Abstract
The death triad, including coagulopathy, hypothermia, and acidosis, is shown to be a strong predictor of mortality in trauma patients. We aimed to investigate whether the inclusion of hypotension, defined as systolic blood pressure (SBP) < 60 mmHg, as a fourth factor in the death triad would comprise a death tetrad to help stratify mortality risk in trauma patients. A total of 3361 adult trauma patients between 1 January 2009 and 31 December 2019 were allocated into groups to investigate whether hypotension matters in determining the mortality outcome of trauma patients who possess 1–3 death triad components compared to those without any component. Hypotension was added to the death tetrad, and the adjusted mortality outcome was compared among groups with 0–4 death tetrad components. Herein, we showed that SBP < 60 mmHg could be used to identify patients at risk of mortality among patients with one or two death triad components. Patients with one, two, and three death tetrad components had respective adjusted mortality rates of 3.69-, 10.10-, and 40.18-fold, determined by sex, age, and comorbidities. The mortality rate of trauma patients with all the four death tetrad components was 100%. The study suggested that hypotension, defined as an SBP < 60 mmHg, may act as a proper death tetrad component to stratify the mortality risk of trauma patients.
Keywords: coagulopathy, hypothermia, acidosis, hypotension, death triad, death tetrad, mortality, trauma
1. Introduction
The death triad includes metabolic acidosis in full blood (potential of hydrogen (PH) < 7.2), hypothermia (temperature < 35 °C measured by tympanic thermometers), and coagulopathy (international normalized ratio [INR] > 1.5) when patients arrive at the emergency department. The death triad observed in trauma patients has been proven to be a strong predictor of mortality [1,2,3,4]. The mortality of trauma patients at 24 h increased with the addition of all three components of the death triad [5]. In patients with multiple traumas, the death triad predicted 24 h mortality in 96% of patients [6]. In patients with abdominal gunshot wounds and major vascular injuries whose mortality rate was as high as 40%, the death triad comprised 85% of mortality [7]. Mitra et al. found that the overall mortality rate would be as high as 47.8% if the trauma patient presented with all three components of the death triad [1]. Thus, the death triad has been used worldwide and it provides important information for risk stratification in dealing with trauma patients.
Each component of the death triad had a negative impact on patient survival, and the relationship between the death triad and mortality was clear and validated [1,8]. Among the three components of the death triad, metabolic acidosis has been proven to be associated with increased mortality [9], coagulopathy was related to a 4- to 5-fold increase in mortality following major trauma [10], and hypothermia contributes not only to mortality associated with the injury but also to fluid requirements and duration of surgery [7,11]. Furthermore, hypothermia is also a significant contributor to coagulopathy, regardless of the condition of metabolic acidosis or fluid infusion [12,13].
Interestingly, although hypotension has been widely recognized as a major contributor of mortality in trauma patients [14,15,16,17,18], the death triad did not use hypotension as a component in determining patient outcomes. This study aimed to investigate whether hypotension matters in determining the mortality outcome of trauma patients with various components of the death triad and to explore whether hypotension could act as a fourth factor besides the death triad to stratify the mortality risk in trauma patients.
2. Materials and Methods
2.1. Study Population and Grouping
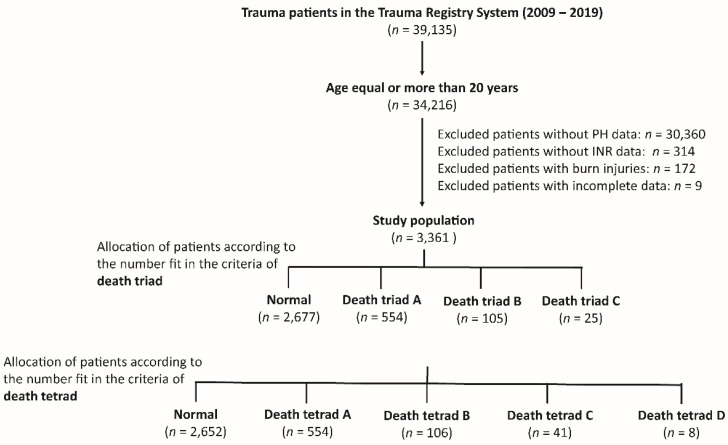
This study reviewed registered medical information from the registered trauma database between 1 January 2009 and 31 December 2019 in a Level I trauma center that provides care to trauma patients in southern Taiwan [19,20,21]. All registered data were prospectively collected from two licensed registers of hospitalized trauma patients. As shown in Figure 1, the eligibility of 39,195 hospitalized trauma patients was assessed. After excluding those aged <20 years, those lacking PH data (n = 30,360), lack of INR data (n = 314), patients with burn injuries (n = 172), and those with incomplete registered data (n = 9), 3361 patients were enrolled in the study for further analysis. First, trauma patients were grouped according to number fit in the components of the death triad. Patients with one, two, and three items fit in the components of the death triad and were allocated into death triad groups A, B, and C, respectively (Figure 1). With hypotension, defined as a non-invasive measurement of systolic blood pressure (SBP) < 60 mmHg at the triage station when patients arrived at the emergency department, as one component of the death tetrad, further grouping was performed according to the number fit in the components of the death tetrad. Patients with one, two, three, and four items fit in the components of the death tetrad and were allocated into the death tetrad groups A, B, C, and D, respectively (Figure 1).
