Abstract
Secreted and surface-exposed antigens of intracellular pathogens are thought to provide target structures for detection by the host immune system. The major secreted product of intracellular Leishmania mexicana amastigotes, a proteophosphoglycan (aPPG), is known to contribute to the establishment of the parasitophorous vacuole and is able to activate complement. aPPG belongs to a novel class of serine- and threonine-rich Leishmania proteins that are extensively modified by phosphodiester-linked phosphooligosaccharides and terminal mannooligosaccharides. Here we show that mice chronically infected with L. mexicana generally do not produce antibodies or Th cells specific for aPPG. Similarly, antibody titers are very low in mice vaccinated with aPPG, and specific CD4+ T cells are undetectable. Comparative analyses of other Leishmania glycoconjugates indicate that L. mexicana-specific carbohydrate structures are poorly immunogenic in mice and that the proteophosphoglycan aPPG behaved immunologically like a carbohydrate. The latter observation is explained by the lack of induction of aPPG-specific CD4+ T cells. In contrast, recombinant aPPG peptides stimulate CD4+ T-cell responses and high titers of specific antibodies are found in the sera of mice vaccinated with these peptides. Native aPPG is highly resistant to proteinases and apparently cannot be degraded by macrophages. It is concluded that conventional CD4+ T cells against the polypeptide backbone of aPPG are not induced because the molecule resists antigen processing due to its extensive and complex carbohydrate modification. The complex glycan chains of aPPG, which exhibit important biological functions for the parasite, may therefore also have evolved to evade detection by the immune system of the host organism.
Leishmania spp. are the etiologic agents of a spectrum of human diseases. These protozoans have a digenetic life cycle and are transmitted by insect vectors as flagellated, extracellular promastigotes to their mammalian host, where they replicate as nonmotile amastigotes in parasitophorous vacuoles derived from the phagolysosomal compartment of parasitized macrophages (2). Healing of the disease and killing of the parasites is dependent on activation of potent microbicidal mechanisms in the host cells. This process is initiated by CD4+ T lymphocytes that secrete lymphokines such as gamma interferon (IFN-γ) and tumor necrosis factors which act via specific receptors on macrophages. The secretion of these activating cytokines by Leishmania antigen-specific CD4+ T lymphocytes is triggered by the interaction of their T-cell receptors with their cognate major histocompatibility complex (MHC) class II peptide complexes (15). The question of whether macrophages harboring an established infection can provide parasite-derived peptides bound to MHC class II molecules on their surface for the interaction with CD4+ T lymphocytes has been studied by using genetically engineered parasites (20, 21). The results of these investigations indicate that the parasitophorous vacuole membrane communicates with the macrophage plasma membrane and may be the compartment where peptides derived from parasite proteins are produced and then loaded onto MHC class II molecules. Provided that MHC class II expression is induced by IFN-γ in infected macrophages, T-cell activation by the host cell is possible. The data further suggest that only the subset of parasite antigens which are secreted or exposed on the parasite surface is efficiently presented by macrophages harboring live parasites, while intracellular proteins are presented only if the parasites are killed (20, 21).
The search for cell surface proteins in amastigotes has proven to be difficult, and, at least for L. mexicana, a major amastigote surface protein has not been identified (19), although such proteins are typical for several parasitic protozoa. Instead, the surface appears to be covered with glycoinositolphospholipids and glycosphingolipids (19). However, L. mexicana amastigotes secrete large amounts of a stage-specific proteophosphoglycan (aPPG) (8). This is so far the only secreted amastigote product that has been identified in infected tissue and has been analyzed in some detail: aPPG belongs to a novel class of serine- and threonine-rich Leishmania proteins that are extensively modified by phosphodiester-linked phosphooligosaccharides and terminal mannooligosaccharides (9). Lesions of L. mexicana-infected mice yield 40 to 100 μg of aPPG per g of tissue, and in the parasite-containing phagolysosome its concentration may reach several milligrams per milliliter (8, 17). Macrophages infected in vitro contain 0.1 to 0.2 pg of aPPG per cell, which is consistent with the in vivo estimate (1, 17). Since purified aPPG has an apparent molecular mass ranging from 4 × 105 to 2 × 106 Da, the number of aPPG molecules per infected macrophage (assuming an average molecular mass of 106 Da) is calculated to be about 105. The continuous secretion of aPPG into the phagolysosome may contribute to the enlargement of this compartment, which is typical for macrophages infected with L. mexicana (17). Occasionally, aPPG is detected by immunocytochemistry in macrophage vesicles, suggesting that aPPG is also exported from the parasitophorous vacuole and, possibly, secreted by viable infected host cells (8). Upon rupture of infected macrophages, aPPG is released and can be taken up by other phagocytes, most probably by receptor-mediated endocytosis.
As a highly abundant and secreted parasite product, aPPG could provide an ideal target for the cellular immune response of the host in L. mexicana infections. In the present study, we investigated the immune response to this secreted parasite product in mice infected with L. mexicana as well as in those immunized with the purified molecule. We demonstrated that in spite of the very high local concentration and the large amounts present in infected tissue, aPPG elicited no B-cell response in most infected mice and was not recognized by conventional CD4+ T cells. Likewise, in immunized animals, the purified native compound was a very poor B-cell antigen and was not CD4+ T-cell immunogenic, in contrast to the Escherichia coli-expressed recombinant form. These results suggest that L. mexicana amastigotes avoid the stimulation of the immune system of their mammalian host by heavy glycosylation of their major secretory product, aPPG, which appears to minimize its immunogenicity.
