Abstract
The continued circulation of SARS-CoV-2 virus in different parts of the world opens up the possibility for more virulent variants to evolve even as the coronavirus disease 2019 transitions from pandemic to endemic. Highly transmissible and virulent variants may seed new disruptive epidemic waves that can easily put the healthcare system under tremendous pressure. Despite various nucleic acid-based diagnostic tests that are now commercially available, the wide applications of these tests are largely hampered by specialized equipment requirements that may not be readily available, accessible and affordable in less developed countries or in low resource settings. Hence, the availability of lateral flow immunoassays (LFIs), which can serve as a diagnostic tool by detecting SARS-CoV-2 antigen or as a serological tool by measuring host immune response, is highly appealing. LFI is rapid, low cost, equipment-free, scalable for mass production and ideal for point-of-care settings. In this review, we first summarize the principle and assay format of these LFIs with emphasis on those that were granted emergency use authorization by the US Food and Drug Administration followed by discussion on the specimen type, marker selection and assay performance. We conclude with an overview of challenges and future perspective of LFI applications.
Keywords: dipstick, immunochromatography, antigen, antibody, diagnostic, serology, point-of-care, immunosensor
1. Introduction
It has been almost three years since the onset of the coronavirus disease 2019 (COVID-19) pandemic but global case incidence has remained high with over 2.9 million new cases and 8300 fatalities reported in the week ending on 2 October 2022 [1]. All these add to the staggering number of 615 million confirmed cases and 6.5 million deaths that were attributed to the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus globally as of 2 October 2022 [1]. The virus can be transmitted from an infected person via infective respiratory droplets and fomites in the immediate environment [2]. Following a mean incubation period of 5 days, the most common clinical presentation of COVID-19 includes fever, cough, and shortness of breath with typical imaging features consisting of bilateral pneumonia, multiple mottling, and ground-glass opacity [3,4]. Although the majority of COVID-19 cases are mild, serious complications may develop in a subset of patients including acute respiratory distress syndrome, acute cardiac injury, acute kidney injury, and septic shock [4,5,6]. The disease can progress rapidly from mild to severe: the median times from onset of symptoms to intensive care unit admission and death were 10.5 days [5] and 14 days [7], respectively.
Given that COVID-19 affects all age groups with a spectrum of illnesses ranging from asymptomatic to fatal [6], the availability of rapid, sensitive and specific diagnostic tests that can accurately triage and identify COVID-19 patients at first point of contact will be central to concomitant measures to control the spread of this disease. The swift release of the SARS-CoV-2 genome sequence early in the outbreak had allowed highly specific and sensitive nucleic acid tests to be developed and used for diagnostic, screening and surveillance purposes. Presently, a range of nucleic acid amplification tests (NAATs) have been granted emergency use authorization (EUA) status by the US Food and Drug Administration (FDA). These include non-isothermal- and isothermal-based amplification technologies with various amplicon detection methods being employed such as fluorometric, colorimetric, electrochemical, magnetic resonance, clustered regularly interspaced short palindromic repeats/Cas systems, matrix-assisted laser desorption ionization time-of-flight and sequencing [8,9]. However, the technical intricacies and sophisticated instrument requirements of FDA-EUA nucleic acid tests confine most of them to Clinical Laboratory Improvement Amendments (CLIA)-certified, high-complexity laboratories.
Compared to nucleic acid tests, lateral flow immunoassays (LFIs) that detect SARS-CoV-2 antigen are more suited for decentralized testing to identify acute or early infection as they are relatively cheap to produce, easy to use, yield a rapid visual result and are virtually equipment-free. LFIs that fulfill the World Health Organization’s ASSURED criteria (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free and Deliverable to end users) [10] can rapidly expand testing capabilities for this virus, particularly in middle- and low-income countries. At point-of-care settings, the on-site detection and same-day reporting features of these paper-based diagnostic tools will greatly help physicians in making evidence-based COVID-19 patient management decisions. On the other hand, serological LFIs that assess the immune status to COVID-19 by detecting antibodies against SARS-CoV-2 provides important epidemiological information such as the cumulative incidence of infection, the proportion of mild and asymptomatic cases, the proportion of severe and fatal cases among those who are infected, and the immune status of the population [11].
The merits of LFI make it an attractive first-line test against COVID-19 as this technology platform does not suffer from the many drawbacks associated with real-time reverse transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA), i.e., multiple liquid-handling steps, high equipment requirements and longer turnaround time. A general comparison between LFI, RT-PCR and ELISA is presented in Table 1. Although the applications of LFI for COVID-19 testing have been covered in recent reviews [12,13,14,15], there were no attempts to provide a comprehensive overview of LFIs that have received EUA from the US FDA. As of 11 October 2022, a total of 69 LFIs have been granted EUA: 44 are antigen diagnostic tests and the remaining 25 are serological tests that detect antibodies, such as immunoglobulin (Ig) M and/or IgG, against SARS-CoV-2. In this review, we discuss the principle and different assay formats of the LFIs that detect SARS-CoV-2 antigen(s) and anti-SARS-CoV-2 antibodies in detail. We also compare the performance of these LFIs and conclude with the challenges and future perspective of LFI applications beyond the COVID-19 pandemic.
Table 1.
An overview of differences between RT-PCR, LFI for the detection of SARS-CoV-2 antigen, ELISA and serological LFI.
| RT-PCR | LFI for the Detection of SARS-CoV-2 Antigen | ELISA | Serological LFI | |
|---|---|---|---|---|
| Detection Target | SARS-CoV-2 nucleic acid | SARS-CoV-2 antigen | Antibodies against SARS-CoV-2 | Antibodies against SARS-CoV-2 |
| Sample Type | Upper and lower respiratory specimens | NS and NP swabs | Serum, plasma, venous whole blood | Serum, plasma, venous whole blood, finger-prick whole blood |
| Sensitivity | High | Moderate | High | Moderate |
| Specificity | High | High | High | High |
| Turnaround Time | 2–3 h | 10–15 min | 2–5 h | 10–15 min |
| Instrumentation | Required | Not required except for fluorescent LFI | Required | Not required |
| Test Complexity | Most are complex | Easy-to-use | Most are complex | Easy-to-use |
| Laboratory Requirements | High | Average | High | Average |
| Usage | To detect active infection | To detect active infection | To detect past infection | To detect past infection |
NS, nasal; NP, nasopharyngeal.
2. LFI: An Overview
2.1. Principle of LFI
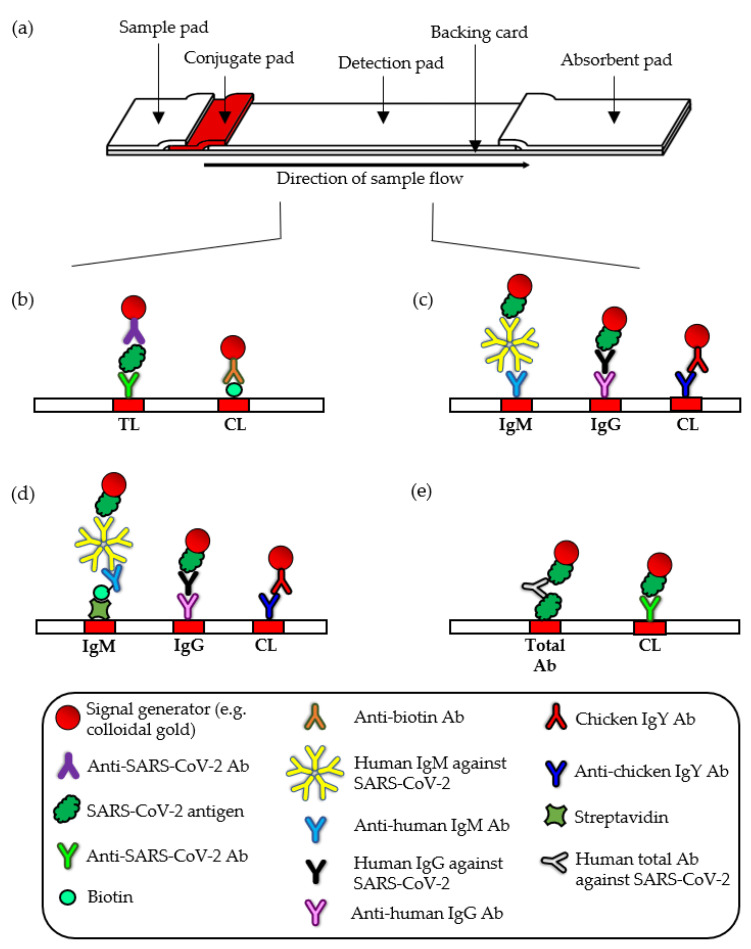
LFIs are self-contained and self-operating devices: properties that are made possible by the LFI configuration and capillary action. By mounting several components of different materials (a sample pad, conjugate pad, detection pad and an absorbent pad) in an overlapping manner, a device that functions as a single test system is created wherein an aqueous medium can flow continuously from proximal to distal ends of the strip (Figure 1a). Briefly, when an aqueous biological sample (or its extract) is introduced to the sample pad, the sample moves through the interstitial space by capillary action to the conjugate pad that holds the detector agent. The target analyte, if present, will bind to the detector agent that is conjugated with a signal generator. The immunocomplexes will be anchored to the test line(s) where capture agents are coated on the detection pad. Accumulation of the signal generator at the test line(s) enables the immunocomplexes to be detected while unreacted substances continue to migrate to the absorbent pad.
Figure 1.
(a) Assembly of the different components of LFI. Assay formats for the detection of SARS-CoV-2 antigen (b), IgM and IgG (c,d), and total antibody (e). TL, test line; CL, control line; Ab, antibody.
2.2. Components of LFI
2.2.1. Sample Pad
As the first component of the LFI to encounter the sample, the sample pad plays a crucial role in flow regulation because flooding of the device will adversely affect the assay performance. Woven meshes or cellulose fiber is typically used but the sample pad material can be suited for specific tasks such as the FUSION 5 membrane (Whatman, Kent, UK) that can act as an effective blood separator and prevent red blood cells from obscuring the signal generated on the detection pad [16]. Additionally, modifying agents such as proteins, viscosity enhancers, surfactants and/or buffer salts can be introduced at the sample pad to promote optimal test conditions. Different modifying agents may be used to serve different purposes such as to block the detection pad, to alter the sample viscosity, to aid in the rehydration and release of signal generator and to facilitate antigen–antibody binding by altering the chemical nature of the sample [17].
2.2.2. Conjugate Pad
The conjugate pad is made of a porous material, such as glass fiber filter, to provide a matrix onto which desiccated signal generator can be held in a stable and functional state. The signal generator is functionalized with detector agents, allowing the formation of complete immunocomplexes to be detected visually or measured using a benchtop/handheld optical reader. Colloidal gold is usually employed as the signal generator in LFIs because it is relatively inexpensive, non-toxic, easy to synthesize and functionalize, stable in both liquid and dried forms, can be visualized with the naked eye and does not succumb to photodecomposition [16,18,19]. Functionality of the conjugates is preserved in the desiccated state with the help of a stabilizer such as sucrose or trehalose sugar. These water substitutes maintain the structural integrity of dried proteins through hydrogen bonding [20]. The amount of analyte in a sample that can be detected by a lateral flow strip is dictated by the volume of sample that is needed to release all the rehydrated signal generators from the conjugate pad because the subsequent formation of incomplete immunocomplexes would not contribute to the signal generation on the detection pad. A pink to red colored signal is generally obtained with gold conjugates but signal generation in other colors can also be achieved such as by using colored latex particles. Other signal generators such as carbon, selenium, liposomes, chemiluminescent and fluorescent nanoparticles are used less often [21]. In the case of fluorescent nanoparticles, a fluorescence analyzer is required for analysis, but the automated result interpretation eliminates the potential bias arising from the subjective judgement of the operator.
