Abstract
Yersinia pestis, the etiologic agent of plague, secretes a set of environmentally regulated, plasmid pCD1-encoded virulence proteins termed Yops and V antigen (LcrV) by a type III secretion mechanism (Ysc). LcrV is a multifunctional protein that has been shown to act at the level of secretion control by binding the Ysc inner-gate protein LcrG and to modulate the host immune response by altering cytokine production. LcrV also is essential for the unidirectional targeting of Yops to the cytosol of infected eukaryotic cells. In this study, we constructed an in-frame deletion within lcrG (ΔlcrG3) to further analyze the requirement of LcrV in Yop targeting. We confirmed the essentiality of LcrV and found that LcrG may have a facilitative role, perhaps by promoting efficient secretion of LcrV. We also constructed mutants of lcrV expressing LcrV truncated at the N or C terminus. Both the N and C termini of LcrV were required for the secretion of LcrV into the medium and targeting of Yops. LcrV was detected in punctate zones on the surface of fixed Y. pestis by laser-scanning confocal microscopy, and this localization required a functional Ysc. However, the truncated LcrV proteins were not found on the bacterial surface. Finally, we tested the ability of LcrV-specific Fab antibody fragments or full-length antibody to interfere with Yop targeting and found no interference, even though this antibody protects mice against plague. These results indicate that LcrV may function in Yop targeting at the extracellular surface of yersiniae and that the protective efficacy of LcrV-specific antibodies can be manifested without blocking Yop targeting.
Pathogenic members of the Yersinia genus include the etiologic agent of plague, Y. pestis, and the enteropathogenic species Y. pseudotuberculosis and Y. enterocolitica. The ability of these facultative intracellular pathogens to cause disease in mammalian hosts is conferred, in part, by components of the coordinately regulated low-Ca2+ response stimulon (LCR) encoded on a common 70-kb virulence plasmid. The virulence of Y. pestis is enhanced by two additional, unique plasmids, pMT1 (encoding murine toxin and capsular fraction 1 protein) and pPCP1 (encoding the plasminogen activator serine protease Pla). The proteinaceous capsule enhances resistance to phagocytosis by monocytes (6), while Pla is an outer membrane protease (66) that is required for full virulence from peripheral infectious routes (4). LCR components encoded on the conserved plasmid (pCD1 in Y. pestis) include a set of secreted antihost proteins termed Yops and V antigen (LcrV) and a specialized apparatus for the secretion and deployment of these proteins during infection. LcrV and most Yops are absolutely essential for virulence in a mouse model (reviewed in references 9, and 44), and the yersiniae employ an elegant mechanism which links expression of these proteins to their subsequent secretion and delivery to respective host targets.
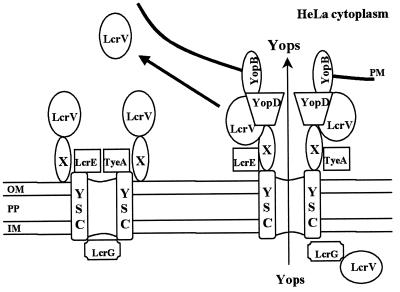
The LCR is thermally regulated by the transcriptional activator LcrF (28), with maximal expression possible at 37°C. Maximal induction of Yops and LcrV, however, can occur only when bacteria are activated for secretion of these proteins by cultivation in contact with eukaryotic cells (10) or in a Ca2+-deficient medium (9, 44, 70, 80), reflecting an intimate link between regulation of the LCR and the ability to secrete proteins. Secretion occurs via a type III mechanism and is mediated by a Yop secretion apparatus (Ysc) consisting of at least 22 gene products (reviewed in references 9 and 44). Numerous studies demonstrated that Y. pestis ysc strains could not be fully induced at 37°C, and it was subsequently surmised that the Ysc mediates secretion of a negative regulator. In a currently accepted model, Ysc secretion channels remain blocked in the presence of Ca2+ or in the absence of cell contact by at least three regulatory proteins. Blockage mediated by LcrE (also called YopN) (15) and TyeA (24) at the outer surface and by LcrG (43) at the inner surface enforces a negative feedback mechanism by causing retention of the secretable negative regulator LcrQ (49, 54, 68) and an accessory protein, YopD (77). Under conditions that relieve the Ysc secretion block, LcrQ is purged from the bacteria, resulting in derepression of Yops and LcrV expression and secretion.
Yops and LcrV lack cleavable signal sequences and are secreted without processing by the Ysc (10, 33). Work by Anderson and Schneewind (1) demonstrated that YopE and YopN (LcrE) secretion can be mediated by a signal contained within their respective mRNAs. In addition, specific Yop chaperones (Sycs) have been identified for some Yops. These are necessary for the secretion of their respective Yops (75) and may function by donating the Yop to the secretion machinery (7). Whether this theme applies to all Yops remains unclear. LcrV has not been systematically mapped for secretion determinants, and no Syc has been identified for it. However, LcrV does bind to the cytoplasmic Ysc gate protein LcrG (43). During contact-induced release, at least six Yops (YopE, YopT, YopH, YpkA, YopM, and YopJ) are targeted, without being released into the surrounding medium, into the cytoplasm of associated eukaryotic cells, where they function as direct antihost effectors. Several of these effector Yops are susceptible to the Y. pestis-specific Pla protease and do not accumulate on the surface of the bacteria (44). Targeting of the secreted effector Yops across the eukaryotic plasma membrane is accomplished by at least three additional Yops (YopB, YopD, and YopK) and LcrV. YopK influences the size (22) of the YopB-containing pore which inserts into the eukaryotic membrane. Although both YopD and LcrV are required for targeting, their precise roles are unclear. YopD has been shown to bind several Yops, including YopB (40), and LcrV may be required for YopB to reach the plasma membrane and form a pore. Yop targeting has been characterized mainly for the enteropathogenic yersiniae, but it is clear that Y. pestis, which has an essentially identical set of LCR genes (9, 46), targets Yops into eukaryotic cells in a similar, contact-activated manner (see, e.g., references 13, 41, and 63). The specific adhesin(s) that functions to promote contact has not been identified, but the resulting attachment lasts at least 4 h. Importantly, the Pla protease, unique to Y. pestis, has not been found to prevent Yop targeting (63), although it can degrade surface-exposed Yops (67). Once targeted, three effector Yops disrupt host cell signaling by interfering with intracellular cascades (YopJ) (57, 58), dephosphorylating focal adhesion components (YopH) (2, 47), and phosphorylating unidentified host proteins (YpkA) (18). YopE (55, 56) and YopT (23) cause disruption of actin microfilaments. The activities of YopE, YopT, and YopH can be directly visualized as the rounding-up or cytotoxic phenotype of infected eukaryotic cells (10). The intracellular role of YopM is unidentified, but the protein does localize to the nuclei of infected cells (63).
V antigen has been associated with full virulence of Y. pestis for decades (5, 52). LcrV is a 327-residue soluble protein (53) whose only known homolog is PcrV of Pseudomonas aeruginosa (78). Unlike most Yops, LcrV is relatively resistant to the Pla protease (69). Evidence that implicates LcrV as a multifunctional protein has accumulated. Within Y. pestis, LcrV is required for induction of the LCR (52, 65), and this activity is manifested by the noted ability of LcrV to associate with cytoplasmic LcrG (43) and titrate the LcrG-mediated inner Ysc secretion block (41). Nilles et al. (41) and Sarker et al. (60) have also reported that LcrV functions in Yop targeting through an effect on YopB. This requirement was demonstrated in the absence of LcrG, thereby separating LcrV’s function in LCR induction from that in targeting of Yops (41).
The observation that LcrV-specific antibodies confer resistance to experimental plague (3, 73, 74) prompted the hypothesis that LcrV also promotes virulence outside the bacteria, and it has been proposed that LcrV acts directly to compromise innate host immune responses. In support of this idea, an LcrV-containing fusion protein (in the absence of yersiniae) was shown to prevent production of the proinflammatory cytokines tumor necrosis factor alpha and gamma interferon in mice (37). This immunosuppressive effect was not limited to Yersinia infections, since exogenous LcrV exacerbated heterologous infections of mice with Listeria monocytogenes or Salmonella typhimurium (37). Nedialkov et al. (38) have proposed that this effect arises from the ability of LcrV to upregulate the negatively acting cytokine interleukin-10. Interestingly, others have shown that LcrV inhibits chemotaxis of neutrophils (76).
This complex array of functions has made the elucidation of LcrV’s mechanisms of action during infection difficult. It is likely that different domains of LcrV are required for its various functions. Genetic analysis indicated that residues 224 to 266 are required for binding to LcrG (60), and a protective epitope lies between residues 176 and 276 (21, 35). The N terminus of LcrV is dispensable for the immunosuppressive activity, because the fusion protein used by Nakajima et al. (37) lacked residues 1 to 67 of LcrV. However, the first 125 residues likely are important for secretion of LcrV (65). How LcrV carries out its multiple functions remains obscure, and its molecular targets are unknown.
This study focuses on LcrV’s functions in Yop targeting and virulence. We found that both the N and C termini of LcrV are required for its role in Yop targeting but that the N terminus was dispensable for LcrV’s role in activation of the Ysc for Yop secretion. LcrV localized to the surface of non-contact-activated Yersinia, but the truncated versions did not, suggesting the hypothesis that LcrV may exist on the bacterial surface in a pretargeting complex. However, LcrV-specific antibody did not prevent Yop targeting, suggesting that antibody can be protective against plague without blocking the delivery of Yops.
