Abstract
Cognitive impairments and emotional lability are common long-term consequences of traumatic brain injury (TBI). How TBI affects interactions between sensory, cognitive, and emotional systems may reveal mechanisms that underlie chronic mental health comorbidities. Previously, we reported changes in auditory-emotional network activity and enhanced fear learning early after TBI. In the current study, we asked whether TBI has long-term effects on fear learning and responses to novel stimuli. Four weeks following lateral fluid percussion injury (FPI) or sham surgery, adult male rats were fear conditioned to either white noise-shock or tone-shock pairing, or shock-only control and subsequently were tested for freezing to context and to the trained or novel auditory cues in a new context. FPI groups showed greater freezing to their trained auditory cue, indicating long-term TBI enhanced fear. Interestingly, FPI-Noise Shock animals displayed robust fear to the novel, untrained tone compared with Sham-Noise Shock across both experiments. Shock Only groups did not differ in freezing to either auditory stimulus. These findings suggest that TBI precipitates maladaptive associative fear generalization rather than non-associative sensitization. Basolateral amygdala (BLA) α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAr) subunits GluA1 and GluA2 levels were analyzed and the FPI-Noise Shock group had increased GluA1 (but not GluA2) levels that correlated with the level of tone fear generalization. This study illustrates a unique chronic TBI phenotype with both a cognitive impairment and increased fear and possibly altered synaptic transmission in the amygdala long after TBI, where stimulus generalization may underlie maladaptive fear and hyperarousal.
Keywords: AMPA receptor, amygdala, associative learning, defensive behavior, fear conditioning, post-traumatic stress
Introduction
Traumatic brain injury (TBI) is a significant risk factor for long-term mental health conditions such as anxiety disorders1–3 and post-traumatic stress disorder (PTSD).4 In both military and civilian populations, the presence of mental health comorbidities is associated with increased rates of persistent post-concussive symptoms,5 even in adolescents.6 Learning and memory impairments are common after TBI, and can last years or even decades.7 The lasting effect of a TBI on neural systems that coordinate sensory, emotional, and cognitive function could have a significant impact on how threats and stressors are perceived, encoded, and generalized.8 Disruptions to these systems could therefore influence the development of later stress- and trauma-related comorbidities.
When a cue reliably signals danger, species-specific defense reactions are engaged to promote survival.9 Heightened or enhanced defense reactions in the absence of a true threat however, can be maladaptive as they are energetically costly and occur at the expense of adaptive activity, that is, foraging, mating, etc.10 Fear generalization occurs when a defensive response previously associated with a particular stimulus and an aversive experience transfers to another stimulus. Sometimes referred to as overgeneralization, this enhanced and inappropriate fear response is dependent on associative mechanisms related to the initial event and is a feature of anxiety- and stress-related disorders.11–13
Learning protocols such as Pavlovian fear conditioning are useful in both clinical14–17 and animal model settings in understanding how aversive experiences are encoded and can evolve into generalized and exaggerated fear. An alternative basis for heightened defense reactions in response to innocuous stimuli is known as sensitization. Sensitization, a non-associative process, is related to a hyperarousal effect from the initial trauma rather than stimulus generalization. Either way, heightened fear and anxiety in the presence of safe or novel stimuli can interfere with daily function. Individuals with TBI often have lasting physical symptoms of heightened stimulus sensitivity,18,19 which may influence the perception, encoding, and reaction to a subsequent aversive experience, permitting for increased vulnerability to fear generalization or sensitization. Uncovering differences in the behavioral and neural mechanisms of heightened defense reactions after TBI will help us in determining optimal neurobiological targets to treat these common and troubling sequelae.20
Previously, we reported that when auditory fear conditioning with white noise occurs within 3 days of lateral fluid percussion injury (FPI), fear learning and defensive behavior are heightened.21,22 In our model, we have reliably replicated an enhanced fear phenotype in the early days after FPI. However, it is important to determine the long-lasting impact of TBI on fear learning and defensive phenotypes. Many clinical studies report long-term consequences on mental health following a TBI.1,23–26 Further, we have seen enhanced dendritic complexity in principal neurons in the amygdala within a day and enduring at least 4 weeks after a single FPI,27 suggesting a lasting impact on the neural substrates of fear and defensive behavior.28,29 Structural imaging in service members with TBI found increased amygdala volume in patients long after the injury was sustained.30 Therefore, in the current study we asked whether and how associative and non-associative fear learning is impacted in the chronic phase following lateral FPI in adult male rats. We also sought to determine if there were changes in proteins related to increased plasticity in the amygdala during a heightened defensive phenotype in the chronic phase after FPI.
Methods
Subjects and lateral fluid percussion injury (FPI)
Young adult male Sprague-Dawley rats (Envigo, 250–275 g upon arrival, approximately 9–10 weeks old) were pair housed in standard, non-enriched sedentary housing and maintained on a 12-h light/dark cycle with ad libitum food and water. All animal procedures were approved by the University of California, Los Angeles (UCLA) Animal Care and Use Committee. Young adult males were used due to epidemiological data indicating that males are at a significantly higher risk for TBI, with the highest male-to-female ratios occurring in young adulthood.31 All groups were handled for 1min/day for 4 days prior to either mild-moderate lateral FPI or sham surgery. Lateral FPI was induced using a previously published protocol21,22,32–35 used in our laboratory. Animals were anesthetized under a 1–2% isoflurane-oxygen mixture.
A midline incision was made followed by a left hemisphere 3-mm diameter craniotomy centered 3 mm posterior and 6 mm lateral to bregma. A plastic injury cap was adhered to the skull with silicone gel and dental cement and filled with sterile saline. The animal was removed from anesthesia and the injury cap was attached to the FPI device (Virginia Commonwealth University, Richmond, VA, USA). Upon toe-pinch response, a brief fluid pulse (∼20 msec) of saline was administered directly to the dura mater. Apnea and loss of consciousness (LOC; measured by toe-pinch response) were measured to determine injury severity. Rats were then placed back on anesthesia to remove the injury cap and suture the scalp. Sham animals received the same surgical procedures except fluid pulse impact. Upon completion of surgery, animals were placed in a heated recovery chamber until normal behavior resumed and then were returned to the vivarium. Injury severity was measured by toe-pinch withdrawal and was within the mild-moderate range, similar across experiments and balanced within experiments comparing different fear conditioning protocols. For experiment 1, injury severities were 31.3 ± 22.8 sec (apnea) and 290.4 ± 101.1 sec (toe pinch), with injury input averaging 2.94 ± 0.36 atm. For experiment 2, injury severities averaged 19.1 ± 10.6 sec (apnea) and 314.9 ± 89.9 sec (toe pinch), with injury input of 1.81 ± 0.2 atm. Rats were weighed daily for one week after surgery to monitor recovery.
Fear conditioning and behavior
To determine chronic effects of TBI on fear learning and expression, behavioral testing began 4 weeks following FPI. Because of the long period between manipulations, all groups were handled daily for 4 days and received transport habituation for 2 days prior to behavioral testing to reduce the stress of leaving the vivarium prior to behavioral testing. For transport habituation, rats were transported from the vivarium to the behavioral testing room for 10 min and returned to the vivarium. Training and auditory cue testing occurred in two distinct conditioning chambers (context A and context B) that differed in transport mode, location, odor, lighting, chamber shape, and flooring (Med Associates Inc., Georgia, VT, USA). For context A, animals were transported from the vivarium on a cart in their home cage, testing room and chamber lights were illuminated, and the test chamber had flat standard shock grid floors and was scented and cleaned with 50% Windex. For context B, animals were transported in an opaque black tub to a testing room and test chamber that had lights off, smooth Plexiglas floors, curved back wall inserts, and was cleaned and scented with 1% acetic acid. Percent time freezing to auditory stimuli and context were recorded as measures of auditory cued and contextual fear, respectively. Auditory stimuli were produced by Med Associates speakers mounted to the chamber. The frequency range for white noise was 10 Hz to 20 kHz broadband white noise. Behavioral testing protocols differed slightly depending on the goals of the experiments, as outlined below.
