Abstract
Nosocomial infections that result in the formation of biofilms on the surfaces of biomedical implants are a leading cause of sepsis and are often associated with colonization of the implants by Staphylococcus epidermidis. Biofilm formation is thought to require two sequential steps: adhesion of cells to a solid substrate followed by cell-cell adhesion, creating multiple layers of cells. Intercellular adhesion requires the polysaccharide intercellular adhesin (PIA), which is composed of linear β-1,6-linked glucosaminylglycans and can be synthesized in vitro from UDP-N-acetylglucosamine by products of the intercellular adhesion (ica) locus. We have investigated a variety of Staphylococcus aureus strains and find that all strains tested contain the ica locus and that several can form biofilms in vitro. Sequence comparison with the S. epidermidis ica genes revealed 59 to 78% amino acid identity. Deletion of the ica locus results in a loss of the ability to form biofilms, produce PIA, or mediate N-acetylglucosaminyltransferase activity in vitro. Cross-species hybridization experiments revealed the presence of icaA in several other Staphylococcus species, suggesting that cell-cell adhesion and the potential to form biofilms is conserved within this genus.
Chronic nosocomial infections by gram-positive bacteria have become more prevalent in recent years with the increased use of prosthetic biomedical implants. Chronic infection of a prosthetic implant can serve as a septic focus that can lead to osteomyelytis, acute sepsis, and death, particularly in immunocompromised patients (5, 12). Bacteria colonize prosthetic implants as a biofilm, multiple layers of sessile cells that adhere to the implant surface as well as to each other. Once a biofilm has formed, it can be very difficult to treat clinically because the bacteria on the interior of the biofilm are well protected from the host immune response as well as antibiotic agents (16).
Biofilm formation is thought to be a two-step process that requires the adhesion of bacteria to a substrate surface followed by cell-cell adhesion, forming the multiple layers of the biofilm (13, 14, 30). This latter process is associated with the polysaccharide intercellular adhesin (PIA), which is composed of linear β-1,6-linked glucosaminylglycans in Staphylococcus epidermidis (22). The intercellular adhesion (ica) locus, icaADB and C, was identified and shown to mediate cell-cell adhesion and PIA production in S. epidermidis (15). It was further demonstrated that icaA and icaD together mediate the synthesis of sugar oligomers in vitro, using UDP-N-acetylglucosamine as a substrate. This N-acetylglucosaminyltransferase activity together with the activity of icaC produces a product in vitro that is recognized by an antibody raised against PIA (8).
The organism most frequently isolated in association with certain types of infections related to biomedical implants, including central venous catheters, cerebrospinal fluid shunts, prosthetic heart valves, and ocular lens implants, is S. epidermidis (2, 5, 6, 18, 24). Staphylococcus aureus is more frequently isolated in association with peripheral intravascular catheters, endotracheal and tracheotomy tubing, peritoneal dialysis tubing, and corneal infections related to contact lens wear (2, 7, 24, 31, 37, 38). Coagulase-negative staphylococci, primarily S. epidermidis, and S. aureus are isolated in approximately equal numbers in association with prosthetic joint and vascular graft infections (18, 35); however, the infections that are associated with S. aureus represent a more serious clinical hazard due to the higher morbidity and mortality associated with this organism compared to those of S. epidermidis.
We set out to investigate whether S. aureus can form biofilms in vitro, and if so, whether it is able to mediate cell-cell adhesion and PIA synthesis via the ica locus. We found not only that the function of the ica locus is conserved between S. epidermidis and S. aureus but also that the ica locus is present in several other Staphylococcus species as well, implying that the cell-cell adhesion function mediated by this locus may be conserved within this genus.
MATERIALS AND METHODS
Staphylococcus strains.
Most of the strains used for this work are listed in Tables 1 and 2 along with national strain collection reference numbers where applicable. S. epidermidis O-47 is a clinical isolate (13), and strain 5179 is a biofilm- and PIA-negative strain (23). Staphylococcus carnosus TM300 is a wild-type plasmid and cloning host strain (9, 33). Bacteria were cultured under standard conditions in B medium (1% tryptone [Gibco BRL Life-Technologies GmbH, Eggenstein, Germany], 0.5% yeast extract [Gibco BRL], 0.5% NaCl, 0.1% K2HPO4, 0.1% glucose) or Luria-Bertani medium (1% tryptone [Gibco BRL], 0.5% yeast extract, 0.5% NaCl) except as noted below. Media were supplemented when appropriate with chloramphenicol (10 μg/ml), tetracycline (10 μg/ml), or ampicillin (100 μg/ml) except where otherwise noted.
TABLE 1.