Figure 1.
Flowchart illustrating the included hospitalized adult trauma patients from the registered trauma database, and the assignment of the study patient populations into three or four groups according to the number fit in the components of death triad or tetrad, respectively. In comparison with the death triad, the death tetrad has an additional component, hypotension, defined as systolic blood pressure < 60 mmHg. PH, potential of hydrogen; INR, international normalized ratio.
2.2. Study Parameter
The study included information on age, sex, comorbidities (including coronary artery disease (CVA), congestive heart failure (CHF), hypertension (HTN), diabetes mellitus (DM), and end-stage renal disease (ESRD)), vital signs, PH, INR, Glasgow Coma Scale (GCS) score, abbreviated injury scale (AIS) in each body region, injury severity score (ISS), in-hospital mortality, length of hospital stay (LOS), and admission into the intensive care unit (ICU). The polytrauma is defined as the trauma patients have injuries of AIS ≥ 3 in more than one body region [22,23].
2.3. Statistical Analysis
Data processing and analysis were performed using IBM SPSS Statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA). Pearson chi-square tests were employed to compare categorical variables and are presented as frequencies and percentages with odds ratios (OR) and 95% confidence intervals (CI). Continuous variables were estimated using Levene’s test for homogeneity of variance. Normally distributed continuous variables are presented as mean and standard deviation, and the unpaired Student’s t-test was used to compare continuous variables that were normally distributed. The Mann–Whitney U test was used to compare non-normally distributed data, which are presented as medians and interquartile ranges (IQR). In this study, in-hospital mortality was the primary outcome, while hospital LOS and ICU admission were the secondary outcomes. The adjusted odds ratio of mortality was computed using logistic regression adjusted for variables with significant differences in patients’ injury characteristics. Statistical significance was set at a calculated p-value of <0.05.
3. Results
3.1. Clinical Characteristics and Outcomes of Trauma Accident Divided According to Death Triad
According to the death triad, the trauma patients were divided into four groups: death triad A group (n = 554), which are those who fulfilled one component of the death triad; death triad B (n = 105) and death triad C (n = 25) groups, which are those who fulfilled two and three components of the death triad, respectively. The remaining patients were allocated to a normal group (n = 2677). As shown in Table 1, the groups of patients showed significant differences in sex, age, and comorbidities of CVA, HTN, and DM. Regarding the consciousness level, the group with more components of the death triad had a lower GCS score than those in the normal group. The median (IQR) GCS of death triad A group was 9 (4–15), death triad B was 4 (3–8), and death triad C was 3 (3–7), in comparison with those in the normal group, which was 15 (8–15). Compared with those patients in the normal group, the group who had component(s) of the death triad had a higher percent of patients with AIS ≥ 3 injuries in the head, thorax, and abdomen as well as patients with polytrauma. Additionally, the group who had more components of the death triad tended to have a higher ISS compared with those in the normal group. The median (IQR) ISS of death triad A group was 22 (16–29), death triad B was 25 (21–34), and death triad C was 25 (17–38), in comparison with those in the normal group, which was 16 (924). With the increased mortality rate for patients with more components of the death triad, the mortality rate in the death triad A (29.1%), B (57.1%), and C (84.0%) groups was significantly higher than that in the normal group (10.6%, all p < 0.001). The requirement for admission into the ICU was higher for those with components of the death triad than for those in the normal group (p < 0.001), while in the hospital LOS, there was no significant difference among these groups (p = 0.060).
Table 1.
The trauma patients grouped according to the number fit in the components of the death triad. The patients with one, two, and three items that fit in the components of the death triad were allocated into the group of death triad A, B, C, respectively.