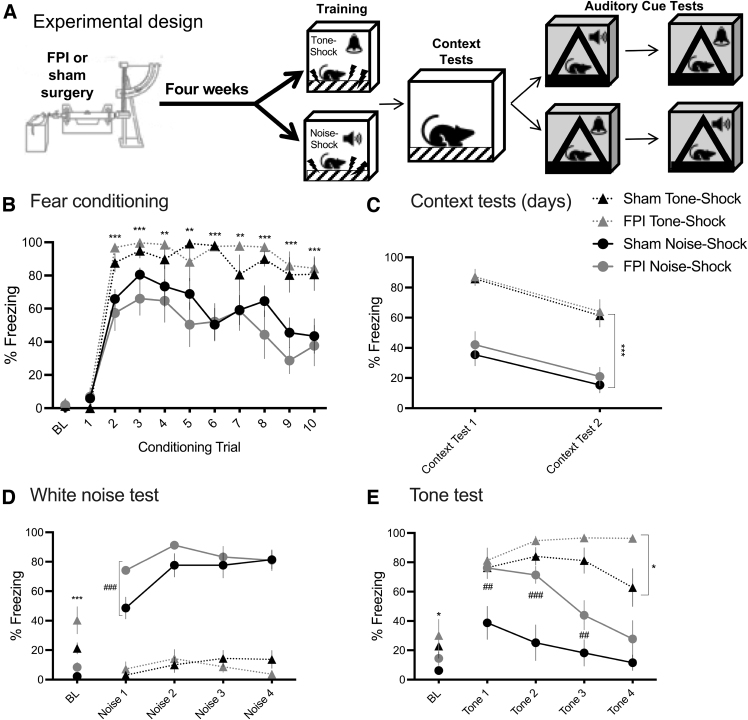
Experiment 1 examined the chronic effects of FPI on fear conditioning to different auditory stimuli. Where CS refers to the conditional stimulus and US refers to the unconditional stimulus, following a 180-sec baseline period, rats received 10 CS-US pairings (30 sec/75 dB CS; 2 sec/0.9 mA footshock US) with 210-sec fixed inter-trial-interval in context A. Auditory CSs were either white noise (Noise Shock) or 2800 Hz pure tone (Tone Shock) of the same intensity (75 dB). Over the following 2 days, all groups were tested for context fear and extinction (20 min/day, context A). All groups were then tested for fear expression to both their trained and untrained auditory cue in a novel context (4 trials each [30 sec]; context B). Stimulus cue testing order was counterbalanced over 2 days (see Fig. 1A).
FIG. 1.
Stimulus-specific fear conditioning leads to increased freezing to novel cue chronically after FPI. (A) Experimental design; n = 8–9/group. (B) Noise-shock or tone-shock fear conditioning 4 weeks after lateral FPI. Increased freezing in tone-shock versus noise-shock fear conditioning, regardless of FPI (***p < 0.001, **p < 0.01 for Tone Shock vs. Noise Shock group). (C) Increased context freezing in Tone Shock groups during context tests (***p < 0.001 Tone Shock vs. Noise Shock). Both groups decreased freezing to context across 2 days of testing. (D) White noise cue test. Tone Shock groups showed elevated baseline freezing (***p < 0.001 Tone Shock vs. Noise Shock). Noise Shock groups showed greater freezing to their trained white noise cue compared with Tone Shock groups, and FPI-Noise Shock animals showed significantly higher freezing to the white noise versus Sham-Noise Shock (##p < 0.01, FPI-Noise Shock vs. Sham-Noise Shock). (E) Tone cue test. Again, Tone Shock groups showed higher baseline freezing compared with Noise Shock groups (*p < 0.05 Tone Shock vs. Noise Shock). A significant three-way interaction showed that FPI-Tone Shock animals showed greater freezing to the trained tone versus Sham-Tone Shock animals during the tone test. Also, FPI-Noise Shock animals showed robust freezing to the untrained, novel tone during the first two trials (##p < 0.01, #p < 0.05 FPI-Noise Shock vs. Sham-Noise Shock). All data represented as mean ± SEM. BL, baseline; FPI, fluid percussion injury; SEM, standard error of the mean.
Experiment 2 Associative generalization versus non-associative sensitization
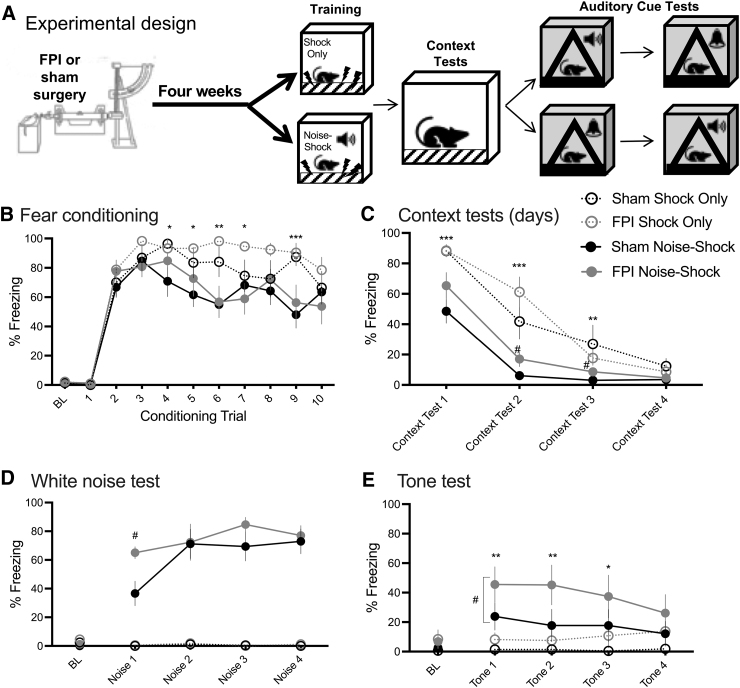
Here we sought to determine whether FPI in the chronic phase affects fear expression as generalization to other stimuli and/or sensitization as non-associative reactivity to novel stimuli, both responses of which are reflective of heightened defense. FPI and Sham groups were fear conditioned with either white noise-shock (Noise Shock) or an unsignaled footshocks (Shock Only) training protocol. Noise-shock fear conditioning was identical to experiment 1 (10 trials; 30 sec/75 dB CS; 2 sec/0.9 mA footshock US), and the Shock Only conditioning protocol was the same but with the auditory CSs removed. In this experiment, all groups received 4 days of 30-min context tests to produce full context extinction. Groups were then tested for percent freezing to both white noise (75 dB) and pure tone (2800 Hz/75 dB) cues in a novel context (4 trials each [30 sec]; context B). Therefore, the Noise Shock group was tested with their trained CS (white noise) and a generalization stimulus (tone), whereas both auditory stimuli were novel for the Shock Only group. Stimulus test order was counterbalanced across 2 days (see Fig. 2A).
FIG. 2.
Increased fear generalization to novel cue chronically after FPI. (A) Experimental design; n = 7–10/group. (B) Noise shock or shock only fear conditioning 4 weeks after lateral FPI. Increased freezing in shock only versus noise-shock fear conditioning, regardless of FPI (***p < 0.001, **p < 0.01, *p < 0.05 for Shock Only vs. Noise Shock group). (C) Increased context freezing in Shock Only groups during context tests (***p < 0.001, **p < 0.01 Shock Only vs. Noise Shock). Both groups decreased freezing to under 10% in the conditioning context across 4 days of testing. FPI-Noise Shock groups showed increased freezing to the conditioning context on test days 2 and 3 compared with Sham-Noise Shock (#p < 0.05 FPI-Noise Shock vs. Sham-Noise Shock). (D) White noise cue test. FPI-Noise Shock animals showed significantly higher freezing to their trained white noise cue on trial 1 versus Sham-Noise Shock (#p < 0.05, FPI-Noise Shock vs. Sham-Noise Shock). Shock Only groups did not show freezing behavior to the novel white noise cue. (E) Tone test. Noise Shock groups showed more freezing to the novel tone than Shock Only groups (**p < 0.01, *p < 0.05 Noise Shock vs. Shock Only). FPI-Noise Shock animals froze significantly more to the tone than Sham-Noise Shock (#p < 0.05 FPI-Noise Shock vs. Sham-Noise Shock), suggesting FPI increased fear generalization in the chronic phase after injury. All data represented as mean ± SEM. BL, baseline; FPI, fluid percussion injury; SEM, standard error of the mean.