Strains used in this work
| Organism | Strain collection no.
|
Common name | Origin, other information | Wella | |
|---|---|---|---|---|---|
| ATCC | NCTC, DSM | ||||
| S. aureus | ATCC 35556 | SA113 | Derived from NCTC 8325, restriction deficient | 1a | |
| ATCC 25904 | NCTC 10833 | Newman D2C | Clumping factor-positive variant of Newman D2C | 1b | |
| ATCC 49832 | 3A | Produces restriction endonuclease Sau3AI | 1c | ||
| ATCC 12600 | NCTC 8532 | Type Strain | Pleural fluid | 1d | |
| DSM 20232 | Copenhagen | Cell wall teichoic acid | 1e | ||
| ATCC 12601 | NCTC 6131 | 2a | |||
| RN 4220 | Derived from 8325-4, restriction deficient | 2b | |||
| ATCC 10832 | Wood 46 | FDA, produces dermatoxin | 2c | ||
| ATCC 12598 | NCTC 8530 | Cowan 1 | Septic arthritis | 2d | |
| ATCC 31153 | NCTC 8178 | Newman | Throat swab, produces clumping factor | 2e | |
| S. epidermidis | ATCC 35984 | RP62A | Catheter sepsis | 3b | |
| O-47 | Clinical isolate | 3c | |||
| S. carnosus | TM300 | Plasmid host | 3d | ||
Corresponding well in Fig. 1.
TABLE 2.
Staphylococcus species used in this study and presence of icaA
| Species | Hybridization | Stock center no. | Name |
|---|---|---|---|
| S. aureus | ++++ | ATCC 35556 | SA113 |
| S. auricularis | +++ | ATCC 33753 | |
| S. capitis capitis | +++ | CCM 2734 | |
| S. carnosus | − | TM300 | |
| S. caseolyticus | − | ||
| S. cohnii cohnii | − | CCM 2736 | |
| S. epidermidis | ++++ | ATCC 35984 | |
| S. gallinarum | − | Tü3928/47 | |
| S. haemolyticus | − | CCM 2737 | |
| S. hominis | − | DSM 20328 | |
| S. hyicus hyicus | − | NCTC 10350 | |
| S. intermedius | ++ | CCM 5739 | |
| S. lentus | − | ||
| S. lugdunensis | ++ | ATCC 43809 | |
| S. pasteuri | + | ATCC 51129 | |
| S. piscifermentans | ++ | SK02 | |
| S. saprophyticus | − | NT219 | |
| S. sciuri | − | SC116 | |
| S. schleiferi schleiferi | − | ATCC 43808 | |
| S. simulans | − | ATCC 27848 | |
| S. warneri | − | ||
| S. xylosus | − | C2a |
Hybridization probes were specific to icaA from both S. aureus and S. epidermidis. ++++, signal with hybridization at 60°C; +++, signal with hybridization at 50°C; ++, signal with hybridization at 40°C; +, weak signal with hybridization at 40°C; −, no signal at 40, 50, or 60°C.
Biofilm assay.
Bacteria were grown overnight in tryptic soy broth (TSB; Gibco BRL) supplemented with 0.25% glucose. Cultures were then diluted 1:200 and incubated overnight in stationary U-bottom well polystyrol microtiter plates (Greiner Labortechnik, Frickenhausen, Germany) at 37°C. Microtiter wells were washed twice with phosphate-buffered saline (7 mM Na2HPO4, 3 mM NaH2PO4, 130 mM NaCl [pH 7.4]), dried in an inverted position, and stained with 0.1% safranin (Serva Feinbiochemica GmbH & Co. KG, Heidelberg, Germany) (13).
PIA detection.
PIA production in S. aureus was detected, with modifications, as described by Gerke et al. (8). Briefly, cells were grown overnight in TSB supplemented with 0.25% glucose, the optical density was determined, and the same number of cells (2 to 4 ml) from each culture was resuspended in 50 μl of 0.5 M EDTA (pH 8.0). Cells were then incubated for 5 min at 100°C and centrifuged to pellet the cells, and 40 μl of the supernatant was incubated with 10 μl of proteinase K (20 mg/ml; Boehringer GmbH, Mannheim, Germany) for 30 min at 37°C. After addition of 10 μl of Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl [pH 7.4]) containing 0.01% bromphenol blue, 4 μl was spotted on a nitrocellulose filter, dried, blocked with 3% bovine serum albumin, and incubated overnight with an anti-S. epidermidis PIA antibody (gift from D. Mack, Hamburg, Germany) (23) absorbed as described by Gerke et al. (8) and diluted 1:5,000. Bound antibodies were detected with an absorbed biotin-conjugated anti-rabbit immunoglobulin G (IgG) antibody (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) diluted 1:5,000, horseradish peroxidase-conjugated streptavidin (Amersham Buchler GmbH & Co. KG, Braunschweig, Germany) diluted 1:3,000, and the Amersham ECL (enhanced chemiluminescence) Western blotting system.
N-Acetylglucosaminyltransferase assay.
Crude membranes were prepared by disrupting cells with glass beads as described previously (8). Protein concentrations were determined by the method of Bradford (3). The basis for the N-acetylglucosaminyltransferase assays has been described previously (8). Assays with crude membranes from plasmid-bearing S. carnosus were performed with 5 mg of protein per ml incubated with 2 mM UDP-N-acetylglucosamine (10 μM 14C labeled) and 4 μM dithiothreitol. For assays with crude membranes from S. epidermidis and S. aureus strains, conditions were modified to 10 μM UDP-N-acetylglucosamine (all 14C labeled), 0.4 μM dithiothreitol, and a protein concentration of 1 mg/ml. Products were analyzed by thin-layer chromatography (NH2-HPTLC plates; Merck, Darmstadt, Germany), 1 μl each, using acetonitrile-water (65:35, vol/vol). N-Acetylglucosamine (10 Bq) was used as a reference compound. Fuji HR-E30 film was exposed for 12 weeks.