MATERIALS AND METHODS
Mice and parasites.
Specific-pathogen-free female C57BL/6, CBA/J, and BALB/c mice were purchased from Charles River (Sulzfeld, Germany); maintained in the animal facility of the Max-Planck-Institut für Biologie, Tübingen, Germany; and used at 8 to 16 weeks of age. Mice were infected with 3 × 106 promastigotes of L. mexicana (strain MNYC/BZ/62/M379; obtained originally from James Alexander, Glasgow, United Kingdom) at the tail base. L. mexicana amastigotes isolated from lesions were cultured axenically at 34°C in Schneider’s Drosophila medium (Serva, Heidelberg, Germany) supplemented with 20% heat-inactivated fetal calf serum (iFCS; Kraeber, Hamburg, Germany) and 3.9 g of 2-(N-morpholino)ethanesulfonic acid (Serva) per liter.
Reagents.
The purification of the Leishmania products lipophophoglycan (LPG), secreted acid phosphatase (sAP), and aPPG and the generation and specificity of the monoclonal antibodies (MAbs) AP3 and LT22 have been described previously (8, 10–12). MAb WIC79.3 which recognizes a glycan epitope unique to L. major LPG, has also been described in an earlier study (4, 13). The cloning and sequencing of the ppg2 gene, which encodes the aPPG protein backbone, will be described elsewhere (4a). Portions of the ppg2 open reading frame encoding the N-terminal and central part (289 amino acids) and the C-terminal part (270 amino acids) of the aPPG protein backbone were expressed in E. coli as His6 fusion proteins and purified by Ni-nitrilotriacetic acid-agarose chromatography (Qiagen, Hilden, Germany).
Immunizations of mice.
C57BL/6 mice were immunized subcutaneously on both sides of the lower back with ovalbumin (10 μg/mouse; Sigma, Deisenhofen, Germany) or with the purified L. mexicana antigens, aPPG, LPG, or sAP, all emulsified in complete Freund’s adjuvant (CFA). The dose per animal of aPPG, LPG, or sAP was normalized for phosphorus content, and amounts corresponding to 500 ng of phosphorus/mouse were injected. Mice immunized with aPPG, LPG, or sAP were boosted intraperitoneally by injecting an identical dose emulsified in incomplete Freund’s adjuvant (IFA) 28 days after the first immunization. Animals were bled on day 14 after the first immunization and on day 10 after the second injection. To investigate the immune responses to unglycosylated aPPG, C57BL/6 mice were immunized by the immunization scheme outlined above with either 10 μg of the recombinant C-terminal part of the aPPG protein backbone per mouse for the assessment of the T-cell response or 20 μg of the recombinant N-terminal and central part of the aPPG protein backbone per mouse for the generation of mouse antisera.
Determination of the relative titers of antigen-specific serum antibodies.
Blood was collected from individual mice by retroorbital bleeding, and serum was prepared from coagulated blood by centrifugation. Sera were diluted in phosphate-buffered saline (PBS)–5% milk powder containing 0.05% Tween 20, and 100-μl volumes of the diluted samples were incubated in duplicate in microtiter plates (Microtest III; Falcon, Becton Dickinson, Oxnard, Calif.) coated with sAP (at 2 μg/ml in 50 mM NaHCO3–100 mM NaCl [pH 8.2]) or LPG (at 10 μg/ml in 50 mM NaHCO3–100 mM NaCl [pH 8.2]). For aPPG, Maxi-Sorb plates (Nunc, Wiesbaden, Germany) coated with 10 μg of aPPG per ml in 50 mM NaHCO3–100 mM NaCl (pH 8.2) were used. Bound antibodies were detected by adding goat anti-mouse immunoglobulin (Ig) polyclonal antibody conjugated to alkaline phosphatase (Dianova, Hamburg, Germany) and p-nitrophenylphosphate as the substrate. Relative titers were determined from serum dilutions giving the same optical density at 405 nm. To assess total anti-L. mexicana antibody titers, microtiter plates were coated with cultured amastigotes (100 μl of 107 parasites/ml in PBS), adherent parasites were fixed with PBS containing 4% paraformaldehyde and washed, and free aldehyde groups were reacted with NH4Cl before blocking with PBS–5% milk powder containing 0.05% Tween 20. After incubation with sera and MAbs, bound antibodies were detected by adding goat anti-mouse Ig polyclonal antibody conjugated to alkaline phosphatase as described above.
T-lymphocyte selection and in vitro restimulation.
