to the opposite side of the filter (data not shown). The HaCaT cells stratified, forming several cell layers that morphologically resembled spinous cells in the suprabasal layer of skin (Fig. 3A). Although we were unable to localize adult skin suprabasal keratin 10 in these cocultures, we were able to detect foreskin suprabasal keratin 13 in immunoblots of coculture lysates as well as in lysates of HaCaT cells harvested from monolayers and tissue culture inserts (data not shown). Since the cells remained submerged in media, the HaCaT cells did not fully differentiate to form a keratinized layer, an important aspect of this model because H. ducreyi does not infect keratinized skin. Furthermore, the HaCaT cells formed desmosomes and contained epidermal keratin filaments, structures rarely observed in HaCaT cells grown in monolayers (data not shown) but characteristic of polarized cells (Fig. 3A).
FIG. 3.
Transmission electron micrographs of H. ducreyi-infected HS27 cell-HaCaT cell cocultures. (A) Uninfected cocultures exhibit healthy cellular morphology. Layers of HaCaT cells resemble suprabasal layers of keratinocytes in skin characterized by spines (S) that connect individual cells. Well-formed desmosomes (D) and epidermal filaments (E) are also apparent. Magnification, ×4,500; bar, 1 μm. (B) H. ducreyi organisms (Hd) adhere to pedestals on HaCaT cells. The H. ducreyi membrane and the HaCaT cell membrane are closely associated (arrowhead). This is a representative micrograph taken 24 h postinoculation. Magnification, ×36,000; bar, 1 μm. (C) An H. ducreyi “school of fish” adheres to the remnants of an HaCaT cell membrane 24 h postinoculation. Necrotic (N) HaCaT cells are evident opposite the large clump of bacteria. Magnification, ×1,800; bar, 1 μm. (D) H. ducreyi organisms (arrowheads) are found in vacuoles within HaCaT cells at 24 h postinoculation. Junctions between cells are disrupted (compare with panel A). Magnification, ×7,200; bar, 1 μm. (E) H. ducreyi organisms (arrowheads) are localized inside dead HaCaT cells (Ha) 24 h postinoculation. Magnification, ×4,500; bar, 1 μm.
Approximately 5 × 105 H. ducreyi organisms were added to the surfaces of the cocultures. At 2, 4, 8, 24, 48, and 72 h postinoculation, nonadherent bacteria were washed away and the cells were fixed and processed for electron microscopy. Individual H. ducreyi organisms were found closely associated with the HaCaT cell surface 2 h postinoculation (data not shown) and throughout the time course (Fig. 3B). H. ducreyi was not found in association with HS27 cells or the collagen matrix. As bacterial numbers increased over time, we observed extracellular clumps of H. ducreyi exhibiting the characteristic “school-of-fish” appearance (29) (Fig. 3C). HaCaT cells near large numbers of H. ducreyi organisms appeared necrotic. It was difficult to find H. ducreyi inside HaCaT cells 2 h postinoculation, consistent with the low numbers of H. ducreyi organisms protected from gentamicin at this time. It was easier to locate intracellular H. ducreyi 24 h or more postinoculation, although this observation was still rare. Intracellular H. ducreyi appeared predominantly in vacuoles (Fig. 3D and E) and was commonly found within dead HaCaT cells within 24 h postinoculation (Fig. 3E). H. ducreyi within dead HaCaT cells appeared healthy, as evidenced by the integrity of double membranes surrounding the bacteria. Finding H. ducreyi within dead HaCaT cells combined with the gentamicin protection data suggests that prolonged culturing of H. ducreyi-infected HaCaT cells results in cytotoxicity and subsequent access of the antibiotic to intracellular organisms.
H. ducreyi induces IL-8 but not TNF-α expression by HS27 cell-HaCaT cell cocultures.
We previously showed that H. ducreyi induced IL-6 and IL-8 but not TNF-α expression from cells in an in vitro skin model (19). Since we were interested in whether the HS27 cell-HaCaT cell coculture model responded similarly to H. ducreyi, we verified that the IL-8 and TNF-α induction profiles in cocultures mimicked those in the in vitro skin model.