MATERIALS AND METHODS
Bacterial strains, eukaryotic cell lines, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. This study employed the human HeLa epithelium-derived cell line. Unless otherwise noted, Escherichia coli strains were cultivated in Luria-Bertani medium (34) at 37°C or on Luria-Bertani agar. Y. pestis strains were grown in the defined medium TMH (70) as described for physiological studies. Briefly, Y. pestis cultures were cultivated with shaking at 200 rpm overnight at 26°C for about eight generations. Cultures were diluted into fresh 26°C TMH to an optical density at 620 nm (OD620) of 0.1, initially incubated at 26°C, and shifted to 37°C when cultures had reached an OD620 of ca. 0.2. Cultures then were harvested 6 h after the shift to 37°C. For infection of eukaryotic cell lines, Y. pestis was grown in heart infusion broth (HIB) (Difco Laboratories, Detroit, Mich.) at 26°C for at least six generations in exponential phase. During construction of the ΔlcrG3 strain, Y. pestis was cultivated on tryptose blood agar (Difco) supplemented with chlorotetracycline HCl (Sigma) and fusaric acid (32), as described by Nilles et al. (41). When appropriate, bacteria were grown in the presence of antibiotics, which were used at 15 μg/ml for tetracycline, 25 μg/ml for chloramphenicol, 50 μg/ml for kanamycin, or 100 μg/ml for ampicillin and streptomycin (Sigma, St. Louis, Mo.). The HeLa cell line was maintained in RPMI (GIBCO-BRL, Grand Island, N.Y.) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) (GIBCO-BRL) at 37°C, with CO2 maintained at 5%. During experiments to determine the partitioning of LcrV and Yops within cultures of infected HeLa cells, infection was done in RPMI lacking FBS. When arabinose induction was required, infection was done in Leibovitz’s L15 medium (L15) (GIBCO-BRL) lacking FBS.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Properties | Source or reference |
|---|---|---|
| E. coli K-12 | ||
| DH5α | F−φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− rM+) phoA supE44 λ− thi-1 gyrA96 relA1 | GIBCO-BRL |
| DH5α(λpir) | F−φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− rM+) phoA supE44 thi-1 gyrA96 relA1 λpir | Laboratory stock |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacIq ZΔM15 Tn10 (Tcr)] | Stratagene |
| Y. pestisa | ||
| KIM6 | pPCP1, pMT1 | R. R. Brubaker |
| KIM5-3001 | Smr; pCD1 (Lcr+), pPCP1, pMT1 | 30 |
| KIM5-3001.12 | Smr; pCD1 yscC [YscCΔ141–454]b, pPCP1, pMT1 | 51 |
| KIM5-3241 | pCD1 lcrV [LcrVΔ18–215], yopJ::MudI1734 (Kmr Lac+), pPCP1, pMT1 | 52 |
| KIM8-3241 | pCD1 lcrV [LcrVΔ18–215], yopJ::MudI1734 (Kmr Lac+), pMT1, pOS50; KIM5-3241 was cured of pPCP1 as described in Materials and Methods | This study |
| KIM8-3001.12 | Smr; pCD1 yscC [YscCΔ141–454], pMT1, pOS50; KIM5-3001.12, constructed previously (51), was cured of pPCP1 as described in Materials and Methods | This study |
| KIM8-3002 | Smr; pCD1 (Lcr+), pMT1 | 37 |
| KIM8-3002.7 | Smr; pCD1 ΔlcrG3[LcrGΔ6–86], pMT1 | This study |
| KIM8-3002.8 | Smr; pCD1 ΔlcrG2[LcrGΔ6–95] lcrV [LcrVΔ1–268], pMT1 | 42 |
| Plasmids | ||
| pProEX-1 | Apr; expression vector for creation of His6-fusion to N terminus of protein expressed from cloned open reading frame | GIBCO-BRL |
| pLD55 | Apr Tcr; suicide vector for allelic exchange | 32 |
| pBAD18-Kan | Kmr; ParaBAD cloning vector | 17 |
| pTrc99A | Apr; Ptrc cloning vector | Pharmacia |
| pOS50 | Tcr; competitive plasmid for pPCP1 | J. Goguen |
| Constructs | ||
| pES6-1 | HindIII-G of Y. pestis pCD1 cloned into pUC19 (lcrGVH yopBD′)c | 65 |
| pHT-V | PCR-amplified lcrV cloned in pProEX-1 in frame with leader sequence encoding 23 amino acids, including 6 His (lcrV) | This study |
| pHT-VN68 | Subcloned lcrV in pProEX-1 with a 29-residue His6 leader sequence fused at the EcoRV site in lcrV (′lcrV [HT-V Δ1–67 ≡ HT-VN68; same as HT-′V previously described (14)]) | This study |
| pHT-′V2 | EcoRV fragment of pES6-1 in pProEX-1 with the same 29-residue His6 leader sequence as in pHT-VN68 fused at the EcoRV site in lcrV (′lcrV [HT-VΔ1–67 ≡ HT-′V] lcrH yopB′ YopB1–87]) | 14 |
| pTrcV | BbvI/NcoI fragment of pES6-1 cloned into SmaI-cut pTrc99A (lcrV) | This study |
| pAraG18K | lcrG cloned into pBAD18-Kan (lcrG) | 41 |
| pAraV18K | lcrV cloned into pBAD18-kan (lcrV) | 41 |
| pAraGV18K | lcrGand lcrV cloned into pBAD18-Kan (lcrG lcrV) | 41 |
| pMNΔlcrG3 | ΔlcrG3 allele [lcrGΔ6–86] cloned into pLD55 | This study |
| pVC216 | PCR-amplified lcrV encoding amino acids 1–216 in pTrc99A (lcrV′ [LcrVΔ217–327]) | This study |
| pE15VN68 | YopE mRNA secretion signal (45 nt) fused to EcoRV site in lcrV; expression from yopE promoter (yopE′ ′lcrV [LcrVΔ1–67]) | This study |
All strains are Pgm− (73).
Numbers in brackets indicate residues deleted from gene product.
Descriptions in parentheses give relevant genes present on construct.
DNA methods.
Template DNA was isolated with midi-prep or spin-prep columns from Qiagen, Inc. (Studio City, Calif.), and cloning procedures were performed essentially as described previously (31). Amplification of specific DNA was achieved by PCR in a Perkin-Elmer Cetus (Foster City, Calif.) GeneAmp model 2400 thermocycler with Pfu (Stratagene, La Jolla, Calif.) or Vent (New England Biolabs, Beverly, Mass.) DNA polymerase and oligonucleotide primers synthesized by Genosys Biotechnologies (The Woodlands, Tex.) or the Macromolecular Structure Analysis Facility (University of Kentucky). Typical PCR conditions included a 5-min preincubation at 94°C followed by 30 amplification cycles. Denaturation, annealing, and extension were done at 94, 55, and 72°C, respectively, for 15 to 30 s each. Restriction fragments excised from agarose gels and PCR products were purified by using a Qiaquick gel extraction or DNA purification kit (Qiagen), respectively. Transformation of E. coli was achieved by the CaCl2 method (31) or the frozen-storage-based protocol as described previously (19), and that of Y. pestis was achieved by electroporation as described by Perry et al. (45). Double-stranded DNA from pHT-V was sequenced by the method of Sanger et al. (59) with the Sequenase version 2.0 kit (United States Biochemical Corp., Cleveland, Ohio) and α-35S-dATP (NEN Research Products, Boston, Mass.).
Plasmid construction.
The plasmids pHT-V (encoding N-terminally histidine-tagged LcrV) and pHT-VN68 (encoding N-terminally histidine-tagged, N-terminally truncated LcrV) were constructed by insertion of portions of lcrV (PCR amplified with Pfu polymerase) into the expression plasmid pPROEX-1. For pHT-V, lcrV was amplified from pES6-1 DNA. The 5′ end of the sense primer (5′-TATATAGGCGCCATGATTAGAGCCTACGAACAAAACCC-3′) was a non-Yersinia-encoded sequence containing a NarI restriction site; the antisense primer (5′-CCCCCTCCTTTTAGG-3′) annealed immediately downstream of lcrV. PCR products were 5′ end phosphorylated with T4 polynucleotide kinase (Promega, Madison, Wis.) and then digested with NarI. The resulting DNA was ligated into NarI- and StuI-cut pProEX-1 and transformed into E. coli DH5α. PCR fidelity was verified by sequencing the cloned lcrV. pHT-VN68 was derived from pHT-′V2, in which the encoded ′LcrV lacks the first 67 amino acids of LcrV. A sense primer (5′-ACGATATCCCAACGACCG-3′) complementary to pPROEX-1 DNA and an antisense primer (5′-CCCCCTCCTTTTAGG-3′) positioned 68 nucleotides (nt) into lcrH (also called sycD) were used to amplify only ′lcrV and the polyhistidine tag-coding region. The PCR product was digested with KasI and 5′ end phosphorylated with T4 kinase. Treated fragments were ligated into KasI- and StuI-digested pProEX-1 and used to transform E. coli DH5α. The resulting leader sequences fused to LcrV and VN68 were similar (HT-V, MGHHHHHHDYDIPTTENLY FQGA; HT-VN68, MGHHHHHHDYDIPTTENLYFQGAHMGIQR).
pE15VN68 (encoding YopE [amino acids 1 to 15] fused to N-terminally truncated LcrV) was generated by fusing the mRNA secretion signal sequence described by Anderson and Schneewind (1) to a 5′-truncated lcrV. The secretion signal contained within the yopE promoter and first 15 codons was amplified by PCR with Vent DNA polymerase, a sense primer (5′-AAGAATTCTCCTAATAGTTAGATAAAATATCAAC-3′) containing an EcoRI site at the 5′ end (not used in this construct), and an antisense primer (5′-TAACCCGGGTGCCGGCAGGGG-3′) possessing a SmaI site positioned in frame with the yopE coding sequence. The 237-nt product extended from position −187 upstream of yopE to position +45 within yopE. The PCR product was treated with SmaI and used to replace DNA removed from pTrcV by EcoRV digestion. Treatment of pTrcV with EcoRV removed the 5′ end of lcrV (encoding the first 67 amino acids) and upstream pTrc99A vector sequence including a portion of lacIq and the entire trc promoter. Ligation products were used to transform E. coli XL1-Blue. The PCR insert was oriented so that yopE and lcrV sequences were translationally fused but separated by one proline codon derived from the SmaI restriction site.
Both pTrcV (encoding LcrV) and pVC216 (encoding LcrV C-terminally truncated after residue 216) were derived from pES6-1. pTrcV was constructed by restriction digestion of pES6-1 with BbvI (within the 3′ end of lcrG) and NcoI (within lcrH) followed by treatment of the lcrV-containing DNA with mung bean exonuclease (New England Biolabs). The fragment was then purified, ligated into SmaI-cut pTrc99A and used to transform E. coli DH5α. pVC216 was derived by PCR amplification of lcrV with Pfu polymerase and sense (5′-TATATAGGCGCCATGATTAGAGCCTACGAACAAAACCC-3′) and antisense (5′-TTAGAGAATTTTGTACTCTGCGC-3′) primers. The product, containing nt 1 to 648 of lcrV (encoding amino acids 1 to 216), was purified, ligated into SmaI-digested pTrc99A, and used to transform E. coli XL1-Blue. In both constructs, lcrV was oriented such that expression was driven by the inducible trc promoter.
pMNΔlcrG3 carries a PCR-amplified insert beginning in lcrD (1,060 bp upstream of the lcrG start), incorporating a deletion of codons 6 to 86 of lcrG, and ending past the lcrH start (1,305 bp downstream of the lcrG start). The construction included the introduction of a stop codon (TAA) at codon 6 of lcrG to ensure that no peptide longer than 5 amino acids would be made from lcrG. The insert for the final clone was generated in two pieces by PCR amplification of upstream flanking DNA by using primers ΔlcrG-US (5′ CGCGGATCCGCTATCTGCTCGAACAGA 3′) and lcrG1-5KPN (5′ CGGGGTACCTTAATGGGAAGACTTCATAATCTA 3′) and of downstream flanking DNA by using ΔG3-KPN (5′ CGGGGTACCCCAACGATGATGCGAGGGCAA 3′) and ΔlcrG-DSII (5′ GATATCAGTGTCTGTCGTCTCTTG 3′). The upstream fragment was digested sequentially with BamHI and KpnI, while the downstream fragment was digested with KpnI. Purified, digested fragments were combined, ligated into BamHI- and SmaI-digested pLD55, and used to transform E. coli DH5α(λ pir). In the resulting fusion junction (5′ … TCC CAT TAA GGT ACC CCA GCG … 3′) the stop at position 6 (TAA) was followed by codons for glycine and threonine not contained in lcrG and then by codons 87 to 95 of lcrG.
Strain construction.