| Variables | Grouping by Number of Components of Death Triad | ||||
|---|---|---|---|---|---|
| Death Triad A n = 554 |
Death Triad B n = 105 |
Death Triad C n = 25 |
Normal n = 2677 |
p | |
| Gender | <0.001 | ||||
| Male, n (%) | 385 (69.5) | 90 (85.7) | 20 (80.0) | 1698 (63.4) | |
| Female, n (%) | 169 (30.5) | 15 (14.3) | 5 (20.0) | 979 (36.6) | |
| Age, years (SD) | 53.8 ± 20.3 | 52.7 ± 18.8 | 47.2 ± 18.4 | 57.6 ± 19.9 | <0.001 |
| Comorbidities | |||||
| CVA, n (%) | 14 (2.5) | 1 (1.0) | 0 (0.0) | 156 (5.8) | 0.001 |
| HTN, n (%) | 159 (28.7) | 24 (22.9) | 5 (20.0) | 967 (36.1) | <0.001 |
| CAD, n (%) | 41 (7.4) | 4 (3.8) | 0 (0.0) | 173 (6.5) | 0.291 |
| CHF, n (%) | 8 (1.4) | 0 (0.0) | 1 (4.0) | 21 (0.8) | 0.115 |
| DM, n (%) | 84 (15.2) | 10 (9.5) | 1 (4.0) | 543 (20.3) | <0.001 |
| ESRD, n (%) | 18 (3.2) | 1 (1.0) | 1 (4.0) | 108 (4.0) | 0.364 |
| GCS, median (IQR) | 9 (4–15) | 4 (3–8) | 3 (3–7) | 15 (8–15) | <0.001 |
| Head AIS ≥ 3, n (%) | 345 (62.3) | 70 (66.7) | 16 (64.0) | 1497 (55.9) | 0.008 |
| Thorax AIS ≥ 3, n (%) | 153 (27.6) | 43 (41.0) | 12 (48.0) | 519 (19.4) | <0.001 |
| Abdomen AIS ≥ 3, n (%) | 75 (13.5) | 22 (21.0) | 8 (32.0) | 154 (5.8) | <0.001 |
| Extremities AIS ≥ 3, n (%) | 132 (23.8) | 28 (26.7) | 4 (16.0) | 652 (24.4) | 0.723 |
| External AIS ≥ 3, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0.968 |
| Polytrauma, n (%) | 161 (29.1) | 51 (48.6) | 13 (52.0) | 418 (15.6) | <0.001 |
| ISS, median (IQR) | 22 (16–29) | 25 (21–34) | 25 (17–38) | 16 (9–24) | <0.001 |
| 1–15, n (%) | 131 (23.6) | 9 (8.6) | 5 (20.0) | 1066 (39.8) | <0.001 |
| 16–24, n (%) | 165 (29.8) | 28 (26.7) | 4 (16.0) | 956 (35.7) | 0.003 |
| ≥25, n (%) | 258 (46.6) | 68 (64.8) | 16 (64.0) | 655 (24.5) | <0.001 |
| Mortality, n (%) | 161 (29.1) | 60 (57.1) | 21 (84.0) | 283 (10.6) | <0.001 |
| Hospital LOS, days (SD) | 18.6 ± 16.9 | 17.7 ± 20.2 | 13.5 ± 18.8 | 16.7 ± 15.7 | 0.060 |
| Admitted into ICU, n (%) | 469 (84.7) | 99 (94.3) | 19 (76.0) | 1793 (67.0) | <0.001 |
CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; CVA = cerebral vascular accident; DM = diabetes mellitus; ESRD = end-stage renal disease; GCS = Glasgow Coma Scale; HTN = hypertension; ICU = intensive care unit; IQR = interquartile range; ISS = injury severity score; LOS = length of stay; OR= odds ratio; SD = standard deviation.
3.2. Outcomes of Trauma Patients with or without Hypotension and Those with One to Three Components of the Death Triad
This study investigated whether hypotension is important in determining the mortality outcome of trauma patients with one to three components of the death triad. Patients in the death triad A, B, and C groups were divided into those with SBP < 60 mmHg and those with SBP ≥ 60 mmHg. Among the death triad A group patients (Table 2), there was no difference in sex, age, comorbidities, and ISS between the patients with SBP < 60 mmHg and those with SBP ≥ 60 mmHg; however, the patients with SBP < 60 mmHg had a lower GCS (median, IQR: 3 (3–6) vs. 9 (4–15), p < 0.001) and higher mortality rate than those with SBP ≥ 60 mmHg (48.0% vs. 28.2%, p = 0.033). No differences in hospital LOS and ICU admission rates were found between the two groups of patients.
Table 2.
The injury characteristics and outcome of trauma patients in death triad A with or without systolic blood pressure less than 60 mmHg.