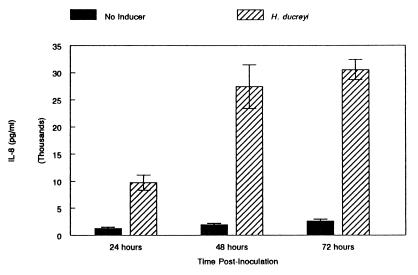
RPMI-10 containing approximately 5 × 105 H. ducreyi organisms or RPMI-10 without bacteria was added to the apical surface of established cocultures. The subnatant medium was collected and replaced with fresh RPMI-10 at 2, 6, 24, 48, or 72 h postinoculation, and the samples were assayed for both IL-8 and TNF-α production by an enzyme-linked immunosorbent assay (Fig. 4). The patterns of IL-8 and TNF-α expression of cocultures in response to H. ducreyi were similar to those of in vitro skin. IL-8 expression levels in H. ducreyi-infected and uninfected cocultures were indistinguishable 2 and 6 h postinoculation. However, by 24 h, cocultures expressed significantly more IL-8 in response to H. ducreyi than did uninfected cocultures (Fig. 4; P, 0.002). Maximum IL-8 expression occurred by 48 h postinoculation, with no significant increase in expression by 72 h postinoculation (Fig. 4; P, <0.05). Consistent with our earlier finding (19), H. ducreyi-infected cocultures that expressed IL-8 did not express TNF-α above the limit of detection of the assay, 15.6 pg/ml, at any time point examined. However, the cocultures were capable of expressing TNF-α, as E. coli ORN200 induced detectable levels of TNF-α (62.6 ± 9.07 pg/ml) 24 h postinoculation (inoculum of 5 × 105 E. coli organisms).
FIG. 4.
H. ducreyi induces IL-8 expression from HS27 cell-HaCaT cell cocultures. Results are expressed as means ± SEM and were obtained from three independent experiments performed in triplicate.
To determine if HS27 or HaCaT cells alone exhibited a cytokine profile similar to that of cocultures in response to H. ducreyi, HS27 and HaCaT cells were cultured separately on tissue culture inserts for 9 to 11 days and inoculated with H. ducreyi. Subnatant medium was assayed for cytokine production. HS27 cells produced a large amount of IL-8 24 and 48 h postinoculation in response to H. ducreyi, whereas HaCaT cells produced a small but significant amount of IL-8 at these times (Table 1). TNF-α was not expressed by HaCaT cells inoculated with H. ducreyi, as was observed with cocultures and the in vitro skin model. In contrast, H. ducreyi induced a detectable amount of TNF-α from HS27 cells 24 h postinoculation (32.8 ± 3.72 pg/ml). A direct interaction between H. ducreyi and HS27 cells may be required for IL-8 and TNF-α expression from HS27 cells, since filtered medium from H. ducreyi-infected HaCaT cells 24 h postinfection did not induce IL-8 or TNF-α expression from HS27 cells (data not shown). The fact that H. ducreyi did not associate with HS27 cells in the coculture system may account for the lack of TNF-α expression and the decreased IL-8 expression by cocultures versus HS27 cells alone in response to H. ducreyi.
TABLE 1.
IL-8 expression by HS27 or HaCaT cells in response to H. ducreyi
| Cell line | Inducer | Mean amt (± SEM) of IL-8 expressed (pg/ml) at:
|
|
|---|---|---|---|
| 24 h | 48 h | ||
| HaCaT | Mock | 328.8 ± 37.8 | 189.0 ± 36.7 |
| H. ducreyi | 530.1 ± 10.7a | 541.5 ± 53.8b | |
| HS27 | Mock | 56.6 ± 14.0 | 70.3 ± 35.5 |
| H. ducreyi | 144,000± 19,000c | 255,000 ± 24,000a | |
P value for comparison with control (mock), 0.002.
P value for comparison with control, 0.014.
P value for comparison with control, 0.007.
H. ducreyi LOS is partially responsible for IL-8 induction in HS27 cell-HaCaT cell cocultures.
LPS and LOS induce IL-8 expression from macrophages (42) and, to some extent, from epithelial cells (18). Furthermore, LPS and LOS are known to be potent inducers of TNF-α expression from macrophages (42) and induce TNF-α from a variety of epithelial cells, including keratinocytes (21). We wondered why live E. coli but not H. ducreyi induced TNF-α expression from cocultures, even though both organisms contain the minimal LPS or LOS requirements for bioactivity (50), one aspect of which is the induction of TNF-α expression (42). Thus, we investigated whether H. ducreyi LOS in the absence of bacterial proteins could induce IL-8 and TNF-α expression from HS27 cell-HaCaT cell cocultures.