Lymph nodes draining the injection sites of infected or immunized mice were removed, and single-cell suspensions were prepared by mechanical disruption and passage through a steel mesh in balanced salt solution-EDTA. Cells were passed through cotton wool plugs and washed with balanced salt solution-EDTA. CD4+ cells were enriched to more than 90% by depletion of CD8α+, CD11b+, CD16/CD32+, and CD45R+ cells on a MACS separation column as specified by the manufacturer (Miltenyi Biotech, Bergisch Gladbach, Germany). CD4+ T lymphocytes (2 × 105/well) were restimulated in 96-well round-bottom microtiter plates (Falcon, Becton Dickinson, Heidelberg, Germany) for 48 h by being mixed with irradiated syngeneic low density spleen cells (2 × 105/well) from naive mice and cultured in the presence or absence of the specific antigens or concanavalin A (ConA) (2.5 μg/ml) in Dulbecco modified Eagle Medium (DME) (CCpro, Neustadt, Germany) supplemented with 1% heat inactivated mouse serum, 2 mM l-glutamine, 1% nonessential amino acids (Gibco, Eggenstein, Germany), and 50 μM β-mercaptoethanol. The specific antigens used were native aPPG (at 10 μg/ml), mild-acid-treated and neutralized aPPG (at a concentration equivalent to 10 μg of native aPPG per ml), or ovalbumin (at 100 μg/ml).
Secretion of interleukin-3 (IL-3), IL-4, and IFN-γ into culture supernatants was assayed as described previously (16) with the specific lymphokine-responsive cell lines 32D clone 3 (IL-3), CT.4S (IL-4), and WEHI-279 (IFN-γ). The sensitivity of the assays was 0.1, 0.3, and 0.1 U/ml for IL-3, IL-4, and IFN-γ, respectively. The aPPG preparations did not interfere with the bioassay because standard amounts of the cytokines were detected with the same sensitivity in the presence and absence of these antigens.
Pulsing of bone marrow macrophages with purified aPPG.
Bone marrow macrophages were derived from the bone marrow cells of C57BL/6 mice by culture and at 37°C with 5% CO2 in air for 6 days in DME containing 20% iFCS and 15 ng of recombinant macrophage colony-stimulating factor per ml on non-tissue-culture petri dishes (Greiner, Nürtingen, Germany). After this time, nonadherent cells were removed and adherent macrophages were detached by incubation in PBS containing 3 mM EDTA and 10 mM glucose. The cells were plated and cultured overnight at 106 cells/well in DME supplemented with 10% iFCS in 12-well plates (Costar, Bodenheim, Germany). Medium was removed the next morning and replaced with 1 ml of DME supplemented with 10% iFCS containing 10 μg of aPPG per ml. After 6 h, the macrophage monolayers were washed twice with 2 ml of warm DME containing 10% iFCS and then incubated at 37°C with 5% CO2 in air for the indicated times in 2 ml of DME containing 10% iFCS. Culture supernatants were removed, and adherent cells were lysed in 1 ml of PBS containing 1% Triton X-100. Cell lysates were collected, and the nuclei were pelleted by centrifugation. An aliquot of the lysate supernatant was taken to determine the aPPG content by two-site enzyme-linked immunosorbent assay (ELISA) with purified aPPG as a standard. The rest of the supernatants were extracted with phenol, and the aqueous phase was concentrated on Centricon-10 (Amicon, Witten, Germany). Concentrated samples were electrophoretically separated on 1% agarose gels in Tris-borate-EDTA (TBE) buffer. The separated material was blotted onto Zetaprobe membranes (Bio-Rad, Munich, Germany) by capillary transfer. Immobilized aPPG was detected by using a mouse IgM MAb, AP3 (12). Binding of the antibody was visualized with polyclonal anti-mouse Ig-alkaline phosphatase conjugates and 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Sigma). Alternatively, agarose gels were stained directly with Stains All (Sigma).
RESULTS
Humoral immune response to L. mexicana aPPG, LPG, and sAP.
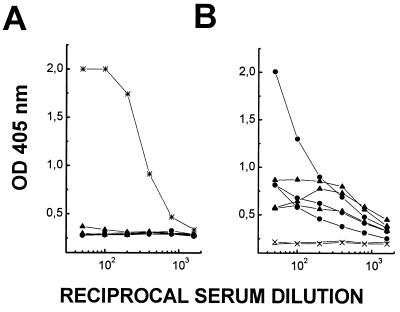
C57BL/6 and CBA/J mice (three each) were infected with L. mexicana promastigotes at the base of their tails. These strains, like most inbred strains, are susceptible to infection with L. mexicana, and the mice developed large nodular lesions (>1 cm in diameter) at the injection site within 4 to 5 months after infection. At this stage, their serum was analyzed by ELISA for the presence of antibodies specific for aPPG. In infected animals of both strains, aPPG was not recognized by serum antibodies from most infected mice. Only one of six mice showed a very low aPPG-specific antibody titer (Fig. 1A). Similar results were obtained with sera from several (more than 20) BALB/c mice with long-term L. mexicana infections. In contrast, antibodies against fixed amastigotes were readily detectable with titers as high as 103 in infected animals of all mouse strains investigated (Fig. 1B). These results indicate that while parasite aPPG does not elicit a specific antibody production in the majority of infected mice and only occasionally leads to very low titers in some animals, this effect is not part of a generalized suppression of the host B-cell response.
FIG. 1.