Y. pestis strains lacking the plasminogen activator protease (Pla) were created by curing strains of the encoding plasmid pPCP1. Y. pestis KIM5-3241 and KIM5-3001.12 were transformed with a plasmid, pOS50 (a gift of J. Goguen, University of Massachusetts, Worcester), containing pPCP1’s origin of replication. Transformants were transferred on tryptose blood agar containing tetracycline to allow curing of pPCP1 via competition with pOS50. Potential plasmid-cured strains were screened for the loss of pPCP1 by analysis of plasmid DNA as previously described (25) and for the lack of plaminogen activator activity by assaying for activation of thrombin-mediated cleavage of fibrin film as previously described (71). Strains cured in this way then retained the pOS50 plasmid. Y. pestis KIM8-3002.7 (ΔlcrG3) was created by allelic exchange of the parent lcrG on pCD1 with the ΔlcrG3 allele carried on pMNΔlcrG3. Introduction of pMNΔlcrG3 into Y. pestis, selection for crossover events, and screens for successful replacements were done as described previously (41). For physiological studies characterizing the phenotype of Y. pestis KIM8-3002.7, bacteria were cultivated in TMH as described above (70), and the cultures were separated into whole cells and cell-free culture supernatants as done previously (41).
Antibody preparations.
LcrV-specific antibodies (α-HTV) were raised against purified HT-V in female New Zealand White rabbits as previously described (50). A nonspecific, irrelevant antibody pool (α-NS) was prepared from rabbit preimmune serum harvested prior to hyperimmunization with HT-V. α-Yersinia was a preparation of polyclonal rabbit antibodies that had been raised against whole cells of Y. pestis KIM6 grown at 26°C in HIB and partially purified by ammonium sulfate precipitation. In one set of experiments YopM-specific rabbit polyclonal antibody (α-YopM) (39) was used as an irrelevant antibody control. All antibodies other than α-Yersinia were purified from serum by using an Econo Pac protein A cartridge (Bio-Rad Laboratories, Hercules, Calif.) and subsequently dialyzed into phosphate-buffered saline (PBS) (135 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH7.4) as described previously (39). For some experiments, α-HTV or α-NS was dialyzed into buffer containing 100 mM sodium acetate (pH 5.5) and used to generate antibody Fab fragments. Antibodies were digested with papain as described previously (20) for 18 h, and Fab fragments were purified by collecting flowthrough fractions from a protein A cartridge. Fab preparations were dialyzed into PBS and concentrated by centrifugation in a Centricon 30 concentrator (Amicon, Inc., Beverly, Mass.), and the protein content was quantitated by the bicinchoninic acid assay (Pierce Chemical, Rockford, Ill.). When resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by immunoblot analysis, Fab fragments were of predicted sizes, and when used as primary antibodies in immunoblots, they were able to detect LcrV (data not shown). α-HTV was absorbed twice with nonspecific antigens for use in immunostaining of samples to be analyzed by confocal microscopy. Briefly, spleens were excised from female BALB/c mice (Harlan Sprague-Dawley, Indianapolis, Ind.), minced, and extracted with ice-cold acetone. Five hundred micrograms of precipitated, dried material was combined with 500 μl of α-HTV in the presence of a protease inhibitor cocktail (Pefabloc, leupeptin, and aprotinin at 20 μg/ml each; all from Boehringer Mannheim Corp., Indianapolis, Ind.) and incubated overnight on ice. Solid material was then pelleted at 4°C by centrifugation at 20,800 × g and discarded.
Passive immunization of mice with α-HTV.
Female BALB/c mice (7 to 8 weeks old) were passively immunized with 500 μg of α-HTV or with 333 μg of α-NS Fab fragments as a negative control. There were four mice per group in one experiment and five per group in a repetition. Single antibody doses were given intraperitoneally in 100- to 250-μl volumes of PBS. Forty-eight hours after passive immunization, mice were challenged with a lethal dose of Y. pestis KIM5-3001. Bacteria were diluted into PBS and injected intravenously into the retro-orbital sinus, and mice were observed for 14 days postinfection. The actual infecting doses were determined by enumerating CFU from samples of the doses given to the mice. These were 746 CFU in one experiment and 215 in a replicate experiment.
Infection assays.
Prior to infection, eukaryotic cells were subcultured into six-well 35-mm-diameter tissue culture plates in RPMI with 10% (vol/vol) FBS and incubated at 37°C with 5% CO2 for roughly 72 h or to a density of 5 × 105 to 8 × 105 per well. Cells were washed twice with RPMI or L15 medium lacking FBS immediately prior to infection. Bacteria were cultivated at 26°C in HIB and harvested at an OD620 of ca. 1.0. Arabinose or isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 0.2% or 0.1 mM, respectively, at 30 to 60 min prior to harvest for strains harboring constructs with inducible promoters. Harvested bacteria were diluted directly into 37°C L15 or RPMI lacking FBS (containing arabinose or IPTG where appropriate) and transferred to duplicate wells containing eukaryotic cells. Bacteria were added at a 10-fold excess per well over the number of eukaryotic cells (nominal multiplicity of infection [MOI] = 10). The plates were then centrifuged at 200 × g for 5 min to achieve contact between bacteria and target cells and incubated at 37°C with 5% CO2 (for RPMI) or at 37°C (for L15) for 4 h. After infection, one replicate well per infecting bacterial strain was treated for 5 min at 37°C (5% CO2) with trypsin (100 μg/ml in RPMI or L15), and the trypsin treatment was terminated by addition of the protease inhibitor cocktail described above. The contents of the wells were subsequently harvested and fractionated following lysis of the HeLa cells in ice-cold water containing protease inhibitors at 2 μg/ml, essentially as described previously (13, 41). Three fractions were collected: (i) large cellular debris plus yersiniae recovered by centrifugation at 20,800 × g, (ii) proteins precipitated with 10% (wt/vol) trichloroacetic acid (TCA) from filtered media, and (iii) TCA-precipitated soluble proteins released by water lysis plus small organelles not removed by centrifugation at 20,800 × g. The fractions were solubilized in electrophoresis sample buffer containing 2.3% (wt/vol) SDS, 5% (vol/vol) β-mercaptoethanol, 60 mM Tris (pH 6.8), and 25% (vol/vol) glycerol.
Infection assays were also carried out in the presence of LcrV-specific antibodies. For these experiments, we estimated the amount of LcrV in a typical infection assay. Briefly, one well of HeLa cells infected with Y. pestis KIM8-3002 for 4 h was harvested, and total proteins in the culture were TCA precipitated and solubilized in electrophoresis buffer. We estimated that the concentration of LcrV was ca. 20 ng/ml, or 0.53 nM, by comparing immunoblots of serially diluted samples to immunoblots of serial dilutions of a known concentration of HT-V. This value was used to estimate fold excesses of LcrV-specific antibodies in neutralization experiments. HeLa cells and Y. pestis KIM8-3002 were cultivated as described above. In one set of experiments, full-length α-HTV or α-YopM was added at 175 μg/ml to yersiniae that had been grown at 26°C and diluted into 37°C RPMI, and this suspension was layered onto washed HeLa cells without centrifugation. The antibody was present at an estimated 2,000-fold molar excess over the total amount of LcrV that typically is produced in each infected well (4,000-fold molar excess of antigen-binding sites). The slower initiation of infection without centrifugation was used to allow the antibody time to interact with both bacteria and HeLa cells. In a variation of this protocol, α-NS was added to HeLa cells at 500 μg/ml to block any Fc receptors. After 30 min, the cells were washed and overlayered with yersiniae in the presence of α-HTV or α-YopM at 175 μg/ml and α-NS, also at 175 μg/ml. At various times after infection, the cultures were observed by phase-contrast microscopy for cytotoxicity (retraction and rounding up). After 3 h of infection, they were photographed through a green filter with Kodak ASA100 black-and-white film.
In a second type of experiment, bacteria were diluted into 37°C RPMI containing α-HTV Fab fragments at 26.6 μg/ml and incubated for 10 min prior to addition to the HeLa monolayer. This concentration was an estimated 1,000-fold molar excess over the amount of LcrV present in each well. Infection of monolayers by centrifugation and harvesting of samples were performed as described above. To assess antibody neutralization of LcrV in culture supernatants, we immunoprecipitated LcrV-Fab complexes from the medium fraction of a replicate infected culture as follows. The medium was removed, passed through a 0.2-μm-pore-size filter, and combined with a twofold molar excess of donkey anti-rabbit whole-molecule immunoglobulin G (IgG) (Accurate Chemical and Scientific Corp., Westbury, N.Y.) bound to protein A-Sepharose beads (Sigma). The slurry was mixed for 2 h at room temperature. Beads were subsequently pelleted and washed multiple times with PBS. Void and flowthrough fractions were combined, and proteins were precipitated by overnight treatment with TCA at a 10% (wt/vol) final concentration. Beads and pelleted precipitated proteins were suspended in equal volumes of electrophoresis sample buffer to solubilize proteins.
In a third protocol, the yersiniae were preincubated at 37°C without HeLa cells to allow surface expression of LcrV. For this treatment, they were washed once with PBS, diluted to give an MOI of 10, dispensed at 2 ml/well in six-well dishes, and centrifuged onto the wells for 5 min at 200 × g. The dishes were incubated for 1.5 h at 37°C with 5% CO2. They were then resuspended in each well, and 350 μg of α-HTV, 350 μg of α-YopM (as an irrelevant antibody treatment), or a corresponding volume (ca. 0.2 ml) of PBS was added to each well, and the dishes were incubated for 30 min at 37°C with CO2 to allow the α-HTV antibody (present at 175 μg/ml) time to bind to LcrV on the bacterial surface. The bacteria were then resuspended and used to infect HeLa cells. Prior to infection, the HeLa cells had been washed once with PBS and incubated for 30 min at 37°C with 5% CO2 in RPMI containing 1% (vol/vol) mouse serum (MS) (Sigma) to block any Fc receptors. This medium was removed, 2 ml of bacterial suspension containing antibody or PBS was added, and the infection was initiated by centrifugation as described earlier. One set of wells was photographed as described above at hourly intervals for 4 h. Duplicate pairs of wells were harvested and fractionated as described above for immunoblot analysis after 4 h of infection. In a repetition of this experiment, the bacterial MOI was reduced to 2, and the concentration of blocking antibody was correspondingly reduced fivefold to 35 μg/ml. In both experiments, the ratio of antigen-binding sites on α-HTV to LcrV was ca. 4,000.
Protein electrophoresis and immunoblot analysis.
Proteins from fractionated bacterial cultures and from infection assays were resolved in polyacrylamide gels (12% [wt/vol] acrylamide) by SDS-PAGE (27). Samples were loaded so that lanes containing different culture fractions represented equivalent volumes of the original cultures. Resolved proteins were transferred to Immobilon-P (Millipore, Corp., Bedford, Mass.) in carbonate buffer (pH 9.9) (64) when LcrG was analyzed or in Tris-glycine (72) in all other cases. Specific proteins were detected by using polyclonal antibodies specific for His-tagged LcrV (α-HTV), His-tagged YopD (α-YopD) (76), His-tagged YopE (α-YopE) (a gift of G. Plano, University of Miami) or glutathione S-transferase (GST)-tagged LcrG (α-GST-G) (43). Alkaline phosphatase or horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma) was used to visualize proteins by development with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) (GIBCO-BRL) or enhanced chemiluminescence (ECL) substrate (Pierce), respectively.
Confocal analysis.