DNA blots.
Chromosomal DNA was prepared, with modifications, by the method of Marmur (25) as follows. Bacteria were grown to an optical density at 578 nm of 1 to 2 and washed in 5× Tris-EDTA. Cells were then resuspended in 100 μl of 5× Tris-EDTA, 50 μl of lysostaphin (0.5 mg/ml; Sigma), and 1 μl of RNase A (20 mg/ml; Boehringer) and incubated at 37°C until viscous. After cell lysis, 150 μl of 2% sodium dodecyl sulfate was added, the mixture incubated for 5 min at 37°C, and 50 μl of proteinase K (20 mg/ml) added for at last 30 min. After incubation for 5 min at room temperature with 150 μl of 5 M NaClO4, the mixture was extracted with phenol-chloroform-isoamyl alcohol (25:24:1) (Merck) and precipitated with an equal volume isopropanol. DNA digests and Southern blotting were performed by standard methods (32). Prehybridization and hybridization were performed with DIG (digoxigenin) Easy Hyb solution (Boehringer) at 60, 50, and 40°C. Washes were performed in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate at 50°C. DIG-labeled probes were made by using PCR, DIG nucleotide labeling mix (Boehringer), and Taq polymerase (AGS, Heidelberg, Germany) as recommended by the manufacturers, using a MiniCycler PTC-150 (MJ Research, Inc., Watertown, Mass.).
Operon identification.
The existence of an ica operon in S. aureus was postulated based on the ability to form an in vitro biofilm similar to that of S. epidermidis. A search of public nucleotide sequence data libraries using the S. epidermidis ica gene sequences (15) revealed no significant homologies to the ica locus in S. aureus. The genes were detected, however, with Pathoseq, S. aureus contig sequence information available from a commercial provider (Incyte Pharmaceuticals, Palo Alto, Calif.). The S. aureus clones showing homologies to S. epidermidis icaA to icaC were SAU1c0610, SAU1c0627, SAU1c0071, SAU1c0377, SAU1c0511, and SAU1c0012. Due to the sequence errors caused by single-read shotgun sequencing and gaps due to missing or incomplete contigs, it was necessary to subclone and resequence the S. aureus ica locus. The Pathoseq information was used to design PCR primer SA12 (see below) and some sequencing primers.
PCR amplification, cloning, and sequencing.
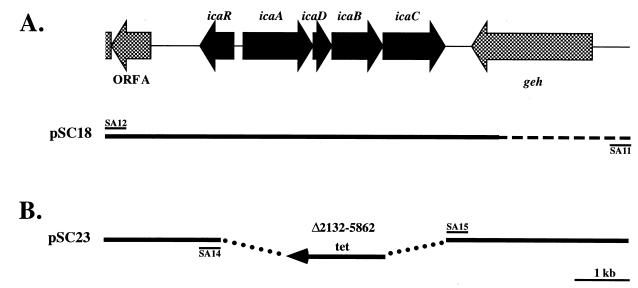
Chromosomal DNA from S. aureus ATCC 35556 was amplified via PCR using primers SA11 and SA12 and the Expand Long Template PCR System (Boehringer) as recommended by the manufacturer and cloned into the KpnI site of shuttle vector pBT5, a derivative of pBT2 lacking the EcoRI restriction site in the multiple cloning site (4), creating plasmid pSC18. Primers SA14 and SA15 were used to amplify plasmid pSC18, which deleted nucleotides 2132 to 5862 (mid-icaR through icaC). The tetracycline resistance cassette from pT181mcs (1) was then ligated by using XhoI restriction sites into the deleted region, creating plasmid pSC23. Cloning was performed in Escherichia coli DH5α. Portions of pSC18 and chromosomal DNA from S. aureus ATCC 35556 were sequenced by using a LI-COR DNA sequencer Long Readir 4200 (Lincoln Corporation, Inc., Lincoln, Neb.). Computer sequence analysis was performed with MacDNASIS Pro (Hitachi Software Engineering, San Bruno, Calif.). Enzymes used for cloning were purchased from Gibco BRL, Boehringer, or New England Biolabs GmbH (Schwalbach, Germany). Primers were obtained from MWG-Biotech (Ebersberg, Germany) or Interactiva (Ulm, Germany). The primers used for DNA amplification in S. aureus were SA11 (CGGGGTACCTGCAGGATGGTCATTATGAGTGC), SA12 (AGGGGTACCGAGCTCGCTAATAGGTGACTTTGG), SA14 (ATTTCTCGAGAAGGGGTATGACGGTACAAC), and SA15 (GTAAATGCTCGAGGGAGTGGGACAGAAA). The primers used to amplify the tetracycline resistance cassette from plasmid pT181mcs were tet-2 (GAGCTCGAGTGGCAAAATGCTAGCCAC) and tet-6 (GCGCTCGAGTTCGCCAGCGATTAACGGA). The KpnI restriction site at the beginning of the sequence indicated by a solid line in Fig. 2A is a cloning artifact introduced via the primer used for PCR amplification and is not found at this position on the chromosome. Plasmids were transformed into staphylococci via protoplast transformation (10, 11) or electroporation (21).
FIG. 2.