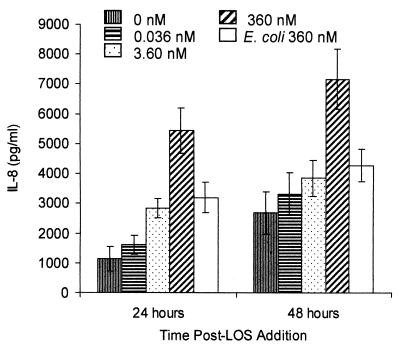
We added 360, 3.6, or 0.036 nM purified H. ducreyi LOS (corresponding to approximately 5 × 107, 5 × 105, or 5 × 103 bacteria, respectively) in RPMI-10 to HS27 cell-HaCaT cell cocultures and assayed the subnatant medium for IL-8 (Fig. 5) and TNF-α as described above. Consistent with results obtained with live H. ducreyi, 360 nM purified H. ducreyi LOS induced significantly greater IL-8 expression from cocultures than that seen in controls 24 and 48 h post-LOS addition (Fig. 5; P, <0.05). There was no significant IL-8 expression in response to LOS 6 h postinoculation. In contrast to the finding that cocultures expressed more IL-8 by 48 h than by 24 h after the addition of live H. ducreyi, maximum expression of IL-8 in response to 360 nM LOS occurred by 24 h postinoculation. Furthermore, 360 nM LOS did not induce IL-8 expression to the levels induced by live H. ducreyi (compare the y axes of Fig. 4 and 5). By 24 h, 360 nM H. ducreyi LOS and 360 nM E. coli LPS induced IL-8 expression from cocultures; however, H. ducreyi LOS induced a significantly higher concentration of IL-8 than did E. coli LPS at this time (Fig. 5; P, <0.05). H. ducreyi LOS but not E. coli LPS induced significantly more IL-8 than that seen in controls by 48 h (Fig. 5). Combined, these data suggest that H. ducreyi LOS induces a specific response from cocultures.
FIG. 5.
H. ducreyi LOS is partially responsible for H. ducreyi-induced IL-8 expression from HS27 cell-HaCaT cell cocultures. H. ducreyi LOS (0 to 360 nM) or E. coli LPS (360 nM) was added to cocultures, and subnatant medium was removed and replaced with 1 ml of fresh medium. Results are expressed as means ± SEM and were obtained from four independent experiments performed in duplicate.
Neither 360 nM H. ducreyi LOS nor 360 nM E. coli LPS induced TNF-α expression from HS27 cell-HaCaT cell cocultures (data not shown), suggesting either that LPS or LOS is not the sole mechanism for TNF-α induction in this system or that the system lacks cell types or molecules necessary for LOS- or LPS-induced TNF-α expression.
DISCUSSION
The study of H. ducreyi pathogenesis has been hampered by the scarcity of relevant in vitro models with which to study the roles of the putative virulence determinants of this obligate human pathogen. In this report, we describe a coculture model for examining H. ducreyi adherence to and entry into keratinocytes. In addition, we have used the cocultures to assess the host cell cytokine response to H. ducreyi infection in an attempt to begin to understand the inflammatory response to H. ducreyi infection in vivo.
It is unclear whether H. ducreyi interacts with fibroblasts or keratinocytes upon introduction into breaks in the skin. We constructed a coculture system consisting of HS27 dermal foreskin fibroblasts grown on a collagen I-coated tissue culture insert and HaCaT keratinocytes to more closely mimic in vivo skin structure than keratinocyte monolayers. Collagen I was used as the growth substrate because it is the most abundant collagen type in the dermis. Cocultures exhibited the same cytokine profile in response to H. ducreyi as that of a commercially available in vitro skin system, whereas a different cytokine profile was induced when the cell types were cultured separately. The commercially available in vitro skin model used previously in our laboratory was constructed similarly, except that the cells were fed only from the bottom and the keratinocytes were exposed to the air, facilitating terminal differentiation of the keratinocytes and keratinization (19). Since H. ducreyi does not infect keratinized skin (12, 17, 53), it was necessary to first abrade the surface of the keratinized layer of the in vitro skin model to establish H. ducreyi infection. The fact that our modified coculture model does not require this step, as the keratinocytes remain submerged in medium and do not terminally differentiate, adds a particularly attractive feature to this model.