Serum antibody response to aPPG and amastigotes in mice chronically infected with L. mexicana. Sera from CBA/J (circles) and C57BL/6 (triangles) mice were collected. Total antibody responses to solid-phase-bound aPPG (A) and L. mexicana amastigotes (B) in serially diluted sera were compared by ELISA. Antibodies were detected with goat anti-mouse IgG or IgM conjugated to alkaline phosphatase. Hybridoma supernatant containing MAb LT22 (∗) was used as positive control in panel A. Preinfection sera (×) served as negative controls. Data points correspond to mean OD405 values of duplicate determinations of individual sera and are plotted against reciprocal serum dilutions. Lines link data points of individual sera at different dilutions and represent the variation seen between mice.
aPPG shares structural features with LPG, the major glycoconjugate of promastigotes (9, 11). LPG of different species also have common structural motifs, e.g., the glycolipid core, phosphodisaccharide repeats, or Manα1-2Man-caps (14). It has been reported previously that mice infected with L. major LRC-L137-V121 have high antibody titers against LPG from promastigotes of the same species (5). Our results with individual sera from L. major vaccine strain-infected mice and analysis of their content of LPG-specific antibody on homologous and heterologous LPG by ELISA confirm these observations (Fig. 2). However, the anti-L. major LPG antibodies reacted only very weakly with LPG from L. mexicana (Fig. 2). Furthermore, sera from L. mexicana-infected mice recognized neither the homologous nor the heterologous LPG.
FIG. 2.
Serum antibody responses to LPG in mice chronically infected with L. mexicana or L. major. Sera from CBA/J (circles; L. mexicana only), C57BL/6 (triangles), or BALB/c (squares) mice infected with L. mexicana (solid symbols) or L. major (open symbols) were analyzed by ELISA as described in the legend to Fig. 1. Lines link data points of individual sera at different dilutions. Plates were coated with L. major LPG (A) or L. mexicana LPG (B). Hybridoma supernatant containing MAb WIC79.3 (∗), an L. major LPG-specific antibody, and a polyclonal serum raised in mice against L. mexicana sAP (⧫) cross-reacting with L. mexicana LPG were used as positive controls.
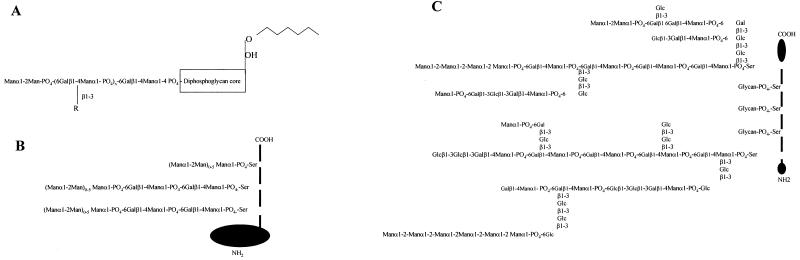
This finding prompted us to compare the immunogenicity of L. mexicana aPPG, LPG, and another phosphoglycosylated product of L. mexicana, sAP, a polymeric enzyme that contains at its C-terminal end similar arrays of phosphodisaccharide repeats and Manα1-2Man-caps (Fig. 3). MAbs directed against some of these glycan structures (AP3 and LT22) cross-react with all three molecules. Antigen doses for immunizations were based on the phosphorus content, which is indicative of the number of phosphoglycan moieties present. The serum antibody responses in mice immunized with the individual antigens were compared, after the first immunization and after a boosting injection, by ELISA with serial dilutions of the respective sera on microtiter plates coated with sAP, LPG, or aPPG. The primary antibody response was weak for all antigens (Table 1). In sera of mice immunized with sAP, the relative antibody titer against homologous antigen increased more than 600-fold after the booster injection, indicating a T-cell-driven B-cell response. Notably, cross-reactive antibodies in these sera recognizing epitopes shared with LPG or aPPG increased only by a factor of 10 to 20. Sera of LPG- or aPPG-immunized animals did not show a prominent increase in antibody titers against the homologous antigen after the boosting injection. Secondary humoral immune responses to these two antigens were only about 6- to 15-fold higher than the primary responses. These results suggest that the B-cell response to the protein-containing aPPG was similar to that of a nonproteinaceous antigen, exemplified here by LPG, and that L. mexicana carbohydrate structures are of very low immunogenicity in mice.
FIG. 3.
Schematic drawing of Leishmania LPG (A) as well as sAP (B) and aPPG (C) from L. mexicana. x, 15 to 30 (average number of repeats in LPG); R, in L. mexicana Glc; in L. major Gal, Galβ1-3Gal, Galβ1-3Galβ1-3Gal, Araβ1-2Gal, Araβ1-2Galβ1-2Gal, or Glcβ1-3Gal. Black areas in panels B and C symbolize the protein backbone.
TABLE 1.