Map of the ica locus and surrounding sequence in S. aureus ATCC 35556. (A) Genomic organization of the ica locus and surrounding chromosomal region. The region between primers SA12 and SA11 was amplified by PCR and cloned into vector pBT5, creating plasmid pSC18. The solid line indicates the region sequenced and submitted to the EMBL/GenBank/DDBJ nucleotide sequence data libraries; the dashed line indicates previously published sequence (database accession no. M90693). (B) Schematic of pSC23 diagramming the knockout construct. Plasmid pSC18 was amplified by inverse PCR and primers SA14 and SA15, which deleted the sequence between the middle of icaR and the end of icaC. The tetracycline resistance cassette from pT181 (tet) was then ligated into the deleted ica gene locus.
Homologous recombination.
Wild-type S. aureus ATCC 35556 containing plasmid pSC23 was grown overnight in B medium at 30°C with chloramphenicol (10 μg/ml), diluted 1:1,000 and grown again at 30°C with antibiotic selection, diluted 1:1,000 and grown at 42°C without antibiotic selection twice, diluted 1:100, and plated on TSB plates containing tetracycline (2.5 μg/ml). Homologous recombination and plasmid curing of chloramphenicol-sensitive, tetracycline-resistant colonies were then confirmed by PCR and Southern blotting.
Nucleotide sequence accession number.
The sequence indicated by a solid line in Fig. 2A has been submitted to the EMBL/GenBank/DDBJ nucleotide sequence data libraries under accession no. AF086783.
RESULTS
S. aureus forms biofilms in vitro and contains the ica locus.
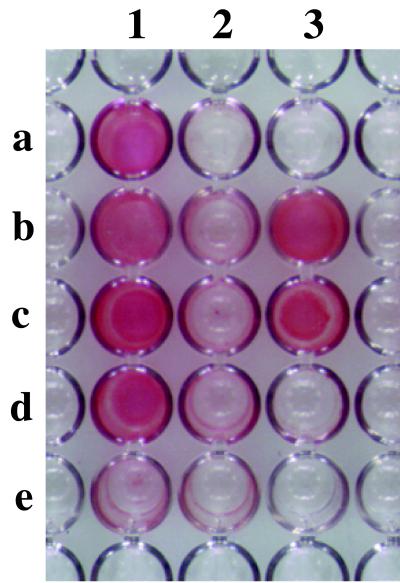
Ten commonly studied S. aureus strains, listed in Table 1, were tested for the ability to form biofilms in polystyrol microtiter plates (Fig. 1). As is often seen with heterogeneous S. epidermidis strains, some strains were able to form a strong biofilm and others formed a weak or no biofilm. Two biofilm-forming S. epidermidis strains were included for comparison (Fig. 1, wells 3b and 3c), in addition to the non-biofilm-forming S. carnosus (well 3d).
FIG. 1.
Biofilm formation in S. aureus strains. The strains listed in Table 1 were grown overnight in polystyrol microtiter wells in TSB supplemented with 0.25% glucose. The cells that adhered to the plate after washing were then visualized by staining with safranin.
All strains shown in Fig. 1 and listed in Table 1 exception those of S. carnosus contain icaA, icaD, icaB, and icaC as detected with individual gene probes on Southern blots (data not shown). As is commonly seen with S. epidermidis isolates, in vitro biofilm formation is fairly sensitive to growth conditions; for example, the addition of glucose or glucosamine to the media may be required even for “strong” biofilm-forming strains. Since several of the strains fail to form an in vitro biofilm despite the presence of the ica genes and growth conditions that allow biofilm formation in other strains, it is possible that these strains contain point mutations within the ica locus, that the production of PIA is otherwise negatively regulated, or that biofilm formation is influenced by some other, as yet unidentified factor(s). None of these strains contain insertional elements near the ica locus of a size that could be detected by PCR amplification using primers SA11 and SA12 (Fig. 2A) (data not shown) (39).
Sequence of the ica locus in S. aureus and sequence comparison with S. epidermidis.
Sequence information available from a commercial provider (Incyte Pharmaceuticals) was used to design a PCR primer, and the ica locus from S. aureus ATCC 35556 was cloned and sequenced (Fig. 2A). The organization of the locus itself is identical to that of S. epidermidis; however, other than the presence of a lipase gene downstream and in the opposite orientation, the surrounding sequence shows no similarity. The predicted gene labeled icaR, located upstream and transcribed in the opposite direction from icaA, icaD, icaB, and icaC is also present in S. epidermidis, but its function is not yet known. A sequence similarity comparison between S. aureus ATCC 35556 and S. epidermidis ATCC 35984 is shown in Table 3.
TABLE 3.
Sequence comparison of the ica locus between S. aureus and S. epidermidis
| Locusa | Size of predicted polypeptide (amino acids) | DNA | Protein
|
|
|---|---|---|---|---|
| % Identity | % Similarity | |||
| icaA | ||||
| S.a. | 412 | 76 | 78 | 89 |
| S.e. | 412 | |||
| icaD | ||||
| S.a. | 101 | 72 | 59 | 79 |
| S.e. | 101 | |||
| icaB | ||||
| S.a. | 290 | 77 | 62 | 82 |
| S.e. | 289 | |||
| icaC | ||||
| S.a. | 350 | 71 | 67 | 79 |
| S.e. | 355 | |||
| icaR | ||||
| S.a. | 186 | 89 | 65 | 83 |
| S.e. | 185 | |||
Deletion of the ica locus in S. aureus eliminates biofilm formation and PIA production.