2.2.3. Detection Pad
The detection pad represents one of the most important components of a LFI because reactions that occurred on this pad form the basis for result interpretation. Various materials that differ in pore size, porosity, thickness, and structural characteristics can be used as the detection pad including nylon, polyethersulfone, polyethylene, and glass fiber but nitrocellulose is still the material of choice because proteins interact and bind readily to the hydrophobic, neutral membrane via electrostatic interactions [21]. Nitrocellulose membranes with different capillary flow rates are commercially available but LFI developers would need to strike a balance between speed, cost, and assay performance as the sensitivity and total assay time decreases while specificity and reagent consumption increases with increasing capillary flow rate [17].
The test line is always dedicated towards the capture of the target analyte and a control line located downstream of the test line is also an integral part of all LFIs because it serves as built-in functionality and operational controls for each device. The control line ensures the reliability of the assay as multiple factors can negatively impact the assay performance including characteristic and composition of the sample, temperature variations during transport and storage, the use of faulty lateral flow devices or reagents, and improper execution of the assay procedure. The control line can be constructed with species-specific anti-Ig antibodies if the antibodies raised by the host are conjugated to the signal generator or with antibodies against a particular antigen if the said antigen is conjugated to the signal generator. However, the majority of FDA-EUA LFIs incorporate another set of signal generators that are conjugated with other antibodies (such as anti-biotin antibody, rabbit IgG and chicken IgY) or small molecules (such as dinitrophenyl) instead of the detector agent. This particular set of signal generators will only be captured at the control line in order to serve as a procedure control. Although such a control line would not be able to rule out false negative result due to dysfunctional detector agent–signal generator conjugates, factors influencing the development and validation of novel LFIs, such as cost, time and availability of supplies, during this public health crisis would need to be taken into consideration. Regardless of the target analyte which may or may not be present, a signal must be obtained at the control line. If the control line does not appear, the LFI result will be considered invalid and the sample has to be retested with a new lateral flow device.
2.2.4. Absorbent Pad
The absorbent pad acts as a sink to accumulate unreacted substances including excess sample and reagents. Typically made of cellulose fibers, the absorbent pad drives the capillary action and must be able to accommodate the sample and buffer volume that are needed to perform the assay.
2.2.5. Backing Card and Cassette
Proper lamination of the LFI materials on an adhesive backing card is a key factor in achieving consistent and uniform flow front. The backing card provides tensile strength and structural support for handling purposes but errors during the lamination process can lead to an irregular flow front and may even halt capillary flow. LFIs tend to be housed in a plastic cassette with internal pressure bars or pins to hold the device in position and to protect it from physical damage. Despite the additional cost incurred to house the strip within a cassette, there are several advantages to be gained. For example, the location of the sample loading site, the test line and the control line can be indicated on the cassette in order to facilitate proper assay execution and accurate result interpretation.
3. LFIs for SARS-CoV-2 Antigen Detection
3.1. Assay Format
SARS-CoV-2 has four major structural proteins: the spike (S), membrane (M), and envelope (E) that constitute the surface proteins and the nucleocapsid (N) protein that forms the ribonucleoprotein core inside the viral envelope. Among the 44 FDA-EUA antigen-detecting LFIs, 42 are directed against the N antigen whereas only two target both N antigen and receptor binding domain (RBD) of the S protein (Table 2). Almost half (47.7%) of the FDA-EUA antigen-detecting LFIs are over-the-counter, fully at-home diagnostic tests for COVID-19 [22] that can be purchased, conducted and interpreted at home without the involvement of a healthcare provider or a laboratory. The remaining LFIs are authorized to be used at patient care settings operating under a CLIA Certificate of Waiver as well as in CLIA-certified, high- or moderate-complexity laboratories. Notably, only two are multiplexed antigen tests namely Sofia 2 Flu + SARS Antigen FIA (Quidel) and Status COVID-19/Flu (Princeton BioMeditech Corporation, NJ, USA) that simultaneous detect and differentiate between SARS-CoV-2, influenza A and influenza B.
Table 2.
Characteristics of FDA-EUA LFIs for the detection of SARS-CoV-2 antigen.
| Developer | Test Name | Diagnostic Marker(s) | Time-to-Result | Sample Indicated for Testing | LoD (TCID50/mL) | Authorized Setting(s) |
|---|---|---|---|---|---|---|
| InBios International Inc. | SCoV-2 Ag Detect Rapid Self-Test | N antigen | 20–25 min | Anterior nasal (nares) swab samples | 6.3 × 103 | Home, H, M, W |
| Quidel Corporation | QuickVue At-Home OTC COVID-19 Test | N antigen | 10–15 min | Anterior nasal (nares) swab samples | 1.91 × 104 | Home, H, M, W |
| ACON Laboratories, Inc. | Flowflex COVID-19 Antigen Home Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 2.5 × 103 | Home, H, M, W |
| Xiamen Boson Biotech Co., Ltd. | Rapid SARS-CoV-2 Antigen Test Card | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 1.4 × 102 | Home, H, M, W |
| Access Bio, Inc. | CareStart COVID-19 Antigen Home Test | N antigen | 10–15 min | Anterior nasal (nares) swab samples | 2.8 × 103 | Home, H, M, W |
| ANP Technologies, Inc. | NIDS COVID-19 Antigen Rapid Test Kit | N antigen | 15–30 min | Mid-turbinate (MT) nasal swabs | 3.11 × 102 | H, M, W |
| Genabio Diagnostics Inc. | Genabio COVID-19 Rapid Self-Test Kit | N antigen | 15–20 min | Anterior nasal (nares) swab samples | 1.78 × 104 | Home, H, M, W |
| OSANG LLC | OHC COVID-19 Antigen Self Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 1.4 × 104 | Home, H, M, W |
| PHASE Scientific International, Ltd. | INDICAID COVID-19 Rapid Antigen At-Home Test | N antigen | 20–25 min | Anterior nasal (nares) swab samples | 2.8 × 103 | Home, H, M, W |
| PHASE Scientific International, Ltd. | INDICAID COVID-19 Rapid Antigen Test | N antigen | 20–25 min | Anterior nasal (nares) swab samples | 2.8 × 103 | H, M, W |
| Celltrion USA, Inc. | Celltrion DiaTrust COVID-19 Ag Rapid Test | N and RBD antigens | 15–20 min | Mid-turbinate (MT) nasal swabs | 3.2 × 101 | H, M, W |
| GenBody Inc. | GenBody COVID-19 Ag | N antigen | 15–20 min | Nasopharyngeal (NP) or anterior nasal (AN) swab specimens | 1.11 × 102 | H, M, W |
| Siemens Healthineers | CLINITEST Rapid COVID-19 Antigen Self-Test | N antigen | 15–20 min | Anterior nasal (nares) swab samples | 7.0 × 103 | Home, H, M, W |
| OraSure Technologies, Inc. | InteliSwab COVID-19 Rapid Test Rx | N antigen | 30–40 min | Anterior nasal (nares) swab samples | 2.5 × 102 | Home, H, M, W |
| OraSure Technologies, Inc. | InteliSwab COVID-19 Rapid Test | N antigen | 30–40 min | Anterior nasal (nares) swab samples | 2.5 × 102 | Home, H, M, W |
| OraSure Technologies, Inc. | InteliSwab COVID-19 Rapid Test Pro | N antigen | 30–40 min | Anterior nasal (nares) swab samples | 2.5 × 102 | H, M, W |
| Maxim Biomedical, Inc. | MaximBio ClearDetect COVID-19 Antigen Home Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 7.5 × 102 | Home, H, M, W |
| SD Biosensor, Inc. | Pilot COVID-19 At-Home Test | N antigen | 20–30 min | Anterior nasal (nares) swab samples | 1.4 × 103 | Home, H, M, W |
| Ellume Limited | Ellume COVID-19 Home Test | N antigen | 15 min | Mid-turbinate (MT) nasal swabs | 6.31 × 103 | Home, H, M, W |
| iHealth Labs, Inc. | iHealth COVID-19 Antigen Rapid Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 20 × 103 | Home, H, M, W |
| Watmind USA | Speedy Swab Rapid COVID-19 Antigen Self-Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 2.8 × 102 | Home, H, M, W |
| Celltrion USA, Inc. | Celltrion DiaTrust COVID-19 Ag Home Test | N and RBD antigens | 15–20 min | Mid-turbinate (MT) nasal swabs | 2.8 × 101 | Home, H, M, W |
| Salofa Oy | Sienna-Clarity COVID-19 Antigen Rapid Test Cassette | N antigen | 10–20 min | Nasopharyngeal (NP) swab specimens | 1.25 × 103 | H, M, W |
| QIAGEN GmbH | QIAreach SARS-CoV-2 Antigen | N antigen | 2–15 min | Nasopharyngeal (NP) or anterior nasal (AN) swab specimens | 5 × 104 | H, M |
| InBios International, Inc. | SCoV-2 Ag Detect Rapid Test | N antigen | 20–25 min | Anterior nasal (nares) swab samples | 6.3 × 103 | H, M, W |
| Abbott Diagnostics Scarborough, Inc. | BinaxNOW COVID-19 Ag Card Home Test | N antigen | 15 min | Anterior nasal (nares) swab samples | 1.41 × 102 | Home, H, M, W |
| Abbott Diagnostics Scarborough, Inc. | BinaxNOW COVID-19 Antigen Self Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 7 × 102 | Home, H, M, W |
| Oceanit Foundry LLC | ASSURE-100 Rapid COVID-19 Test | N antigen | 20–30 min | Anterior nasal (nares) swab samples | 1.41 × 102 | H, M, W |
| Becton, Dickinson and Company (BD) | BD Veritor At-Home COVID-19 Test | N antigen | 15–20 min | Anterior nasal (nares) swab samples | 0.88 × 102 | Home, H, M, W |
| Luminostics, Inc. | Clip COVID Rapid Antigen Test | N antigen | 45 s | Anterior nasal (nares) swab samples | 1.87 × 102 | H, M, W |
| Abbott Diagnostics Scarborough, Inc. | BinaxNOW COVID-19 Ag Card | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 1.41 × 102 | H, M, W |
| Abbott Diagnostics Scarborough, Inc. | BinaxNOW COVID-19 Ag 2 Card | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 1.41 × 102 | H, M, W |
| Nano-Ditech Corp. | Nano-Check COVID-19 Antigen Test | N antigen | 15–20 min | Nasopharyngeal (NP) swab specimens | 7 × 102 | H, M, W |
| iHealth Labs, Inc. | iHealth COVID-19 Antigen Rapid Test Pro | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 20 × 103 | H, M, W |
| Becton, Dickinson and Company (BD) | BD Veritor System for Rapid Detection of SARS-CoV-2 | N antigen | 15–20 min | Anterior nasal (nares) swab samples | 1.4 × 102 | H, M, W |
| Access Bio, Inc. | CareStart COVID-19 Antigen test | N antigen | 10–15 min | Nasopharyngeal (NP) or anterior nasal (AN) swab specimens | 8 × 102 | H, M, W |
| Quidel Corporation | QuickVue SARS Antigen Test | N antigen | 10–15 min | Anterior nasal (nares) swab samples | 7.57 × 103 | H, M, W |
| Princeton BioMeditech Corp. | Status COVID-19-Flu A&B | N antigen | 15–20 min | Nasopharyngeal (NP) or anterior nasal (AN) swab specimens | 2.7 × 103 | H, M, W |
| Xtrava Health | SPERA COVID-19 Ag Test | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 1.56 × 103 | H, M, W |
| Ellume Limited | ellume.lab COVID Antigen Test | N antigen | 3–15 min | Mid-turbinate (MT) nasal swabs | 7.16 × 103 | H, M, W |
| Quidel Corporation | Sofia SARS Antigen FIA | N antigen | 15–30 min | Anterior nasal (nares) swab samples | 2.8 × 102 | H, M, W |
| Becton, Dickinson and Company (BD) | BD Veritor System for Rapid Detection of SARS-CoV-2 & Flu A + B | N antigen | 15–20 min | Anterior nasal (nares) swab samples | 1.13 × 102 | H, M, W |
| Quidel Corporation | QuickVue At-Home COVID-19 Test | N antigen | 10–15 min | Anterior nasal (nares) swab samples | 1.91 × 104 | Home, H, M, W |
| Quidel Corporation | Sofia 2 Flu + SARS Antigen FIA | N antigen | 15–30 min | Nasopharyngeal (NP) or anterior nasal (AN) swab specimens | 9.17 × 101 | H, M, W |
Monoclonal antibodies are prized for their high specificity and in the context of SARS-CoV-2 testing, the incidence of false-positive results and cross-reactivity with structurally similar antigens will be less likely as compared to polyclonal antibodies. Monoclonal antibodies in LFI are easier to optimize due to the homogeneity of the molecular species. However, the production process is not only tedious and costly but also requires technical expertise and specialized facilities. Unlike monoclonal antibodies, polyclonal antibodies are cheaper, quicker, and easier to produce but different batches of polyclonal antibodies will vary in quantity and quality. Polyclonal antibodies targeting different epitopes may capture the antigen more effectively, but assay performance can be compromised by the presence of non-specific antibodies and proteins. Heterogeneity of the polyclonal composition can complicate LFI optimization process as each Ig class and isotype may require slightly different conditions for binding and conjugation. Furthermore, potential structural and steric problems can arise from species such as IgA and IgM [16].