| Variables | Death Triad A | |||
|---|---|---|---|---|
| SBP < 60 mmHg n = 25 |
SBP ≥ 60 mmHg n = 529 |
OR (95% CI) | p | |
| Gender | 0.243 | |||
| Male, n (%) | 20 (80.0) | 365 (69.0) | 1.80 (0.66–4.87) | |
| Female, n (%) | 5 (20.0) | 164 (31.0) | 0.56 (0.21–1.51) | |
| Age, years (SD) | 52.7 ± 17.8 | 53.8 ± 20.4 | - | 0.787 |
| Comorbidities | ||||
| CVA, n (%) | 1 (4.0) | 13 (2.5) | 1.65 (0.21–13.17) | 0.631 |
| HTN, n (%) | 7 (28.0) | 152 (28.7) | 0.97 (0.40–2.36) | 0.937 |
| CAD, n (%) | 1 (4.0) | 40 (7.6) | 0.51 (0.07–3.86) | 0.506 |
| CHF, n (%) | 0 (0.0) | 8 (1.5) | - | 0.536 |
| DM, n (%) | 3 (12.0) | 81 (15.3) | 0.75 (0.22–2.58) | 0.652 |
| ESRD, n (%) | 1 (4.0) | 17 (3.2) | 1.26 (0.16–9.83) | 0.828 |
| GCS, median (IQR) | 3 (3–6) | 9 (4–15) | - | <0.001 |
| ISS, median (IQR) | 25 (17–28) | 22 (16–29) | - | 0.245 |
| 1–15, n (%) | 4 (16.0) | 127 (24.0) | 0.60 (0.20–1.79) | 0.357 |
| 16–24, n (%) | 7 (28.0) | 158 (29.9) | 0.91 (0.37–2.23) | 0.842 |
| ≥25, n (%) | 14 (56.0) | 244 (46.1) | 1.49 (0.66–3.34) | 0.333 |
| Mortality, n (%) | 12 (48.0) | 149 (28.2) | 2.35 (1.05–5.28) | 0.033 |
| Hospital LOS, days (SD) | 18.0 ± 18.0 | 18.6 ± 16.8 | - | 0.873 |
| Admitted into ICU, n (%) | 20 (80.0) | 449 (84.9) | 0.71 (0.26–1.95) | 0.508 |
CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; CVA = cerebral vascular accident; DM = diabetes mellitus; ESRD = end-stage renal disease; GCS = Glasgow Coma Scale; HTN = hypertension; IQR = interquartile range; ISS = injury severity score; OR= odds ratio; SBP = systolic blood pressure; SD = standard deviation.
Among the death triad B patients (Table 3), there was no difference in age, comorbidities, GCS, and ISS between the patients with SBP < 60 mmHg and those with SBP ≥ 60 mmHg; however, the patients with SBP < 60 mmHg predominantly had more males and higher mortality rates than those with SBP ≥ 60 mmHg (79.2% vs. 50.6%, p = 0.013). No differences in hospital LOS and ICU admission rates were found between the two groups of patients.
Table 3.
The injury characteristics and outcome of trauma patients in death triad B with or without systolic blood pressure less than 60 mmHg.
| Variables | Death Triad B | |||
|---|---|---|---|---|
| SBP < 60 mmHg n = 24 |
SBP ≥ 60 mmHg n = 81 |
OR (95% CI) | p | |
| Gender | 0.018 | |||
| Male, n (%) | 17 (70.8) | 73 (90.1) | 0.27 (0.09–0.84) | |
| Female, n (%) | 7 (29.2) | 8 (9.9) | 3.76 (1.20–11.79) | |
| Age, years (SD) | 54.8 ± 17.6 | 52.1 ± 19.2 | - | 0.541 |
| Comorbidities | ||||
| CVA, n (%) | 0 (0.0) | 1 (1.2) | - | 0.584 |
| HTN, n (%) | 8 (33.3) | 16 (19.8) | 2.03 (0.74–5.58) | 0.164 |
| CAD, n (%) | 0 (0.0) | 4 (4.9) | - | 0.267 |
| CHF, n (%) | 0 (0.0) | 0 (0.0) | - | - |
| DM, n (%) | 3 (12.5) | 7 (8.6) | 1.51 (0.36–6.35) | 0.572 |
| ESRD, n (%) | 0 (0.0) | 1 (1.2) | - | 0.584 |
| GCS, median (IQR) | 3 (3–6) | 5 (3–9) | - | 0.230 |
| ISS, median (IQR) | 25 (17–36) | 25 (22–34) | - | 0.296 |
| 1–15, n (%) | 4 (16.7) | 5 (6.2) | 3.04 (0.75–12.38) | 0.107 |
| 16–24, n (%) | 6 (25.0) | 22 (27.2) | 0.89 (0.31–2.54) | 0.833 |
| ≥25, n (%) | 14 (58.3) | 54 (66.7) | 0.70 (0.28–1.78) | 0.453 |
| Mortality, n (%) | 19 (79.2) | 41 (50.6) | 3.71 (1.26–10.89) | 0.013 |
| Hospital LOS, days (SD) | 14.8 ± 21.2 | 18.5 ± 20.0 | - | 0.426 |
| Admitted into ICU, n (%) | 24 (100) | 75 (92.6) | - | 0.170 |
CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; CVA = cerebral vascular accident; DM = diabetes mellitus; ESRD = end-stage renal disease; GCS = Glasgow Coma Scale; HTN = hypertension; IQR = interquartile range; ISS = injury severity score; OR= odds ratio; SBP = systolic blood pressure; SD = standard deviation.
Among the death triad C patients (Table 4), there was no difference in sex, age, comorbidities, GCS, and ISS between the patients with SBP < 60 mmHg and those with SBP ≥ 60 mmHg. The mortality rate (100.0% vs. 76.5%, p = 0.134), hospital LOS, and ICU admission rate were not significantly different between the death triad C patients with SBP < 60 mmHg and those with SBP ≥ 60 mmHg.
Table 4.