Tissue collection
Two days after the last auditory cue test, animals in experiment 2 were anesthetized with isoflurane and brains were rapidly removed. Basolateral amygdala (BLA) units were immediately microdissected and frozen separately on dry ice, then stored at −80°C until analyzed. Tissue samples were prepared using the Syn-PER Reagent (ThermoFisher) and manufacturers protocol to isolate BLA-enriched synaptic fractions. Briefly, tissue was homogenized in Syn-PER reagent with a pestle homogenizer. Samples were then spun in a refrigerated centrifuge at 1200g for 10 min at 4°C to spin down large cellular debris. The supernatant was then collected in a fresh tube and spun at 15,000g for 20 min at 4C°. The supernatant was discarded, and the remaining pellet was resuspended in Syn-PER reagent and stored at −80°C as the enriched synaptic fraction for further analysis.
Western blot and analysis
Western blots were performed using a standard protocol in our laboratory.34,36,37 Total protein for each sample was determined with a Pierce BCA Protein Assay Kit. Due to the limitation of lanes per gel (n = 24), groups were divided between two blots, whereas one control group (Sham-Shock Only) was replicated across both blots. Blot 1 was loaded with Shock Only groups (Sham-Shock Only, FPI-Shock Only), blot 2 was loaded with Noise Shock groups (Sham-Noise Shock, FPI-Noise Shock) and a replicate of Sham-Shock Only. Due to the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAr) subunits (GluA1 and GluA2) both being near 110 kDA, this pattern was duplicated in third and fourth blots. Samples were counterbalanced across lanes. Normalized protein was pseudorandomly loaded at a concentration of 0.33 mg/mL on 10% Tris-HCl gels and run at 160 V for 50 min. Proteins were then transferred from the gel to a nitrocellulose membrane at 0.4 amps for 120 min.
Total protein was imaged on a Bio-Rad imager with filter using SYPRO™ Ruby Protein Stain solution (Bio-Rad Laboratories, Inc.). The membrane was washed then blocked in 5% milk for 60 min. Primary antibody (GluA1, 1:5000, Abcam, AB31232; GluA2, 1:5000, Millipore Sigma, MABN1189) was added and incubated overnight at 4°C. Blots were washed and incubated in secondary antibody (1:10 k) in 1% milk buffer solution. Blots were washed and protein bands were developed using Bio-Rad ECL and exposed for 1 to 5 min on a Bio-Rad MP imager. Raw values for the proteins of interest were normalized to total protein within each lane/sample. Synaptic protein enrichment and antibody specificity was validated for BLA tissue and are illustrated in Supplementary Figure S1.
Behavioral measures
Freezing behavior, an active defensive response, was measured as an index of fear and defined as complete cessation of movement except that required for respiration.38–40 Percent time freezing to auditory stimuli and context were recorded and measured by an automated system calibrated to a highly trained observer (MSF; VideoFreeze, Med Associates).
Statistical analysis
Behavioral data (n = 7–10/group) were analyzed using mixed factors analysis of variance (ANOVA) for injury group (Sham, FPI) and training protocol (Shock Only, Noise-Shock) across trials. Western blot data were analyzed by one-way ANOVA across groups and blots. Pearson correlations were performed for western blot data and freezing during the first trials of the tone generalization test. Statistical significance was determined at a p-value of 0.05 or less, and when significant interactions were detected, post hoc analyses were performed for simple main effects and multiple comparisons (western blot).
Results
Experiment 1: Stimulus-specific fear conditioning in the chronic phase after FPI
There were no differences between groups in freezing at pre-shock baseline (injury factor, F[1,29] = 1.549, p > 0.1; conditional stimulus factor, F[1,29] = 0.771, p > 0.1). A mixed factors ANOVA for conditioning group and injury across 10 fear conditioning trials revealed a significant trial × conditional stimulus interaction (F[9,261] = 3.843, p < 0.001), indicating differences in freezing between Tone Shock and Noise Shock groups depended on trial, regardless of injury 4 weeks prior. Post hoc analyses showed that Tone Shock groups showed higher levels of freezing across fear conditioning trials (trials 2–10; p < 0.01, Fig. 1B).
Over the next 2 days all rats were placed back into the training context (context A) with no other stimuli for 20 min and tested for context fear, measured by percent time freezing in the context. All groups decreased freezing from context test 1 to context test 2 (F[1,29] = 81.277, p < 0.001), indicating context fear extinction (Fig. 1C). We also saw a significant main effect of conditional stimulus group, showing that Tone Shock groups regardless of FPI had higher amounts of freezing compared with the Noise Shock groups (F[1,29] = 55.115, p < 0.001; Fig. 1C).
Across stimulus test days, all groups were placed in a novel context (context B) and tested for freezing to the trained or novel auditory cue (4 × 30 sec presentations of tone or white noise), with test stimulus order counterbalanced across groups. Both tests occurred in the same context, which was distinct from the conditioning context. For both tests, baseline freezing showed that Tone Shock groups had significantly higher levels of freezing relative to the Noise Shock conditioning group (noise test baseline freezing: F[1,29] = 16.82, p < 0.001; Fig. 1D; tone test baseline freezing: F[1,29] = 7.148, p < 0.05; Fig. 1E).
In the noise test (Fig. 1D), Noise Shock groups showed more freezing during noise trials compared with animals that were trained with Tone Shock (F[1,29] = 250.707, p < 0.001). A mixed factors ANOVA for conditioning group and injury across the four noise trials revealed both a significant trial × injury interaction (F[3,87] = 5.75, p < 0.001) as well as a trial × conditioning group interaction (F[3,87] = 4.245, p < 0.01). Post hoc analyses for simple main effects showed that the FPI-Noise Shock group showed greater freezing on noise trial 1 compared with the Sham-Noise Shock group (t[14] = 3.439, p < 0.01; Fig. 1C).
In the tone test (Fig. 1E), we found a significant trial × conditioning group × injury three-way interaction (F[3,87] = 3.793, p < 0.05), where the effect of conditioning group on freezing to tone was dependent on injury and trial. Post hoc analyses revealed that within Tone Shock groups, FPI-Tone Shock showed higher and persistent freezing levels to the trained tone during trial 4 (p < 0.05; Fig. 1D). We also found that when the animals were trained with white noise, FPI-Noise Shock animals showed robust freezing to the novel tone during the first two tone trials (Sham-Noise Shock vs. FPI-Noise Shock for freezing to tone, trial 1: p < 0.01; trial 2: p < 0.001; trial 3: p < 0.05; Fig. 1E).
The main findings from experiment 1 are that although overall tone-shock fear conditioning leads to increased levels of freezing, FPI-Noise Shock animals show increased freezing to both the conditioned (white noise) and novel auditory stimulus (pure tone) relative to uninjured controls (Sham). Group sizes for experiment 1 were Sham-Tone Shock n = 9, Sham-Noise Shock n = 8, FPI-Tone Shock n = 8, and FPI-Noise Shock n = 8.