3.2. Specimen Type
The FDA-EUA LFIs for the detection of SARS-CoV-2 antigen are recommended to be performed within 5 to 12 days of symptom onset. The indicated specimen types for these antigen tests are currently limited to nasal swab and/or nasopharyngeal swab whereas a much wider range of specimens from the upper and lower respiratory tract can be tested with most of the FDA-EUA NAATs [8]. Nasal swab is the only acceptable specimen type in more than three-quarters of the FDA-EUA LFIs, followed by nasal and nasopharyngeal swabs in seven tests and nasopharyngeal swabs only in two of the tests. Unlike a nasopharyngeal specimen that requires a trained healthcare provider to collect, nasal specimens (anterior nares and mid-tubinate swabs) can be self-collected at the testing site or at home [23] with the procedure being less invasive and pain-free. Nonetheless, improper specimen collection and handling procedures can still adversely affect the test performance. For example, swabbing the nostril too quickly, swabbing only one of the nostrils and simply twirling or leaving the swab in the nose for several seconds may result in insufficient specimen for viral antigen detection [24].
The studies by Mak and colleagues highlighted the impacts on LFI sensitivity when different specimen types, including those that were outside of the manufacturer’s instructions for use (IFU), were tested in combination or alone [25,26]. In one of the studies, the highest sensitivity was obtained with nasopharyngeal and throat swabs (45.7%), followed by throat saliva (40%), nasopharyngeal aspirate and throat swab (34.3%) and sputum (11%) [26]. Given that the characteristics of these specimens differed substantially from one to another, the testing of specimen types outside of the manufacturer’s IFU may result in over dilution or a sub-optimal condition for antigen–antibody binding due to the changes in the chemical nature, matrix and viscosity of the specimen. In addition to the respiratory tract specimens, the detection of SARS-CoV-2 antigen in serum (n = 11/13) and urine specimen (n = 14/19) of COVID-19 cases with LFI have been reported in two separate preprints [27,28].
3.3. Diagnostic Marker
Unlike NAAT, LFI only detects antigens that are originally present in the specimen. Hence, the selection of target antigen is important as it relates directly to the test performance. Similar to SARS-CoV antigen detection [29,30], the N protein is the preferred target in FDA-EUA SARS-CoV-2 antigen-detecting LFIs because of its relative abundance during active infection [31]. Based on the clinical performance of several FDA-EUA antigen-detecting LFIs, the N protein can be detected as early as one day after symptom onset with specimen positivity ranging from 11.1% (n = 4/36) [32] to 43.8% (n = 14/32) [33]. Furthermore, positive correlations (r2 = 0.66–0.90) between SARS-CoV-2 N protein levels and RT-PCR Ct values in nasopharyngeal specimens have been reported in several studies [34,35,36].
The N protein is 419-amino acid in length and contains three distinct domains that interact with the viral genomic RNA via positively charged amino acid residues: the N-terminal domain (NTD; 46–176 residues), a serine/arginine-rich domain (SR-rich; 184–204 residues) in the linkage region and the C-terminal domain (CTD; 247–364 residues) [37,38]. With a size of 46 kDa, the N protein can be detected with the double antibody sandwich format. However, cross-reactivity between SARS-CoV-2 and SARS-CoV is frequently reported due to the high level of identity in the whole amino acid sequence (90.5%) [37] and epitope region sequence (78–100%) of the N protein [39]. Hence, positive results of FDA-EUA N antigen-detecting LFIs do not differentiate between SARS-CoV and SARS-CoV-2. Amino acid homology of the N protein between SARS-CoV-2 and other human coronaviruses shared a much lesser degree of identity that ranged from 28% (HCoV-229E) to 49% (MERS-CoV) [40].
3.4. Performance
In general, all the reagents and materials that are needed to perform the test are provided with the exception of a timer. A two-step procedure is typically adopted wherein a swab specimen is placed into the assay extraction buffer or reagent before the solution is introduced into the sample well of the lateral flow device followed by visual interpretation of the result within 10 to 30 min. While most of the lateral flow devices consist of a rectangular plastic cassette that houses the strip, the BinaxNOW COVID-19 Ag Card differs by housing the strip in a cardboard, book-shaped hinged test card. After the extraction reagent has been loaded and the swab specimen inserted into card, the extracted sample only comes into contact with the strip when the card is closed. The test results are categorized as either positive when both test and control lines appear, negative when only the control line appears or invalid when the control line does not appear. A positive result indicates the presence of SARS-CoV-2 antigens and confirmatory NAAT testing may be warranted based on the tested individual’s clinical and epidemiological characteristics. Negative results for individuals showing clinical signs and symptoms that are consistent with COVID-19 or with symptom onset beyond five days should be treated as presumptive and confirmed with a FDA-authorized NAAT.
For LFI that generates non-visually interpreted results, such as the Sofia SARS Antigen FIA (Quidel Corporation, San Diego, CA, USA), Sofia 2 Flu + SARS Antigen FIA (Quidel Corporation, San Diego, CA, USA), BD Veritor System for Rapid Detection of SARS-CoV-2 (BD) and Clip COVID Rapid Antigen Test (Luminostics, Fremont, CA, USA), the additional reader or analyzer required will entail a higher cost. However, these tests are generally more sensitive than naked eye detection and automation of the signal measurement permit additional analysis to be performed such as to correct for non-specific binding and to set the threshold to be applied for result interpretation. Automation of the result interpretation also reduces variability and eliminates the potential bias arising from the subjective judgement of the operator. Some analyzers, such as the BD Veritor Plus Analyzer (BD) and Sofia/Sofia2 (Quidel), offer the options of analyzing the LFI device after the test development has been timed manually (Analyze Now mode) or automated test development timing and analysis (Walk Away mode) by inserting the device immediately into the analyzer after sample application. The Ellume COVID-19 Home Test (Ellume) is the only FDA-EUA antigen-detecting LFI that integrates the analyzer (an optoelectronics reader system) within the housing of the lateral flow strip. In addition to the reagents and materials provided, the user must have a smartphone to download the Ellume COVID-19 Test App in order to connect with the LFI device. The user is also guided by a self-paced, step-by-step instructions to perform the test and the result will be automatically sent to the user’s smartphone and displayed via the downloaded application.
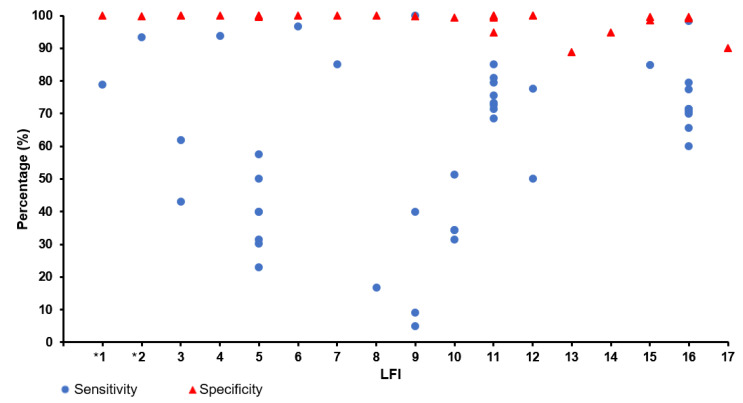
Compared to the chemically labile RNA that is prone to degradation, viral antigen is a more robust analyte for SARS-CoV-2 testing and the use of LFI obviates tedious liquid-handling steps such as RNA extraction and amplification reaction mixture preparation. This simplicity in operation, however, comes at the expense of sensitivity. At present, the limit of detection (LoD) attained by FDA-EUA LFIs for SARS-CoV-2 antigen testing ranged from 28 to 50,000 TCID50/mL whereas the LoD of NAAT is generally well below 1 TCID50/mL [8]. The implication of selecting a LFI with a high LoD is an increased risk of misdiagnosis as the test would generate a false-negative test result when the level of antigen in the sample is below the LoD. Among the FDA-EUA LFIs, only the performance characteristics of BD Veritor System for Rapid Detection of SARS-CoV-2 and BinaxNOW COVID-19 Ag Card have been evaluated in peer and/or non-peer reviewed studies [41,42,43]. While both LFIs showed high specificity (99.9–100%), the sensitivity of BD Veritor System for Rapid Detection of SARS-CoV-2 (94.1%; 95% CI: 71.1–100%) was found to be higher than that of the BinaxNOW COVID-19 Ag Card (93.4%; 95% CI: 68.1–99.8%) [42,43]. In an attempt to compare the analytical sensitivity of BinaxNOW COVID-19 Ag Card to RT-PCR in terms of viral RNA copies, Perchetti and colleagues established that the LoD of the LFI was equivalent to 4.04–8.06 × 104 copies/swab which corresponded to a CT value of approximately 30 [41]. However, this LoD was determined with contrived specimens stored in phosphate-buffered saline and, hence, may not reflect the real life sensitivity of the assay with direct nasal swab. The comparison of sensitivity and specificity of various commercial LFIs for the detection SARS-CoV-2 antigen is presented in Figure 2, with the details available in Supplementary Table S1. The performance varied greatly between the LFIs with some assays demonstrating sensitivity below 50% but the specificity for all the LFIs ranged from 88.9 to 100%.