The injury characteristics and outcome of trauma patients in death triad C with or without systolic blood pressure less than 60 mmHg.
| Variables | Death Triad C | |||
|---|---|---|---|---|
| SBP < 60 mmHg n = 8 |
SBP ≥ 60 mmHg n = 17 |
OR (95% CI) | p | |
| Gender | 0.668 | |||
| Male, n (%) | 6 (75.0) | 14 (82.4) | 0.64 (0.09–4.89) | |
| Female, n (%) | 2 (25.0) | 3 (17.6) | 1.56 (0.21–11.83) | |
| Age, years (SD) | 52.3 ± 16.4 | 44.8 ± 19.4 | - | 0.359 |
| Comorbidities | ||||
| CVA, n (%) | 0 (0.0) | 0 (0.0) | - | - |
| HTN, n (%) | 2 (25.0) | 3 (17.6) | 1.56 (0.21–11.83) | 0.668 |
| CAD, n (%) | 0 (0.0) | 0 (0.0) | - | - |
| CHF, n (%) | 1 (12.5) | 0 (0.0) | - | 0.137 |
| DM, n (%) | 0 (0.0) | 1 (5.9) | - | 0.484 |
| ESRD, n (%) | 1 (12.5) | 0 (0.0) | - | 0.137 |
| GCS, median (IQR) | 3 (3–3) | 3 (3–12) | - | 0.048 |
| ISS, median (IQR) | 25 (18–37) | 29 (15–38) | - | 0.725 |
| 1–15, n (%) | 1 (12.5) | 4 (23.5) | 0.46 (0.04–5.00) | 0.520 |
| 16–24, n (%) | 2 (25.0) | 2 (11.8) | 2.50 (0.28–22.04) | 0.400 |
| ≥25, n (%) | 5 (62.5) | 11 (64.7) | 0.91 (0.16–5.20) | 0.915 |
| Mortality, n (%) | 8 (100) | 13 (76.5) | - | 0.134 |
| Hospital LOS, days (SD) | 7.1 ± 10.1 | 16.5 ± 21.3 | - | 0.151 |
| Admitted into ICU, n (%) | 4 (50.0) | 15 (88.2) | 0.13 (0.02–1.01) | 0.037 |
CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; CVA = cerebral vascular accident; DM = diabetes mellitus; ESRD = end-stage renal disease; GCS = Glasgow Coma Scale; HTN = hypertension; IQR = interquartile range; ISS = injury severity score; OR= odds ratio; SBP = systolic blood pressure; SD = standard deviation.
3.3. Clinical Characteristics and Outcomes of Trauma Accidents with Different Death Tetrad Score
Based on our hypothesis, we added SBP < 60 mmHg as the fourth component to stratify the risk of mortality. According to the death tetrad, the trauma patients can be divided into five groups: death tetrad A (n = 554) group, those who fulfilled one component of the death tetrad; death tetrad B (n = 106), C (n = 41), and D (n = 8) groups, those who fulfilled two, three, and four components of the death tetrad, respectively. The remaining patients were allocated to the normal group (n = 2652). These groups of patients were significantly different in terms of sex, age, and comorbidities of CVA, HTN, CHF, DM, GCS, and ISS (Table 5). With the increased mortality rate for those patients with more components of the death tetrad, the mortality rate in the death tetrad A (28.2%), B (50.0%), C (78.0%), and D (100%) groups was significantly higher than that in the normal group (10.4%, all p < 0.001). Controlling for the underlying patient characteristics of sex, age, and comorbidities (CVA, HTN, CHF, and DM), patients in the death tetrad A, B, and C groups had a respective adjusted mortality rate of 3.69-, 10.10-, and 40.18-fold than those in the normal group. There was 100% mortality for all the eight trauma patients with all four components of the death tetrad. The requirement for admission into the ICU was higher for those with components of the death tetrad than for those in the normal group (p < 0.001), while there was no significant difference in the hospital LOS among these groups of patients (p = 0.052).
Table 5.
The trauma patients grouped according to the number fit in the components of death tetrad. The patients with one, two, three, four items that fit in the components of the death tetrad were allocated into the group of death tetrad A, B, C, D, respectively.