Experiment 2: Associative or non-associative fear as generalization or sensitization chronically after FPI
In experiment 1, we found that when white noise-shock fear conditioning occurred 4 weeks after FPI, injured animals displayed robust freezing to both the trained (75 dB white noise) and a novel, untrained cue (75 dB, 2800 Hz tone). It is unclear whether the elevated level of freezing to the novel tone was an effect of stimulus generalization, which would be reliant on associative mechanisms based on similarity of stimulus modality between the trained and novel cue. An alternative explanation is that the enhanced defensive response to the novel stimulus arose through sensitization, a non-associative mechanism whereby the aversive experience (fear conditioning) led to elevated defensive response to any novel, intense external stimulus or change in the environment.41 Either way, the enhanced defense response to a novel cue may be considered inappropriate and maladaptive, reflecting increased fear and hyperarousal observed in anxiety- and stress-related disorders.42 However, the nature of the difference may source from divergent neurobiological mechanisms, which will be important to understand in developing approaches to clinical management. To test whether the enhanced defense response to a novel cue in the chronic phase after FPI is due to stimulus generalization or sensitization, 4 weeks after FPI or sham surgery, rats were fear conditioned with either white noise paired with shock (the same protocol from experiment 1) or shock only conditioning with no auditory cue. All groups were then tested for freezing to cues of 75-dB white noise or 2800-Hz tone.
There were no group differences in freezing at baseline prior to the fear conditioning trials. To analyze acquisition, we measured the percent time freezing during each 30-sec presentation of the white noise stimulus for the Noise Shock groups or the equivalent 30-sec period in the context prior to each shock for the Shock Only groups. During acquisition, we found a significant trial × conditioning group interaction (F[9,261] = 2.153, p < 0.05), indicating that regardless of FPI there were different levels of freezing across trials depending on the conditioning protocol. Post hoc analyses showed that Shock Only groups showed higher levels of freezing compared with the Noise Shock groups during trials 4–7 and 9 (p < 0.05; Fig. 2B). Freezing in the Shock Only group occurs because of conditioning to contextual cues.43
In experiment 1, we saw reductions in freezing across two 20-min context tests; however, we did find elevated and significant group differences in baseline freezing during the auditory cue tests in a new context. Differences in baseline freezing may influence the defensive response recruited in reaction to the stimulus cue presentation,44 or occlude group and stimulus interactions. Because of the elevated freezing at baseline during the cue test sessions, in this experiment we extinguished freezing to context for all groups prior to the cue tests in a new context. We analyzed contextual fear as percent time freezing in the first 10 min of each session to avoid false scoring of inactivity or sleeping as freezing. Freezing to the context was below 10% for all groups by four 30-min sessions of context testing. A mixed factors ANOVA for conditioning group and injury across the 4 days of context tests showed that all groups reduced freezing in the conditioning context across test days (F[3,87] = 148.856, p < 0.001), and a significant time × conditioning group interaction (F[3,87] = 9.686, p < 0.001), where pairwise post hoc tests showed that Shock Only groups had higher freezing than Noise Shock groups initially on test days 1–3 (p < 0.01; Fig. 2C).
We also found a significant time × injury interaction (F[3,87] = 2.919, p < 0.05). Post hoc pairwise tests showed increased context freezing in the FPI groups during test day 2 compared with sham groups (p = 0.05, Fig. 2C), an FPI enhanced fear effect similar to our previous findings acutely after FPI.21,22 Because we did not observe significant increases in contextual fear under the same conditions for the FPI-Noise Shock group in experiment 1, we speculate that the effect of FPI on noise-shock paired conditioning is not as robust in the chronic phase as in our earlier studies after acute FPI.
Following extinction of freezing to the conditioning context, there were no group differences in baseline freezing in the novel context prior to auditory cue presentation for either tone or white noise test.
During the white noise cue test, we found a significant trial × conditioning group × injury three-way interaction for freezing during the noise presentations (F[3,87] = 3.845, p < 0.05). This revealed that the FPI-Noise Shock group had significantly higher freezing during the first noise presentation compared with the Sham-Noise Shock group (p < 0.001), indicating a robust elevation in defensive behavior to the cue associated with the aversive event. Shock Only groups did not show any freezing to the novel white noise cues during this test.
During the tone test we found a significant trial × conditioning group interaction (F[3,87] = 2.838, p < 0.05) where Noise Shock groups showed more freezing to the novel tone during the first three tone trials (p < 0.05; Fig. 2E), indicating some stimulus generalization. Interestingly, we also found a significant main effect of injury, showing that FPI groups were freezing more to the novel tone than sham controls (F[1,29] = 6.568, p < 0.05), suggesting that FPI groups showed increased freezing overall to the novel tone. However, when analyzed separately, FPI-Noise Shock groups had significantly increased freezing to the novel tone compared with Sham-Noise Shock groups (F[1,15] = 5.822, p < 0.05), whereas there was no significant difference between Shock Only groups (FPI vs. Sham: F[1,14] = 1.022, p > 0.1). These data support the hypothesis that in the chronic phase after TBI, enhanced freezing to a novel stimulus reflects fear stimulus generalization rather than sensitization.
The major takeaways from experiment 2 are that like in experiment 1, FPI-Noise Shock animals show increased freezing to both the trained (white noise) and novel (pure tone) stimulus, compared with both Sham-Noise Shock and Shock Only groups, indicating fear generalization and not sensitization. Group sizes for experiment 2 were Sham-Shock Only n = 7, Sham-Noise Shock n = 10, FPI-Shock Only n = 9, and FPI-Noise Shock n = 7.
Experiment 2: Chronic FPI alters AMPAr subunits in the BLA
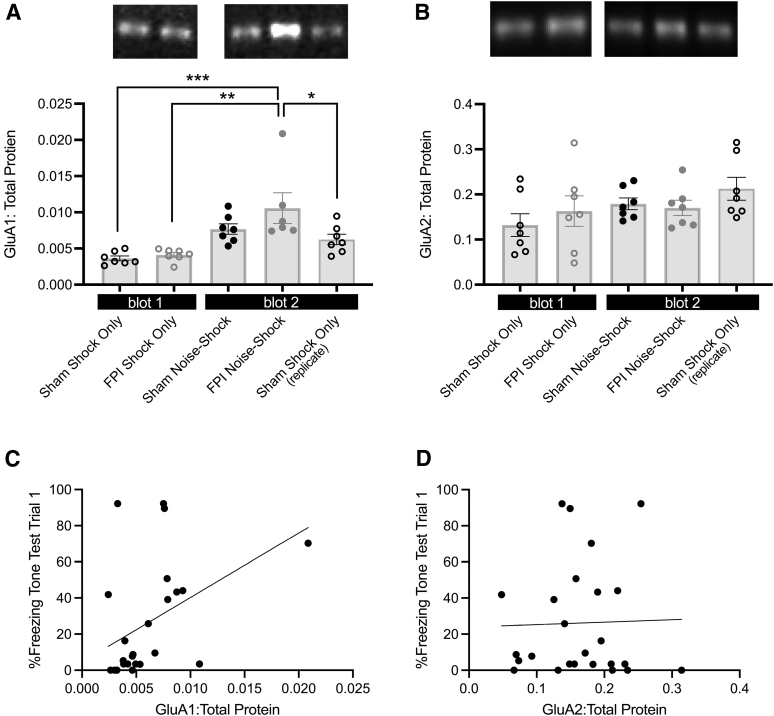
At the completion of behavioral testing in experiment 2, we analyzed BLA tissue ipsilateral to the FPI or craniotomy to determine synaptic levels of AMPAr subunits (GluA1 and GluA2). AMPArs that are homomeric with GluA1 subunits are calcium permeable, whereas the AMPAr heteromeric GluA1-GluA2 subunit composition is calcium impermeable.45 Higher levels of GluA1 protein provide indirect evidence for the potential of increased plasticity at those synapses. Due to the limited number of lanes per gel, groups were divided between two blots with one control group (Sham-Shock Only) replicated across both blots. Normalized GluA1:total protein and GluA2:total protein were analyzed by one-way ANOVA for groups, including the control replicate (Sham-Shock Only, FPI-Shock Only, Sham Noise-Shock, FPI-Noise Shock, and Sham-Shock Only replicate). Group sizes for western blot samples were n = 6–7/group based on optimal samples across gel lane capacity. There was a significant effect of group for GluA1 (F[4,33] = 7.931, p < 0.001; Fig. 3A), but not GluA2 (F[4,34] = 1.463, p > 0.1; Fig. 3B). Bonferroni corrected post hoc comparisons revealed that BLA GluA1 was significantly increased in FPI-Noise Shock compared with Sham-Shock Only (blot 1; p < 0.001), FPI-Shock Only (p < 0.001), and Sham-Shock Only (blot 2; p < 0.05; Fig. 3A).