Figure 2.
Sensitivity and specificity of commercial LFIs for the detection of SARS-CoV-2 antigen. (1). BD Veritor System for Rapid Detection of SARS-CoV-2 (VRD) (Becton, Dickinson and Company, Bergen, NJ, USA); (2). BinaxNOW COVID-19 Ag Card (Abbott Diagnostics, Chichago, IL, USA), (3). Biocredit COVID-19 Ag Detection Kit (RapiGEN, Anyang, South Korea); (4). Bioeasy 2019-Novel Coronavirus (2019-nCoV) Fluorescence Antigen Rapid Test Kit (fluorescence immunochromatographic assay) (Bioeasy Biotechnology, Shenzhen, China); (5). COVID-19 Ag Respi-Strip (Coris Bioconcept, Gembloux, Belgium); (6). COVID-VIRO (AAZ, Boulogne Billancourt, France); (7). Diagnostic Kit for 2019-nCoV Ag Test (Bioeasy Biotechnology, Shenzhen, China); (8). Huaketai New Coronavirus (Savant Biotechnology, Beijing, China); (9). Innova Lateral Flow Device (Innova Medical Group, Pasadena, CA, USA); (10). NADAL COVID-19 Ag Test (Nal Von Minden GmbH, Moers, Germany); (11). Panbio COVID-19 Ag Rapid Test Device (Abbott Rapid Diagnostics, Cologne, Germany); (12). Rapid antigen test provided by R-Biopharm; (13). Rapid COVID-19 Antigen Test (Healgen, Houston, TX, USA); (14). RIDA QUICK SARS-CoV-2 Antigen (R-Biopharm, Darmstadt, Germany); (15). SD Biosensor SARS-CoV-2 Rapid Antigen Test (Roche Diagnostics, Basel, Switzerland); (16). STANDARD Q COVID-19 Ag (SD Biosensor, Suwon-si, Republic of Korea); (17). StrongStep COVID-19 Antigen Test (Liming Bio-Products, Nanjing, China). * FDA-EUA.
4. LFIs for the Detection of Anti-SARS-CoV-2 Antibodies
4.1. Assay Format
Serological LFIs that detect the presence of specific antibodies, such as IgM and IgG, against SARS-CoV-2 provide an indirect proof of COVID-19 infection as the individual has mounted an adaptive immune response to the infection. Although serological LFI is unsuitable to be an early diagnostic tool because it lags behind the molecular detection of viral genome, evidence of antibody seroconversion can be used to supplement the result of RT-PCR, to predict disease outcome, to identify eligible COVID-19 convalescent plasma donors as well as for epidemiological investigation and surveillance purposes [44,45,46]. The presence of IgM and IgG can be distinguished in a single lateral flow strip by constructing two test lines: one with anti-human IgM and another one with anti-human IgG. A selection of SARS-CoV-2 antigens such as N, S and fragments of the S protein (S1 subunit and RBD), which can be used individually or in combination, serve as the detector agent(s) that will be conjugated to a signal generator. Therefore, the target antibody would be sandwiched between the anti-human IgM/IgG and the SARS-CoV-2 antigen that is coupled to a signal generator (Figure 1c). A deviation from this format is seen in the CareStart COVID-19 IgM/IgG [47] wherein anti-SARS-CoV-2 IgM, if present, would form an immunocomplex with biotinylated anti-human IgM and SARS-CoV-2 antigens conjugated to colored particles. The biotinylated immunocomplex will be captured at the test line that is coated with streptavidin via affinity binding between biotin and streptavidin (Figure 1d).
Of the 20 FDA-EUA LFIs that detect IgM and/or IgG against SARS-CoV-2, only three LFIs were designed for the sole detection of IgG (RapCov Rapid COVID-19 Test [48], SCoV Detect IgG Rapid Test [49] and SGTi-flex COVID-19 IgG [50]) whereas the rest incorporate the detection of both IgM and IgG (Table 3). On the other hand, LFIs that are designed to detect total antibody against SARS-CoV-2 do not seek to differentiate between the different classes of Ig. At the time of writing, only one LFI has been granted FDA-EUA status for the detection of total antibody against SARS-CoV-2. The WANTAI SARS-CoV-2 Ab Rapid Test [51] uses the double-antigen sandwich format wherein RBD of the S protein is employed as both capture and detector agents. Antibodies that bind to the antigens coated on the test line and to the gold conjugates will result in a signal generation (Figure 1e).
Table 3.
Characteristics of FDA-EUA serological LFIs for the detection of antibodies against SARS-CoV-2.
| Developer | Test Name | Serological Marker | Antigenic Target(s) | Time-to-Result | Sample Indicated for Testing | Combined Sensitivity (95% CI) | Combined Specificity (95% CI) | Authorized Setting(s) |
|---|---|---|---|---|---|---|---|---|
| Innovita (Tangshan) Biological Technology Co., Ltd. | Innovita 2019-nCoV Ab Test (Colloidal Gold) | IgM and IgG | S1 and N | 10–15 min | Serum, plasma, venous whole blood | 93.3% (78.7–98.2%) | 98.8% (93.3–99.8%) | H, M |
| Megna Health, Inc. | Rapid COVID-19 IgM/IgG Combo Test Kit | IgM and IgG | S1 and N | 15–20 min | Serum, plasma, fingerprick whole blood | 100% (88.7–100%) | 95% (87.8–98%) | H, M, W |
| Jiangsu Well Biotech Co., Ltd. | Orawell IgM/IgG Rapid Test | IgM and IgG | RBD | 10–15 min | Serum, plasma | 100% (93.8–100%) | 94.8% (88.5–97.8%) | H, M |
| QIAGEN, GmbH | QIAreach Anti-SARS-CoV-2 Total Test | Total Antibody | S1 | 10 min | Serum, plasma | 100% (88.7–100%) | 97.5% (91.3–99.3%) | H, M |
| Hangzhou Laihe Biotech Co., Ltd. | LYHER Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Combo Test Kit (Colloidal Gold) | IgM and IgG | S1 | 10–15 min | Serum, plasma | 100% (88.7–100%) | 98.8% (93.3–99.8%) | H, M |
| NOWDiagnostics, Inc. | ADEXUSDx COVID-19 Test | Total Antibody | RBD | 15–30 min | Serum, plasma, venous whole blood, fingerprick whole blood | 93.3% (78.7–98.2%) | 100% (95.4–100%) | H, M, W |
| Assure Tech. (Hangzhou Co., Ltd.) | Assure COVID-19 IgG/IgM Rapid Test Device | IgM and IgG | S1 and N | 15–30 min | Serum, plasma, venous whole blood, fingerprick whole blood | 100% (88.7–100%) | 98.8% (93.3–98.8%) | H, M, W |
| Healgen Scientific LLC | COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) | IgM and IgG | S1 | 10–15 min | Serum, plasma, venous whole blood | 100% (88.7–100%) | 97.5% (91.3–99.3%) | H, M |
| Biohit Healthcare (Hefei) Co. Ltd. | Biohit SARS-CoV-2 IgM/IgG Antibody Test Kit | IgM and IgG | N | 15–20 min | Serum, plasma, venous whole blood | 96.7% (83.3–99.4%) | 95% (87.8–98%) | H, M |
| ACON Laboratories, Inc. | ACON SARS-CoV-2 IgG/IgM Rapid Test | IgM and IgG | - | 15–20 min | Serum, plasma, venous whole blood | 100% (88.7–100%) | 97.5% (91.3–99.3%) | H, M |
| NanoEntek America, Inc. | FREND COVID-19 total Ab | Total Antibody | N | 3–4 min | Plasma | 96.7% (83.3–99.4%) | 98.8% (93.3–99.8%) | H, M |
| Sugentech, Inc. | SGTi-flex COVID-19 IgG | IgG | N and RBD | 10–30 min | Serum, plasma, venous whole blood, fingerprick whole blood | 96.7% (83.3–99.4%) | 100% (95.4–100%) | H, M, W |
| Nirmidas Biotech, Inc. | MidaSpot COVID-19 Antibody Combo Detection Kit | IgM and IgG | - | 18–25 min | Serum, plasma, fingerprick whole blood | 100% (88.7–100%) | 96.2% (89.5–98.7%) | H, M, W |
| InBios International, Inc. | SCoV-2 Detect IgG Rapid Test | IgG | S | 20–25 min | Serum, plasma, venous whole blood, fingerprick whole blood | 100% (88.7–100%) | 100% (95.4–100%) | H, M, W |
| Access Bio, Inc. | CareStart COVID-19 IgM/IgG | IgM and IgG | N and RBD | 10–15 min | Serum, plasma, venous whole blood, fingerprick whole blood | 100% (88.7–100%) | 97.5% (91.3–99.3%) | H, M, W |
| Diabetomics, Inc. | CovAb SARS-CoV-2 Ab Test | Total Antibody | S1 | 15–20 min | Oral fluid (gingival crevicular fluid) | - | - | H, M, W |
| Salofa Oy | Sienna-Clarity COVIBLOCK COVID-19 IgG/IgM Rapid Test Cassette | IgM and IgG | RBD | 10–20 min | Serum, plasma, venous whole blood, fingerprick whole blood | 93.3% (78.7–98.2%) | 98.8% (93.3–99.8%) | H, M, W |
| Access Bio, Inc. | CareStart EZ COVID-19 IgM/IgG | IgM and IgG | N and RBD | 15–20 min | Serum, plasma, venous whole blood, fingerprick whole blood | 100% (88.7–100%) | 100% (95.4–100%) | H, M, W |
| ADVAITE, Inc. | RapCov Rapid COVID-19 Test | IgG | N | 15–20 min | fingerprick whole blood | 90% (73.6–97.3%) | 95.2% (89.2–97.9%) | H, M, W |
| Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. | WANTAI SARS-CoV-2 Ab Rapid Test | Total Antibody | RBD | 15–20 min | Serum, plasma, venous whole blood | 100% (88.7–100%) | 98.8% (93.3–99.8%) | H, M |
| Hangzhou Biotest Biotech Co., Ltd. | RightSign COVID-19 IgG/IgM Rapid Test Cassette | IgM and IgG | RBD | 10–20 min | Serum, plasma, venous whole blood, fingerprick whole blood | 100% (88.7–100%) | 100% (95.4–100%) | H, M, W |
| Xiamen Biotime Biotechnology Co., Ltd. | BIOTIME SARS-CoV-2 IgG/IgM Rapid Qualitative Test | IgM and IgG | - | 20–30 min | Serum, plasma, venous whole blood | 96.7% (83.3–99.4%) | 97.5% (91.3–99.3%) | H, M |
| Nirmidas Biotech, Inc. | Nirmidas COVID-19 (SARS-CoV-2) IgM/IgG Antibody Detection Kit | IgM and IgG | S1 and RBD | 10–15 min | Serum, plasma | - | - | H, M |
| TBG Biotechnology Corp. | TBG SARS-CoV-2 IgG/IgM Rapid Test Kit | IgM and IgG | S and N | 15–20 min | Serum, plasma | 93.3% (78.7–98.2%) | 95% (87.8–98%) | H, M |
| Biocan Diagnostics Inc. | Tell Me Fast Novel Coronavirus (COVID-19) IgG/IgM Antibody Test | IgM and IgG | S and N | 10–15 min | Serum, plasma, venous whole blood | 100% (94.9–100%) | 99.4% (96.6–99.9%) | H, M |
4.2. Specimen Type
The types of specimens indicated for use with the FDA-EUA serological LFIs include venous whole blood, serum, plasma, oral fluid and/or finger-prick whole blood. While the time of specimen collection is not stated in the IFU of most FDA-EUA serological LFIs, there are some LFIs that are only intended for specimens that were collected after a specific period of time following the onset of symptoms and these time periods ranged from >7 days [52,53] to >14 days [54] after symptom onset. Among the indicated specimens, fingerprick whole blood is the easiest to be obtained without the need for a phlebotomist but only 11 serological LFIs are designed to accept fingerprick whole blood at the time of writing. The fingerprick whole blood has to be tested immediately, whereas the remaining abovementioned specimen types may be stored for a given duration at appropriate temperatures. Of note, only one serological LFI [55] is designed to work with oral fluid (gingival crevicular fluid). On the other hand, venous whole blood is an acceptable specimen in more than half of the FDA-EUA serological LFIs and it is collected using standard phlebotomy protocols into a blood collection tube containing anticoagulant such as sodium citrate, sodium heparin, and dipotassium EDTA. Since whole blood is only recommended to be stored at 2 to 8 °C for 2 to 3 days and not frozen for prolonged storage, the immediate collection of plasma is required if the test could not be run within 2 to 3 days of collection. The plasma can then be stored frozen at −20 °C or lower for one month.