Bacteria were tested for surface localization of LcrV by cultivating Yersinia strains at 26°C in HIB as done in infection assays to an OD620 of ca. 1.0. Bacteria were diluted into 37°C RPMI lacking FBS (containing 0.1 mM IPTG where appropriate) to a density of 106/ml, and 1.0-ml aliquots were centrifuged at 200 × g for 5 min onto 13-mm-diameter glass coverslips, each bearing ca. 105 HeLa cells, in 24-well cluster dishes (Costar, Cambridge, Mass.). After incubation for 2 h at 37°C with 5% CO2, wells were washed once with 1.0-ml volumes of Hanks balanced salt solution (GIBCO-BRL) and subsequently treated with 2% (wt/vol) paraformaldehyde (Sigma) in PBS (pH 7.4) for 30 min. Fixed bacteria were blocked for 1 h at room temperature with 10% (vol/vol) FBS plus 1% MS in PBS and then washed and incubated for 1 h in PBS plus 1% (vol/vol) MS with α-HTV, α-YopE, or α-Yersinia. Samples were then washed with PBS and incubated for 1 h with anti-rabbit IgG antibodies coupled to the fluorochrome Oregon green (Molecular Probes, Eugene, Oreg.). After staining, coverslips were mounted by using SlowFade mounting medium (Molecular Probes) and analyzed by using laser-scanning confocal microscopy. Specimens were visualized by differential interference contrast with a Leica DM IRB/E inverted microscope with Nomarski optics and a 100× objective. Fluorescence images were derived from a single 0.5-μm-thick optical plane with the Leica TCS NT confocal system (Ar-Kr laser), and image size was recorded at 1,024 by 1,024 pixels with an additional zoom factor of between 4.5 and 5.5.
In a control experiment for Fig. 7 to assay for LcrV expression differences between the parent Y. pestis KIM8-3002 and the yscC Y. pestis KIM8-3001.12, the yersiniae were grown and diluted into 37°C RPMI. Two milliliters per well was centrifuged as described above in six-well cluster dishes and incubated for 2 h at 37°C with 5% CO2. The contents of the well were removed, the well was washed with 1.0 of ml PBS which was combined with the harvested bacteria, and total proteins were recovered by TCA precipitation. Proteins were neutralized and resuspended in electrophoresis sample buffer and boiled for 5 min. Serial twofold dilutions were prepared and subjected to immunoblot analysis. Duplicate blots were probed with α-HTV and α-Yersinia (to ensure comparable loading of the lanes).
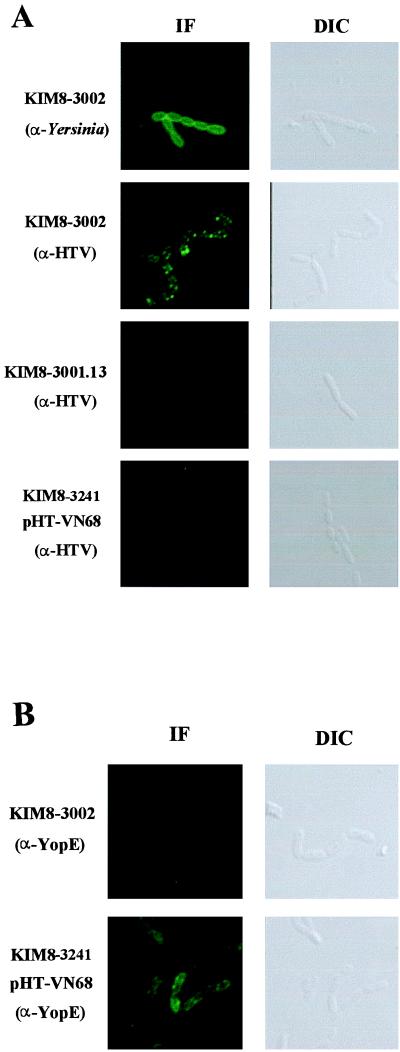
FIG. 7.
LcrV can localize to the surface of Y. pestis. Surface deposition of LcrV and YopE was tested in Y. pestis strains lacking pPCP1. Parent Y. pestis (KIM8-3002), secretion-negative Y. pestis (yscC KIM8-3001.12), and the lcrV-null strain (KIM8-3241) complemented with pHT-VN68 were fixed on coverslips after incubation for 2 h at 37°C in RPMI. Bacteria were stained with α-Yersinia or α-HTV (A) or α-YopE (B). The respective proteins were then visualized by treatment with Oregon green-conjugated secondary antibody followed by confocal laser-scanning microscopy. Immunofluorescence (IF) confocal and differential interference contrast (DIC) images are shown.
RESULTS
LcrV is essential for Yop targeting, while LcrG has a facilitative role.
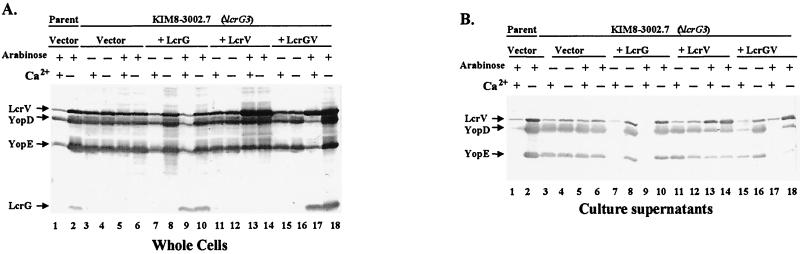
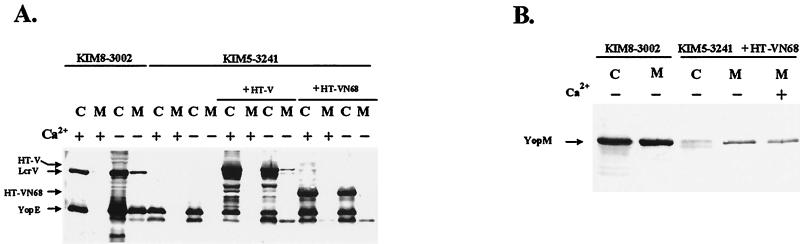
The goal of this study was to gain insight into how LcrV promotes virulence of Y. pestis. We began by clarifying the essentiality of LcrV for Yop targeting. Our previous report had shown that LcrV was required for the targeting of Yops into infected eukaryotic cells, but our data (41) and those of Sarker et al. (60) suggested that LcrG also may be needed. Importantly, LcrV and LcrG have been shown to interact (43, 60), raising the possibility that they may function synergistically to promote Yop targeting. To determine how LcrG might influence LcrV’s activity in targeting, we constructed a Y. pestis strain possessing an in-frame, nonpolar deletion within lcrG that did not remove the ribosome-binding site for the downstream gene (lcrV) and therefore was predicted not to decrease LcrV expression. Consistent with the case for other Yersinia strains lacking lcrG (41, 61, 64), the ΔlcrG3 Y. pestis KIM8-3002.7 displayed a “Ca2+-blind” growth phenotype in defined medium (79): i.e., the bacteria ceased growth prematurely at 37°C whether Ca2+ was present or not (data not shown). This phenotype is often indicative of strong induction of the LCR and secretion of LcrV and Yops, regardless of the presence of Ca2+. In agreement with these predictions, the levels of proteins detected in whole-cell samples (Fig. 1A) from the ΔlcrG3 strain were elevated irrespective of the presence of Ca2+ (lanes 3 to 6), whereas the parent possessing wild-type pCD1 displayed significant induction of LcrV, YopD, and YopE only in the absence of Ca2+ (lanes 1 and 2). Importantly, the induced levels of protein encoded by the adjacent downstream gene (lcrV) and by the last gene of the polycistronic operon (yopD) confirmed the lack of polarity of the lcrG mutation in ΔlcrG3 Y. pestis. When complemented with pAraG18K in the absence of arabinose induction, LcrG was not detectable in the ΔlcrG3 strain by this assay, but the small amount present was sufficient to restore near-wild-type Ca2+ regulation of all gene products tested (Fig. 1A, lanes 7 and 8). Addition of arabinose, however, resulted in detectable levels of LcrG and complete restoration of Ca2+ regulation (lanes 9 and 10), demonstrated by the decreased levels of LcrV and Yops D and E when Ca2+ was present. Providing excess LcrV (lanes 11 to 14) did not appreciably alter the constitutively induced levels of YopD and YopE, supporting the observation by Nilles et al. (43) that the inductive role of LcrV in Ca2+ regulation is dependent on its interaction with LcrG. Finally, providing both lcrG and lcrV in the ΔlcrG3 mutant restored the wild-type regulation of expression in the presence (Fig. 1A, lanes 17 and 18) but not the absence (lanes 15 and 16) of arabinose induction. Unlike the presumed small amount of LcrG present in the absence of arabinose (lane 7), LcrG was unable to mediate a significant reduction of YopD or YopE in the presence of Ca2+ (lane 15), possibly because excess LcrV provided on the same construct could prevent LcrG-mediated blockage of the Ysc secretion channel.
FIG. 1.
KIM8-3002.7 (ΔlcrG3) is defective for negative regulation of LcrV and Yops, and LcrG is required for efficient secretion of LcrV. Y. pestis KIM8-3002 (parent) containing plasmid pBAD18-Kan (vector) (lanes 1 and 2) or Y. pestis KIM8-3002.7 possessing pBAD18-Kan (lanes 3 to 6), pAraG18K (+LcrG) (lanes 7 to 10), pAraV18K (+LcrV) (lanes 11 to 14), or pAraGV18K (+LcrGV) (lanes 15 to 18) was grown in TMH at 37°C in the presence (odd-numbered lanes) or absence (even-numbered lanes) of Ca2+. Arabinose was added to 0.2% (wt/vol) prior to a shift to 37°C for vector controls (lanes 1, 2, 5, and 6) or to achieve induction of lcrG (lanes 9 and 10), lcrV (lanes 13 and 14), or lcrG and lcrV (lanes 17 and 18). Cultures were harvested after 6 h of growth at 37°C, and samples were fractionated into whole cells (A) and cell-free culture supernatants (B). Material corresponding to 0.03 OD620 unit · ml was resolved by SDS-PAGE in a 12% polyacrylamide gel and analyzed by immunoblotting with an antibody cocktail containing α-HTV, α-YopD, α-YopE, and α-GST-G. Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies followed by development with NBT-BCIP.
LcrV, YopD, and YopE secreted into culture supernatants during growth (Fig. 1B) correlated with induction detected in whole-cell samples (Fig. 1A). LcrV, YopD, and YopE were secreted by the ΔlcrG3 mutant at significant levels (Fig. 1B, lanes 3 to 6), even under conditions that normally cause secretion to be blocked (lanes 3 and 5). Interestingly, even though secretion was constitutive, levels of protein were lower (lanes 3 to 6) than those of respective proteins secreted in the absence of Ca2+ by the parent strain (lane 2). This was an effect not seen in our previous strains defective in lcrG or lacking lcrG but also having weak lcrV expression (43, 64). As with expression in the cells, complementation with LcrG also restored downregulation of secretion in the presence of Ca2+ (Fig. 1B, lanes 7 to 10), presumably by restoring a functional inner gate to the Ysc (43). Comparison of the levels of protein in lanes 3 to 6 to that in lane 10 revealed that LcrG expressed in trans did not completely restore the wild-type level of Yop secretion, but it did appear to increase the secretion of LcrV more than that of YopD or YopE. As expected, providing LcrV under arabinose induction (lanes 13 and 14) resulted in elevated LcrV being released at essentially wild-type levels into the culture medium without an increase in the level of YopD or YopE secretion. Complementing with lcrG and lcrV under arabinose induction (lanes 17 and 18) resulted in normal regulation of secretion. In summary, the largest effect of the ΔlcrG3 mutation was to decrease secretion of LcrV. This was almost completely alleviated by complementation with lcrG and was overcompensated by overexpression of lcrV. The mutation had smaller effects on secretion of YopE and YopD, which were not changed by complementation, perhaps revealing a limitation of complementing in trans.