Using a quantitative adherence assay and TEM, we found that H. ducreyi 35000 adhered to HaCaT cells cultured in monolayers and in cocultures with fibroblasts. These data are consistent with those of previous studies that examined H. ducreyi adherence to primary keratinocytes grown as monolayers (7, 16, 58), suggesting that HaCaT cells are relevant for studies of H. ducreyi-keratinocyte interactions. We found that approximately 13% of H. ducreyi 35000 adhered to HaCaT cells and that maximum adherence occurred approximately 2 h postinoculation. Totten et al. (58), using an uncharacterized strain of H. ducreyi (LA228R), and Brentjens et al. (7), using H. ducreyi 35000, also found that 2 h was required for maximum adherence to primary keratinocytes and that 30 to 50% of H. ducreyi LA228R or 15 to 23% of H. ducreyi 35000 adhered at this time. Gibson et al. (16) also found that 15% of H. ducreyi 35000 adhered to primary keratinocytes 2 h postinoculation. The significantly higher percentage of H. ducreyi adherence found by Totten et al. (58) may be accounted for by two facts. First, the H. ducreyi strain used in their study was passaged in the temperature-dependent animal model and might be more adherent and invasive than the commonly used laboratory strain 35000. Second, the percentage of adherence in their study was based on the number of H. ducreyi organisms that adhered versus the input number of H. ducreyi organisms and thus did not account for replication of the population during the assay.
Using TEM, we observed H. ducreyi adherent to HaCaT cell processes or pedestals emanating from the cell surface. Similar pedestals have also been observed under epithelial cell-adherent enteropathogenic E. coli, and their synthesis requires induction by a bacterial protein and subsequent actin polymerization (43). Cytochalasin D, a potent inhibitor of actin polymerization, at low concentrations (≤5 ng/ml) that did not cause rounding up of the HaCaT cells, had no effect on H. ducreyi adherence (data not shown). Higher concentrations of cytochalasin D (≥5 ng/ml) had a severe effect on HaCaT cell morphology. Thus, the data cannot exclude the possibility that actin polymerization is important for H. ducreyi adherence.
We found that only 0.005% of H. ducreyi bacteria entered HaCaT cells in our system, whereas Totten et al. (58) found that 0.11% of H. ducreyi 35000 entered primary keratinocytes. The differences observed here may be explained by differences in experimental protocols or in vitro systems. Nevertheless, the percentage of H. ducreyi bacteria protected from gentamicin in our experiments was remarkably low, even though we had little difficulty locating intracellular bacteria by electron microscopy. A plausible explanation for this finding is that gentamicin is able to gain access to the intracellular milieu of HaCaT cells, killing intracellular H. ducreyi. One likely mechanism by which this may occur is if H. ducreyi were cytotoxic or caused minor membrane damage to HaCaT cells. Although H. ducreyi does not cause the destruction of HaCaT cell layers at least up to 72 h postinoculation (unpublished results), some degree of cytopathology, such as vacuolation, has been observed by us (unpublished results) and others (26). H. ducreyi secretes a cytotoxin (cytolethal distending toxin) that exhibits a cytopathic effect on HaCaT cells (26). Finding necrotic HaCaT cells in the vicinity of extracellular clumps of H. ducreyi (Fig. 3C) and H. ducreyi within dead HaCaT cells (Fig. 3E) supports the notion that H. ducreyi is cytotoxic for these cells.
Another possible explanation for why gentamicin may enter HaCaT cells is that HaCaT cells may be naturally leaky. The gentamicin protection assay is widely used to quantitate bacterial invasion into epithelial cells and is based on the premise that gentamicin does not enter epithelial cells; thus, intracellular bacteria are protected from gentamicin exposure. However, it is known that aminoglycoside antibiotics, such as gentamicin, may enter some epithelial cells by endocytosis (47).
Finally, it is possible that H. ducreyi enters only keratinocytes of a specific differentiation type in vivo, a cell type that may be present in a small percentage in the HaCaT cell population. Staining of HaCaT cells with markers for specific stages of keratinocyte differentiation may help address this issue. The observation of large numbers of H. ducreyi organisms adherent to some HaCaT cells but not others is consistent with the possibility that H. ducreyi exhibits selective cell tropism.