4.3. Serological Marker
Given that IgM is generally the first class of Ig to be produced in response to an infection before class-switching to IgG, the presence of SARS-CoV-2-specific IgM provides an indication that the tested individual is at the early stage of infection. Nevertheless, IgM seroconversion occurring later than that of IgG and synchronous seroconversion of IgG and IgM among COVID-19 patients have also been observed [56]. The pentameric IgM has ten antigen-binding sites that contribute to its higher avidity towards antigen although the affinity is lower than that of IgG [57]. In contrast, IgG has two antigen-binding sites, exhibits higher specificity than IgM and is usually detectable after years of infection. In addition to IgM and IgG, IgA is also a potential marker for serological identification of SARS-CoV-2 infection. The kinetics of IgA was investigated in a longitudinal study of 19 COVID-19 patients and IgA levels were found to be consistently higher and persisted longer than IgM [46].
The immune response towards SARS-CoV-2 is generally similar to that of SARS-CoV although significant time dependences have been observed between the two viruses. After infection with SARS-CoV-2, IgM level peaks around 14 days post-symptom onset followed by a rapid declination in the third week whereas with SARS-CoV, the IgM peaks around three weeks post-symptom onset [58,59]. SARS-CoV-specific IgG also peaked later (around the fifth week of post-symptom onset) as compared to SARS-CoV-2-specific IgG that peaks around the second or third week post-symptom onset and remained high up to the fifth week [58,59]. In a study involving 285 COVID-19 patients, the median day of seroconversion for both IgG and IgM was 13 days post-symptom onset with 100% IgG seroconversion and 94.1% IgM seroconversion observed within 19 and 22 days post-symptom onset, respectively [56].
The N and S proteins of SARS-CoV-2 are known to be highly immunogenic and the main targets for antibody responses. A profiling study of anti-SARS-CoV-2 antibody seroconversion against the N protein revealed that IgM, IgA and IgG levels increased gradually within 1 to 3 weeks post-symptom onset with IgM and IgA peaking in the second and first week, respectively, whereas IgG continued to increase before reaching a plateau in the third week [60]. Notably, IgM, IgA and IgG could be detected as early as day 1 post-symptom onset, but the median time of appearance of IgM and IgA was at day 5 while for IgG was at day 14 [60]. A similar anti-SARS-CoV-2 antibody profile against the N and S proteins was described in another study. IgM and IgG were observed to share a similar dynamic pattern and level in the first two weeks post-symptom onset before IgG level continued to increase and surpassed that of IgM in the third week [61]. A meta-analysis has showed that the combined detection of IgG and IgM in LFIs resulted in greater sensitivity (78–83%) as compared to IgM and IgG alone (53–66%) but remained lower than those of ELISA- and chemiluminescent immunoassay-based tests (90–96%) [62]. In a separate study, greater sensitivity was attained by detecting total antibody against SARS-CoV-2 as compared to the detection of IgM and IgG alone or in combination [63]. However, less information may be derived from assays that do not distinguish the classes of Ig that were detected.
A previous study on SARS patients found that the antibody response was frequently and predominantly directed to the N protein instead of the S protein [64]. Compared to antibodies against other viral components (S, E and M proteins), anti-N antibodies were found to be more persistent and occurred in greater abundance [65]. Results from the study by Burbelo et al. [66] indicated that the detection of anti-N antibodies may be more sensitive for early identification of the infection as compared to those of the S protein counterparts, although contrasting results were reported in other studies [67,68]. The combined detection of N and S proteins by their IgM and IgG can potentially increase the SARS-CoV-2 detection rate in early infections [61]. Although cross-reactivity of anti-N and anti-S antibodies between SARS-CoV and SARS-CoV-2 have been reported in multiple studies [40,56,60,69] due to the high level of shared amino acid sequence identity (N, 90%; S, 77%), false-positive results due to cross-reactivity of SARS-CoV-specific antibodies has been postulated to be unlikely given that there were no SARS outbreaks since 2003 and as such, SARS-CoV-specific antibodies are unlikely to be present in the population [40].
4.4. Performance
The performance of some FDA-EUA LFIs in detecting anti-SARS-CoV-2 antibodies has been evaluated in several studies. The sensitivity of Healgen COVID-19 IgG/IgM Rapid Test Cassette was found to range from 67.7 to 100% with a specificity of 99% [70,71,72] whereas Biohit SARS-CoV-2 IgM/IgG Antibody Test Kit had a sensitivity and specificity of 94.6% (95% CI: 87.8–100%) and 92.6% (95% CI: 84.7–100%), respectively [73]. In another study, the WANTAI SARS-CoV-2 Ab Rapid Test was found to have a sensitivity and specificity of 83.1% (95% CI: 72.0–90.5%) and 98.0% (95% CI: 94.7–99.4%), respectively [70]. Greater variations in performance were observed for Innovita SARS-CoV-2 IgG/IgM antibody test kit whereby sensitivity and specificity of the assay ranged from 50 to 93% and 49 to 91%, respectively [74,75,76]. The comparison of sensitivity and specificity of various commercial LFIs for the detection of anti-SARS-CoV-2 antibodies is presented in Supplementary Figure S1 with the details available in Supplementary Table S2. Similar to antigen-detecting LFIs, the sensitivity of these serological LFIs varied greatly but specificity was more than 90% for most of the serological LFIs.
5. Conclusions and Future Perspective
As COVID-19 transitions from pandemic to endemic, the emergence of new variants of SARS-CoV-2 is expected [77]. Despite the significant progress that has been achieved in the development of medication, vaccines and diagnostics in the fight against COVID-19, new variants with increased transmissibility and/or disease severity can lead to a rapid surge in cases and quickly strain the finite healthcare resources. Accurate and rapid diagnosis continue to a crucial component to control COVID-19 outbreaks and to minimize the detrimental impacts on the healthcare system and economy. Although nucleic acid-based RT-PCR remained as the gold-standard diagnostic test, wide adoption and implementation of this technically intricate and equipment-dependent test are greatly hindered in countries and/or regions with weak or scarce laboratory infrastructure. Hence, the LFI platform provides an avenue to increase the capacity of COVID-19 testing and, more importantly, the rapid test can be deployed in both laboratory and non-laboratory settings and costs only a fraction of the price of RT-PCR test.
The availability of LFIs for the general population to perform self-testing at home has led to a tremendous increase in accessibility to COVID-19 testing while simultaneously allows appropriate containment measures to be taken early on to minimize the spread of COVID-19. Whereas LFI that detects SARS-CoV-2 antigen facilitate the diagnosis of infection early in its course, serological LFI can supplements nucleic acid-based diagnostics and assists in contact tracing, particularly among asymptomatic individuals. The feasibility of using LFI for SARS-CoV-2 detection by self-testing at home has been investigated in a large-scale study involving 1022 participants. The finding that 96% and 97% of the participants were able to perform the test without supervision and obtained a valid test result, respectively, advocates the suitability of LFI for mass self-testing [78]. The many advantages of LFI, which appeal to both end-users and manufacturers, make it a tool of choice in the field of point-of-care diagnostics but the platform is not without its own drawbacks. A major disadvantage of LFI is the subjective interpretation of the test result whereby ambiguity may arise in the interpretation of weak positive result even if the end users were skilled. A dedicated reader that is capable of imaging and quantifying the LFI result can be used to overcome operator bias [79] but this may lead to a substantial increase in the assay cost. Recent efforts to circumvent the need for a specialized readout device for LFI have focused on repurposing the smartphone as a reader by leveraging the phone’s built-in camera and capability to run mobile applications [80]. Furthermore, the smartphone also holds the potential for a test result to be uploaded in real-time to health-care related information systems to facilitate rapid reporting or for electronic record keeping purposes, but an active internet connection would become a requirement. The Ellume COVID-19 Home Test represents one such example, wherein the test incorporates the use of a smartphone (but not its camera) and a mobile application (Ellume COVID-19 Test App) to connect with the analyzer that is housed within the lateral flow device.
Another challenge associated with LFI lies in the improvement of the sensitivity and specificity of the assay. Some of the strategies that have been proposed focused on replacing colloidal gold as the signal generator and these include the use of lanthanide-doped polystyrene nanoparticles [79,81], multi-functional nanocomposite with a combination of magnetic-adhesion-color-nanozyme properties [82], and composite polymer beads that capitalize on two-wavelength imaging [83]. A nanoelectrokinetic-based sample enrichment step prior to the LFI [84] was also recently described but all these methods entailed the use of additional devices. Other strategies that have successfully increased the sensitivity of LFI without compromising the simplicity and practically of the platform were directed towards promoting the formation of the immune-complex on the lateral flow strip itself. For example, the addition of a macromolecular crowding agent, such as Ficoll MW 400,000 and Ficoll MW 70,000, led to a 5–10-fold improvement of the signal on commercially available LFIs [85]. A soluble time-delay wax barrier that selectively and temporarily accumulate the target and label nanoparticles on top of the test line was reported to generate a 51.7-fold and 96% enhancement in sensitivity and signal, respectively. Enhancement in the sensitivity and LoD of the LFI may also be attained via manufacturing technique. The laser-direct write technique, which was used to dispense a liquid photopolymer at specific regions of the nitrocellulose membrane, followed by photopolymerization to create impermeable walls inside the volume of the membrane was demonstrated to improve sensitivity and LoD by 62 and 30 times, respectively, as compared to conventional LFI [86]. Although LFI is a relatively old technology, various modifications and strategies have been described to improve its performance especially for clinical applications [87]. The adoption of these advances may improve the robustness, reliability and performance of LFI as a diagnostic tool beyond the COVID-19 pandemic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12112854/s1, Figure S1: Sensitivity and specificity of the commercial LFIs for the detection of antibody against SARS-CoV-2, Table S1: Comparison of performance evaluation between commercial LFIs for the detection of SARS-CoV-2 antigen [25,41,42,43,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113], Table S2: Comparison of performance evaluation between commercial serology LFIs for the detection of antibody against SARS-CoV-2 [70,71,72,73,74,75,76,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131].