FIG. 3.
Fear generalization in chronic TBI is associated with increased synaptic GluA1 in the BLA. (A) Synaptic GluA1 is significantly elevated in FPI-Noise Shock groups compared with groups that received shock only conditioning. (*p < 0.05, **p < 0.01, ***p < 0.001; top: representative blot images). (B) There were no differences among groups for synaptic GluA2 in the BLA (top: representative blot images). (C) Significant correlation between BLA GluA1 and freezing during the tone test. (D) No relationship between freezing during the tone test and BLA GluA2 levels. (A,B) Data are represented as mean ± SEM. BLA, basolateral amygdala; FPI, fluid percussion injury; SEM, standard error of the mean; TBI, traumatic brain injury.
To determine the relationship between BLA AMPAr subunits and fear generalization, Pearson correlations were performed for BLA GluA1 and GluA2 with percent time freezing during the generalization tone test (trial 1). Correlations revealed a significant positive relationship between BLA GluA1 and freezing during the tone test (r = 0.564, p < 0.01; Fig. 3C). There was no significant relationship with BLA GluA2 and tone test freezing (r = 0.28, p > 0.1; Fig. 3D). These data suggest a possible contribution of BLA GluA1 in chronic TBI and fear generalization.
Additional analyses: Chronic phonophobia phenotype after FPI
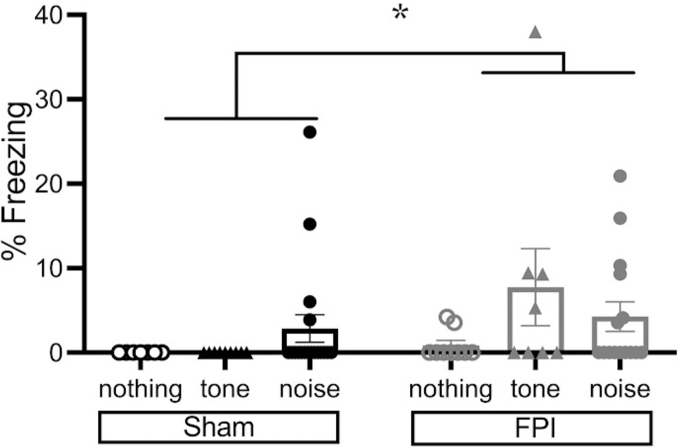
Although the purpose of the study was to investigate how FPI animals differ following different fear learning protocols in defensive freezing behavior to a novel auditory cue, we were also interested whether FPI animals exhibit a phonophobia-like phenotype 4 weeks after injury. We have shown stimulus-elicited defensive behavior (freezing) 48 h after FPI when rats were exposed to 75-dB white noise alone as well as 2800-Hz tones albeit to a lesser degree, whereas sham controls are unbothered by either.21,35 FPI led to freezing to white noise alone and engaged auditory fear neurocircuitry within the auditory thalamus-amygdala pathway that led to robust enhanced fear following noise-shock fear conditioning. Whereas this phenotype is more pronounced during the inter-stimulus-intervals, it is also significant during the stimulus presentation itself. Therefore, we wanted to take a closer look at our data to determine whether either auditory stimulus elicited defensive freezing in FPI groups during the first trial of fear conditioning (or the equivalent 30-sec period in the Shock Only group), prior to the first footshock.
A two-factor ANOVA for injury and stimulus showed a marginal effect of injury (F[1,60] = 3.743, p = 0.058); however, Levene's test for equal variances was statistically significant indicating unequal variances across conditions (F = 4.489, p < 0.05). Therefore, we conducted the non-parametric Mann-Whitney U test for the injury factor and found a significant effect of injury (p < 0.05; Fig. 4). One third of the FPI animals (7/21) showed freezing of at least 9% in the first auditory trial (tone and noise combined). Whereas 2/18 sham rats displayed freezing above 9% to the first trial of white noise, no sham rats showed freezing to the 2800-Hz tone. We also looked at whether freezing during the first tone or white noise trial of fear conditioning correlated with amount of freezing during the generalization tone test or was related to injury severity metrics in FPI groups, but these relationships were not significant (p > 0.1). These data suggest that the auditory sensitivity effects observed after FPI may be long-lasting in some animals, which may contribute to subsequent enhanced fear learning and a heightened defensive phenotype and will be the basis of future investigation.
FIG. 4.
Phonophobia phenotype in chronic FPI. During the first trial of either tone or white noise fear conditioning (or the equivalent time period for Shock Only groups) prior to the first footshock, we observed a significant proportion of FPI groups showing increased freezing defensive behavior to an auditory stimulus (*p < 0.05, Mann-Whitney U test for unequal variances). Group data are represented as mean ± SEM. FPI, fluid percussion injury; SEM, standard error of the mean.
Discussion
This study investigated the chronic effects of TBI on fear learning and expression to both trained and novel stimuli. We found that when fear conditioning occurred 4 weeks after lateral FPI, adult male rats that were trained with either white noise-shock or tone-shock paired fear conditioning displayed enhanced fear to their trained cue relative to sham controls. Additionally, when tested with the alternative cue, the FPI Noise-Shock group displayed robust fear to the 2800-Hz pure tone, suggesting inappropriate fear to an untrained, novel auditory stimulus. To determine whether this effect was due to associative (generalization) or non-associative (sensitization) factors, we tested fear expression to a novel pure tone following either white noise-shock paired or unsignaled footshocks (shock only) 4 weeks after FPI. We again saw enhanced fear to the trained noise cue in the FPI Noise-Shock group compared with sham, and enhanced fear to the novel pure tone.
During the auditory cue tests, we saw little freezing behavior in groups that were fear conditioned with shock only to either stimulus, confirming that the enhanced fear to a novel tone in the FPI Noise-Shock group reflects fear generalization rather than sensitization. We also looked at protein expression levels of synaptic AMPAr subunits in the BLA and found increased levels of BLA GluA1, but not GluA2 in the FPI Noise-Shock group that showed the highest level of fear during cue testing. BLA GluA1 levels positively correlated with fear generalization. Additional analyses revealed a greater proportion of animals that had FPI displayed an increased defensive response during the first trial of auditory fear conditioning, prior to the first shock. This effect may suggest lasting sensitivity to auditory stimuli in chronic TBI; however, this did not appear to contribute to the amount of fear generalization after conditioning. Findings from our study indicate a lasting injury-induced increase in auditory stimulus generalization, a unique phenotype following diffuse TBI. This novel finding illustrates both a learning deficit and increased fear in the chronic phase after TBI, where stimulus generalization may underlie maladaptive fear and hyperarousal common to anxiety-and stress-related comorbidities.