By TEM, we observed intracellular H. ducreyi predominantly within vacuoles, consistent with the findings of Totten et al. (58). This location may protect H. ducreyi from a necrotic environment, an idea supported by the finding of apparently healthy H. ducreyi bacteria within dead HaCaT cells (Fig. 3E). It has been suggested that H. ducreyi entry into keratinocytes may be an important factor in the tissue destruction observed in chancroid ulcers (58). Although we were able to locate H. ducreyi organisms within HaCaT cells cultured in the coculture system by TEM, this finding was rare. Furthermore, we found more necrotic HaCaT cells associated with adherent, extracellular H. ducreyi than containing intracellular H. ducreyi. These data suggest that a prolonged intracellular stage may not be a significant factor in the pathogenesis of H. ducreyi. Other factors, such as toxins and the inflammatory response, may be primarily responsible for tissue necrosis in chancroid lesions.
Epidermal cells are important immune mediators, as they are the first line of defense against invading pathogens. Consistent with our previous study with an in vitro skin model, we found that IL-8 expression was induced in HS27 cell-HaCaT cell cocultures in response to H. ducreyi. IL-8 is a chemokine that is a potent inducer of neutrophil chemotaxis and neutrophil activation and is also capable of inducing the chemotaxis of T cells (31). During natural H. ducreyi infection, neutrophils accumulate in the ulcer and remain a prominent inflammatory cell type in the lesion over the duration of the infection (15). Since neutrophils have a short life span, their continued presence in the ulcer must be due to the constant taxis of new neutrophils to the site of H. ducreyi infection. Our findings that H. ducreyi induces IL-8 expression from cocultures 24 h postinoculation, that maximum expression occurs at 48 h, and that expression continues at that level at least up to 72 h postinoculation suggest that the continuous expression of IL-8 may occur in vivo and may mediate constant PMN chemotaxis and recruitment of T cells.
H. ducreyi LOS added to cocultures at 360 nM, correlating with approximately 5 × 107 bacteria, also induced IL-8 expression 24 h postinoculation. However, the level of IL-8 expression at 24 h was only half that induced by live H. ducreyi, even though the number of live H. ducreyi bacteria present 24 h postinoculation was approximately 5 × 107 (data not shown). These data suggest that other bacterial components may be necessary for the maximum induction of IL-8. However, it is also possible that purified LOS interacts with eukaryotic cells in a manner different from that of LOS present in the H. ducreyi membrane. H. ducreyi LOS did not induce increased IL-8 expression between 24 and 48 h postinoculation, as was observed for live organisms, suggesting that bacterial replication or LOS shedding from H. ducreyi during replication may account for the observed increase in IL-8 expression during this time frame. The finding that H. ducreyi LOS induced significantly more IL-8 than E. coli LPS at the same concentration suggests that a unique interaction between H. ducreyi LOS and cocultured cells may occur. We are currently investigating the role of H. ducreyi-mediated IL-8 expression in neutrophil migration to the site of infection and the bacterial factors responsible for IL-8 induction. The results of these experiments will provide information about the events occurring early during H. ducreyi infection.
TNF-α is an important inflammatory mediator that is induced, among other ways, in response to gram-negative bacterial pathogens upon stimulation of macrophages with LPS (42). Surprisingly, H. ducreyi did not induce TNF-α expression from cocultures or the in vitro skin system, whereas E. coli did. This result was unexpected, since the LOS of H. ducreyi contains all of the structural requirements necessary to induce TNF-α expression (42, 50). Even more surprising was our finding that purified E. coli LPS did not induce TNF-α from cocultures, even when present at levels higher than those physiologically necessary to induce TNF-α from macrophages. Higher levels of E. coli LPS (100 μg) have been found to induce TNF-α expression from keratinocytes (21); however, we chose to use physiologically relevant LPS levels for our studies. Our findings suggest that LPS is not the mediator of E. coli-induced TNF-α expression in the coculture system. The TNF-α expression observed in cocultures in the presence of whole E. coli may be due to the fact that E. coli begins to kill cells in cocultures 24 h postinoculation, and HaCaT cellular lysis has been shown to trigger TNF-α expression (14). Another possible explanation is that E. coli, unlike H. ducreyi, is capable of gaining access to fibroblasts in cocultures, eliciting a TNF-α response by these cells. The finding that H. ducreyi induces TNF-α from HS27 cells cultured separately suggests that if H. ducreyi were capable of directly interacting with HS27 cells in cocultures, a TNF-α response might be elicited.