Antibody titers in C57BL/6 mice immunized with sAP, LPG, or aPPG
| Immunogena | Antibody titerb for plate coated with:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| sAP
|
LPG
|
aPPG
|
|||||||
| First bleed | Second bleed | Increase (fold)c | First bleed | Second bleed | Increase (fold) | First bleed | Second bleed | Increase (fold) | |
| sAP | 18 | 1.6 × 103 | 602 | 50 | 7.2 × 102 | 13 | 23 | 3.0 × 102 | 24 |
| 10 | 6.3 × 104 | 1.0 × 102 | 5.7 × 102 | 10 | 3.0 × 102 | ||||
| 10 | 4.1 × 103 | 35 | 2.0 × 102 | 23 | 7.9 × 102 | ||||
| LPG | 12 | 66 | 2 | 25 | 1.4 × 102 | 6 | 10 | 28 | 7 |
| 10 | 10 | 30 | 2.6 × 102 | 10 | 20 | ||||
| 10 | 20 | 30 | 80 | 10 | 5.7 × 102 | ||||
| aPPG | 23 | 1.2 × 102 | 5 | 1.2 × 102 | 5.0 × 102 | 8 | 10 | 1.8 × 102 | 15 |
| 12 | 1.3 × 102 | 40 | 2.9 × 102 | 15 | 2.2 × 102 | ||||
| 10 | 40 | 40 | 5.7 × 102 | 1.0 × 102 | 1.2 × 103 | ||||
Mice were immunized with sAP, LPG, or aPPG emulsified in CFA and boosted 1 month later with a second injection of antigens in IFA.
Vaccinated mice were bled on day 14 after priming and on day 10 after boosting. Values are mean reciprocal serum dilutions giving identical OD405 values of duplicate determinations of individual sera.
Geometric mean of the increase in antibody titer in sera from immunized and boosted mice. Each value applies to all three titers given for the immunogen.
Native aPPG fails to induce specific CD4+ T lymphocytes.
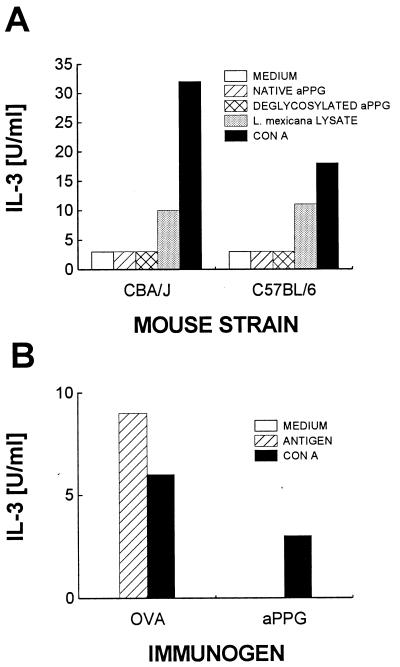
To investigate the T-cell response to aPPG, CD4+ T lymphocytes enriched from lymph nodes draining the lesion site of chronically infected C57BL/6 and CBA/J mice were restimulated in vitro with syngeneic low-density splenocytes as antigen-presenting cells. Purified native aPPG or aPPG treated with mild acid was added at a concentration corresponding to 5 μg/ml to stimulate specific CD4+ T cells. Alternatively, a freeze-thaw lysate of L. mexicana promastigotes was added as a source of parasite antigens. The lymphocytes were also restimulated with ConA as a positive control. T-cell stimulation was determined indirectly by measuring lymphokine secretion. CD4+ cells from both strains of mice secreted only background levels of IL-3 (Fig. 4A) and undetectable levels of IL-4 or IFN-γ (data not shown) in response to native aPPG or deglycosylated aPPG. Lack of reactivity with aPPG in the above system could be due to very low frequencies of antigen-specific T cells, to inappropriate concentrations of aPPG used in vitro, or to suppressed T-cell responses in long-term-infected mice (1a). However, increasing the number of CD4+ cells in the assays, using repeated rounds of restimulation to expand the numbers of rare specific cells, or adding larger amounts of aPPG (up to 50 μg/ml) did not change the results (data not shown). In contrast, the purified CD4+ cells secreted IL-3 (and IL-4 [results not shown]) when restimulated in the presence of L. mexicana freeze-thaw lysate. Furthermore, Th cells from infected mice immunized with ovalbumin in CFA responded to ovalbumin restimulation in vitro as well as did T cells from immunized, noninfected controls (data not shown). In summary, CD4+ cells of L. mexicana-infected mice lack T cells against aPPG, and this is not a consequence of a generalized suppression of their T cell responses.
FIG. 4.
CD4+ T cells from infected or immunized mice do not respond to restimulation with aPPG in vitro. CD4+ cells were enriched from pooled draining lymph nodes of three each of L. mexicana-infected C57BL/6 or CBA/J mice (A) or C57BL/6 mice (B) immunized with aPPG or ovalbumin. The cells (2 × 105) were restimulated in the presence of antigen-presenting cells in vitro with either native aPPG, deglycosylated aPPG, a freeze-thaw lysate of L. mexicana, promastigotes, or ConA. In panel B, cells were restimulated with ovalbumin, aPPG, or ConA. Stimulation was determined indirectly by measuring bioactive IL-3 released from activated CD4+ T cells. Data shown are mean IL-3 concentrations in supernatants of duplicate cultures. Results in panel A are representative of two and three experiments performed with CD4+ cells prepared from CBA and C57BL/6 mice, respectively. In panel B, one of two experiments is shown.
This result is corroborated by the analysis of CD4+-T-cell responses in mice vaccinated with aPPG. C57BL/6 mice were injected with 10 μg of aPPG per mouse in CFA. One week later, lymph nodes draining the site of injection were removed and CD4+ cells were enriched and restimulated in vitro as described above. The supernatants of these cultures contained no IL-3 (Fig. 4B). For comparison, CD4+ lymphocytes isolated from mice immunized with ovalbumin in adjuvants secreted IL-3 when restimulated in vitro in the presence of the immunogen (Fig. 4B). Therefore, even in immunized mice, aPPG-specific CD4+ T cells were undetectable.