To show that the ica locus in S. aureus is required for biofilm formation, a deletion mutant was constructed in biofilm-forming strain ATCC 35556 (Fig. 1, well 1a). The ica genes (icaADBC and the beginning of icaR) were replaced with the tetracycline resistance cassette from plasmid pT181 as diagrammed in Fig. 2B and described in more detail in Materials and Methods. This temperature-sensitive plasmid construct (pSC23) was used to replace the wild-type ica locus via homologous recombination, creating the knockout strain ATCC 35556Δica::tet.
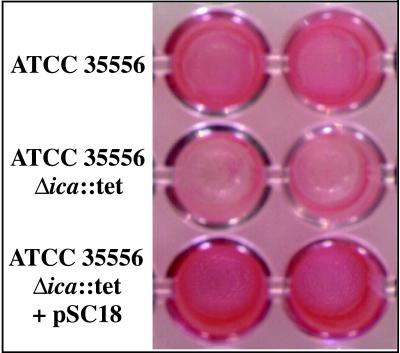
The deletion mutant was then tested for the ability to form a biofilm in vitro. As shown in Fig. 3, the ica knockout mutant is not able to form a strong biofilm compared to the wild-type parent strain; however, when the strain is complemented with plasmid pSC18, carrying the wild-type ica locus (see Fig. 2A), biofilm formation is restored. This finding demonstrates that the ica locus is required for biofilm formation in S. aureus.
FIG. 3.
Loss of biofilm formation in S. aureus ATCC 35556Δica::tet. The ica locus in S. aureus ATCC 35556 was deleted and replaced with a tetracycline resistance cassette by homologous recombination. The knockout strain, ATCC 35556Δica::tet, is unable to form a biofilm in vitro; however, the ability to form a biofilm is restored when the knockout strain is complemented with pSC18, carrying the wild type ica genes. The assay for each strain is shown in duplicate.
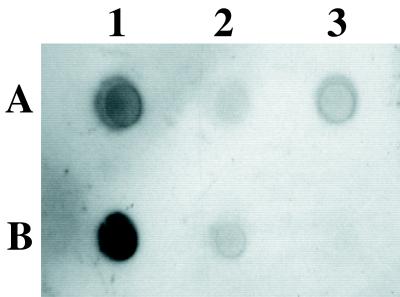
The same three strains, wild type, knockout, and complemented knockout, were then assayed for the ability to produce PIA, which is mediated by the ica locus. Figure 4 shows the production of PIA as detected with an antibody raised against S. epidermidis PIA (gift from D. Mack). Cell surface extracts were treated with proteinase K before spotting on a nitrocellulose filter to eliminate the cross-reaction of S. aureus protein A with the IgGs used for detection. The antibody was then able to recognize the remaining sugar polymer in the wild-type S. aureus ATCC 35556 (spot A1). The deletion mutant was no longer able to produce PIA, but PIA production was restored in the complemented mutant (spots A2 and A3, respectively). The faint signal remaining in the knockout (spot A2) may represent undigested protein A or another cross-reaction not affected by deletion of the ica locus. PIA-producing S. epidermidis ATCC 35984 (RP62A) and O-47 as well as the non-PIA-producing S. carnosus strain TM300 were included for comparison (spots B1, B2, and B3, respectively).
FIG. 4.
Loss of PIA production in S. aureus ATCC 35556Δica::tet. Cell surface extracts from overnight cultures of S. aureus ATCC 35556 (A1), ATCC 35556Δica::tet (A2), ATCC 35556Δica::tet carrying pSC18 (A3), S. epidermidis ATCC 35984 (RP62A) (B1), S. epidermidis O-47 (B2), and S. carnosus TM300 (B3) were treated with proteinase K, and PIA production was detected with an anti-S. epidermidis PIA antibody, showing that PIA is no longer produced in the ica knockout strain and is restored in the complemented mutant.
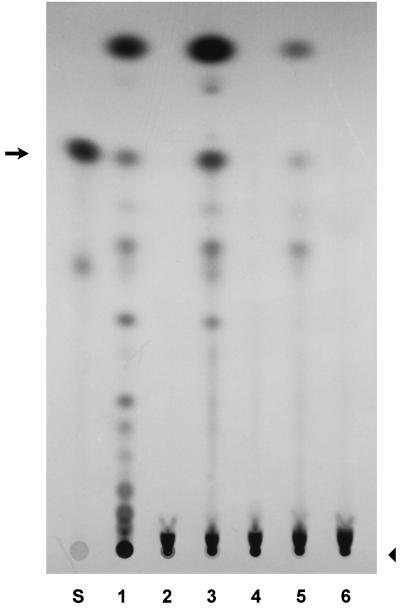
To further show that the ica genes in S. aureus mediate PIA production, we applied crude membrane extracts to an in vitro assay using UDP-N-acetylglucosamine as a substrate (8). Figure 5 shows in vitro-synthesized N-acetylglucosaminyltransferase products mediated by crude membrane extracts from S. carnosus containing a plasmid carrying the S. epidermidis ica genes, from the biofilm- and PIA-producing S. epidermidis strain ATCC 35984 (RP62A), and from the wild-type S. aureus strain ATCC 35556 (lanes 1, 3, and 5, respectively). It is significant that extracts from wild-type S. epidermidis and S. aureus strains are able to mediate detectable activity in vitro, as had previously been shown for the S. epidermidis ica genes on an inducible plasmid expressed in S. carnosus (8). The synthesis products were separated on an NH2-HPTLC plate. Negative controls were crude membrane extracts from S. carnosus carrying the vector alone corresponding to the construct in lane 1 (lane 2) and from a non-biofilm- and non-PIA-producing S. epidermidis strain, 5179 (lane 4). The S. aureus knockout strain (lane 6) did not show N-acetylglucosaminyltransferase activity in vitro, demonstrating that the ica genes are required for the synthesis of these sugar oligomers in S. aureus.