LOS- or LPS-mediated TNF-α expression requires either the CD14 cellular receptor (64) or the soluble form of this receptor (sCD14) (40) plus a serum protein, LPS binding protein (LBP) (49). LBP binds to LOS or LPS and transports LOS or LPS to either sCD14 in serum or CD14 on the cell surface, which interacts with another unknown receptor responsible for initiating signal transduction events, leading to TNF-α expression (60). Most human epithelial cells do not express CD14 but can still be stimulated with LOS or LPS to express TNF-α if the culture medium contains serum that has sCD14 and LBP (40), both of which can be supplied by FBS (used in our studies) but are not as active as their human counterparts (27). One reason for the absence of a TNF-α response in our coculture system may be that the HS27 or HaCaT cells do not express CD14 and/or that the serum does not contain active sCD14 or LPB. We are currently investigating these possibilities.
In conclusion, we have developed a new in vitro model with which to study H. ducreyi-host cell interactions and the host cell immune response to H. ducreyi. The coculture described in this report represents a relevant system that will be useful for examining potential virulence factors of H. ducreyi that are responsible for ulcer formation. Furthermore, this system will enable us to examine the role of H. ducreyi products that are responsible for the host cell cytokine response to infection. Since the system is amenable to the addition of inflammatory cells, such as PMN, we will be able to examine mechanisms of immune cell chemotaxis to infected keratinocytes in vitro and may gain insight into events that occur early in natural H. ducreyi infection. The results of these experiments will provide a basis for an understanding of the epithelial and neutrophil immune responses to H. ducreyi infection and insight into ways to modify the immune response to efficiently clear infection.
ACKNOWLEDGMENTS
This work was supported by NIAID grant AI42824 to T.H.K.
We thank Vicky Madden and Bob Bagnell for help with TEM and Janne Cannon, Paul Orndorff, and past and present members of the Kawula laboratory (Marcia Hobbs, Gina Donato, Lani San Mateo, and Kristen Toffer) for many helpful ideas and discussions throughout the course of this work.
REFERENCES
- 1.Abeck D, Johnson A P, Mensing H. Binding of Haemophilus ducreyi to extracellular matrix proteins. Microb Pathog. 1992;13:81–84. doi: 10.1016/0882-4010(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 2.Alfa M J. Cytopathic effect of Haemophilus ducreyi for human foreskin cell culture. J Med Microbiol. 1992;37:43–50. doi: 10.1099/00222615-37-1-43. [DOI] [PubMed] [Google Scholar]
- 3.Alfa M J, DeGagne P. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb Pathog. 1997;22:39–46. doi: 10.1006/mpat.1996.0089. [DOI] [PubMed] [Google Scholar]
- 4.Alfa M J, DeGagne P, Hollyer T. Haemophilus ducreyi adheres to but does not invade cultured foreskin cells. Infect Immun. 1993;61:1735–1742. doi: 10.1128/iai.61.5.1735-1742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens R J, Spinola S M, Campagnari A A. Haemophilus ducreyi adheres to human keratinocytes. Microb Pathog. 1994;16:243–247. doi: 10.1006/mpat.1994.1025. [DOI] [PubMed] [Google Scholar]
- 8.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellazzo A, Shero M, Apicella M A, Spinola S M. Expression of pili by Haemophilus ducreyi. J Infect Dis. 1992;165:S198–S199. doi: 10.1093/infdis/165-supplement_1-s198. [DOI] [PubMed] [Google Scholar]
- 9a.Centers for Disease Control and Prevention. STD surveillance. U.S. Atlanta, Ga: Department of Health and Human Services; 1997. [Google Scholar]
- 10.Chen C Y, Mertz K J, Spinola S M, Morse S A. Comparison of enzyme immunoassays for antibodies to Haemophilus ducreyi in a community outbreak of chancroid in the United States. J Infect Dis. 1997;175:1390–1395. doi: 10.1086/516471. [DOI] [PubMed] [Google Scholar]
- 11.Cope L D, Lumbley S, Latimer J L, Klesney-Tate J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dienst R B. Virulence and antigenicity of Haemophilus ducreyi. Am J Syph Gonorrhea Vener Dis. 1948;32:289–291. [PubMed] [Google Scholar]
- 13.Elkins C, Totten P A, Olsen B, Thomas C E. Role of the Haemophilus ducreyi Ton system in internalization of heme from hemoglobin. Infect Immun. 1998;66:151–160. doi: 10.1128/iai.66.1.151-160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezepchuk Y V, Leung D Y M, Middleton M H, Bina P, Reiser R, Norris D A. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-alpha from human keratinocytes. J Investig Dermatol. 1996;107:603–609. doi: 10.1111/1523-1747.ep12583377. [DOI] [PubMed] [Google Scholar]