Author Contributions
Conceptualization, C.Y.Y. (Choo Yee Yu), K.G.C., C.Y.Y. (Chan Yean Yean) and G.Y.A.; formal analysis, C.Y.Y. (Choo Yee Yu) and G.Y.A.; resources, C.Y.Y. (Choo Yee Yu), K.G.C., C.Y.Y. (Chan Yean Yean) and G.Y.A.; writing—original draft preparation, C.Y.Y. (Choo Yee Yu) and G.Y.A.; writing—review and editing, C.Y.Y. (Choo Yee Yu), K.G.C., C.Y.Y. (Chan Yean Yean) and G.Y.A.; visualization, C.Y.Y. (Choo Yee Yu) and G.Y.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Weekly Epidemiological Update on COVID-19. [(accessed on 5 October 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19.
- 2.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAloon C., Collins A., Hunt K., Barber A., Byrne A.W., Butler F., Casey M., Griffin J., Lane E., McEvoy D., et al. Incubation period of COVID-19: A rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10:e039652. doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu C.Y., Chan K.G., Yean C.Y., Ang G.Y. Nucleic Acid-Based Diagnostic Tests for the Detection SARS-CoV-2: An Update. Diagnostics. 2021;11:53. doi: 10.3390/diagnostics11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan K.G., Ang G.Y., Yu C.Y., Yean C.Y. Harnessing CRISPR-Cas to Combat COVID-19: From Diagnostics to Therapeutics. Life. 2021;11:1210. doi: 10.3390/life11111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kettler H., White K., Hawkes S.J. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections: Key Findings and Recommendations. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 11.World Health Organization Serology and Early Investigation Protocols. 2020. [(accessed on 5 October 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/serology-in-the-context-of-covid-19#:~:text=The%20use%20of%20serology%20in,of%20infection%20among%20different%20populations%3B&text=the%20proportion%20of%20the%20population,against%20infection%20in%20the%20future.
- 12.Castrejon-Jimenez N.S., Garcia-Perez B.E., Reyes-Rodriguez N.E., Vega-Sanchez V., Martinez-Juarez V.M., Hernandez-Gonzalez J.C. Challenges in the Detection of SARS-CoV-2: Evolution of the Lateral Flow Immunoassay as a Valuable Tool for Viral Diagnosis. Biosensors. 2022;12:728. doi: 10.3390/bios12090728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filchakova O., Dossym D., Ilyas A., Kuanysheva T., Abdizhamil A., Bukasov R. Review of COVID-19 testing and diagnostic methods. Talanta. 2022;244:123409. doi: 10.1016/j.talanta.2022.123409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ince B., Sezginturk M.K. Lateral flow assays for viruses diagnosis: Up-to-date technology and future prospects. Trends Analyt. Chem. 2022;157:116725. doi: 10.1016/j.trac.2022.116725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey S.K., Mohanta G.C., Kumar V., Gupta K. Diagnostic Tools for Rapid Screening and Detection of SARS-CoV-2 Infection. Vaccines. 2022;10:1200. doi: 10.3390/vaccines10081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang G.Y., Yu C.Y., Chan Y.Y. The hurdles in making diagnostics accessible to the bottom billions: Can lateral flow immunoassays make a difference? In: Asma I., Norazmi M.N., Jafri M.A., Armando A., Maria E.S., editors. Sustainable Diagnostics for Low Resources Areas. Penerbit Universiti Sains Malaysia; Penang, Malaysia: 2017. [Google Scholar]
- 17.Merck Millipore . Rapid Lateral Flow Test Strips: Considerations for Product Development. EMD Millipore Corporation; Billerica, MA, USA: 2013. [Google Scholar]
- 18.Seydack M. Nanoparticle labels in immunosensing using optical detection methods. Biosens. Bioelectron. 2005;20:2454–2469. doi: 10.1016/j.bios.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Thobhani S., Attree S., Boyd R., Kumarswami N., Noble J., Szymanski M., Porter R.A. Bioconjugation and characterisation of gold colloid-labelled proteins. J. Immunol. Methods. 2010;356:60–69. doi: 10.1016/j.jim.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Paek S.H., Lee S.H., Cho J.H., Kim Y.S. Development of rapid one-step immunochromatographic assay. Methods. 2000;22:53–60. doi: 10.1006/meth.2000.1036. [DOI] [PubMed] [Google Scholar]
- 21.Posthuma-Trumpie G.A., Korf J., van Amerongen A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration Home Diagnostic Test for COVID-19. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-antigen-test-first-over-counter-fully-home-diagnostic.
- 23.Centers for Disease Control and Prevention Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. [(accessed on 5 October 2022)];2020 Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html.
- 24.Centers for Disease Control and Prevention Interim Guidance for Antigen Testing for SARS-CoV-2. [(accessed on 5 October 2022)];2020 Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html.
- 25.Mak G.C., Lau S.S., Wong K.K., Chow N.L., Lau C.S., Lam E.T., Chan R.C., Tsang D.N. Analytical sensitivity and clinical sensitivity of the three rapid antigen detection kits for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;133:104684. doi: 10.1016/j.jcv.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T., Chan R.C., Tsang D.C. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;129:104500. doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diao B., Wen K., Chen J., Liu Y., Yuan Z., Han C., Chen J., Pan Y., Chen L., Dan Y., et al. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv. 2020 doi: 10.1101/2020.03.07.20032524. [DOI] [Google Scholar]
- 28.McAulay K., Kaleta E., Grys T. Rapid Detection of SARS-CoV-2 Antigen from Serum in a Hospitalized Population. medRxiv. 2020 doi: 10.1101/2020.12.21.20248140. [DOI] [Google Scholar]
- 29.Di B., Hao W., Gao Y., Wang M., Wang Y.-D., Qiu L.-W., Wen K., Zhou D.-H., Wu X.-W., Lu E.-J., et al. Monoclonal antibody-based antigen capture enzyme-linked immunosorbent assay reveals high sensitivity of the nucleocapsid protein in acute-phase sera of severe acute respiratory syndrome patients. Clin. Diagn. Lab. Immunol. 2005;12:135–140. doi: 10.1128/CDLI.12.1.135-140.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Che X.-Y., Hao W., Wang Y., Di B., Yin K., Xu Y.-C., Feng C.-S., Wan Z.-Y., Cheng V.C., Yuen K.-Y. Nucleocapsid protein as early diagnostic marker for SARS. Emerg. Infect. Dis. 2004;10:1947–1949. doi: 10.3201/eid1011.040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutta N.K., Mazumdar K., Gordy J.T. The Nucleocapsid Protein of SARS–CoV-2: A Target for Vaccine Development. J. Virol. 2020;94:e00647-20. doi: 10.1128/JVI.00647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luminostics Clip COVID Rapid Antigen Test. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/144256/download.
- 33.Quidel Sofia 2 Flu + SARS Antigen FIA. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/142704/download.
- 34.Hirotsu Y., Maejima M., Shibusawa M., Amemiya K., Nagakubo Y., Hosaka K., Sueki H., Hayakawa M., Mochizuki H., Tsutsui T., et al. Analysis of a persistent viral shedding patient infected with SARS-CoV-2 by RT-qPCR, FilmArray Respiratory Panel v2.1, and antigen detection. J. Infect. Chemother. 2020;27:406–409. doi: 10.1016/j.jiac.2020.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirotsu Y., Maejima M., Shibusawa M., Nagakubo Y., Hosaka K., Amemiya K., Sueki H., Hayakawa M., Mochizuki H., Tsutsui T., et al. Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients. Int. J. Infect. Dis. 2020;99:397–402. doi: 10.1016/j.ijid.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock N.R., Savage T.J., Wardell H., Lee R., Mathew A., Stengelin M., Sigal G.B. Correlation of SARS-CoV-2 nucleocapsid antigen and RNA concentrations in nasopharyngeal samples from children and adults using an ultrasensitive and quantitative antigen assay. medRxiv. 2020 doi: 10.1128/JCM.03077-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng W., Liu G., Ma H., Zhao D., Yang Y., Liu M., Mohammed A., Zhao C., Yang Y., Xie J., et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem. Biophys. Res. Commun. 2020;527:618–623. doi: 10.1016/j.bbrc.2020.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman M.S., Islam M.R., Alam A., Islam I., Hoque M.N., Akter S., Rahaman M.M., Sultana M., Hossain M.A. Evolutionary dynamics of SARS-CoV-2 nucleocapsid protein and its consequences. J. Med. Virol. 2020;93:2177–2195. doi: 10.1002/jmv.26626. [DOI] [PubMed] [Google Scholar]
- 39.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D., et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perchetti G.A., Huang M.-L., Mills M.G., Jerome K.R., Greninger A.L. Analytical Sensitivity of the Abbott BinaxNOW COVID-19 Ag CARD. J. Clin. Microbiol. 2020;59:e02880-20. doi: 10.1128/JCM.02880-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilarowski G., Lebel P., Sunshine S., Liu J., Crawford E., Marquez C., Rubio L., Chamie G., Martinez J., Peng J., et al. Performance characteristics of a rapid SARS-CoV-2 antigen detection assay at a public plaza testing site in San Francisco. medRxiv. 2020 doi: 10.1101/2020.11.02.20223891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Moeren N., Zwart V.F., Lodder E.B., Van den Bijllaardt W., Van Esch H.R.J.M., Stohr J.J.J.M., Pot J., welschen I., Van Mechelen P.M.F., Pas S.D., et al. Evaluation of the test accuracy of a SARS-CoV-2 rapid antigen test in symptomatic community dwelling individuals in the Netherlands. PLoS ONE. 2021;16:e0250886. doi: 10.1371/journal.pone.0250886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ota M. Will we see protection or reinfection in COVID-19? Nat. Rev. Immunol. 2020;20:351. doi: 10.1038/s41577-020-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao L., Deng W., Gao H., Xiao C., Liu J., Xue J., Lv Q., Liu J., Yu P., Xu Y., et al. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.03.13.990226. [DOI] [Google Scholar]
- 46.Padoan A., Sciacovelli L., Basso D., Negrini D., Zuin S., Cosma C., Faggian D., Matricardi P., Plebani M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin. Chim. Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Access Bio CareStart COVID-19 IgM/IgG. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/140447/download.
- 48.ADVAITE Inc. RapCov Rapid COVID-19 Test. [(accessed on 5 October 2022)];2021 Available online: https://www.fda.gov/media/145080/download.