FIG. 5.
N-Acetylglucosaminyltransferase activity is mediated by the ica locus. Crude membrane extracts were incubated with radiolabeled UDP-N-acetylglucosamine, and synthesized oligomers were separated on an NH2-HPTLC plate. Lane S contains the standard, N-acetylglucosamine, alone. The remaining lanes contain products synthesized by crude membrane extracts from S. carnosus carrying pTXicaADBC, an inducible expression plasmid containing S. epidermidis icaA, icaD, icaB, and icaC (lane 1), S. carnosus harboring the vector, pTX16, alone (lane 2), S. epidermidis ATCC 35984 (RP62A), which produces PIA (lane 3), S. epidermidis 5179, a strain that does not produce PIA and does not form biofilms (lane 4), S. aureus wild-type strain ATCC 35556 (lane 5), and S. aureus ica knockout strain ATCC 35556Δica::tet (lane 6). An arrow indicates the monomer. Oligomers of increasing size are seen as a ladder-like series of spots that descend on the plate toward the origin (arrowhead) at the bottom. The smear just above the origin is unreacted UDP-N-acetylglucosamine.
The ica locus is also present in other Staphylococcus species.
Since the sequence of the ica locus is well conserved between S. epidermidis and S. aureus (Table 3), we examined whether other Staphylococcus species carry these genes. Curiously, cross-hybridization between S. aureus and S. epidermidis on DNA blots was weak (data not shown). Therefore, icaA DNA probes from both S. epidermidis and S. aureus were used simultaneously to hybridize cross-species Southern blots of decreasing stringency containing DNA from 22 different Staphylococcus species (Table 2). Cross-species hybridization was detected with S. auricularis and S. capitis, to a lesser extent with S. intermedius, S. lugdunensis, S. piscifermentans, and weakly with S. pasteuri. For those species that showed no cross-hybridization with these probes, one can conclude that an icaA homologue, however distantly related, is not detectable under these conditions, but one cannot go so far as to say that no homologue is present in the genome of these species. The result does show that the ica locus is conserved in some members of this genus, and it suggests that intercellular adhesion mediated by the ica locus may be a general phenomenon that is conserved among staphylococci.
In agreement with the presence of an icaA homolog, S. auricularis and S. capitis react weakly with the antibody raised against S. epidermidis PIA, as do S. haemolyticus and S. saprophyticus, though the latter two strains do not cross-hybridize with icaA DNA probes, and none of these species form a biofilm in vitro. Conversely, our S. simulans representative forms a very strong biofilm in vitro but does not produce a product that cross-reacts with our anti-PIA antibody. S. gallinarum, S. lentus, and S. sciuri were also able to form weak biofilms in vitro, but a PIA-like product was not detectable (data not shown).
DISCUSSION
Intercellular adhesion is conserved between S. epidermidis and S. aureus.
We have shown that there is a functional conservation of intercellular adhesion mediated by the ica locus between S. epidermidis and S. aureus. Although nosocomial infections related to certain types of biomedical implants are commonly associated with S. epidermidis, S. aureus infections occur at a high frequency in association with other types of prosthetic devices and have more serious clinical consequences due to the expression of various virulence factors and the frequent presence of genes encoding antibiotic resistance. The prevalence of S. epidermidis and other coagulase-negative staphylococci in patients with some types of biomedical implant-associated infections may be due to (i) the organisms’ increased proximity, and therefore access, to surgical incisions and/or (ii) transmission via skin contact between patients and/or hospital staff. S. aureus, in contrast to typical members of the human epidermal flora, resides predominantly in aural and nasal tissues, which are usually remote from surgical implant sites. It is likely that with better access, this species would be found even more frequently in association with all types of biomedical implant-related infections.
The ability to mediate intercellular adhesion and the formation of biofilms in both of these species is unlikely to have arisen recently in conjunction with the invention of prosthetic medical devices. Rather, it must have had a function much earlier in the evolution and survival of these organisms. The presence of the icaA gene in other Staphylococcus species also supports the notion that the locus has or had a more general function in the survival of this genus in a variety of environments.
Differences in the ability to form biofilms among related S. aureus strains.