Heightened defensive behavior in chronic TBI
The current study expands on a growing body of work supporting increased defensive behaviors after TBI. Our lab has shown that lateral FPI leads to enhanced fear when fear conditioning occurred within the first days after injury in adult rats.21,22,35 Our current data support a lasting effect of TBI, enhancing fear behavior when the stressor (fear conditioning) occurred in the chronic phase after injury. Other studies have found long-lasting enhancement in defensive freezing after fear conditioning and TBI. When fear conditioning occurred 3 weeks following controlled cortical impact (CCI) in mice, even following successful fear extinction CCI mice have shown strong resurgence of fear several weeks later.46 Additionally, studies that used repetitive blast injury have shown increased freezing in injured groups when fear conditioning occurred 4–9 months after injury.47–49
These findings have clinical implications for the lasting effects of TBI on PTSD development. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, a clinical PTSD diagnosis cannot be made until at least 6 months following a traumatic event,50 and TBI has been shown to be strongly associated with PTSD in U.S. service members months after deployment in Iraq and Afghanistan.4 We also observed persistent freezing across tone test trials in the FPI-Tone Shock group in experiment 1 (Fig. 1E), whereas other groups show reduction in freezing across trials. This observation is related to impaired fear extinction after TBI,51,52 with clinical relevance to challenges and resistance in fear reduction during exposure therapy. Although the current study did not assess baseline differences in anxiety-like behavior, FPI changes in anxiety levels in the chronic phase53 may also influence fear learning and freezing behavior. Our data support lasting effects of brain trauma on defensive and hyperarousal phenotypes after TBI in the rat.
Our prior work suggests that noise sensitivity reflected an acute physical symptom of the injury that we found engaged auditory fear networks and exerted an influence on subsequent fear learning.21 In the current study, when we looked at freezing during the first auditory trial in the current experiments 4 weeks after FPI, we saw that a significant proportion of FPI animals exhibited freezing to both 75-dB tone and white noise. Whether the increased amount of freezing to novel auditory stimuli in FPI animals 4 weeks following the injury reflects a persistent physical phonophobia symptom or a defensive reaction to novelty54 is still unknown. This question plagues the clinical field regarding how to properly treat and disentangle the symptoms attributed to a history of TBI or a psychiatric diagnosis when the conditions are comorbid.55 In a military study on TBI and PTSD comorbidity, an interesting finding is that at least 5 months after deployment post-concussive symptoms were increased in those with comorbid PTSD.56 It will be important to continue this work to better understand the lasting effects of TBI on physical symptoms and mental health.
Stimulus specificity of white noise on freezing behavior
Across two experiments, we replicated that chronically after FPI, auditory fear conditioning (white noise-shock) increased fear generalization to a novel tone. The difference in magnitude of fear generalization between experiments 1 and 2 (Fig. 1E vs. Fig. 2E) is likely due to the amount of extinction to the conditioning context. In human studies, fear generalization increases with respect to increasing fear intensity.57 Without full extinction to the conditioning context, during the baseline period in the novel context we see contextual fear generalization in the Tone Shock groups, which was even more pronounced in the FPI group (Fig. 1D,E).
However, even in the case where contextual fear was fully extinguished and context fear did not generalize to a novel context (Fig. 2D,E, baseline), FPI-Noise Shock groups showed robust fear to the novel tone. This finding is akin to a recent clinical study in patients with anxiety that found that in the patients, emotional stimuli shaped sensory representations during conditioning, which altered these representations later to result in less stimulus discrimination, even in a safe context.13 Fear generalization can be modulated by external factors including type and intensity of stimuli in the aversive experience,58 or perhaps history of TBI such as in the current study. A study in active duty military personnel reported that TBI was associated with an increased response to threat cues and more PTSD symptoms.59 Therefore, how the injured brain encoded the noise-shock association during conditioning may have shaped the representation of the novel tone during the generalization test.
An important point to consider regarding the stimuli used in our study is that white noise encompasses all audible frequencies, and when groups were fear conditioned with white noise, stimulus generalization occurred to a novel 2800-Hz tone. This effect was most pronounced and robust in the FPI-Noise Shock groups, where levels of freezing were similar to the level of the trained cue (Fig. 1E). However, in experiment 1, for both Tone Shock groups there was no apparent generalization when the novel cue was white noise (Fig. 1D). Therefore, the acquired auditory fear memory was easier to transfer from white noise to 2800 Hz, but not the other way around. Because white noise contains 2800-Hz frequency as part of its makeup, one explanation is that the frequency was more strongly encoded in chronically after TBI. One may speculate that the observed TBI fear stimulus generalization may reflect an encoding deficit reflecting poor pattern separation between trained and untrained stimuli upon retrieval, which is often associated with hippocampal damage.60 An alternative interpretation is that all frequencies were conditioned in the Noise Shock groups, where both sham and FPI groups showed some generalization, but to a substantially greater magnitude for FPI groups. We did not test other alternative frequencies, but it will be important in future studies to determine the degree of generalization that this heightened defensive phenotype manifests across a frequency gradient.
Maladaptive fear after TBI is associated with increased excitatory-related proteins in the amygdala
We found that chronic TBI enhanced fear generalization was associated with elevated synaptic GluA1 protein in the BLA. We have reported structural changes in the amygdala by dendritic hypertrophy in BLA principal neurons lasting at least 28 days after injury.27 Further, others have shown altered glutamatergic signaling in the amygdala chronically after FPI.61 AMPArs are the primary mediators of fast excitatory transmission. GluA1-containing homomers (GluA2-lacking AMPArs) are calcium-permeable and facilitate synaptic transmission.45 It is interesting that we found a specific increase in only GluA1, but not GluA2 in the BLA in the FPI-Noise Shock group that was associated with maladaptive fear generalization. In a well-established model of traumatic stress that causes lasting fear sensitization known as stress-enhanced fear learning, GluA1 but not GluA2 in the BLA is also increased and that increase is dependent on stress hormones during the traumatic event.62
We recently reported a significant decrease in BLA GluA2 48 h after FPI,37 which may contribute to interactive TBI effects on excitatory transmission during subsequent stressors. Other studies have reported increased BLA GluA1 following stress,63 which facilitates maladaptive associative learning.64 The synaptic protein analysis in the current study included tissue from BLA ipsilateral to the injury. Recently, we observed bilateral effects across different excitatory- and inhibitory-related proteins in the BLA, most of which returned to control levels within one week.37 Some reports suggest there may be lateralized effects of the amygdala on fear learning behaviors in rodents65,66 as well as in humans.67,68 It will be important in future studies to tease apart the effects of amygdala laterality and lateralized effects of TBI on neural circuits and corresponding behavior. Taken together, our study and others support the hypothesis that TBI and stress interactions affect amygdala function that may facilitate the encoding of subsequent traumatic events and increase the susceptibility of hyperarousal and chronic stress-related comorbidities.28,29
The current study revealed that heightened levels of fear after FPI were associated with synaptic protein changes in the amygdala, a known regulator of fear and negative valence processing. Whereas GluA1 in the BLA has shown to be a player in enhanced fear expression across our studies and studies discussed above, other synaptic proteins and brain regions across fear and memory circuitry may play a role in fear generalization and hyperarousal after TBI. Other excitatory-related proteins including the balance of NMDA (N-methyl-D-aspartate) receptors, synaptophysin, calmodulin, and/or cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signaling could not only influence neuronal signaling, but also have an influence on enhanced activity in the BLA, which could in turn affect behavior and fear generalization.69–71 Further, TBI has wide ranging effects across neural circuits involved in fear, anxiety, and learning and memory. A balance between excitatory and inhibitory signaling across the amygdala, prefrontal and insular cortex, hippocampus, and thalamic areas is important for adaptive fear responses while an imbalance across these regions is implicated in fear generalization.72,73 TBI and/or stress may influence activity across these nodes in fear neurocircuitry and lead to sensitized21,74 or blunted activity75,76 to influence downstream circuits including the hypothalamic-pituitary-adrenal (HPA) axis8 and processing of stressful events.
Conclusions
Understanding the effects of significant stress after TBI is an important area of ongoing research. This was the first study to show chronic effects of FPI leading to enhanced auditory fear generalization, which was associated with increased excitatory-related proteins in the amygdala. Our study has important clinical implications for the lasting effects of TBI on subsequent traumatic stress exposure and stress-related symptom development. We also open the question that long term after TBI, increased defensive reactions to sounds may reflect long-lasting auditory sensitivity or increased hyperarousal to novelty. In future studies it will be important to disentangle the chronic physical symptoms after a history of TBI from an elevated baseline defense state for both sexes, and their underlying mechanisms to provide effective treatments for the heterogenous TBI patient population.