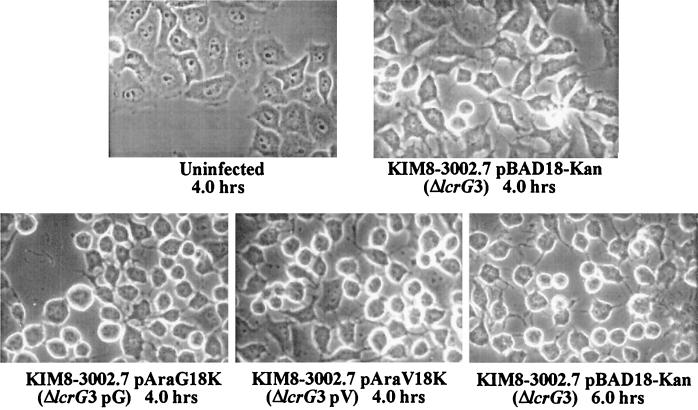
We tested the ability of ΔlcrG3 Y. pestis and complemented strains to induce cytotoxicity in infected HeLa cells (Fig. 2) as a very sensitive indicator of Yop targeting (10, 41). HeLa cells infected with the ΔlcrG3 mutant were only beginning to show evidence of cytotoxicity after 4 h of incubation. This defect in Yop targeting was circumvented by reintroducing LcrG from pAraG18K, since strong cytotoxicity (cell rounding) was shown in this culture after 4 h of infection. These results are consistent with those reported by Sarker et al. (61) and the hemolysis test of Nilles et al. (41), which indicated that LcrG contributes to the mechanism for targeting of Yops. Interestingly, HeLa cells infected with the noncomplemented ΔlcrG3 mutant showed a strong cytotoxic effect by 6 h postinfection (Fig. 2), indicating that LcrG is not essential for Yop targeting. Because our analysis of these strains in TMH had indicated that LcrG may be required for the efficient secretion of LcrV, an effect that was overcome by overexpressing lcrV in trans, and since we had previously demonstrated that LcrV was required for Yop targeting, we extended our analysis to include ΔlcrG3 Y. pestis carrying pAraV18K. This strain, like the parent, was able to induce strong cytotoxicity by 4 h postinfection. Taken together, these results indicate that LcrG plays a facilitative role in Yop targeting, while LcrV is essential, a finding that agrees with the conclusion of Sarker et al. (61). However, our data further suggest that LcrG may be necessary for efficient secretion of LcrV. If true, this may indicate that LcrV functions to promote Yop targeting outside the bacteria, possibly at the interface between bacteria and infected cells.
FIG. 2.
LcrG facilitates targeting of Yops into infected HeLa cells. Y. pestis KIM8-3002 (parent) or Y. pestis KIM8-3002.7 (ΔlcrG3) possessing pBAD18-Kan, pAraG18K (ΔlcrG3 pG), or pAraV18K (ΔlcrG3 pV) was used to infect HeLa cell monolayers at an MOI of 10. Expression of LcrG was induced by addition of arabinose to 2% (wt/vol), and LcrV was induced by addition of IPTG to 0.1 mM. Cell morphology was visualized by phase-contrast microscopy and recorded at 3.5 or 6 h after infection by photography through a green filter.
Both N and C termini of LcrV are required for Yop-targeting activity.
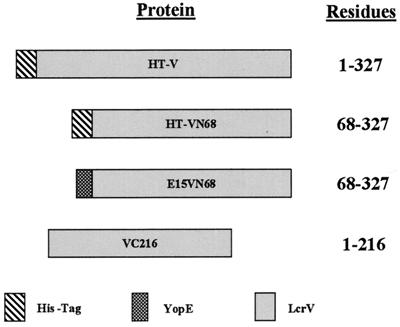
We initiated the identification of regions of LcrV that are necessary for targeting of Yops by testing the ability of several altered versions of LcrV to support this function. The proteins used in these assays are shown diagrammatically in Fig. 3. HT-V represents full-length LcrV, while HT-VN68 and E15VN68 represent N-terminal truncations which lacked the first 67 residues of LcrV. VC216 was truncated at the C terminus and lacked the final 111 residues of LcrV, a domain which displays a high degree of sequence similarity (27.9% similarity and 49.5% identity) to PcrV of P. aeruginosa.
FIG. 3.
Schematic representation of LcrV proteins. Portions of lcrV were subcloned into expression vectors possessing IPTG-inducible promoters. HT-V (full-length LcrV) and HT-VN68 (N-terminally truncated LcrV) possesses an N-terminal leader sequence containing six His residues. Fusion of the YopE mRNA secretion signal to LcrV in pE15VN68 resulted in the addition of amino acids 1 to 15 of YopE to N-terminally truncated LcrV, yielding the protein E15VN68. It was expressed from the yopE promoter. VC216 represents a C-terminal truncation and lacks the last 111 residues of LcrV.
We first tested whether HT-V and HT-VN68 could restore the ability of a Y. pestis lcrV-null strain, KIM5-3241, to target Yops. The YopJ− mutation, also present in this strain, has been studied previously for its effect on LcrV function and LCR regulation (65). It was not expected to complicate our findings in any significant way. To confirm this and provide an initial characterization, the strains were analyzed for their growth at 37°C in the defined medium TMH and for their control of Yop expression and secretion in response to exogenous calcium (Fig. 4). As noted previously (65), Y. pestis KIM5-3241 expressed YopE weakly whether calcium was present or not and secreted very little (an amount not detectable at the loading used) in the absence of calcium. HT-V did not allow significant induction of Yop expression in the absence of calcium, but it did restore weak secretion of YopE, which was calcium regulated as in our Lcr+ parent strains KIM5-3002 (data not shown) and KIM8-3002 (Fig. 4A). HT-V itself was weakly secreted in the absence of calcium (Fig. 4A), showing that the His tag leader did not prevent LcrV secretion. HT-VN68 itself was never secreted, and it also did not allow strong induction of Yop expression in the absence of calcium, but it did permit weak secretion of YopE (Fig. 4A) and YopM (Fig. 4B). Curiously, some (weaker) secretion always occurred in the presence of calcium, indicating that Y. pestis KIM5-3241(pHT-VN68) is weakly constitutive, or depolarized, for secretion of Yops. Consistent with this, Y. pestis KIM5-3241(pHT-VN68) showed a “calcium-blind” growth phenotype (79); i.e., it entered growth restriction whether calcium was present or not (data not shown). These data indicate that although HT-VN68, like HT-V, can bind to LcrG and weakly activate the Ysc for Yop secretion by releasing the inner-gate block (Fig. 4 and reference 12), the HT-VN68–LcrG complex is unable to respond normally to the downregulating state of the Ysc in the presence of Ca2+ and to fully close the Ysc channel. Accordingly, the N terminus of LcrV is necessary for the normal regulation of a Ysc activation function.
FIG. 4.
Yop and V antigen expression and secretion in defined medium by lcrV yopJ Y. pestis KIM5-3241 complemented with pHT-V and pHT-VN68. Yersiniae pregrown at 26°C in the defined medium TMH containing (+) or lacking (−) 2.5 mM CaCl2 were shifted to 37°C, and IPTG was added to cultures containing pHT-V and pHT-VN68 to induce expression of the complementing lcrV genes. After 5 h, cultures were harvested, and proteins in whole cells (C) and the cell-free culture medium (M) were analyzed in immunoblots probed with a mixture of antibodies specific for LcrV and YopE (A) or anti-YopM (B). Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies followed by development with NBT-BCIP.
lcrV yopJ Y. pestis KIM5-3241 alone (as a negative control) and expressing HT-VN68 or HT-V (as a positive control) was used to infect HeLa cell monolayers in RPMI (Fig. 5). After 4 h of infection, HeLa cells infected with Y. pestis carrying pHT-V had rounded up, while those infected with lcrV yopJ Y. pestis alone or carrying pHT-VN68 had not (data not shown), suggesting that targeting of Yops was occurring only in the presence of HT-V. To confirm localization of Yops, we fractionated infected monolayers and looked for targeted proteins in the HeLa cell soluble fraction and for secreted proteins in the cell-free medium. Detection of YopE by immunoblot analysis revealed that YopE was indeed targeted by Y. pestis KIM5-3241(pHT-V), since it was detected in soluble fractions (Fig. 5, lanes 2 and 4) and was not susceptible to degradation by trypsin (lane 4). In contrast, Y. pestis KIM5-3241 lacking pHT-V released only a small amount of YopE into the culture medium (Fig. 5, lane 1) and did not target Yops into the HeLa cells (Fig. 6A, lanes 7 and 8, and data not shown). In agreement with the cytotoxicity observations, YopE was not targeted by Y. pestis KIM5-3241(pHT-VN68). Instead, the majority of YopE from this strain was released into the medium (Fig. 5, lane 7). Although a small portion was cell associated (lane 6), it was completely susceptible to trypsin (lane 8), indicating extracellular localization. The strong release of YopE into the medium (lane 7) compared to that from the lcrV yopJ strain lacking pHT-VN68 (lane 1) indicates that HT-VN68 was able to carry out LcrV’s function of Ysc activation for Yop secretion, as was seen in defined medium (Fig. 4). We also examined LcrV localization. HT-V was detected in the medium (Fig. 5, lane 3) and soluble (lanes 2 and 4) fractions, indicating again that the His tag leader did not interfere with LcrV secretion or its entry into cells. In contrast, HT-VN68 was not detected in either of the analyzed fractions (lanes 6 to 9); however, it was detected in debris fractions containing contact-activated yersiniae in repetitions of this experiment (data not shown). Taken together, these results indicate that the first 67 residues of LcrV are necessary for release of LcrV from bacteria and for LcrV that can function in Yop targeting, although they are dispensable for LcrV’s ability to promote secretion of Yops to the bacterial surface.
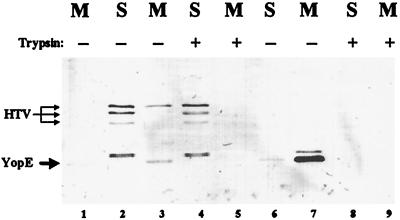
FIG. 5.
HT-VN68 is not secreted by Y. pestis and cannot complement Yop targeting. Y. pestis KIM5-3241 (lcrV yopJ) (lane 1) or Y. pestis KIM5-3241 carrying pHT-V (lanes 2 to 5) or pHT-VN68 (lanes 6 to 9) was used to infect HeLa cells, in duplicate, at an MOI of 10 in the presence of 0.1 mM IPTG. After 4 h, one replicate for each was treated with trypsin (lanes 4, 5, 8, and 9) at 100 μg/ml to assess protease accessibility of HT-V, HT-VN68, and YopE. The presence of proteins in cell-free medium (M) or H2O-released HeLa cell soluble (S) fractions was analyzed by resolving samples representing 20% of the original cultures in a 12% polyacrylamide gel followed by immunoblotting. Blots were probed with α-HTV and α-YopE, and proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies followed by development with NBT-BCIP.
FIG. 6.