Recombinant aPPG peptides are immunogenic in C57BL/6 mice.
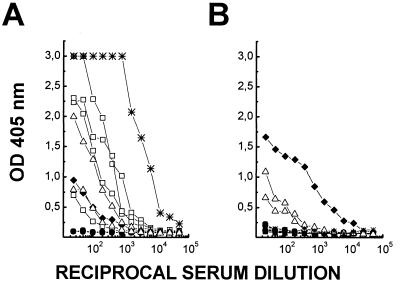
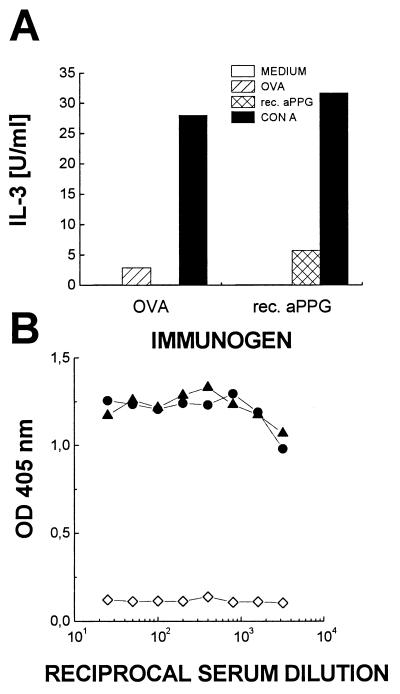
Failure to detect aPPG-specific CD4+ T-cell responses in vaccinated mice might reflect a paucity of T-cell epitopes that can be generated from the protein backbone of aPPG in C57BL/6 mice. To test this possibility, C57BL/6 mice were immunized with a purified E. coli-expressed C-terminal protein fragment (∼30 kDa) of the aPPG protein backbone, which had been emulsified in 50% CFA–PBS. CD4+ T cells were purified from the draining lymph nodes and restimulated in vitro with the recombinant protein at 2 μg/ml in the presence of syngeneic spleen cells. CD4+ cells from mice immunized with ovalbumin served as controls. Vaccination with recombinant aPPG induced specific CD4+ cells that proliferated in response to the immunogen in vitro (results not shown) and secreted IL-3 (Fig. 5A). This response is not due to possible E. coli lipopolysaccharide contamination, since CD4+ cells from ovalbumin-immunized animals do not react at all to restimulation with recombinant aPPG. Therefore, the protein backbone of aPPG contains immunogenic epitopes for C57BL/6 mice that can be generated from corresponding nonglycosylated, recombinant polypeptides. In addition, C57BL/6 mice were immunized with the purified E. coli-expressed N-terminal part of the aPPG protein backbone. One booster immunization with protein emulsified in 50% IFA–PBS resulted in antibody titers in serum of more than 3 × 103, which indicated a normal secondary humoral immune response to the aPPG protein backbone (Fig. 5B).
FIG. 5.
Recombinant (rec.) aPPG induces conventional CD4+ cells and high-titer antibodies. (A) CD4+ cells were enriched from draining lymph nodes of C57BL/6 mice immunized with aPPG or ovalbumin (OVA) in 50% CFA–PBS. The cells (2 × 105) were restimulated in the presence of antigen-presenting cells in vitro either with the recombinant C-terminal fragment of aPPG or with ovalbumin. Stimulation was determined indirectly by measuring the amount of bioactive IL-3 released from activated CD4+ T cells. Data shown are mean IL-3 concentrations in supernatants of duplicate cultures. (B) C57BL/6 mice were immunized with the recombinant N-terminal fragment of aPPG in 50% CFA–PBS and boosted once with the antigen in 50% IFA–PBS. Total antibody responses against solid-phase-bound antigen in serially diluted individual sera (solid symbols) were compared by ELISA as described in the legend Fig. 1. Preimmune serum (open symbols) was used as a negative control.
aPPG is not degraded by macrophages.
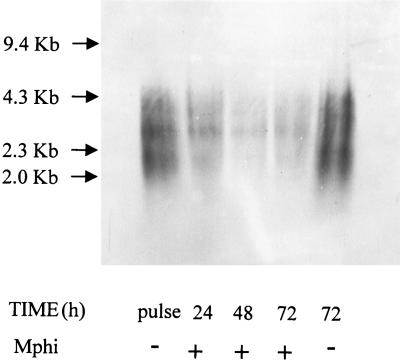
Lack of proteolytic cleavage of aPPG and the ensuing failure to generate peptidic fragments presented on MHC class II molecules is another possible reason for the absence of a detectable aPPG-specific CD4+ T-cell response in immunized and infected mice. We have previously reported that the electrophoretic migration of purified aPPG is not altered by proteinase K digestion (8). Therefore, we tested the resistance of aPPG to degradation by macrophages. Bone marrow-derived macrophage were pulsed in vitro for 6 h with aPPG at 10 μg/ml. Uptake of aPPG during this time results in vacuolization of the macrophages, which was observed microscopically (data not shown) (17). Pulsed cultures were washed twice to remove free aPPG and then incubated for 24, 48, or 72 h at 37°C. After these intervals, the cells were lysed with Triton X-100. The aPPG content in the lysates was quantitated by two-site ELISA by comparison with a standard of purified aPPG with MAb LT22, an antibody recognizing [PO4-6(Glcβ1-3)Galβ1-4Manα1-] residues. The total amount of aPPG per monolayer averaged 170 ng/106 cells directly after the pulse. The lysates were deproteinized by phenol extraction and concentrated, and the crude aPPG-containing fractions were electrophoretically separated on 1.2% agarose gels. The separated samples were blotted onto positively charged nylon membranes, and the bound material was detected with MAb AP3 directed against [(Manα1-2)1,2Manα1-PO4] (12). The electrophoretic mobility of aPPG did not change over the total length of the incubation and was identical to the aPPG used to pulse the cells (Fig. 6). This indicates that aPPG is not subject to proteolytic cleavage by lysosomal enzymes in macrophages.