| Variables | Trauma Death Tetrad | |||||
|---|---|---|---|---|---|---|
| Death Tetrad A n = 554 |
Death Tetrad B n = 106 |
Death Tetrad C n = 41 |
Death Tetrad D n = 8 |
Normal n = 2652 |
p | |
| Gender | <0.001 | |||||
| Male, n (%) | 381 (68.8) | 93 (87.7) | 31 (75.6) | 6 (75.0) | 1682 (63.4) | |
| Female, n (%) | 173 (31.2) | 13 (12.3) | 10 (24.4) | 2 (25.0) | 970 (36.6) | |
| Age, years (SD) | 53.7 ± 20.3 | 52.2 ± 18.8 | 50.6 ± 18.8 | 52.3 ± 16.4 | 57.6 ± 19.9 | <0.001 |
| Comorbidities | ||||||
| CVA, n (%) | 13 (2.3) | 2 (1.9) | 0 (0.0) | 0 (0.0) | 156 (5.9) | 0.002 |
| HTN, n (%) | 157 (28.3) | 23 (21.7) | 11 (26.8) | 2 (25.0) | 962 (36.3) | <0.001 |
| CAD, n (%) | 42 (7.6) | 5 (4.7) | 0 (0.0) | 0 (0.0) | 171 (6.4) | 0.282 |
| CHF, n (%) | 8 (1.4) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 21 (0.8) | 0.003 |
| DM, n (%) | 83 (15.0) | 10 (9.4) | 4 (9.8) | 0 (0.0) | 541 (20.4) | 0.001 |
| ESRD, n (%) | 18 (3.2) | 2 (1.9) | 0 (0.0) | 1 (12.5) | 107 (4.0) | 0.269 |
| GCS, median (IQR) | 9 (4–15) | 4 (3–8) | 3 (3–9) | 3 (3–3) | 15 (8–15) | <0.001 |
| ISS, median (IQR) | 22 (16–29) | 25 (21–33) | 25 (16–38) | 25 (18–37) | 16 (9–24) | <0.001 |
| 1–15, n (%) | 134 (24.2) | 9 (8.5) | 8 (19.5) | 1 (12.5) | 1059 (39.9) | <0.001 |
| 16–24, n (%) | 164 (29.6) | 29 (27.4) | 8 (19.5) | 2 (25.0) | 950 (35.8) | 0.005 |
| ≥25, n (%) | 256 (46.2) | 68 (64.2) | 25 (61.0) | 5 (62.5) | 643 (24.2) | <0.001 |
| Mortality(%) | 156 (28.2) | 53 (50.0) | 32 (78.0) | 8 (100) | 276 (10.4) | <0.001 |
| AOR of Mortality | 3.69 (2.93–4.65) | 10.10 (6.65–15.35) | 40.18 (18.73–86.22) | - | - | <0.001 |
| Hospital LOS, days (SD) | 18.4 ± 16.8 | 18.4 ± 19.5 | 15.5 ± 21.0 | 7.1 ± 10.1 | 16.7 ± 15.6 | 0.052 |
| Admitted into ICU, n (%) | 468 (84.5) | 95 (89.6) | 39 (95.1) | 4 (50.0) | 1774 (66.9) | <0.001 |
AOR= Adjusted odds ratio; CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; CVA = cerebral vascular accident; DM = diabetes mellitus; ESRD = end-stage renal disease; GCS = Glasgow Coma Scale; HTN = hypertension; IQR = interquartile range; ISS = injury severity score; OR= odds ratio; SD = standard deviation.
4. Discussion
This study revealed that SBP < 60 mmHg could be further used to identify patients at risk of mortality with one or two components of the death triad. However, there was no significant difference in the mortality rate for those who had all components of the death triagles. This may be due to the relatively small number of patients. The study also suggested that an SBP < 60 mmHg may act as a proper component of the death tetrad of trauma patients to stratify the risk of mortality. The mortality rate of trauma patients with all three components of the death triad was 84%, while in patients with all four components of the death tetrad, the mortality rate was 100%. In addition to coagulopathy, hypothermia, and acidosis, the addition of hypotension as a death tetrad may be applied for mortality risk stratification in patients with trauma.
To improve the prediction of mortality in trauma patients, the addition of hypocalcemia on the lethal triage had been proposed to become “lethal diamond” [24]. The study found that a lower calcium level on admission to the emergency department was associated with increased mortality [25]. Calcium is also thought to play a key role in resuscitation [24]. However, the measurement of calcium level is not routinely performed, and it takes time to obtain the result of serum calcium levels. Additionally, although ISS was able to predict mortality [26] and the necessity for hospitalization and ICU [27], ISS is not suitable as a predictor for patients’ outcome assessment in the emergency room because ISS was calculated only after a whole-body examination was performed and was not immediately available in the emergency room. However, GCS may be considered as a choice for add-on in the death triad to stratify patients who are at risk of mortality. One study used GCS instead of temperature as the BIG (base deficit [B], international normalized ratio [I], and GCS [G]) score for predicting mortality in pediatric trauma [23]. The predictive power was comparable with that of more complex systems, such as pediatric logistic organ dysfunction, Pediatric Index of Mortality 2, and Pediatric Risk of Mortality III [28]. However, most GCS evaluations may be reserved for patients with head injury. In contrast, SBP could be detected in patients injured by all causes of trauma and is easily monitored repeatedly, non-invasively, inexpensively, and rapidly.