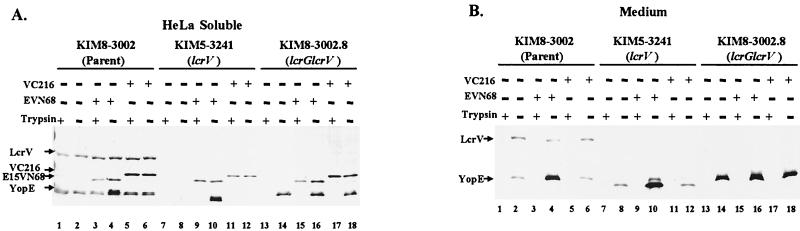
Both the N and C termini of LcrV are required to mediate Yop targeting and for release of LcrV from bacteria. Y. pestis KIM8-3002 (parent), KIM5-3241 (lcrV yopJ), and KIM8-3002.8 (lcrG lcrV) alone (lanes 1, 2, 7, 8, 13, and 14), carrying pVC216 (lanes 5, 6, 11, 12, 17, and 18) or carrying pE15VN68 (lanes 3, 4, 9, 10, 15, and 16) were used to infect HeLa cells, in duplicate, at an MOI of 10 in the presence of 0.2 mM IPTG. After 4 h, trypsin was added to replicate wells at 100 μg/ml to assess protease accessibility of LcrV and YopE. Samples were fractionated, and portions representing 12% of the original cultures were resolved in 12% polyacrylamide gels. Native LcrV, truncated LcrVs, and YopE in the HeLa cell soluble fraction (A) or released into culture medium (B) were detected by probing blots with α-HTV and α-YopE. Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies followed by development with NBT-BCIP.
One possible explanation for why HT-VN68 did not mediate targeting of Yops was that although the truncated protein had the capacity to function in Yop targeting, it needed to be secreted to have this effect. In an attempt to accomplish secretion of N-terminally truncated LcrV, we constructed E15VN68 by fusing the YopE mRNA-dependent secretion signal to LcrVN68, because this secretion signal can mediate secretion of a heterologous protein by yersiniae (1, 7). We also tested the function of C-terminally truncated VC216. E15VN68 and VC216 were expressed in the parent Y. pestis KIM8-3002 and in two strains defective in the ability to target Yops: KIM5-3241 (lcrV yopJ) and KIM8-3002.8 (lcrG lcrV). The ability of these truncated versions of LcrV to be secreted and support Yop targeting was tested in the HeLa cell infection assay (Fig. 6). After 4 h of infection, localization of E15VN68, VC216, and YopE in HeLa cell soluble (Fig. 6A) and culture medium (Fig. 6B) fractions was analyzed. As expected, the parent Y. pestis targeted YopE (Fig. 6A, lanes 1 and 2), whereas Y. pestis lacking lcrV (lanes 7 and 8) or lacking lcrG and lcrV (lanes 13 and 14) did not. When expressed in the parent Y. pestis strain, neither of the truncated LcrVs interfered with the ability of the strain to target YopE. YopE was detected in the HeLa cell soluble fraction (lanes 3 to 6) and was protected from degradation by trypsin (lanes 3 and 5). Expression of either E15VN68 or VC216 in lcrV and lcrG lcrV Y. pestis failed to complement the Yop-targeting defect. Although YopE was detected in soluble fractions (Fig. 6A, lanes 10, 14, 16, and 18), it was susceptible to degradation by trypsin (lanes 9, 11, 15, and 17), indicating an extracellular localization prior to lysis of the cells. In agreement with these data, infections with these strains did not result in cytotoxicity of HeLa cells (data not shown). Interestingly, both truncated versions of LcrV became associated with the HeLa cells, regardless of the strain in which they were expressed (Fig. 6A, lanes 3, 4, 9, 10, 15, and 16 for E15VN68 and lanes 5, 6, 11, 12, 17, and 18 for VC216). However, although native LcrV was secreted by the parent strain and appeared in the culture medium (Fig. 6B, lanes 2, 4, and 6), neither E15VN68 (lane 4) nor VC216 (lane 6) was detected in the culture medium. Secretion of the truncated proteins was not detected even from lcrG lcrV Y. pestis, a strain depolarized for Yop secretion (Fig. 6B, lanes 15 to 18). In other experiments, these Y. pestis strains failed to secrete E15VN68 and VC216 into the medium when the yersiniae were grown in vitro at 37°C in the defined medium TMH lacking Ca2+ (data not shown). Levels of YopE in medium fractions from the parent (Fig. 6B, lane 4) or lcrV (Fig. 6B, lane 10) strain carrying E15VN68 were elevated compared to amounts released by those host strains alone (Fig. 6B, lanes 2 and 8, respectively), indicating that E15VN68 (like HT-VN68) functioned to induce Yop secretion to the bacterial surface, even though it could not sponsor targeting of Yops into HeLa cells. We speculate that this results from the previously described ability of LcrV to bind LcrG (41).
Surface localization of LcrV on Y. pestis.
Nilles et al. (41) and Sarker et al. (60) had speculated that LcrV is a dynamic participant in the secretion process and may even mediate assembly of a surface structure for the delivery of Yops. According to this hypothesis, LcrV may be present on the outer surface of Y. pestis. We tested this possibility by staining bacteria with α-HTV and analyzing LcrV distribution by laser-scanning confocal microscopy (Fig. 7). Y. pestis was incubated in RPMI, a medium that does not promote secretion of LcrV or Yops in the absence of contact with eukaryotic cells (13). Surface deposition of LcrV was tested by probing fixed but unpermeabilized bacteria with α-HTV. Staining with antibodies specific for Yersinia lacking pCD1 was used as a positive control and produced an even, homogeneous surface staining of the bacteria. However, probing with α-HTV showed that LcrV was present in punctate zones on the surface of the bacteria. This deposition was not affected by Pla, since this surface-associated pattern of LcrV was also detected in Y. pestis lacking the plasmid pPCP1 (data not shown). We also tested localization of LcrV in a strain lacking the outer membrane secretin YscC, which is essential for Ysc function. A control experiment verified that the amounts of LcrV in the bacteria were the same for the parent and yscC strains after 2 h in RPMI at 37°C with 5% CO2; i.e., the yscC mutation did not prevent thermal induction of LcrV expression under these conditions (data not shown). No fluorescence signal was detected on the surface of yscC Y. pestis KIM8-3001.12 (Fig. 7A), indicating that LcrV requires the Ysc machinery to gain access to the bacterial surface.
We used this surface localization assay to test whether the truncated forms of LcrV could gain access to the bacterial surface when expressed in Y. pestis. To detect only the truncated proteins, HT-VN68, E15VN68, and VC216 were expressed in the lcrV-null strain. In this test, we used Y. pestis KIM8-3241, a Pla− derivative of this strain, to ensure detection of the truncated LcrV proteins at the bacterial surface even if they should have increased sensitivity to Pla. All three strains failed to produce detectable signals, indicating that the truncated proteins were not on the surface of Y. pestis (Fig. 7A and data not shown). The parent Y. pestis strain was also stained with α-YopE as a negative control (Fig. 7B). No signal was detected by confocal analysis. YopE could be detected by this method, however, when the depolarized strain KIM8-3241(pHT-VN68) was tested as a positive control (Fig. 7B). These results indicate that LcrV, but not YopE, is present on the surface of non-contact-induced Y. pestis after passing through the Ysc. We believe that this is a stable localization, because surface localization of LcrV was detected in the absence of conditions sufficient to allow secretion of LcrV and Yops into the culture medium. LcrV’s linkage to the surface is probably not through Pla-susceptible proteins, since LcrV was detected on the surface of Y. pestis possessing pPCP1 (not shown). Finally, N- and C-terminally truncated LcrVs are neither localized to the bacterial surface nor secreted into the medium, even though they apparently are targeted to the HeLa cell interior by contact-activated yersiniae.
LcrV-specific antibody does not block targeting of Yops and LcrV.
Our previous results suggested that LcrV may need to be secreted to have its role in targeting of Yops, raising the possibility that the surface-localized LcrV may be important for Yop targeting. Since we were able to detect surface-localized LcrV with antibody, we tested whether LcrV-specific antibodies would interfere with Yop targeting (Fig. 8). In one experimental design, α-HTV or α-YopM was present at an estimated 2,000-fold molar excess over the amount of LcrV that typically is expressed in the infected monolayer (see Materials and Methods). The α-YopM served as an irrelevant antibody against a target not expected to be at the bacterial surface to control for nonspecific effects of immunoglobulin on the assay. The antibody was added to Y. pestis KIM8-3002 just prior to infection of washed HeLa cells, but the bacteria were allowed to settle by gravity onto the monolayer, instead of being rapidly brought into contact by centrifugation, to allow the antibody time to interact with bacteria and HeLa cells. In some wells, the HeLa cells were pretreated for 30 min with antibody from preimmune serum (α-NS), and α-NS was also present along with α-HTV or α-YopM during the infection. The monolayers were observed for retraction and rounding up (cytotoxicity), which are indicative of targeting of YopE. Figure 8A shows that by 3 h, all infected monolayers were showing clear signs of cytotoxicity (data not shown for wells containing α-NS). By 4 h, the infected cells were further rounded up. This test indicated that LcrV-specific antibody did not block targeting of Yops by Y. pestis.
FIG. 8.
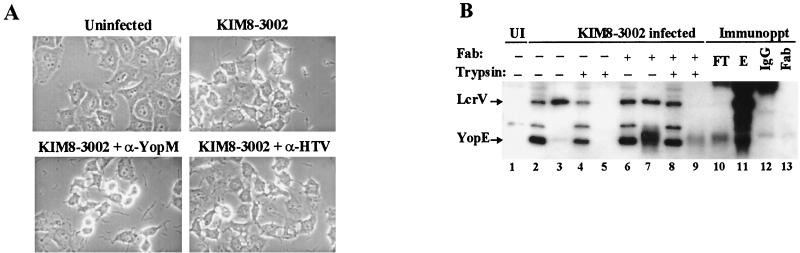
LcrV-specific antibody does not neutralize Yop-targeting activity by nonpreinduced Y. pestis. (A) α-HTV or α-YopM antibody was added at 175 μg/ml to warm RPMI containing Y. pestis KIM8-3002, and the mixture was added without centrifugation to monolayers of HeLa cells at an MOI of 10. After incubation for 3 h at 37°C with 5% CO2, the cultures were visualized by phase-contrast microscopy and photographed through a green filter. (B) HeLa cell monolayers were untreated (UI) (lane 1) or infected with Y. pestis KIM8-3002 at an MOI of 10 in the presence (lanes 6 to 9) or absence (lanes 2 to 5) of LcrV-specific Fab antibody fragments (α-HTV Fab). For this experiment, Fab antibody fragments were present at 26.5 μg/ml, corresponding to a 1,000-fold molar excess over estimated LcrV levels. After 4 h, replicate wells were treated with trypsin at 100 μg/ml prior to harvesting (lanes 4, 5, 8, and 9) or were harvested directly. Samples were fractionated into cell-free supernatants (lanes 3, 5, 7, and 9) and HeLa cell soluble fractions (lanes 1, 2, 4, 6, and 8). To verify that released LcrV could be quantitatively bound by the Fab fragments, LcrV bound by α-HTV Fab was immunoprecipitated (Immunoppt) from a nontrypsinized supernatant fraction by using anti-IgG and protein A-agarose beads. The combined void and protein A column washes (FT), elution (E) fractions, and fractionated culture samples were analyzed by immunoblotting with α-HTV and α-YopE. Preparations of whole antibody (IgG) (lane 12) and Fab fragments (lane 13) were also resolved as references. Proteins were visualized by probing with horseradish peroxidase-coupled secondary antibody, developed with ECL reagent, and exposed to film.