- 15.Freinkel A L. Histological aspects of sexually transmitted genital lesions. Histopathology. 1987;11:819–831. doi: 10.1111/j.1365-2559.1987.tb01885.x. [DOI] [PubMed] [Google Scholar]
- 16.Gibson B W, Campagnari A A, Melaugh W, Phillips N J, Apicella M A, Grass S, Wang J, Palmer K L, Munson R S., Jr Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 1997;179:5062–5071. doi: 10.1128/jb.179.16.5062-5071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenblatt R B, Sanderson E S, Kupperman H S, Hair Q, Fried P. The experimental prophylaxis of chancroid disease-II. Am J Syph Gonorrhea Vener Dis. 1944;28:165–178. [Google Scholar]
- 18.Hedges S R, Agace W W, Svanborg C. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 1995;3:266–270. doi: 10.1016/s0966-842x(00)88941-6. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs M M, Paul T R, Wyrick P B, Kawula T H. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction profile in an in vitro skin model. Infect Immun. 1998;66:2914–2921. doi: 10.1128/iai.66.6.2914-2921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karkhanis Y D, Zeitner J Y, Jackson J J, Carlo D J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram negative bacteria. Anal Biochem. 1977;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 21.Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel J C, Luger T A. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagergard T. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb Pathog. 1992;13:203–217. doi: 10.1016/0882-4010(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 23.Lagergard T, Purven M. Neutralizing antibodies to Haemophilus ducreyi cytotoxin. Infect Immun. 1993;61:1589–1592. doi: 10.1128/iai.61.4.1589-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammel C J, Dekker N P, Palefsky J, Brooks G F. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J Infect Dis. 1993;167:642–650. doi: 10.1093/infdis/167.3.642. [DOI] [PubMed] [Google Scholar]
- 25.Le-Bacq F, Mason P R, Gwanzura L, Robertson V J, Latif A S. HIV and other sexually transmitted diseases at rural hospitals in Zimbabwe. Genitourin Med. 1993;69:352–356. doi: 10.1136/sti.69.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumbley S R, Latimer J L, Hansen E J. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Haemophilus ducreyi cytolethal distending toxin kills human keratinocytes but not human foreskin fibroblasts, abstr. B-288; p. 104. [Google Scholar]
- 27.Meszaros K, Aberle S, White M, Parent J B. Immunoreactivity and bioactivity of lipopolysaccharide-binding protein in normal and heat-inactivated sera. Infect Immun. 1995;63:363–365. doi: 10.1128/iai.63.1.363-365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamadzadeh M, Muller M, Hultsch T, Enk A, Saloga J, Knop J. Enhanced expression of IL-8 in normal human keratinocytes and human keratinocyte cell line HaCaT in vitro after stimulation with contact sensitizer, toleragens and irritants. Exp Dermatol. 1994;3:298–303. doi: 10.1111/j.1600-0625.1994.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 29.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nzila N, Laga M, Thiam M A, Mayimona K, Edidi B, Ban-Dyck E, Behets F, Hassig S, Nelson A, Mokwa K, Ashley R L, Piot P, Ryder R W. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991;5:715–721. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Oppenheim J J, Zachariae C O C, Mukaiada N, Matsushima K. Properties of the novel proinflammatory supergene intercrine cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 32.Palmer K L, Goldman W E, Munson R S., Jr An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 33.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 35.Palmer K L, Thornton A C, Fortney K R, Hood A F, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 36.Pepin J, Dunn D, Gaye I, Alonso P, Egboga R, Tedder R, Piot P, Berry N, Schellenberg D, Whittle H. HIV-2 infection among prostitutes working in The Gambia: association with serological evidence of genital ulcer diseases and with generalized lymphadenopathy. AIDS. 1991;5:69–75. [PubMed] [Google Scholar]