Strains ATCC 35556 (SA113) and RN4220 (Fig. 1, wells 1a and 2b, respectively) are both derivatives of S. aureus NCTC 8325 and, in effect, cousins. Strain NCTC 8325 underwent a chemical mutagenesis to produce restriction-negative strain SA113 (ATCC 35556) (17). Strain NCTC 8325 (RN1) was also treated twice with UV light to remove three prophages, producing strain 8325-4 (RN450) (28, 29). This strain was then subjected to a chemical mutagenesis, producing the restriction-negative strain RN4220 (20). While strain SA113 is able to form a strong biofilm, neither strain 8325-4 (not shown) nor strain RN4220 shows this phenotype. Instead, the two latter strains leave a thin layer of cells on the bottom of the microtiter plate well. This same phenotype is also seen in S. epidermidis transposon-induced ica mutants (13, 15). In each case, cells are able to adhere to the substrate surface, the genetically distinct first step in biofilm formation, but are not able to build a multilayered biofilm due to a defect in cell-cell adhesion. This implies that the multiple mutageneses that separate S. aureus SA113 and its cousins, 8325-4 and RN4220, have altered the regulation or expression, either directly or indirectly, of genes required for cell-cell adhesion, and therefore biofilm formation. While RN4220 leaves only a thin layer of cells on the bottom of the microtiter plate well in a biofilm assay (Fig. 1, well 2b), RN4220 carrying pSC18 is able to form a multilayered biofilm (data not shown). In addition, RN4220 carrying plasmid pCN27, containing icaA to icaC from S. epidermidis, was reported to form a strong biofilm (26). This implies that the expression or activity of the ica genes or gene products in S. aureus RN4220 may be less than for its cousin SA113, but that a plasmid carrying the ica region is able to rescue this phenotype. Similarly, the ica knockout mutant in S. aureus ATCC 35556 described here fails to form cell clusters when grown in culture, unlike the wild-type and complemented mutant strains. Cells from all three strains are able to attach to a polystyrene surface in a primary adhesion assay, however, supporting results for S. epidermidis showing that a mutation in the ica locus affects cell-cell adhesion but does not affect adhesion to a solid substrate (13).
As can be seen in Fig. 3 and 4, the complementation of the ATCC 35556 ica knockout strain is not complete. This phenomenon might be due to an as yet uncharacterized regulatory function in the region that is included on the complementation plasmid. A correlation can, however, be seen between the level of PIA production and the thickness of the biofilm formed (compare wild-type, knockout, and complemented knockout in Fig. 3 and 4).
Presence of the ica locus in other Staphylococcus species.
The Staphylococcus species that cross-hybridized with icaA DNA probes from S. aureus and S. epidermidis were the species that are phylogenetically the most closely related. The so-called epidermidis phylogenetic group, based on DNA comparisons as well as some biochemical properties (19, 34), includes S. auricularis and S. capitis, both of which appear to carry a copy of the icaA gene. Other, more distantly related members of this group, S. haemolyticus, S. hominis, and S. warneri, failed to cross-hybridize under the conditions used. Coagulase-positive S. intermedius was so named to reflect a sequence composition that places it phylogenetically between S. aureus and S. epidermidis (27), and accordingly, this species was also able to cross-hybridize with icaA probes. The remaining three icaA-positive species, S. lugdunensis, S. pasteuri and S. piscifermentans have not been classified into any of the larger phylogenetic groupings based on sequence comparisons (19, 34, 36).
Tests on the 22 different Staphylococcus species to detect biofilm formation and PIA production were inconclusive. As seen with both S. epidermidis and S. aureus (e.g., Fig. 1), strain representatives within the same species behave very differently, and a single tested strain from each species is unlikely to be representative of the species as a whole. In addition, even if these strains produce PIA, there is no guarantee that the available antibody raised against S. epidermidis PIA can recognize sugar moieties that may be modified in a different manner, for example, deacetylated or succinated (26), in other, albeit related, species. In addition, nonspecific cross-reactions such as that seen between IgGs and protein A in S. aureus cannot be discounted. Those species that appear to carry no icaA-like gene yet are able to form a biofilm in vitro or produce a product that reacts with the anti-S. epidermidis PIA antibody may well have an entirely different and as yet unidentified mechanism mediating biofilm formation. Studies that include a larger number of representatives for each species are clearly required in order to show that functional intercellular adhesion, and not just the presence of the ica locus, occurs in Staphylococcus species other than S. epidermidis and S. aureus.
S. aureus and S. epidermidis are the gram-positive bacteria most often associated with medical implant-related infections. We have shown that both species mediate the cell-cell adhesion step of biofilm formation via the ica locus and that deletion of the ica genes eliminates the ability to produce PIA and form a biofilm in vitro. Due to the high level of morbidity and mortality associated with S. aureus infections, as well as the high frequency of infection by both organisms, the ica locus represents an important potential clinical target for the prevention of chronic infections associated with prosthetic medical devices.
ACKNOWLEDGMENTS
We thank Phuong Lan Huynh, Elisabeth Knorpp, and Ulrike Pfitzner and for photography.
S.E.C. was supported by NRSA postdoctoral fellowship AI09626 from the National Institute of Allergy and Infectious Diseases. This project was supported by grant DLR: 01KI9751/1 from the German Bundesministerium für Bildung Wissenschaft, Forschung und Technologie.