FIG. 6.
Bone marrow macrophages are unable to degrade aPPG. Macrophages (Mphi) were pulsed with purified aPPG. Free aPPG was removed by washing, and the cells were cultured for the indicated times. The cells were then lysed, and aPPG was repurified and concentrated. The purified material was separated electrophoretically on 1.2% agarose gels, transferred to cationized membranes, and detected with the mannooligosaccharide-specific MAb, AP3. The fragments of HindIII-digested λ-DNA were used for size comparison. The purified aPPG used for pulsing (pulse) and the same material incubated for 72 h in medium in the absence of Mphi (72, −) were separated on the same gel for comparison. No material was detected on the lower-molecular-mass parts of the gel by immunostaining of the blotted samples or by direct staining of the gel with Stains All (results not shown).
DISCUSSION
In this study we have investigated the immune response of L. mexicana-infected mice to a secreted product of amastigotes, aPPG. Our results show that despite its abundance in parasitized tissue (8), its highly unusual glycan structures (9), and the lack of homology of its protein backbone to mouse proteins (4a), aPPG does not induce any antibody response in most infected animals while some individuals show a very low antibody titer. In addition, no specific CD4+, MHC class II-restricted T-cell reactivity with aPPG was detected. It appears unlikely that this unresponsiveness of mice to aPPG is the result of a generalized immune suppression caused by the infection, because both T-cell and B-cell reactivities against proteins in a Leishmania lysate as well as T-cell reactivity with ovalbumin (introduced by vaccination) can be easily detected in infected mice.
Infection of mice with L. major and vaccination of mice with purified L. major LPG elicits a glycoconjugate-specific antibody response. Interestingly, these antibodies do not react or react only very weakly with L. mexicana LPG (Fig. 2 and 3A), although the two molecules share most structural elements. The species-specific galactosylated and arabinosylated side chains (Fig. 3A) appear to be the immunodominant epitopes on L. major LPG. In contrast, L. mexicana LPG and aPPG are poor B-cell antigens in mice. It is possible that this result is due to an inherent property of L. mexicana glycan structures, which are low in galactosylated oligosaccharides and completely devoid of arabinosylated oligosaccharides.
Comparative investigation of the immune responses of mice vaccinated with L. mexicana glycoconjugates sAP, LPG, and aPPG demonstrated that aPPG shows no marked secondary humoral response and therefore behaves like a carbohydrate antigen, i.e., like LPG. The absence of aPPG-specific, conventional CD4+ T-cell reactivity in vaccinated mice is consistent with this interpretation. The surprisingly complex glycosylation of aPPG compared to promastigote phosphoglycan antigens (9) may have evolved to prevent antigen processing of this molecule by the host cell, thereby evading the T-cell response. This is supported by the findings that, in contrast to sAP, aPPG was resistant to degradation by proteinase K (reference 8 and unpublished results) or by lysosomal proteases (see above) and that recombinant peptides corresponding to different parts of the aPPG protein backbone induced specific CD4+ T cells and strong secondary-antibody responses upon immunization. Glycans appear to cover the protein backbone completely, since sera raised in C57BL/6 mice against native aPPG do not react with the deglycosylated product and sera raised against the deglycosylated material do not bind to native molecules (5a).
Cell surface and secreted antigens of parasite and bacterial pathogens are thought to be prime targets of the host immune response (6, 7, 21). The intracellular mammalian stage of the protozoan parasite L. mexicana, the amastigote, appears to minimize the risk of detection by the host immune system in several ways. (i) The amastigote surface does not appear to display a major cell surface protein (3, 19). (ii) The molecules that are abundant on the surface of L. mexicana amastigotes, glycoinositolphospholipids and glycosphingolipids, either are not immunogenic in mice or are derived from the host cell itself, respectively (19). (iii) As shown in this study, the dominant secretory product of L. mexicana amastigotes induces neither a B-cell nor a T-cell response in its model mammalian host, thereby also avoiding immune system recognition at this level.
While being invisible to the host immune system, aPPG may have some crucial functions for the parasites: we have previously shown that aPPG induces vacuole formation in mammalian macrophages (17). In addition, purified aPPG activates the complement cascade via the mannose binding protein pathway (18). Triggering the complement cascade will release anaphylactic peptides that are known chemoattractants for monocytes and thus support lesion development in L. mexicana infections by eliciting a constant influx of new host cells. In summary, secretion of aPPG by the parasites could be critical for the establishment and subsistence of L. mexicana infections. The recent cloning of the genes encoding the aPPG protein backbone (4a) allows the creation of deletion mutants which will provide information about whether this molecule is essential for the virulence of this parasite in its mammalian hosts.