This study has some limitations. First, there was a small number of trauma patients who had all components of the death tetrad. Second, selection bias may exist due to the retrospective nature of the study. Additionally, because this study only evaluated in-hospital mortality but not long-term mortality, a selection bias may have occurred in the outcome measurement. Moreover, some pre-hospital interventions, such as damage control, blood and fluid transfusion, and resuscitation, may result in different patient outcomes, and these factors were not controlled for further analysis. Additionally, the definition of hypotension with SBP < 60 mmHg is arbitrary, and the regular blood pressure of the patients prior to the trauma injury is unknown. The study regarding how many of those hypotensive patients who develop the worst outcome have an increase in circulating lactate and an alteration in the base excess may help illustrate the relationship of these components of tetrad [29]; however, the lack of lactate data in most of the patient population deterred such an investigation in this study. Furthermore, the inclusion of the patients with different proportions of injuries to each body region may lead to bias in the outcome measurement. Finally, the results of this study were limited to a single urban trauma center, which may not be generalizable to other areas.
5. Conclusions
The study suggested that hypotension, defined as an SBP < 60 mmHg, may act as a proper component of the death tetrad to stratify the mortality risk of trauma patients.
Acknowledgments
We appreciate the assistance of the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Author Contributions
Writing—original draft preparation, W.-J.T.; writing—review and editing, H.-Y.T.; resources T.-Y.H.; validation, S.-E.C. and W.-T.S.; formal analysis, S.-Y.H.; conceptualization, C.-H.H.; funding acquisition, C.-H.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Chang Gung Memorial Hospital (protocol code 202201535B0).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study under the regulation of institutional review board.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Chang Gung Memorial Hospital, grant number CORPG8M0291 to Ching-Hua Hsieh.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mitra B., Tullio F., Cameron P.A., Fitzgerald M. Trauma patients with the ‘triad of death’. Emerg. Med. J. 2012;29:622–625. doi: 10.1136/emj.2011.113167. [DOI] [PubMed] [Google Scholar]
- 2.Keane M. Triad of death: The importance of temperature monitoring in trauma patients. Emerg. Nurse. 2016;24:19–23. doi: 10.7748/en.2016.e1569. [DOI] [PubMed] [Google Scholar]
- 3.Mikhail J. The trauma triad of death: Hypothermia, acidosis, and coagulopathy. AACN Clin. Issues. 1999;10:85–94. doi: 10.1097/00044067-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Muthukumar V., Karki D., Jatin B. Concept of Lethal Triad in Critical Care of Severe Burn Injury. Indian J. Crit. Care Med. 2019;23:206–209. doi: 10.5005/jp-journals-10071-23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith A., Hendrix V., Shapiro M., Duchesne J., Taghavi S., Schroll R., Tatum D., Guidry C. Is the “Death Triad” a Casualty of Modern Damage Control Resuscitation. J. Surg. Res. 2021;259:393–398. doi: 10.1016/j.jss.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Bozorgi F., Khatir I.G., Ghanbari H., Jahanian F., Arabi M., Ahidashti H.A., Hosseininejad S.M., Ramezani M.S., Montazer S.H. Investigation of Frequency of the Lethal Triad and Its 24 Hours Prognostic Value among Patients with Multiple Traumas. Open Access Maced. J. Med. Sci. 2019;7:962–966. doi: 10.3889/oamjms.2019.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feliciano D.V. Abdominal vascular injuries. Surg. Clin. N. Am. 1988;68:741–755. doi: 10.1016/S0039-6109(16)44583-4. [DOI] [PubMed] [Google Scholar]
- 8.Samuels J.M., Moore H.B., Moore E.E. Damage Control Resuscitation. Chirurgia. 2017;112:514–523. doi: 10.21614/chirurgia.112.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corwin G.S., Sexton K.W., Beck W.C., Taylor J.R., Bhavaraju A., Davis B., Kimbrough M.K., Jensen J.C., Privratsky A., Robertson R.D. Characterization of Acidosis in Trauma Patient. J. Emerg. Trauma Shock. 2020;13:213–218. doi: 10.4103/jets.Jets_45_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brohi K., Singh J., Heron M., Coats T. Acute traumatic coagulopathy. J. Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 11.Jurkovich G.J., Greiser W.B., Luterman A., Curreri P.W. Hypothermia in trauma victims: An ominous predictor of survival. J. Trauma. 1987;27:1019–1024. doi: 10.1097/00005373-198709000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Valeri C.R., Feingold H., Cassidy G., Ragno G., Khuri S., Altschule M.D. Hypothermia-induced reversible platelet dysfunction. Ann. Surg. 1987;205:175–181. doi: 10.1097/00000658-198702000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini W.Z. Coagulopathy by hypothermia and acidosis: Mechanisms of thrombin generation and fibrinogen availability. J. Trauma. 2009;67:202–208. doi: 10.1097/TA.0b013e3181a602a7. [DOI] [PubMed] [Google Scholar]