REFERENCES
- 1.Augustin J, Rosenstein R, Wieland B, Schneider U, Schnell N, Engelke G, Entian K-D, Götz F. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur J Biochem. 1992;204:1149–1154. doi: 10.1111/j.1432-1033.1992.tb16740.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker A S, Schein O D. Ocular infections. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: ASM Press; 1994. pp. 111–134. [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. doi: 10.1111/j.1574-6968.1997.tb10387.x. [DOI] [PubMed] [Google Scholar]
- 5.Christensen G D, Baldassarri L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: American Society for Microbiology; 1994. pp. 45–78. [Google Scholar]
- 6.Dickinson G, Bisno A L. Infections associated with indwelling devices: concepts of pathogenesis; infections associated with intravascular devices. Antimicrob Agents Chemother. 1989;33:597–601. doi: 10.1128/aac.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson G M, Bisno A L. Infections associated with indwelling devices: infections related to extravascular devices. Antimicrob Agents Chemother. 1989;33:602–607. doi: 10.1128/aac.33.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerke C, Kraft A, Süßmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin (PIA) J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 9.Götz F. Staphylococcus carnosus. A new host for gene cloning and protein production. Soc Appl Bacteriol Symp Ser. 1990;19:49S–53S. doi: 10.1111/j.1365-2672.1990.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 10.Götz F, Kreutz B, Schleifer K H. Protoplast transformation of Staphylococcus carnosus by plasmid DNA. Mol Gen Genet. 1983;189:340–342. [Google Scholar]
- 11.Götz F, Schumacher B. Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett. 1987;40:285–288. [Google Scholar]
- 12.Gristina A G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;23:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 13.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilmann C, Götz F. Further characterization of Staphylococcus epidermidis transposon mutants deficient in primary attachment or intercellular adhesion. Zentbl Bakteriol. 1998;287:69–83. doi: 10.1016/s0934-8840(98)80149-7. [DOI] [PubMed] [Google Scholar]
- 15.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoyle B D, Costerton J W. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 17.Iordanescu S, Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC 8325. J Gen Microbiol. 1976;96:277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- 18.Karchmer A W, Gibbons G W. Infections of prosthetic heart valves and vascular grafts. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: ASM Press; 1994. pp. 213–250. [Google Scholar]
- 19.Kloos W E. Systematics and the natural history of staphylococci. 1 Soc. Appl Bacteriol Symp Ser. 1990;19:25S–37S. doi: 10.1111/j.1365-2672.1990.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 20.Kreiswirth B N, Lofdahl S, Betley M J, O’Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee J C. Electrotransformation of staphylococci. Methods in Mol Biol. 1995;47:209–216. doi: 10.1385/0-89603-310-4:209. [DOI] [PubMed] [Google Scholar]
- 22.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maki D G. Infections caused by intravascular devices used for infusion therapy: pathogenesis, prevention, and management. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: ASM Press; 1994. pp. 155–212. [Google Scholar]
- 25.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:202–218. [Google Scholar]
- 26.McKenney D, Hübner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble W C. Systematics and the natural history of staphylococci. 2. Soc Appl Bacteriol Symp Ser. 1990;19:39S–48S. doi: 10.1111/j.1365-2672.1990.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 28.Novick R P. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 29.Novick R P, Richmond M H. Nature and interactions of the genetic elements governing penicillinase synthesis in Staphylococcus aureus. J Bacteriol. 1965;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters G, Locci R, Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentbl Bakteriol Hyg I Abt Orig Reihe B. 1981;172:293–299. [PubMed] [Google Scholar]
- 31.Robinson D L, Fowler V G, Sexton D J, Corey R G, Conlon P J. Bacterial endocarditis in hemodialysis patients. Am J Kidney Dis. 1997;30:521–524. doi: 10.1016/s0272-6386(97)90311-5. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schleifer K H, Fischer U. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int J Syst Bacteriol. 1982;32:153–156. [Google Scholar]
- 34.Schleifer K H, Kroppenstedt R M. Chemical and molecular classification of staphylococci. Soc Appl Bacteriol Symp Ser. 1990;19:9S–24S. doi: 10.1111/j.1365-2672.1990.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 35.Steckelberg J M, Osmon D R. Prosthetic joint infection. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: ASM Press; 1994. pp. 259–290. [Google Scholar]
- 36.Takahashi T, Kaneko M, Mori Y, Tsuji M, Kikuchi N, Miramune T. Phylogenetic analysis of Staphylococcus based on the 16S rDNA sequence and assignment of clinical isolates from animals. J Vet Med Sci. 1997;59:775–783. doi: 10.1292/jvms.59.775. [DOI] [PubMed] [Google Scholar]
- 37.Tiruvilauamala P, Johanson W G., Jr . Infections associated with endotracheal intubation and tracheostomy. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: ASM Press; 1994. pp. 135–154. [Google Scholar]
- 38.Vychytil A, Lorenz M, Schneider B, Horl W H, Haag-Weber M. New criteria for management of catheter infections in peritoneal dialysis patients using ultrasonography. J Am Soc Nephrol. 1998;9:290–296. doi: 10.1681/ASN.V92290. [DOI] [PubMed] [Google Scholar]
- 39.Ziebuhr W, Krimmer V, Rachid S, Lößner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 1999;32:345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]