Supplementary Material
Acknowledgments
The authors thank Yan Cai, Sima Ghavim, and Ellen Hsieh for their technical assistance with this project.
Authors' Contributions
The authors contributed as follows: AH: conceptualization (lead); design (lead); investigation (lead); visualization (lead) and writing-original draft (lead); SW: investigation (lead), visualization (supporting) and writing-review and editing (supporting); NC: investigation (supporting); writing-review and editing (supporting); JL: investigation (supporting); writing-review and editing (supporting); DH: conceptualization (lead); resources (lead); funding acquisition (lead); writing-review and editing (supporting); CG: conceptualization (lead); resources (lead); funding acquisition (lead); writing-review and editing (supporting); MF: conceptualization (lead); design (lead); resources (lead); funding acquisition (lead); writing-review and editing (supporting).
Funding Information
This work was supported in part by F32NS098694: AH; NARSAD Young Investigator Grant: AH; R01MH062122: MSF; UCLA Depression Grand Challenge Fellowship Fund: MSF; Staglin Center for Brain and Behavioral Health: MSF; UCLA Brain Injury Research Center; 1R01NS27544: DH, CG; UCLA Steve Tisch BrainSPORT: CG, and Easton Labs for Brain Health: CG.
Author Disclosure Statement
No conflicting financial interests exist related to this project. AH and CG have received past research funding from Avanir Pharmaceuticals. MSF is director of research for Neurovation Labs. CG receives consulting fees from NFL-Neurological Care Program, NHLPA, and serves on the advisory panel for the Major League Soccer, NBA, and U.S. Soccer Federation. CG received consultation fees and stock options for serving on the medical board of Highmark International and has received book royalties from Blackwell Publishing (Prioritized Neurological Differential Diagnosis).
Supplementary Material
References
- 1. Mallya S, Sutherland J, Pongracic S, et al. The manifestation of anxiety disorders after traumatic brain injury: a review. J Neurotrauma 2015;32(7):411–421; doi: 10.1089/neu.2014.3504. [DOI] [PubMed] [Google Scholar]
- 2. Scholten AC, Haagsma JA, Cnossen MC, et al. Prevalence of and risk factors for anxiety and depressive disorders after traumatic brain injury: a systematic review. J Neurotrauma 2016;33(22):1969–1994; doi: 10.1089/neu.2015.4252. [DOI] [PubMed] [Google Scholar]
- 3. Moore EL, Terryberry-Spohr L, Hope DA. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj 2006;20(2):117–132; doi: 10.1080/02699050500443558. [DOI] [PubMed] [Google Scholar]
- 4. Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med 2008;358(5):453–463; doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 5. McAllister TW, Arciniegas D. Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation 2002;17(4):265–283. [PubMed] [Google Scholar]
- 6. Rosenbaum PE, Locandro C, Chrisman SPD, et al. Characteristics of pediatric mild traumatic brain injury and recovery in a concussion clinic population. JAMA Netw Open 2020;3(11):e2021463; doi: 10.1001/jamanetworkopen.2020.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol 2005;27(8):977–1021; doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman AN, Taylor AN. Stress reactivity after traumatic brain injury: implications for comorbid post-traumatic stress disorder. Behav Pharmacol 2019;30(2 and 3-Spec Issue)115–121; doi: 10.1097/FBP.0000000000000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fanselow MS, Lester LS. A Functional Behavioristic Approach to Aversively Motivated Behavior: Predatory Imminence as a Determinant of the Topography of Defensive Behavior. In: Evolution and Learning. (Bolles RC, Beecher MD. eds.) Erlbaum: HIllsdale, NJ; 1988; pp. 185–211. [Google Scholar]
- 10. Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model for fear and pain. Behav Brain Sci 1980;3:291–323. [Google Scholar]
- 11. Dunsmoor JE, Paz R. Fear generalization and anxiety: behavioral and neural mechanisms. Biological psychiatry 2015;78(5):336–343; doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 12. Lissek S, Rabin S, Heller RE, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry 2010;167(1):47–55; doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laufer O, Israeli D, Paz R. Behavioral and neural mechanisms of overgeneralization in anxiety. Curr Biol 2016;26(6):713–722; doi: 10.1016/j.cub.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 14. Fullana MA, Dunsmoor JE, Schruers KRJ, et al. Human fear conditioning: from neuroscience to the clinic. Behav Res Ther 2020;124:103528; doi: 10.1016/j.brat.2019.103528. [DOI] [PubMed] [Google Scholar]
- 15. Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol 2006;73(1):39–48; doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 16. Craske MG. Anxiety Disorders: Psychological Approaches to Theory and Treatment. Westview: Boulder, CO; 1999. [Google Scholar]
- 17. Hermans D, Craske MG, Mineka S, et al. Extinction in human fear conditioning. Biol Psychiatry 2006;60(4):361–368; doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 18. Landon J, Shepherd D, Stuart S, et al. Hearing every footstep: noise sensitivity in individuals following traumatic brain injury. Neuropsychol Rehabil 2012;22(3):391–407; doi: 10.1080/09602011.2011.652496. [DOI] [PubMed] [Google Scholar]
- 19. Callahan ML, Binder LM, O'Neil ME, et al. Sensory sensitivity in Operation Enduring Freedom/Operation Iraqi Freedom veterans with and without blast exposure and mild traumatic brain injury. Appl Neuropsychol Adult 2018;25(2):126–136; doi: 10.1080/23279095.2016.1261867. [DOI] [PubMed] [Google Scholar]
- 20. Kostelnik C, Lucki I, Choi KH, et al. Translational relevance of fear conditioning in rodent models of mild traumatic brain injury. Neurosci Biobehav Rev 2021;127:365–376; doi: 10.1016/j.neubiorev.2021.04.037. [DOI] [PubMed] [Google Scholar]
- 21. Hoffman AN, Lam J, Hovda DA, et al. Sensory sensitivity as a link between concussive traumatic brain injury and PTSD. Sci Rep 2019;9(1):13841; doi: 10.1038/s41598-019-50312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reger ML, Poulos AM, Buen F, et al. Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol Psychiatry 2012;71(4):335–343; doi: 10.1016/j.biopsych.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson L, Stewart W, Dams-O'Connor K, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 2017;16(10):813–825; doi: 10.1016/S1474-4422(17)30279-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016;124(2):511–526; doi: 10.3171/2015.2.JNS14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bryant RA, Harvey AG. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J Nerv Ment Dis 1999;187(5):302–305; doi: 10.1097/00005053-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 26. Sariaslan A, Sharp DJ, D'Onofrio BM, et al. Long-term outcomes associated with traumatic brain injury in childhood and adolescence: a nationwide Swedish cohort study of a wide range of medical and social outcomes. PLoS Med 2016;13(8):e1002103; doi: 10.1371/journal.pmed.1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoffman AN, Paode PR, May HG, et al. Early and persistent dendritic hypertrophy in the basolateral amygdala following experimental diffuse traumatic brain injury. J Neurotrauma 2017;34(1):213–219; doi: 10.1089/neu.2015.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weis CN, Webb EK, deRoon-Cassini TA, et al. Emotion dysregulation following trauma: shared neurocircuitry of traumatic brain injury and trauma-related psychiatric disorders. Biol Psychiatry 2022;91(5):470–477; doi: 10.1016/j.biopsych.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCorkle TA, Barson JR, Raghupathi R. A role for the amygdala in impairments of affective behaviors following mild traumatic brain injury. Front Behav Neurosci 2021;15:601275; doi: 10.3389/fnbeh.2021.601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tate DF, Wade BS, Velez CS, et al. Volumetric and shape analyses of subcortical structures in United States service members with mild traumatic brain injury. J Neurol 2016;263(10):2065–2079; doi: 10.1007/s00415-016-8236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frost RB, Farrer TJ, Primosch M, et al. Prevalence of traumatic brain injury in the general adult population: a meta-analysis. Neuroepidemiology 2013;40(3):154–159; doi: 10.1159/000343275. [DOI] [PubMed] [Google Scholar]
- 32. Fineman I, Giza CC, Nahed BV, et al. Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J Neurotrauma 2000;17(9):739–749. [DOI] [PubMed] [Google Scholar]