- 14.Lai W.H., Rau C.S., Hsu S.Y., Wu S.C., Kuo P.J., Hsieh H.Y., Chen Y.C., Hsieh C.H. Using the Reverse Shock Index at the Injury Scene and in the Emergency Department to Identify High-Risk Patients: A Cross-Sectional Retrospective Study. Int. J. Environ. Res. Public Health. 2016;13:357. doi: 10.3390/ijerph13040357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo S.C., Kuo P.J., Hsu S.Y., Rau C.S., Chen Y.C., Hsieh H.Y., Hsieh C.H. The use of the reverse shock index to identify high-risk trauma patients in addition to the criteria for trauma team activation: A cross-sectional study based on a trauma registry system. BMJ Open. 2016;6:e011072. doi: 10.1136/bmjopen-2016-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang J.F., Rau C.S., Wu S.C., Liu H.T., Hsu S.Y., Hsieh H.Y., Chen Y.C., Hsieh C.H. Use of the reverse shock index for identifying high-risk patients in a five-level triage system. Scand. J. Trauma Resusc. Emerg. Med. 2016;24:12. doi: 10.1186/s13049-016-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai W.H., Wu S.C., Rau C.S., Kuo P.J., Hsu S.Y., Chen Y.C., Hsieh H.Y., Hsieh C.H. Systolic Blood Pressure Lower than Heart Rate upon Arrival at and Departure from the Emergency Department Indicates a Poor Outcome for Adult Trauma Patients. Int. J. Environ. Res. Public Health. 2016;13:528. doi: 10.3390/ijerph13060528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S.C., Rau C.S., Kuo S.C.H., Chien P.C., Hsieh H.Y., Hsieh C.H. The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. Int. J. Environ. Res. Public Health. 2018;15:2346. doi: 10.3390/ijerph15112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh C.H., Hsu S.Y., Hsieh H.Y., Chen Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017;40:113–120. doi: 10.1016/j.bj.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh C.H., Liu H.T., Hsu S.Y., Hsieh H.Y., Chen Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017;40:121–128. doi: 10.1016/j.bj.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh C.H., Chen Y.C., Hsu S.Y., Hsieh H.Y., Chien P.C. Defining polytrauma by abbreviated injury scale ≥ 3 for a least two body regions is insufficient in terms of short-term outcome: A cross-sectional study at a level I trauma center. Biomed. J. 2018;41:321–327. doi: 10.1016/j.bj.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S.C., Chou S.E., Liu H.T., Hsieh T.M., Su W.T., Chien P.C., Hsieh C.H. Performance of Prognostic Scoring Systems in Trauma Patients in the Intensive Care Unit of a Trauma Center. Int. J. Environ. Res. Public Health. 2020;17:7226. doi: 10.3390/ijerph17197226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh C.H., Wu S.C., Chou S.E., Su W.T., Tsai C.H., Li C., Hsu S.Y., Hsieh C.H. Geriatric Nutritional Risk Index as a Tool to Evaluate Impact of Malnutrition Risk on Mortality in Adult Patients with Polytrauma. Int. J. Environ. Res. Public Health. 2020;17:9233. doi: 10.3390/ijerph17249233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wray J.P., Bridwell R.E., Schauer S.G., Shackelford S.A., Bebarta V.S., Wright F.L., Bynum J., Long B. The diamond of death: Hypocalcemia in trauma and resuscitation. Am. J. Emerg. Med. 2021;41:104–109. doi: 10.1016/j.ajem.2020.12.065. [DOI] [PubMed] [Google Scholar]
- 25.Ditzel R.M., Jr., Anderson J.L., Eisenhart W.J., Rankin C.J., DeFeo D.R., Oak S., Siegler J. A review of transfusion- and trauma-induced hypocalcemia: Is it time to change the lethal triad to the lethal diamond? J. Trauma Acute Care Surg. 2020;88:434–439. doi: 10.1097/TA.0000000000002570. [DOI] [PubMed] [Google Scholar]
- 26.Sewalt C.A., Venema E., Wiegers E.J.A., Lecky F.E., Schuit S.C.E., den Hartog D., Steyerberg E.W., Lingsma H.F. Trauma models to identify major trauma and mortality in the prehospital setting. Br. J. Surg. 2020;107:373–380. doi: 10.1002/bjs.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orhon R., Eren S.H., Karadayi S., Korkmaz I., Coskun A., Eren M., Katrancioglu N. Comparison of trauma scores for predicting mortality and morbidity on trauma patients. Ulus. Travma Acil Cerrahi Derg. 2014;20:258–264. doi: 10.5505/tjtes.2014.22725. [DOI] [PubMed] [Google Scholar]
- 28.Bolstridge J., O’Neil E.R., Aden J.K., Muisyo T., Spinella P.C., Borgman M.A. Use of the BIG score to predict mortality in pediatric trauma. Am. J. Emerg. Med. 2021;45:472–475. doi: 10.1016/j.ajem.2020.09.060. [DOI] [PubMed] [Google Scholar]
- 29.Paladino L., Sinert R., Wallace D., Anderson T., Yadav K., Zehtabchi S. The utility of base deficit and arterial lactate in differentiating major from minor injury in trauma patients with normal vital signs. Resuscitation. 2008;77:363–368. doi: 10.1016/j.resuscitation.2008.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.