- 37.Pepin J, Quigley M, Todd J, Gaye I, Janneh M, Van-Dyck E, Piot P, Whittle H. Association between HIV-2 infection and genital ulcer diseases among male sexually transmitted disease patients in The Gambia. AIDS. 1992;6:489–493. doi: 10.1097/00002030-199205000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Phillips D M, Tan X. HIV-1 infection of the trophoblast cell line BeWo: a study of virus uptake. AIDS Res Hum Retroviruses. 1992;8:1683–1691. doi: 10.1089/aid.1992.8.1683. [DOI] [PubMed] [Google Scholar]
- 39.Plummer F A, Simonsen J N, Cameron D W, Ndinya-Achola O, Kreiss J K, Gakinya M N, Waiyaki P, Cheang M, Piot P, Ronald A R, Ngugi E N. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 40.Pugin J, Schurer-Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purven M, Lagergard T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 43.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz N, Wang B, Pentland A, Caparon M. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol Microbiol. 1998;27:337–346. doi: 10.1046/j.1365-2958.1998.00681.x. [DOI] [PubMed] [Google Scholar]
- 45.Ryle C M, Breitkreutz D, Stark H J, Leigh I M, Steinert P M, Roop D, Fusenig N E. Density-dependent modulation of synthesis of keratins 1 and 10 in the human keratinocyte cell line HACAT and in ras-transfected tumorigenic clones. Differentiation. 1989;40:42–54. doi: 10.1111/j.1432-0436.1989.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 46.San Mateo L R, Hobbs M M, Kawula T H. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol Microbiol. 1998;27:391–404. doi: 10.1046/j.1365-2958.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 47.Sandoval R, Leiser J, Molitoris B A. Aminoglycoside antibiotics traffic to the Golgi complex in LLC-PK1 cells. J Am Soc Nephrol. 1998;9:167–174. doi: 10.1681/ASN.V92167. [DOI] [PubMed] [Google Scholar]
- 48.Schoop V M, Mirancea N, Fusenig N E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J Investig Dermatol. 1999;112:343–353. doi: 10.1046/j.1523-1747.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 49.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 50.Schweda E K, Jonasson J A, Jansson P E. Structural studies of lipooligosaccharides from Haemophilus ducreyi ITM 5535, ITM 3147, and a fresh clinical isolate, ACY1: evidence for intrastrain heterogeneity with the production of mutually exclusive sialylated or elongated glycoforms. J Bacteriol. 1995;177:5316–5321. doi: 10.1128/jb.177.18.5316-5321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spinola S M, Castellazzo A, Shero M, Apicella M A. Characterization of pili expressed by Haemophilus ducreyi. Microb Pathog. 1990;9:417–426. doi: 10.1016/0882-4010(90)90060-4. [DOI] [PubMed] [Google Scholar]
- 52.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 53.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 54.St. Geme J W, III, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990;58:4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens M K, Hassett D J, Radolf J D, Hansen E J. Cloning and sequencing of the gene encoding the Cu, Zn-superoxide dismutase of Haemophilus ducreyi. Gene. 1996;183:35–40. doi: 10.1016/s0378-1119(96)00417-9. [DOI] [PubMed] [Google Scholar]
- 56.Stevens M K, Procella S, Klesney-Tait J, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Syrjanen S, Mikola H, Nykanen M, Hukkanen V. In vitro establishment of lytic and nonproductive infection by herpes simplex virus type 1 in three-dimensional keratinocyte culture. J Virol. 1996;70:6524–6528. doi: 10.1128/jvi.70.9.6524-6528.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Totten P A, Lara J C, Norn D V, Stamm W E. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect Immun. 1994;62:5632–5640. doi: 10.1128/iai.62.12.5632-5640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Totten P A, Norn D V, Stamm W E. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulevitch R J, Tobias P S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Opin Immunol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 61.Wang B, Ruiz N, Pentland A, Caparon M. Keratinocyte proinflammatory responses to adherent and nonadherent group A streptococci. Infect Immun. 1997;65:2119–2126. doi: 10.1128/iai.65.6.2119-2126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wasserheit J N. Epidemiological synergy: interrelationships between human immunodeficiency virus and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 63.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. In: Whistler R L, editor. Methods in carbohydrate chemistry. New York, N.Y: Academic Press; 1965. pp. 83–91. [Google Scholar]
- 64.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]