- 33. Ip EY, Giza CC, Griesbach GS, et al. Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J Neurotrauma 2002;19(5):573–585; doi: 10.1089/089771502753754055. [DOI] [PubMed] [Google Scholar]
- 34. Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience 2004;128(2):305–322; doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 35. Hoffman AN, Watson SL, Makridis AS, et al. Sex differences in behavioral sensitivities after traumatic brain injury. Front Neurol 2020;11(553190); doi: 10.3389/fneur.2020.553190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sta Maria NS, Reger ML, Cai Y, et al. D-cycloserine restores experience-dependent neuroplasticity after traumatic brain injury in the developing rat brain. J Neurotrauma 2017;34(8):1692–1702; doi: 10.1089/neu.2016.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffman AN, Watson S, Fanselow MS, et al. Region-dependent modulation of neural plasticity in limbic structures early after traumatic brain injury. Neurotrauma Rep 2021;2(1):200–213; doi: 10.1089/neur.2020.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fanselow MS. Opiate modulation of the active and inactive components of the postshock reaction: parallels between naloxone pretreatment and shock intensity. Behav Neurosci 1984;98(2):269–277. [DOI] [PubMed] [Google Scholar]
- 39. Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol 1979;93(4):736–744. [DOI] [PubMed] [Google Scholar]
- 40. Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol 1969;67(3):370–375. [DOI] [PubMed] [Google Scholar]
- 41. Haddad AD, Pritchett D, Lissek S, et al. Trait anxiety and fear responses to safety cues: stimulus generalization or sensitization? J Psychopathol Behav Assess 2012;34:323–331. [Google Scholar]
- 42. Lissek S, Grillon C. Overgeneralization of conditioned fear in the anxiety disorders Zeitschrift fur Psychologie/J Psychol 2010;218(2):146–148; doi: 10.1027/0044-3409/a000022. [DOI] [Google Scholar]
- 43. Fanselow MS. Signaled shock-free periods and preference for signaled shock. J Exp Psychol: Animal Behav Processes 1980;6(1):65–80. [Google Scholar]
- 44. Fanselow MS, Hoffman AN, Zhuravka I. Timing and the transition between modes in the defensive behavior system. Behav Processes 2019;166:103890; doi: 10.1016/j.beproc.2019.103890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA—gated glutamate receptor channels depends on subunit composition. Science 1991;252(5007):851–853; doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 46. Corne R, Leconte C, Ouradou M, et al. Spontaneous resurgence of conditioned fear weeks after successful extinction in brain injured mice. Prog Neuropsychopharmacol Biol Psychiatry 2019;88:276–286; doi: 10.1016/j.pnpbp.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 47. Elder GA, Dorr NP, De Gasperi R, et al. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J Neurotrauma 2012;29(16):2564–2575; doi: 10.1089/neu.2012.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perez-Garcia G, De Gasperi R, Gama Sosa MA, et al. PTSD-related behavioral traits in a rat model of blast-induced mTBI are reversed by the mGluR2/3 receptor antagonist BCI-838. eNeuro 2018;5(1); doi: 10.1523/ENEURO.0357-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perez-Garcia G, Gama Sosa MA, De Gasperi R, et al. Blast-induced "PTSD": evidence from an animal model. Neuropharmacology 2019;145(Pt B):220–229; doi: 10.1016/j.neuropharm.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 50. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Publishing: Arlington, VA; 2013. [Google Scholar]
- 51. Davies DR, Olson D, Meyer DL, et al. Mild traumatic brain injury with social defeat stress alters anxiety, contextual fear extinction, and limbic monoamines in adult rats. Front Behav Neurosci 2016;10:71; doi: 10.3389/fnbeh.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao J, Huynh J, Hylin MJ, et al. Mild traumatic brain injury reduces spine density of projection neurons in the medial prefrontal cortex and impairs extinction of contextual fear memory. J Neurotrauma 2018;35(1):149–156; doi: 10.1089/neu.2016.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jones NC, Cardamone L, Williams JP, et al. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma 2008;25(11):1367–1374; doi: 10.1089/neu.2008.0641. [DOI] [PubMed] [Google Scholar]
- 54. Perusini JN, Fanselow MS. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem 2015;22(9):417–425; doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McAllister TW. Psychopharmacological issues in the treatment of TBI and PTSD. Clin Neuropsychol 2009;23(8):1338–1367; doi: 10.1080/13854040903277289. [DOI] [PubMed] [Google Scholar]
- 56. Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol 2008;167(12):1446–1452; doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- 57. Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learn Mem 2009;16(7):460–469; doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem 2004;81(3):162–166; doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 59. Glenn DE, Acheson DT, Geyer MA, et al. Fear learning alterations after traumatic brain injury and their role in development of posttraumatic stress symptoms. Depress Anxiety 2017;34(8):723–733; doi: 10.1002/da.22642. [DOI] [PubMed] [Google Scholar]
- 60. Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci 2011;34(10):515–525; doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Beitchman JA, Griffiths DR, Hur Y, et al. Experimental traumatic brain injury induces chronic glutamatergic dysfunction in amygdala circuitry known to regulate anxiety-like behavior. Front Neurosci 2019;13:1434; doi: 10.3389/fnins.2019.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perusini JN, Meyer EM, Long VA, et al. Induction and expression of fear sensitization caused by acute traumatic stress. Neuropsychopharmacology 2016;41(1):45–57; doi: 10.1038/npp.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuniishi H, Yamada D, Wada K, et al. Stress induces insertion of calcium-permeable AMPA receptors in the OFC-BLA synapse and modulates emotional behaviours in mice. Transl Psychiatry 2020;10(1):154; doi: 10.1038/s41398-020-0837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cai YQ, Wang W, Hou YY, et al. Central amygdala GluA1 facilitates associative learning of opioid reward. J Neurosci 2013;33(4):1577–1588; doi: 10.1523/JNEUROSCI.1749-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 2001;8(3):148–155; doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci 2004;118(1):15–23; doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- 67. Hardee JE, Thompson JC, Puce A. The left amygdala knows fear: laterality in the amygdala response to fearful eyes. Soc Cogn Affect Neurosci 2008;3(1):47–54; doi: 10.1093/scan/nsn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Braem S, De Houwer J, Demanet J, et al. Pattern analyses reveal separate experience-based fear memories in the human right amygdala. J Neurosci 2017;37(34):8116–8130; doi: 10.1523/JNEUROSCI.0908-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 2012;35(1):24–35; doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Krabbe S, Grundemann J, Luthi A. Amygdala inhibitory circuits regulate associative fear conditioning. Biol Psychiatry 2018;83(10):800–809; doi: 10.1016/j.biopsych.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 71. Ghosh S, Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci 2015;18(1):112–120; doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- 72. Asok A, Kandel ER, Rayman JB. The neurobiology of fear generalization. Front Behav Neurosci 2018;12:329; doi: 10.3389/fnbeh.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pollack GA, Bezek JL, Lee SH, et al. Cued fear memory generalization increases over time. Learn Mem 2018;25(7):298–308; doi: 10.1101/lm.047555.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ghosh S, Laxmi TR, Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J Neurosci 2013;33(17):7234–7244; doi: 10.1523/JNEUROSCI.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tapp ZM, Cornelius S, Oberster A, et al. Sleep fragmentation engages stress-responsive circuitry, enhances inflammation and compromises hippocampal function following traumatic brain injury. Exp Neurol 2022;353:114058; doi: 10.1016/j.expneurol.2022.114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McKlveen JM, Moloney RD, Scheimann JR, et al. "Braking" the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry 2019;86(9):669–681; doi: 10.1016/j.biopsych.2019.04.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.