In a second experiment, Fab fragments derived from α-HTV were used to avoid any artificial association of bacteria through whole antibodies binding to Fc receptors on HeLa cells (Fig. 8B). The Fab fragments were added at an estimated 1,000-fold molar excess over the total amount of LcrV that was anticipated to be present in each well. Analysis of extracts from monolayers infected with Y. pestis in the absence (Fig. 8B, lanes 2 to 5) and presence (lanes 6 to 9) of excess α-HTV Fab fragments revealed that targeting occurred in the presence of antibody fragments; comparable levels of trypsin-resistant YopE were detected in the absence (lane 4) or presence (lane 8) of Fab fragments. Partitioning of LcrV among culture fractions was also unaffected in this assay. LcrV was released into the medium (lanes 3 and 7) and became cell associated (lanes 4 and 8), regardless of the presence of Fab fragments. To be certain that all available LcrV was associated with antibody, we immunoprecipitated Fab fragments (and associated LcrV) from the culture medium. The elution (Fig. 8B, lane 11) but not the flowthrough (lane 10) from this column contained LcrV, indicating that all extracellular LcrV had been bound by antibody fragments.
In the experiments of Fig. 8, the yersiniae had come into contact with the HeLa cells after being at 37°C for a relatively short time following pregrowth at 26°C, a temperature that supports only weak expression of LcrV and no secretion. After contact, the yersiniae may have developed full induction and expression of their Yop delivery mechanism, and antibody might potentially have had only limited access to the functional targeting complexes at the junction between the bacterium and the HeLa cell. Accordingly, these experiments may have shown only that antibody cannot block targeting once yersiniae have become attached to host cells.
To address this limitation, we preincubated the yersiniae under the exact conditions used to demonstrate surface LcrV. During the last 30 min of the 2-h induction period, the yersiniae were in the presence of α-HTV or α-YopM (as an irrelevant antibody), as in Fig. 8A, or no antibody. Then they were used to infect HeLa cells that had been pretreated with 1% MS to block Fc receptors. This experiment was done for MOIs of 10 and 2 with similar results. With these preinduced yersiniae, cytotoxicity developed more quickly than had been seen with nonpreinduced bacteria. Cytotoxicity at 1 h of infection (the earliest we checked) was fully developed for an MOI of 10 and had clearly begun to develop for an MOI of 2. The degree of cytotoxicity seen at this or longer infection times was not affected by either antibody treatment, indicating that Yop targeting was occurring, despite the presence of α-HTV or the control antibody α-YopM (Fig. 9A). In the test using an MOI of 10 and antibodies at 175 μg/ml, both antibodies did cause a significant reduction in the amount of YopE in the trypsin-inaccessible soluble fraction as determined by immunoblotting; however, this was a nonspecific effect that was equivalent for the two antibodies (not shown). When the MOI used was 2, we correspondingly reduced the antibody concentration to 35 μg/ml, in hopes of eliminating this nonspecific effect while maintaining the same high ratio of antibody to LcrV in the assay. Figure 9B shows immunoblot analysis of the fractionated infected cultures from that experiment. Equivalent amounts of YopE were inaccessible to trypsin in the HeLa cell soluble fraction in all infected cultures, whether or not either antibody was present.
FIG. 9.
LcrV-specific antibody does not neutralize Yop-targeting activity of Y. pestis preinduced for expression of LcrV and Yops. Y. pestis KIM8-3002 was grown overnight at 26°C, diluted into warm RPMI, centrifuged in six-well dishes, and incubated for 2 h at 37°C with 5% CO2 to allow surface expression of LcrV. After 1.5 h of the incubation, the bacteria were resuspended, and α-YopM or α-HTV was added to some wells. The yersiniae were used to infect HeLa cells at an MOI of 2, and some wells were photographed at hourly intervals, up to 4 h, through phase-contrast optics and a green filter. (A) Cultures after 2 h of infection. (B) After 4 h of infection, replicate cultures were treated (+) or not (−) with trypsin, harvested, and fractionated as described in Materials and Methods, and TCA-precipitated proteins from cell-free medium (Med) and HeLa cell soluble (Sol) fractions were analyzed by immunoblotting with a mixture of α-HTV and α-YopE antibodies as a probe. The rightmost two lanes contain 0.2 and 2.0 μg of α-HTV as a reference for the size of the predominant band due to antibody. The proteins were visualized by probing with horseradish peroxidase-coupled secondary antibody followed by development with ECL reagent and exposure to film.
These experiments showed that our α-HTV preparation does not block Yop targeting, despite being present in a large concentration. This antibody had previously been shown to confer protection against Y. pestis KIM5 (39). In this study, we confirmed that this antibody preparation protects mice against intravenous challenge with a lethal dose (ca. 5 and 18 50% lethal doses, respectively, in two experiments) of Y. pestis KIM5-3001. In the two trials, all mice that received α-HTV survived, whereas all that had been treated with Fab fragments of α-NS died. Accordingly, our findings showed that our α-HTV preparation was protective against experimental plague, even though it was not able to block targeting of Yops.
DISCUSSION
In this study, we have found that LcrV’s N and C termini are required for Yop targeting. We also demonstrated a novel surface localization of LcrV that did not occur for truncated forms of LcrV unable to sponsor Yop targeting, and we speculate that the secretion of LcrV is required for targeting to occur. However, LcrV’s function in targeting of Yops was not blocked by our protective LcrV-specific polyclonal antibody.
Our initial tests for requirements of the targeting reaction stemmed from our previous finding that LcrV was required for targeting of Yops into eukaryotic cells but that LcrG was necessary for maximal hemolysis by Y. pestis, which was an indirect assay for a functional Yop delivery channel (41). In that study we were not able to clearly demonstrate LcrG’s role in targeting, because the LcrG− strain we used expressed LcrV only weakly, and supplying LcrG in trans without also supplying LcrV caused downregulation and blockage of secretion. Accordingly, we wanted to know whether Y. pestis was like Y. enterocolitica (61) in that LcrV may function more efficiently in Yop targeting in the presence of LcrG. Our new, nonpolar ΔlcrG3 strain indeed induced cytotoxicity significantly more slowly than did the parent Y. pestis strain, but its Yop-targeting defect could be alleviated by complementation with lcrG or by providing excess LcrV, indicating that LcrG is not essential for Yop targeting but is indirectly involved. Curiously, LcrV was secreted at significantly lower levels by the ΔlcrG3 mutant than by the parent strain. Release of LcrV into the medium could be increased to near-wild-type levels when lcrV or lcrG was expressed in trans. Clearly, LcrG is not essential for LcrV secretion, but it may function to make secretion of LcrV more efficient. Future studies will determine whether it mediates this effect within the bacteria by modulating Ysc activity or at the bacterial surface, where it conceivably could be acting in association with LcrV.
To begin to dissect how LcrV carries out its multiple functions, we tested whether two versions of LcrV could support Yop secretion and targeting. HT-VN68 was able to support secretion of YopE, consistent with the idea that LcrV participates in Ysc activation by binding to the inner-gate protein LcrG, since this N-terminally truncated version retains a domain required for association with LcrG (60), and cross-linking experiments have shown that HT-VN68 and LcrG can interact (12). However, HT-VN68 did not support targeting of YopE into the HeLa cell cytoplasm. We believe that this defect in targeting was probably not due to the His-containing N-terminal leader, since a similarly tagged full-length LcrV supported targeting of YopE. Curiously, HT-VN68 itself was not secreted into the medium by Y. pestis during infection of HeLa cells, an observation consistent with its inability to be secreted during growth in defined medium under inductive conditions (Fig. 4). Therefore, the inability of HT-VN68 to promote targeting of YopE could be due either to the altered localization of LcrV or to the lack of a domain directly required for LcrV’s activity in Yop targeting. In an attempt to achieve secretion of N-terminally truncated LcrV, we fused the mRNA secretion signal of YopE to 5′-truncated lcrV. However, the resulting fusion protein, E15VN68, still was not secreted in defined medium by parent, lcrV, or lcrG lcrV Y. pestis (data not shown), nor was it released into the culture medium in the tissue culture infection model, and it failed to complement the Yop-targeting defects in Y. pestis lcrV or lcrG lcrV strains (Fig. 6). Although we did not test this, we predict that E15VN68 can productively associate with LcrG, because when E15VN68 was expressed in parent or lcrV Y. pestis, significantly more YopE was released into the medium than was released by these strains lacking E15VN68. The C-terminally truncated VC216 also was not released into defined medium or into the medium of infected HeLa cells by parent, lcrV, or lcrG lcrV Y. pestis, and VC216 was unable to support Yop targeting (Fig. 6 and data not shown). However, it did not promote YopE secretion into the medium (Fig. 6B). Interestingly, VC216 does cross-link to LcrG when lcrV Y. pestis KIM5-3241 expressing pVC216 is grown in the defined medium TMH at 37°C in the absence of calcium and whole-cell fractions are treated with dithiobis(succinimidylpropionate) (12). VC216 lacks LcrV residues 224 to 266, which, when deleted from GST-LcrV, prevented this fusion protein from binding LcrG in a pull-down experiment where the two proteins were overexpressed in E. coli (60). Accordingly, GST-LcrVΔ224–266 and VC216 may differ significantly in conformation, such that VC216 can bind LcrG but LcrVΔ224–266 cannot. However, the VC216-LcrG interaction apparently is not functional in activating the Ysc. In Yersinia, the functional LcrV-LcrG interaction involves components of the Ysc to achieve activation or blocking of secretion, and the binary interaction of LcrV and LcrG is only part of the picture. Our truncated LcrV proteins have revealed differential roles of LcrV domains in these processes. HT-VN68 is able to weakly activate the Ysc but is defective in secretion shutdown, indicating a role for the N terminus of LcrV in secretion shutdown, and VC216 cannot activate the Ysc, indicating a role for the C terminus in Ysc activation. Further investigation will be required to ascertain the respective roles of each of these domains in secretion of LcrV and to learn why YopE’s mRNA secretion signal did not work for secretion of HT-VN68.
Our experiments have shown that both the N and C termini are required for LcrV’s role in targeting of Yops. While this paper was being revised, a study by Pettersson et al. (48) appeared, confirming LcrV’s role in Yop targeting and assessing the function of LcrV with the internal residues 160 to 171 deleted or lacking the C-terminal 10, 20, and 30 residues. These LcrV variants were not able to support Yop secretion in vitro when expressed in trans in lcrV Y. pseudotuberculosis. These data support the idea that the C terminus of LcrV (as well as residues 160 to 171) is necessary for Ysc activation. The LcrV variants also could not support Yop targeting when expressed in trans in an lcrV yopN background, even though the yopN mutation was expected to relieve any lcrV-derived defect in Yop expression and secretion. Interestingly, these LcrV variants could be secreted into the medium by the lcrV yopN double mutant in vitro, removing any argument that lack of secretion of the variant LcrVs per se underlay their inability to promote Yop targeting. Accordingly, the failure of the LcrV variants to support Yop targeting suggests that the C terminus of LcrV and residues 160 to 171 function directly in the targeting process.
Intriguingly, both E15VN68 and VC216 gained access to the HeLa cell cytoplasm. Entry of E15VN68 into HeLa cells did not involve the Syc-dependent YopE-derived secretion signal, since E15VN68 lacks the SycE-binding domain necessary for targeting of YopE (62), and E15VN68 still gained access to HeLa cytoplasm when expressed in a Y. pestis yopB strain (11). We believe that this entry is not an artifact of the fractionation protocol but is the result of a novel secretion pathway that is described elsewhere (13).