- 49.InBios International SCoV Detect IgG Rapid Test. [(accessed on 5 October 2022)];2021 Available online: https://www.fda.gov/media/151817/download.
- 50.Sugentech SGTi-flex COVID-19 IgG. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/141891/download.
- 51.Beijing Wantai Biological Pharmacy WANTAI SARS-CoV-2 Ab Rapid Test. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/140030/download.
- 52.Biotech N. MidaSpot COVID-19 Antibody Combo Detection Kit. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/144877/download.
- 53.ACON Laboratories ACON SARS-CoV-2 IgG/IgM Rapid Test. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/144562/download.
- 54.TBG Biotechnology TBG SARS-CoV-2 IgG/IgM Rapid Test Kit. [(accessed on 5 October 2022)];2020 Available online: https://www.fda.gov/media/141773/download.
- 55.Diabetom ics CovAb SARS-CoV-2 Ab Test. [(accessed on 5 October 2022)];2021 Available online: https://www.fda.gov/media/149943/download.
- 56.Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 57.Schroeder H.W., Jr., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125((Suppl. 2)):S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li G., Chen X., Xu A. Profile of Specific Antibodies to the SARS-Associated Coronavirus. N. Engl. J. Med. 2003;349:508–509. doi: 10.1056/NEJM200307313490520. [DOI] [PubMed] [Google Scholar]
- 59.Woo P.C.Y., Lau S.K.P., Wong B.H.L., Chan K.-H., Chu C.-M., Tsoi H.-W., Huang Y., Peiris J.S.M., Yuen K.-Y. Longitudinal Profile of Immunoglobulin G (IgG), IgM, and IgA Antibodies against the Severe Acute Respiratory Syndrome (SARS) Coronavirus Nucleocapsid Protein in Patients with Pneumonia Due to the SARS Coronavirus. Clin. Diagn. Lab. Immunol. 2004;11:665. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., Dela Cruz C.S., Wang Y., Wu C., Xiao Y., et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19) Clin. Infect. Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., Peng P., Liu X., Chen Z., Huang H., et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kontou P.I., Braliou G.G., Dimou N.L., Nikolopoulos G., Bagos P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics. 2020;10:319. doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lou B., Li T.-D., Zheng S.-F., Su Y.-Y., Li Z.-Y., Liu W., Yu F., Ge S.-X., Zou Q.-D., Yuan Q., et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur. Respir. J. 2020;56:2000763. doi: 10.1183/13993003.00763-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leung D.T., Tam F.C., Ma C.H., Chan P.K., Cheung J.L., Niu H., Tam J.S., Lim P.L. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J. Infect. Dis. 2004;190:379–386. doi: 10.1086/422040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li D., Li J. Immunologic testing for SARS-CoV-2 infection from the antigen perspective. J. Clin. Microbiol. 2020;59:e02160-20. doi: 10.1128/JCM.02160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burbelo P.D., Riedo F.X., Morishima C., Rawlings S., Smith D., Das S., Strich J.R., Chertow D.S., Davey R.T., Cohen J.I. Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019. J. Infect. Dis. 2020;222:206–213. doi: 10.1093/infdis/jiaa273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L. Wu, W.; Tang, S.; et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58:e00461-20. doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lv H., Wu N.C., Tak-Yin Tsang O., Yuan M., Perera R.A.P.M., Leung W.S., So R.T.Y., Chun Chan J.M., Yip G.K., Hong Chik T.S., et al. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020;31:107725. doi: 10.1016/j.celrep.2020.107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tollånes M.C., Jenum P.A., Kierkegaard H., Abildsnes E., Bævre-Jensen R.M., Breivik A.C., Sandberg S. Evaluation of 32 rapid tests for detection of antibodies against SARS-CoV-2. Clin. Chim. Acta. 2021;519:133–139. doi: 10.1016/j.cca.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., Bogers S., Embregts C.W.E., Laksono B.M., Leijten L., Rokx C., Rijnders B., Rahamat-Langendoen J., et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. Commun. 2020;11:3436. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lagerqvist N., Maleki K.T., Verner-Carlsson J., Olausson M., Dillner J., Wigren Byström J., Monsen T., Forsell M., Eriksson J., Bogdanovic G., et al. Evaluation of 11 SARS-CoV-2 antibody tests by using samples from patients with defined IgG antibody titers. Sci. Rep. 2021;11:7614. doi: 10.1038/s41598-021-87289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trombetta B.A., Kandigian S.E., Kitchen R.R., Grauwet K., Webb P.K., Miller G.A., Jennings C.G., Jain S., Miller S., Kuo Y., et al. Evaluation of serological lateral flow assays for severe acute respiratory syndrome coronavirus-2. BMC Infect. Dis. 2021;21:580. doi: 10.1186/s12879-021-06257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Liu Y.-P., Diao B., Ding J.-Y., Yuan M.-X., Ren F.-F., Wang Y., Huang Q.-C. Diagnostic indexes of a rapid immunoglobulin G/immunoglobulin M combined antibody test for severe acute respiratory syndrome coronavirus 2. Chin. Med. J. 2021;134:475–477. doi: 10.1097/CM9.0000000000001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yong G., Yi Y., Tuantuan L., Xiaowu W., Xiuyong L., Ang L., Mingfeng H. Evaluation of the auxiliary diagnostic value of antibody assays for the detection of novel coronavirus (SARS-CoV-2) J. Med. Virol. 2020;92:1975–1979. doi: 10.1002/jmv.25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boum Y., Fai K.N., Nicolay B., Mboringong A.B., Bebell L.M., Ndifon M., Abbah A., Essaka R., Eteki L., Luquero F., et al. Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: A clinical, prospective, diagnostic accuracy study. Lancet Infect. Dis. 2021;21:1089–1096. doi: 10.1016/S1473-3099(21)00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu C.Y., Wong S.Y., Liew N.W.C., Joseph N., Zakaria Z., Nurulfiza I., Soe H.J., Kairon R., Amin-Nordin S., Chee H.Y. Whole genome sequencing analysis of SARS-CoV-2 from Malaysia: From alpha to Omicron. Front. Med. 2022;9:1001022. doi: 10.3389/fmed.2022.1001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iruzubieta P., Fernández-Lanas T., Rasines L., Cayon L., Álvarez-Cancelo A., Santos-Laso A., García-Blanco A., Curiel-Olmo S., Cabezas J., Wallmann R., et al. Feasibility of large-scale population testing for SARS-CoV-2 detection by self-testing at home. Sci. Rep. 2021;11:9819. doi: 10.1038/s41598-021-89236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng M., Chen J., Xun J., Dai R., Zhao W., Lu H., Xu J., Chen L., Sui G., Cheng X. Development of a Sensitive Immunochromatographic Method Using Lanthanide Fluorescent Microsphere for Rapid Serodiagnosis of COVID-19. ACS Sens. 2020;5:2331–2337. doi: 10.1021/acssensors.0c00927. [DOI] [PubMed] [Google Scholar]
- 80.Tian R., Ji J., Zhou Y., Du Y., Bian X., Zhu F., Liu G., Deng S., Wan Y., Yan J. Terminal-conjugated non-aggregated constraints of gold nanoparticles on lateral flow strips for mobile phone readouts of enrofloxacin. Biosens. Bioelectron. 2020;160:112218. doi: 10.1016/j.bios.2020.112218. [DOI] [PubMed] [Google Scholar]
- 81.Chen Z., Zhang Z., Zhai X., Li Y., Lin L., Zhao H., Bian L., Li P., Yu L., Wu Y., et al. Rapid and Sensitive Detection of anti-SARS-CoV-2 IgG, Using Lanthanide-Doped Nanoparticles-Based Lateral Flow Immunoassay. Anal. Chem. 2020;92:7226–7231. doi: 10.1021/acs.analchem.0c00784. [DOI] [PubMed] [Google Scholar]
- 82.Dou L., Bai Y., Liu M., Shao S., Yang H., Yu X., Wen K., Wang Z., Shen J., Yu W. ‘Three-To-One’ multi-functional nanocomposite-based lateral flow immunoassay for label-free and dual-readout detection of pathogenic bacteria. Biosens. Bioelectron. 2022;204:114093. doi: 10.1016/j.bios.2022.114093. [DOI] [PubMed] [Google Scholar]
- 83.Miller B.S., Thomas M.R., Banner M., Kim J., Chen Y., Wei Q., Tseng D.K., Göröcs Z.S., Ozcan A., Stevens M.M., et al. Sub-picomolar lateral flow antigen detection with two-wavelength imaging of composite nanoparticles. Biosens. Bioelectron. 2022;207:114133. doi: 10.1016/j.bios.2022.114133. [DOI] [PubMed] [Google Scholar]
- 84.Kim C., Yoo Y.K., Lee N.E., Lee J., Kim K.H., Lee S., Kim J., Park S.J., Lee D., Lee S.W., et al. Nanoelectrokinetic-assisted lateral flow assay for COVID-19 antibody test. Biosens. Bioelectron. 2022;212:114385. doi: 10.1016/j.bios.2022.114385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christopoulou N.-M., Kalogianni D.P., Christopoulos T.K. Macromolecular crowding agents enhance the sensitivity of lateral flow immunoassays. Biosens. Bioelectron. 2022;218:114737. doi: 10.1016/j.bios.2022.114737. [DOI] [PubMed] [Google Scholar]
- 86.Katis I.N., He P.J.W., Eason R.W., Sones C.L. Improved sensitivity and limit-of-detection of lateral flow devices using spatial constrictions of the flow-path. Biosens. Bioelectron. 2018;113:95–100. doi: 10.1016/j.bios.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Di Nardo F., Chiarello M., Cavalera S., Baggiani C., Anfossi L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors. 2021;21:5185. doi: 10.3390/s21155185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.García-Fiñana M., Hughes D.M., Cheyne C.P., Burnside G., Stockbridge M., Fowler T.A., Fowler V.L., Wilcox M.H., Semple M.G., Buchan I. Performance of the Innova SARS-CoV-2 antigen rapid lateral flow test in the Liverpool asymptomatic testing pilot: Population based cohort study. BMJ. 2021;374:n1637. doi: 10.1136/bmj.n1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abdelrazik A.M., Elshafie S.M., Abdelaziz H.M. Potential Use of Antigen-Based Rapid Test for SARS-CoV-2 in Respiratory Specimens in Low-Resource Settings in Egypt for Symptomatic Patients and High-Risk Contacts. Lab. Med. 2022;52:e46–e49. doi: 10.1093/labmed/lmaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corman V.M., Haage V.C., Bleicker T., Schmidt M.L., Mühlemann B., Zuchowski M., Jo W.K., Tscheak P., Möncke-Buchner E., Müller M.A., et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. The Lancet Microbe. 2021;2(7):e311-e9. Lancet Microbe. 2021;2:e311–e319. doi: 10.1016/S2666-5247(21)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weitzel T., Legarraga P., Iruretagoyena M., Pizarro G., Vollrath V., Araos R., Munita J.M., Porte L. Comparative evaluation of four rapid SARS-CoV-2 antigen detection tests using universal transport medium. Travel Med. Infect. Dis. 2021;39:101942. doi: 10.1016/j.tmaid.2020.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R., Pizarro G., Vial P., Iruretagoyena M., Dittrich S., et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mertens P., De Vos N., Martiny D., Jassoy C., Mirazimi A., Cuypers L., Wijngaert S.V.D., Monteil V., Melin P., Stoffels K., et al. Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context. Front. Med. 2020;7:225. doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambert-Niclot S., Cuffel A., Le Pape S., Vauloup-Fellous C., Morand-Joubert L., Roque-Afonso A.-M., Le Goff J., Delaugerre C. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-CoV-2 Antigen in Nasopharyngeal Swabs. J. Clin. Microbiol. 2020;58:e00977-20. doi: 10.1128/JCM.00977-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blairon L., Wilmet A., Beukinga I., Tré-Hardy M. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: Experiences of a general hospital. J. Clin. Virol. 2020;129:104472. doi: 10.1016/j.jcv.2020.104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scohy A., Anantharajah A., Bodéus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020;129:104455. doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Courtellemont L., Guinard J., Guillaume C., Giaché S., Rzepecki V., Seve A., Gubavu C., Baud K., Helloco C.L., Cassuto G.N., et al. High performance of a novel antigen detection test on nasopharyngeal specimens for diagnosing SARS-CoV-2 infection. J. Med. Virol. 2021;93:3152–3157. doi: 10.1002/jmv.26896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferguson J., Dunn S., Best A., Mirza J., Percival B., Mayhew M., Megram O., Ashford F., White T., Moles-Garcia E., et al. Validation testing to determine the sensitivity of lateral flow testing for asymptomatic SARS-CoV-2 detection in low prevalence settings: Testing frequency and public health messaging is key. PLoS Biol. 2021;19:e3001216. doi: 10.1371/journal.pbio.3001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mak G.C.K., Lau S.S.Y., Wong K.K.Y., Chow N.L.S., Lau C.S., Lam E.T.K., Chan R.C.W., Tsang D.N.C. Evaluation of rapid antigen detection kit from the WHO Emergency Use List for detecting SARS-CoV-2. J. Clin. Virol. 2020;134:104712. doi: 10.1016/j.jcv.2020.104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M.., Martínez M., Poujois S., Forqué L., Valdivia A., et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 2020;27:472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Linares M., Pérez-Tanoira R., Carrero A., Romanyk J., Pérez-García F., Gómez-Herruz P., Arroyo T., Cuadros J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020;133:104659. doi: 10.1016/j.jcv.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gremmels H., Winkel B.M.F., Schuurman R., Rosingh A., Rigter N.A.M., Rodriguez O., Ubijaan J., Wensing A.M.J., Bonten M.J.M., Hofstra L.M. Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2020;31:100677. doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fenollar F., Bouam A., Ballouche M., Fuster L., Prudent E., Colson P., Tissot-Dupont H., Million M., Drancourt M., Raoult D., et al. Evaluation of the Panbio Covid-19 rapid antigen detection test device for the screening of patients with COVID-19. J. Clin. Microbiol. 2020;59:e02589-20. doi: 10.1128/JCM.02589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bulilete O., Lorente P., Leiva A., Carandell E., Oliver A., Rojo E., Pericas P., Llobera J., COVID-19 Primary Care Research Group Panbio™ rapid antigen test for SARS-CoV-2 has acceptable accuracy in symptomatic patients in primary health care. J. Infect. 2021;82:391–398. doi: 10.1016/j.jinf.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agulló V., Fernández-González M., de la Tabla V.O., Gonzalo-Jiménez N., García J.A., Masiá M., Gutiérrez F. Evaluation of the rapid antigen test Panbio COVID-19 in saliva and nasal swabs in a population-based point-of-care study. J. Infect. 2020;82:186–230. doi: 10.1016/j.jinf.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., Corman V.M. Evaluation of a SARS-CoV-2 rapid antigen test: Potential to help reduce community spread? J. Clin. Virol. 2021;135:104713. doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nalumansi A., Lutalo T., Kayiwa J., Watera C., Balinandi S., Kiconco J., Nakaseegu J., Olara D., Odwilo E., Serwanga J., et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. International Journal of Infectious Diseases. Int. J. Infect. Dis. 2021;104:282–286. doi: 10.1016/j.ijid.2020.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cerutti F., Burdino E., Milia M.G., Allice T., Gregori G., Bruzzone B., Ghisetti V. Urgent need of rapid tests for SARS CoV-2 antigen detection: Evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J. Clin. Virol. 2020;132:104654. doi: 10.1016/j.jcv.2020.104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaimayo C., Kaewnaphan B., Tanlieng N., Athipanyasilp N., Sirijatuphat R., Chayakulkeeree M., Angkasekwinai N., Sutthent R., Puangpunngam N., Tharmviboonsri T., et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020;17:177. doi: 10.1186/s12985-020-01452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Igloi Z., Velzing J., van Beek J., van de Vijver D., Aron G., Ensing R., Benschop K., Han W., Boelsums T., Koopmans M., et al. Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands. Emerg Infect. Dis. 2021;27:1323. doi: 10.3201/eid2705.204688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamayoshi S., Sakai-Tagawa Y., Koga M., Akasaka O., Nakachi I., Koh H., Maeda K., Adachi E., Saito M., Nagai H., et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses. 2020;12:1420. doi: 10.3390/v12121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindner A.K., Nikolai O., Kausch F., Wintel M., Hommes F., Gertler M., Krüger L.J., Gaeddert M., Tobian F., Lainati F., et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021;57:2003961. doi: 10.1183/13993003.03961-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Escrivá B.F., Mochón M.D.O., González R.M., García C.S., Pla A.T., Ricart A.S., García M.M., Aranda I.T., García F.G., Cardona C.G. The effectiveness of rapid antigen test-based for SARS-CoV-2 detection in nursing homes in Valencia, Spain. J. Clin. Virol. 2021;143:104941. doi: 10.1016/j.jcv.2021.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Churiwal M., Lin K.D., Khan S., Chhetri S., Muller M.S., Tompkins K., Smith J., Litel C., Whittelsey M., Basham C., et al. Assessment of the Field Utility of a Rapid Point-of-Care Test for SARS-CoV-2 Antibodies in a Household Cohort. Am. J. Trop. Med. Hyg. 2022;106:156–159. doi: 10.4269/ajtmh.21-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velay A., Gallais F., Benotmane I., Wendling M.J., Danion F., Collange O., de Sèze J., Schmidt-Mutter C., Schneider F., Bilbault P., et al. Evaluation of the performance of SARS-CoV-2 serological tools and their positioning in COVID-19 diagnostic strategies. Diagn. Microbiol. Infect. Dis. 2020;98:115181. doi: 10.1016/j.diagmicrobio.2020.115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrey D.O., Cohen P., Meyer B., Torriani G., Yerly S., Mazza L., Calame A., Arm-Vernez I., Guessous I., Stringhini S., et al. Head-to-Head Accuracy Comparison of Three Commercial COVID-19 IgM/IgG Serology Rapid Tests. J. Clin. Med. 2020;9:2369. doi: 10.3390/jcm9082369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mercado M., Malagón-Rojas J., Delgado G., Rubio V.V., Galindo L.M., Barrera E.L.P., Gaviria P., Zabaleta G., Alarcon Z., Arévalo A., et al. Evaluation of nine serological rapid tests for the detection of SARS-CoV-2. Rev. Panam. Salud. Publica. 2020;44:e149. doi: 10.26633/RPSP.2020.149. [DOI] [Google Scholar]
- 118.Bond K., Nicholson S., Lim S.M., Karapanagiotidis T., Williams E., Johnson D., Hoang T., Sia C., Purcell D., Mordant F., et al. Evaluation of Serological Tests for SARS-CoV-2: Implications for Serology Testing in a Low-Prevalence Setting. J. Infect. Dis. 2020;222:1280–1288. doi: 10.1093/infdis/jiaa467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tuaillon E., Bolloré K., Pisoni A., Debiesse S., Renault C., Marie S., Groc S., Niels C., Pansu N., Dupuy A.M., et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J. Infect. 2020;81:e39–e45. doi: 10.1016/j.jinf.2020.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee Y., Liao C., Liu P., Cheng C., Chung M., Liu C., Chang S., Hsueh P. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J. Infect. 2020;81:e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pérez-García F., Pérez-Tanoira R., Romanyk J., Arroyo T., Gómez-Herruz P., Cuadros-González J. Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: A prospective single-center study. J. Clin. Virol. 2020;129:104473. doi: 10.1016/j.jcv.2020.104473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen B., Zheng Y., Zhang X., Zhang W., Wang D., Jin J., Lin R., Zhang Y., Zhu G., Zhu H., et al. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am. J. Transl. Res. 2020;12:1348–1354. [PMC free article] [PubMed] [Google Scholar]
- 123.Hoffman T., Nissen K., Krambrich J., Rönnberg B., Akaberi D., Esmaeilzadeh M., Salaneck E., Lindahl J., Lundkvist A. Evaluation of a COVID-19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS-CoV-2. Infect. Ecol. Epidemiol. 2020;10:1754538. doi: 10.1080/20008686.2020.1754538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dellière S., Salmona M., Minier M., Gabassi A., Alanio A., Le Goff J., Delaugerre C., Chaix M.-L. Evaluation of the COVID-19 IgG/IgM Rapid Test from Orient Gene Biotech. J. Clin. Microbiol. 2020;58:e01233-20. doi: 10.1128/JCM.01233-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J., Long X., Guo S., Zhao Z., Liu Y., et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J. Infect. 2020;81:e28–e32. doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Imai K., Tabata S., Ikeda M., Noguchi S., Kitagawa Y., Matuoka M., Miyoshi K., Tarumoto N., Sakai J., Ito T., et al. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J. Clin. Virol. 2020;128:104393. doi: 10.1016/j.jcv.2020.104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiang J., Yan M., Li H., Liu T., Lin C., Huang S., Shen C. Evaluation of Enzyme-Linked Immunoassay and Colloidal Gold-Immunochromatographic Assay Kit for Detection of Novel Coronavirus (SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19) MedRxiv. 2020 doi: 10.1101/2020.02.27.20028787. [DOI] [Google Scholar]
- 128.Cassaniti I., Novazzi F., Giardina F., Salinaro F., Sachs M., Perlini S., Bruno R., Mojoli F., Baldanti F. Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J. Med. Virol. 2020;92:1724–1727. doi: 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paradiso A.V., De Summa S., Loconsole D., Procacci V., Sallustio A., Centrone F., Silvestris N., Cafagna V., De Palma G., Tufaro A., et al. Rapid Serological Assays and SARS-CoV-2 Real-Time Polymerase Chain Reaction Assays for the Detection of SARS-CoV-2: Comparative Study. J. Med. Internet. Res. 2020;22:e19152. doi: 10.2196/19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spicuzza L., Montineri A., Manuele R., Crimi C., Pistorio M.P., Campisi R., Vancheri C., Crimi N. Reliability and usefulness of a rapid IgM-IgG antibody test for the diagnosis of SARS-CoV-2 infection: A preliminary report. J. Infect. 2020;81:e53–e54. doi: 10.1016/j.jinf.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Montesinos I., Gruson D., Kabamba B., Dahma H., van den Wijngaert S., Reza S., Carbone V., Vandenberg O., Gulbis B., Wolff F., et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020;128:104413. doi: 10.1016/j.jcv.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.