ACKNOWLEDGMENTS
We thank Peter Overath for support and helpful comments on the manuscript and Monika Demar for excellent technical assistance.
REFERENCES
- 1.Aebischer, T. Unpublished data.
- 1a.Afonso L C, Scott P. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61:2952–2959. doi: 10.1128/iai.61.7.2952-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander J, Russell D G. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–249. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 3.Bahr V, Stierhof Y D, Ilg T, Demar M, Quinten M, Overath P. Expression of lipophosphoglycan, high-molecular-weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol Biochem Parasitol. 1993;58:107–121. doi: 10.1016/0166-6851(93)90095-f. [DOI] [PubMed] [Google Scholar]
- 4.de Ibarra A A, Howard J G, Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982;85:523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]
- 4a.Goepfert, U., N. Goehring, C. Klein, and T. Ilg. Submitted for publication.
- 5.Handman E, Hocking R E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982;37:28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Harbecke, D., and T. Aebischer. Unpublished data.
- 6.Hess J, Dietrich G, Gentschev I, Miko D, Goebel W, Kaufmann S H. Protection against murine listeriosis by an attenuated recombinant Salmonella typhimurium vaccine strain that secretes the naturally somatic antigen superoxide dismutase. Infect Immun. 1997;65:1286–1292. doi: 10.1128/iai.65.4.1286-1292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilg T, Stierhof Y D, McConville M J, Overath P. Purification, partial characterization and immunolocalization of a proteophosphoglycan secreted by Leishmania mexicana amastigotes. Eur J Cell Biol. 1995;66:205–215. [PubMed] [Google Scholar]
- 9.Ilg T, Craik D, Currie G, Multhaup G, Bacic A. Stage-specific proteophosphoglycan from Leishmania mexicana amastigotes. Structural characterization of novel mono-, di-, and triphosphorylated phosphodiester-linked oligosaccharides. J Biol Chem. 1998;273:13509–13523. doi: 10.1074/jbc.273.22.13509. [DOI] [PubMed] [Google Scholar]
- 10.Ilg T, Stierhof Y D, Etges R, Adrian M, Harbecke D, Overath P. Secreted acid phosphatase of Leishmania mexicana: a filamentous phosphoglycoprotein polymer. Proc Natl Acad Sci USA. 1991;88:8774–8778. doi: 10.1073/pnas.88.19.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilg T, Etges R, Overath P, McConville M J, Thomas-Oates J, Thomas J, Homans S W, Ferguson M A. Structure of Leishmania mexicana lipophosphoglycan. J Biol Chem. 1992;267:6834–6840. [PubMed] [Google Scholar]
- 12.Ilg T, Harbecke D, Wiese M, Overath P. Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur J Biochem. 1993;217:603–615. doi: 10.1111/j.1432-1033.1993.tb18283.x. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher M, Curtis J M, Sacks D L, Handman E, Bacic A. Epitope mapping of monoclonal antibodies directed against lipophosphoglycan of Leishmania major promastigotes. Mol Biochem Parasitol. 1994;66:187–200. doi: 10.1016/0166-6851(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 14.McConville M J, Ferguson M A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondino A, Jenkins M K. Surface proteins involved in T cell costimulation. J Leukoc Biol. 1994;55:805–815. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- 16.Morris L, Troutt A B, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–2721. [PubMed] [Google Scholar]
- 17.Peters C, Stierhof Y D, Ilg T. Proteophosphoglycan secreted by Leishmania mexicana amastigotes causes vacuole formation in macrophages. Infect Immun. 1997;65:783–786. doi: 10.1128/iai.65.2.783-786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters C, Kawakami M, Kaul M, Ilg T, Overath P, Aebischer T. Secreted proteophosphoglycan of Leishmania mexicana amastigotes activates complement by triggering the mannan binding lectin pathway. Eur J Immunol. 1997;27:2666–2672. doi: 10.1002/eji.1830271028. [DOI] [PubMed] [Google Scholar]
- 19.Winter G, Fuchs M, McConville M J, Stierhof Y D, Overath P. Surface-antigens of Leishmania mexicana amastigotes—characterization of glycoinositol phospholipids and a macrophage-derived glycosphingolipid. J Cell Sci. 1994;107:2471–2482. doi: 10.1242/jcs.107.9.2471. [DOI] [PubMed] [Google Scholar]
- 20.Wolfram M, Ilg T, Mottram J C, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells specific for amastigote cysteine proteinases requires intracellular killing of the parasites. Eur J Immunol. 1995;25:1094–1100. doi: 10.1002/eji.1830250435. [DOI] [PubMed] [Google Scholar]
- 21.Wolfram M, Fuchs M, Wiese M, Stierhof Y D, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T-cells by a model parasite antigen secreted into the parasitophorous vacuole or expressed on the amastigote surface. Eur J Immunol. 1996;26:3153–3162. doi: 10.1002/eji.1830261248. [DOI] [PubMed] [Google Scholar]