The correlation between the abilities to secrete LcrV and to target Yops suggests that secretion of LcrV is required for this protein to function in Yop targeting. LcrV can associate with the Yop translocator proteins YopB and YopD, which are thought to function at the interface between bacteria and associated eukaryotic cells, and there has been speculation that an LcrV-containing structure on the bacterial surface may mediate Yop delivery (60). Cornelis (8) speculated that LcrV may be a component of an extracellular “injectisome” similar to the apparatus visualized in Salmonella (26). Therefore, we tested whether LcrV could have a surface localization consistent with this hypothesis. Analysis of uninduced lcrV yopJ yersiniae by confocal microscopy revealed an LcrV-specific, punctate outer surface staining pattern (Fig. 7). This localization was unique to LcrV, since the effector protein YopE was not detected under similar conditions. However, we did detect a similar staining pattern for YopE on bacteria that had been rendered depolarized for secretion by expression of HT-VN68, suggesting that the punctate staining may correlate to Ysc secretion channels. An intact Ysc was required for this localization, since LcrV was not detected on the surface of a Y. pestis yscC strain, despite being present within the yersiniae. Similar findings were made by Pettersson et al. (48). Consistent with its inability to be secreted, HT-VN68 was not detected on the surface of Y. pestis, indicating that the N terminus is required to traverse the Ysc. This defect was also seen for the C-terminally truncated VC216 (data not shown). It is significant that surface-localized LcrV was detected on bacteria cultivated under conditions that do not promote Yop secretion (Fig. 7) as well as on the distal side of yersiniae bound to HeLa cells (data not shown). In both cases, we speculate that LcrV is stably anchored to nonsecreting channels. How LcrV is anchored to the surface is unclear, but it is probably not through a previously characterized Yop, since LcrV had a surface localization on Pla+ Y. pestis (data not shown). When the Ysc becomes activated, LcrV is released into the medium. It is possible that LcrV is secreted directly into the medium by the Ysc; alternatively, the hypothetical LcrV-containing structures may be destabilized. Perhaps the Yop-targeting mechanism is a dynamic structure, assembling and disassembling, and this process releases at least some of the components of the Yop-targeting complex into the surrounding medium. Consistent with this idea, YopB and YopD, which also are thought to function in Yop targeting (9), have been found to be released in the medium by Y. enterocolitica infecting HeLa cells (29).
LcrV-specific antibodies have previously been thought to neutralize the diffusible immunomodulatory activity of LcrV, since Nakajima and Brubaker (36) showed that α-LcrV antibody administered during infections of mice with Y. pestis restored production of tumor necrosis factor alpha and gamma interferon. However, given LcrV’s role in Yop targeting and our speculation that LcrV needs to be secreted to have its effect, we wondered whether LcrV-specific antibodies might be protective because they block the Yop-targeting activity of LcrV. We were unable to attenuate Yop targeting by either full-length LcrV-specific antibody or α-LcrV Fab fragments, even though our polyclonal LcrV-specific IgG preparation has been shown to provide protection against plague in mice (reference 39 and this study). This is in contrast to the conclusion reached by Pettersson et al., who observed decreased cytotoxicity when HeLa cells were infected for 3 h with Y. pseudotuberculosis in the presence of an LcrV-specific polyclonal antiserum or a protective monoclonal antibody directed against LcrV residues 135 to 275 (48). The MOI of 2 used for those experiments is low compared to usual practice, but this may be crucial to see the partial effect on cytotoxicity. In our test at an MOI of 2, we still saw no effect of α-HTV on cytotoxicity or targeting of YopE. Evidence indicates that there are at least two protective epitopes on LcrV, lying in residues 2 to 135 and 135 to 275 (21, 35). These may be differentially represented in the antibody preparations used by Pettersson et al. and in the present study. Evidence for a qualitative difference between the antibody preparations is that the anti-LcrV antiserum used by Pettersson et al. did not recognize PcrV in immunoblots (48), whereas α-HTV does (16). We do not dispute that antibody to LcrV can diminish targeting of Yops and that this might contribute to protection. However, we speculate that in our protection experiments, interference with Yop targeting was a minor effect of LcrV-specific antibody and that there are other ways that antibody acts to protect against plague. Future work is required to determine whether our antibodies might protect by opsonizing through antibody association with surface LcrV or by neutralizing a diffusible activity of LcrV.
In conclusion, we propose a model (Fig. 10) in which LcrV associates with the extracellular surface of yersiniae at Ysc secretion channels either directly or through a non-Pla-susceptible protein(s). Surface-localized LcrV may mediate assembly of a Yop delivery apparatus which provides a link between the Ysc channel and the associated eukaryotic cell. We did not formally prove that LcrV must be secreted to function in targeting, although we established several correlations that suggest this hypothesis. Future work will define the role of LcrV secretion through the Ysc and determine whether LcrV functions as an integral structural element of the Yop-targeting apparatus, as a chaperone, or within the yersiniae as part of a Ysc gateway for proteins destined to be targeted. As a related issue, the significance of LcrV’s release into the medium needs to be determined. It could reflect a dynamic instability of the Yop delivery structure, which is inherent in the targeting mechanism of the activated Ysc. Finally, it is important to determine whether the released LcrV is a diffusible antihost protein with a significant immunomodulatory effect during infection.
FIG. 10.
Model for the role of surface-localized LcrV in Yop targeting. The Ysc creates a secretion channel through the bacterial inner membrane (IM), across the periplasm (PP), and through the outer membrane (OM). These pores remain blocked in the absence of direct contact with the eukaryotic cell plasma membrane (PM) by LcrE, TyeA, and LcrG. LcrV is associated with the extracellular surface of these closed pores directly or through an additional protein (X). Upon cell contact, LcrE, TyeA, and LcrG are displaced, and LcrV mediates formation of a delivery apparatus for the transfer of Yops directly from the bacteria to the eukaryotic cell cytoplasm.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI21017.
We gratefully thank Jon Goguen (University of Massachusetts) for providing the plasmid pOS50 and Gregory Plano (University of Miami) for the gift of α-YopE. We thank Andrew Williams and Greta Fowler for their work in generating Y. pestis KIM8-3241 from KIM5-3241 and acknowledge Mike Russ, University of Kentucky Macromolecular Structure Analysis Facility, for the synthesis of some of the oligonucleotides used in this study.
REFERENCES
- 1.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 2.Black D S, Bliska J B. Identification of p130cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker R R. The V antigen of yersiniae: an overview. Contrib Microbiol Immunol. 1991;12:127–133. [PubMed] [Google Scholar]
- 4.Brubaker R R, Beesley E D, Surgalla M J. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science. 1965;149:422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- 5.Burrows T W, Bacon G A. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956;37:481–493. [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanaugh D C, Randall R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of fleaborne plague. J Immunol. 1959;83:348–363. [PubMed] [Google Scholar]
- 7.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 11.Fields, K. A., and S. C. Straley. Unpublished data.
- 12.Fields K A. Ph.D. thesis. Lexington: University of Kentucky; 1999. [Google Scholar]
- 13.Fields K A, Straley S C. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect Immun. 1999;67:4801–4813. doi: 10.1128/iai.67.9.4801-4813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields K A, Williams A W, Straley S C. Failure to detect binding of LcrH to the V antigen of Yersinia pestis. Infect Immun. 1997;65:3954–3957. doi: 10.1128/iai.65.9.3954-3957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsberg Å, Viitanen A M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 16.Frank, D. W. Personal communication.
- 17.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 20.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 21.Hill J, Leary S E C, Griffin K F, Williamson E D, Titball R W. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect Immun. 1997;65:4476–4482. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fallman M, Magnusson K E, Wolf-Watz H, Forsberg Å. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Micrbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 23.Iriarte M, Cornelis G R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 24.Iriarte M, Sory M P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubori T, Matsushima Y, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lambert de Rouvroit C L, Sluiters C, Cornelis G R. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol Microbiol. 1992;6:395–409. [PubMed] [Google Scholar]
- 29.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindler L E, Klempner M S, Straley S C. Yersinia pestis pH6 antigen: genetic, biochemical, and virulence characterization of a protein involved in pathogenesis in bubonic plague. Infect Immun. 1990;58:2569–2577. doi: 10.1128/iai.58.8.2569-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 32.Metcalf W W, Jiang W, Daniels L L, Kim S-K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Mol Microbiol. 1996;24:73–91. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 33.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G R. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 35.Motin V L, Nakajima R, Smirnov G B, Brubaker R R. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect Immun. 1994;62:4192–4201. doi: 10.1128/iai.62.10.4192-4201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:21–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedialkov Y A, Motin V L, Brubaker R R. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect Immun. 1997;65:1196–1203. doi: 10.1128/iai.65.4.1196-1203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth J, Straley S C. Effect of Yersinia pestis YopM on experimental plague. Infect Immun. 1997;65:924–930. doi: 10.1128/iai.65.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neyt C, Cornelis G R. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopB. Mol Microbiol. 1999;31:143–156. doi: 10.1046/j.1365-2958.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 41.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilles, M. L., and S. C. Straley. Unpublished data.
- 43.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry R D, Pendrak M, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F R. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130cas and FAK, and the associated accumulation of these proteins in focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersson J, Holmström A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg Å, Wolf-Watz H. The V-antigen of Yersinia is surface-exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 49.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 50.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price S B, Cowan C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rimpilainen M, Forsberg Å, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosqvist R, Forsberg Å, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 56.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kohler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvment in the induction of programmed cell death and in the suppression of macrophage TNF-α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1998;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 59.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarker M, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarker M, Sory M, Boyd A P, Iriarte M, Cornelis G R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skrzypek E, Cowan C, Straley S C. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol Microbiol. 1998;30:1051–1065. doi: 10.1046/j.1365-2958.1998.01135.x. [DOI] [PubMed] [Google Scholar]
- 64.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on the secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sodeinde O A, Goguen J D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988;56:2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sodeinde O, Sample A K, Brubaker R R, Goguen J D. Plasminogen activator/coagulase of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56:2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing down regulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 69.Straley S C. The plasmid-encoded outer-membrane proteins of Yersinia pestis. Rev Infect Dis. 1988;10(Suppl. 2):S323–S326. doi: 10.1093/cid/10.supplement_2.s323. [DOI] [PubMed] [Google Scholar]
- 70.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Surgalla M J, Beesley E D, Brubaker R R. Influence of unsaturation on fibrinolytic activity of salts of fatty acids. Proc Soc Exp Biol Med. 1967;126:256–258. doi: 10.3181/00379727-126-32416. [DOI] [PubMed] [Google Scholar]
- 72.Towbin H, Staehelin T, Gordon J. Electophoretic transfer of proteins from polacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Une T, Brubaker R R. Role of V antigen in promoting virulence in yersiniae. Contrib Microbiol Immunol. 1987;9:179–185. [PubMed] [Google Scholar]
- 75.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaparones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welkos S, Friedlander A, McDowell D, Weeks J, Tobery S. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb Pathol. 1998;24:185–196. doi: 10.1006/mpat.1997.0188. [DOI] [PubMed] [Google Scholar]
- 77.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yahr T L, Mende-Mueller L M, Friese M B, Frank D W. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahorchak R J, Charnetsky W T, Little R V, Brubaker R R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979;139:792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]